Abstract
Purpose
Overexpression of miR-100 in stem cells derived from basal-like breast cancers causes loss of stemness, induction of luminal breast cancer markers and response to endocrine therapy. We, therefore, explored miR-100 as a novel biomarker in patients with luminal breast cancer.
Methods
miR-100 expression was studied in 90 patients with oestrogen-receptor-positive/human-epidermal growth factor receptor 2-negative breast cancer enrolled in a prospective study of endocrine therapy given either preoperatively, or for the treatment of de novo metastatic disease. Response was defined as a Ki67 ≤2.7% after 21±3 days of treatment. The prognostic role of miR-100 expression was evaluated in the Molecular Taxonomy of Breast Cancer International Consortium (METABRIC) and The Cancer Genome Atlas (TCGA) breast cancer datasets. Additionally, we explored the correlation between miR-100 and the expression its targets reported as being associated with endocrine resistance. Finally, we evaluated whether a signature based on miR-100 and its target genes could predict the luminal A molecular subtype.
Results
Baseline miR-100 was significantly anticorrelated with baseline and post-treatment Ki67 (p<0.001 and 0.004, respectively), and independently associated with response to treatment (OR 3.329, p=0.047). In the METABRIC dataset, high expression of miR-100 identified women with luminal A tumours treated with adjuvant endocrine therapy with improved overall survival (HR 0.55, p<0.001). miR-100 was negatively correlated with PLK1, FOXA1, mTOR and IGF1R expression, potentially explaining its prognostic effect. Finally, a miR-100-based signature developed in patients enrolled in the prospective study outperformed Ki67 alone in predicting the luminal A phenotype.
Conclusions
Our findings suggest that miR-100 should be further explored as a biomarker in patients with luminal breast cancer.
Keywords: miR-100, luminal breast cancer, endocrine therapy, prognosis, response prediction
Key questions.
What is already known about this subject?
We demonstrated that miR-100 levels are associated with response to endocrine therapy in women with operable luminal breast cancers.
What does this study add?
We found miR-100 levels to stratify luminal A tumours into two prognostically different subgroups.
The role of miR-100 as a post-transcriptional regulator of genes implicated in endocrine resistance and prognosis in luminal breast cancer may explain miR-100 predictive and prognostic ability. A signature based on four biomarkers, including miR-100, was accurate and reproducible in predicting the luminal A phenotype.
How might this impact on clinical practice?
miR-100 appears as a promising biomarker in early, luminal breast cancer and should be studied further.
Introduction
Breast cancer is the most frequent malignancy and the second cause of cancer death in the female sex.1 About 70% of newly diagnosed operable breast cancers express hormone receptors and have a normal human-epidermal growth factor receptor 2 (HER2) status.2 By gene expression analysis, these tumours can be further stratified into two molecular phenotypes. So-called luminal A tumours are slowly proliferating, carry a good prognosis, and are highly sensitive to endocrine therapy. Conversely, luminal B tumours present higher proliferation, relative endocrine resistance, and often require chemotherapy as part of postsurgical therapy.3 4 Despite substantial benefits of adjuvant endocrine therapy on disease-free and overall survival (OS), several women with luminal tumours still experience metastatic recurrence.5 Identifying biomarkers of sensitivity or resistance to endocrine therapy is, therefore, a priority in breast cancer research.
MicroRNAs (miRNAs) are short, non-coding RNA molecules that act as negative regulators of gene expression at the post-transcriptional level. Alterations in miRNA functions have been implicated in a variety of human diseases, including cancer.6 Additionally, due to high stability in serum, circulating miRNAs are emerging as promising tumour biomarkers.7–10 MiR-100, a member of the miR-99 family, has been found to post-transcriptionally suppress several downstream effectors involved in cancer cell proliferation, apoptosis, invasion and cell cycle arrest.11 Clinical data point at a potential of either tumour or circulating miR-100 in cancer detection and outcome prediction.12–15 Interestingly, miR-100 has been found to post-transcriptionally downregulate mTOR, FOXA1, PLK1, FGFR3 and IGF1R.16–19 These genes, in turn, have been described as mediators of resistance to endocrine therapy in luminal cancers.20–26
Added to this background, we previously demonstrated that the ectopic expression of miR-100 in cancer stem cells derived from aggressive, basal-like breast cancers (hormone receptors and HER2 negative) caused loss of stemness, and induction of cytokeratins 8–18 (markers of luminal breast cancer cells) and oestrogen receptor (ER) expression.27 Not only was this evolution phenotypical, but also functional because the acquisition of hormone receptor expression resulted in an endocrine-sensitive status. We, therefore, hypothesised that miR-100 could be a clinically exploitable biomarker in women with luminal breast cancer. To substantiate this hypothesis, we designed a prospective study to address the following questions: (1) are increasing tumour levels of miR-100 associated with response to endocrine therapy administered in the preoperative setting?; (2) are increasing levels of miR-100 prognostic beyond the tumour molecular phenotype (luminal A and luminal B); (3) is miR-100 expression correlated with the expression of genes that are known to influence response to endocrine therapy?; (4) could the expression on miR-100 and its target genes help to distinguish between luminal A and luminal B tumours in the clinic? Here, we present the results of our study.
Methods
Patients
The ‘REporting recommendations for tumour MARKer prognostic studies’ checklist for this study is found in online supplemental material.28
esmoopen-2020-000937supp001.pdf (112.3KB, pdf)
A single-arm, non-randomised, monoinstitutional clinical study was designed to explore miR-100 as a predictor of response to endocrine therapy (BC-P1-13, (online supplemental table 1). To be eligible for the study women had to be at their first diagnosis of hormone-receptor-positive, HER2-negative breast cancer with a baseline Ki67 ≥5% and to belong to one of the following three groups: (1) candidates to immediate breast surgery; (2) candidates to neoadjuvant endocrine therapy to induce tumour regression before surgery; (3) candidates to first-line endocrine therapy for primary breast cancer with synchronous distant metastases (de novo metastatic). Treatment consisted of tamoxifen 20 mg/day or letrozole 2.5 mg/day in premenopausal and postmenopausal women, respectively. Ovarian function suppression was allowed in premenopausal patients in groups 2 and 3. Treatment was planned to be 3 weeks in women in group 1, 4–6 months in women in group 2, and as long as clinically indicated in women in group 3. All women included in this study were managed by the multidisciplinary breast clinic of the Candiolo Cancer Institute, FPO-IRCCS.
Two baseline tumour core biopsies were obtained for all patients. In one of the baseline core biopsies, ER, PgR, HER2, and tumour proliferation (Ki67 score) were determined at our Pathology Department by conventional immunohistochemistry (IHC) on routinely processed, formalin-fixed, paraffin-embedded tumour specimens. Patients with HER2 2+status could enter the study if fluorescence in situ hybridisation excluded HER2 amplification. The second baseline biopsy was preserved in RNAlater and processed for miRNA and gene expression evaluation at our Cancer Molecular Biology Unit.
Subsequently, Ki67 was evaluated on surgical specimens in patients in group 1, and on tumour core biopsy taken after 21±3 days of treatment in women in groups 2 and 3. Therefore, all patients were planned to have tumour proliferation assessed after a short exposure of about 3 weeks of endocrine therapy.
The key findings from the BC-P1-13 study were further explored in two publicly available gene-expression datasets (METABRIC and TCGA),29 30 and in an Institutional retrospective cohort consisting of 77 randomly selected archival primary luminal tumour samples from women treated with surgery followed by adjuvant treatments including endocrine therapy at the Candiolo Cancer Institute between 2006 and 2010 (online supplemental table 2). This latter cohort was assembled to test whether formalin-fixed, paraffine-embedded archival material could be suitable for the evaluation of miR-100 and its relationship with the luminal subtypes.
Evaluation of miR-100 levels, target gene expression and PAM50
For the BC-P1-13 prospective study, total RNA was extracted from fresh tissue of core biopsies (median tumour cellularity 60%, range 25%–85%) using the miRNeasy Mini Kit (Qiagen) and following manufacturer’s instructions. For the archival cohort, total RNA was extracted from formalin-fixed paraffine-embedded (FFPE) sample sections after dissection of tumour tissue, using the miRNeasy FFPE Kit (Qiagen). For miRNA evaluation, 20 nanograms of RNA from each sample were reverse-transcribed using the microRNA Reverse Transcription Kit, and miRNA levels were assessed by quantitative qRT-PCR using the specific TaqMan microRNA Assay (Applied Biosystems). The microRNA let-7d-5p was used as an endogenous control to normalise miR-100 expression in each sample since, from an in silico analysis conducted on the TCGA breast cancer dataset, it showed high expression and low IQR among patients’ tumours.31 Total RNA extracted from a breast cancer cell line (MDA-MB-486) was used as a reference RNA and analysed in every qRT-PCR in parallel with tumour samples. MiR-100 expression in each tumour sample was then compared with miR-100 expression in the reference RNA and reported as log2 fold change (-∆∆CT sample/reference) in all the statistical analyses. For the evaluation of FOXA1, PLK1, mTOR, IGF1R and FGFR3 expression, 200 ng of RNA from each sample were used for reverse transcription with the high capacity cDNA reverse transcription Kit, and gene expression was assessed using specific TaqMan probes (Applied Biosystems). Beta-actin was used as a housekeeping gene. Gene expression in each sample was compared with gene expression in the reference RNA and reported as log2 fold change, as described for miR-100 analysis.
The molecular subtype classification was carried out on the BC-P1-13 and archival cohorts using a minimum of 150 ng/sample of total RNA with the PAM50 gene signature panel on the nCounter FLEX Analysis System (NanoString Technologies), following manufacturer’s instructions. Intrinsic subtype classification was performed by the NanoString Technologies’ service.
Statistical methods
Because the role of miR-100 as a determinant of endocrine responsiveness is exploratory, we sized the BC-P1-13 study on the primary end-point of complete cell cycle arrest (CCCA, defined as a post-treatment Ki67 ≤2.7%).32 Because this threshold corresponded to the lowest tertile of the normal logarithm of Ki67 scores after a short treatment, it is suggested that about 30%–35% of hormone receptor-positive tumours could be defined as endocrine responsive at this time point. Therefore, the clinical trial was sized to confirm a 30% proportion of endocrine-responsive patients with a beta error of 10% and an alpha error of 5%. Considering a possible dropout rate of 10%, the final trial size was established at 88 patients to be enrolled in 24 months. After a first interim analysis, because no patient on tamoxifen achieved a CCCA, the protocol was amended to include only postmenopausal patients assigned to letrozole 2.5 mg/day. We conducted post hoc analyses using different definitions of proliferative response, as log-fold of Ki67 change, percentage reduction of Ki67 (exploring various cutoffs), and geometric mean reduction. All the participants had to sign an informed consent before being considered for the study procedures.
The predictive value of miR-100 for CCCA after short-term endocrine therapy was evaluated by logistic regression analysis considering baseline Ki67 expression, histological type, grade, ER expression, progesterone receptor expression, and molecular phenotype as co-variates. Because the baseline Ki67 values were not distributed normally, they were log2-transformed. A dichotomised post-treatment Ki67 variable was generated using 2.7% of positivity as the cut-off. Pretreatment variables that resulted statistically significant in the univariable setting were entered in the multivariable analyses. Logistic regression analysis was also used to build a prediction model of luminal A molecular subtype; in this case, the dichotomous dependent variable was created according to the samples’ PAM50 classification as luminal A or not. Variables identified as statistically significant in univariable analysis were then evaluated in the multiple logistic regression analysis combining miR-100 and its target genes expression level into a score. Expression level scores for the prediction of the luminal A molecular phenotype were generated by subtracting either the single target gene or the mean of the two target genes expression values from miR-100 level as follows:
The best model was selected as the one with the minimum Akaike information criterion value and was tested via analysis of variance (ANOVA) test. To evaluate the predictive performance of the model, the area under the receiver operating characteristic curve (AUC-ROC) and the Youden’s index (J=sensitivity + specificity −1) were used as accuracy estimators.
The developed optimal predictive model was validated in an institutional archival cohort (online supplemental table 2) as well as in the public METABRIC dataset (EGAD00010000434 and EGAD00010000438 for normalised mRNA and miRNA expression, respectively). Clinical data including PAM50 subtyping were downloaded from cBioPortal. Due to methodological differences in assessing predictive variables (miR-100, PLK1, FOXA1 and Ki67 were analysed by using gene microarrays in the public study), METABRIC data were split in training (N=509) and validation sets (N=261) focusing on luminal patients (N=770) according to PAM50 classification. In this case, the model was first fitted in the training set and then used to predict the luminal A subtype in the validation set using the same covariates of the best model previously described in the BC-P1-13 prospective study. The AUC was evaluated in both sets and in the retrospective study as a readout of the model performance.
Kaplan-Meier survival estimate was employed to assess the difference in survival rates between patients with high and low miR-100 expression using the median as cut-off. This survival analysis was performed in hormonal therapy-treated patients with ER positive status (assessed by IHC) or luminal PAM50 subtype (A vs B). The significance was determined using the log-rank test and HRs were estimated using Cox regression analysis.
All analyses were performed using the statistical software R V.3.4.4 (http://www.R-project.org) and its packages ‘ROCR’, ‘Survival’ and ‘Survminer’. Where appropriate, statistical tests were two sided. Statistical significance was defined as p<0.05.
Results
Patient disposition in the BC-P1-13 cohort
The predictive value of baseline tumour levels of miR-100 with respect to response to endocrine therapy was explored in a prospective study of presurgical endocrine therapy (BC-P1-13, details in the Methods section). A total of 124 patients were screened between May 2014 and October 2017 and, of these, 90 patients and 90 tumours were evaluable for this analysis. (online supplemental material, figure 1) describes the enrolment flow and the reasons for the exclusion of 34 patients from further analyses. Online supplemental table 1 summarises the relevant patient demographics and tumour characteristics.
esmoopen-2020-000937supp002.pdf (4.1MB, pdf)
Molecular subtype classification was performed by PAM50 analysis on pretreatment biopsies in 89 tumours and revealed that 41 tumours were luminal A (46%), 44 luminal B (50%), 3 basal-like (3%) and 1 HER2 enriched (1%). There was no difference in the relative frequency of luminal A and luminal B tumours in premenopausal and postmenopausal patients (χ2 test, p=0.315). The four non-luminal tumours were all in postmenopausal patients.
MiR-100 expression level is an independent predictor of response to endocrine therapy
After short-term treatment with either tamoxifen or letrozole (21 days±3), the median Ki67 was 4.9% (range 0.2%–77.6%). Online supplemental figure 2 shows Ki67 before and after treatment in all 90 tumours (A) and in tumours treated with either letrozole or tamoxifen (B and C, respectively).
Overall, 35 patients (39%) achieved a CCCA (95% CI 29% to 50%), 23 of whom had a luminal A (56%), and 12 a luminal B tumour (25%, χ2 test, p=0.003). No tumours in premenopausal patients treated with tamoxifen achieved a CCCA. Of the four non-luminal cancers, none showed CCCA.
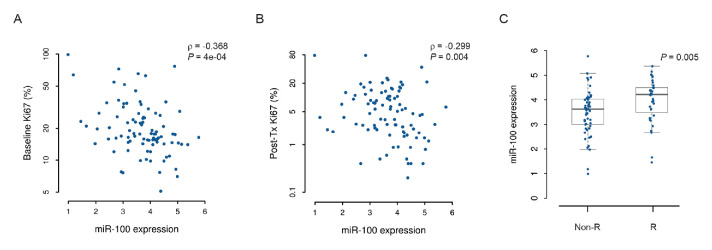
In 90 tumours analysed, the median baseline miR-100 value was 3.736 (log2 fold change with respect to the reference, range 0.986–5.771). Values were not significantly different between premenopausal and postmenopausal patients (3.727 vs 3.760, respectively, p=0.777). MiR-100 levels showed a statistically significant negative correlation with both baseline and post-treatment Ki67 values in the overall population (figure 1A, B) and in postmenopausal patients receiving letrozole (online supplemental figure 3A, B). Correlations between miR-100 and other histopathological variables are shown in online supplemental table 3).
Figure 1.
MiR-100 expression negatively correlates with proliferation and is higher in patients achieving complete cell cycle arrest. Scatter plots with Spearman correlation coefficients (ρ) between miR-100 expression in baseline tumour biopsies and either (A) baseline Ki67 or (B) post-treatment Ki67 (post-Tx Ki67); the logarithmic scale is used on the ordinate axis. (C) Boxplots with p value from Mann-Whitney U test for baseline miR-100 expression in responder and non-responder patients. The line within the box, boundaries and whiskers indicate median, IQR and 1.5 times the IQR, respectively. Non-R, non responder; R, responder.
Median miR-100 values were significantly higher in CCCA responders compared with non-responders, both in the overall population (figure 1C) and in postmenopausal patients receiving letrozole (online supplemental figure 3C). Indeed, univariable logistic regression analysis showed that the increase of miR-100 expression was significantly associated with CCCA in the overall population (OR 1.852, 95% CI 1.094 to 3.134, p=0.022). Corresponding values in the postmenopausal population receiving letrozole were OR 2.119, 95% CI 1.208 to 3.719 (p=0.009). We then looked for a suitable miR-100 cut-off that could discriminate between responders and non-responders. We identified the value of 4.264, corresponding to the 75th percentile of miR-100 values, and used it to dichotomise miR-100 (upper quartile vs others). We performed univariable and multivariable logistic regression analysis looking for predictors of CCCA (table 1). From these analyses, we excluded women treated with tamoxifen as no CCCA was observed in premenopausal patients receiving tamoxifen. Only baseline Ki67 values and miR-100 (upper quartile vs others) resulted significantly and independently associated with CCCA in multivariable analysis (table 1). Finally, in the overall population, we found a statistically significant higher Ki67 geometric mean reduction after treatment in patients in the upper quartile of miR-100 expression (−78.1%, 95% CI −68.1% to −89.7%), compared with the others (−40.5%, 95% CI −29.7% to −55.0%, p=0.018). Notably, the per cent reduction in Ki67 was not correlated with baseline Ki67 (online supplemental figure 4).
Table 1.
Univariable and multivariable analysis of predictors of complete cell cycle arrest
| Variable | OR | 95% CI | P value |
| Univariable | |||
| Histology | 0.811 | ||
| Ductal | 1 | ||
| Lobular | 1.418 | 0.473 to 4.225 | |
| Other | 1.261 | 0.232 to 6.842 | |
| Grade | 0.001 | ||
| G1 | 1 | ||
| G2/G3 | 0.258 | 0.113 to 0.588 | |
| ER (continuos) | 1.414 | 0.222 to 8.999 | 0.713 |
| PgR (continuous) | 1.010 | 0.998 to 1.022 | 0.118 |
| Subtype | |||
| Non-Luminal A | 1 | <0.001 | |
| Luminal A | 6.389 | 2.302 to 17.732 | |
| Baseline Ki67* | 0.250 | 0.112 to 0.559 | <0.001 |
| miR-100 (upper quartile vs others) | 5.143 | 1.822 to 14.518 | 0.002 |
| Multivariable | |||
| Baseline Ki67* | 0.289 | 0.127 to 0.656 | 0.003 |
| miR-100 (upper quartile vs others) | 3.329 | 1.015 to 12.974 | 0.047 |
*Ki67 values were log2 transformed.
ER, oestrogen receptor.
MiR-100 is associated with prognosis in patients with breast cancer treated with adjuvant hormonal therapy
The correlation of miR-100 with the response to short-term endocrine therapy, suggests a potential association with long-term clinical outcomes in patients with Luminal breast cancer receiving adjuvant endocrine therapy. We interrogated the METABRIC dataset to evaluate the prognostic value of miR-100 expression in 719 ER-positive patients with breast cancer who underwent systemic hormonal therapy. As shown in the Kaplan-Meier plot (figure 2A), a high expression of miR-100 (above the median value) was associated with improved OS (HR 0.69; 95% CI 0.57 to 0.85; log-rank p<0.001). Notably, miR-100 levels identified two prognostically different luminal A patient subgroups (HR 0.55; 95% CI 0.41 to 0.76; p<0.001, figure 2B), whereas no effect was seen in luminal B patients (figure 2C). MiR-100 levels were prognostic in the ER-positive population of the TCGA dataset as well (HR 0.5; 95% CI 0.29 to 0.86; p=0.01; online supplemental figure 5).
Figure 2.
MiR-100 predicts prognosis in endocrine therapy treated patients and identifies two subpopulations of luminal A patients with different survival. Kaplan-Meier curves of overall survival in (A) the ER-positive overall population (as determined by IHC), (B) luminal A and (C) luminal B patient subgroups (as determined by PAM50) who underwent endocrine therapy in the METABRIC dataset, stratified by median miR-100 expression level. P values were calculated by the log-rank test. ER, oestrogen receptor; IHC, immunohistochemistry.
MiR-100 regulates the expression of genes involved in resistance to hormonal therapy
After observing that baseline tumour miR-100 expression predicts response to short-term hormonal therapy and long-term prognosis, we were interested in identifying potential molecular mechanisms that could explain its predictive and prognostic value. Previously published data demonstrated that mTOR, FOXA1, PLK1, FGFR3 and IGF1R are implicated in endocrine resistance and prognosis in HR-positive patients with breast cancer.20–26 All these genes have been reported to be miR-100 targets.16–19 In this scenario, we hypothesised that high miR-100 levels could downregulate the expression of mTOR, FOXA1, PLK1, FGFR3 and IGF1R, thus contributing to a better therapeutic response and outcome. We assessed the expression of the five genes in baseline tumour biopsies by quantitative RT-PCR and performed a Spearman correlation analysis with miR-100 levels. In line with our hypothesis, the expression levels of miR-100 and PLK1, FOXA1 and mTOR were inversely correlated with high statistical significance (figure 3). Of note, the outlier sample in the FOXA1/miR-100 anti-correlation plot was a basal-like tumour. IGF1R expression was also negatively correlated with miR-100, although the anticorrelation did not reach statistical significance. Finally, no significant correlation was seen with FGFR3 (not shown). None of these genes was significantly correlated with baseline Ki67, except for PLK1, which showed a significant positive correlation (Spearman’s correlation coefficient 0.703, p<0.001).
Figure 3.
MiR-100 negatively correlates with the expression of target genes involved in endocrine therapy resistance. Scatter plots with Spearman correlation coefficients (ρ) and p values between the expression of miR-100 and its target genes in baseline tumour biopsies. The outlier sample in the miR-100/FOXA1 plot corresponds to a basal-like tumour according to the PAM50 gene classifier.
Association of a miR-100-based signature with the luminal A molecular subtype
When genomic tests are not available, the expression of Ki67 is used to approximate the tumour subtype (luminal A, low vs luminal B, high values) in women with HR-positive/HER2-negative early breast cancer to tailor treatments.2 33 We hypothesised that miR-100 and its correlated genes could provide additional predictive value to distinguish between luminal A and luminal B tumours. As expected, miR-100 expression was significantly higher in luminal A than in luminal B tumours stratified by PAM50 analysis, both in the BC-P1-13 patients, and in an archival institutional cohort of 77 breast cancer specimens from patients with luminal cancers (figure 4A). Similar results were obtained by analysing the METABRIC dataset (figure 4B).
Figure 4.
A miR-100-based classifier predicts the luminal A molecular subtype. (A) Boxplots of miR-100 expression in Luminal A and B tumour biopsies classified by the PAM50 gene signature in the BC-P1-13, archival (B) and in the METABRIC (C) cohorts. The line within the box, boundaries and whiskers indicate median, IQR and 1.5 times the IQR, respectively. P values were calculated by the Mann-Whitney U test. (C, D) Receiver operating characteristic curves for the prediction of the luminal A subtype according to the multivariable logistic regression model (based on baseline Ki67 and miR100/target score) in the BC-P1-13 (C) and archival (D) cohorts. The performance of each model was evaluated as the area under the curve (AUC) and Youden’s index (J).
We then performed univariable and multivariable analyses of predictors of the luminal A molecular subtype, including histology, tumour grade, ER expression, PgR expression, baseline Ki67, miR-100 and the five miR-100 target genes. Baseline Ki67, miR-100, PLK1 and FOXA1 were significant predictors of the luminal A subtype (online supplemental table 4). By combining these markers, we developed a model for predicting the luminal A subtype that, based on ROC curve analyses, outperformed Ki67 with a 15% higher sensitivity and an AUC of 0.91 vs 0.87 in the BC-P1-13 cohort (Youden’s index 0.74 vs 0.63; figure 4C and online supplemental figure 6A). This improvement in predicting power was statistically significant (ANOVA p<0.001). The predictive ability of the model was independently confirmed in the institutional archival cohort with an AUC of 0.93 vs 0.81 for Ki67 alone (Youden’s index 0.73 vs 0.47; figure 4D and online supplemental figure 6B), highlighting that the model performs well also on routinely processed FFPE samples. The miR-100-based model was also challenged in the METABRIC dataset, which was split in a training and a validation set. Despite all the variables being quantified with different techniques compared with our prospective and retrospective studies, the ROC curves suggested the reproducibility of the model (online supplemental figure 6C).
Discussion
In this study, we sought to explore miR-100 as a predictor of endocrine responsiveness and prognosis in HR-positive/HER2-negative patients with breast cancer. We derived the rationale from our previous experimental evidence suggesting that ectopic expression of this miRNA was able to induce a phenotypic and functional change in models of basal-like breast cancer stem cells.27 These included loss of stemness, expression of hormone receptor, and sensitivity to endocrine therapy. To pursue our aims, we used a widely adopted and validated clinical model consisting of the evaluation of tumour proliferation by Ki67 after a short exposure to endocrine therapy.32 The rate of CCCA (post-treatment Ki67 ≤2.7%) found in postmenopausal patients was similar to what reported in the medical literature, with the additional information that no CCCA was observed in premenopausal patients receiving tamoxifen alone. One main finding of the study was that miR-100 is inversely correlated with both baseline and post-treatment Ki67. In parallel, miR-100 expression was also higher in non-ductal histology, lower-grade tumours and luminal A tumours (online supplemental table 3). This corroborates the hypothesis that high levels of miR-100 characterise biologically less aggressive and more endocrine-responsive HR-positive tumours. Indeed, miR-100 was significantly and independently correlated with CCCA in postmenopausal patients receiving aromatase inhibitors. The multivariable analysis also suggested that the Ki67 score before treatment is a strong predictor of a CCCA, meaning that tumours starting at lower levels of proliferation are more likely to achieve the post-treatment cut-off for CCCA. Notably, although the molecular phenotype (luminal A vs non-luminal A) was a strong predictor of CCCA in univariable analysis, it was not retained in the final multivariable model. Being Ki67 and miR-100 anticorrelated, there is concern that our multivariable analysis might not accurately resolve their respective contribution to the predictive power of the model. To partially overcome this issue and to include in the analysis the whole population enrolled in the BC-P1-13 study, we explored other definitions of proliferative response. In particular, we considered the percent reduction in Ki67 values during short term exposure, which showed no correlation with baseline Ki67 (online supplemental figure 4). We found a significantly larger geometric mean reduction in the Ki67 score in tumours with high expression of miR-100 (≥the 75th percentile), providing further evidence of the predictive role of this miRNA with respect to response to endocrine therapy independently of baseline Ki67.
In line with the observed predictive role of miR-100, analyses conducted in the METABRIC and TCGA populations of patients with ER-positive breast cancer confirmed a strong association of high miR-100 expression and improved OS. Additionally, in the METABRIC dataset, which provides the PAM50 molecular subtypes, we found that miR-100 could prognostically stratify patients with luminal A tumours exposed to endocrine therapy. In these patients, miR-100 levels above the median were associated with a 45% reduction in the risk of death, an outstanding finding that deserves further investigation. Indeed, in the clinical practice, patients with operable luminal A tumours are considered as having an excellent long-term prognosis with endocrine therapy alone. However, here we show that the Luminal A patients can be stratified into prognostically different subgroups, and, according to our observation, miR-100 appears to be an informative biomarker to this aim. The anticorrelation between miR-100 levels and the expression of four out of five target genes previously reported to sustain endocrine therapy resistance suggests that the predictive role of miR-100 may result from the simultaneous downregulation of multiple genes individually mediating tumour resistance. Moreover, we found evidence that high levels of miR-100 concur to the luminal A phenotype and we were able to develop and validate a signature based on miR-100, two of its target genes (PLK1 and FOXA1) and baseline Ki67 that predicted the luminal A molecular subtype with better performance than Ki67 alone. This is underlined by a higher and more reproducible Youden’s index, which captures the performance of a diagnostic test, associated to the miR-100-based classifier compared with the Ki67 score alone. We, thus believe, that this signature deserves to be further developed and validated in larger and independent series for its prognostic value in patients with HR-positive breast cancer receiving adjuvant treatment.
While our results are encouraging, pointing at miR-100 as a potential new biomarker, we have to acknowledge some limitations that need being considered. First, a defined cut-off for miR-100 expression level able to dichotomise patients in responders and non-responders to primary endocrine therapy remains to be refined and confirmed in larger and independent patient cohorts. Second, the clinical translatability of miR-100 and its target genes as biomarkers relies on procedures which are not routinely performed in many Pathology Departments and that need to be standardised.
In conclusion, we demonstrated that high levels of miR-100 are associated with an endocrine-responsive phenotype in patients with luminal tumours undergoing primary endocrine therapy. We suggest that endocrine responsiveness likely results from the simultaneous low expression of genes that are related to endocrine resistance, and that miR-100 could be an important player in modulating their expression. Being strongly related to endocrine responsiveness, miR-100 results also associated with long-term survival in patients with early breast cancer submitted to adjuvant endocrine therapy. The finding in molecularly defined luminal A tumours is striking and has relevant potential clinical implications. Finally, the expression of miR-100 and its target genes could be integrated in a simple and reproducible signature that was able to distinguish between molecularly defined luminal A and B tumours. Our findings support confirmatory studies in larger and independent datasets. Additionally, due to the suggested role as a biomarker for human cancers, like glioblastoma,13 oesophageal cancer14 or bladder carcinoma,15 34 a formal evaluation of serum miR-100 is warranted to explore this non-invasive modality in the management of in women with luminal breast cancer.
Acknowledgments
We thank M. Milanesio and B. Martinoglio for their technical support.
Footnotes
Twitter: @FilippoMontemu1
AP and SEB contributed equally.
FM and SG contributed equally.
Contributors: I hereby declare that all the authors listed above have provided substantial contribution fo this work and fulfill the ICMJE criteria to claim contributorship. Furthermore, each contributor is aware of other author’s specific contribution to the manuscript.
Funding: This study makes use of data generated by the Molecular Taxonomy of Breast Cancer International Consortium. Funding for the project was provided by Cancer Research UK and the British Columbia Cancer Agency Branch (Curtis et al., Nature 486;346–52, 2012). Funded by Associazione Italiana per la Ricerca sul Cancro (AIRC) I.G. 2013 and 2016, project code 1451 and 19 174.
Competing interests: FM has received personal speaker’s fees from Roche, Novartis, Pfizer, Pierre Fabre, Eli Lilly, Daiichi Sankyo, Astra Zeneca. EB is the recipient of a Ph.D. fellowship from the Department of Medical Sciences, University of Torino ('Dipartimenti di Eccellenza 2018–2022', Project no. D15D18000410001).
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available on reasonable request. Anonymised patient data are available on reasonable request by contacting the corresponding author.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7–30. 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 2.Burstein HJ, Curigliano G, Loibl S, et al. Estimating the benefits of therapy for early-stage breast cancer: the St. Gallen international consensus guidelines for the primary therapy of early breast cancer 2019. Ann Oncol 2019;30:1541–57. 10.1093/annonc/mdz235 [DOI] [PubMed] [Google Scholar]
- 3.Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature 2000;406:747–52. 10.1038/35021093 [DOI] [PubMed] [Google Scholar]
- 4.Sorlie T, Tibshirani R, Parker J, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A 2003;100:8418–23. 10.1073/pnas.0932692100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 2015;386:1341–52. 10.1016/S0140-6736(15)61074-1 [DOI] [PubMed] [Google Scholar]
- 6.Lin S, Gregory RI. MicroRNA biogenesis pathways in cancer. Nat Rev Cancer 2015;15:321–33. 10.1038/nrc3932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bottani M, Banfi G, Lombardi G. Circulating miRNAs as diagnostic and prognostic biomarkers in common solid tumors: focus on lung, breast, prostate cancers, and osteosarcoma. J Clin Med 2019;8. 10.3390/jcm8101661. [Epub ahead of print: 11 Oct 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Cosimo S, Appierto V, Pizzamiglio S, et al. Early modulation of circulating microRNAs levels in HER2-positive breast cancer patients treated with trastuzumab-based neoadjuvant therapy. Int J Mol Sci 2020;21. 10.3390/ijms21041386. [Epub ahead of print: 18 Feb 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Cosimo S, Appierto V, Pizzamiglio S, et al. Plasma miRNA levels for predicting therapeutic response to neoadjuvant treatment in HER2-positive breast cancer: results from the NeoALTTO trial. Clin Cancer Res 2019;25:3887–95. 10.1158/1078-0432.CCR-18-2507 [DOI] [PubMed] [Google Scholar]
- 10.Zou X, Xia T, Li M, et al. MicroRNA profiling in serum: potential signatures for breast cancer diagnosis. Cancer Biomark 2020. 10.3233/CBM-201547. [Epub ahead of print: 21 Aug 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li C, Gao Y, Zhang K, et al. Multiple roles of MicroRNA-100 in human cancer and its therapeutic potential. Cell Physiol Biochem 2015;37:2143–59. 10.1159/000438572 [DOI] [PubMed] [Google Scholar]
- 12.Wang J, Yu M, Guan S, et al. Prognostic significance of microRNA-100 in solid tumors: an updated meta-analysis. Onco Targets Ther 2017;10:493–502. 10.2147/OTT.S122774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang H, Wang J, Wang Z, et al. Serum miR-100 is a potential biomarker for detection and outcome prediction of glioblastoma patients. Cancer Biomark 2019;24:43–9. 10.3233/CBM-181416 [DOI] [PubMed] [Google Scholar]
- 14.Jamali L, Tofigh R, Tutunchi S, et al. Circulating microRNAs as diagnostic and therapeutic biomarkers in gastric and esophageal cancers. J Cell Physiol 2018;233:8538–50. 10.1002/jcp.26850 [DOI] [PubMed] [Google Scholar]
- 15.Motawi TK, Rizk SM, Ibrahim TM, et al. Circulating microRNAs, miR-92a, miR-100 and miR-143, as non-invasive biomarkers for bladder cancer diagnosis. Cell Biochem Funct 2016;34:142–8. 10.1002/cbf.3171 [DOI] [PubMed] [Google Scholar]
- 16.Doghman M, El Wakil A, Cardinaud B, et al. Regulation of insulin-like growth factor-mammalian target of rapamycin signaling by microRNA in childhood adrenocortical tumors. Cancer Res 2010;70:4666–75. 10.1158/0008-5472.CAN-09-3970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi W, Alajez NM, Bastianutto C, et al. Significance of Plk1 regulation by miR-100 in human nasopharyngeal cancer. Int J Cancer 2010;126:2036–48. 10.1002/ijc.24880 [DOI] [PubMed] [Google Scholar]
- 18.Catto JWF, Miah S, Owen HC, et al. Distinct microRNA alterations characterize high- and low-grade bladder cancer. Cancer Res 2009;69:8472–81. 10.1158/0008-5472.CAN-09-0744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drayton RM, Peter S, Myers K, et al. MicroRNA-99a and 100 mediated upregulation of FOXA1 in bladder cancer. Oncotarget 2014;5:6375–86. 10.18632/oncotarget.2221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ross-Innes CS, Stark R, Teschendorff AE, et al. Differential oestrogen receptor binding is associated with clinical outcome in breast cancer. Nature 2012;481:389–93. 10.1038/nature10730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu X, Jeselsohn R, Pereira R, et al. FOXA1 overexpression mediates endocrine resistance by altering the ER transcriptome and IL-8 expression in ER-positive breast cancer. Proc Natl Acad Sci U S A 2016;113:E6600–9. 10.1073/pnas.1612835113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomlinson DC, Knowles MA, Speirs V. Mechanisms of FGFR3 actions in endocrine resistant breast cancer. Int J Cancer 2012;130:2857–66. 10.1002/ijc.26304 [DOI] [PubMed] [Google Scholar]
- 23.Knowlden JM, Hutcheson IR, Barrow D, et al. Insulin-like growth factor-I receptor signaling in tamoxifen-resistant breast cancer: a supporting role to the epidermal growth factor receptor. Endocrinology 2005;146:4609–18. 10.1210/en.2005-0247 [DOI] [PubMed] [Google Scholar]
- 24.Bhola NE, Jansen VM, Bafna S, et al. Kinome-wide functional screen identifies role of PLK1 in hormone-independent, ER-positive breast cancer. Cancer Res 2015;75:405–14. 10.1158/0008-5472.CAN-14-2475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller TW, Hennessy BT, González-Angulo AM, et al. Hyperactivation of phosphatidylinositol-3 kinase promotes escape from hormone dependence in estrogen receptor-positive human breast cancer. J Clin Invest 2010;120:2406–13. 10.1172/JCI41680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.deGraffenried LA, Friedrichs WE, Russell DH, et al. Inhibition of mTOR activity restores tamoxifen response in breast cancer cells with aberrant Akt activity. Clin Cancer Res 2004;10:8059–67. 10.1158/1078-0432.CCR-04-0035 [DOI] [PubMed] [Google Scholar]
- 27.Petrelli A, Carollo R, Cargnelutti M, et al. By promoting cell differentiation, miR-100 sensitizes basal-like breast cancer stem cells to hormonal therapy. Oncotarget 2015;6:2315–30. 10.18632/oncotarget.2962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McShane LM, Altman DG, Sauerbrei W, et al. Reporting recommendations for tumor marker prognostic studies (REMARK). J Natl Cancer Inst 2005;97:1180–4. 10.1093/jnci/dji237 [DOI] [PubMed] [Google Scholar]
- 29.Viré E, Curtis C, Davalos V, et al. The breast cancer oncogene EMSY represses transcription of antimetastatic microRNA miR-31. Mol Cell 2014;53:806–18. 10.1016/j.molcel.2014.01.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lánczky A, Nagy Ádám, Bottai G, et al. miRpower: a web-tool to validate survival-associated miRNAs utilizing expression data from 2178 breast cancer patients. Breast Cancer Res Treat 2016;160:439–46. 10.1007/s10549-016-4013-7 [DOI] [PubMed] [Google Scholar]
- 31.Curtis C, Shah SP, Chin S-F, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 2012;486:346–52. 10.1038/nature10983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dowsett M, Smith IE, Ebbs SR, et al. Prognostic value of Ki67 expression after short-term presurgical endocrine therapy for primary breast cancer. J Natl Cancer Inst 2007;99:167–70. 10.1093/jnci/djk020 [DOI] [PubMed] [Google Scholar]
- 33.Cheang MCU, Chia SK, Voduc D, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst 2009;101:736–50. 10.1093/jnci/djp082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qureshi A, Fahim A, Kazi N, et al. Expression of miR-100 as a novel ancillary non-invasive biomarker for early detection of bladder carcinoma. J Pak Med Assoc 2018;68:759–63. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2020-000937supp001.pdf (112.3KB, pdf)
esmoopen-2020-000937supp002.pdf (4.1MB, pdf)