Abstract
This experiment studied the effect of broiler breeder nutritional strategies on uniformity, carcass traits, tibia parameters, and behavior during rearing and prebreeder periods (up to 22 wk of age). One-day-old pullets (n = 384) were randomly assigned to 4 treatments arranged as a 2 × 2 factorial, with 2 fiber levels (control vs. fibrous diet, 15% diluted in AMEn and nutrient content) and 2 vitamin C feed inclusions (0 vs. 200 mg/kg). At 6, 15, and 22 wk, blood sampling was carried out (4 birds/replicate) to determine serum alkaline phosphatase (ALP) levels, and behavior was observed by visual scan sampling. At 22 wk, carcass traits, tibia parameters, and intestinal morphology were assessed (2 birds/replicate), and tail- and wing-feather integrity of all birds were scored. Fibrous diet did not modify BW uniformity, mortality, or tibia growth when compared with control diet. Pullets fed the fibrous diet had lower tibia breaking strength, elastic modulus, and ash content values (P < 0.05). They also had lower ALP serum level at 6 and 22 wk (P < 0.05), their breast muscle was less developed (18.5 vs. 19.8%, P < 0.05), and their abdominal fat deposition was higher (1.14 vs. 0.87%, P < 0.05). At 15 and 22 wk, they performed, on average, 97% less grasping feather pecking and 45% less non–food object pecking behaviors, and their wing-feather score was lower (P < 0.05) at 22 wk. Tail- and wing-feather scores of the control treatments were reduced by vitamin C inclusion (tail: 0.30 vs. 1.15, P < 0.05; wing: 0.98 vs. 1.26, P < 0.05) at 22 wk. In conclusion, fibrous diet improves carcass traits (reduces breast muscle and increases abdominal fat deposition), deteriorates bone mineral deposition and thus skeletal strength, and reduces stereotypic behaviors, improving wing-feather integrity. Vitamin C inclusion improves tail- and wing-feather integrity of lower in feed allowance.
Key words: broiler breeder, fibrous diet, vitamin C, skeletal strength and behavior
Introduction
Correct management of broiler breeders is essential to prevent having uneven flocks, where larger or aggressive pullets are likely to outcompete smaller or timid pullets, resulting in unequal access to feed and increasing flock BW variation (Zuidhof et al., 2015). Low uniformity may result in broiler breeder pullets being under the advised standard BW and not having received the nutrients required, thus leading to poor body development and stressful situations, which, respectively, might cause leg health and stereotypic behavior issues.
Fibrous (FBR) diets used to dilute the daily distributed ration, by adding different concentrations of raw material sources of insoluble fiber, might be a useful nutritional strategy to increase daily feed allocation and cleanup time. In this way, it might be possible to obtain more uniform flocks and reduce pullets under the standard BW with poor skeletal development and under stress. Likewise, González-Alvarado et al. (2007) found that the gizzard contents were greater in broilers chicks fed oat and soy hulls, sources of insoluble fiber, than in chicks fed the control (CTR) diets, indicating that fiber was retained longer in this organ; Hetland and Svihus (2001) also found that inclusion of 4% oat hulls increased the fresh contents of the gizzard in chicks. In broiler breeders, longer filling of the gizzard, in particular, and of the gastrointestinal tract, in general, might promote a feeling of satiety and reduce stress mainly during the period of rearing when weekly feed increases are lower.
Vitamin C, which is not normally included in broiler breeder premixes, has to be studied as another nutritional strategy. Regarding body development, when vitamin C is supplemented, it enhances 1,25-dihydroxyvitamin D (1,25-D) production (Weiser et al., 1988), which influences calcium metabolism and thus affects bone growth. In addition, its deficiency results in widespread connective tissue abnormalities, whose origin comes from a disruption in collagen synthesis (Whitehead and Keller, 2003). Vitamin C supplementation might also be useful because, although poultry can biosynthesize it, its endogenous synthesis may not be adequate to always meet the full poultry requirements, specifically during stress periods, when they may exceed the synthesizing ability (Gous and Morris, 2005). Furthermore, vitamin C may also help the broiler breeders to cope with stressful situations (Satterlee et al., 1993, Satterlee et al., 1994, Jones et al., 1996, Jones et al., 1999), mainly when management is suboptimal during the rearing period.
To evaluate whether skeletal development of the broiler breeders during the rearing phase is correct, pullet BW has to be weekly compared with the BW profiles provided by the breeding companies; however, this is an indirect form to assess correct skeletal growth. Alkaline phosphatase (ALP) is a serological marker directly related to skeletal development and, in particular, to bone formation and growth (Seibel, 2005). ALP is a ubiquitous enzyme attached on the outer cell surface (Stinson and Hamilton, 1994); the precise function of the enzyme is still unknown, but it obviously plays an important role in osteoid formation and mineralization (Harris, 1990). This serological marker is now an important tool in the assessment and differential diagnosis of metabolic bone diseases in humans; nevertheless, it has never been used in broiler breeder pullets to assess body development during the rearing period.
Physiological indicators of stress, such as plasma corticosterone concentrations, heterophil-to-lymphocyte ratio, and white-blood-cell frequencies, have been used to evaluate the welfare of broiler breeder pullets (Savory et al., 1996, Hocking et al., 1998, Hocking et al., 1999, de Jong et al., 2002, Sandilands et al., 2006). Apart from these indicators, a noninvasive measurement would be practical as a test to quickly assess the ease or uneasiness of a flock, for example, stress of the broiler breeder pullets in the rearing farms. Morrissey et al. (2014) used feather pecking as a parameter to determine stress and welfare, and their study showed that broiler breeders fed FBR diluted diets feather-pecked significantly less often. Therefore, because feather pecking results in damaged feather cover, the assessment of tail- and wing-feather integrity might be a practical way to evaluate stress and, if necessary, to quickly apply corrective management actions.
The aim of the present study is to assess the effect of FBR diet (15% diluted in AMEn and nutrient content) and vitamin C supplementation (200 mg/kg) on BW uniformity, mortality, carcass traits, tibia parameters, and behavior of broiler breeders in the rearing and prebreeder phases. We hypothesized that there would be greater skeletal strength and lower stereotypic behaviors than those in control pullets. Furthermore, ALP and tail- and wind-feather scores have been proposed to assess skeletal development and stress, respectively, in broiler breeder pullets.
Materials and methods
Birds and Facility
All of the animal experimentation procedures used in the experiment were approved by the Animal Ethics Committee of the Universitat Autònoma de Barcelona (UAB, Barcelona, Spain) and were in compliance with the European Union guidelines for the care and use of animals in research (European Parliament, 2010).
A total of 384 one-day-old Ross 308 female broiler breeder chicks were randomly distributed into 32 pens (12 chicks/pen) in the experimental facility Granja Solé (Vila-rodona, Tarragona, Spain) during a period from 0 to 22 wk of age. Each pen had an area of 1.5 m2 and contained a bell drinker. Feed and drinker space was sufficient for all the hens to reach feed and water at the same time. New wood shavings were utilized as litter material, and litter was topped up weekly to keep it dry.
A vaccination program adapted to the epidemiology of the farm area was followed throughout the rearing period. Management applied was in accordance with the guidelines of the Ross Parent Stock Management Handbook (Aviagen, 2013).
Experimental Design
The experiment included 4 treatments arranged as a 2 × 2 factorial with 2 fiber levels, CTR diet andFBR diet, and 2 vitamin C inclusions, diet not vitamin C–supplemented (C−) and diet vitamin C–supplemented (200 mg/kg) (C+). Therefore, the treatments were CTR/C−, CTR/C+, FBR/C−, and FBR/C+, and each one was replicated 8 times.
Birds were randomly allotted to the different replicates, with an initial BW of 43.2 g ± 2.94 g and a BW coefficient of variation (CV) of 7.1 ± 0.51%.
Feeding Program, Diets, and Feed Intake
Feed was produced in the feed mill of Institut de Recerca i Tecnologia Agroalimentaria Mas Bové (Constantí, Tarragona, Spain). A four-phase diet-feeding program was followed: Starter I (the first week), Starter II (from 2 to 6 wk), Grower (from 7 to 15 wk) and Prebreeder (from 16 to 22 wk). Starter I was the only diet shared by the 4 treatments. Feed-form presentation was crumble for Starter I, short pellet for Starter II, and regular pellet for Grower and Prebreeder. Feed was always distributed on the floor.
Ingredients and nutrient composition of the experimental diets are shown in Tables 1 and 2, respectively. The CTR diets were formulated to achieve the nutrient specifications of the broiler breeders during rearing and prebreeder periods as per Ross 308 Parent Stock Nutrition Specifications (Aviagen, 2016). Raw materials, source of insoluble fiber, were included in the FBR diets to dilute 15% of their AMEn and nutrient content (crude protein and digestible amino acids, digestible phosphorous and calcium, and vitamin and mineral premix). Among these, a forage pellet, containing 67% ray grass and 33% barley straw, was used. The AMEn estimated value of the forage pellet was 892 kcal, and its nutritional analyzed values were as follows: dry matter, 89.7%; CP, 11.6%; crude fiber (CF), 24.8%; neutral detergent fiber (NDF), 48.1%; acid detergent fiber (ADF), 30.1%; acid detergent lignin (ADL), 8.1%; crude fat, 1.3%; and ash, 11.7%. In the diets supplemented with vitamin C, 200 mg/kg of a stabilized (phosphorylated) Na/Ca salt of L-ascorbic acid was included.
Table 1.
Ingredients (%) of the experimental diets.
| Ingredients | Starter I | Starter II |
Grower |
Prebreeder |
|||
|---|---|---|---|---|---|---|---|
| CTR | FBR | CTR | FBR | CTR | FBR | ||
| Wheat | 35.2 | 39.4 | - | 43.8 | - | 43.5 | - |
| Corn | 24.9 | 24.9 | 36.9 | 16.3 | 22.9 | 24.8 | 21.8 |
| Soybean meal 48% | 25.2 | 25.5 | 10.5 | - | - | 6.3 | - |
| Rye | - | - | 12.0 | - | 28.7 | - | 33.1 |
| Forage pellet1 | - | - | 5.4 | - | 16.3 | - | 11.5 |
| Sunflower 28–30% | 5.0 | - | 15.0 | 17.0 | 8.7 | 12.0 | 9.7 |
| Wheat bran (high starch) | 3.0 | 5.0 | 16.1 | 17.6 | 20.0 | 8.0 | 20.0 |
| Dicalcium phosphate | 2.33 | 2.17 | 1.70 | 1.83 | 1.40 | 1.53 | 0.97 |
| Soybean oil | 2.00 | 0.50 | - | 0.98 | - | 0.57 | - |
| Limestone | 0.97 | 0.97 | 0.80 | 0.90 | 0.63 | 1.97 | 1.63 |
| Premix2 | 0.30 | 0.50 | 0.42 | 0.50 | 0.42 | 0.50 | 0.43 |
| Sodium bicarbonate | 0.27 | 0.31 | 0.41 | 0.36 | 0.40 | 0.29 | 0.37 |
| L-lysine sulfate 54.6% | 0.24 | - | - | - | - | - | - |
| L-Lysine HCL 65% | - | 0.19 | 0.32 | 0.22 | 0.16 | 0.12 | 0.15 |
| Salt | 0.23 | 0.20 | 0.05 | 0.17 | 0.05 | 0.22 | 0.06 |
| DL-Methionine | 0.20 | 0.22 | 0.16 | 0.11 | 0.11 | 0.11 | 0.10 |
| Choline Cl-60% | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.07 | 0.08 |
| L-Threonine | 0.05 | 0.09 | 0.09 | 0.10 | 0.06 | 0.03 | 0.03 |
| Xylanase + ß- glucanase | - | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Vitamin C mg/kg3 | 200 | ||||||
| Vitamin C mg/kg C+4 | 200 | 200 | 200 | 200 | 200 | 200 | |
| Vitamin C mg/kg C−5 | 0 | 0 | 0 | 0 | 0 | 0 | |
Abbreviations: CTR, control diet; FBR, fibrous diet.
Forage pellet composition: Ray grass (67%) and barley straw (33%).
Premix provided the following per kilogram of diet: vitamin A (retinyl acetate), 11,000 IU; vitamin D3 (cholecalciferol), 3500 IU; vitamin E (DL-α-tocopheryl acetate), 100 IU; vitamin K3, 3.00 mg; vitamin B1 (thiamin), 3.00 mg; vitamin B2 (riboflavin), 6.00 mg; vitamin B5 (pantothenic acid), 13.00 mg; vitamin B6 (pyridoxine hydrochloride), 4.00 mg; vitamin B12 (cyanocobalamin), 0.02 mg; vitamin B3 (nicotinic acid), 30.00 mg; vitamin B9 (folic acid), 1.50 mg; vitamin B7 (biotin), 0.20 mg; iodine (calcium iodate), 1.25 mg; Mn (manganous oxide), 120.00 mg; Cu (cupric sulfate), 16.00 mg; Zn (zinc oxide), 110 mg; Se (sodium selenite), 0.30 mg; Fe (iron sulphate), 40.00 mg.
Vitamin C product source: Rovimix STAY–C 35 (0.0571% inclusion).
C+: Diets vitamin C supplemented.
C−: Diets not vitamin C supplemented.
Table 2.
Metabolizable energy and nutrient composition (% as fed-basis, unless otherwise indicated) of the experimental diets.
| Item | Starter I | Starter II |
Grower |
Prebreeder |
|||
|---|---|---|---|---|---|---|---|
| CTR | FBR | CTR | FBR | CTR | FBR | ||
| AMEn, kcal/kg | 2,824 | 2,804 | 2,399 | 2,600 | 2,240 | 2,700 | 2,295 |
| Nutrient composition | |||||||
| Dry matter1 | 87.7 | 88.0 | 87.8 | 88.5 | 87.4 | 88.4 | 88.1 |
| Crude protein1 | 17.9 | 18.3 | 15.7 | 13.1 | 11.8 | 13.8 | 11.8 |
| dig Lys | 0.95 | 0.96 | 0.81 | 0.54 | 0.43 | 0.54 | 0.43 |
| dig Met | 0.47 | 0.48 | 0.40 | 0.34 | 0.28 | 0.33 | 0.27 |
| dig M + C | 0.74 | 0.76 | 0.63 | 0.56 | 0.45 | 0.56 | 0.45 |
| dig Thre | 0.64 | 0.67 | 0.56 | 0.46 | 0.37 | 0.43 | 0.34 |
| dig Trp | 0.20 | 0.20 | 0.16 | 0.14 | 0.11 | 0.15 | 0.11 |
| Crude fiber1 | 4.5 | 3.2 | 8.1 | 7.2 | 8.8 | 5.0 | 7.5 |
| Neutral detergent fiber1 | 11.6 | 10.3 | 17.6 | 17.5 | 19.9 | 12.2 | 18.4 |
| Acid detergent fiber1 | 5.0 | 3.7 | 8.2 | 7.9 | 9.2 | 5.2 | 8.0 |
| Acid detergent lignin1 | 0.93 | 0.76 | 2.42 | 2.34 | 2.56 | 1.60 | 2.64 |
| Crude fat1 | 4.2 | 2.5 | 2.9 | 3.2 | 2.3 | 2.7 | 2.4 |
| Ash1 | 6.4 | 5.9 | 6.4 | 5.7 | 6.1 | 6.4 | 6.5 |
| Ca | 1.06 | 1.00 | 0.88 | 0.90 | 0.78 | 1.22 | 1.02 |
| dig P | 0.48 | 0.45 | 0.40 | 0.42 | 0.35 | 0.36 | 0.29 |
| Phytate | 0.99 | 0.93 | 1.30 | 1.29 | 1.14 | 1.08 | 1.18 |
| Vitamin C, mg/kg1 | 117.0 | ||||||
| Vitamin C, mg/kg C+1,2 | 134.0 | 164.0 | 152.0 | 131.5 | 150.0 | 116.5 | |
| Vitamin C, mg/kg C−1,3 | <5 | <5 | <5 | <5 | <5 | <5 | |
Abbreviations: CTR, control diet; FBR, fibrous diet.
Analyzed values.
C+: Diets vitamin C supplemented.
C−: Diets not vitamin C supplemented.
Feed was provided ad libitum during the first week to ensure a correct initial development. From this moment on, to follow the same standard BW profile in the 4 treatments, the total daily feed distributed per pen was calculated by multiplying all of the pullets present at that moment in the pen by the daily AMEn allowance per bird and divided by the AMEn of the diet. Both, standard BW profile and daily AMEn allowance per pullet, were based on Ross 308 Parent Stock Performance Objectives (Aviagen, 2016).
Collected Data, Sampling, and Analytical Determinations
Body Weight, Uniformity, and Mortality
Pullets were weekly individually weighed, and average BW and BW CV, calculated per pen. Mortality was recorded daily and accumulated mortality calculated to make comparisons among the different treatments.
Feed
Experimental feed samples were collected from the different diets supplied during the rearing and prebreeder periods of the trial. These samples were ground and stored at 5°C until they were analyzed in duplicate. Diet proximate analyses were performed in accordance with the method of the Association of Official Agricultural Chemists (AOAC) International (2005): dry matter (Method 934.01), crude protein (Method 968.06), CF (Method 962.09), NDF/ADF/ADL (Method 973.18), crude fat (Method 2,003.05), and ash content (Method 942.05). All analyses were carried out in the Servei de Nutrició i Benestar Animal (SNiBA) laboratory of the UAB.
Carcass Traits
At 22 wk, after weighing all of the pullets, the 2 closest birds to the average BW of each pen were euthanized to perform measurements and take samples to study carcass traits, intestinal morphology, and tibia parameters.
Breast muscle (pectoralis major plus pectoralis minor), abdominal fat (surrounding the gizzard and pericloacal), empty digestive tract (from proventriculus to cloaca, both included), and oviduct were extracted and weighed to calculate their BW percentage.
Intestinal Morphology
Tissue samples that were 3-cm long were taken from the jejunum, at the midpoint between the bile duct entry and Meckel's diverticulum (2 birds/pen). Samples were fixed in 10% buffered formalin solution, dehydrated in a graded ethanol series, and embedded in paraffin. Four cross sections (4-μm-thick each) were cut separately from each sample and included in the same preparation. Sections were stained with hematoxylin and eosin, with the method of Hampson (1986) being taken into account. Histomorphological measurements of the intestinal mucosa were carried out: at least the height of 30 intact villi (distance from the villus tip to the villus–crypt junction) and the depth of 30 crypts of Lieberkühn (depth of the invagination between adjacent villi) of each preparation were measured, and the ratio between both was calculated. The measurements were taken from digital photographs of the preparations, which were captured in a Leica SCN400 scanner (Leica Microsystems CMS GmbH, Wetzlar, Germany) at 40×. The images obtained were analyzed by the Slide Path Digital Image Hub, version 4.0.7 software (Leica Biosystems GmbH, Nussloch, Germany). This analysis was carried out in the Universidad CEU Cardenal Herrera, Valencia, Spain.
Tibia Parameters
Tibias of right legs (2 birds/pen) were used to determine tibia length (TL), tibia width (TW), tibia breaking strength (BS) (maximum load endurance), apparent elastic modulus (EM), and ash content. TL was measured from the intercondylar eminence to the tip of the lateral malleolus, and TW was measured in the narrowest part of the tibia diaphysis. To perform these 2 measurements, a calliper (0.03-mm precision and 0.01-mm resolution) was used. To determine tibia BS and EM, the method of 3-point flexural bending test (Fleming et al., 1998) was taken into account; measurements were made by using an MTS Alliance RT/5 material testing system (MTS Systems Corp.; Eden Prairie, MN 55344) (force resolution, 0.001 N and distance resolution, 0.001 mm). These studies were carried out at the Institut de Recerca i Tecnologia Agroalimentaria (Girona, Spain). To determine the percentage of ash content, right tibias were boiled and any adherent tissue was cleaned off; they were weighed, dried (109°C 12 h), chemically cleaned (in acetone for 48 h), dried (109°C 12 h), weighed again, and burned in a muffle oven overnight (550°C). These analyses were carried out in the SNiBA laboratory of the UAB.
Alkaline Phosphatase
To determine ALP, the 4 closest pullets to the average BW of each pen were chosen at 6 wk and blood was sampled at 6, 15, and 22 wk of age. Blood samples were spun at 1,107 g for 20 min to obtain the serum, which was stored at −20°C until the moment to be analyzed. To test ALP, a colorimetric method based on catalytic transformation of p-nitrophenylphosphate as substrate (International Federation of Chemical Chemistry method) on the Beckman Coulter AU analyzer (Beckman Coulter, Inc.; Brea, CA 92821) was used. ALP analyses were carried out by the Servei de Bioquímica Clínica Veterinària of the UAB.
Behavior
At 6, 15, and 22 wk of age, behavior of the pullets was observed by using an instantaneous, visual scan-sampling technique, and the method followed by Hocking et al. (2004) was taken into account. Grasping feather pecking (GFP) (grasping tail and back feathers of other pullets) and non–food object pecking (NFOP) (pecking at drinkers and pen walls) behaviors were observed during two 20-min sessions a day, once during the morning (9h.30– 12h.30) and once in the afternoon (13h.30– 16h.30). Observations were made per block of 4 pens at 1-min intervals, in a random order, after all feeding-related activities had ceased and after allowing 5 min for the birds to adjust to the presence of the observer. The number of pullets engaged in each behavioral category was recorded and expressed in proportion to the total number of active animals. All observations were performed by the same observer.
The integrity of the tail and wing feathers of all of the pullets was scored at 22 wk of age. A feather score between 0 and 5 was used: (0) fully feathered, (1) rough, (2) some broken feathers and small bald areas, (3) heavily broken feathers and some bald areas, (4) almost bald/large bald areas, and (5) bald (no feather cover). To perform this scoring, the Protocol for Scoring the Feather Cover of Broiler Breeders (Aviagen, 2010) was taken into account.
Statistical Analysis
The statistical analysis was performed with the version 3.5.1 R program (R Core Team, 2018). Factorial ANOVA was carried out to analyze BW, BW CV, intestinal morphology, BS, EM, tibia ash content, TL, and TW. The factors were fiber level (CTR and FBR) and vitamin C inclusion (C− and C+). Mortality was analyzed by contrasting fiber level (CTR and FBR), vitamin C inclusion (C− and C+), and their combinations (CTR/C−, CTR/C+, FBR/C−, and FBR/C+). Several contingency tables were created and solved through a Fisher's exact test for count data. To analyze GFP, NFOP, and tail- and wing-feather integrity status, fiber level (CTR and FBR) and vitamin C inclusion (C− and C+) effects were contrasted through a Wilcoxon rank test (Mann–Whitney test). The 4 combinations of fiber level and vitamin C inclusion effects (CTR/C−, CTR/C+, FBR/C−, and FBR/C+) were contrasted through a Kruskal–Wallis test; when this test result was significant, pairwise combinations were contrasted by the Wilcoxon rank test.
Results
Body Weight, Uniformity, and Mortality
Table 3 shows BW and BW CVs at 6, 15, and 22 wk. BW and BW CVs were not affected by fiber level or vitamin C inclusion, nor was there any interaction (P > 0.05). BW CVs between the pullets fed the CTR and FBR diets were similar at 6 wk (11.36 and 11.59%, respectively, P = 0.818), 15 wk (9.71 and 10.32%, respectively, P = 0.449), and 22 wk (8.68 and 9.02%, respectively, P = 0.714). Cumulative mortality (%) at 22 wk was not affected by fiber level (CTR: 2.22% and FBR: 0.56%, P = 0.372) or vitamin C inclusion (C−: 0.52% and C+: 2.38%, P = 0.189); the combination of effects (fiber level/vitamin C inclusion) was not significant either (P = 0.240).
Table 3.
Effects of fiber level and vitamin C inclusion on body weight (BW) and body weight coefficient of variation (CV) at 6, 15, and 22 wk of age.
| Effects | BW (g)1 |
BW CV (%)2 |
||||
|---|---|---|---|---|---|---|
| 6 wk | 15 wk | 22 wk | 6 wk | 15 wk | 22 wk | |
| Fiber level | ||||||
| CTR | 700 | 1,831 | 2,693 | 11.36 | 9.71 | 8.68 |
| FBR | 687 | 1,818 | 2,684 | 11.59 | 10.32 | 9.02 |
| Vitamin C inclusion | ||||||
| C− | 695 | 1,831 | 2,692 | 10.56 | 10.32 | 9.24 |
| C+ | 691 | 1,818 | 2,685 | 12.39 | 9.72 | 8.46 |
| SEM | 5.2 | 12.0 | 16.0 | 0.213 | 0.572 | 0.644 |
| P-values | ||||||
| Fiber level | 0.085 | 0.460 | 0.692 | 0.818 | 0.449 | 0.714 |
| Vitamin C inclusion | 0.565 | 0.443 | 0.804 | 0.081 | 0.461 | 0.393 |
| Interaction | 0.309 | 0.892 | 0.730 | 0.281 | 0.180 | 0.258 |
Abbreviations: C−, not vitamin C–supplemented diet; C+, vitamin C–supplemented diet; CTR, control diet; FBR, fibrous diet.
P < 0.05 was considered significant.
n = 192 for fiber level and vitamin C effects and n = 96 for the interaction.
n = 16 for fiber level and vitamin C effects and n = 8 for the interaction.
Feed and Nutrient Intake
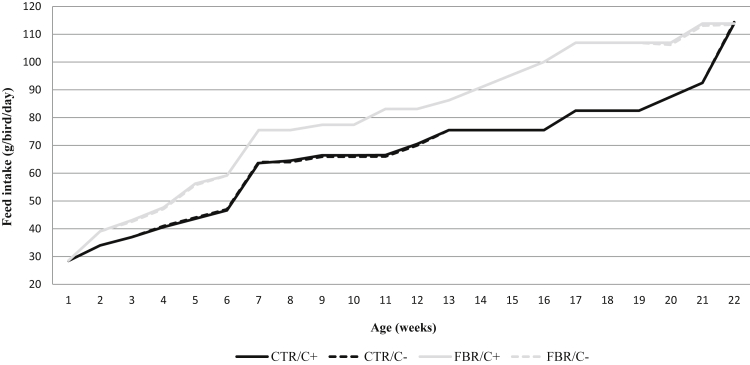
Feed intake between 0 and 22 wk of age is shown in Figure 1. After weekly adjustments to follow the standard BW profile in all treatments, accumulated feed intake (g) at 22 wk of the FBR treatments was 17.3% higher than that of the CTR (FBR/C−: 12,431 ± 9.9 and FBR/C+: 12,407 ± 8.8 vs. CTR/C−: 10,268 ± 52.5 and CTR/C+: 10,264 ± 36.0) (P < 0.01).
Figure 1.
Weekly feed intake of the broiler breeder pullets from 0 to 22 wk of age, depending on fiber level and vitamin C inclusion. Values are pooled means of 8 replicates per treatment. CTR/C−: control diet not vitamin C supplemented, CTR/C+: control diet vitamin C supplemented, FBR/C−: fibrous diet not vitamin C supplemented, and FBR/C+: fibrous diet vitamin C supplemented. Pooled SEM = 5.01.
The analyzed values of the experimental diets showed that, on average, the FBR diets had 12.9% less CP, 8.5% less crude fat, 37.5% more CF, 29.1% more NDF, 34.7% more ADF, and 38.9% more ADL. Vitamin C–supplemented diets of the treatments CTR/C+ and FBR/C+ presented an average vitamin C concentration (mg/kg) of 138 ± 18.2, whereas in the nonsupplemented diets of the treatments, CTR/C− and FBR/C−, vitamin C concentration (mg/kg) was <5 (mg/kg).
Carcass Traits
The effects of fiber level and vitamin C inclusion on carcass traits (relative to BW) at 22 wk of age are shown in Table 4. There was no vitamin C inclusion effect, but pullets fed the FBR diet, compared with those fed the CTR diet, had less breast muscle (18.5 vs. 19.8%, P = 0.001) and higher abdominal fat deposition (1.14 vs. 0.87%, P = 0.031), total empty digestive tract (5.07 vs. 4.63%, P = 0.001), proventriculus plus gizzard (2.04 vs. 1.94%, P = 0.035), and caecum plus rectum (0.90 vs. 0.64%, P < 0.001). However, there was no significant difference between the pullets fed the FBR diet and those fed the CTR diet, for small intestine (2.13 and 2.05%, P = 0.136) or for oviduct (12.5 g and 16.2 g/10 kg BW, P = 0.227).
Table 4.
Effects of fiber level and vitamin C inclusion on carcass traits (% BW, oviduct: g/10 Kg BW) at 22 wk of age.
| Effects | Carcass traits |
||||||
|---|---|---|---|---|---|---|---|
| Breast muscle | Abdominal fat | Total digestive tract | Proventriculus + gizzard | Small intestine | Caecum + rectum | Oviduct | |
| Fiber level | |||||||
| CTR | 19.8 | 0.87 | 4.63 | 1.94 | 2.05 | 0.64 | 16.2 |
| FBR | 18.5 | 1.14 | 5.07 | 2.04 | 2.13 | 0.90 | 12.5 |
| Vitamin C inclusion | |||||||
| C− | 19.0 | 1.03 | 4.88 | 1.97 | 2.12 | 0.79 | 13.4 |
| C+ | 19.3 | 0.98 | 4.81 | 2.02 | 2.08 | 0.74 | 15.3 |
| SEM (n = 32) | 0.23 | 0.082 | 0.081 | 0.033 | 0.042 | 0.041 | 2.12 |
| P-values | |||||||
| Fiber level | 0.001 | 0.031 | 0.001 | 0.035 | 0.136 | <0.001 | 0.227 |
| Vitamin C inclusion | 0.345 | 0.601 | 0.526 | 0.368 | 0.265 | 0.343 | 0.536 |
| Interaction | 0.595 | 0.105 | 0.897 | 0.530 | 0.365 | 0.895 | 0.439 |
Abbreviations: C−, not vitamin C–supplemented diet; C+, vitamin C–supplemented diet; CTR, control diet; FBR, fibrous diet.
P < 0.05 was considered significant.
Intestinal Morphology
Histomorphological parameters of the intestinal mucosa of the middle portion of the jejunum are shown in Table 5. At 22 wk of age, there were neither fiber level nor vitamin C inclusion effects on the intestinal morphology: villus height, Lieberkühn crypt depth, and their ratio.
Table 5.
Effects of fiber level and vitamin C inclusion on the histomorphological parameters of the intestinal mucosa of the middle portion of the jejunum at 22 wk of age.
| Effects | Villi height (μm) | Lieberkühn crypt depth (μm) | Ratio height/depth |
|---|---|---|---|
| Fiber level | |||
| CTR | 974 | 160 | 6.12 |
| FBR | 935 | 166 | 5.67 |
| Vitamin C inclusion | |||
| C− | 982 | 162 | 6.00 |
| C+ | 925 | 165 | 5.73 |
| SEM (n = 21) | 32.6 | 3.5 | 0.250 |
| P-values | |||
| Fiber level | 0.471 | 0.411 | 0.323 |
| Vitamin C inclusion | 0.318 | 0.618 | 0.570 |
| Interaction | 0.955 | 0.884 | 0.905 |
Abbreviations: C−, not vitamin C–supplemented diet; C+, vitamin C–supplemented diet; CTR, control diet; FBR, fibrous diet.
P < 0.05 was considered significant.
Tibia Parameters
The effects of fiber level and vitamin C inclusion on tibia BS, EM, and ash content at 22 wk are shown in Table 6. There was no vitamin C inclusion effect, but tibias from the pullets fed the CTR diet, compared with those fed the FBR diet, were able to endure more weight before breaking, their BS values were higher (45.1 vs. 41.4 kgf/mm2, P = 0.009), they were also more rigid or less ductile, their EM values were higher (43.3 vs. 36.4 kgf/mm, P = 0.001), and they had higher ash content (41.8 vs. 40.0%, P = 0.001).
Table 6.
Effects of fiber level and vitamin C inclusion on tibia breaking strength (BS), elastic modulus (EM), and ash content at 22 wk of age.
| Effects | BS (kgf/mm2) | EM (kgf/mm) | Ash content (%) |
|---|---|---|---|
| Fiber level | |||
| CTR | 45.1 | 43.3 | 41.8 |
| FBR | 41.4 | 36.4 | 40.0 |
| Vitamin C inclusion | |||
| C− | 43.5 | 40.7 | 41.2 |
| C+ | 43.0 | 39.0 | 40.6 |
| SEM (n = 32) | 0.97 | 1.26 | 0.37 |
| P-values | |||
| Fiber level | 0.009 | 0.001 | 0.001 |
| Vitamin C inclusion | 0.732 | 0.351 | 0.240 |
| Interaction | 0.988 | 0.632 | 0.760 |
Abbreviations: C−, not vitamin C–supplemented diet; C+, vitamin C–supplemented diet; CTR, control diet; FBR, fibrous diet.
P < 0.05 was considered significant.
Regarding tibia measurements, no effects of fiber level or vitamin C inclusion were found. Pullets fed the CTR diet, compared with those fed the FBR diet, had no significant differences in TL and TW values (TL: 130 mm and 131 mm, P = 0.147; TW: 7.01 mm and 7.09 mm, P = 0.277).
Alkaline Phosphatase
The effects of fiber level and vitamin C inclusion on ALP serological levels at 6, 15, and 22 wk are shown in Table 7. At 6 and 22 wk, there was no vitamin C inclusion effect, but pullets fed the CTR diet, compared with those fed the FBR diet, had higher ALP levels (6 wk: 10,045 vs. 8,042 IU, P = 0.001; 22 wk: 1,107 vs. 775 IU, P < 0.001). However, at 15 wk, there was neither fiber level nor vitamin C inclusion effect on serum ALP levels.
Table 7.
Effects of fiber level and vitamin C inclusion on alkaline phosphatase (ALP) serological level at 6, 15, and 22 wk of age.
| Effects | ALP (IU) |
||
|---|---|---|---|
| 6 wk | 15 wk | 22 wk | |
| Fiber level | |||
| CTR | 10,045 | 1,371 | 1,107 |
| FBR | 8,042 | 1,344 | 775 |
| Vitamin C inclusion | |||
| C− | 8,989 | 1,408 | 987 |
| C+ | 9,098 | 1,304 | 890 |
| SEM (n = 64) | 424.5 | 61.4 | 41.7 |
| P-values | |||
| Fiber level | 0.001 | 0.765 | <0.001 |
| Vitamin C inclusion | 0.856 | 0.247 | 0.114 |
| Interaction | 0.058 | 0.104 | 0.220 |
Abbreviations: C−, not vitamin C–supplemented diet; C+, vitamin C–supplemented diet; CTR, control diet; FBR, fibrous diet.
P < 0.05 was considered significant.
Behavior
The effects of fiber level and vitamin C inclusion on bird behavior (GFP and NFOP) at 6, 15, and 22 wk and on tail- and wing-feather scores at 22 wk of age are shown in Table 8.
Table 8.
Effects of fiber level and vitamin C inclusion on the percentage of birds performing grasping feather pecking (GFP) and non–food object pecking (NFOP) at 6, 15, and 22 wk and on tail- and wing-feather score at 22 wk of age.
| Effects | GFP1 |
NFOP1 |
Tail-feather score2 |
Wing-feather score2 |
||||
|---|---|---|---|---|---|---|---|---|
| 6 wk | 15 wk | 22 wk | 6 wk | 15 wk | 22 wk | 22 wk | 22 wk | |
| Fiber level | ||||||||
| CTR | 0 | 7.74 | 5.17 | 12.4 | 24.0 | 35.1 | 0.73 | 1.12 |
| FBR | 0 | 0.29 | 0.05 | 12.9 | 14.1 | 18.5 | 0.20 | 0.70 |
| Vitamin C inclusion | ||||||||
| C− | 0 | 5.83 | 3.73 | 13.4 | 18.4 | 28.2 | 0.66 | 1.01 |
| C+ | 0 | 2.20 | 1.49 | 11.9 | 19.7 | 25.4 | 0.25 | 0.80 |
| Fiber level/vitamin C | ||||||||
| CTR/C− | 0 | 11.53a | 7.47a | 14.6 | 22.4a,b | 35.2a | 1.15a | 1.26a |
| CTR/C+ | 0 | 3.96b | 2.88a | 10.2 | 25.7a | 35.1a | 0.30b | 0.98b |
| FBR/C− | 0 | 0.14b | 0b | 12.2 | 14.4a,b | 21.2b | 0.19b | 0.76c |
| FBR/C+ | 0 | 0.45b | 0.10b | 13.6 | 13.7b | 15.8b | 0.21b | 0.63c |
| P-values | ||||||||
| Fiber level | 0 | 0.016 | 0.008 | 0.773 | 0.005 | <0.001 | <0.001 | <0.001 |
| Vitamin C inclusion | 0 | 0.104 | 0.828 | 0.707 | 0.778 | 0.537 | <0.001 | 0.029 |
| Fiber level/vitamin C | 0 | 0.009 | 0.029 | 0.924 | 0.043 | 0.003 | <0.001 | <0.001 |
a-cMeans within a column and within a source with no common superscript differ significantly.
Abbreviations: CTR, control diet; C−, not vitamin C–supplemented diet; C+, vitamin C–supplemented diet; FBR, fibrous diet.
Treatments: CTR/C−: control diet not vitamin C supplemented, CTR/C+: control diet vitamin C supplemented, FBR/C−: fibrous diet not vitamin C supplemented, and FBR/C+: fibrous diet vitamin C supplemented.
Tail- and wing-feather scores: (0) fully feathered, (1) rough, (2) some broken feathers and small bald areas, (3) heavily broken feathers and some bald areas, (4) almost bald/large bald areas, and (5) bald (no feather cover).
n = 32 for fiber level and vitamin C effects and n = 16 for the combinations fiber level/vitamin C.
n = 192 for fiber level and vitamin C effects and n = 96 for the combinations fiber level/vitamin C.
At 6 wk, pullets did not perform GFP at all, and there were no fiber level or vitamin C inclusion effects on NFOP. At 15 wk, the combinations of fiber level and vitamin C effects showed a significantly higher percentage of GFP in the CTR/C− treatment than in the other treatments (P = 0.009); there were no significant differences among the CTR/C+, FBR/C−, and FBR/C+ treatments. Regarding NFOP, the CTR/C+ treatment had a significantly higher percentage than the FBR/C+ treatment (P = 0.043); the CTR/C− and FBR/C− treatments had intermediate values. At 22 wk, and for GFP and NFOP, the combination of effects showed no significant differences between CTR/C− and CTR/C+, nor between FBR/C− and FBR/C+ treatments; nevertheless, the percentage values of the CTR treatments were significantly higher than were those of the FBR treatments (GFP: P = 0.029; NFOP: P = 0.003).
Tail-feather score results at 22 wk showed a significantly higher value of the CTR/C− treatment than with other treatments (P < 0.001); there were no significant differences among the CTR/C+, FBR/C−, and FBR/C+ treatments. Wing-feather score results showed that the CTR/C− treatment had a significantly higher value at 22 wk (P < 0.001). The CTR/C+ treatment had an intermediate value significantly higher than did those of the FBR/C− and FBR/C+ treatments, which showed no difference between them.
Discussion
Effect of Fibrous Diet
In the present study, it was expected that feeding broiler breeder pullets with FBR diets, which increased feed allocation and was supposed to lessen competition, might result in higher BW uniformity and lower mortality. Opposite to our expectations, the FBR diet did not improve uniformity or reduce mortality. These results are congruent with those of other authors who fed broiler breeder pullets with diluted diets in rearing and found neither uniformity improvement (Zuidhof et al., 2015, de Los Mozos et al., 2017) nor mortality reduction (de Los Mozos et al., 2017). It would be interesting to study the effect of FBR diets on these parameters in field conditions, where management is poorer and, thus, competition is higher.
Lower breast muscle mass of the birds fed the FBR diet might be related to its higher insoluble fiber inclusion. Kluth and Rodehutscord (2009) studied the inevitable endogenous amino acid loss at the terminal ileum of broilers fed diets with 2 different insoluble fiber (cellulose) levels. The results showed a significant increase in CP and amino acid flow at the terminal ileum when the cellulose level in the diet was increased. In another study carried out with roosters force-fed N-free diets, Parsons et al. (1983) found that amino acid excretion was higher when high-insoluble fiber diet (with 17.5% cellulose) was used, in comparison with a low-insoluble fiber diet. As noted by de Lange et al. (1989), the effect of cellulose might be mediated by the mechanical effect of fiber. Likewise, Leterme et al. (1998) reported that fiber has an abrasive effect on the intestinal wall and may result in an increase in endogenous cell losses and nutrients to the lumen. Therefore, the birds fed the FBR diet might have had higher amino acid endogenous losses, and this might impair breast muscle deposition. Furthermore, the more developed digestive tract of the birds fed the FBR diet found in this study might also be a cause of their less developed breast muscle mass because amino acid allocation for the development and turnover of this higher digestive tract might have been to the detriment of breast muscle growth.
The relevance of phytate–protein complexes should also be highlighted in lowering protein utilization in monogastric animals. In the present study, diets were not phytase supplemented, and pullets fed the FBR diet consumed 19.2% more phytate throughout rearing and prebreeder periods (up to 22 wk of age) than did those of the control (FBR diet: 146 g vs. control: 118 g, P < 0.01). Phytate levels were not analyzed but are based on formulation levels. The study carried out with broilers by Ravindran et al. (2001) showed a linear improvement in ileal digestibility of nitrogen and all amino acids after the addition of increasing levels of supplemental phytase to a lysine-deficient diet. Regarding lysine, its percentages of improvement in digestibility with 500 and 1000 FTU/kg were 4.5 and 5.9%, respectively.
The higher amino acid endogenous losses and requirements to develop and maintain the digestive tract might be related to the more important abdominal fat deposition found in the birds fed the diluted diet. Yalçin et al. (2010) found that feeding broiler chickens diets containing 19.2, 16.6, and 15.5% CP (low protein) led to an increase in the total-carcass fat deposition, as compared with chickens fed diets containing 22.9, 19.9, and 18.2% CP in the starter, grower, and finisher phases, respectively. Corzo et al. (2006) and Zhan et al. (2006) reported significant increases in the abdominal fat percentage after feeding chickens methionine-deficient diets. Berri et al. (2008) and Nasr and Kheiri (2011) showed that dietary L-lysine supplementation lowered fat deposition in broiler chickens. In fact, lysine promotes lean meat production by reducing carcass fatness via lipogenesis inhibition (Grisoni et al., 1991).
Nowadays, the target is to control an excess of breast muscle and to stimulate abdominal fat deposition before the onset of production because fat content in the rearing period is related to egg persistence in the second half of the production phase (van Emous et al., 2015). Therefore, the fact of having the pullets fed the FBR diet, with less breast muscle and more abdominal fat, is an important advantage of this feed strategy.
The higher development of the proventriculus plus gizzard of the birds fed the FBR diet might be related to the high lignin content of most insoluble fiber sources, which leads to a longer retention of the feed in the gizzard, improving its muscular development and thus its function (Hetland and Svihus, 2001, Hetland et al., 2003, Jiménez-Moreno et al., 2010). Likewise, Sacranie et al. (2012) studied broiler chickens exposed to FBR diets diluted with coarse hulls or the same hulls finely ground. The large particle size of the coarse hulls and their hardness as a result of the insoluble fiber content explained why birds consuming the coarse hull diet developed the heaviest gizzards. However, birds fed the fine hull diet also exhibited heavier gizzards than did the control group, although less so than those of the coarse hull group.
In the present study, it was hypothesized that there would be greater skeletal strength in the broiler breeder fed the FBR diet than in the control pullets. However, the pullets fed the diluted diet had tibias with significantly lower BS, EM, and ash content. These results might signal poor bone mineral deposition early in rearing because it is stated that 90% of the skeleton of the broiler breeders is already developed at 11–13 wk of age (Ross Parent Stock Management Handbook, Aviagen, 2013). Nevertheless, results from this study are not congruent with those of Oikeh et al. (2019), who found that broilers fed diluted diets with insoluble fiber and lignin (lignocellulose) improved femur BS and femur and tibia ash weights. BS represents maximum load endurance: less mineralized bones have lower values; EM reflects the intrinsic stiffness or rigidity of the bone: less mineralized bones also have lower values (Turner and Burr, 1993); and because bone mineralization provides compressional strength to bone, the bone ash content has been used as an indicator of bone strength (Rath et al., 2000). The results of the present study suggest that skeletal strength of the pullets fed the FBR diet is poorer and thus mineral absorption might have been impaired. This would not be related to changes in the intestinal morphology produced by the FBR diet because this research did not show effects of fiber level on villus height, Lieberkühn crypt depth, and their ratio. These results are in agreement with those from some broiler studies which have not reported a relationship between intestinal morphology parameters and the presence of structural components (raw materials source of fiber or whole cereals) in broiler diets (Wu et al., 2004, Wu and Ravindran, 2004, Husvéth et al., 2015). However, high-insoluble fiber inclusion might have accelerated the passage time of the digesta through the digestive tract and reduce Ca and P absorption. The study by Enting et al. (2007a) compared a normal-density diet with low-density diets in broiler breeders at 22 wk. They reported a shorter mean retention time of the digesta in the jejunum and ileum, the difference being significant between normal- and low-density diets in the jejunum. In accordance with the results of the study by Leeson et al. (1991), the shorter retention time seems to be related to an increase in the insoluble fiber content of the low-density diets. The less time the digesta spend in the jejunum and ileum is negatively related to the amount of Ca and P absorbed by paracellular absorption, a passive and nonsaturable way (Fujita et al., 2008, Adedokun and Adeola, 2013), which, in turn, may impair bone mineral deposition and skeletal strength. Likewise, it should be highlighted that the formation of Ca–phytate complexes reduces phytate hydrolysis and Ca and phosphorus digestibility (Sebastian et al., 1996, Tamim et al., 2004, Plumstead et al., 2008), and, as was explained before, in the present study, pullets fed the FBR diet consumed a higher, significant amount of phytate than did those fed the CTR diet. Future research should consider the impact on bone mineralization of phytate intake in FBR diets.
There are no studies that have used ALP to assess skeletal development (bone mineral deposition) in broiler breeder pullets in rearing. However, in chickens, elevated ALP activity has been predominantly related to increased osteoblastic activity and used as a marker for evaluating skeletal health and bone disease, such as skeletal growth, rickets, fracture repair, and osteomyelitis (Lumeij and Westerhof, 1987). More recently, Ekmay et al. (2012) used ALP serological levels to assess broiler breeder growth and bone mineral deposition during transition into sexual maturity (24–26 wk of age). In the present study, pullets fed the FBR diet had lower ALP serological levels at 6 and 22 wk, which suggests that their bone mineral deposition during the starter and prebreeder periods was poorer than that of the control. It has to be emphasized that the ALP serological level is directly related to bone development and mineral deposition; thus, it is a better indicator of skeletal development than is BW, which is an indirect indicator.
In this study, the FBR diet did not affect bone growth, as TL and TW were not influenced. However, ALP serological levels at 6 wk and BS, EM, and ash content at 22 wk of age signal poorer bone mineral deposition of the birds fed the FBR diet (lower ALP serum values and lower BS and EM parameters and ash content of the tibias). Therefore, the FBR diet did not influence skeletal growth; nonetheless, mineral deposition was affected.
In the present study, it was hypothesized that there would be lower stereotypic behaviors in the broiler breeders fed the FBR diet than in the control pullets. As we expected, pullets fed the diluted diet showed a reduction in GFP and NFOP, stereotypic behaviors, at 22 wk of age. The reduction of GFP and NFOP in the birds fed the FBR diet may be explained by the increasing satiety coming from its insoluble fiber content. Oat hulls, a source of insoluble fiber, have been used in several studies to dilute the energy of the diets (Zuidhof et al., 1995, Hocking et al., 2004, Sandilands et al., 2005, Sandilands et al., 2006, Hocking, 2006, Enting et al., 2007a, Enting et al., 2007b, Nielsen et al., 2011); however, this compound was primarily designed to increase gastric satiety through delaying crop and gizzard emptying. Other studies showed a reduction in stereotypic behaviors in broiler breeders fed FBR diluted diets (Zuidhof et al., 1995, de Jong et al., 2005). Hocking et al. (2004) observed decreased spot pecking in broiler breeders fed diets with 20% oat hulls and 5% sugar beet pulp.
The wing-feather score at 22 wk of the pullets fed the FBR diet was lower than in those fed the CTR diet. Therefore, the results of the present study show, at 22 wk, a reduction of GFP and at the same time a lower wing-feather score of the birds fed the FBR diet; there might be a positive correlation to be further studied.
Effect of Vitamin C
In the present study, it was hypothesized that a positive effect of vitamin C might be obtained on the skeletal strength of broiler breeder pullets. However, vitamin C supplementation did not affect tibia BS, EM, or ash content. Our expectations were based on vitamin C involvement in the normal proliferation and differentiation of chondrocytes (essential for the initial formation of bone and later remodeling) and on the fact that its deficiency results in widespread connective tissue abnormalities coming from a disruption in collagen synthesis (Whitehead and Keller, 2003). In a similar manner, Weiser et al., 1988 reported the enhancement of 1,25-D production in vitamin C–supplemented chicks. One of the possible causes of not obtaining any vitamin C effect on skeletal strength might have been its low inclusion in the feed of our experiment. It was designed to supplement 200 mg/kg of vitamin C (after Whitehead et al. (1993) recommendation), but the average analyzed value of the different diets was 138 mg/kg. In a similar way, in the present study, the premix provided an adequate level of vitamin D3, which might have shaded the effect of vitamin C on the enhancement of 1,25-D production. Therefore, higher levels of vitamin C supplementation and a vitamin D3–deficient diet might be options for the experimental design of a future study. Another reason to study the vitamin C effect on skeletal strength was the fact that its endogenous synthesis in poultry may not be sufficient during stress periods, when the requirements may exceed the synthesizing ability (Gous and Morris, 2005). However, in experimental conditions, the management is correct, and thus, the stress is low. It would be interesting to study the vitamin C effect in field conditions, where management is poorer and thus competition and stress are higher.
In this study, it was hypothesized that there would be lower stereotypic behaviors in vitamin C–supplemented broiler breeders. At 15 wk, vitamin C inclusion reduced GFP of the pullets fed the CTR diet (lower in feed allowance) to the same level as the FBR diet did. As was explained previously, vitamin C recovery was lower than expected, and in experimental conditions, the birds may be little subjected to stress; these may also be the causes that vitamin C supplementation did not show a more important effect on reducing stereotypic behaviors. Nevertheless, a better feather-cover integrity was found; vitamin C reduced tail- and wing-feather scores of the pullets fed the CTR diet, in the case of tail score at the same level as in the FBR diet. In fact, studies show that vitamin C might help birds to cope with stressful situations (Satterlee et al., 1993, Satterlee et al., 1994, Jones et al., 1996, Jones et al., 1999). It has to be highlighted that feather cover protects female broiler breeders from being naturally damaged by the males during mating; therefore, it is important that the females finish the rearing period with good feather-cover integrity to obtain correct hatchability during production.
Conclusions
Fibrous diets, formulated with raw material source of insoluble fiber, do not modify broiler breeder pullet uniformity or mortality. Pullets fed the FBR diet have less breast muscle and more abdominal fat, which is a great advantage for later production; at 22 wk, their GFP and NFOP behaviors are lower and their wing feather integrity is better, which means they undergo less stress; however, even with their skeletal growth not being modified, their bone mineral content and thus skeletal strength are lower. It can be concluded that the FBR diet improves carcass traits and reduces stereotypic behaviors. Vitamin C inclusion does not influence skeletal strength, but it is effective in improving tail- and wing-feather integrity of the pullets fed the CTR diets, lower in feed allowance. Moreover, ALP could be a direct way to assess bone mineral deposition, and the tail- and wing-feather scores could be noninvasive practical tests to quickly assess stress in rearing farms.
Acknowledgments
All co-authors participated equally in the research and the writing of this manuscript. The English of this manuscript was proofread by Chuck Simmons, a native English-speaking, retired university Instructor of English.
Conflict of Interest Statement: The authors did not provide a conflict of interest statement.
References
- AOAC International . 18th ed. AOAC International; Gaithersburg, MD, US: 2005. Official Methods of Analysis of AOAC International. [Google Scholar]
- Adedokun S.A., Adeola O. Calcium and phosphorus digestibility: metabolic limits. J. Appl. Poult. Res. 2013;22:600–608. [Google Scholar]
- Aviagen . Aviagen, Ltd.; Newbridge, UK: 2010. Protocol for Scoring the Feather Cover of Broiler Breeders during the Laying Period. [Google Scholar]
- Aviagen . Aviagen, Ltd.; Newbridge, UK: 2013. Ross Parent Stock Management Handbook. [Google Scholar]
- Aviagen . Aviagen, Inc.; Huntsville, AL, US: 2016. Ross 308 Parent Stock Nutrition Specifications. [Google Scholar]
- Aviagen . Aviagen, Inc.; Huntsville, AL, US: 2016. Ross 308 Parent Stock Performance Objectives. [Google Scholar]
- Berri C., Besnard J., Relandeau C. Increasing dietary lysine increases final pH and decreases drip loss of broiler breast meat. Poult. Sci. 2008;87:480–484. doi: 10.3382/ps.2007-00226. [DOI] [PubMed] [Google Scholar]
- Corzo A., Kidd M.T., Dozier W.A., Shack L.A., Burgess S.C. Protein expression of pectoralis major muscle in chickens in response to dietary methionine status. Br. J. Nutr. 2006;95:703–708. doi: 10.1079/bjn20051716. [DOI] [PubMed] [Google Scholar]
- de Jong I.C., Enting H., van Voorst A., Blokhuis H.J. Do low-density diets improve broiler breeder welfare during rearing and laying? Poult. Sci. 2005;84:194–203. doi: 10.1093/ps/84.2.194. [DOI] [PubMed] [Google Scholar]
- de Jong I.C., van Voorst S., Ehlhardt D.A., Blokhuis H.J. Effects of restricted feeding on physiological stress parameters in growing broiler breeders. Br. Poult. Sci. 2002;43:157–168. doi: 10.1080/00071660120121355. [DOI] [PubMed] [Google Scholar]
- de Lange C.F., Sauer W.C., Mosenthin R., Souffrant W.B. The effect of feeding different protein-free diets on the recovery and amino acid composition of endogenous protein collected from the distal ileum and feces in pigs. J. Anim. Sci. 1989;67:746–754. doi: 10.2527/jas1989.673746x. [DOI] [PubMed] [Google Scholar]
- de Los Mozos J., García-Ruiz A.I., den Hartog L.A., Villamide M.J. Growth curve and diet density affect eating motivation, behavior, and body composition of broiler breeders during rearing. Poult. Sci. 2017;96:2708–2717. doi: 10.3382/ps/pex045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekmay R.D., Salas C., England J., Cerrate S., Coon C.N. The effects of pullet body weight, dietary nonpyhtate phosphorus intake, and breeder feeding regimen on production performance, chick quality, and bone remodeling in broiler breeders. Poult. Sci. 2012;91:948–964. doi: 10.3382/ps.2011-01931. [DOI] [PubMed] [Google Scholar]
- Enting H., Veldman A., Verstegen M.W.A., van der Aar P.J. The effect of low-density diets on broiler breeder development and nutrient digestibility during the rearing period. Poult. Sci. 2007;86:720–726. doi: 10.1093/ps/86.4.720. [DOI] [PubMed] [Google Scholar]
- Enting H., Kruip T.A., Verstegen M.W.A., van der Aar P.J. The effect of low-density diets on broiler breeder performance during the laying period and on embryonic development of their offspring. Poult. Sci. 2007;86:850–856. doi: 10.1093/ps/86.5.850. [DOI] [PubMed] [Google Scholar]
- European Parliament . Official Journal of the European Union; Strasbourg, France: 2010. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. [Google Scholar]
- Fleming R.H., McCormack H.A., McTeir L., Whitehead C.C. Medullary bone and humeral breaking strength in laying hens. Res. Vet. Sci. 1998;64:63–67. doi: 10.1016/s0034-5288(98)90117-5. [DOI] [PubMed] [Google Scholar]
- Fujita H., Sugimoto K., Inatomi S., Maeda T., Osanai M., Uchiyama Y., Yamamoto Y., Wada T., Kojima T., Yokozaki H., Yamashita T., Kato S., Sawada N., Chiba H. Tight junction proteins claudin-2 and -12 are critical for vitamin D-dependent Ca2+ absorption between enterocytes. Mol. Biol. Cell. 2008;19:1912–1921. doi: 10.1091/mbc.E07-09-0973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Alvarado J.M., Jiménez-Moreno E., Lázaro R., Mateos G.G. Effect of type of cereal, heat processing of the cereal, and inclusion of fiber in the diet on productive performance and digestive traits of broilers. Poult. Sci. 2007;86:1705–1715. doi: 10.1093/ps/86.8.1705. [DOI] [PubMed] [Google Scholar]
- Gous R.M., Morris T.R. Nutritional interventions in alleviating the effects of high temperatures in broiler production. Worlds. Poult. Sci. J. 2005;61:463–475. [Google Scholar]
- Grisoni M.L., Uzu G., Larbier M., Geraert P.A. Effect of dietary lysine on lipogenesis in broilers. Reprod. Nutr. Dev. 1991;31:683–690. doi: 10.1051/rnd:19910608. [DOI] [PubMed] [Google Scholar]
- Hampson D.J. Alterations in piglet small intestinal structure at weaning. Res. Vet. Sci. 1986;40:32–40. [PubMed] [Google Scholar]
- Harris H. The human alkaline phosphatases: what we know and what we don’t know. Clin. Chim. Acta. 1990;186:133–150. doi: 10.1016/0009-8981(90)90031-m. [DOI] [PubMed] [Google Scholar]
- Hetland H., Svihus B. Effect of oat hulls on performance, gut capacity and feed passage time in broiler chickens. Br. Poult. Sci. 2001;42:354–361. doi: 10.1080/00071660120055331. [DOI] [PubMed] [Google Scholar]
- Hetland H., Svihus B., Krogdalhl Å. Effects of oat hulls and wood shaving on digestion in broilers and layers fed diets based on whole or ground wheat. Br. Poult. Sci. 2003;44:275–282. doi: 10.1080/0007166031000124595. [DOI] [PubMed] [Google Scholar]
- Hocking P.M., Maxwell M.H., Mitchell M.A., Robertson G.W. Welfare and productivity of restricted broiler breeder females fed ad libitum or restricted after the peak of egg production. Br. Poult. Sci. 1998;39:S16–S17. doi: 10.1080/00071669888115. [DOI] [PubMed] [Google Scholar]
- Hocking P.M., Maxwell M.H., Mitchell M.A. Welfare of food restricted male and female turkeys. Br. Poult. Sci. 1999;40:19–29. doi: 10.1080/00071669987782. [DOI] [PubMed] [Google Scholar]
- Hocking P.M., Zaczek V., Jones E.K.M., Macleod M.G. Different concentrations and sources of dietary fibre may improve the welfare of female broiler breeders. Br. Poult. Sci. 2004;45:9–19. doi: 10.1080/00071660410001668806. [DOI] [PubMed] [Google Scholar]
- Hocking P.M. High-fibre pelleted rations decrease water intake but do not improve physiological indexes of welfare in food-restricted female broiler breeders. Br. Poult. Sci. 2006;47:19–23. doi: 10.1080/00071660500468041. [DOI] [PubMed] [Google Scholar]
- Husvéth F., Pál L., Galamb E., Ács K.C., Bustyaházai L., Wágner L., Dublecz F., Dublecz K. Effects of whole wheat incorporated into pelleted diets on the growth performance and intestinal function of broiler chickens. Anim. Feed Sci. Technol. 2015;210:144–151. [Google Scholar]
- Jiménez-Moreno E., González-Alvarado J.M., González-Sánchez D., Lázaro R., Mateos G.G. Effects of type and particle size of dietary fiber on growth performance and digestive traits of broilers from 1 to 21 days of age. Poult. Sci. 2010;89:2197–2212. doi: 10.3382/ps.2010-00771. [DOI] [PubMed] [Google Scholar]
- Jones R.B., Satterlee D.G., Moreau J., Waddington D. Vitamin C supplementation and fear-reduction in Japanese quail: short-term cumulative effects. Br. Poult. Sci. 1996;37:33–42. doi: 10.1080/00071669608417834. [DOI] [PubMed] [Google Scholar]
- Jones R.B., Satterlee D.G., Cadd G.G. Timidity in Japanese quail: effects of vitamin C and divergent selection for adrenocortical response. Physiol. Behav. 1999;67:117–120. doi: 10.1016/s0031-9384(99)00039-6. [DOI] [PubMed] [Google Scholar]
- Kluth H., Rodehutscord M. Effect of inclusion of cellulose in the diet on the inevitable endogenous amino acid losses in the ileum of broiler chicken. Poult. Sci. 2009;88:1199–1205. doi: 10.3382/ps.2008-00385. [DOI] [PubMed] [Google Scholar]
- Leeson A., Summers J.D., Caston L.J. Diet dilution and compensatory growth in broilers. Poult. Sci. 1991;70:867–873. doi: 10.3382/ps.0700867. [DOI] [PubMed] [Google Scholar]
- Leterme P., Froidmont E., Rossi F., Thewis A. The high water-holding capacity of pea inner fibers affects the ileal flow of endogenous amino acids in pigs. J. Agric. Food Chem. 1998;46:1927–1934. [Google Scholar]
- Lumeij J.T., Westerhof I. Blood chemistry for the diagnosis of hepatobiliary disease in birds. A. Review. Vet. Q. 1987;9:255–261. doi: 10.1080/01652176.1987.9694110. [DOI] [PubMed] [Google Scholar]
- Morrissey K.L.H., Widowski T., Leeson S., Sandilands V., Arnone A., Torrey S. The effect of dietary alterations during rearing on growth, productivity, and behavior in broiler breeder females. Poult. Sci. 2014;93:285–295. doi: 10.3382/ps.2013-03265. [DOI] [PubMed] [Google Scholar]
- Nasr J., Kheiri F. Effect of different lysine levels on Arian broiler performance. Ital. J. Anim. Sci. 2011;10:170–174. [Google Scholar]
- Nielsen B.L., Thodberg K., Malmkvist J., Steenfeldt S. Proportion of insoluble fibre in the diet affects behaviour and hunger in broiler breeders growing at similar rates. Animal. 2011;5:1247–1258. doi: 10.1017/S1751731111000218. [DOI] [PubMed] [Google Scholar]
- Oikeh I., Sakkas P., Taylor J., Giannenas I., Blake D.P., Kyriazakis I. Effects of reducing growth rate via diet dilution on bone mineralization, performance and carcass yield of coccidia-infected broilers. Poult. Sci. 2019;98:5477–5487. doi: 10.3382/ps/pez400. [DOI] [PubMed] [Google Scholar]
- Parsons C.M., Potter L.M., Brown R.D. Effects of dietary carbohydrate and of intestinal microflora on excretion of endogenous amino acids by poultry. Poult. Sci. 1983;62:483–489. doi: 10.3382/ps.0620483. [DOI] [PubMed] [Google Scholar]
- Plumstead P.W., Leytem A.B., Maguire R.O., Spears J.W., Kwanyuen P., Brake J. Interaction of calcium and phytate in broiler diets. 1. Effects on apparent prececal digestibility and retention of phosphorus. Poult. Sci. 2008;87:449–458. doi: 10.3382/ps.2007-00231. [DOI] [PubMed] [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; Vienna, AT: 2018. R: A Language and Environment for Statistical Computing. [Google Scholar]
- Rath N.C., Huff G.R., Huff W.E., Balog J.M. Factors regulating bone maturity and strength in poultry. Poult. Sci. 2000;79:1024–1032. doi: 10.1093/ps/79.7.1024. [DOI] [PubMed] [Google Scholar]
- Ravindran V., Selle P.H., Ravindran G., Morel P.C.H., Kies A.K., Bryden W.L. Microbial phytase improves performance, apparent metabolizable energy, and ileal amino acid digestibility of broilers fed a lysine-deficient diet. Poult. Sci. 2001;80:338–344. doi: 10.1093/ps/80.3.338. [DOI] [PubMed] [Google Scholar]
- Sacranie A., Svihus B., Denstadli V., Moen B., Iji P.A., Choct M. The effect of insoluble fiber and intermittent feeding on gizzard development, gut motility, and performance of broiler chickens. Poult. Sci. 2012;91:693–700. doi: 10.3382/ps.2011-01790. [DOI] [PubMed] [Google Scholar]
- Sandilands V., Tolkamp B.J., Kyriazakis I. Behaviour of food restricted broilers during rearing and lay - effects of an alternative feeding method. Physiol. Behav. 2005;85:115–123. doi: 10.1016/j.physbeh.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Sandilands V., Tolkamp B.J., Savory C.J., Kyriazakis I. Behaviour and welfare of broiler breeders fed qualitatively restricted diets during rearing: are there viable alternatives to quantitative restriction? Appl. Anim. Behav. Sci. 2006;96:53–67. [Google Scholar]
- Satterlee D.G., Jones R.B., Ryder F.H. Short-latency stressor effects on tonic immobility fear reactions of Japanese quail divergently selected for adrenocortical responsiveness to immobilization. Poult. Sci. 1993;72:1132–1136. doi: 10.3382/ps.0721132. [DOI] [PubMed] [Google Scholar]
- Satterlee D.G., Jones R.B., Ryder F.H. Effects of ascorbyl-2-polyphosphate on adrenocortical activation and fear-related behavior in broiler chickens. Poult. Sci. 1994;73:194–201. doi: 10.3382/ps.0730194. [DOI] [PubMed] [Google Scholar]
- Savory C.J., Hocking P.M., Mann J.S., Maxwell M.H. Is broiler breeder welfare improved by using qualitative rather than quantitative food restriction to limit growth rate? Anim. Welf. 1996;5:105–127. [Google Scholar]
- Sebastian S., Touchburn S.P., Chavez E.R., Laguë P.C. Efficacy of supplemental microbial phytase at different dietary calcium levels on growth performance and mineral utilization of broiler chickens. Poult. Sci. 1996;75:1516–1523. doi: 10.3382/ps.0751516. [DOI] [PubMed] [Google Scholar]
- Seibel M.J. Biochemical markers of bone turnover: part I: biochemistry and variability. Clin. Biochem. Rev. 2005;26:97–122. [PMC free article] [PubMed] [Google Scholar]
- Stinson R.A., Hamilton B.A. Human liver plasma membranes contain an enzyme activity that removes membrane anchor from alkaline phosphatase and converts it to a plasma-like form. Clin. Biochem. 1994;27:49–55. doi: 10.1016/0009-9120(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Tamim N.M., Angel R., Christman M. Influence of dietary calcium and phytase on phytate phosphorus hydrolysis in broiler chickens. Poult. Sci. 2004;83:1358–1367. doi: 10.1093/ps/83.8.1358. [DOI] [PubMed] [Google Scholar]
- Turner C.H., Burr D.B. Basic biomechanical measurements of bone: a tutorial. Bone. 1993;14:595–608. doi: 10.1016/8756-3282(93)90081-k. [DOI] [PubMed] [Google Scholar]
- van Emous R.A., Kwakkel R.P., van Krimpen M.M., Hendriks W.H. Effects of dietary protein levels during rearing and dietary energy levels during lay on body composition and reproduction in broiler breeder females. Poult. Sci. 2015;94:1030–1042. doi: 10.3382/ps/pev079. [DOI] [PubMed] [Google Scholar]
- Weiser H., Schlachter M., Bachmann H. Proceedings of the Seventh Workshop on Vitamin D, Rancho Mirage, California. US. Walter de Gruyter; Berlin, DE: 1988. The importance of vitamin C for hydroxylation of Vitamin D 3 to 1 a, 25 (OH)z DJ and of 24R, 25 (OH)2 D3 to a more active metabolite; pp. 644–653. [Google Scholar]
- Whitehead C.C., Rennie J.S., McCormack H.A., Hocking P.M. Defective down syndrome in chicks is not caused by riboflavin deficiency in breeders. Br. Poult. Sci. 1993;34:619–623. doi: 10.1080/00071669308417618. [DOI] [PubMed] [Google Scholar]
- Whitehead C.C., Keller T. An update on ascorbic acid in poultry. Worlds. Poult. Sci. J. 2003;59:161–184. [Google Scholar]
- Wu Y.B., Ravindran V. Influence of whole wheat inclusion and xylanase supplementation on the performance, digestive tract measurements and carcass characteristics of broiler chickens. Anim. Feed Sci. Technol. 2004;116:129–139. [Google Scholar]
- Wu Y.B., Ravindran V., Thomas D.G., Birtles M.J., Hendriks W.H. Influence of method of whole wheat inclusion and xylanase supplementation on the performance, apparent metabolisable energy, digestive tract measurements and gut morphology of broilers. Br. Poult. Sci. 2004;45:385–394. doi: 10.1080/00071660410001730888. [DOI] [PubMed] [Google Scholar]
- Yalçin S., Özkul H., Özkan S., Gous R., Yaşa I., Babacanoğlu E. Effect of dietary protein regime on meat quality traits and carcase nutrient content of broilers from two commercial genotypes. Br. Poult. Sci. 2010;51:621–628. doi: 10.1080/00071668.2010.520302. [DOI] [PubMed] [Google Scholar]
- Zhan X.A., Li J.X., Xu Z.R., Zhao R.Q. Effects of methionine and betaine supplementation on growth performance, carcase composition and metabolism of lipids in male broilers. Br. Poult. Sci. 2006;47:576–580. doi: 10.1080/00071660600963438. [DOI] [PubMed] [Google Scholar]
- Zuidhof M.J., Holm D.E., Renema R.A., Jalal M.A., Robinson F.E. Effects of broiler breeder management on pullet body weight and carcass uniformity. Poult. Sci. 2015;94:1389–1397. doi: 10.3382/ps/pev064. [DOI] [PubMed] [Google Scholar]
- Zuidhof M.J., Robinson F.E., Feddes J.J.R., Hardin R.T., Wilson J.L., McKay R.I., Newcombe M. The effects of nutrient dilution on the well-being and performance of female broiler breeders. Poult. Sci. 1995;74:441–456. doi: 10.3382/ps.0740441. [DOI] [PubMed] [Google Scholar]