Abstract
Our patented protease A–digested crude chalaza hydrolysates (CCH) show antioxidant abilities in vitro. The prophylactic effects of CCH on cognitive dysfunction and brain oxidative damages were investigated via a D-galactose (DG)–injected mouse model in this study. Fifty-four mice were randomly divided into the following: (1) CON, 0.1 mL 0.9% saline (subcutaneous injection [SC] on the back)+distilled water (oral gavage); (2) DG, 100 mg/kg BW/day D-galactose (Bio-Serv Co., Flemington, NJ, USA) (SC on the back)+distilled water (oral gavage); (3) DG_LCH, 100 mg/kg BW/day D-galactose (SC on the back) + 50 mg CCH/kg BW/day in 0.1 ml distilled water (oral gavage); (4) DG_MCH, 100 mg/kg BW/day D-galactose (SC on the back) + 100 mg CCH/kg BW/day (oral gavage); (5) DG_HCH, 100 mg/kg BW/day D-galactose (SC on the back) + 200 mg CCH/kg BW/day (oral gavage); (6) DG_AG, 100 mg/kg BW/day D-galactose (SC on the back) + 100 mg aminoguanidine hydrochloride/kg BW/day (oral gavage). The experiment lasted for 84 D. CCH, containing antioxidant-free amino acids and anserine, restored (P < 0.05) DG-injected memory injury in the Morris water maze test and attenuated the neuronal degenerations and nucleus shrinkages in the dentate gyrus area. CCH supplementation also reduced amyloid β-peptide protein levels and accumulation of advanced glycation end products (AGE) in the brain of DG-injected mice, whereas the brain antioxidant capacity was reversed (P < 0.05) by supplementing CCH. Furthermore, AGE receptor (RAGE), NFκb, IL-6, and TNF-α gene expressions were downregulated (P < 0.05) by supplementing CCH. Therefore, CCH show prophylactic effects on the development of oxidative stress-induced cognitive dysfunction.
Key words: antioxidant/anti-inflammatory capacity, crude chalaza hydrolysate, cognitive dysfunction, D-galactose, hippocampus morphology
Introduction
As the global aging population increases rapidly, dementia, the best-known factor of which is aging, has caused a heavy social burden. Dementia, which is caused by damages of brain cells, is associated with a decline in memory or other thinking skills severe enough to interfere with the daily life. Oxidative stress is thought to be a main cause of dementia (Praticò, 2008). The reactive oxygen species (ROS) overproduction, which exceeds the endogenous antioxidant system ability, can result in oxidative injuries. In addition, the hippocampus, which plays a major role in forming and storing memories (Phelps, 2004), is one of the first regions in the brain to suffer oxidative damage, as a result of memory impairments and cognitive dysfunction (Seib and Martin-Villalba, 2015).
D-galactose (DG) is a reducing sugar, which can generate ROS and advanced glycation end products (AGE) during its metabolism in vivo, which leads to inflammatory response and neurodegenerative changes consequently (Ho et al., 2003). It has been reported that rodents chronically treated with DG show oxidative damages in the brain and cognitive dysfunction, accompanied by several age-related hallmarks such as mitochondria dysfunction, neuronal apoptosis, and neuro-inflammation (Cui et al., 2006). Ho et al. (2003) indicated that the formation of AGE is one of the possible aging mechanisms in the DG-induced aging model. Therefore, the DG-induced aging mouse model has widely been used in brain aging studies and antiaging pharmacology research studies. A recent report revealed that age-dependent changes in NO synthase (NOS) activity and expression attributed to inducible NOS (Lourenço et al., 2017). Aminoguanidine hydrochloride (AG), a novel inhibitor of inducible NOS, was proven to ameliorate formation of AGE in DG-treated mice (Song et al., 1999). Thus, the AG was determined as a referential positive control agent in this study.
Within recent decades, bioactive effects of food-derived protein hydrolysates have been attracted an attention (Chou et al., 2014, Abeyrathne et al., 2015, Lin et al., 2017). These protein hydrolysates obtained from plants or animals possess some or better beneficial bioactivities when compared with their parent proteins (Kim et al., 2012, Chou et al., 2014, Yang et al., 2019). In Taiwan, approximately 7 billion chicken eggs are produced per year, and for the 6% market share of liquid egg industries (Council of Agriculture, Executive Yuan, Taiwan 2015), it is estimated that about 400 ton chicken egg chalazae are produced in the liquid egg industry per year, which are regarded as byproducts and always abandoned. Our previous study has indicated that protease A–digested crude chalaza hydrolysates (CCH) are rich in antioxidant-free amino acids (i.e. leucine, arginine, lysine, phenylalanine) and dipeptides (carnosine/anserine), as well as possess free radical scavenging and metal ion chelating activities (Yang et al., 2019). Moreover, the administration of CCH exhibits a hepatoprotective effect against chronic alcohol consumption via ameliorating lipid accumulation, enhancing antioxidant capacities and decreasing the inflammatory cytokine values in livers. In this study, we aimed to investigate the prophylactic effects of CCH on cognitive dysfunction and oxidative damage in the brain of DG-injected mice. The main analyses in this study included the following: (1) the free amino acid profile and carnosine/anserine contents in raw chalazae and CCH; (2) learning and memory abilities via Morris water maze; (3) neuron protective effects in the hippocampus; (4) antioxidant and anti-inflammatory activities in brains of DG-injected mice.
Materials and methods
Preparation of FunctionalCCH and Free Amino Acid Profile Analyses
The chalazae were collected from the liquid egg production of Daiegg Co., Ltd. (Tainan City, Taiwan), which had passed the certification of Certified Agricultural Standards, and were immediately transported to our laboratory at −20 C. The CCH were prepared according to the previous method from our Taiwan patent (Chen and Lin, 2017). Briefly, the suitable amount of CCH powders for this study were manufactured by protease A (63,000 U/g; Amano Enzyme Ltd., Nagoya, Japan) digestion and then stored at −20 C for further experiments. The free amino acid contents of lyophilized crude chalazae and CCH powder were analyzed in the Food Industry Research and Development Institute (Hsinchu, Taiwan). The lyophilized chalazae and CCH were homogenized in 20 mL trichloroacetic acid (7%, v/v) for 2 min and then filtered. The precipitates were also homogenized twice with trichloroacetic acid as described previously. After filtering through the 0.45-μm filtration membrane, free amino acids were identified and quantified by using an amino acid analyzer (Hitachi L8800, Hitachi High-Technologies Co., Tokyo, Japan). The amounts of free amino acids were detected under 570 nm and 440 nm wavelength and calculated based on the peak area in comparison with amino acid and carnosine/anserine standards (Amino acids mixture standard solution, Type AN-II and Type B) (Wako Pure Chemical Industries, Ltd., Osaka, Japan). The data were presented as milligrams of free amino acids per 100 g lyophilized chalaza powders and CCH powders.
Animals and Treatments
Fifty-four 8-week-old male Institute of Cancer Research mice (approximately 28–30 g weight) were purchased from BioLASCO Taiwan Co., Ltd., Taipei, Taiwan. Three mice with an ear tag (No.1, 2, and 3) were housed in one cage in an animal room under a condition of 22 ± 2 C, 60 to 80% relatively humidity, and 12/12 h light–dark cycle. Chow diets (LabDiet 5001, PMI Nutrition International/Purina Mills LLC, Richmond, IN, USA) and distilled water were provided ad libitum for mice during the experimental period. According to our previous study, the administration of 100 mg CCH/kg BW/day could pose the hepatoprotective effects in C57BL/6 mice against chronic alcohol consumption (Yang et al., 2019). Hence, dosages as 50, 100, and 200 mg CCH/kg BW/day for low, medium, and high dosages, respectively, were given to the DG-treated mice.
After 4 wk of acclimation, 54 12-week-old mice were randomly divided into 6 groups (n = 9 in each group), which are as follows: (1) CON, 0.1 ml 0.9% saline (subcutaneous injection [SC] on the back) + 0.1 ml distilled water (oral gavage); (2) DG, 100 mg/kg BW/day D-galactose (Bio-Serv Co., Flemington, NJ, USA) in 0.1 mL saline (SC on the back) + 0.1 mL distilled water (oral gavage); (3) DG_LCH, 100 mg/kg BW/day D-galactose in 0.1 mL saline (SC on the back) + 50 mg CCH/kg BW/day in 0.1 mL distilled water (oral gavage); (4) DG_MCH, 100 mg/kg BW/day D-galactose in 0.1 mL saline (SC on the back) + 100 mg CCH/kg BW/day in 0.1 mL distilled water (oral gavage); (5) DG_HCH, 100 mg/kg BW/day D-galactose in 0.1 mL saline (SC on the back) + 200 mg CCH/kg BW/day in 0.1 mL distilled water (oral gavage); and (6) DG_AG, 100 mg/kg BW/day D-galactose in 0.1 mL saline (SC on the back) + 100 mg AG (Sigma-Aldrich Co., St. Louis, MO, USA)/kg BW/day in 0.1 mL distilled water (oral gavage). All mice were received each treatment for 84 D. On the 78th day of the experiment, mice were subjected to the behavioral test (Morris water maze) continuously for 5 D. The animal use and protocol were reviewed and approved by the Institution Animal Care and Use Committee (IACUC No.: NTU105-EL-00163) of National Taiwan University, Taiwan.
Behavioral Test (Morris Water Maze)
The Morris water maze test was started on the 78th day of the experiment, and the testing procedure was according to Tu et al. (2018). A circular water pool (100 cm in diameter, 80 cm in depth) and a movable escape platform (4.3 cm in diameter, 16 cm in height) were used in the experiment, as well as the pool was divided into 4 quadrants (z1: right; z2: target; z3: left; z4: opposite) with 4 different visual cues located above the water surface and placed on each quadrant on the interior side of the pool. Pool water was equilibrated to room temperature (23 ± 1°C) and mixed with skim milk powder and food coloring agent (Blue, Ever Style Foodstuff Industrial Co., Ltd., Taipei City, Taiwan) to reduce odor trail interference and make water opaque. The testing procedures were as follows: (1) Mice were placed into water gently facing the edge of the pool. If the mice found the platform in 60 s, they were allowed to stay on the platform for 15 s, returned to their cage to rest for 60 s, and then received the test again. (2) If the mice could not find the platform, they were gently guided to the platform and were allowed to stay on the platform for 15 s, and they received the test again after resting in the cage for 60 s. The same testing procedure was repeated 4 times in a row per day, and the swimming routs of mice were recorded by the video recorder right above the pool. All data were analyzed by the animal behavior monitor software (Singa Trace mouse II, Diagnostic & Research Instruments Co., Ltd., Taipei City, Taiwan). On the 77th day of the experiment (the end of 11th week experiment), the mice were first transferred to the test room to acclimatize the environment for at least 30 min before testing. Then, the escape platform, which was tied using red rubber bands on the top to increase its visibility, emerged 1 to 1.5 cm above the water surface and was placed in the middle of the pool for training for the behavioral test. Mice were placed into water to search the escape platform. On the starting day of the reference memory test (78th day), the escape platform without red rubber bands was hidden in the midpoint of z2 quadrant while additional water was added to the pool to submerge the platform to 1 to 1.5 cm below water surface. Then, mice were placed in the z3 quadrant as the starting quadrant, and the starting quadrant would be changed after 3 D (79th∼81st day) in a counterclockwise direction (z4, z1 to z2). The escape latency, the period needed to reach the platform, was recorded in 4 consecutive days (78th∼81st day), and each mouse would be received 4 trials per day. Meanwhile, the swimming speed per mouse on each testing day was also calculated. For the probe test (82nd day), the escape platform was removed from the z2 quadrant, and the mice were placed in the z3 quadrant to swim freely for 60 s. The searching period in the target quadrant (The hidden platform was previously placed in z2 quadrant) and the number of crossing over the z2 quadrant were recorded in every trial.
Sample Collection and Serum Biochemical Values
During the experimental period, food and water intakes and body weights were recorded weekly. At the end of the experiment (84th day), mice were fasted overnight and then sacrificed the next day. The blood samples were collected through the orbital sinus and kept at room temperature for 1 h to allow clotting. Then the sera were collected by centrifugation of the collected blood samples at 3,000 × g at 4 C for 15 min (Centrifuge 3,700; Kubota Co., Tokyo, Japan) and then stored at −20°C for further analyses. The brain from each mouse were removed and weighted individually. To determine the physiological change by the injection of DG and the CCH administration, the serum aspartate transferase, alanine transferase, triglyceride (TG), and total cholesterol levels were analyzed by using commercial enzymatic kits with a SPOTCHEMTM EZ SP-4430 automatic dry biochemistry analyzer (ARKRAY Inc., Kyoto, Japan).
Assignment of Brain Tissues for Further Analyses
Three brains from different mice per group (No. 1 mouse in each cage per group) were sectioned by using the rodent brain matrix (Sunpoint Co., Taoyuan City, Taiwan); the sectioned brain tissues involved all of the hippocampus area and most of the cerebral cortex area. The sectioned brain tissues per treatment group were placed in 10% formaldehyde solution (Merck Millipore Co., Darmstadt, Hesse, Germany) for a histopathological examination. The other 6 sectioned brain tissues per group were separated into 2 hemispheres. The left hemispheres were preserved in 1.0 mL RNA later (Qiagen Co., Valencia, CA, USA) at 4°C overnight and then stored at −80°C for further gene expression analyses, and the right ones were equally divided into 2 sections where one was stored in 1.5 mL tube at −80°C for further Western blotting analyses, and the other section was stored in 2.0 mL tube at −80°C for the preparation of brain homogenates, which were used for further antioxidative capacity analyses.
Histopathological Sections and Staining
The sectioned brain tissues were fixed in 10% neutral-buffered formalin solution (Merck Millipore Co., Darmstadt, Hesse, Germany) up to 24 h, dehydrated in graded alcohol (30, 50, 70, 95, and 99.5%; Sigma-Aldrich), cleared in xylene (Merck Millipore Co., Darmstadt, Hesse, Germany), and embedded in paraffin wax (Leica Microsystems, Singapore) via an embedding plate (Electron Microscopy Sciences, Hatfield, USA) and a plastic ware (Simport, Beloeil, Canada) by the Digital Dry Bath Incubator (Genepure Technology, Taipei, Taiwan) at 63°C. The blocks were then sliced into 5-μm-thick slices by using a microtome (Model#: HM315 R, Thermo Fisher Scientific, Inc., Waltham, MA, USA), spread out in a water bath at 43°C, and dried by electronic heating plate at 35 C. Importantly, all of the slices per sample were in the same section where the dentate gyrus and Ammon's horn of the hippocampus can be observed clearly. For hematoxylin and eosin staining, the slides were deparaffinized in xylene for 20 min, rehydrated in graded alcohol, and stained with hematoxylin (Merck Millipore Co., Darmstadt, Hesse, Germany) and eosin (Merck Millipore Co., Darmstadt, Hesse, Germany) for an optimal period (20 s for hematoxylin, 20 min for eosin). After dehydration and mounting, the photomicrographs of each slide were taken under a LEICA DM500 microscope (Leica Microsystems, Singapore) with IHD-4600 camera system (Sage Vision Co., LTD, New Taipei City, Taiwan) and Toup View 3.7 software (ToupTek Co., LTD, Hangzhou, China).
Immunohistochemical Analysis for β-Amyloid Accumulation in the Dentate Gyrus
The commercial kit (Mouse IgG, PK-6102) of the immunochemistry assay was purchased from VECTOR Laboratories, Inc. (Burlingame, CA, USA), and the procedure was in accordance with the instruction manual. First, the slides of each group were deparaffinized and hydrated by xylene and graded alcohol, and were washed with tap water and PBS buffer (pH 7.4). Then, antigen retrieval step was followed for antigen presenting; the slides were sunk into sodium citrate buffer (10 mM citric acid [Sigma-Aldrich], 0.05% Tween-20 [Sigma-Aldrich], pH 6.0) and heated by using an autoclave (120°C∼122°C) for 2 min. After cooling the slides with tap water, endogenous peroxidase activity blocking procedure was performed using 1% H2O2/methanol buffer (Sigma-Aldrich) for 15 min and avidin/biotin blocking kit (SP-2100; VECTOR Laboratories, Inc., Burlingame, CA, USA) for 30 min. Before antibody incubation, the blocking buffer that contains animal sera was added on the tissue area for an optimal period (8 h). Then, the slides were incubated with primary antibody (anti–Amyloid beta (Aβ) A4 protein, clone MM26-2.1.3) (Merck Millipore Co., Darmstadt, Hesse, Germany), which was diluted to an optimal concentration (1:100) overnight. Next, the diluted biotinylated secondary antibody buffer was added for an optimal reaction period (30 min), and the slides were incubated with VECTASTAIN Elite ABC reagent for 30 min. Finally, slides were hybridized with DAB substrate reagent (Cat. No. SK-4100, VECTOR Laboratories, Inc., Burlingame, CA, USA) for an optimal period (3.5 min) until desired stain intensity develops, and the slides were rinsed in tap water immediately. PBS buffer was added to wash the slides for 5 min 3 times between different buffers in the whole experiment. After all the aforementioned steps, counterstaining with hematoxylin would be performed. After dehydration and mounting, the photomicrographs of each slide were obtained under a LEICA DM500 microscope (Leica Microsystems, Singapore) with IHD-4600 camera system (Sage Vision Co., LTD, New Taipei City, Taiwan) and Toup View 3.7 software (ToupTek Co., LTD Hangzhou, China).
Preparation of Brain Homogenates
The weighted right hemisphere of the brain from the rest of the mice (No. 2 and 3 mouse in each cage per group) was homogenized on ice with 9-fold volume of PBS (pH 7.4, including 0.25 M sucrose) by a homogenizer (Polytron, PT-2100; Kinematica AG, Lucerne, Switzerland) and centrifuged at 1,000 × g at 4°C for 15 min. The supernatant was collected for further antioxidative analyses. The protein concentration in the supernatant was qualified by a Bio-Rad protein assay kit (catalog # 500-0006; Bio-Rad Laboratories, Inc.) via using bovine serum albumin as a standard.
Western Blotting for Aβ and AGE Protein in Brain Tissues
Before Western blotting, the protein extraction of the brain homogenates had been performed. The brain homogenate (300 μL) was homogenized in the same amount of RIPA (Radioimmunoprecipitation assay) buffer (Merck Millipore Co., Darmstadt, Hesse, Germany) containing protein inhibitors and was incubated on ice for 30 min. The lysate was then centrifuged (13,200 × g, 4°C, 15 min), and the supernatants were collected. All the samples, which was diluted to the same protein concentration that was measured by using Bio-Rad protein assay kit (catalog # 500-0006; Bio-Rad Laboratories, Inc., Hercules, CA, USA) were mixed with 5X sample buffer containing β-mercaptoethanol (Amresco, LLC., Solon, OH, USA) to a ratio of 1:4 and heated at 100 C for 10 min. After cooling, the samples were stored at −80 C for Western blotting. Equal amounts of proteins (40 μg) with different molecular mass were separated by 10% sodium dodecyl sulphate–polyacrylamide gel electrophoresis and then transferred to the polyvinylidene difluoride membrane (Amersham Hybond-P, No. RPN303 F, GE Healthcare, Buckinghamshire, UK) via the TE77XP semidry blotter (Hoefer, Inc., Richmond, USA) or the Mini Trans-Blot electrophoretic transfer cell (Bio-Rad Laboratories, Inc.). The membrane was first blocked with 5% skim milk for 1 h at room temperature and then incubated with primary antibodies against β-actin (1:5000 dilution, Level Biotechnology, Inc., New Taipei City, Taiwan), AGE polyclonal antibody (bs-1158 R, 1:1,000 dilution, Bioss Inc., Woburn, MA, USA), and anti-Aβ A4 protein (clone MM26-2.1.3, 1:1,000 dilution, Merck Millipore Co., Darmstadt, Hesse, Germany) overnight at 4 C. After washing with PBST (0.1% Tween-20 in PBS) for 5 min 3 times, the membrane was incubated with conjugated anti–mouse IgG–horseradish peroxidase (1:10,000 dilution, Thermo Fisher Scientific Taiwan Co., Ltd., Taipei, Taiwan) at room temperature for 1 h. The protein bands were detected with the chemiluminescence kit (Immobilon Western, Millipore Co., Billerica, MA, USA) and analyzed by MultiGel-21 (TOP BIO CO., New Taipei City, Taiwan). AGE are known as Amadori, Schiff base, and Maillard products, which could be of various molecular weight owing to the random site of glycation. Therefore, the whole bands in each lane were quantified in this study (Lu et al., 2011). ImageJ software (National Institutes of Health, Bethesda, MD, USA) was used to quantify the optical density of protein bands with the value of the β-actin band as a reference.
Antioxidative Capacity Analyses in Sera and Brains
The thiobarbituric acid reactive substances (TBARS), trolox equivalent antioxidant capacity (TEAC) and reduced glutathione (GSH) levels in sera or brain tissues were measured according to the previous procedure (Lin et al., 2017). Sixty microliter sera or brain homogenate (PBS for blank) was mixed with 90 μL TBA (J.T. Baker; Mallinckrodt Baker, Inc., Philipsburg, NJ, USA) solution and 510 μL trichloroacetic acid–hydroxyl chloride reagent (Sigma-Aldrich) in order. The samples were heated in a water bath (95°C) for 30 min, cooled on ice for 10 min, and centrifuged at 1,000 × g at 4 C for 5 min. The TBARS values of serum and brain tissue were calculated by considering the extinction coefficients of malondialdehyde to be 1.56 × 105 M−1 cm−1 at 535 nm. The TEAC value was measured by the decrease in absorption at 734 nm after the addition of reactant via a standard curve for trolox on scavenging 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS+) capacity. The decrease in absorption at 734 nm after the addition of reactant was used to calculate the TEAC value. The TEAC values in samples were calculated based on a standard curve plotted for trolox on scavenging 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) capacity. The higher TEAC value of a sample results in stronger antioxidative activity. The cycsteine group among reduced GSH can bind to 5,5′-dithiobis (2-nitrobenzoic acid) and undergo a color reaction to produce a yellow product (5-thio-2-nitrobenzoic acid), which can be measured at the absorption peak of 412 nm wavelength. The reduced GSH contents in serum and brain tissue were calculated by taking the extinction coefficients of 5-thio-2-nitrobenzoic acid to be 1.36 × 104 M−1 cm−1 at 412 nm.
The activities of superoxide dismutase (SOD), catalase (CAT), and GSH peroxidase (GPx) in brain tissues were assayed as per the previous methods (Tu et al., 2018). The SOD activities of brain tissues were measured by the inhibition of SOD on pyrogallol autoxidation. The alteration of absorbance per min under 420 nm wavelength was calculated to obtain inhibition (%) by the following formula: 1-[(sample or standardΔA/min)/(max speedΔA/min)], where max speed was set to reaction of ddH2O loading, indicating nearly no inhibition of pyrogallol autoxidation. A standard curve was plotted for SOD, which was used for calculation of activities of SOD in liver tissues. Regarding the measurement of CAT, a mixture of 50 μL brain homogenate and 450 μL PBS (pH 7.4, 0.25 M sucrose) was reacted with 250 μL H2O2 (30 mM). The difference in absorbances between the initial time period and 60 s were measured at 240 nm wavelength. The CAT activity was calculated by considering the extinction coefficient of H2O2 (0.0394 mM−1 cm−1). One unit of CAT was expressed as the amount of enzyme that decomposed one μmole H2O2 in 1 min at 25 C. The enzymatic activity of GPx was measured by using the RANSEL assay kit (Randox Laboratories Ltd., Crumlin, UK). The principle of assay is based on the following: GPx catalyses the oxidation of GSH by cumene hydroperoxide. In the presence of GSH reductase and NADPH, the oxidized GSH is immediately transformed to the reduced form with a concomitant oxidation to NADP+. Four microliter brain homogenate was mixed with 8 μL cumene hydroperoxide and 200 μL reagents. The GPx activities of brain tissues were calculated by the decrease of absorbance between the initial time period and 1.5 min at 340 nm wavelength.
mRNA Expression Analyses
The total RNA in the left hemisphere of brain tissues of rest of the mice (No. 2 and 3 mouse in each cage per group) were extracted by using a commercial kit (TrisureTM, Bioline, London, UK), and the cDNA was synthesized from total RNA by a reverse transcriptional reaction as per the manufacturer's protocol offered by GoSript Reverse Transcriptase (Promega Co., Fitchburg, WI, USA). As per the principle of quantitative real-time PCR, all the primers were designed by using the National Center for Biotechnology Information primer blast for the specific gene sequences to amplify the specific cDNA fragments: glyceraldehyde 3-phosphate dehydrogenase (Gapdh, F: 5′-AACCTGCCAAGTATGATGA-3′, R: 5′-GGAGTTGCTGTTGAAGTC-3’; Accession No.: NM_001289726.1); receptor for advanced glycation end-products (Rage, F: 5′-CAGCATCAGGGTCACAGAAAC-3′; R: 5′-CGTTTTCGCCACAGGATAGC-3′; Accession No.: NM_007425.3); nicotinamide adenine dinucleotide phosphate-oxidase 2 (Nox2, F: 5′-AGCTATGAGGTGGTGATGTTAGTGG-3′; R: 5′-CACAATATTTGTACCAGACAGACTTGAG-3′; Accession No.: FJ168469.1); nuclear factor kappa B (Nfκb, F: 5′-TGGGGCCTGCAAAGGTTATC-3′; R: 5′-TTTGCAAAGCCAACCACCAT-3′; Accession No.: NM_001276711.1); interleukin 1 beta (IL-1β, F: 5′-GCCACCTTTTGACAGTGATG-3′; R: 5′-AGTGATACTGCCTGCCTGAA-3′; Accession No.: NM_008361.3); interleukin 6 (IL-6, F: 5′-GCCATTGCACAACTCTTTTCTC-3′; R: 5′- GCCATTGCACAACTCTTTTCTC-3′; Accession No.: NM_031168.1); tumor necrosis factor alpha (Tnfα, F: 5′-CCCCACTCTGACCCCTTTAC-3′; R: 5′-ACTGTCCCAGCATCTTGTGT-3′; Accession No.: NM_013693.3). The quantitative PCR was performed as per the instructions of ABI Fast SYBR Green Master Mix (Applied Biosystems; Thermo Fisher Scientific, Inc., Foster, CA, USA). The sizes of the reaction products are as follows: Gapdh, 119 bp; Rage, 150 bp; Nox2, 88 bp; Nfκb, 145 bp; IL-1β, 165 bp; IL-6, 149 bp; and Tnfα, 157 bp. The fluorescence signals of each target gene were detected by using the StepOne Real time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The mouse Gapdh gene was used as an internal control, and the values of all genes in DG, DG_LCH, DG_MCH, DG_HCH, and DG_AG groups were expressed in relation to the average value of CON group, which was set to 1.0.
Statistical Analyses
All of the experimental data were analyzed by SAS 9.4 software (SAS Institute Inc., Cary, NC, USA). When a significant difference was detected at the 0.05 probability level by using an analysis of variance, differences between treatments were tested by using the Least Significant Difference test.
Results
Effects of CCH on Growth Performance, Relative Sizes of Brain, Liver, and Fat Tissues and Serum Biochemical Values of DG-Injected Mice
The contents of all free amino acids in functional CCH used in this study were 11,534.76 mg/100 g dried CCH powder when compared with the 178.16 mg/100 g of lyophilized chalazae, which is 64.7 times higher (Table 1). The major amino acids in CCH are leucine, arginine, lysine, and phenylalanine, which account for 13.84, 11.22, 9.92, and 7.78% in the total free amino acids, respectively. Besides, the histidine-containing dipeptide (anserine) was not assayed in lyophilized crude chalazae but in CCH (309.06 mg/100 g dried CCH powder), whereas carnosine contents were analyzed in neither lyophilized crude chalazae nor CCH. After 12 wk of experiment, body weights and food and water intakes were not affected (P > 0.05) among all groups (Table 2). Regarding serum biochemical values, no (P > 0.05) differences on serum aspartate transaminase, TG, and total cholesterol values were recorded among all groups, but lower (P < 0.05) alanine transaminase values were measured in both the DG_HCH and DG_AG groups than in the other groups (Table 2). However, a higher tendency toward serum TG values in mice with DG injection, except the cotreatment of aminoguanidine (DG_AG group) than those without DG injection (CON group) was observed. Besides, there were no (P > 0.05) differences in the relative sizes of the brain among all groups (Table 2).
Table 1.
Contents of free amino acids and dipeptides (anserine and carnosine) in lyophilized chalazae and functional chalazae hydrolysates (CCH).
| Amino acid | Item |
|
|---|---|---|
| Chalazae (mg/100g dried wt.) | CCH (mg/100 g dried wt.) | |
| Leucine | 23.21 ± 4.49 | 1,596.08 ± 81.33 |
| Arginine | 6.36 ± 3.02 | 1,294.72 ± 83.93 |
| Lysine | 10.05 ± 2.66 | 1,144.80 ± 64.18 |
| Phenylalanine | 9.71 ± 1.83 | 896.92 ± 65.81 |
| Valine | 12.58 ± 6.11 | 691.46 ± 59.52 |
| Isoleucine | 4.30 ± 1.80 | 641.03 ± 42.14 |
| Threonine | 10.83 ± 1.01 | 417.06 ± 23.37 |
| Methionine | 4.59 ± 2.62 | 332.94 ± 29.23 |
| Tryptophan | N.D. | 323.54 ± 49.18 |
| Histidine | N.D. | 268.40 ± 15.38 |
| Total EAA | 81.61 ± 11.58 | 7,606.95 ± 500.20 |
| Total BCAA | 40.09 ± 10.61 | 2,928.57 ± 182.41 |
| L-Alanine | 10.61 ± 6.85 | 515.83 ± 29.25 |
| Asparagine | N.D. | 505.50 ± 39.38 |
| Tyrosine | 4.26 ± 2.25 | 464.26 ± 45.04 |
| Glutamic acid | 33.36 ± 6.54 | 410.70 ± 33.95 |
| Serine | 4.56 ± 2.78 | 396.77 ± 31.69 |
| 3-Aminoisobutyric acid | N.D. | 388.27 ± 151.48 |
| o-Phosphorserine | 4.84 ± 0.77 | 325.35 ± 40.66 |
| Glycine | 6.77 ± 2.50 | 154.93 ± 9.10 |
| Proline | 10.18 ± 0.31 | 146.25 ± 11.35 |
| β-Alanine | 0.67 ± 0.06 | 128.74 ± 10.44 |
| Aspartic acid | 3.63 ± 2.48 | 110.52 ± 6.76 |
| 2-Aminoadipic acid | N.D. | 100.71 ± 11.18 |
| o-Phosphoethanolamine | N.D. | 94.11 ± 9.45 |
| Ornithine | 8.24 ± 2.88 | 82.05 ± 2.31 |
| Cystine | 8.89 ± 1.35 | 51.33 ± 8.35 |
| Sarcosine | N.D. | 48.02 ± 5.88 |
| DL-plus allo-δ-Hydroxylsine | 0.55 ± 0.48 | 4.45 ± 0.21 |
| Total NEAA | 96.55 ± 15.75 | 3,927.81 ± 308.60 |
| Anserine | N.D. | 309.06 ± 26.55 |
| Carnosine | N.D. | N.D. |
Data are given as mean ± SEM (n = 3).
Abbreviations: BCAA, branched-chain amino acids; EAA, essential amino acid; N.D., not detectible; NEAA, non-essential amino acid.
Table 2.
Growth performance, relative sizes of brains, serum biochemical values, and serum and brain antioxidative statuses of the experimental mice.
| Parameter | Treatment |
|||||
|---|---|---|---|---|---|---|
| CON | DG | DG_LCH | DG_MCH | DG_HCH | DG_AG | |
| Growth performance | ||||||
| Initial body weight (g) | 37.44 ± 0.47a | 37.60 ± 0.74a | 37.39 ± 0.59a | 38.04 ± 0.56a | 36.87 ± 0.65a | 37.22 ± 0.59a |
| Final body weight (g) | 40.81 ± 1.98a | 39.94 ± 1.36a | 40.49 ± 1.42a | 41.60 ± 1.46a | 40.11 ± 1.00a | 39.84 ± 1.07a |
| Food intake (g/mouse/day) | 7.17 ± 0.20a | 7.61 ± 0.13a | 7.12 ± 0.57a | 7.14 ± 1.10a | 7.14 ± 0.37a | 7.06 ± 0.32a |
| Water intake (g/mouse/day) | 6.90 ± 0.26a | 6.25 ± 0.45a | 6.47 ± 0.44a | 6.43 ± 0.38a | 7.04 ± 0.22a | 6.28 ± 0.27a |
| Relative size (g/100 g BW) | ||||||
| Brain | 1.30 ± 0.07a | 1.30 ± 0.06a | 1.31 ± 0.04a | 1.29 ± 0.06a | 1.32 ± 0.03a | 1.33 ± 0.04a |
| Serum biochemical value | ||||||
| AST (IU/l) | 94.63 ± 4.49a | 97.33 ± 11.67a | 102.29 ± 7.56a | 83.75 ± 2.43a | 84.67 ± 13.81a | 80.33 ± 6.13a |
| ALT (IU/l) | 33.86 ± 2.82a | 32.29 ± 4.03a | 33.78 ± 3.16a | 25.89 ± 3.57a,b | 22.43 ± 2.68b | 19.80 ± 2.15b |
| TG (mg/dL) | 65.63 ± 5.51a | 87.43 ± 10.90a | 85.43 ± 6.41a | 80.89 ± 5.32a | 83.60 ± 6.49a | 68.6 ± 6.17a |
| T-Cho (mg/dL) | 136.44 ± 6.36a | 131.78 ± 9.51a | 139.44 ± 7.45a | 142.25 ± 8.13a | 122.00 ± 7.69a | 131.33 ± 10.11a |
| Serum antioxidative status | ||||||
| TBARS (nmol MDA eq./ml) | 28.03 ± 1.78c | 39.75 ± 3.03a | 33.76 ± 1.29b | 29.57 ± 1.30b,c | 27.55 ± 1.94c | 24.13 ± 1.32c |
| TEAC (μmol/mL) | 8.45 ± 0.24a,b | 7.96 ± 0.22b | 8.36 ± 0.09a,b | 8.39 ± 0.15a,b | 8.52 ± 0.15a | 8.56 ± 0.22a |
| Reduced GSH (nmol/mL) | 78.11 ± 8.23b | 56.39 ± 2.74c | 66.28 ± 5.36b,c | 95.66 ± 5.47a | 94.80 ± 6.42a | 101.07 ± 5.53a |
| Brain antioxidative status | ||||||
| TBARS (nmol MDA eq./mg protein) | 6.45 ± 0.37b | 7.34 ± 0.50a | 6.76 ± 0.18a,b | 5.96 ± 0.11b,c | 5.52 ± 0.22c | 6.14 ± 0.19b,c |
| TEAC (nmol/mg protein) | 463.34 ± 16.70a,b | 385.58 ± 11.87c | 399.82 ± 7.88b,c | 440.84 ± 17.00a | 445.73 ± 11.93a | 469.85 ± 9.88a |
| Reduced GSH (nmol/mg protein) | 376.34 ± 14.06b | 359.20 ± 16.48b | 371.63 ± 10.04b | 424.58 ± 7.94a | 425.99 ± 8.51a | 429.46 ± 9.89a |
| SOD activity (unit/mg protein) | 2.87 ± 0.20b | 1.94 ± 0.22c | 4.55 ± 0.02a | 4.25 ± 0.23a | 4.15 ± 0.12a | 3.35 ± 0.10b |
| CAT activit (munit/mg protein) | 4.33 ± 0.21a | 2.88 ± 0.23c | 3.67 ± 0.19b | 3.96 ± 0.20a,b | 4.45 ± 0.10a | 4.37 ± 0.24a |
| GPx activity (munit/mg protein) | 151.85 ± 3.79a,b | 128.38 ± 2.79c | 139.09 ± 4.03b,c | 158.14 ± 7.03a | 156.11 ± 2.41a | 162.43 ± 5.51a |
Data are given as mean ± SEM (n = 9, except food intake and water intake, n = 3, as well as brain antioxidative status, n = 6).
a-cMean values without the common letter in each test parameter indicate a significant difference (P < 0.05).
Abbreviations: ALT, alanine transferase; AST, aspartate transferase; CAT, catalase; GPx, glutathione peroxidase; GSH, glutathione; MDA, malondialdehyde; SOD, superoxide dismutase; TBARS, trolox equivalent antioxidant capacity; T-Cho, total cholesterol; TEAC, trolox equivalent antioxidant capacity; TG, triglyceride
Effects of CCH on Spatial Learning and Memory Abilities in Morris Water Maze of DG-Injected Mice
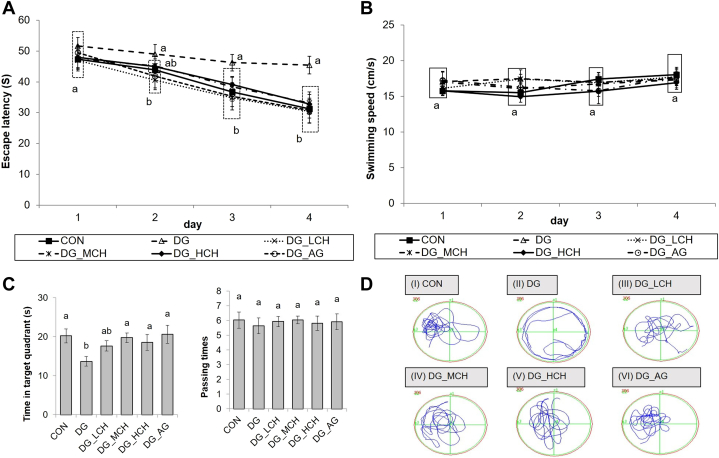
In the reference memory test (Figure 1A), the DG group exhibited significantly longer (P < 0.05) escape latencies on day 3 and day 4 (day 3: 46.23 ± 2.69 s; day 4: 45.45 ± 2.90 s) than those of the CON group (day 3: 36.71 ± 3.01 s; day 4: 31.25 ± 3.04 s). However, the administration of CCH and aminoguanidine shortened (P < 0.05) the escape latencies (day 3, DG_LCH: 34.78 ± 3.84 s; DG_MCH: 38.36 ± 3.15 s; DG_HCH: 39.07 ± 2.69 s; DG_AG: 35.25 ± 3.32 s; day 4, DG_LCH: 30.37 ± 2.90 s; DG_MCH: 33.11 ± 3.80 s; DG_HCH: 32.75 ± 3.65 s; DG_AG:30.72 ± 3.96 s) in the DG-injected mice (day 3∼4), and their escape latencies were similar (P > 0.05) to that in the CON group (day 2∼4). Meanwhile, there were no (P > 0.05) differences in the swimming speeds among all groups in the reference memory test (Figure 1B). In the probe test (Figure 1C), the DG group significantly spent less (P < 0.05) searching time in the target quadrant (13.69 ± 1.19 s) than that of the CON group (20.21 ± 1.82 s), whereas the administration of middle and high dosages of CCH (100 and 200 mg/kg BW/day) or aminoguanidine significantly increased (P < 0.05) the searching time spent in the target quadrant (DG_MCH: 19.75 ± 1.23 s; DG_HCH: 18.53 ± 2.03 s; DG_AG: 20.62 ± 2.27 s) compared with DG group. Besides, no (P > 0.05) differences in the number of crossing over the target quadrant were recorded among all groups (Figure 1C). According to the illustrations of swimming routes among groups, the DG group swam along the edge of the pool compared with the control group, but the DG-and AG-supplemented group swam around the center of pool (Figure 1D).
Figure 1.
Effects of crude chalazae hydrolysates on learning and memory abilities in Morris water maze of D-galactose injected mice. (A) Escape latency in the reference memory test. Mice were treated with 4 trials per day for continuous 4 D. (B) The average swimming speeds of the experimental mice during the reference memory test. (C) The spent periods in the target quadrant and the numbers of crossing over the target quadrant in the probe test (day 5). The hidden platform was previously placed in the target quadrant (z2). (D) The swimming route of each group was recorded in the probe test (I ∼ VI). 1Data are given as mean ± SEM (n = 9). 2Data points within the same testing day or data bars without the common letter indicate a significant difference (P < 0.05).
Effects of CCH on Histological Pathology and Aβ Depositions in the Hippocampus of DG-Injected Mice
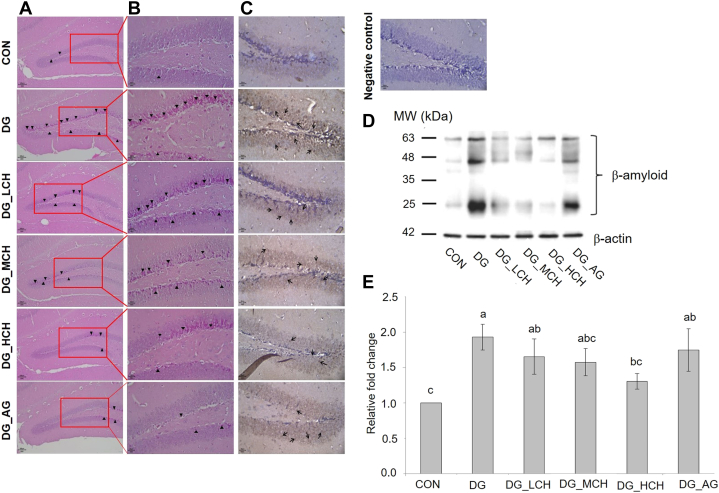
The morphological features of the dentate gyrus area in the mouse hippocampus from each group were evaluated by using hematoxylin and eosin staining (Figure 2A and 2B). In comparison with the CON group, the DG group exhibited large amounts of contracted neuron bodies with condensed cytoplasm where the whole neurons were entirely stained by hematoxylin in the granule cell layer (GCL) and the subgranular zone (SGZ, the border between the hilus and GCL). These contracted neuron bodies were referred as dark neurons and were presented as black triangles. With the increased dosages of CCH, the dark neurons in the dentate gyrus area were markedly reduced, and the morphological feature of the dentate gyrus area in the DG_HCH group was quite similar to that in CON and DG_AG groups. Furthermore, via an immunohistochemical staining of Aβ, the extracellular depositions of Aβ in the dentate gyrus area were stained and observed with brown dye in each group (Figure 2C). In the DG group, the extracellular depositions of Aβ (black arrows) were easily observed in the dentate gyrus area in comparison with the CON group, whereas the administration of CCH or aminoguanidine did not completely reduce the Aβ depositions in the dentate gyrus area. Hence, the Aβ protein expressions in the mouse brain were further analyzed by using Western blotting (Figure 2D). Because the anti-Aβ antibody used in this study was suitable for the detection of different oligomers of Aβ in tissues as protocol indicated (Merck Millipore Co., Darmstadt, Hesse, Germany), several proteins bands for Aβ were illustrated. However, when DG group exhibited higher (P < 0.05) Aβ levels in the brain tissues than in the CON group, the elevated Aβ levels were reduced on the increased dosages of CCH administrations (Figure 2E). In addition, the high dosage of CCH (200 mg/kg BW/day) significantly reduced (P < 0.05) the Aβ levels in the brains of DG-injected mice, and the Aβ levels in the brains of DG_HCH group were even similar (P > 0.05) to that of the CON group. Besides, no (P > 0.05) significant reduction of the Aβ expression was assayed in aminoguanidine cotreated group.
Figure 2.
Morphological features of the dentate gyrus area in mouse hippocampus from each group were evaluated by hematoxylin and eosin staining at 100X (A) and 400X (B) magnifications. The presence of dark neurons characterized by contracted neuron bodies (black triangles). (C) β-amyloid accumulations in the dentate gyrus area from each group were shown as brown dye by using DAB-revealed horseradish peroxidase immunohistochemistry. Brain sections were examined with primary antibody (except negative control) and hematoxylin stained at 400X magnification. The black arrows indicate the depositions of β-amyloid. Western blotting analysis (D) and quantification (E) of the relative amounts of β-amyloid in the mice brains. 1Data bars are given as mean ± SEM (n = 6). 2Not-sharing the common letter on data bars indicates a significant difference (P < 0.05).
Effects of CCH on Serum and Brain Antioxidant Capacities, and Inflammatory Responses in the Brains of DG Injected Mice
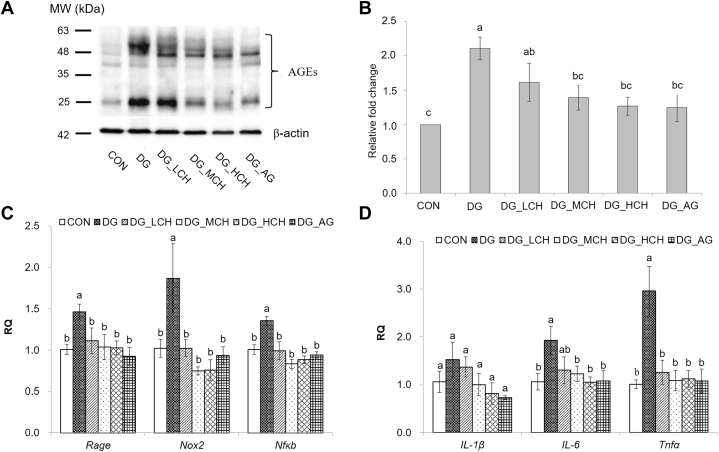
Serum and brain antioxidant capacities of the experimental mice were demonstrated in Table 2. The (P < 0.05) serum TBARS value measured in the DG group was higher than that of the CON group, but the administration of CCH or aminoguanidine significantly decreased (P < 0.05) those values in DG-injected mice. In comparison with the CON group, only a lower tendency toward serum TEAC value was detected in the DG group, whereas the administration of CCH or aminoguanidine reversed the depleted values and increased (P < 0.05) effects were observed in DG_HCH and DG_AG groups than in the DG group. In addition, the DG group had the lower (P < 0.05) GSH content in sera than the CON group, but the cotreatments of CCH (middle and high dosages) or aminoguanidine increased (P < 0.05) them. Regarding the brain antioxidant capacities, the DG group also exhibited the higher (P < 0.05) TBARS value than CON group, whereas the administration of CCH (middle and high dosages) or aminoguanidine reduced (P < 0.05) the elevated values compared with DG group. In contrast, TEAC values in the brains were reduced (P < 0.05) in DG group when compared with the CON group, but no (P > 0.05) differences on reduced GSH contents in brains were measured between CON and DG groups. However, the administration of CCH (middle and high dosages) or aminoguanidine increased (P < 0.05) both values. In addition, DG group showed the lower (P < 0.05) SOD, CAT, and GPx activities than those of CON group, but the administration of CCH or aminoguanidine increased (P < 0.05) those activities except GPx activities in DG_LCH group (Table 2). To evaluate the effects of CCH on brain inflammatory responses via AGE/RAGE/NFκB pathway, the protein expressions of AGE in mouse brains were analyzed by using the Western blotting method (Figure 3A). DG group exhibited the higher (P < 0.05) AGE protein expression in the brains than that of the CON group; the elevated AGE protein expressions were alleviated (P < 0.05) by the administration of CCH (middle and high dosages) or aminoguanidine, and those values were even similar (P > 0.05) to that of the CON group (Figure 3B). Moreover, the relative expressions of genes related to inflammatory responses in brains of the experimental mice were analyzed as well (Figure 3C and 3D). Higher (P < 0.05) Rage, Nox2 (NADPH oxidase), and Nfκb gene expressions were detected in the DG group than in the CON group; however, the CCH or aminoguanidine administration indeed downregulated (P < 0.05) those gene expressions (Figure 3C). Regarding the expressions of proinflammatory genes, although no (P > 0.05) differences on the IL-1β gene expressions in brains were detected among all groups, there was a tendency toward the lower IL-1β gene expression in DG-injected mice cotreated with CCH or aminoguanidine (Figure 3D). Higher (P < 0.05) IL-6 and Tnfα gene expressions were observed in the DG group than in the CON group, but the administration of CCH or aminoguanidine downregulated (P < 0.05) them except IL-6 gene expression in the DG_LCH group (Figure 3D).
Figure 3.
Western blotting analysis (A) and quantification (B) of the relative amounts of advanced glycation end products (AGE) in the mouse brains. (C) and (D) The relative expression of genes involved in inflammation of brain cells of the experimental mice. Total RNA was isolated and analyzed by qPCR for the expressions of inflammation relative genes. (C) Rage, Nox2, and NFκb. (D) IL-1β, IL-6, and Tnfα. The values of mRNA were normalized to the value of Gapdh. 1Data are given as mean ± SEM (n = 6). 2Not-sharing the common letter on data bars indicate a significant difference (P < 0.05).
Discussion
Oxidative stress is thought to be a main cause of dementia (Praticò, 2008). The ROS overproduction, which exceeds the endogenous antioxidant system ability, can result in oxidative injuries. An increasing number of studies on antioxidant properties of food-derived hydrolysates or peptides have been available recently (Rajapakse et al., 2005, Hsieh et al., 2009, Chou et al., 2014, Lee et al., 2017). Based on the free amino acid profile in CCH, some major amino acids including leucine, phenylalanine, valine, isoleucine, and alanine all belong to hydrophobic amino acids. After protease A hydrolyzation, the total amount of hydrophobic amino acids increased 64.6 folds compared with that in lyophilized chalazae and accounted for as much as 45.94% in the total free amino acids. It has been reported that the hydrophobic amino acids can facilitate interaction with free radicals by increasing their solubilities in lipids (Qian et al., 2008). Scott and Bolton (2000) also indicated that arginine can suppress the brain inflammation and reduce free radical production in the experimental allergic encephalomyelitis rat model. In addition, the aromatic amino acids (phenylalanine, tryptophan, histidine, and tyrosine) account for 17.29% in CCH. Besides, aromatic amino acids can also act as proton donors to scavenge free radicals (Rajapakse et al., 2005). The amount of acidic amino acids (glutamic and aspartic acids) can be significantly increased (14.09 folds) after protease-A hydrolyzation. Moreover, the contents of total branched-chain amino acids (BCAA) (leucine, valine, and isoleucine) increased to 73.05 folds after protease-A hydrolyzation, which account for 25.39% in the total free amino acids. The BCAA antioxidative activities have also attracted more attention and been investigated within recent years. The administration of BCAA has a prophylactic effect on liver function in a nonalcoholic steatohepatitis mouse model by reducing oxidative stress (Tanaka et al., 2016). On the other hand, histidine-containing dipeptides, that is carnosine, anserine, and homocarnosine, possess peroxyl radical-trapping and metal ion chelating activities owing to the histidine's imidazole ring (Maikhunthod and Intarapichet, 2005). Importantly, these free amino acids and dipeptides can pass through the blood–brain barrier via specific transporter proteins in different transport pathways to contribute to antioxidative abilities in the brain (Campos-Bedolla et al., 2014). Our previous study reported that even an injection of a high DG dosage (1.2 g/kg BW/day) for 6 wk does not affect the body weight and food/water intakes of C57BL/6J mice as well (Chou et al., 2014). Regarding the tendency towards the higher serum TG values in DG, DG_LCH, DG_MCH, and DG_HCH groups than in the CON group in this study, an injection with 500 mg DG/kg BW/day for 7 wk significantly increased serum TG values of mice which is due to the accumulation of AGE in sera, causing a pathogenic modification of lipoprotein and a dysfunction to clear lipid from the circulatory system (Zhu et al., 2014). Besides, aminoguanidine can be applied as an inhibitor of AGE in DG-induced mice (Tu et al., 2018). Based on the investigation of serum biochemical values of Institute of Cancer Research mice from Charles River Laboratories, Japan (2013), serum TG values in each group are still within the normal range. Hence, the administration of DG, CCH, and aminoguanidine did not have any adverse effect on physiological manifestation in this study.
The Morris water maze test has played an important role in the validation of rodent models for neurocognitive disorders and the investigation of possible neurocognitive treatments (Tu et al., 2018). D-galactose has been frequently used to induce cognitive dysfunction in rodent models. It was reported that a daily injection of 100 mg or 200 mg DG/kg BW to the 12-week-old C57BL/6 J mice or 8-10-week-old Kunming strain mice for 8 wk significantly prolongs the escape latencies in the reference memory test and reduces the searching time in the target quadrant in the probe test of mice (Wei et al., 2005). The current results indicated that the CCH administration significantly ameliorates impaired spatial learning and memory abilities of DG-injected mice and has a similarly ameliorative effect with the administration of aminoguanidine (positive control). Moreover, as shown in the Figure 1D, in comparison with the DG group, which tends to swim around the whole swimming pool with a directionless search, CON and CCH/aminoguanidine-administrated groups primarily swam back and forth in the target quadrant in the probe test. Besides, no differences in the swimming speeds were observed among all groups in the Morris water maze test, which indicates no influences of the motor function in the experimental mice (Figure 1B). Taken together, the CCH administration significantly ameliorates (P < 0.05) the cognitive dysfunction of DG-injected mice in the behavioral test. The hippocampus, located in the medial temporal lobe of the brain, belongs to the limbic system and plays an important role in forming and storing memories (Phelps, 2004). It was reported that the hippocampus is composed of 2 different neuron cells: (1) the pyramidal neurons form Ammon's horn and (2) the granule neurons form dentate gyrus. The dentate gyrus area is one of the few regions where neurogenesis takes place and composed of 4 main regions, which include molecular layer, GCL, SGZ, and hilus (Liaury et al., 2012). A high rate of neurogenesis in SGZ increases learning and memory abilities, but in contrast, a decrease in neurogenesis results in neurodegeneration and also shows a deleterious effect on cognitive function (Shors et al., 2001). It has been well known that aging and oxidative stress are the greatest risk factors for neurodegeneration (Seib and Martin-Villalba, 2015). A daily injection of 100 mg DG/kg BW to the C57BL/6J mice for 7 wk decreased the proliferation of endogenous progenitor cells in the SGZ and reduced the migration and survival of new neurons in the GCL, which leads to cognitive dysfunction in the Morris water maze test (Cui et al., 2006). In addition, cells with severe cytoplasmic condensations were observed in the SGZ and GCL of DG-injected mice. The contracted cells are also referred to as dark neurons, which are mainly characterized with the following electron microscopic characteristics: (1) a marked condensation of cytoplasm, (2) intact nuclear membrane and cytoplasmic organelles, (3) mitochondrial swelling, and (4) an aggregation of the nuclear chromatin (Ooigawa et al., 2006). The results of hippocampal histology indicated that the CCH administration especially in the highest dosage (200 mg/kg BW/day) could markedly reduce the large amounts of dark neurons in the SGZ and GCL of DG-injected mice (Figure 2A and 2B). Hence, the improved cognitive function of CCH in DG-injected mice in the Morris water maze test might be highly corresponding to the reduction of neurodegeneration in SGZ and GCL. Moreover, the extracellular deposition of Aβ is one of the pathological hallmarks in the progress of Alzheimer's disease. Aβ itself has an oxidative ability and creates a positive feedback on amyloid precursor protein (APP) levels by inducing more oxidative stress; with time, the accumulation of Aβ deposits in the brain and triggers further oxidative stress reactions and inflammatory responses, which ultimately translate into irreversible neurodegeneration of brains' neurons (Praticò, 2008). It was indicated that the Aβ depositions in the hippocampus of 15-month-old human APP V717 F transgenic mice (with overexpressing mutant APP) are mainly in diffuse but not compact form and mostly distributed in the dentate gyrus area (Reilly et al., 2003). According to aforementioned references, the Aβ depositions in the dentate gyrus area of DG-injected mice in our study could be confirmed, and they were supposed to be classified as the diffuse form. Although the administration of CCH or aminoguanidine did not obviously reduce the Aβ depositions in the dentate gyrus area, decreased Aβ levels in the whole brain tissues could be observed (Figure 2C–2E). In addition, it was reported that soy isoflavones can reduce the elevated Aβ levels in the DG (120 mg/kg BW/day, 50 D)-treated mouse brains via attenuating oxidative stress in the brains (Hsieh et al., 2009). Hence, the reduced effects on Aβ levels in brain tissues of DG-injected mice by cotreated with CCH might contribute to their antioxidant capacities. Besides, it seems that CCH (especially the high dosage) had more significantly reduced effects on Aβ protein levels than by aminoguanidine (Figure 2E). It has been proven that an arginine-containing small compound (Arg-Arg-coumarin) could inhibit the formations and aggregations of 42-residue beta amyloid in vitro (Khaidar et al., 1994). Accordingly, it is assumed that arginine, one of the major free amino acids in our CCH, might take part in reducing Aβ protein levels in mouse brains, but it still needs further investigation. Moreover, it was reported that the main damage induced by Aβ depositions in the brain is the oxidative stress reaction and inflammatory response (Praticò, 2008).
The brain is highly susceptible to oxidative stress because of the following reasons: (1) The brain consumes about 20% of the total oxygen consumption for its relatively small weight (2%), but it possesses a relatively paucity of antioxidant systems compared with other organs; (2) The brain is enriched with polyunsaturated fatty acids and contains high contents of transition metals, which act as potent pro-oxidants (Floyd, 1999). Our previous report indicated that a daily injection of 1.2 g DG/kg BW to the 12-week-old C57BL/6J mice for 6 wk significantly increased serum and brain TBARS values and decreased brain TEAC values (Chou et al. 2014). Meanwhile, they reported that the administration of pepsin-digested chicken liver hydrolysates, which possess high contents of antioxidant-free amino acids and dipeptides, significantly increases in vivo antioxidative capacities in DG-treated mice. The similar results from present study should be supposed that the antioxidant-free amino acids (e.g., leucine, arginine, lysine, phenylalanine, and valine) and dipeptides (anserine) in our CCH are able to pass through the blood–brain barrier via specific transporters (Campos-Bedolla et al., 2014) to contribute their antioxidant capacities and attenuate oxidative stress in brains caused by DG treatment. Furthermore, the reduced Aβ protein levels in brains (Figure 2C–2E) and ameliorative neurodegeneration in the dentate gyrus area (Figure 2A and 2B) of DG-injected mice cotreated with CCH were corresponding to the reduction of oxidative stress in brains. It has been mentioned that the deleterious effects of AGE in Alzheimer's disease are attributed to their pro-oxidant and inflammatory responses, which is related to AGE/RAGE/NFκB pathway (Ahmed, 2005). The interaction of AGE with the RAGE (the receptor of AGE) triggers the activation of transcription factor NFκB (nuclear factor kappa B), thus leading to the production of inflammatory promoters such as tumor necrosis factor α and interleukin 6. Besides, AGE-RAGE interaction activates NADPH oxidase (a complex of enzymes which produces superoxide) and increases intracellular oxidative stress that also activate NFκB (Luevano-Contreras and Chapman-Novakofski, 2010). Moreover, a daily injection of 50 mg DG/kg BW to the 11-week-old Kunming strain mice for 8 wk could significantly increase AGE, RAGE, and NFκB protein levels and the expressions of inflammatory biomarkers in mouse brains (Lu et al., 2010). In this study, the administration of CCH reduced the elevated AGE protein levels in DG-injected mouse brains and further ameliorated inflammatory responses via inhibiting AGE/RAGE/NFκB pathway activation (Figure 3C and 3D). Besides the antioxidant abilities, some major free amino acids (i.e. arginine and lysine) and dipeptide (anserine) in our CCH were reported to possess antiglycation abilities (Menzel and Reihsner, 1991, Kawasaki and Kamijo, 2012). Besides, carnosine and anserine can act as competitive glycation agents in decomposition of aldose-derived Schiff bases, which are the first intermediates of the nonenzymatic glycation cascade and lead to the reduction of AGE formations (Szwergold, 2005). Hence, it is supposed that the reduced AGE protein levels in brains of DG-injected mice cotreated with CCH might be due to the antioxidative and antiglycation capacities of CCH. Therefore, it could be reasonably drawn that the reduced effects of CCH on AGE protein levels of the brain would further ameliorate oxidative stress and inflammatory responses in brains and reflect the correlations of improved learning and memory abilities of DG-injected mice.
Conclusion
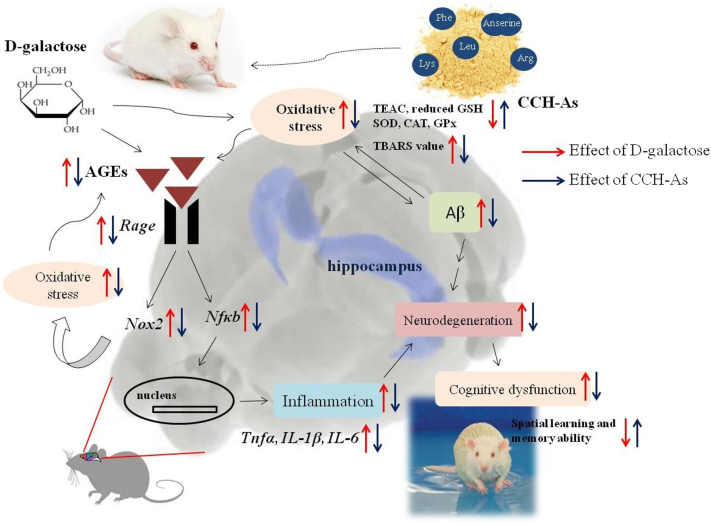
After protease A hydrolyzation, the total amount of free amino acids and dipeptides (anserine) dramatically increased in CCH than in raw materials (lyophilized chalazae). In the in vivo antioxidant experiment, CCH exhibited the protective effects on cognitive dysfunction and oxidative damage in the brain of DG-injected mice (Figure 4). The administration of CCH (mainly the middle or high dosage) significantly attenuates oxidative stress in the brain induced by DG injection, which further results in decreased AGE and Aβ protein levels in the brain, downregulates brain inflammation–related genes via inhibiting AGE/RAGE/NFκB pathway, ameliorates neurodegeneration in the dentate gyrus area of the hippocampus, and enhances cognitive learning and memory abilities of DG-injected mice. These protective effects could be attributed to the great amounts of bioactive free amino acids (i.e. leucine, arginine, lysine, and phenylalanine) and dipeptides (anserine) in the CCH. In addition, the application of chicken egg chalazae not only increases the byproducts' value from liquid egg industries but also reduces the environmental pollution caused by its byproduct properties. Taken together, our CCH can be a potential functional food ingredient to improve antioxidant effects and combat oxidative stress–induced brain dysfunction.
Figure 4.
Protective mechanisms of functional chalaza hydrolysates (CCH) on cognitive dysfunction and oxidative damage in the brain of D-galactose injected mice.
Acknowledgments
We acknowledge the funding of this research by the Ministry of Science and Technology, Taiwan (Project: MOST 106-2313-B-002-040-MY3).
Conflict of Interest Statement: The authors declare no conflicts of interest.
References
- Abeyrathne E.D.N.S., Lee H.Y., Jo C., Suh J.W., Ahn D.U. Enzymatic hydrolysis of ovomucoid and the functional properties of its hydrolysates. Poult. Sci. 2015;94:2280–2287. doi: 10.3382/ps/pev196. [DOI] [PubMed] [Google Scholar]
- Ahmed N. Advanced glycation end products - role in pathology of diabetic complications. Diabetes Rese. Clin. Pract. 2005;67:3–21. doi: 10.1016/j.diabres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Campos-Bedolla P., Walter F.R., Veszelka S., Deli M.A. Role of the blood-brain barrier in the nutrition of the central nervous system. Arch. Med. Res. 2014;45:610–638. doi: 10.1016/j.arcmed.2014.11.018. [DOI] [PubMed] [Google Scholar]
- Charles River Laboratories, Japan 2013. http://www.crj.co.jp/cms/pdf/info_common/ICR-IGS_CRLJ_nov_2013.pdf (in Japanese)
- Chen Y.C., Y.X Lin. 2019. Assignee: National Taiwan University. Egg chalaza hydrolysate, method for preparing the same and usage of the same. US Pat. No. US10, 456, 426 B2. [Google Scholar]
- Chou C.H., Wang S.Y., Lin Y.T., Chen Y.C. Antioxidant activities of chicken liver hydrolysates by pepsin treatment. Int. J. Food Sci. Technol. 2014;49:1654–1662. [Google Scholar]
- Council of Agriculture, Executive Yuan, Taiwan. 2015. https://eng.coa.gov.tw/ws.php?id=2505271
- Cui X., Zou P., Zang Q., Li X., Hu Y., Long J., Packer L., Liu J. Chronic systemic D-galactose exposure induces memory loss, neurodegeneration, and oxidative damage in mice: protective effects of R-alpha-lipoic acid. J. Neurosci. Res. 2006;83:1584–1590. doi: 10.1002/jnr.20845. [DOI] [PubMed] [Google Scholar]
- Floyd R.A. Neuroinflammatory processes are important in neurodegenerative diseases: a hypothesis to explain the increased formation of reactive oxygen and nitrogen species as major factors involved in neurodegenerative disease development. Free Radic. Biol. Med. 1999;26:1346–1355. doi: 10.1016/s0891-5849(98)00293-7. [DOI] [PubMed] [Google Scholar]
- Hsieh H.M., Wu W.M., Hu M.L. Soy isoflavones attenuate oxidative stress and improve parameters related to aging and Alzheimer’s disease in C57BL/6J mice treated with D-galactose. Food Chem. Toxicol. 2009;47:625–632. doi: 10.1016/j.fct.2008.12.026. [DOI] [PubMed] [Google Scholar]
- Ho S.C., Liu J.H., Wu R.Y. Establishment of the mimetic aging effect in mice caused by D-galactose. Biogerontology. 2003;4:15–18. doi: 10.1023/a:1022417102206. [DOI] [PubMed] [Google Scholar]
- Kawasaki T., Kamijo S. Inhibition of aggregation of amyloid β42 by arginine-containing small compounds. Biosci. Biotechnol. Biochem. 2012;76:762–766. doi: 10.1271/bbb.110879. [DOI] [PubMed] [Google Scholar]
- Khaidar A., Marx M., Lubec B., Lubec G. L-arginine reduces heart collagen accumulation in the diabetic db/db mouse. Circulation. 1994;90:479–483. doi: 10.1161/01.cir.90.1.479. [DOI] [PubMed] [Google Scholar]
- Kim J., Moon S.H., Ahn D.U., Paik H.D., Park E. Antioxidant effects of ovotransferrin and its hydrolysates. Poult. Sci. 2012;91:274–2754. doi: 10.3382/ps.2012-02150. [DOI] [PubMed] [Google Scholar]
- Lee D., Bamdad F., Khey K., Sunwoo H.H. Antioxidant and anti-inflammatory properties of chicken egg vitelline membrane hydrolysates. Poult. Sci. 2017;96:3510–3516. doi: 10.3382/ps/pex125. [DOI] [PubMed] [Google Scholar]
- Liaury K., Miyaoka T., Tsumori T., Furuya M., Wake R., Ieda M., Tsuchie K., Taki M., Ishihara K., Tanra A.J., Horiguchi J. Morphological features of microglial cells in the hippocampal dentate gyrus of Gunn rat: a possible schizophrenia animal model. J. Neuroinflammation. 2012;9:56. doi: 10.1186/1742-2094-9-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.L., Tai S.Y., Chen J.W., Chou C.H., Fu S.G., Chen Y.C. Ameliorative effects of pepsin-digested chicken liver hydrolysates on development of alcoholic fatty livers in mice. Food Funct. 2017;8:1763–1774. doi: 10.1039/c7fo00123a. [DOI] [PubMed] [Google Scholar]
- Lourenço C.F., Ledo A., Barbosa R.M., Laranjinha J. Neurovascular-neuroenergetic coupling axis in the brain: master regulation by nitric oxide and consequences in aging and neurodegeneration. Free Radic. Bio. Med. 2017;108:668–682. doi: 10.1016/j.freeradbiomed.2017.04.026. [DOI] [PubMed] [Google Scholar]
- Lu J., Wu D.M., Zheng Y.L., Hu B., Zhang Z.F., Ye Q., Liu C.M., Shan Q., Wang Y.J. Ursolic acid attenuates D-galactose-induced inflammatory response in mouse prefrontal cortex through inhibiting AGEs/RAGE/NF-κB pathway activation. Cereb. Cortex. 2010;20:2540–2548. doi: 10.1093/cercor/bhq002. [DOI] [PubMed] [Google Scholar]
- Lu L., Peng W.H., Wang W., Wang L.J., Chen Q.J., Shen W.F. Effects of atorvastatin on progression of diabetic nephropathy and local RAGE and soluble RAGE expressions in rats. J. Zhejiang Univ. Sci. B. 2011;12:652–659. doi: 10.1631/jzus.B1101004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luevano-Contreras C., Chapman-Novakofski K. Dietary advanced glycation end products and aging. Nutrients. 2010;2:1247–1265. doi: 10.3390/nu2121247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maikhunthod B., Intarapichet K.O. Heat and ultrafiltration extraction of broiler meat carnosine and its antioxidant activity. Meat Sci. 2005;71:364–374. doi: 10.1016/j.meatsci.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Menzel E.J., Reihsner R. Alterations of biochemical and biomechanical properties of rat tail tendons caused by non-enzymatic glycation and their inhibition by dibasic amino acids arginine and lysine. Diabetologia. 1991;34:12–16. doi: 10.1007/BF00404018. [DOI] [PubMed] [Google Scholar]
- Ooigawa H., Nawashiro H., Fukui S., Otani N., Osumi A., Toyooka T., Shima K. The fate of Nissl-stained dark neurons following traumatic brain injury in rats: difference between neocortex and hippocampus regarding survival rate. Acta Neuropathol. 2006;112:471–481. doi: 10.1007/s00401-006-0108-2. [DOI] [PubMed] [Google Scholar]
- Phelps E.A. Human emotion and memory: interactions of the amygdale and hippocampal complex. Curr. Opin. Neurobiol. 2004;14:198–202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Praticò D. Oxidative stress hypothesis in Alzheimer’s disease: a reappraisal. Trends Pharmacol. Sci. 2008;29:609–615. doi: 10.1016/j.tips.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Qian Z.J., Jung W.K., Kim S.K. Free radical scavenging activity of a novel antioxidative peptide purified from hydrolysates of bullfrog skin, Rana catesbeiana Shaw. Bioresour. Technol. 2008;99:1690–1698. doi: 10.1016/j.biortech.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Rajapakse N., Mendis E., Jung W.K., Je J.Y., Kim S.K. Purification of a radical scavenging peptide from fermented mussel sauce and its antioxidant properties. Food Res. Int. 2005;38:175–182. [Google Scholar]
- Reilly J.F., Games D., Ryde R.D., Freedman S., Schenk D., Young W.G., Morrison J.H., Bloom F.E. Amyloid deposition in the hippocampus and entorhinal cortex: quantitative analysis of a transgenic mouse model. Proc. Natl. Acad. Sci. USA. 2003;100:4837–4842. doi: 10.1073/pnas.0330745100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott G.S., Bolton C. L-arginine modifies free radical production and the development of experimental allergic encephalomyelitis. Inflamm. Res. 2000;49:720–726. doi: 10.1007/s000110050652. [DOI] [PubMed] [Google Scholar]
- Seib D.R., Martin-Villalba A. Neurogenesis in the normal ageing hippocampus: a mini-review. Gerontology. 2015;61:327–335. doi: 10.1159/000368575. [DOI] [PubMed] [Google Scholar]
- Shors T.J., Miesegaes G., Beylin A., Zhou M., Rydel T. Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Song X., Bao M., Li D., Li Y.M. Advanced glycation in D-galactose induced mouse aging model. Mech. Aging Dev. 1999;108:239–251. doi: 10.1016/s0047-6374(99)00022-6. [DOI] [PubMed] [Google Scholar]
- Szwergold B.S. Carnosine and anserine act as effective transglycating agents in decomposition of aldose-derived Schiff bases. Biochem. Biophys. Res. Commun. 2005;336:36–41. doi: 10.1016/j.bbrc.2005.08.033. [DOI] [PubMed] [Google Scholar]
- Tanaka H., Fukahori S., Baba S., Ueno T., Sivakumar R., Yagi M., Asagiri K., Ishii S., Tanaka Y. Branched-chain amino acid–rich supplements containing microelements have antioxidant effects on nonalcoholic steatohepatitis in mice. J. Parenter. Enter. Nutr. 2016;40:519–528. doi: 10.1177/0148607114555160. [DOI] [PubMed] [Google Scholar]
- Tu D.G., Chang Y.L., Chou C.H., Lin Y.L., Chiang C.C., Chang Y.Y., Chen Y.C. Preventive effects of taurine against D-galactose-induced cognitive dysfunction and brain damages. Food Funct. 2018;9:124–133. doi: 10.1039/c7fo01210a. [DOI] [PubMed] [Google Scholar]
- Wei H., Li L., Song Q., Ai H., Chu J., Li W. Behavioural study of the D-galactose induced aging model in C57BL/6J mice. Behav. Brain Res. 2005;157:245–251. doi: 10.1016/j.bbr.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Yang K.T., Lin Y.L., Lin Y.X., Wang S.Y., Wu Y.H.S., Chou C.H., Fu S.G., Chen Y.C. Protective effects of antioxidant egg-chalaza hydrolysates against chronic alcohol-consumption induced liver steatosis in mice. J. Sci. Food Agric. 2019;99:2300–2310. doi: 10.1002/jsfa.9426. [DOI] [PubMed] [Google Scholar]
- Zhu S.Y., Dong Y., Tu J., Zhou Y., Zhou X.H., Xu B. Silybum marianum oil attenuates oxidative stress and ameliorates mitochondrial dysfunction in mice treated with D-galactose. Pharmacogn. Mag. 2014;10:S92–S99. doi: 10.4103/0973-1296.127353. [DOI] [PMC free article] [PubMed] [Google Scholar]