Abstract
Purpose
Optical coherence tomography (OCT) captures retinal damage in neuromyelitis optica spectrum disorders (NMOSD). Previous studies investigating OCT in NMOSD have been limited by the rareness and heterogeneity of the disease. The goal of this study was to establish an image repository platform, which will facilitate neuroimaging studies in NMOSD. Here we summarise the profile of the Collaborative OCT in NMOSD repository as the initial effort in establishing this platform. This repository should prove invaluable for studies using OCT to investigate NMOSD.
Participants
The current cohort includes data from 539 patients with NMOSD and 114 healthy controls. These were collected at 22 participating centres from North and South America, Asia and Europe. The dataset consists of demographic details, diagnosis, antibody status, clinical disability, visual function, history of optic neuritis and other NMOSD defining attacks, and OCT source data from three different OCT devices.
Findings to date
The cohort informs similar demographic and clinical characteristics as those of previously published NMOSD cohorts. The image repository platform and centre network continue to be available for future prospective neuroimaging studies in NMOSD. For the conduct of the study, we have refined OCT image quality criteria and developed a cross-device intraretinal segmentation pipeline.
Future plans
We are pursuing several scientific projects based on the repository, such as analysing retinal layer thickness measurements, in this cohort in an attempt to identify differences between distinct disease phenotypes, demographics and ethnicities. The dataset will be available for further projects to interested, qualified parties, such as those using specialised image analysis or artificial intelligence applications.
Keywords: neuro-ophthalmology, neurology, medical retina, radiology & imaging
Strengths and limitations of this study.
The Collaborative OCT in neuromyelitis optical spectrum disorders repository cohort comprises the largest number of retinal optical coherence tomography (OCT) images from patients with a neuromyelitis spectrum disorder, a rare autoimmune disease of the central nervous system.
Besides imaging data, information on clinical and functional scores as well as laboratory parameters were assessed.
We collect OCT images as original files, allowing for a detailed quality reading and standardised, device-independent OCT analysis.
Despite standardised image analysis, the image heterogeneity due to different OCT machines and scan protocols remains a challenge.
Introduction
Neuromyelitis optical spectrum disorders (NMOSD) are rare autoimmune neuroinflammatory diseases spanning a broad age range. They are clinically characterised by recurrent attacks of optic neuritis (ON), myelitis and less frequently by the brainstem and cerebral attacks, and profoundly impact patients’ quality of life.1–4 The current concepts of NMOSD are rapidly changing. An international panel of experts published the latest NMOSD diagnostic criteria in 2015.5 Pathogenic serum autoantibodies against aquaporin-4 (AQP4-IgG), an astrocytic water channel protein, can be detected in 60%–80% of patients with NMOSD.6 7 The diagnostic criteria differentiate patients with positive or negative/unknown AQP4-IgG status. In the latter case, strict rules apply to the clinical presentation and paraclinical findings, in particular those from MRI for diagnosing NMOSD.5 8 In some AQP4-IgG seronegative patients within the NMO disease spectrum, for example with isolated recurrent ON or myelitis, serum antibodies against myelin oligodendrocyte glycoprotein (MOG) can be detected.9–12 As a different cellular target is involved, most experts consider MOG-IgG autoimmunity, or MOG-IgG-associated encephalomyelitis, as a separate disease entity, pathogenetically distinct from classic AQP4-IgG-associated NMOSD.13–15 The term myelin oligodendrocyte glycoprotein antibody disease has recently been proposed for this disorder.16
ON is one of the most common clinical manifestation of NMOSD. It frequently results in severe structural optic nerve damage and visual impairment, often occurs bilaterally, with common relapses.3 17 18 Patients often develop a visual disability as a result of decreased high-contrast visual acuity (HCVA) and low-contrast visual acuity, as well as colour and visual field defects. These functional limitations result in impaired vision-related quality of life and a high incidence of legal blindness.19–21
Optical coherence tomography (OCT) acquires high-resolution retinal images and plays an important role in assessing ON-associated damage in NMOSD22 and other neuroinflammatory disorders associated with ON.23 24 After transection of optic nerve axons following acute ON, retrograde neurodegeneration leads to neuroaxonal damage in the retina.25
Retinal post-inflammatory neuroaxonal degeneration typically progresses for about 6 months after the onset of acute idiopathic ON. It can be assessed by OCT as peripapillary retinal nerve fibre layer thickness (pRNFL) or ganglion and inner plexiform layer thickness (GCIP).26 Due to the rarity of NMOSD, adequate disease-specific studies on temporal ON dynamics are scant.27 Thus, it remains uncertain which time frame accurately reflects the disease. For example, in a recent population-based study of all acute ON in Southern Denmark, there was not a single AQP4-IgG seropositive case and only two with MOG-IgG in the 50 patients presenting with de novo acute ON during study duration.28 In MOG-IgG seropositive patients, ON appears to be milder than in patients with detectable AQP4-IgG29 and leads to a better outcome despite equally severe retinal thinning.30 However, MOG-IgG seropositive patients display a higher relapse frequency, potentially leading to comparable cumulative retinal damage and loss of vision in the two subgroups.31 32 Furthermore, MOG-IgG seropositive patients have a more pronounced pRNFL thinning in the temporal quadrant, while temporal and nasal quadrants are equally affected in AQP4-IgG positive disease.19 33 It is currently unclear, to what extent the retina and vision are affected by NMOSD independently of ON.22 In multiple sclerosis (MS), disease-associated and progressive neurodegeneration can occur in eyes unaffected by ON.34 35 Studies of NMOSD have led to conflicting results. ON in NMOSD tends to be more posterior and closer to the chiasm than that occurring in MS, and chiasmal affection could lead to carry-over effects in the less affected companion eye.36 37 However, recently described microstructural changes in the retina also suggest a primary retinopathy in NMOSD.38–41 This is potentially mediated by AQP4-expressing retinal Müller cells and could be independent of ON. Longitudinal data demonstrating progressive GCIP loss in AQP4-IgG positive patients with NMOSD independent of ON further supports this notion.42 43 These observations remain to be independently confirmed. In contrast, a recent exploratory longitudinal study in MOG-IgG seropositive patients suggested only progressive RNFL loss but not longitudinal GCIP reduction.44
Besides neuroaxonal damage, macroscopic retinal findings have been reported in NMOSD. Macular microcysts occur in the inner nuclear layer and are associated with severe ON in approximately 20% of patients with NMOSD.45–47 Pathology and clinical significance of macular microcysts, sometimes called microcystic macular oedema, remain unclear; however, macular processes appear to occur more frequently than other processes such as vitreous traction, which was found in one MOG-IgG positive patient with microcysts.48
Investigating OCT for clinically and pathologically meaningful information is hampered by the rareness and heterogeneity of NMOSD.49 In 2015, the Guthy Jackson Charitable Foundation International Clinical Consortium (GJCF-ICC) agreed to establish an image repository platform (neuromyelitis optical imaging repository (NOIR)), the purpose of which is to facilitate multinational and multicentre neuroimaging studies in NMOSD. NOIR is intended to help identify imaging pitfalls in NMOSD, develop and clinically validate imaging biomarkers of the disease, clinical disability and to define imaging endpoints for clinical trials. Here we report the outcome of the Collaborative OCT in NMOSD repository study (CROCTINO) as the initial effort in establishing a platform for investigating retinal abnormalities using OCT in NMOSD.
Cohort description
Study design
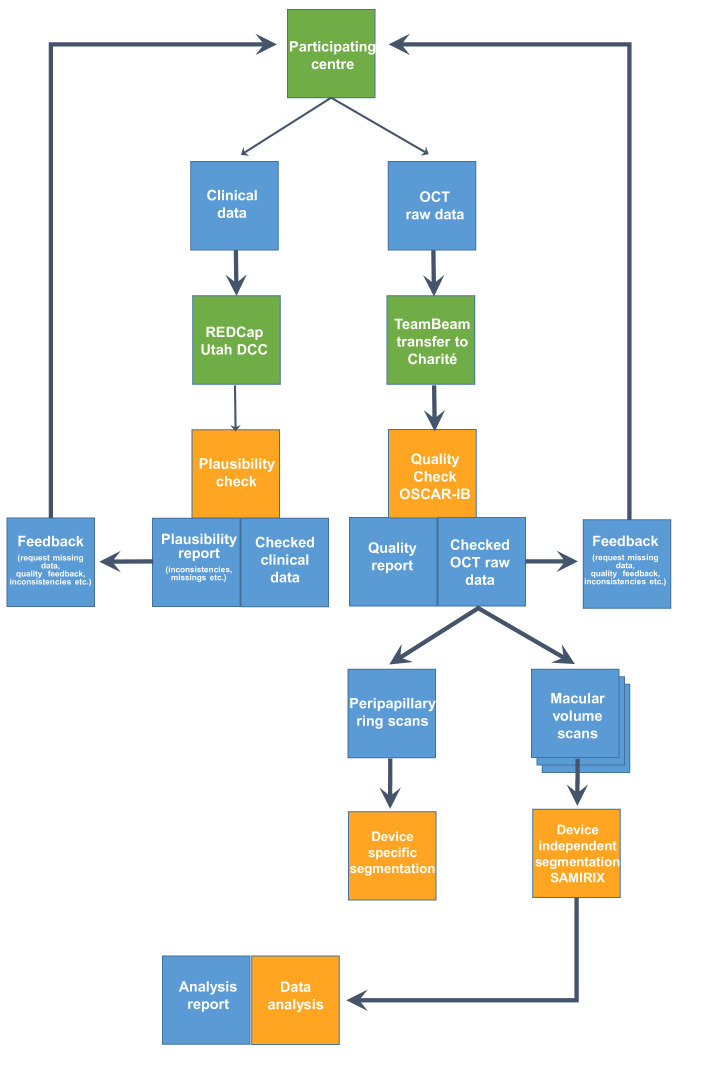
The study was designed as a multinational and multicentre repository study collecting longitudinal OCT data as well as relevant clinical data from patients with NMOSD and healthy controls (HCs). Participating centres were asked to contribute both retrospective and prospective data that was collected over a defined period extending from 2000 to 2018. Scientific coordination and OCT reading were performed at the Charité—Universitätsmedizin Berlin Translational Neuroimaging Group. Participating centres were mainly recruited from the GJCF-ICC, which includes international researchers and clinicians focusing on NMOSD. Additional experts who had previously published studies using OCT in NMOSD but who were not members of the GJCF-ICC were contacted and invited to participate. For this purpose, a questionnaire screening each centre for the number of eligible patients and the type of OCT instruments used at their site was sent to the entire GJCF-ICC and to other identified experts. Centres giving a positive response to the recruitment questionnaire received further instructions on how to contribute OCT images and the associated demographical and clinical data of their patients and HCs. The overall study design—including the workflow—is illustrated in figure 1.
Figure 1.
Flow chart explaining the overall study design and information technology (IT) infrastructure. DCC, Data Coordinating Center; OCT, optical coherence tomography; REDCap, Research Electronic Data Capture; SAMIRIX is a custom-developed intraretinal segmentation pipeline55. OSCAR-IB are validated consensus quality criteria for retinal OCT reading53.
Inclusion and exclusion criteria
Subjects were included with (a) a diagnosis of NMOSD as specified by the 2015 International Panel criteria5 or (b) longitudinal extensive transverse myelitis and/or (c) recurrent ON or (d) HCs (without matching requirements). OCT source data, demographic and clinical metadata from patients and HCs were required, including age, sex, disease subtype, autoantibody status and clinical history including details of ON.
Data collection and workflow
All demographic and clinical data were assessed in an electronic repository based on Research Electronic Data Capture (REDCap),50 located at the University of Utah Data Coordination Center. OCT images were transferred by participating centres via TeamBeam (Skalio GmbH, Hamburg, Germany), a commercial web-based medical data exchange service certified for secure data transfer. OCT images were then stored and analysed using a secure server at Charité—Universitätsmedizin Berlin, Berlin, Germany.
Study preparation began in January 2016 by identifying potential participating centres, setting up the information technology (IT) infrastructure and developing the electronic case report forms (eCRFs) in REDCap. eCRFs were sent to all participants for review and were revised accordingly. The data collection was launched on 1 September 2016, when the final repository was released, and centres received REDCap accounts for data entry. Data collection officially closed on 30 September 2018. After a detailed plausibility check, identified missing data could be submitted until 31 December 2018. A detailed timeline is illustrated in figure 2.
Figure 2.
Detailed study timeline including all study phases with start and end dates, as well as a brief description of each phase. eCRFs, electronic case report forms; OCT, optical coherence tomography.
Collected dataset
The data elements that were collected are listed in online supplemental file 1 (including indications of which data elements were mandatory or optional) and are summarised as follows:
bmjopen-2019-035397supp001.pdf (318.7KB, pdf)
Demographic and clinical data
In addition to age and sex, ethnicity was assessed based on published NIH categories (https://grants.nih.gov/grants/guide/notice-files/not-od-15-089.html). Possible selections comprised ‘American Indian or Alaska Native’, ‘Asian’, ‘Black or African American’, ‘Hispanic or Latino’, ‘Native Hawaiian or Other Pacific Islander’, ‘White’ and ‘Other’; up to two selections were possible. Height and weight were collected. Information concerning the presence of ophthalmological comorbidities other than those related to NMOSD was requested. The possible answers were: ‘Unknown’, ‘No (excluded by examination)’, ‘No (excluded by history taking)’ and ‘Yes’. In case of ‘Yes’, the condition had to be specified. Furthermore, the presence of other comorbidities was assessed. Information about NMOSD diagnosis was mandatory, with possible selections of ‘NMO (2006 criteria)’, ‘AQP4-IgG seropositive NMOSD (2015 criteria)’, ‘AQP4-IgG seronegative NMOSD (2015 criteria)’, ‘MOG-IgG-associated encephalomyelitis/NMOSD’, ‘RON/CRION (Recurrent Optic Neuritis)’, ‘LETM (Longitudinal Extensive Transverse Myelitis)’. We inquired about the AQP4 and MOG antibody statuses separately (both mandatory) with possible answers ‘Seropositive’, ‘Currently seronegative, but at least one previous test was positive’, ‘Seronegative’, ‘Not known/Never assessed’. Patients were considered to be seropositive for AQP4-IgG or MOG-IgG if antibodies were detected in at least one assay. Centres were also asked which assay they used for antibody testing. Provision of the Expanded Disability Status Scale (EDSS) score was optional.51 History of clinical attacks was requested with a focus on ON. Information concerning each eye was recorded separately as was the number of ON attacks and date of last episode. History of other NMOSD defining attacks, such as transverse myelitis, area postrema syndrome, brainstem syndromes, symptomatic narcolepsy or acute diencephalic clinical syndrome with NMOSD-typical diencephalic MRI lesions, and symptomatic cerebral syndrome with NMOSD-typical brain lesions was requested without dates and with the option ‘unknown’. Immunotherapy was assessed with a selection of common NMOSD treatments52 and possible selections ‘Current’ and ‘Previous use’.
Optical coherence tomography
Scans from all common OCT machines were accepted, resulting in scans from three different types of machines: (a) Spectralis SD-OCT (Heidelberg Engineering, Heidelberg, Germany), (b) Cirrus HD-OCT (Carl Zeiss Meditec, Dublin, California, USA) and (c) Topcon 3D OCT-1 (Topcon, Tokyo, Japan). We requested that centres upload two scan types per eye from each visit:
A peripapillary ring scan with 12° or approximately 3.4 mm diameter around the optic disc. For those using Cirrus and Topcon, an optic disc volume scan was uploaded since peripapillary data are extracted from this scan.
A macular volume scan, centred on the fovea, with a minimum size of 20°×20° (approximately 6 mm×6 mm).
There were no restrictions regarding OCT image averaging or quality. At least one scan per visit was required to be included in the repository. After images were transferred, their quality was assessed based on modified OSCAR-IB criteria.53 54 Modifications were made for better applicability to macular scans (see online supplemental file 2). pRNFL segmentation was performed with device-internal algorithms, intraretinal layer segmentation with a device-independent segmentation pipeline.55
bmjopen-2019-035397supp002.pdf (38.2KB, pdf)
Visual function
HCVA was requested with a selection menu ranging from 20/10 (−0.3 logMAR) to ‘no light perception’. Information on the method of testing was not requested, but details of whether testing was performed best corrected, habitually corrected or uncorrected were recorded. Monocular HCVA was mandatory while binocular HCVA was optional. Additionally, low contrast letter acuity (LCLA) from 2.5% contrast Sloan charts, normal/abnormal classifications of visual evoked potentials (VEP) P100 latencies and mean deficit and pattern standard deviation from visual fields could be entered as optional information.
Statistical analysis
Cohort and statistical description for this cohort profile were performed with R V.3.4.456 in R Markdown and RStudio (RStudio, Boston, Massachusetts, USA).
Cohort overview
Centres from North and South America, Asia and Europe participated in CROCTINO. In total, data from 539 patients from 22 centres and 114 HCs from 5 centres were collected. Centres and the size of their patient and HC cohorts are listed alphabetically in table 1.
Table 1.
Participating centres with number of patients, number of HCs and used OCT device
| Centre | Patients | HC | OCT device |
| Bangkok, Thailand | 25 | 0 | Cirrus |
| Barcelona, Spain | 13 | 13 | Spectralis or Cirrus |
| Belo Horizonte, Brazil | 57 | 0 | Spectralis |
| Berlin, Germany | 76 | 39 | Spectralis |
| Duesseldorf, Germany | 11 | 28 | Spectralis |
| Goyang-si, Korea | 50 | 0 | Topcon OCT |
| Isfahan, Iran | 40 | 18 | Spectralis |
| Istanbul, Turkey | 8 | 0 | Cirrus |
| Liverpool, UK | 8 | 0 | Spectralis |
| Lyon, France | 10 | 0 | Spectralis |
| Mangalore, India | 40 | 16 | Spectralis |
| Maracaibo, Venezuela | 3 | 0 | Spectralis |
| Michigan, USA | 5 | 0 | Spectralis |
| Milan, Italy | 30 | 0 | Spectralis |
| Munich, Germany | 11 | 0 | Spectralis |
| New York, USA | 6 | 0 | Spectralis and Cirrus |
| Odense, Denmark | 9 | 0 | Spectralis |
| Oxford, UK | 48 | 0 | Spectralis |
| Petah-Tikva, Israel | 25 | 0 | Cirrus |
| Sao Paulo, Brazil | 9 | 0 | Spectralis |
| Seattle, USA | 30 | 0 | Cirrus |
| Strasbourg, France | 25 | 0 | Spectralis |
HC, healthy control; OCT, optical coherence tomography.
In total, we collected 1868 peripapillary ring scans/optic nerve volume scans and 1672 macular volume scans fulfilling defined specifications. Only scans with corresponding clinical data were considered. Device and scan specifications are depicted in table 2.
Table 2.
Technical and scan specifications
| Protocol* | Axial resolution (µm) | Acquisition (A-scans/s) | n | B-scans | A-scans per B-scan | Size (mm)† |
| Spectralis Mac-1 | 3.962 | 40 00062 | 651 | 61 | 768 | 6×6 |
| Spectralis Mac-2 | 240 | 25 | 512 | 6×6 | ||
| Spectralis Mac-3 | 225 | 25 | 1024 | 7.5×9 | ||
| Spectralis Ring-1 | 1143 | 1 | 1536 | 3.4 | ||
| Spectralis Ring-2 | 226 | 1 | 768 | 3.5 | ||
| Cirrus Mac | 5.063 | 27 00063 | 293 | 128 | 512 | 6×6 |
| Cirrus Ring | 388 | 200 | 200 | 6×6/3.4‡ | ||
| Topcon Mac | 6.064 | 50 00064 | 263 | 128 | 512 | 6×6 |
| Topcon Ring | 111 | 128 | 512 | 6×6/3.4‡ |
*Only protocols making up more than 5% of the total macular scan rates were considered.
†Scan size can vary as it depends on the eye length.
‡Peripapillary ring scan extracted from optic nerve head volume scan.
Mac, macular volume.
A cohort characterisation is depicted in table 3. The mean patient age (mean±SD) was 43.1±14.8 years with 444 (82%) women. The age of HCs (mean±SD) was 32.1±9.8 years with 72 (63%) women. The majority of patients were White or Middle Eastern (n=315), followed by Asian (n=128) and Black or African American (n=27).
Table 3.
Cohort description
| Healthy controls | Patients | ||
| Subjects (n) | 114 | 539 | |
| Centres (n) | 5 | 22 | |
| Age (years; mean±SD) | 32.1±9.8 | 43.1±14.8 | |
| Sex (woman; n (%)) | 72 (63.2) | 444 (82.4) | |
| Time since disease onset (years; mean±SD) | – | 5.5±19.5 | |
| Age at initial symptom onset (years; mean±SD) | – | 36.2±15.1 | |
| Ethnicity (n (%)) |
White or Middle Eastern | 96 (84.2) | 315 (58.4) |
| Asian | 17 (14.9) | 128 (23.7) | |
| Black or African American | 0 (0) | 27 (5.0) | |
| Hispanic or Latino | 1 (0.9) | 11 (2.0) | |
| Other | 0 (0) | 23 (4.3) | |
| Not reported | 0 (0) | 35 (6.5) | |
| Patients fulfilling the 2015 diagnostic criteria for NMOSD (fulfilled; n (%)) | – | 515 (95.5) | |
| AQP4-IgG seropositive NMOSD (n (%)) | – | 369 (68.5) | |
| MOG-IgG seropositive NMOSD (n (%)) | – | 54 (10.0) | |
| Double-negative NMOSD (n (%)) | – | 34 (6.3) | |
| NMOSD with unknown antibody-status (n (%)) | – | 58 (11.0) | |
| Patients with a history of optic neuritis (n (%)) | – | 400 (74.2) | |
| Patients with a history of myelitis (n (%)) | – | 410 (76.1) | |
AQP4-IgG, aquaporin-4 IgG antibodies; MOG-IgG, myelin oligodendrocyte glycoprotein IgG antibodies; NMOSD, neuromyelitis optica spectrum disorders.
Among all patients, 515 (95.5%) fulfilled the 2015 diagnostic criteria for NMOSD.5 Of those not meeting the 2015 criteria, 21 (3.9%) carried the diagnosis of LETM, and 3 (0.6%) recurrent ON. Among those fulfilling NMOSD diagnostic criteria, 369 (72%) were AQP4-IgG seropositive, leaving 146 (28%) with negative or unknown AQP4-IgG status. Among the AQP4-IgG seronegative patients, 54 (37%) were MOG-IgG seropositive, 34 (23%) were double-negative for AQP4-IgG and MOG-IgG and 58 (40%) had an unknown antibody status. In the entire cohort, 369 (68%) were AQP4-IgG seropositive, 54 (10%) MOG-IgG seropositive, 52 (10%) had double negative and 64 (12%) had an unknown antibody status. There were 400 (74%) patients with at least one episode of ON, and 410 (76%) patients with a history of myelitis. The mean time since disease onset (mean±SD) was 5.47±19.47 years and mean age at initial symptom onset (mean±SD) was 36.18±15.05 years. Information about current N, N-Dimethyltryptamine (DMT) was available in 398 (74%) patients. Ninety-seven patients (18.0%) received a combination of multiple DMTs. Most frequent DMT options comprised rituximab (n=128; 24%), azathioprine (n=121; 22%), oral prednisolone (n=98; 18%), mycophenolate mofetil (n=77; 14%) and methotrexate (n=11; 2%).
The majority of the centres provided OCT source data from a Spectralis machine (17 centres), followed by Cirrus (6 centres) and one centre contributed Topcon OCT images (table 1).
HCVA was available for 529 (98%) patients and 83 (73%) HCs. Most of the patients received best-corrected HCVA (47%), 28% were habitually corrected and 25% not corrected. LCLA was obtained from 99 (18%) patients and 40 (35%) HCs. VEP were available for 177 (33%), and visual fields for 90 (17%) patients applying devices at the discretion of each centre.
Longitudinal data were available for 157 (29%) patients from 11 centres. Mean follow-up time (mean±SD) was 27±17 months.
Patient and public involvement
Patients and the Public were not explicitly involved in the design or conduct of this study. However, the results of this study will be presented on a Guthy-Jackson Charitable Foundation’s International NMO Patient Day, giving patients, relatives and researchers the opportunity to share ideas for projects based on this cohort.
Findings to date
To our knowledge, the CROCTINO repository comprises the largest dataset on retinal OCT in NMOSD. Cohort demographics are in line with previous epidemiological NMOSD studies: gender ratio, age at symptom onset and prevalence of AQP4-IgG and MOG-IgG antibodies are similar to previously published studies and suggest a cohort that is representative of the disease spectrum.57–59 OCT quality and quantitative analyses are currently being investigated as first scientific derivatives of the work and will be published in the near future. During the study, many challenges in conducting an investigator-driven academic repository study were experienced. First, this study was implemented without centre reimbursement. While we are unaware of specific guidelines in this regard, we felt that data quality would potentially be higher when centres receive no reimbursement. Although without reimbursement we expected a lower motivation for interested centres to participate at all, we reckoned that data quality might be higher from participating centres contributing out of sole scientific interest. Despite the absence of financial compensation, centre motivation was excellent, and we were able to include substantially more datasets (n=539) than initially anticipated (n=200). Lack of reimbursement did preclude the participation of some centres, particularly in North America. Sharing of data requires legal compliance, including the conclusion of data transfer agreements (DTA). Although we provided an initial DTA template for this study, almost all centres required modifications, the consequence of different legal systems in this multinational setting. This process resulted in a substantially delayed data collection. On the other hand, the DTA templates for each of the involved centres in combination with concluded DTA for this repository now provide a powerful platform and network for future studies in NMOSD.
During the conduct of this study, we developed several technical solutions, which will be made available beyond the scope of this repository. First, the technical infrastructure and newly established workflows will be instrumental in conducting future neuroimaging studies in NMOSD. We further developed a device-independent intraretinal layer segmentation pipeline based on an established segmentation algorithm.60 This pipeline is capable of importing and segmenting OCT scans from all devices included in the study and will presumably lead to better comparability of OCT data obtained from different devices.55 Finally, we modified the widely used OSCAR-IB criteria53 for assessing OCT image quality to be applicable to macular volume scans and all OCT devices. The modified criteria will be validated using data from our repository.
Strengths and limitations
With 539 patients, CROCTINO comprises the largest dataset of OCT images from patients with NMOSD that we are aware of. The cohort resembles epidemiological studies published earlier and includes relevant demographic and clinical data. Further, the autoantibody status is available for many datasets, including not only AQP4-IgG but also MOG-IgG, thus distinguishing this cohort from many earlier studies where MOG-IgG was either unknown or unavailable. Inclusion of different ethnicities and geographical regions should allow analysis and comparison with patients with NMOSD in different cohorts, who are usually investigated only in smaller separate analyses from one or few centres. Finally, this study collected raw OCT imaging data thereby allowing detailed quality reading and image analysis, also with advanced technologies in the future.
The study has several limitations, many of which are inherent to multicentre settings. First, the inclusion of retrospective data precluded application of a standardised, homogeneous imaging protocol. Heterogeneity in the dataset is further caused by (a) use of different OCT machines, (b) different scan protocols and (c) no homogeneous visual function measures. Several geographic regions are not represented in the repository (eg, Australia, Africa), and HC scans are not matched demographically and are only available for some ethnicities and from certain regions. Although great care was taken in collecting, completing and verifying the data, we could not ensure complete individual data set validity. For example, different methods for antibody testing61 with often uncertain sensitivity and specificity and a non-standardised documentation of patient-reported information could lead to some noise in respective analyses.
Collaboration and future directions
The study coordinating centre is pursuing several scientific projects based on the repository in collaboration with the participating centres, such as the analysis of pRNFL and intramacular layer thickness measurements in this cohort in an attempt to identify differences between distinct disease phenotypes, demographics and ethnicities. We encourage collaboration with interested researchers in additional scientific projects. The CROCTINO dataset will be made available to all participating centres and to qualified investigators on request (details see below). The study coordinators are also interested in further expanding the repository by including (a) additional data from currently underrepresented geographic regions, (b) additional HC data to provide expanded ethnicity comparators and (c) more prospective, longitudinal data, also extending already existing follow-up periods of the included patients. The established NOIR platform and the CROCTINO cohort will serve to investigate retinal imaging biomarkers in NMOSD in the future.
Supplementary Material
Acknowledgments
The authors would like to thank all who contributed to data assessment and collection at the participating centres.
Footnotes
Twitter: @imaynart
AUB and FP contributed equally.
Collaboration and future directions: The study coordinating centre is pursuing several scientific projects based on the repository in collaboration with the participating centres, such as the analysis of pRNFL and intramacular layer thickness measurements in this cohort in an attempt to identify differences between distinct disease phenotypes, demographics and ethnicities. We encourage collaboration with interested researchers in additional scientific projects. The CROCTINO dataset will be made available to all participating centres and to qualified investigators upon request (details see below). The study coordinators are also interested in further expanding the repository by including (a) additional data from currently underrepresented geographic regions, (b) additional healthy control data to provide expanded ethnicity comparators and (c) more prospective, longitudinal data, also extending already existing follow-up periods of the included patients. The established NOIR platform and the CROCTINO cohort will serve to investigate retinal imaging biomarkers in NMOSD in the future.
Funding: The creation of this repository was supported by a grant from the Guthy Jackson Charitable Foundation.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data collected will be electronically stored in a repository and can be accessed by all participants for research purposes upon request. GJCF-ICC and the principal investigators of the repository will coordinate projects and access. Investigators not involved in CROCTINO data collection who desire access to the data should approach the corresponding author or the GJCF-ICC for access.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Jarius S, Wildemann B, Paul F. Neuromyelitis optica: clinical features, immunopathogenesis and treatment. Clin Exp Immunol 2014;176:149–64. 10.1111/cei.12271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sepulveda M, Delgado-García G, Blanco Y, et al. Late-onset neuromyelitis optica spectrum disorder: the importance of autoantibody serostatus. Neurol Neuroimmunol Neuroinflamm 2019;6:e607. 10.1212/NXI.0000000000000607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beekman J, Keisler A, Pedraza O, et al. Neuromyelitis optica spectrum disorder: patient experience and quality of life. Neurol Neuroimmunol Neuroinflamm 2019;6:e580. 10.1212/NXI.0000000000000580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D'Souza M, Papadopoulou A, Levy M, et al. Diagnostic procedures in suspected attacks in patients with neuromyelitis optica spectrum disorders: results of an international survey. Mult Scler Relat Disord 2020;41:102027. 10.1016/j.msard.2020.102027 [DOI] [PubMed] [Google Scholar]
- 5.Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015;85:177–89. 10.1212/WNL.0000000000001729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zekeridou A, Lennon VA. Aquaporin-4 autoimmunity. Neurol Neuroimmunol Neuroinflamm 2015;2:e110 10.1212/NXI.0000000000000110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cook LJ, Rose JW, Alvey JS, et al. Collaborative international research in clinical and longitudinal experience study in NMOSD. Neurol Neuroimmunol Neuroinflamm 2019;6:e583. 10.1212/NXI.0000000000000583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim HJ, Paul F, Lana-Peixoto MA, et al. MRI characteristics of neuromyelitis optica spectrum disorder: an international update. Neurology 2015;84:1165–73. 10.1212/WNL.0000000000001367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim S-M, Woodhall MR, Kim J-S, et al. Antibodies to MOG in adults with inflammatory demyelinating disease of the CNS. Neurol Neuroimmunol Neuroinflamm 2015;2:e163. 10.1212/NXI.0000000000000163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chalmoukou K, Alexopoulos H, Akrivou S, et al. Anti-MOG antibodies are frequently associated with steroid-sensitive recurrent optic neuritis. Neurol Neuroimmunol Neuroinflamm 2015;2:e131. 10.1212/NXI.0000000000000131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Narayan R, Simpson A, Fritsche K, et al. Mog antibody disease: a review of MOG antibody seropositive neuromyelitis optica spectrum disorder. Mult Scler Relat Disord 2018;25:66–72. 10.1016/j.msard.2018.07.025 [DOI] [PubMed] [Google Scholar]
- 12.Waters P, Woodhall M, O'Connor KC, et al. Mog cell-based assay detects non-MS patients with inflammatory neurologic disease. Neurol Neuroimmunol Neuroinflamm 2015;2:e89. 10.1212/NXI.0000000000000089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zamvil SS, Slavin AJ. Does MOG Ig-positive AQP4-seronegative opticospinal inflammatory disease justify a diagnosis of NMO spectrum disorder? Neurol Neuroimmunol Neuroinflamm 2015;2:e62. 10.1212/NXI.0000000000000062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jarius S, Paul F, Aktas O, et al. MOG encephalomyelitis: international recommendations on diagnosis and antibody testing. J Neuroinflammation 2018;15:134. 10.1186/s12974-018-1144-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwon YN, Waters PJ, Kim M, et al. Peripherally derived macrophages as major phagocytes in MOG encephalomyelitis. Neurol Neuroimmunol Neuroinflamm 2019;6:e600. 10.1212/NXI.0000000000000600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wynford-Thomas R, Jacob A, Tomassini V. Neurological update: MOG antibody disease. J Neurol 2019;266:1280–6. 10.1007/s00415-018-9122-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramanathan S, Prelog K, Barnes EH, et al. Radiological differentiation of optic neuritis with myelin oligodendrocyte glycoprotein antibodies, aquaporin-4 antibodies, and multiple sclerosis. Mult Scler 2016;22:470–82. 10.1177/1352458515593406 [DOI] [PubMed] [Google Scholar]
- 18.Bennett JL, de Seze J, Lana-Peixoto M, et al. Neuromyelitis optica and multiple sclerosis: seeing differences through optical coherence tomography. Mult Scler 2015;21:678–88. 10.1177/1352458514567216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneider E, Zimmermann H, Oberwahrenbrock T, et al. Optical coherence tomography reveals distinct patterns of retinal damage in neuromyelitis optica and multiple sclerosis. PLoS One 2013;8:e66151. 10.1371/journal.pone.0066151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Outteryck O, Majed B, Defoort-Dhellemmes S, et al. A comparative optical coherence tomography study in neuromyelitis optica spectrum disorder and multiple sclerosis. Mult Scler 2015;21:1781–93. 10.1177/1352458515578888 [DOI] [PubMed] [Google Scholar]
- 21.Schmidt F, Zimmermann H, Mikolajczak J, et al. Severe structural and functional visual system damage leads to profound loss of vision-related quality of life in patients with neuromyelitis optica spectrum disorders. Mult Scler Relat Disord 2017;11:45–50. 10.1016/j.msard.2016.11.008 [DOI] [PubMed] [Google Scholar]
- 22.Oertel FC, Zimmermann H, Paul F, et al. Optical coherence tomography in neuromyelitis optica spectrum disorders: potential advantages for individualized monitoring of progression and therapy. Epma J 2018;9:21–33. 10.1007/s13167-017-0123-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oertel FC, Zimmermann HG, Brandt AU, et al. Novel uses of retinal imaging with optical coherence tomography in multiple sclerosis. Expert Rev Neurother 2019;19:31–43. 10.1080/14737175.2019.1559051 [DOI] [PubMed] [Google Scholar]
- 24.Oberwahrenbrock T, Traber GL, Lukas S, et al. Multicenter reliability of semiautomatic retinal layer segmentation using OCT. Neurol Neuroimmunol Neuroinflamm 2018;5:e449. 10.1212/NXI.0000000000000449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brandt AU, Specovius S, Oberwahrenbrock T, et al. Frequent retinal ganglion cell damage after acute optic neuritis. Mult Scler Relat Disord 2018;22:141–7. 10.1016/j.msard.2018.04.006 [DOI] [PubMed] [Google Scholar]
- 26.Costello F, Pan YI, Yeh EA, et al. The temporal evolution of structural and functional measures after acute optic neuritis. J Neurol Neurosurg Psychiatry 2015;86:1369–73. 10.1136/jnnp-2014-309704 [DOI] [PubMed] [Google Scholar]
- 27.Stiebel-Kalish H, Hellmann MA, Mimouni M, et al. Does time equal vision in the acute treatment of a cohort of AQP4 and MOG optic neuritis? Neurol Neuroimmunol Neuroinflamm 2019;6:e572. 10.1212/NXI.0000000000000572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soelberg K, Specovius S, Zimmermann HG, et al. Optical coherence tomography in acute optic neuritis: a population-based study. Acta Neurol Scand 2018;138:566–73. 10.1111/ane.13004 [DOI] [PubMed] [Google Scholar]
- 29.Akaishi T, Sato DK, Nakashima I, et al. MRI and retinal abnormalities in isolated optic neuritis with myelin oligodendrocyte glycoprotein and aquaporin-4 antibodies: a comparative study. J Neurol Neurosurg Psychiatry 2016;87:446–8. 10.1136/jnnp-2014-310206 [DOI] [PubMed] [Google Scholar]
- 30.Sotirchos ES, Filippatou A, Fitzgerald KC, et al. Aquaporin-4 IgG seropositivity is associated with worse visual outcomes after optic neuritis than MOG-IgG seropositivity and multiple sclerosis, independent of macular ganglion cell layer thinning. Mult Scler 2019:135245851986492. 10.1177/1352458519864928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pache F, Zimmermann H, Mikolajczak J, et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 4: afferent visual system damage after optic neuritis in MOG-IgG-seropositive versus AQP4-IgG-seropositive patients. J Neuroinflammation 2016;13:282. 10.1186/s12974-016-0720-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borisow N, Mori M, Kuwabara S, et al. Diagnosis and treatment of NMO spectrum disorder and MOG-Encephalomyelitis. Front Neurol 2018;9:888. 10.3389/fneur.2018.00888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Havla J, Kümpfel T, Schinner R, et al. Myelin-oligodendrocyte-glycoprotein (MOG) autoantibodies as potential markers of severe optic neuritis and subclinical retinal axonal degeneration. J Neurol 2017;264:139–51. 10.1007/s00415-016-8333-7 [DOI] [PubMed] [Google Scholar]
- 34.Petzold A, de Boer JF, Schippling S, et al. Optical coherence tomography in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol 2010;9:921–32. 10.1016/S1474-4422(10)70168-X [DOI] [PubMed] [Google Scholar]
- 35.Petzold A, Balcer LJ, Calabresi PA, et al. Retinal layer segmentation in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol 2017;16:797–812. 10.1016/S1474-4422(17)30278-8 [DOI] [PubMed] [Google Scholar]
- 36.Storoni M, Davagnanam I, Radon M, et al. Distinguishing optic neuritis in neuromyelitis optica spectrum disease from multiple sclerosis: a novel magnetic resonance imaging scoring system. J Neuroophthalmol 2013;33:123–7. 10.1097/WNO.0b013e318283c3ed [DOI] [PubMed] [Google Scholar]
- 37.Khanna S, Sharma A, Huecker J, et al. Magnetic resonance imaging of optic neuritis in patients with neuromyelitis optica versus multiple sclerosis. Journal of Neuro-Ophthalmology 2012;32:216–20. 10.1097/WNO.0b013e318254c62d [DOI] [PubMed] [Google Scholar]
- 38.Oertel FC, Kuchling J, Zimmermann H, et al. Microstructural visual system changes in AQP4-antibody-seropositive NMOSD. Neurol Neuroimmunol Neuroinflamm 2017;4:e334. 10.1212/NXI.0000000000000334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jeong IH, Kim HJ, Kim N-H, et al. Subclinical primary retinal pathology in neuromyelitis optica spectrum disorder. J Neurol 2016;263:1343–8. 10.1007/s00415-016-8138-8 [DOI] [PubMed] [Google Scholar]
- 40.Yamamura T, Nakashima I. Foveal thinning in neuromyelitis optica: a sign of retinal astrocytopathy? Neurol Neuroimmunol Neuroinflamm 2017;4:e347. 10.1212/NXI.0000000000000347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Motamedi S, Oertel FC, Yadav SK, et al. Altered fovea in AQP4-IgG–seropositive neuromyelitis optica spectrum disorders. Neurol Neuroimmunol Neuroinflamm 2020;7:e805 10.1212/NXI.0000000000000805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oertel FC, Havla J, Roca-Fernández A, et al. Retinal ganglion cell loss in neuromyelitis optica: a longitudinal study. J Neurol Neurosurg Psychiatry 2018;89:1259–65. 10.1136/jnnp-2018-318382 [DOI] [PubMed] [Google Scholar]
- 43.Pisa M, Ratti F, Vabanesi M, et al. Subclinical neurodegeneration in multiple sclerosis and neuromyelitis optica spectrum disorder revealed by optical coherence tomography. Mult Scler 2020;26:1197–206. 10.1177/1352458519861603 [DOI] [PubMed] [Google Scholar]
- 44.Oertel FC, Outteryck O, Knier B, et al. Optical coherence tomography in myelin-oligodendrocyte-glycoprotein antibody-seropositive patients: a longitudinal study. J Neuroinflammation 2019;16:154. 10.1186/s12974-019-1521-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaufhold F, Zimmermann H, Schneider E, et al. Optic neuritis is associated with inner nuclear layer thickening and microcystic macular edema independently of multiple sclerosis. PLoS One 2013;8:e71145. 10.1371/journal.pone.0071145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sotirchos ES, Saidha S, Byraiah G, et al. In vivo identification of morphologic retinal abnormalities in neuromyelitis optica. Neurology 2013;80:1406–14. 10.1212/WNL.0b013e31828c2f7a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gelfand JM, Cree BA, Nolan R, et al. Microcystic inner nuclear layer abnormalities and neuromyelitis optica. JAMA Neurol 2013;70:629. 10.1001/jamaneurol.2013.1832 [DOI] [PubMed] [Google Scholar]
- 48.Brandt AU, Oberwahrenbrock T, Kadas EM, et al. Dynamic formation of macular microcysts independent of vitreous traction changes. Neurology 2014;83:73–7. 10.1212/WNL.0000000000000545 [DOI] [PubMed] [Google Scholar]
- 49.Mori M, Kuwabara S, Paul F. Worldwide prevalence of neuromyelitis optica spectrum disorders. J Neurol Neurosurg Psychiatry 2018;89:555–6. 10.1136/jnnp-2017-317566 [DOI] [PubMed] [Google Scholar]
- 50.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33:1444–52. 10.1212/WNL.33.11.1444 [DOI] [PubMed] [Google Scholar]
- 52.Trebst C, Jarius S, Berthele A, et al. Update on the diagnosis and treatment of neuromyelitis optica: recommendations of the neuromyelitis optica Study Group (NEMOS). J Neurol 2014;261:1–16. 10.1007/s00415-013-7169-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tewarie P, Balk L, Costello F, et al. The OSCAR-IB consensus criteria for retinal OCT quality assessment. PLoS One 2012;7:e34823. 10.1371/journal.pone.0034823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schippling S, Balk LJ, Costello F, et al. Quality control for retinal OCT in multiple sclerosis: validation of the OSCAR-IB criteria. Mult Scler 2015;21:163–70. 10.1177/1352458514538110 [DOI] [PubMed] [Google Scholar]
- 55.Motamedi S, Gawlik K, Ayadi N, et al. Normative data and minimally detectable change for inner retinal layer thicknesses using a semi-automated OCT image segmentation pipeline. Front Neurol 2019;10:1117. 10.3389/fneur.2019.01117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.R Core Team (2017) R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2017. https://www.R-project.org/ [Google Scholar]
- 57.Pandit L, Asgari N, Apiwattanakul M, et al. Demographic and clinical features of neuromyelitis optica: a review. Mult Scler 2015;21:845–53. 10.1177/1352458515572406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jarius S, Ruprecht K, Wildemann B, et al. Contrasting disease patterns in seropositive and seronegative neuromyelitis optica: a multicentre study of 175 patients. J Neuroinflammation 2012;9:503. 10.1186/1742-2094-9-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Borisow N, Kleiter I, Gahlen A, et al. Influence of female sex and fertile age on neuromyelitis optica spectrum disorders. Mult Scler 2017;23:1092–103. 10.1177/1352458516671203 [DOI] [PubMed] [Google Scholar]
- 60.Lang A, Carass A, Hauser M, et al. Retinal layer segmentation of macular OCT images using boundary classification. Biomed Opt Express 2013;4:1133. 10.1364/BOE.4.001133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Waters P, Reindl M, Saiz A, et al. Multicentre comparison of a diagnostic assay: aquaporin-4 antibodies in neuromyelitis optica. J Neurol Neurosurg Psychiatry 2016;87:1005–15. 10.1136/jnnp-2015-312601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heidelberg Engineering G SPECTRALIS imaging platform technical specifications, 2020. Available: https://www.heidelbergengineering.com/download.php?https://media.heidelbergengineering.com/uploads/Products-Downloads/200279-002-INT-AE18_SPECTRALIS-Technical-Data-Sheet_EN.pdf
- 63.Carl Zeiss Meditec I Cirrus HD-OCT brochure, 2007. Available: http://www.eyecarealliance.com/wp-content/uploads/2018/08/Cirrus-OCT-4000-Brochure_ECA.pdf
- 64.TOPCON Medical Systems I 3D Oct-1 MAESTRO optical coherence tomography brochure, 2017. Available: https://west.visionexpo.com/__novadocuments/491865?v=636682302031730000
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-035397supp001.pdf (318.7KB, pdf)
bmjopen-2019-035397supp002.pdf (38.2KB, pdf)