Abstract
Introduction
Women of reproductive age (WRA) are a high-risk population for anaemia and micronutrient deficiencies. Evidence supports the role of periconceptional nutrition in the development of adverse pregnancy complications. However, in India, there are limited population-based data to guide evidence-based recommendations and priority setting. The objective of this study is to conduct a population-based biomarker survey of anaemia and vitamin B12 and folate status in WRA as part of a periconceptional surveillance programme in Southern India.
Methods
WRA (15–40 years) who are not pregnant or lactating and reside within 50 km2 of our community research site in Southern India will be screened and invited to participate in the biomarker survey at our research facility at Arogyavaram Medical Centre. After informed consent/assent, structured interviews will be conducted by trained nurse enumerators to collect sociodemographic, dietary, anthropometry, health and reproductive history data. Venous blood samples will be collected at enrolment; whole blood will be analysed for haemoglobin. Plasma, serum and red blood cells (RBCs) will be processed and stored <−80°C until batch analysis. Vitamin B12 concentrations will be measured via chemiluminescence, and RBC and serum folate concentrations will be evaluated using the World Health Organisation (WHO)-recommended microbiological assay at our laboratory in Bangalore. A WHO surveillance system will also be established to determine the baseline prevalence of birth defects in this setting.
Ethics and dissemination
This study has obtained clearance from the Health Ministry Screening Committee of the Indian Council of Medical Research. The study protocol was reviewed and approved by the Institutional Review Board at Cornell University and the Institutional Ethics Committees at Arogyavaram Medical Centre and St. John’s Research Institute. Findings from this biomarker survey will establish the burden of anaemia and micronutrient deficiencies in WRA and directly inform a randomised trial for anaemia and birth defects prevention in Southern India. The results of this study will be disseminated at international research conferences and as published articles in peer-reviewed journals.
Trial registration numbers
Clinical trials registration number NCT04048330, NCT03853304 and Clinical Trials Registry of India (CTRI) registration number REF/2019/03/024479.
Keywords: epidemiology, gynaecology, nutrition & dietetics, obstetrics, public health, anaemia
Strengths and limitations of this study.
This population-based biomarker survey is the first study to date to establish the burden of anaemia and micronutrient deficiencies in women of reproductive age in this setting.
This protocol describes the development of an established Centers for Disease Control and Prevention site for the gold standard World Health Organisation (WHO)-recommended microbiological assay for folate and WHO Southeast Asia Regional Office (SEARO) site for newborn and birth defects surveillance.
One limitation of this study is that its cross-sectional design will not enable evaluation of the effects of micronutrients on anaemia or changes in nutritional biomarkers over time.
Findings from this biomarker survey will inform the development of a randomised efficacy trial of quadruple-fortified salt for anaemia and birth defects prevention in Southern India.
Introduction
Women of reproductive age (WRA) are a high-risk population for anaemia and micronutrient deficiencies due to social structures and the physical demands of pregnancy and lactation, particularly in South Asia.1–6 Inadequate periconceptional folate and vitamin B12 status are implicated in the development of birth defects and other pregnancy complications.7 8 Randomised trials established that periconceptional folic acid supplementation can prevent the occurrence and recurrence of neural tube defects (NTDs),9–11 and fortification of staple foods with folic acid has been associated with decreasing NTDs in many countries around the globe.12–14 Red blood cell (RBC) folate has been identified as a biomarker of NTD risk at the population level,15 16 and increases in RBC folate concentrations predicted reductions in NTD risk of up to 10-fold in USA, Chinese and Irish populations.17 18 Emerging evidence has identified maternal vitamin B12 deficiency as a risk factor for NTDs,8 and vitamin B12 status may modify circulating folate biomarkers that predict NTD risk.18 It is estimated that the burden of NTDs in India is among the highest in the world.19 20 However, there is little representative population-level data from Southern India. Surveillance programmes are urgently needed to establish the burden of anaemia and key micronutrient deficiencies in settings such as Southern India to inform interventions for anaemia and birth defects prevention.
The objective of this study is to conduct a biomarker survey of anaemia and vitamin B12 and folate status in WRA as part of a periconceptional surveillance programme in Southern India and to inform the development of a randomised trial for anaemia and birth defects prevention. As part of this surveillance programme, we are also establishing the World Health Organisation (WHO)-recommended microbiological assay (MBA) for folate at our laboratory in Bangalore, India, and a WHO-SEARO site for birth defects surveillance in this setting. Findings from this preintervention biomarker survey will directly inform the development of a randomised efficacy trial of quadruple-fortified salt for the prevention of anaemia and birth defects in Southern India and serve as the foundation for future public health programmes to improve the health of women and young children in this population.
Methods
Study site
This biomarker survey will be conducted as part of a periconceptional surveillance programme in Southern India. This research study is a collaboration between Cornell University, the Centers for Disease Control and Prevention (CDC), Arogyavaram Medical Centre (AMC) and St. John’s Research Institute (SJRI). The community-based surveillance programme is located in Chittoor, Andhra Pradesh, India, approximately 3 hours from Bangalore, India. The catchment area includes approximately 15 000 households and 75 000 individuals. A subset of these households is visited on an annual basis to assess sociodemographic information, health and nutritional status of WRA and utilisation of community health services.
Preparations for field activities
Research facilities at Arogyavaram Medical Centre (AMC)
Prior to the start of data collection, a dedicated research building was identified and renovated, including installation of generators and internet. The renovation included seven rooms for primary data collection, with dedicated stations for collection of biochemical specimens, anthropometry and dietary assessment, private rooms for assessment of women’s health and reproductive history and a room with locked cabinets for storage of consent forms and data collection instruments. A dedicated research team was hired and trained on research protocols, research ethics and data quality assurance/quality control (QA/QC), including a study coordinator, field supervisors, laboratory manager, laboratory technicians, phlebotomists, research assistants and nurse enumerators.
Central and field site research laboratories
A dedicated laboratory space was constructed at our central laboratory at SJRI, for the WHO-recommended MBA for folate, with expert guidance from CDC. Two rooms were renovated for preparation and analysis of samples and equipped with yellow light UV-filters (Lithoprotect, Microchemicals, Germany) to protect folate compounds from white UV light. Laboratory protocols for sample collection and processing and for conducting the folate MBA were developed and adapted in collaboration with the CDC. Laboratory staff participated in a 2-week hands-on training on the folate MBA at the CDC in Atlanta, Georgia. The laboratory is joining the CDC Performance Verification Program for the folate MBA in 2020. Completion of methods and performance of the folate MBA will be reported in future manuscripts on completion and verification of analyses.
A field site research laboratory was established at AMC, including dedicated space for processing samples, portable freezers (ie, −20°C to +10°C), a −80°C freezer for archiving samples, a generator and trained laboratory staff in research and laboratory methods. Yellow light UV-filters (Lithoprotect, Microchemicals, Germany) were also installed in the AMC laboratory.
Birth defects surveillance
A hospital-based birth defects surveillance system was established with local hospitals, including the Government Hospital and AMC, at the direction of the WHO Southeast Asia Regional Office (SEARO). Clinical nurse research assistants and a study coordinator were trained in the Southeast Asia Region Newborn and Birth Defects (SEAR-NBBD) Database at the All India Institute of Medical Science (AIIMS) in New Delhi, India. Training included identification of birth defects, data collection and entry in the online SEAR-NBBD surveillance system, using methods outlined in the WHO/CDC Birth Defects Surveillance Manual21 22 and SEAR-NBBD Manual.23 The SEAR-NBBD system provides support for verification of data reported online to maintain data quality in terms of correctness and completeness. Prospective hospital-based surveillance of monthly delivery data began with deliveries in January 2015 and prospective SEAR-NBBD birth defects surveillance initiated in February 2019. Information on pregnancies resulting in a birth defect are recorded, including date, pregnancy outcome, photograph record of birth defect using standard methods and details of the woman’s previous adverse pregnancy outcomes.
Census
As part of the community-based surveillance programme, a census was conducted of all households within the AMC catchment area. This provided the foundation for the periconceptional surveillance programme and identification and selection of households and WRA for the biomarker survey described below. Houses were contacted by trained nurse enumerators, with household level data collected 6 days per week (Monday–Saturday, beginning July 2017). If an adult (≥18 years) was present, enumerators asked if the household was willing to participate in the census, and informed consent was audio–video recorded. Whenever possible, enumerators returned to all households until they made contact with an adult (≥18 years). If a household did not consent to participate, or if no eligible participant could be located, the location of the house was recorded, and basic demographic information (eg, urban/rural and village/ward) was recorded. As part of the community-based surveillance programme, data collection was conducted in 19 196 households (rural: 10 117; urban: 9079).
Periconceptional surveillance programme: catchment area
A census has been completed for all households within a 50 km2 catchment area (6552 households; rural: 3124; urban: 3428) of AMC (table 1). A total of 5708 WRA (15–40 years; rural: 2649, urban: 3059) were identified residing in 3865 households (rural: 1760; urban: 2105). Findings will be used to directly inform the selection of households for the biomarker survey, with a target sample size of 1500 WRA.
Table 1.
Population characteristics: census of all households in catchment area
| Variables | Rural | Urban | Total |
| GM (95% CI) or n (%) | GM (95% CI) or n (%) | GM (95% CI) or n (%) | |
| Households | |||
| Total households screened* | 3124 | 3428 | 6552 |
| Consent obtained | 3092 (99.0) | 3364 (98.1) | 6456 (98.5) |
| Average household size | 3.4 (3.4 to 3.5) | 3.5 (3.4 to 3.5) | 3.5 (3.4 to 3.5) |
| Households with ≥1 WRA | 1760 (56.9) | 2105 (62.6) | 3865 (59.9) |
| Households with ≥2 WRA | 370 (11.9) | 398 (11.8) | 768 (11.9) |
| Native language Telugu | 2477 (79.3) | 2791 (81.4) | 5268 (80.4) |
| Women of reproductive age | 2649 | 3059 | 5708 |
| Not pregnant or lactating | 2214 (83.6) | 2564 (83.8) | 4778 (83.7) |
| Pregnant | 122 | 127 | 249 |
| Lactating | 313 | 368 | 681 |
| WRA | |||
| Eligible (15–40 years, not pregnant or lactating) | 2214 (83.6) | 2564 (83.8) | 4778 (83.7) |
| Age | 26.5 (26.2 to 26.9) | 26.6 (26.3 to 26.9) | 26.7 (26.3 to 26.8) |
| Marital status | |||
| Currently married | 1469 (66.5) | 1765 (69.1) | 3234 (67.9) |
| Widowed, divorced or separated | 82 (3.7) | 79 (3.1) | 162 (3.4) |
| Never married | 658 (29.8) | 710 (27.8) | 1368 (28.7) |
| Has children | 1326 (60.1) | 1600 (62.4) | 2926 (61.9) |
| Number of children | 1.9 (1.9 to 1.9) | 1.9 (1.9 to 2.0) | 1.9 (1.9 to 1.9) |
| Some formal education | 1762 (79.8) | 2035 (79.5) | 3797 (79.6) |
| Employment status | |||
| Not formally employed | 871 (39.4) | 1107 (43.2) | 1978 (41.5) |
| Full time | 729 (33.0) | 771 (30.1) | 1500 (31.4) |
| Part time or seasonal | 137 (6.2) | 146 (5.7) | 283 (5.9) |
| Student | 474 (21.4) | 535 (20.9) | 1009 (21.2) |
*n=6 houses were not located.
GM, geometric mean; WRA, woman of reproductive age (15–40 years).
Patient and public involvement
No patients were directly involved in the design, planning, conception and conduct of this study.
Biomarker survey in women of reproductive age
Electronic data capture (EDC)
Electronic case report forms (CRFs) will be developed within the ConnEDCt EDC platform. ConnEDCt is a complex, adaptive, relational database that has been previously described.24 ConnEDCt functions in settings with limited or no internet and uses logic rules to adapt CRFs and variables in real-time as data are entered, optimising data quality and streamlining data capture workflows. ConnEDCt is synced daily to an encrypted, third-party cloud server to ensure data integrity, to harmonise data across multiple collection points and to adhere to privacy and data protection regulations in India and the USA. ConnEDCt uses role-based, password-protected user accounts to protect data.
Development of Case Report Forms (CRFs)
Case report forms will be developed to evaluate sociodemographic characteristics of the household and to collect sociodemographic, dietary, anthropometric, health, reproductive history and biochemical data from WRA. The women’s health questionnaire will be developed in collaboration with the CDC, including questions that are developed and adapted based on a CDC programme, Birth Defects Study to Evaluate Pregnancy ExposureS. An interactive 24-hour dietary recall will be developed in collaboration with colleagues at SJRI25; AMC research staff will participate in trainings at SJRI on dietary assessment methods and administration of a 24-hour recall. All questionnaires will be translated to the local language (Telugu), adapted for cultural sensitivity and piloted prior to data collection.
Surveillance sample: biomarker survey
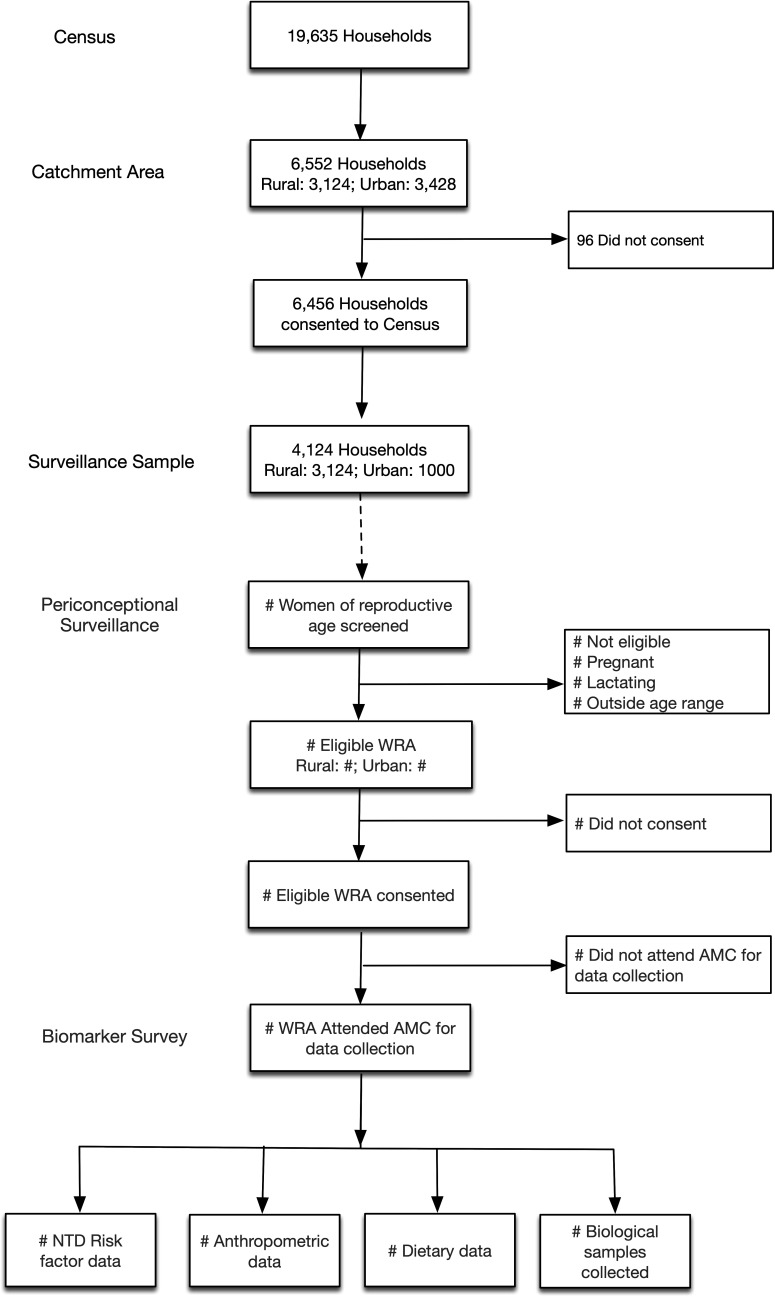
In order to obtain representative, population-level data in this setting, all rural households (n=3124) and a random subset (n=1000) of urban households in the 50 km2 catchment area will be selected for subsequent screening and recruitment of WRA for the biomarker study, with a target sample size of 1500 WRA. The random sample of urban households (n=1000) will be selected from the census, through a simple random sample conducted by a biostatistician from the CDC (CER) otherwise uninvolved with study implementation or data collection. For households with more than one eligible WRA, an a priori algorithm will be developed to randomly select one eligible WRA as the proband for the biomarker survey, for a final sample with one eligible WRA per household. An overview of the census and periconceptional surveillance programme, and household and participant recruitment for the biomarker survey is presented in figure 1.
Figure 1.
Participant flow chart. AMC, Arogyavaram Medical Centre; NTD, neural tube defect; WRA, women of reproductive age.
Inclusion and exclusion criteria and screening
Participants will be female, aged 15–40 years, who are currently not pregnant or lactating. For the biomarker survey, all households will be recontacted to confirm the roster household members, provide information regarding the biomarker study to family members, identify WRA and evaluate eligibility for the biomarker survey. Women who are identified as potentially eligible for the biomarker study will be screened for age and current reproductive status privately by trained nurse enumerators.
Programmatically, all eligible WRA in the household will be invited to participate in the biomarker survey, and research field staff will be blinded to which WRA is the proband. Women who are eligible for the biomarker study will receive information on the study, be asked if they would be interested in participating and be invited to participate in informed consent or assent. Women who are pregnant or who have severe anaemia (<8.0 g/dL) will be immediately referred to a local clinic for follow-up, as per standard of care.
Informed consent or assent
Informed consent will be conducted in a private space in the individual’s home or in a private room at AMC for individuals 18 years of age or older. For participants aged 15–<18 years, informed assent will be conducted at their home, in the presence of their parent or guardian. Informed consent (or assent) forms will be read aloud to all participants by a trained nurse enumerator. Written informed consent or assent will be obtained from all study participants, with audiovisual recording in accordance with regulations of the government of India for clinical trials. If a woman is not able to read, a literate witness will be present to sign the form as a witness of the informed consent/assent process, and the individual will be asked to sign the form via a thumbprint.
Trained nurse enumerators will explain the purpose, study procedures and potential risks and benefits involved in study participation and answer any questions. They will clearly explain to each participant that her participation in the research is entirely voluntary and that she can stop participating at any time after consent is granted without any penalty. Participants will be provided with contact information for key study personnel, including the principal investigator, if any questions arise during or after the study. After completing the informed consent/assent process, individuals who consent to participate will be provided an appointment and invited to participate in data collection.
Data collection
Data will be collected at our central research facility at AMC. Transportation to and from AMC will be provided for participants, as needed. Eligibility and consent will be confirmed before initiation of data collection. Individuals who express an interest in participating in the biomarker study but miss their scheduled appointment for informed consent or data collection will be followed up to reschedule appointments.
Data collection will include sociodemographic characteristics, overall health, dietary assessment (via 24-hour recall), anthropometry, reproductive history and biological specimen collection (table 2).
Table 2.
Data collection
| Data collection forms | Sociodemographic | |
| Dietary | 24-hour recall | |
| Women’s Health Questionnaire | Health status* | |
| Reproductive history | ||
| Risk factors for NTDs | ||
| Anthropometry | Circumference | Waist and hip |
| Mid-upper arm | ||
| Skinfolds | Triceps | |
| Biological samples | Blood, saliva and urine |
| Biomarker assessment | Indicator | Definition |
| Complete blood count | Haemoglobin and anaemia | Hb <12.0 g/dL, <8.0 g/dL |
| Erythrocyte (RBC) folate† | Folate status | RBC folate <305 nmol/L, <748 nmol/L |
| Serum folate† | Serum folate <7.0 nmol/L | |
| Total vitamin B12 | Vitamin B12 status | Vitamin B12 <148 pmol/L, <221 pmol/L |
*Morbidity, signs and symptoms.
†WHO-recommended microbiological assay.
NTDs, neural tube defects; RBC, red blood cell.
Sociodemographic
Structured interviews will be conducted to collect data on sociodemographic characteristics, including age, employment, current marital status, highest level of schooling attained, average monthly income and number of children who are currently living.
Dietary assessment
An interviewer-administered structured interactive multipass CRF will be used to conduct a 24-hour recall, with standard utensils and food containers for prompts and measurement of portion sizes. This questionnaire will include foods consumed (ie, type of food, amount consumed and time) and include items on cooking methods and questions relating to food fortification, salt use and consumption patterns, and specific food items containing iron, vitamin B12 and folate.
Anthropometry
Anthropometric measurements, including weight (recorded via a digital balance to the nearest 0.01 kg), height (using a stadiometer to the nearest 0.1 cm), mid-upper arm, waist and hip circumferences (using a tape measure to the nearest 0.1 cm) and triceps skinfolds (using callipers to the nearest 0.5 mm), will be measured in triplicate by trained research assistants.
Women’s Health Questionnaire
A structured questionnaire will be used to collect data on women’s overall health (including current signs and symptoms), reproductive history, pregnancy history, pregnancy outcomes (including birth defects) and additional questions on current and past exposures that have been linked to increased risk of birth defects, including smoking, obesity and medications.
Biological specimen collection
Biological specimens to be collected include blood, saliva and urine samples. Blood samples will be analysed for haemoglobin, total vitamin B12 concentrations, whole blood folate (in a whole blood lysate, with calculations to determine erythrocyte folate, also called RBC folate) and serum folate concentrations for the primary outcomes (table 2); additional samples will be processed, aliquoted and archived for future laboratory analyses. Blood: venous blood (12 mL) will be collected in three vacutainers (ie, red-top, purple-top dipotassium ethylenediaminetetraacetic acid (K2EDTA) and blue-top metal free K2EDTA) for each participant. After collection, blood samples will be immediately stored in a portable freezer unit (ie, −20°C–10°C) at 4°C–6°C until processing within 4 hours. Urine: urine will be collected from participants (≥8 mL) using the ‘clean catch’ method in a designated bathroom at AMC. Participants will be provided a labelled acid-washed urine collection cup and alcohol-free towelettes. Trained research assistants will describe sample collection to participants, and visual aids will be posted on the walls in the bathroom. After collection, urine samples will be stored at 4°C–6°C until processing within 4 hours. Saliva: prior to saliva collection, participants will be asked if they consumed any foods or beverages in the previous 30 min; if not, participants will be provided instructions on saliva sample collection (≥2 mL) and provided with a straw and/or cotton ball to assist with saliva collection. If participants have consumed foods or beverages in the past 30 min, they will be redirected to other data collection stations until the 30 min window has passed, and then requested to return to the biochemical station for saliva collection.
Sample processing and storage
Samples will be processed and stored using laboratory protocols developed in collaboration with colleagues at the CDC. Red-top vacutainers will be processed first, then purple-top vacutainers, and then blue-top vacutainers. After clotting for 30–40 min at room temperature, the red-top vacutainers will be centrifuged for 10 min (~1400 G) to separate serum from cells, and serum will be aliquoted and archived for future analyses. Purple-top vacutainers will be allowed to reach room temperature, remixed by inversion, and 100 µL of whole blood will be added to 1 mL of 1% ascorbic acid to generate a whole blood lysate for the MBA for erythrocyte folate. Purple-top vacutainers will be analysed for complete blood count; remaining blood will be centrifuged for 10 min (~1400 G), and plasma will be aliquoted and stored for future analyses. Finally, blue-top vacutainers will be allowed to reach room temperature, remixed by inversion, centrifuged for 10 min (~1400 G), and plasma will be aliquoted into metal-free cryovials. Cryovials of blood, serum and plasma samples will be stored ≤−80°C until laboratory analyses in batch. Urine samples will be aliquoted into metal-free and standard cryovials. Urine and saliva samples will be archived ≤−80°C for future analyses.
Laboratory analyses
Complete blood count (Hb) will be analysed with a Coulter counter in real-time at our field site laboratory. Serum total vitamin B12 concentrations will be assessed via chemiluminescence (Elecsys 2010 Roche Diagnostics, Mannheim, Germany). Erythrocyte (RBC) folate and serum folate concentrations will be measured using the WHO-recommended microbiological assay at our laboratory at SJRI in Bangalore, India.
Primary outcomes
The primary outcomes for the biomarker survey are presented in table 2:
Haemoglobin concentrations, g/dL; anaemia and severe anaemia.
Folate status: RBC folate and serum folate concentrations, nmol/L; folate deficiency and insufficiency.
Vitamin B12 status: total vitamin B12 concentrations, pmol/L; vitamin B12 deficiency and insufficiency.
Anaemia and severe anaemia will be defined as haemoglobin <12.0 g/dL and <8.0 g/dL, respectively. Vitamin B12 deficiency and insufficiency will be defined as total vitamin B12 <148 pmol/L and <221 pmol/L,26 27 respectively. Folate deficiency will be defined as erythrocyte folate <305 nmol/L (risk of macrocytic anaemia); folate insufficiency will be defined as erythrocyte folate <748 nmol/L (risk of NTDs), the recommended calibrator-adjusted equivalent of the threshold for optimal prevention of NTDs.16 28
Ethics and dissemination
The study protocol has been reviewed and approved by the Institutional Review Board (IRB) at Cornell University, the Institutional Ethics Committee (IEC) at AMC and the IEC at SJRI. A CDC research determination was conducted, and non-disclosure of personally identifiable information agreement is in place. The protocol for this surveillance project was reviewed in accordance with CDC human research protection procedures and was determined to be non-research, routine surveillance activity. This study has received clearance from the Indian Council of Medical Research Health Ministry Screening Committee. The protocol for this preintervention biomarker study has been registered at ClinicalTrials.gov (NCT04048330). The protocol for the randomised trial was reviewed according to the CDC’s human research protection procedures and was determined to be research, but CDC involvement did not constitute engagement in human subjects research. The randomised trial has been registered at ClincialTrials.gov (NCT03853304) and the Clinical Trials Registry of India (CTRI) (REF/2019/03/024479). The results of this study will be disseminated at international research conferences and as published articles in peer-reviewed journals.
Future directions
As part of our periconceptional surveillance programme, this population-based biomarker survey will establish the burden of anaemia and vitamin B12 and folate deficiencies in women of reproductive age in this setting. Findings will provide critical biomarker data that will directly inform the development of a randomised efficacy trial of quadruple-fortified salt for the prevention of anaemia and birth defects in Southern India.
Supplementary Material
Footnotes
Twitter: @FinkelsteinLab
Funding: This study was supported by the Centers for Disease Control and Prevention; the Division of Nutritional Sciences, Cornell University; and by the University of South Carolina’s Disability Research and Dissemination Center (DRDC) through its cooperative agreement (6U19DD001218) with the Centers for Disease Control and Prevention. AF was supported by the National Institutes of Health (5T32HD087137).
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the Centers for Disease Control and Prevention, DRDC, Eunice Kennedy Shriver National Institute of Child Health and Human Development, or the National Institutes of Health.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.WHO Nutritional anaemias tools for effective prevention and control. Geneva: World Health Organisation, 2017. [Google Scholar]
- 2.WHO The global prevalence of anaemia in 2011. World health organisation report. Geneva: World Health Organisation, 2015. [Google Scholar]
- 3.Kalaivani K. Prevalence & consequences of anaemia in pregnancy. Indian J Med Res 2009;130:627–33. [PubMed] [Google Scholar]
- 4.Allen LH. How common is vitamin B-12 deficiency? Am J Clin Nutr 2009;89:693S–6. 10.3945/ajcn.2008.26947A [DOI] [PubMed] [Google Scholar]
- 5.Allen LH, Rosenberg IH, Oakley GP, et al. . Considering the case for vitamin B12 fortification of flour. Food Nutr Bull 2010;31:S36–46. 10.1177/15648265100311S104 [DOI] [PubMed] [Google Scholar]
- 6.Wirth JP, Woodruff BA, Engle-Stone R, et al. . Predictors of anemia in women of reproductive age biomarkers reflecting inflammation and nutritional determinants of anemia (BRINDA) project. Am J Clin Nutr 2017;106 10.3945/ajcn.116.143073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finkelstein JL, Layden AJ, Stover PJ. Vitamin B-12 and perinatal health. Adv Nutr 2015;6:552–63. 10.3945/an.115.008201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Molloy AM. Should vitamin B12 status be considered in assessing risk of neural tube defects? Ann N Y Acad Sci 2018;1414:109–25. 10.1111/nyas.13574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prevention of neural tube defects: results of the medical Research Council vitamin study. MRC vitamin study Research Group. Lancet 1991;338:131–7. [PubMed] [Google Scholar]
- 10.Czeizel AE, Dudás I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med 1992;327:1832–5. 10.1056/NEJM199212243272602 [DOI] [PubMed] [Google Scholar]
- 11.Berry RJ, Li Z, Erickson JD, et al. . Prevention of neural-tube defects with folic acid in China. N Engl J Med Overseas Ed 1999;341:1485–90. 10.1056/NEJM199911113412001 [DOI] [PubMed] [Google Scholar]
- 12.De Wals P, Tairou F, Van Allen MI, et al. . Spina bifida before and after folic acid fortification in Canada. Birth Defects Res A Clin Mol Teratol 2008;82:622–6. 10.1002/bdra.20485 [DOI] [PubMed] [Google Scholar]
- 13.De Wals P, Tairou F, Van Allen MI, et al. . Reduction in neural-tube defects after folic acid fortification in Canada. N Engl J Med 2007;357:135–42. 10.1056/NEJMoa067103 [DOI] [PubMed] [Google Scholar]
- 14.Sayed A-R, Bourne D, Pattinson R, et al. . Decline in the prevalence of neural tube defects following folic acid fortification and its cost-benefit in South Africa. Birth Defects Res A Clin Mol Teratol 2008;82:211–6. 10.1002/bdra.20442 [DOI] [PubMed] [Google Scholar]
- 15.WHO Guideline: optimal serum and red blood cell folate concentrations in women of reproductive age for prevention of neural tube defects. Geneva: World Health Organisation, 2015. [PubMed] [Google Scholar]
- 16.Cordero AM, Crider KS, Rogers LM, et al. . Optimal serum and red blood cell folate concentrations in women of reproductive age for prevention of neural tube defects: World Health organization guidelines. MMWR Morb Mortal Wkly Rep 2015;64:421–3. [PMC free article] [PubMed] [Google Scholar]
- 17.Crider KS, Devine O, Hao L, et al. . Population red blood cell folate concentrations for prevention of neural tube defects: Bayesian model. BMJ 2014;349:g4554. 10.1136/bmj.g4554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen M-Y, Rose CE, Qi YP, et al. . Defining the plasma folate concentration associated with the red blood cell folate concentration threshold for optimal neural tube defects prevention: a population-based, randomized trial of folic acid supplementation. Am J Clin Nutr 2019;109:1452–61. 10.1093/ajcn/nqz027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zaganjor I, Sekkarie A, Tsang BL, et al. . Describing the prevalence of neural tube defects worldwide: a systematic literature review. PLoS One 2016;11:e0151586. 10.1371/journal.pone.0151586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhide P, Sagoo GS, Moorthie S, et al. . Systematic review of birth prevalence of neural tube defects in India. Birth Defects Res A Clin Mol Teratol 2013;97:437–43. 10.1002/bdra.23153 [DOI] [PubMed] [Google Scholar]
- 21.WHO/CDC/ICBDSR Birth defects surveillance: a manual for programme managers. Geneva: World Health Organization, 2014. [Google Scholar]
- 22.WHO/CDC/ICBDSR Birth defects surveillance: atlas of selected congenital anomalies. Geneva: World Health Organization, 2014. [Google Scholar]
- 23.WHO-SEARO Hospital-Based birth defects surveillance: a guide to establish and operate. New Delhi, India, 2016. [Google Scholar]
- 24.Ruth CJ, Huey SL, Krisher JT, et al. . An electronic data capture framework (ConnEDCt) for global and public health research: design and implementation. J Med Internet Res 2020;22:e18580. 10.2196/18580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dwarkanath P, Soares MJ, Thomas T, et al. . Food frequency questionnaire is a valid tool for the assessment of dietary habits of South Indian pregnant women. Asia Pac J Public Health 2014;26:494–506. 10.1177/1010539512459945 [DOI] [PubMed] [Google Scholar]
- 26.Yetley EA, Pfeiffer CM, Phinney KW, et al. . Biomarkers of vitamin B-12 status in NHANES: a roundtable summary. Am J Clin Nutr 2011;94:313S–21. 10.3945/ajcn.111.013243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allen LH, Miller JW, de Groot L, et al. . Biomarkers of nutrition for development (bond): vitamin B-12 review. J Nutr 2018;148:1995S–2027. 10.1093/jn/nxy201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bailey LB, Stover PJ, McNulty H, et al. . Biomarkers of nutrition for Development-Folate review. J Nutr 2015;145:1636S–80. 10.3945/jn.114.206599 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.