Abstract
Granulomatosis with polyangiitis (GPA) is a rare antineutrophil cytoplasm antibody-associated vasculitis. Several therapeutic advances have occurred over the past two decades, but relapse rate remains high and refractory cases are not uncommon. Here, we present the case of a female patient diagnosed with GPA at the age of 9 years with a severe, multirelapsing disease course which failed to adequately respond to conventional therapies. Avacopan, a novel C5a receptor inhibitor, was started based on phase II studies that showed promise as a steroid-sparing adjunct. The patient was able to successfully reduce her glucocorticoid dose and reduce her immunosuppressive treatments without another flare. She has been on avacopan for 35 months, had no adverse events that required its discontinuation, and her disease is in sustained remission.
Keywords: rheumatology, vasculitis, therapeutic indications, immunology
Background
Granulomatosis with polyangiitis (GPA) is a rare antineutrophil cytoplasm antibody (ANCA)-associated vasculitis (AAV) with an incidence between 2.1 and 15 cases per million and a prevalence of 23.7–160 cases per million across European and North American populations.1–5 The mean age at diagnosis is around 40–50 years in observational cohorts, with no apparent gender predominance, but there are rare reports in children.6
GPA is typically characterised by multiorgan or system involvement including constitutional symptoms (89%), ear, nose and throat involvement (72%), pulmonary nodules or haemorrhage (65%), glomerulonephritis (53%), cutaneous manifestations (28%), peripheral neuropathy (26%) and/or ocular inflammation (22%). ANCA positivity by immunofluorescence is observed in approximately 90% of patients with systemic or diffuse GPA, and 78% of those with more limited forms, with anti-proteinase 3 (PR3) ANCA identified by ELISA in about 80% and 60%, respectively.5–8
Several advances in therapy were made over the past four decades, with up to 90% of the patients achieving initial remission in latest clinical trials. However, relapses have remained common, affecting 18%–40% in the first 2 years and over 50% by 5 years with conventional maintenance treatments such as azathioprine (AZA) with low-dose prednisone.9 Rituximab (RTX), an anti-CD20 monoclonal antibody, has achieved better results as a maintenance agent, at least compared with AZA (5% vs 29% experience major relapses at 28 months).10 Mortality rates in some clinical trials remain high at approximately 11% in the first year after diagnosis, with 60% of these deaths attributable to therapy-associated adverse events such as infection.11 Alternative, effective and safe treatment options, thus, remain needed in order to further improve outcomes, limit the use of glucocorticoids and achieve more sustained remission, including in these patients with refractory or multirelapsing disease.
Here, we describe the case of a young female patient with refractory GPA and recurrent life-threatening relapses despite therapy with various conventional pharmacologic agents. Based on the promising results of small phase II studies, avacopan, a novel C5a receptor inhibitor with potential steroid-sparing benefit, was started and improved the course of her disease.
Case presentation
A previously healthy 9-year-old girl presented in 2007 with progressive fatigue, dyspnoea, stridor, rhinitis, sinusitis, and conjunctivitis (figure 1). Initial CT scan of the chest demonstrated multiple pulmonary nodules and severe stenoses of the main bronchi, left more than right (figure 2). Urinalysis demonstrated proteinuria and hematuria with normal glomerular filtration rate. PR3-ANCA was detected in her serum by ELISA. A nasal mucosal biopsy showed granulomatous inflammation. The diagnosis of GPA was thus established.
Figure 1.
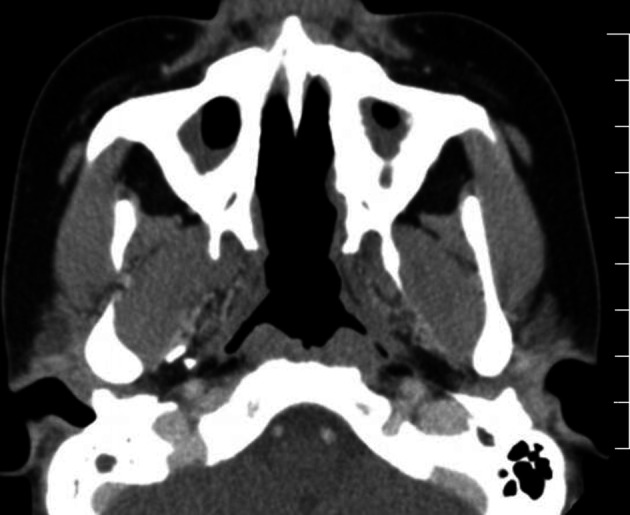
CT of sinuses: bilateral sclerosis of maxillary sinuses and destruction of midline nasal structures and septum.
Figure 2.
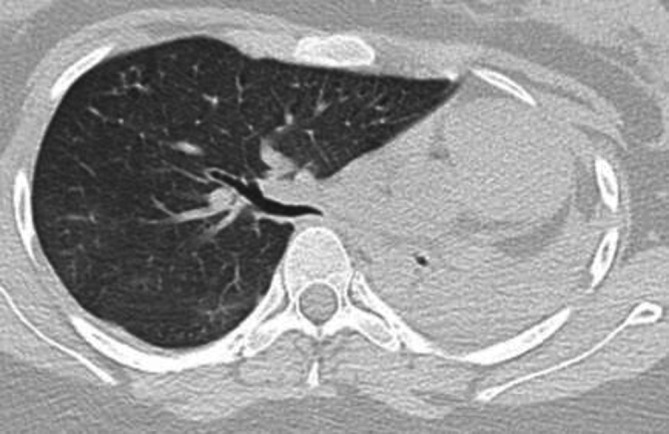
CT chest: severe R main bronchial stenosis (3 mm) with left lung collapse and levo-shift of mediastinal structures and heart.
Induction treatment included high-dose prednisone and 7 pulses of intravenous cyclophosphamide (CYC). Remission was achieved in 2008, and maintenance therapy was started with continuous low-dose prednisone and mycophenolate mofetil. A few months later, she suffered a relapse with recurrent severe bronchial stenosis, pulmonary nodules, as well as recurrent hematuria and proteinuria. Induction was achieved again with a course of RTX (375 mg/m2/week x 4), followed with methotrexate (MTX) for maintenance. However, between 2008 and 2013, she had multiple life-threatening relapses, typically characterised by pulmonary or upper airway involvement that required repeat balloon dilatations and iterative increases in her prednisone dose. Since 2010, she had to remain on no less than 15 mg/day of prednisone, in addition to the MTX.
In 2013, she suffered another severe relapse with acute respiratory distress syndrome, new cavitary lung nodules and a left pneumothorax. She was reinduced with high-dose prednisone and RTX (375 mg/m2/week x 4), in addition to intravenous immunoglobulins. Because the response was only partial, with worsening bronchial stenosis, she received in late 2013 a single dose of eculizumab (anti-C5 monoclonal antibody) with transient improvement. This was followed, for maintenance, by repeat courses of RTX, initially every 6 then every 4 months, in addition to prednisone (>15 mg daily), oral MTX (25 mg/week), monthly intravenous immunoglobulin, and leflunomide 20 mg daily (AZA was tried before, but caused some hypersensitivity reactions).
Until January 2017, despite this regimen, she could not taper the prednisone below 15 mg/day due to recurrent subglottic and bronchial stenoses, which required repeat dilations and eventually led to a complete collapse of her left lung (figure 3). She was declined for lung transplantation because of the unsatisfactory control of her disease, the extent of her stenoses, and her inability to tolerate invasive ventilation. She suffered multiple sequelae of her disease and treatments, including avascular necrosis of hips and vertebrae, osteoporosis, recurrent infections (bacterial pneumonia, Pneumocystis jirovecii pneumonia, septic arthritis, cytomegalovirus-related cytopenia and osteomyelitis of the sinuses. Her poor health and prognosis lead her to sign a ‘Do Not Resuscitate’ order in 2014 despite her young age.
Figure 3.
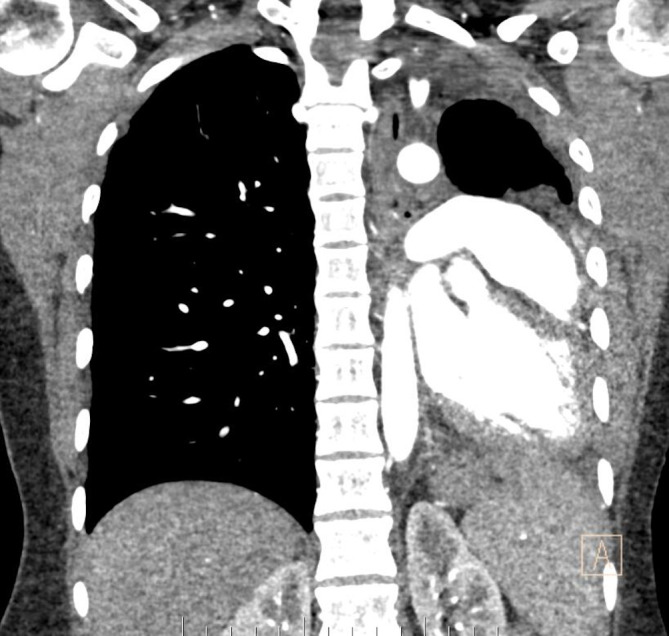
CT chest: left lung collapse and levo-shift of mediastinal structures and heart.
In January 2017, she began treatment with avacopan, 30 mg orally, two times per day, provided compassionately on the ground of an open-label, single patient study, reviewed and approved by the Mount Sinai Hospital (Toronto) research and ethic board (17–0295-E) and by Health Canada (# 201517).
Outcome and follow-up
Since initiating avacopan, she has had no distinct disease flares and was able to gradually decrease her other immunosuppressive agents. Sixteen months after starting avacopan (April 2018), she stopped the intravenous immunoglobulin infusions, and gradually decreased the prednisone down to 5 mg daily (in May 2018). After 18 months of avacopan, she stopped the leflunomide (June 2018), and the RTX 1 g infusions were spaced to every 6 months. At month 30 of avacopan (July 2019), she stopped the MTX. She further tapered the prednisone from 5 mg daily as of month 33 and stopped it at month 35 (November 2019).
She, thus, has been taking avacopan 30 mg two times per day for 35 months to date, and is still on RTX 1 g every 6 months (which will be reduced to 500 mg every 6 months in 6–12 months). In 2017 (ie, during the first year of avacopan treatment), she reported 2 episodes of bronchitis which rapidly resolved with oral antibiotics (one with a 24-hour stay in the hospital), and she had several benign episodes of conjunctivitis, rapidly controlled with antibiotic eyedrops. None of these episodes required an increase in glucocorticoid dosing or other changes in immunosuppressive therapy. She did not require new tracheal or bronchial dilations since 2017 and underwent successful nasal reconstructive surgery in December 2018 (month 24 of avacopan). Her ANCA has remained negative since 2016. Unfortunately, the left lung collapse and left main bronchus stenosis have not improved. She is pursuing a degree in social work.
Discussion
This case illustrates some of the persistent and ongoing challenges in GPA. Refractory forms exist, and some manifestations such as subglottic and bronchial stenosis, which may be more frequent in young female patients, can be extremely difficult to treat.12–14 New treatment options are being investigated in controlled trials, but the use of innovative, non-approved agents sometimes needs to be considered in single or limited numbers of patients with refractory and relapsing disease, who would unlikely be eligible for ongoing trials. Avacopan seems to have helped for this patient with multirelapsing GPA, as her other treatments could be successfully tapered without any relapses.
Conventional induction therapy for severe GPA includes glucocorticoid-based regimens in combination with either RTX or CYC. RTX is preferred over CYC in young patients due to the risk of infertility or cancers later in life with the latter. Maintenance agents included the conventional AZA or MTX, which are less and less used because RTX has been repeatedly shown in studies conducted in the last 5 years to be more effective at preventing major relapses.15 However, relapses and refractory disease are not uncommon, and alternative, new, more effective and/or safer options remain needed.
Investigation into the pathogenesis of GPA has yielded some new potential treatment targets. The alternative complement pathway was shown to play an important role in AAV. Complement 5a (C5a), which binds the C5a receptor, facilitates the activation and maintenance of inflammation. C5a induces surface expression of adhesion molecules, promotes vascular permeability, and directs chemotaxis, activation and margination of neutrophils.16 ANCA-stimulated neutrophils, in turn, release C5a, thereby establishing an inflammatory amplification loop.17 In a mouse model of myeloperoxidase (MPO)-ANCA glomerulonephritis, C5a receptor knock-out mice demonstrated profound amelioration of their vasculitis. Similarly, C5-depleted serum failed to prime neutrophils for ANCA-induced respiratory burst activity.17 Studies in human supported the importance of this pathway in AAV. ANCA stimulation of C5a-primed neutrophils from patients with PR3-ANCA vasculitis demonstrated increased generation of C3bBbP (a surrogate for alternative pathway activation and microparticle release from neutrophils) compared with healthy controls.18 Compared with healthy controls, active MPO-ANCA vasculitis patients demonstrate higher levels of Bb, C3a, C5a, sC5b-9, whereas PR3-ANCA positive patients demonstrate higher levels of C3a, C5a, sC5b-9 and C4d, but without obvious difference in complement fraction levels between new-onset or relapsing disease, or any clear correlation with the Birmingham’s vasculitis activity score.19
Eculizumab is a humanised anti-C5 monoclonal antibody. There have been some case reports in GPA patients. However, it was withdrawn from study in AAV (NCT01275287). Eculizumab interferes with the formation of the membrane attack complex necessary for defence against certain pathogenic bacteria.16 20 Eculizumab is thus associated with respiratory tract infections (16%), nasopharyngitis (15%), neutropenia (13%) and brain abscess (4%).21 22
Avacopan (initially investigated under the code CCX168) is an orally administered, selective C5a receptor inhibitor. Thereby, it does not interfere with the formation of the membrane attack complex.16 Based on primate and phase 1 studies, a phase 2 randomised, placebo-controlled trial of 67 patients with AAV had been conducted. Patients were treated with CYC or RTX and either prednisone 60 mg daily, avacopan (30 mg two times per day for 3 months) and reduced dose prednisone (20 mg), or avacopan without any prednisone for 3 months. Both avacopan groups were non-inferior to the prednisone monotherapy group in achieving clinical response at week 12 (86.4% and 81.0% vs 70.0%). Adverse events were similar between the three groups, including those grade 3 or greater (9% in all groups). The most common serious adverse events which were grade 3 or greater, included pneumonia and renal vasculitis in the prednisone-only group (4% each), deep vein thrombosis and febrile infection in the combination group (5% each), and hepatic/pancreatic enzyme increase and renal impairment in the avacopan-only group (5% each). The most common side effects experienced in the avacopan-only group were lymphopenia (28%) and new-onset or worsening hypertension (36%). There was a lower incidence of adverse effects potentially related to glucocorticoids in the combination group and avacopan-only group compared with the prednisone-only group (18% and 50% vs 65%).23
ADVOCATE (ClinicalTrials.gov; NCT02994927) a recently completed phase 3 randomised, double-blind, active-controlled trial assessing the safety and efficacy of avacopan (for 12 months) vs prednisone (for 6 months) for induction and maintenance of remission in patients with AAV undergoing standard-of-care induction treatment with CYC (followed by AZA) or RTX (without subsequent maintenance agent). Preliminary results from of this trial are promising, and further analyses are ongoing prior to formal publication. Success of this study, if confirmed, would potentially signal the beginning of a paradigm shift in the treatment of AAV away from glucocorticoid-based regimens.
Use in children will need to be further studied.
Patient’s perspective.
My life with systematic vasculitis started in August 2007. I had just turned 9 a month earlier and like any 9-year-old I was active, energetic and chaotic. During the last few weeks of August, I started getting these cold-like symptoms. I hated that I could not enjoy my summer before school start because I was sick.
After a fever that lasted 17 days, and a journey that took me to Toronto’s Hospital for Sick Kids, and a tube down my throat to help me breathe, I learned that I had something called Granulomatosis with Polyangiitis aka GPA. I was terrified out of my mind. All of a sudden, my life came to a halt. I was started on a course of treatment involving steroids, immunosuppressants, and chemotherapy. I lost my long hair that I was proud off, I gained a lot of weight, and worst of all I could no longer play my favourite game: soccer.
After multiple surgeries, loss of most of my sinuses, loss of my left lung, and multiple long-term side effects from medication, I survived. I finally graduated from Toronto Sick Kids Hospital and moved to the ‘adult world’ at Mount Sinai Hospital (Toronto). I was scared that this transition would go wrong. I was worried that in the middle of my transition my GPA would become active again and I would end up being admitted to the hospital with doctors that were unfamiliar with my diagnosis and would treat me wrong. It turned out I had nothing to worry about. My new rheumatologist took exceptionally great care of me and stayed in touch with my pediatricians.
I have been taken care of at Mount Sinai Hospital for 4 years now, and I have never reflared. Almost as soon as I started going there, I started using avacopan. Since then, I have only been admitted once for an infection. This has been the longest period in my life since my diagnosis that I have not spent most my nights in a hospital bed. Thanks to my doctor’s care and treatments, I have been disease free for 4 years, and I will be able to complete my schooling and get my degree in a few more months.
Learning points.
Remission can be achieved in granulomatosis with polyangiitis (GPA) in up to 90% of patients treated with glucocorticoids in addition to either cyclophosphamide or rituximab. However, relapse occurs in over 50% of patients by 5 years with older conventional therapies (AZA, MTX, CYC) and 30% with rituximab.
Potential side effects of these treatments can cause significant morbidity and mortality. The risk of death in antineutrophil cytoplasm antibody-associated vasculitis is around 11% in the first year, with nearly 60% of deaths attributable to therapy-associated adverse events.
The alternative complement pathway, especially fraction complement 5a (C5a) and its receptor, play an important role in the pathogenesis of GPA.
Avacopan (CCX168), an oral C5a receptor inhibitor, has demonstrated value as a steroid-sparing induction therapy when added to cyclophosphamide or rituximab for the treatment of GPA.
At 35 months of treatment with avacopan, a patient with previously refractory, life-threatening, multirelapsing disease was able to taper her glucocorticoids and other immunosuppressive agents without suffering any recurrent relapses or serious adverse events.
Acknowledgments
Chemocentryx provided the avacopan for the treatment of the patient, free-of-charge, on a compassionate basis and on the ground of an open-label, single patient protocol (CCX168 – COMPA-TO- A 001), IRB approval Mount Sinai Hospital (Toronto) 17-0295-E and by Health Canada # 201517.
Footnotes
Contributors: DE and CP contributed equally to the drafting and revising of the manuscript. RSMY contributed to the critical review and finalising of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: CP reports receiving fees for serving on advisory boards from Chemocentryx, GlaxoSmithKline, Sanofi and Hoffman-LaRoche; he also reports lecture fees and research grant support from Hoffman-La Roche and GlaxoSmithKline. RSMY is the site primary investigator for a Roche funded clinical trial.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Gibelin A, Maldini C, Mahr A. Epidemiology and etiology of Wegener granulomatosis, microscopic polyangiitis, Churg-Strauss syndrome and Goodpasture syndrome: vasculitides with frequent lung involvement. Semin Respir Crit Care Med 2011;32:264–73. 10.1055/s-0031-1279824 [DOI] [PubMed] [Google Scholar]
- 2.Mohammad AJ, Jacobsson LTH, Westman KWA, et al. Incidence and survival rates in Wegener's granulomatosis, microscopic polyangiitis, Churg-Strauss syndrome and polyarteritis nodosa. Rheumatology 2009;48:1560–5. 10.1093/rheumatology/kep304 [DOI] [PubMed] [Google Scholar]
- 3.Mohammad AJ, Jacobsson LTH, Mahr AD, et al. Prevalence of Wegener's granulomatosis, microscopic polyangiitis, polyarteritis nodosa and Churg-Strauss syndrome within a defined population in southern Sweden. Rheumatology 2007;46:1329–37. 10.1093/rheumatology/kem107 [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez-Gay MA, Garcia-Porrua C, Guerrero J, et al. The epidemiology of the primary systemic vasculitides in northwest Spain: implications of the chapel Hill consensus conference definitions. Arthritis Rheum 2003;49:388–93. 10.1002/art.11115 [DOI] [PubMed] [Google Scholar]
- 5.Pagnoux C. Updates in ANCA-associated vasculitis. Eur J Rheumatol 2016;3:122–33. 10.5152/eurjrheum.2015.0043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pagnoux C, Carette S, Khalidi NA, et al. Comparability of patients with ANCA-associated vasculitis enrolled in clinical trials or in observational cohorts. Clin Exp Rheumatol 2015;33:S-77–83. [PMC free article] [PubMed] [Google Scholar]
- 7.Comarmond C, Cacoub P. Granulomatosis with polyangiitis (Wegener): clinical aspects and treatment. Autoimmun Rev 2014;13:1121–5. 10.1016/j.autrev.2014.08.017 [DOI] [PubMed] [Google Scholar]
- 8.Stone JH, Wegener's Granulomatosis Etanercept Trial Research Group . Limited versus severe Wegener's granulomatosis: baseline data on patients in the Wegener's granulomatosis etanercept trial. Arthritis Rheum 2003;48:2299–309. 10.1002/art.11075 [DOI] [PubMed] [Google Scholar]
- 9.Mukhtyar C, Flossmann O, Hellmich B, et al. Outcomes from studies of antineutrophil cytoplasm antibody associated vasculitis: a systematic review by the European League against rheumatism systemic vasculitis Task force. Ann Rheum Dis 2008;67:1004–10. 10.1136/ard.2007.071936 [DOI] [PubMed] [Google Scholar]
- 10.Guillevin L, Pagnoux C, Karras A, et al. Rituximab versus azathioprine for maintenance in ANCA-associated vasculitis. N Engl J Med 2014;371:1771–80. 10.1056/NEJMoa1404231 [DOI] [PubMed] [Google Scholar]
- 11.Little MA, Nightingale P, Verburgh CA, et al. Early mortality in systemic vasculitis: relative contribution of adverse events and active vasculitis. Ann Rheum Dis 2010;69:1036–43. 10.1136/ard.2009.109389 [DOI] [PubMed] [Google Scholar]
- 12.Pagnoux C, Stubbe M, Lifermann F, et al. Wegener's granulomatosis strictly and persistently localized to one organ is rare: assessment of 16 patients from the French vasculitis Study Group database. J Rheumatol 2011;38:475–8. 10.3899/jrheum.100518 [DOI] [PubMed] [Google Scholar]
- 13.Pagnoux C, Wolter NE. Vasculitis of the upper airways. Swiss Med Wkly 2012;142:w13541. 10.4414/smw.2012.13541 [DOI] [PubMed] [Google Scholar]
- 14.Quinn KA, Gelbard A, Sibley C, et al. Subglottic stenosis and endobronchial disease in granulomatosis with polyangiitis. Rheumatology 2019;58:2203–11. 10.1093/rheumatology/kez217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGeoch L, Twilt M, Famorca L, et al. CanVasc recommendations for the management of antineutrophil cytoplasm antibody-associated vasculitides. J Rheumatol 2016;43:97–120. 10.3899/jrheum.150376 [DOI] [PubMed] [Google Scholar]
- 16.Bekker P, Dairaghi D, Seitz L, et al. Characterization of pharmacologic and pharmacokinetic properties of CCX168, a potent and selective orally administered complement 5A receptor inhibitor, based on preclinical evaluation and randomized phase 1 clinical study. PLoS One 2016;11:e0164646. 10.1371/journal.pone.0164646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schreiber A, Xiao H, Jennette JC, et al. C5A receptor mediates neutrophil activation and ANCA-induced glomerulonephritis. J Am Soc Nephrol 2009;20:289–98. 10.1681/ASN.2008050497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohlsson S, Holm L, Hansson C, et al. Neutrophils from ANCA-associated vasculitis patients show an increased capacity to activate the complement system via the alternative pathway after ANCA stimulation. PLoS One 2019;14:e0218272. 10.1371/journal.pone.0218272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu EY, McInnis EA, Boyer-Suavet S, et al. Measuring circulating complement activation products in Myeloperoxidase- and proteinase 3-Antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatol 2019;71:1894–903. 10.1002/art.41011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manenti L, Urban ML, Maritati F, et al. Complement blockade in ANCA-associated vasculitis: an index case, current concepts and future perspectives. Intern Emerg Med 2017;12:727–31. 10.1007/s11739-017-1636-6 [DOI] [PubMed] [Google Scholar]
- 21.Howard JF, Utsugisawa K, Benatar M, et al. Safety and efficacy of eculizumab in anti-acetylcholine receptor antibody-positive refractory generalised myasthenia gravis (REGAIN): a phase 3, randomised, double-blind, placebo-controlled, multicentre study. Lancet Neurol 2017;16:976–86. 10.1016/S1474-4422(17)30369-1 [DOI] [PubMed] [Google Scholar]
- 22.Misawa S, Kuwabara S, Sato Y, et al. Safety and efficacy of eculizumab in Guillain-Barré syndrome: a multicentre, double-blind, randomised phase 2 trial. Lancet Neurol 2018;17:519–29. 10.1016/S1474-4422(18)30114-5 [DOI] [PubMed] [Google Scholar]
- 23.Jayne DRW, Bruchfeld AN, Harper L, et al. Randomized trial of C5a receptor inhibitor Avacopan in ANCA-associated vasculitis. J Am Soc Nephrol 2017;28:2756–67. 10.1681/ASN.2016111179 [DOI] [PMC free article] [PubMed] [Google Scholar]


