Abstract
This study investigated the effects of ammonia (NH3) exposure (0, 15, 25, and 35 ppm) on growth performance and cytokines in the serum, trachea, and ileum of broilers. A total of 288 22-day-old male broiler chickens were assigned to 4 treatment groups with 6 replicates of 12 chickens for a 21-D trial period. Growth performance and cytokines (IL-1β, IL-6, and IL-10) concentrations in the serum, trachea, and ileum were measured in response to 3, 7, 14, or 21 D of exposure to NH3. Correlations between cytokines in the serum, trachea, and ileum and growth performance, and between tracheal and ileal cytokines, were also analyzed. Results showed that exposure to 15 ppm NH3 did not influence the growth performance, but exposure to both 25 ppm and 35 ppm NH3 decreased the growth performance compared to that of the control group. Exposure to 15 ppm NH3 for 3 D increased IL-6 concentrations and induced an inflammatory response in the trachea and ileum, whereas exposure to 15 ppm NH3 for 7 D increased IL-10 concentrations and induced an anti-inflammatory response in the ileum. Exposure to 25 ppm NH3 induced an inflammatory response in the serum, trachea, and ileum after 3 D and induced an anti-inflammatory response in the ileum after 7 D. Exposure to 35 ppm NH3 for 3 D induced both inflammatory and anti-inflammatory responses in the trachea and ileum. Furthermore, increases in cytokines in the serum, trachea, or ileum were accompanied by a decrease in BW, ADFI, ADG, and an increase of feed/gain (F/G) from 7 D to 21 D. In addition, tracheal cytokine, especially IL-1β, was positively correlated with ileal cytokine IL-1β. These results indicated that the low growth performance associated with NH3 exposure may be due in part to an increase in cytokines, and the inflammatory response in the trachea and ileum may be related to cross-talk by cytokines such as IL-6, IL-10, and, in particular, IL-1β.
Key words: ammonia, cytokine, growth performance, broiler
Introduction
Ammonia (NH3) is a toxic gas and is recognized as one of the most dominant contaminants in buildings that house broiler chickens (David et al., 2015). In production practices, mean aerial NH3 concentrations in poultry houses are typically between 5 and 30 ppm (Koerkamp et al., 1998, Ni et al., 2012). It has been suggested that NH3 should not exceed 25 ppm in poultry houses (Beker et al., 2004, Miles et al., 2004, David et al., 2015, Wei et al., 2015, Yi et al., 2016). However, levels of NH3 in broiler houses usually exceed 25 ppm and can reach as high as 50 ppm or more (Oyetunde et al., 1978, Deaton et al., 1982, Reece et al., 1982, Miles et al., 2004). Therefore, NH3 emissions have received increasing attention for their potentially negative impacts on farming environments, the ecosystem, and human and animal health (Murphy et al., 2012, Costa, 2017).
One of the problems caused by NH3 in the air is reduced growth performance, which results in lower body weights and higher feed conversion ratios (Miles et al., 2004, Yahav, 2004). Previous studies showed that NH3 levels above 25 ppm inside poultry houses have negative effects on chicken growth performance, including reduced BW gain, feed intake, and feed conversion rates (Reece et al., 1981, Beker et al., 2004, Miles et al., 2004, Miles et al., 2006, Xiong et al., 2016).
It is generally accepted that gaseous NH3 is a severe irritant capable of inhibiting the efficiency of the respiratory system (Zhang et al., 2015b, Xiong et al., 2016). In the birds' respiratory system, the trachea acts as an air channel for breathing and is the first line of defense against pollutants (Puchelle et al., 1995). Shi et al. (2019) found that exposure to NH3 (0–3 wk: 19.5–20.5 ppm; 3–6 wk: 44.5–45.5 ppm) directly affected the chicken tracheal transcriptome, causing an abnormalities in the tracheal immune response in the respiratory system, and that increased T helper cells 2 (Th2) and T helper cells 17 (Th17) accelerate the imbalance of regulatory cell (Treg)/T helper cells 1 (Th1), causing inflammation of the trachea injuries. The study by Xiong et al. (2016) found that exposure to high concentration of atmospheric NH3 (75 ppm) increased IL-1β and IL-6 levels in the trachea. In addition, the study by Wang et al. (2019) had showed that excessive exposure to NH3 (30 ppm) caused an inflammatory response in peripheral blood neutrophils in chickens. These results showed that there was limited information available on inflammatory factors in the trachea and peripheral blood of broilers' exposure to NH3. In addition, the study found that associations between inflammatory factors such as systemic IL-6 in the respiratory and intestinal systems have been clearly identified in the clinical literature, but there have been few basic research studies that have investigated which mechanisms of inflammatory cross-talk are involved (Keely et al., 2012). Moreover, there have been limited studies so far on the secretion of cytokines in intestinal, and also few reports on the relationship between respiratory and intestinal cytokines in broiler chickens' exposure to NH3.
Cytokines play an important role in the course of the inflammatory responses (Osborne and Abraham, 2010, Mucksová et al., 2018). Among the cytokines, IL-1β and IL-6 are potent proinflammatory and immunomodulatory cytokines (Tanaka and Kishimoto, 2012, Ghareeb et al., 2013), whereas IL-10 has been recognized as a tolerogenic and anti-inflammatory cytokine that blocks the production of proinflammatory cytokines (Sabat et al., 2010). It is also clear that overproduction of these molecules could have potentially deleterious effects (Webel et al., 1998), such as decreased feed efficiency and growth rates (Colditz, 2002, Sinkora et al., 2002, Pié et al., 2004). However, there is limited information available on the relationship between cytokines and growth performance under NH3 exposure.
The objective of this study was to determine the effects of NH3 on 1) the growth performance of broiler chickens; 2) IL-1β, IL-6, and IL-10 concentrations in the serum, trachea, and ileum after exposure for different periods; and 3) relationships between cytokines (in the serum, trachea, and ileum) and growth performance, as well as between tracheal cytokines and ileal cytokines.
Materials and methods
Ethical Statement
The protocol was approved by the Animal Experimental Welfare and Ethical Inspection Form of Institute of Animal Science, Chinese Academy of Agricultural Sciences (permit number: IAS 2019-42).
Experimental Animals and Treatments
A total of 380 one-day old male Arbor Acres broiler (AA) chicks were obtained from a commercial hatchery in Beijing (Beijing Arbor Acers Broiler Co., Beijing, China) and placed in an environmentally controlled room under standard brooding practices, receiving ad libitum access to water and a standard corn-soybean-based diet during the first 21 D. At the age of 22 D, a total of 288 birds with close weight were selected and transferred into 4 environmentally controlled exposure chambers (4.5 m length × 3.0 m width × 2.5 m height). Each chamber had 6 one-tier cages (0.82 m long × 0.07 m wide × 0.06 m depth). A cage was a replicate, so each treatment had 6 replicates with 12 broilers in each replicate. Broilers in the control group (<3 ppm) were housed in a separate chamber without NH3 from days 22 to 42, whereas broilers in the treatment groups were exposed to NH3 concentration of 15 ± 3 ppm, 25 ± 3 ppm, 35 ± 3 ppm, respectively, during the experimental period. The concentrated NH3 was delivered in a whole-body animal exposure chamber from days 22 to 42. The chambers were computer-programmed to have the NH3 concentration as required. The concentrations of NH3 in 4 chambers were monitored with a LumaSense Photoacoustic Field Gas-Monitor INNOVA 1412 (Santa Clara, CA) during the entire experiment. Temperature (21°C ± 1°C), relative humidity (60 ± 7%), and airflow were controlled during the exposures to ensure adequate ventilation, minimize buildup of animal-generated contaminants (dander, CO2, H2S), and avoid thermal stress. The manure was removed from the chambers every 3 D to reduce NH3 volatilization. The diet during the experiment was formulated to achieve the National Research Council (NRC, 1994; Table 1)-recommended requirements for all nutrients.
Table 1.
Composition and nutrient levels of the complete diets for broilers.
| Item | 1–3 wk | 4–6 wk |
|---|---|---|
| Ingredients (%) | ||
| Corn | 53.36 | 56.51 |
| Soybean meal | 38.50 | 35.52 |
| Soybean oil | 4.10 | 4.50 |
| NaCl | 0.30 | 0.30 |
| Limestone | 1.15 | 1.00 |
| CaHPO4 | 2.01 | 1.78 |
| DL-Met | 0.22 | 0.11 |
| Premix1 | 0.36 | 0.28 |
| Total | 100.00 | 100.00 |
| Nutrient levels (%)2 | ||
| ME/(MJ kg−1) | 12.46 | 12.73 |
| CP | 21.44 | 20.07 |
| Ca | 1.00 | 0.90 |
| AP | 0.45 | 0.40 |
| Lys | 1.17 | 1.00 |
| Met | 0.56 | 0.42 |
| Met + Cys | 0.91 | 0.78 |
The premix was given for 1–3 wk provided the following nutrients per kilogram of diet: vitamin A, 12,500 IU; vitamin D3, 3,750 IU; vitamin E, 16 IU; vitamin K3, 2.0 mg; vitamin B1, 2.5 mg; vitamin B2, 8 mg; vitamin B6, 2.5 mg; vitamin B12, 0.015 mg, pantothenic acid calcium, 12.5 mg; nicotinic acid, 32.5 mg; folic acid, 1.25 mg; biotin, 0.125 mg; choline, 700 mg; Zn (ZnSO4·7H2O), 60 mg; Fe (FeSO4·7H2O), 80 mg; Cu (CuSO4·5H2O), 8 mg; Mn (MnSO4·H2O), 110 mg; I (KI), 0.35 mg; Se (Na2SeO3), 0.15 mg. The premix was given for 4–6 wk provided the following nutrients per kilogram of diet: vitamin A, 10,000 IU; vitamin D3, 3,400 IU; vitamin E, 16 IU; vitamin K3, 2.0 mg; vitamin B1, 2.0 mg; vitamin B2, 6.4 mg; vitamin B6, 2.0 mg; vitamin B12, 0.012 mg; pantothenic acid calcium, 10 mg; nicotinic acid, 26 mg; folic acid, 1 mg; biotin, 0.1 mg; choline, 500 mg; Zn (ZnSO4·7H2O), 40 mg; Fe (FeSO4·7H2O), 80 mg; Cu (CuSO4·5H2O), 8 mg; Mn (MnSO4·H2O), 80 mg; I (KI), 0.35 mg; Se (Na2SeO3), 0.15 mg.
Metabolizable energy was calculated, whereas the others were measured.
Sample Collection
At the 3, 7, 14, and 21 D of the experiment, all birds were weighted after 12 h of fasting (12-h food withdrawals but with ad libitum water). The growth performance parameters including BW, ADG, ADFI, and feed conversion ratio (F/G) were measured. One broiler was randomly selected from each replication for blood and tissue sample collection. The blood samples were collected in 3 to 4 mL volumes from the wing vein into 5 mL sterile test tubes. Blood samples were centrifuged at 1,500 × g for 15 min to separate the serum. After blood collection, chickens were euthanized by cervical dislocation to obtain the trachea and ileum tissues. The middle trachea tissues (2 cm) were collected and then were washed with ice cold sterilized saline, frozen in liquid nitrogen, and stored at −80°C for further analyses. Ileum midsections of 1 cm between Meckel's diverticulum and ceco-iliac junction were collected and then were washed with saline, frozen in liquid nitrogen, and stored at −80°C for further analyses.
Cytokines Analysis
At the 3, 7, 14, and 21 D of the experiment, IL-1β, IL-6, and IL-10 concentrations in the serum, trachea, and ileum were measured by commercial ELISA kits specific for chicken (NovateinBio, USA), respectively, according to the manufacturer's instructions. Optical densities of kit standards and test samples were read at 450 nm using an ELISA plate reader (Multiskan MK3, Thermo Fisher Scientific).
Statistical Analysis
All statistical analyses were performed using SAS statistical version 9.2 (SAS Institute, 2010). Data on growth performance and cytokine concentrations were analyzed by one-way ANOVA. Differences among means were tested by Duncan multiple range test. Pearson's correlations coefficients (R) between growth performance and cytokines in the serum, trachea, and ileum, and between tracheal cytokines and ileal cytokines, were presented and calculated using the correlation procedure. In addition, linear regression analysis was completed using the regression procedure to examine the relationship between tracheal cytokines and ileal cytokines. The data of growth performance and cytokines concentrations were presented as mean ± standard error. The replicate cage served as the experimental unit, and the P < 0.05 was considered statistically significant.
Results
Growth Performance
Broiler performance data are shown in Table 2. At the 3 D, NH3 treatments (0, 15, 25, and 35 ppm) did not influence (P > 0.05) the growth performance (ADFI, ADG, F/G, and BW). At 7 D, compared to the control group, only 35 ppm group had reduced (P < 0.001) ADG and BW, and both 25 ppm and 35 ppm groups had increased (P < 0.001) F/G, and NH3 treatments did not influence (P > 0.05) the ADFI. At 14 D and 21 D, compared to the control group, only 35 ppm group had reduced (P < 0.001) ADFI and increased (P < 0.001) F/G, respectively, and both 25 ppm and 35 ppm groups had reduced (P < 0.001) ADG and BW, 35 ppm group further reduced (P < 0.05) the ADG and BW compared to the 25 ppm group.
Table 2.
Effects of ammonia (NH3) treatments (0, 15, 25, and 35 ppm) on BW, ADFI, ADG, and feed to gain ratio (F/G) of broiler chickens.
| Time points | Treatment | ADFI/g | ADG/g | F/G | BW/kg |
|---|---|---|---|---|---|
| 3 | 0 ppm | 96.06 ± 5.68 | 78.18 ± 1.88 | 1.23 ± 0.08 | 0.886 ± 0.004 |
| 15 ppm | 100.91 ± 3.98 | 79.39 ± 1.92 | 1.27 ± 0.06 | 0.889 ± 0.005 | |
| 25 ppm | 100.61 ± 5.84 | 76.37 ± 1.82 | 1.33 ± 0.09 | 0.883 ± 0.006 | |
| 35 ppm | 99.70 ± 2.01 | 73.94 ± 1.53 | 1.35 ± 0.03 | 0.876 ± 0.008 | |
| P-value | 0.8736 | 0.1888 | 0.6202 | 0.5079 | |
| 7 | 0 ppm | 104.91 ± 3.08 | 80.39 ± 1.16a | 1.31 ± 0.04c | 1.173 ± 0.008a |
| 15 ppm | 108.48 ± 2.16 | 80.39 ± 1.28a | 1.35 ± 0.04b,c | 1.173 ± 0.008a | |
| 25 ppm | 107.28 ± 2.36 | 75.00 ± 2.37a | 1.44 ± 0.05a,b | 1.142 ± 0.015a | |
| 35 ppm | 101.52 ± 1.44 | 66.67 ± 2.00b | 1.53 ± 0.03a | 1.092 ± 0.017b | |
| P-value | 0.1927 | <0.0001 | 0.0047 | 0.0004 | |
| 14 | 0 ppm | 115.09 ± 2.47a | 76.61 ± 1.11a | 1.50 ± 0.03b | 1.686 ± 0.014a |
| 15 ppm | 114.25 ± 1.74a | 72.63 ± 2.12a,b | 1.58 ± 0.05b | 1.634 ± 0.027a,b | |
| 25 ppm | 114.25 ± 2.33a | 69.59 ± 1.59b | 1.65 ± 0.06b | 1.600 ± 0.021b | |
| 35 ppm | 96.47 ± 1.18b | 51.78 ± 1.30c | 1.87 ± 0.06a | 1.365 ± 0.017c | |
| P-value | <0.0001 | <0.0001 | 0.0004 | <0.0001 | |
| 21 | 0 ppm | 119.55 ± 2.27a | 76.27 ± 1.06a | 1.57 ± 0.02b | 2.216 ± 0.021a |
| 15 ppm | 118.19 ± 1.55a | 73.77 ± 0.95a | 1.60 ± 0.02b | 2.166 ± 0.019a | |
| 25 ppm | 118.62 ± 2.38a | 69.95 ± 1.29b | 1.70 ± 0.02b | 2.091 ± 0.026b | |
| 35 ppm | 99.52 ± 1.40b | 41.51 ± 1.13c | 2.41 ± 0.08a | 1.522 ± 0.026c | |
| P-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
a,bMeans with different letters in the same column indicated a significant difference (P < 0.05). Values were presented as the mean ± standard error and were determined at 4 different time points (3 D, 7 D, 14 D, and 21 D).
Serum Cytokine Concentrations
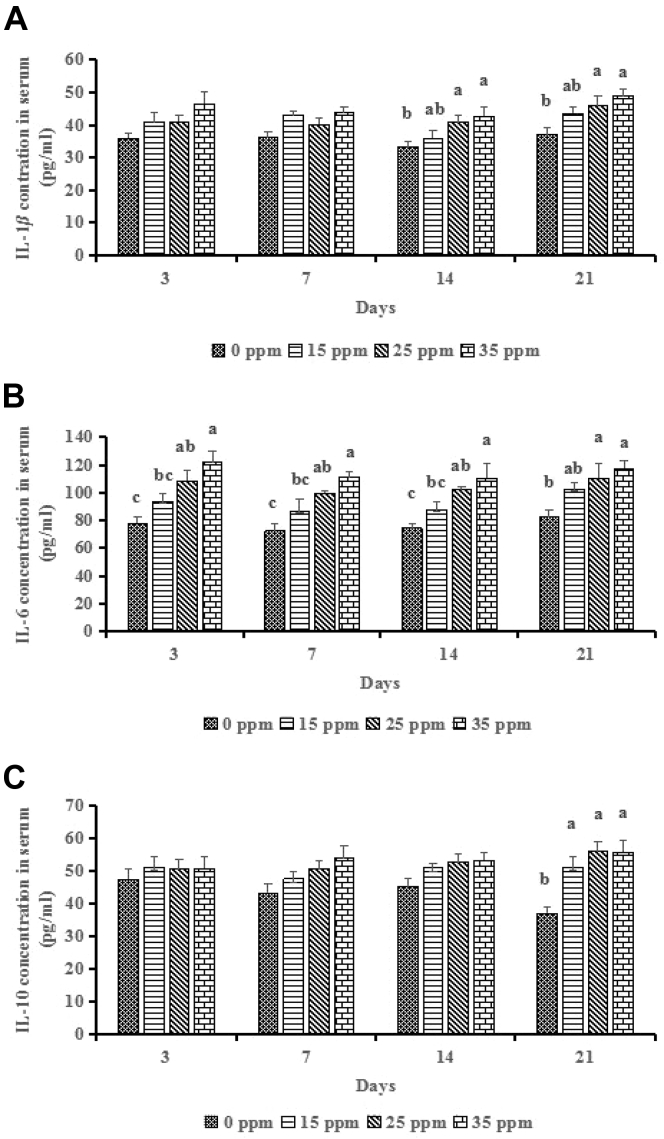
Serum cytokines concentrations are shown in Figure 1. At 3 D and 7 D, NH3 treatments did not influence (P > 0.05) the IL-1β and IL-10 concentrations, and 25 ppm and 35 ppm groups had increased (P < 0.001) the IL-6 concentration compared to the control group. At the 14 D, NH3 treatments did not influence (P > 0.05) the IL-10 concentration, and 25 ppm and 35 ppm groups had increased (P < 0.05) IL-1β and IL-6 concentrations compared to the control group. At the 21 D, compared to the control group, 25 ppm and 35 ppm groups had increased (P < 0.05) the IL-1β and IL-6 concentrations, whereas 15 ppm, 25 ppm, 35 ppm groups had increased (P < 0.001) IL-1β concentration.
Figure 1.
Effects of ammonia (NH3) treatments (0, 15, 25, and 35 ppm) on serum IL-1β (A), IL-6 (B), and IL-10 (C) of broilers at 3, 7, 14, and 21 D. a,bDifferent letters indicate a significant difference at P < 0.05 (n = 6).
Tracheal Cytokine Concentrations
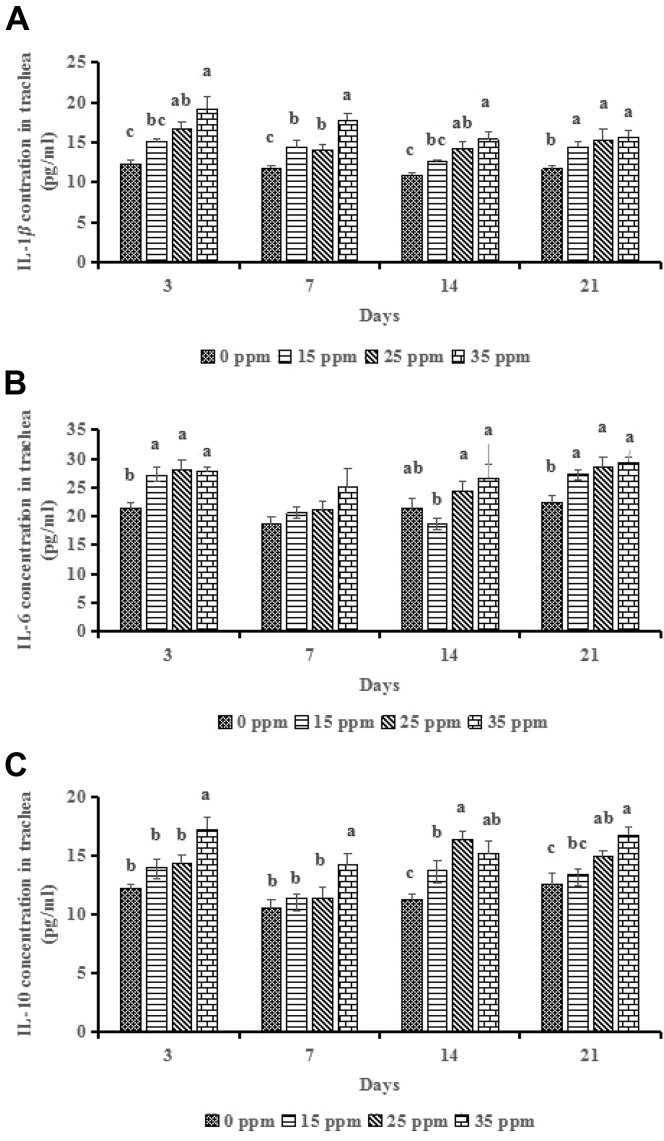
Tracheal cytokine concentrations are shown in Figure 2. At 3 D, compared to the control group, 25 ppm and 35 ppm groups had increased (P < 0.001) the IL-1β concentration, and 15 ppm, 25 ppm, 35 ppm groups had increased (P < 0.001) IL-6 concentration, whereas only 35 ppm group had increased (P < 0.01) the IL-10 concentration. At 7 D, NH3 treatments did not influence (P > 0.05) the IL-6 concentration; however, compared to the control group, 15 ppm, 25 ppm, 35 ppm groups had increased (P < 0.01) IL-1β concentration, and 35 ppm group further increased (P < 0.05) it compared to the 15 ppm and 25 ppm groups, whereas only 35 ppm group had increased (P < 0.05) the IL-10 concentration. At 14 D, compared to the control group, 25 ppm and 35 ppm groups had increased (P < 0.01) the IL-1β concentration, and 15 ppm, 25 ppm, 35 ppm groups had increased (P < 0.01) IL-10 concentration. At 21 D, compared to the control group, 15 ppm, 25 ppm, 35 ppm groups had increased (P < 0.05) IL-1β and IL-6 concentrations, and 25 ppm and 35 ppm groups had increased (P < 0.01) IL-10 concentration.
Figure 2.
Effects of ammonia (NH3) treatments (0, 15, 25, and 35 ppm) on tracheal interleukin (IL)-1β (A), IL-6 (B), and IL-10 (C) of broilers at the 3, 7, 14, and 21 D. a,bDifferent letters indicate significant difference at P < 0.05 (n = 6).
Ileal Cytokines Concentrations
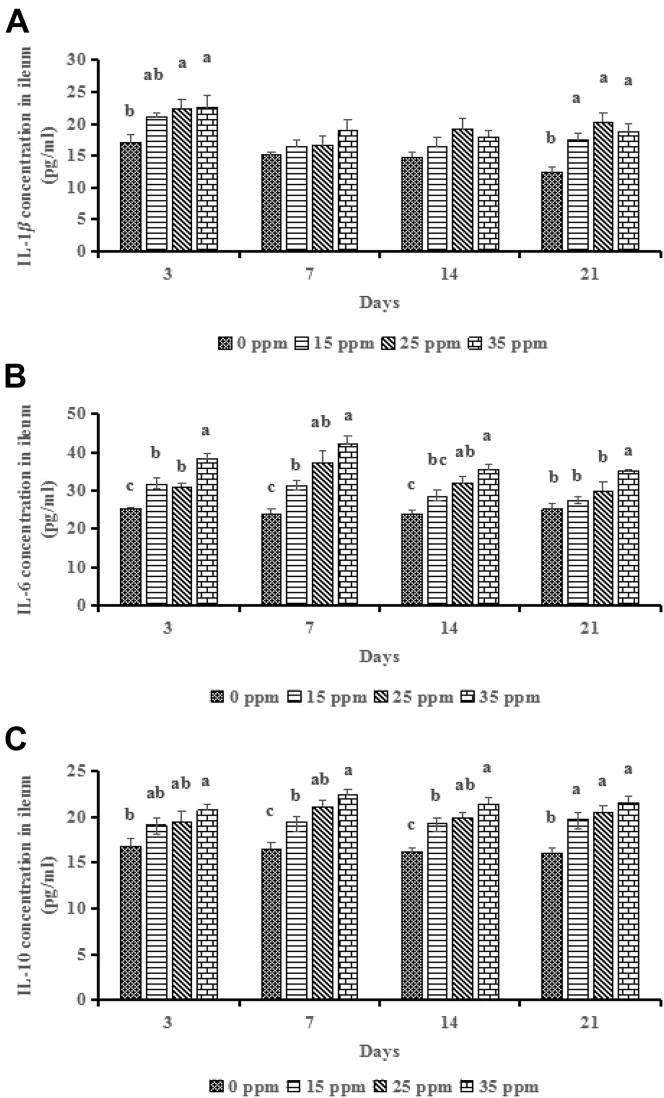
Ileal cytokines concentrations are shown in Figure 3. At 3 D, compared to the control group, 25 ppm and 35 ppm groups had increased (P < 0.05) IL-1β concentration, and 15 ppm, 25 ppm, 35 ppm groups had increased (P < 0.001) IL-6 concentration, and 35 ppm group further increased (P < 0.05) it compared to the 15 ppm and 25 ppm groups, and only 35 ppm group had increased (P < 0.05) the IL-10 concentration. At 7 D, NH3 treatments did not influence (P > 0.05) the IL-1β concentration; however, compared to the control group, 15 ppm, 25 ppm, 35 ppm groups had increased (P < 0.001) both IL-6 and IL-10 concentrations, and 35 ppm group further increased (P < 0.05) them compared to the 15 ppm group. At 14 D, NH3 treatments did not influence (P > 0.05) the IL-1β concentration; however, compared to the control group, 25 ppm and 35 ppm groups had increased (P < 0.01) IL-6 concentration, and 15 ppm, 25 ppm, 35 ppm groups had increased (P < 0.001) IL-10 concentration, and 35 ppm group further increased (P < 0.05) IL-10 concentration compared to the 15 ppm group. At the 21 D, compared to the control group, 15 ppm, 25 ppm, 35 ppm groups had increased (P < 0.001) IL-1β and IL-10 concentrations, and only 35 ppm group had increased (P < 0.01) IL-6 concentration.
Figure 3.
Effects of ammonia (NH3) treatments (0, 15, 25, and 35 ppm) on ileal IL-1β (A), IL-6 (B), and IL-10 (C) of broilers at 3, 7, 14, and 21 D. a,bDifferent letters indicate significant difference at P < 0.05 (n = 6).
Correlation Between Growth Performance and Cytokines in the Serum, Trachea, and Ileum
As shown in Table 3, at 3 D, cytokines (IL-1β, IL-6, and IL-10) concentrations in the serum, trachea, and ileum were not significantly correlated (P > 0.05) with the growth performance. At the 7 D, serum IL-6 concentrations were positively only correlated (P < 0.05) with the F/G, and serum cytokines (IL-1β and IL-10) concentrations were not significantly correlated (P > 0.05) with the growth performance; tracheal IL-1β concentrations were negatively correlated (P < 0.05) with the BW and ADG and positively correlated (P < 0.05) with the F/G, and not significantly correlated (P > 0.05) with the ADFI, and tracheal IL-10 concentrations were only negatively correlated (P < 0.05) with the ADG, and tracheal IL-6 concentrations were not significantly correlated (P > 0.05) with the growth performance; ileal cytokine (IL-1β, IL-6, and IL-10) concentrations were not significantly correlated (P > 0.05) with the growth performance. At 14 D, serum IL-1β concentrations were negatively correlated (P < 0.05) with the BW and ADG and not significantly correlated (P > 0.05) with the ADFI and F/G, and serum IL-6 concentrations were negatively correlated (P < 0.05) with the BW, ADFI, and ADG and not significantly correlated (P > 0.05) with the F/G, and serum IL-10 concentrations were not significantly correlated (P > 0.05) with the growth performance; tracheal IL-1β concentrations were negatively correlated (P < 0.05) with the BW, ADFI, and ADG and not significantly correlated (P > 0.05) with the F/G, and tracheal IL-6 and IL-10 concentrations were not significantly correlated (P > 0.05) with the growth performance; ileal both IL-6 and IL-10 concentrations were negatively correlated (P < 0.05) with the BW, ADFI, and ADG and positively correlated (P < 0.05) with the F/G, and ileal IL-1β concentrations were not significantly correlated (P > 0.05) with the growth performance. At 21 D, serum IL-1β concentrations were negatively correlated (P < 0.05) with the BW and ADG, and positively correlated (P < 0.05) with the F/G, and not significantly correlated (P > 0.05) with the ADFI, and serum IL-6 concentrations were negatively correlated (P < 0.05) with the BW, ADFI, and ADG and positively correlated (P < 0.05) with the F/G, and serum IL-10 concentrations were not significantly correlated (P > 0.05) with the growth performance; tracheal IL-6 concentrations were negatively correlated (P < 0.05) with the BW and ADG and not significantly correlated (P > 0.05) with the ADFI and F/G, and tracheal IL-10 concentrations were negatively correlated (P < 0.05) with the BW, ADFI, and ADG and positively correlated (P < 0.05) with the F/G, and tracheal IL-1β concentrations were not significantly correlated (P > 0.05) with the growth performance; ileal both IL-6 and IL-10 concentrations were negatively correlated (P < 0.05) with the BW, ADFI, and ADG and positively correlated (P < 0.05) with the F/G, and ileal IL-1β concentrations were not significantly correlated (P > 0.05) with the growth performance.
Table 3.
Correlation between cytokines in the serum, trachea, and ileum and growth performance (n = 24).
| Time points | Tissues/cytokines | Correlation coefficient (P-value) |
||||
|---|---|---|---|---|---|---|
| BW | ADFI | ADG | F/G | |||
| 3 D | Serum | IL-1β | 0.0001(0.9995) | −0.38 (0.0680) | −0.09 (0.6735) | −0.28 (0.1850) |
| IL-6 | 0.02 (0.9171) | 0.02 (0.9277) | −0.08 (0.6976) | 0.06 (0.7670) | ||
| IL-10 | 0.31 (0.1372) | −0.11 (0.5997) | 0.33 (0.1163) | −0.25 (0.2418) | ||
| Trachea | IL-1β | −0.25 (0.2472) | 0.03 (0.8895) | −0.16 (0.4516) | 0.11 (0.6159) | |
| IL-6 | −0.29 (0.1751) | 0.07 (0.7600) | −0.35 (0.0917) | 0.22 (0.3095) | ||
| IL-10 | 0.05 (0.8017) | 0.03 (0.9074) | −0.11 (0.6110) | 0.06 (0.7934) | ||
| Ileum | IL-1β | −0.17 (0.4324) | 0.21 (0.3354) | −0.13 (0.5308) | 0.24 (0.2666) | |
| IL-6 | −0.06 (0.7745) | −0.12 (0.5841) | −0.18 (0.3905) | −0.02 (0.9367) | ||
| IL-10 | 0.07 (0.7291) | 0.02 (0.9348) | 0.09 (0.6795) | −0.02 (0.9268) | ||
| 7 D | Serum | IL-1β | −0.036 (0.8662) | 0.17 (0.4238) | −0.08 (0.7146) | 0.17 (0.4164) |
| IL-6 | −0.31 (0.1360) | 0.21 (0.3316) | −0.35 (0.0939) | 0.51 (0.0116) | ||
| IL-10 | −0.07 (0.7468) | −0.13 (0.5526) | −0.12 (0.5800) | 0.03 (0.9025) | ||
| Trachea | IL-1β | −0.59 (0.0026) | −0.21 (0.3188) | −0.64 (0.0009) | 0.56 (0.0040) | |
| IL-6 | −0.21 (0.3196) | 0.08 (0.6957) | −0.25 (0.2317) | 0.33 (0.1191) | ||
| IL-10 | −0.38 (0.0699) | −0.35 (0.0960) | −0.44 (0.0295) | 0.26 (0.2140) | ||
| Ileum | IL-1β | −0.16 (0.4654) | 0.06 (0.7791) | −0.21 (0.3316) | 0.25 (0.2478) | |
| IL-6 | −0.34 (0.1039) | −0.28 (0.1821) | −0.38 (0.0709) | 0.23 (0.2710) | ||
| IL-10 | −0.30 (0.1582) | −0.13 (0.5430) | −0.33 (0.1148) | 0.27 (0.2084) | ||
| 14 D | Serum | IL-1β | −0.43 (0.0343) | −0.32 (0.1287) | −0.43 (0.0342) | 0.37 (0.0792) |
| IL-6 | −0.52 (0.0091) | −0.45 (0.0287) | −0.51 (0.0106) | 0.40 (0.0528) | ||
| IL-10 | −0.26 (0.2230) | −0.15 (0.4715) | −0.25 (0.2329) | 0.25 (0.2423) | ||
| Trachea | IL-1β | −0.52 (0.0093) | −0.48 (0.0166) | −0.50 (0.0126) | 0.38 (0.0651) | |
| IL-6 | −0.29 (0.1724) | −0.35 (0.0917) | −0.30 (0.1601) | 0.16 (0.4619) | ||
| IL-10 | −0.33 (0.1175) | −0.23 (0.2821) | −0.33 (0.1133) | 0.30 (0.1504) | ||
| Ileum | IL-1β | −0.12 (0.5771) | −0.13 (0.5459) | −0.10 (0.6267) | 0.06 (0.7726) | |
| IL-6 | −0.59 (0.0023) | −0.45 (0.0275) | −0.59 (0.0026) | 0.52 (0.0097) | ||
| IL-10 | −0.63 (0.0010) | −0.45 (0.0275) | −0.63 (0.0009) | 0.62 (0.0012) | ||
| 21 D | Serum | IL-1β | −0.44 (0.0318) | −0.38 (0.0666) | −0.44 (0.0298) | 0.43 (0.0338) |
| IL-6 | −0.47 (0.0220) | −0.41 (0.0462) | −0.47 (0.0206) | 0.46 (0.0225) | ||
| IL-10 | −0.37 (0.0794) | −0.29 (0.1747) | −0.37 (0.0748) | 0.34 (0.1063) | ||
| Trachea | IL-1β | −0.34 (0.1032) | −0.27 (0.1959) | −0.34 (0.1007) | 0.33 (0.1203) | |
| IL-6 | −0.42 (0.0427) | −0.40 (0.0521) | −0.42 (0.0395) | 0.36 (0.0835) | ||
| IL-10 | −0.62 (0.0012) | −0.46 (0.0221) | −0.62 (0.0013) | 0.67 (0.0004) | ||
| Ileum | IL-1β | −0.24 (0.2661) | −0.16 (0.4653) | −0.24 (0.2603) | 0.22 (0.3104) | |
| IL-6 | −0.67 (0.0004) | −0.54 (0.0064) | −0.67 (0.0004) | 0.66 (0.0004) | ||
| IL-10 | −0.53 (0.0080) | −0.43 (0.0356) | −0.53 (0.0073) | 0.51 (0.0105) | ||
Correlation Between Tracheal Cytokines and Ileal Cytokines
As shown in Table 4, at 3 D, tracheal IL-1β and IL-6 concentrations were positively (P < 0.05) correlated with the ileal IL-1β and IL-6 concentrations with r = 0.64 and r = 0.48, respectively; and tracheal IL-10 concentrations were not correlated (P > 0.05) with ileal IL-10 concentrations. At the 7 D, tracheal IL-1β concentrations were positively (P < 0.01) correlated with the ileal IL-1β concentrations with r = 0.62; and tracheal IL-6 and IL-10 concentrations were not correlated (P > 0.05) with ileal IL-6 and IL-10 concentrations, respectively. At the 14 D, tracheal IL-1β, IL-6, and IL-10 concentrations were positively (P < 0.05) correlated with the ileal IL-1β, IL-6, and IL-10 concentrations with r = 0.64, r = 0.48, and r = 0.48, respectively. At the 21 D, tracheal IL-1β and IL-6 concentrations were positively (P < 0.05) correlated with the ileal IL-1β and IL-6 concentrations with r = 0.67 and r = 0.41, respectively; and tracheal IL-10 concentrations were not correlated (P > 0.05) with ileal IL-10 concentrations. Regression analysis was also shown in Table 4.
Table 4.
Correlation and regression model between cytokines in the trachea and ileum (n = 24).
| Time points | Cytokines (Y) in the trachea | Cytokines (X) in the ileum |
|
|---|---|---|---|
| Correlation coefficient (P-value) | Regression model (R2) | ||
| 3 D | IL-1β | 0.64 (0.0007) | Y = 8.74 + 0.76X (0.4138) |
| IL-6 | 0.48 (0.0177) | Y = 14.43 + 0.66X (0.2299) | |
| IL-10 | 0.37 (0.0766) | Y = 13.75 + 0.36X (0.1356) | |
| 7 D | IL-1β | 0.62 (0.0013) | Y = 6.42 + 0.71X (0.3821) |
| IL-6 | 0.37 (0.0750) | Y = 21.11 + 0.59X (0.1370) | |
| IL-10 | 0.39 (0.0618) | Y = 14.55 + 0.45X (0.1496) | |
| 14 D | IL-1β | 0.64 (0.0007) | Y = 4.00 + 0.98X (0.4102) |
| IL-6 | 0.48 (0.0168) | Y = 17.71 + 0.54X (0.2333) | |
| IL-10 | 0.48 (0.0172) | Y = 13.38 + 0.41X (0.2318) | |
| 21 D | IL-1β | 0.67 (0.0003) | Y = 2.27 + 1.05X (0.4492) |
| IL-6 | 0.41 (0.0457) | Y = 14.70 + 0.55X (0.1694) | |
| IL-10 | 0.35 (0.0934) | Y = 13.80 + 0.39X (0.1227) | |
Discussion
Cytokines are important soluble signals that mediate cell-to-cell communication during inflammatory and immune responses (Xing et al., 1998, Osborne and Abraham, 2010). Similar to mammals, NH3-induced inflammation in chickens results in the synthesis and release of various cytokines (Osborne and Abraham, 2010). Among these cytokines, IL-1β and IL-6 are potent proinflammatory and immunomodulatory cytokines (Tanaka and Kishimoto, 2012, Ghareeb et al., 2013), whereas IL-10 has been recognized as a tolerogenic and anti-inflammatory cytokine that blocks the production of proinflammatory cytokines (Sabat et al., 2010). In the present study, we investigated the effects of NH3 exposure on growth performance and IL-1β, IL-6, and IL-10 concentrations in the serum, trachea, and ileum of broilers.
Previous studies showed that NH3 levels above 25 ppm inside poultry houses have negative effects on chicken growth performance, including reduced body weight gain, feed intake, and feed conversion rates (Reece et al., 1981, Beker et al., 2004, Miles et al., 2004, Miles et al., 2006). In the present study, birds exposed to 25 and 35 ppm of NH3 showed reduced growth performance, which was consistent with the results from previous studies (Reece et al., 1981, Beker et al., 2004, Miles et al., 2004, Miles et al., 2006).
The trachea is lined with mucociliary epithelium, which allows it to play a defensive role against toxic inhalants. However, because it is part of the upper respiratory tract, it is vulnerable to NH3 exposure. It has been reported that chickens exposed to 100 ppm NH3 exhibited extensive mucus secretion and cilia loss from the tracheal epithelium (Al-Mashhadani and Beck, 1985). The study by Xiong et al. (2016) found that exposure to high concentration of atmospheric NH3 (75 ppm) increased IL-1β and IL-6 levels in trachea. In addition, long-time exposure, even at concentrations as low as 10 ppm, has been shown to lead to excessive mucus production and matted cilia in the trachea of birds (Nagaraja et al., 1983). Furthermore, NH3-induced damage to the trachea increases susceptibility to infections (Anderson et al., 1964, Caveny et al., 1981). In the present study, birds exposed to 15 ppm NH3 for 3 D exhibited increased concentrations of the inflammatory cytokines IL-6. These results indicated that short-term exposure to low NH3 concentration induced an inflammatory response in the trachea. In addition, a question needs to be stressed in this study is that NH3 treatments did not exhibit apparently increased trachea IL-6 levels at the 7 D, which may indicate that broilers adapted to or recovered from the NH3 even 35 ppm stimulus at the 7 D; however, IL-6 concentrations were then increased at 14 and 21 D, which suggested that a longer period of exposure to NH3 even 15 ppm is believed to cause an inflammatory response in the trachea. Similar to the findings in turkeys, long-time exposure, even at concentrations as low as 10 ppm, has been shown to lead to excessive mucus production and matted cilia in the trachea of birds (Nagaraja et al., 1983). IL-10, as an anti-inflammatory cytokine, also increased under NH3 exposure, especially at concentrations of 35 ppm, suggesting that NH3 exposure induced a significant anti-inflammatory cytokine response. IL-10 was originally described as a factor inhibiting cytokine production (Fiorentino et al., 1989) and is known to contribute to the preservation of the immune balance and to suppress the development of autoimmune diseases (Davidson et al., 1996). Taken together, our results suggested that NH3 exposure, especially at concentrations of 35 ppm, may disturb the immune balance of broilers. In addition, we also found that the increase of tracheal cytokines concentrations was negatively correlated with the ADFI, ADG, and BW and positively correlated with the F/G from 7 D until 21 D in the experiment, which suggested that increased cytokines could reduce the growth performance. It is increasingly recognized that cytokines play a role in the control of food intake. As shown by Johnson (1997), an increase in IL-1β and IL-6 concentrations can modulate intermediary metabolism of carbohydrate, fat, and protein substrates; regulate hypothalamic-pituitary outflow; and act in the brain to reduce food intake. It has also been shown that an increase in cytokines IL-1β and IL-6 concentrations could modulate feeding behavior by suppressing feeding during bacterial infections (Konsman et al., 2002). In addition, IL-1β inhibits food intake after peripheral (Swiergiel and Dunn, 2006) or central (Nelson et al., 1999) administration and can act directly in the brain (e.g., the melanocortin system in the hypothalamus), modulate gastrointestinal activities that inhibit feeding, induce the release of hormones that modulate feeding such as leptin and cholecystokinin, and induce alterations in metabolism that impact food intake regulation (Wong and Pinkney, 2004). This suggests that low growth performance under NH3 exposure may be due in part to an increase in tracheal cytokines, which may be explained that, on one hand, the increase of cytokines might suppress feeding, and on the other hand, proinflammatory cytokines cause a shift in nutrient partitioning away from skeletal muscle accretion and toward the metabolic responses necessary to support the immune system, thereby reducing the growth performance.
In addition to increasing the inflammatory response in the upper respiratory tract of the trachea, NH3 exposure is found to have a negative effect on the intestinal tract, such as reducing ileum flora diversity (Yang et al., 2019). Ammonia is a toxin that causes proinflammatory mediators to be released into the circulation, modulating the impact of NH3 on the brain tissue (Skowrońska and Albrecht, 2013), the liver (Zhang et al., 2015a). However, to date, there have been limited reports on the secretions of cytokines in the intestinal tract in response to NH3 exposure. In the present study, the cytokines IL-1β, IL-6, and IL-10 also increased in the ileum after 3 D of NH3 exposure, even at concentrations of 15 ppm, indicating that the ileum immune system plays an important role in protecting against NH3-induced damage. In addition, NH3 exposure for 7 or 14 D apparently did not increase ileal IL-1β levels, whereas ileal IL-1β levels increased after 21 D of NH3 exposure, which may indicate that broilers adapted to or recovered from NH3 exposure, even at concentrations of 35 ppm. However, longer periods of NH3 exposure, even at concentrations of 15 ppm, seem to cause an inflammatory response in the ileum. Moreover, we found that the time broilers adapted to or recovered from the NH3 exposure in the trachea and ileum were different, which might be due to the position and size of the trachea and ileum, but it needs further research and verification. We also investigated the relationship between ileal cytokines and growth performance and found that ileal cytokines concentrations were also negatively correlated with the BW, ADFI, and ADG and positively correlated with the F/G from 14 D until 21 D. These results suggested that NH3 exposure, even at concentrations of 15 ppm, induced an inflammatory response in the ileum, and that low growth performance in broilers exposure to NH3 may be due in part to increases in ileal cytokines.
Moreover, we also found that tracheal cytokines were positively correlated with ileal cytokines in broilers exposed to NH3, which suggested that there may be interactions between the respiratory and intestinal tracts. One possibility is that a loss in epithelial barrier functioning may lead to increased antigen presentation and promote an intestinal inflammatory response. A similar study, which used a whole-body smoke exposure model, found that smoke exposure damaged the epithelial barrier and may have induced an overall increase in antigenic presentation in the intestines, which may contribute to inflammatory bowel disease pathogenesis (Verschuere et al., 2011). Alternatively, research has reported that possible mechanisms of respiratory-gastrointestinal cross-talk include overproduction of proteases during excessive inflammation and changes in immune cell functioning, including increases in cytochrome oxidase expressing lymphocytes and gut-originating T-cell mishoming (Keely et al., 2012). Our results also demonstrated that NH3 exposure induced an excessive inflammatory response in both the trachea and ileum. This suggested that systemic cytokines were the most likely cause of the overlap in inflammatory response in respiratory and intestinal, as is the case with IL-6 in chronic obstructive pulmonary disease and inflammatory bowel diseases (Danese and Gao, 2010, Ruwanpura et al., 2011). Thus, our results demonstrated an important role for the cytokines IL-1β, IL-6, and IL-10 in both the respiratory and intestinal tracts and suggested that these cytokines may link the respiratory and intestinal tracts during NH3 exposure.
In summary, this study showed that exposure to 25 ppm and 35 ppm of NH3 decreased growth performance in broilers. Ammonia exposure in birds, even at concentrations of 15 ppm for 3 D, increased the IL-6 concentrations in the trachea and ileum, resulting in an inflammatory response. In addition, cytokines in the trachea and ileum were negatively correlated with BW, ADFI, and ADG and positively correlated with F/G in broilers, which suggested that low growth performance caused by NH3 exposure might be due in part to an increase in cytokines. Moreover, tracheal cytokines were also positively correlated with ileal cytokines, which suggested that the cross-talk between the respiratory and intestinal tracts may occur via the cytokine IL-1β. This fundamental research will contribute to further understanding of the immunological changes that occur in the upper respiratory and intestinal tracts of broilers during NH3 exposure.
Acknowledgments
This study was supported by the National Key Research and Development Program of China (2016YFD0500509). This research was also supported by the Science and Technology Innovation Project of the Chinese Academy of Agricultural Sciences (ASTIP-IAS09).
Conflict of Interest: The authors did not provide a conflict of interest statement.
References
- Al-Mashhadani E.H., Beck M.M. Effect of atmospheric ammonia on the surface ultrastructure of the lung and trachea of broiler chicks. Poult. Sci. 1985;64:2056–2061. doi: 10.3382/ps.0642056. [DOI] [PubMed] [Google Scholar]
- Anderson D.P., Beard C.W., Hanson R.P. The adverse effects of ammonia on chickens including resistance to infection with New Castle disease virus. Avian Dis. 1964;8:369–379. [Google Scholar]
- Beker A., Vanhooser S.L., Swartzlander J.H., Teeter R.G. Atmospheric ammonia concentration effects on broiler growth and performance. J. Appl. Poult. Res. 2004;13:5–9. [Google Scholar]
- Caveny D.D., Quarles C.L., Greathouse G.A. Atmospheric ammonia and broiler cockerel performance. Poult. Sci. 1981;60:513–516. [Google Scholar]
- Colditz I.G. Effects of the immune system on metabolism: implications for production and disease resistance in livestock. Livest. Prod. Sci. 2002;75:257–268. [Google Scholar]
- Costa A. Ammonia concentrations and emissions from finishing pigs reared in different growing rooms. J. Environ. Qual. 2017;46:255–260. doi: 10.2134/jeq2016.04.0134. [DOI] [PubMed] [Google Scholar]
- Danese S., Gao B. Interleukin-6:a therapeutic Jekyll and Hyde in gastrointestinal and hepatic diseases. Gut. 2010;59:149–151. doi: 10.1136/gut.2008.173534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David B., Mejdell C., Michel V., Lund V., Moe R.O. Air quality in alternative housing systems may have an impact on laying hen welfare. Part II-ammonia. Animals. 2015;5:886–896. doi: 10.3390/ani5030389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson N.J., Leach M.W., Fort M.M., Thompson-Snipes L., Kühn R., Müller W., Berg D.J., Rennick D.M. T helper cell 1-type CD4 T cells, but not B cells, mediate colitis in 10-deficient mice. J. Exp. Med. 1996;184:241–251. doi: 10.1084/jem.184.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deaton J.W., Reece F.N., Lott B.D. Effect of atmospheric ammonia on laying hen performance. Poult. Sci. 1982;61:1815–1817. doi: 10.3382/ps.0611815. [DOI] [PubMed] [Google Scholar]
- Fiorentino D.F., Bond M.W., Mosmann T.R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J. Exp. Med. 1989;170:2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghareeb K., Awad W.A., Soodoi C., Sasgary S., Strasser A., Böhm J. Effects of feed contaminant deoxynivalenol on plasma cytokines and mRNA expression of immune genes in the intestine of broiler chickens. PLoS One. 2013;8:e71492. doi: 10.1371/journal.pone.0071492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R.W. Inhibition of growth by pro-inflammatory cytokines: an integrated view. J. Anim. Sci. 1997;75:1244–1255. doi: 10.2527/1997.7551244x. [DOI] [PubMed] [Google Scholar]
- Keely S., Talley N.J., Hansbro P.M. Pulmonary-intestinal cross-talk in mucosal inflammatory disease. Mucosal Immunol. 2012;5:7–18. doi: 10.1038/mi.2011.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerkamp P.G., Metz J.H.M., Uenk G.H., Phillips V.R., Holden M.R., Sneath R.W., Short J.L., White R.P.P., Hartung J., Seedorf J., Schröder M., Linkert K.H., Pedersen S., Takai H., Johnsen J.O., Wathes C.M. Concentrations and emissions of ammonia in livestock buildings in Northern Europe. J. Agric. Eng. Res. 1998;70:79–95. [Google Scholar]
- Konsman J.P., Parnet P., Dantzer R. Cytokine-induced sickness behaviour: mechanisms and implications. Trends Neurosci. 2002;25:154–159. doi: 10.1016/s0166-2236(00)02088-9. [DOI] [PubMed] [Google Scholar]
- Miles D.M., Branton S.L., Lott B.D. Atmospheric ammonia is detrimental to the performance of modern commercial broilers. Poult. Sci. 2004;83:1650–1654. doi: 10.1093/ps/83.10.1650. [DOI] [PubMed] [Google Scholar]
- Miles D.M., Miller W.W., Branton S.L., Maslin W.R., Lott B.D. Ocular responses to ammonia in broiler chickens. Avian Dis. 2006;50:45–49. doi: 10.1637/7386-052405R.1. [DOI] [PubMed] [Google Scholar]
- Mucksová J., Chalupský K., Plachý J., Kalina J., Rachačová P., Staněk O., Trefil P. Simultaneous detection of chicken cytokines in plasma samples using the Bio-Plex assay. Poult. Sci. 2018;97:1127–1133. doi: 10.3382/ps/pex411. [DOI] [PubMed] [Google Scholar]
- Murphy T., Cargill C., Rutley D., Stott P. Pig-shed air polluted by a-haemolytic cocci and ammonia causes subclinical disease and production losses. Vet. Rec. 2012;171:123. doi: 10.1136/vr.100413. [DOI] [PubMed] [Google Scholar]
- Nagaraja K.V., Emery D.A., Jordan K.A., Newman J.A., Pomeroy B.S. Scanning electron microscopic studies of adverse effects of ammonia on tracheal tissues of turkeys. Am. J. Vet. Res. 1983;44:1530–1536. [PubMed] [Google Scholar]
- National Research Council . 9th rev. ed. Natl. Acad. Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Nelson K.P., Marks N.L., Heyen J.R., Johnson R.W. Behavior of adult and aged mice before and after central injection of interleukin-1. Physiol. Behav. 1999;66:673–679. doi: 10.1016/s0031-9384(98)00339-4. [DOI] [PubMed] [Google Scholar]
- Ni J.Q., Heber A.J., Cortus E.L., Lim T.T., Bogan B.W., Grant R.H., Boehm M.T. Assessment of ammonia emissions from swine facilities in the US-application of knowledge from experimental research. Environ. Sci. Pol. 2012;22:25–35. [Google Scholar]
- Osborne L.C., Abraham N. Regulation of memory T cells by γc cytokines. Cytokine. 2010;50:105–113. doi: 10.1016/j.cyto.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Oyetunde O.O., Thomson R.G., Carlson H.C. Aerosol exposure of ammonia, dust and Escherichia coli in broiler chickens. Can. Vet. J. 1978;19:187–193. [PMC free article] [PubMed] [Google Scholar]
- Pié S., Lallès J.P., Blazy F., Laffitte J., Sève B., Oswald I.P. Weaning is associated with an upregulation of expression of inflammatory cytokines in the intestine of piglets. J. Nutr. 2004;134:641–647. doi: 10.1093/jn/134.3.641. [DOI] [PubMed] [Google Scholar]
- Puchelle E., De Bentzmann S., Zahm J.M. Physical and functional properties of airway secretions in cystic fibrosis-therapeutic approaches. Respiration. 1995;62(Suppl. 1):2–12. doi: 10.1159/000196486. [DOI] [PubMed] [Google Scholar]
- Reece F.N., Lott B.D., Deaton J.W. Low concentrations of ammonia during brooding decrease broiler weight. Poult. Sci. 1981;60:937–940. [Google Scholar]
- Reece F.N., Lott B.D., Deaton J.W. Ammonia in the atmosphere during brooding affects performance of broiler chickens. Poult. Sci. 1982;59:486–488. [Google Scholar]
- Ruwanpura S.M., McLeod L., Miller A., Jones J., Bozinovski S., Vlahos R., Ernst M., Armes J., Bardin P.G., Anderson G.P., Jenkins B.J. Interleukin-6 promotes pulmonary emphysema associated with apoptosis in mice. Am. J. Respir. Cell Mol. Biol. 2011;45:720–730. doi: 10.1165/rcmb.2010-0462OC. [DOI] [PubMed] [Google Scholar]
- Sabat R., Grütz G., Warszawska K., Kirsch S., Witte E., Wolk K., Geginat J. Biology of interleukin-10. Cytokine Growth Factor Rev. 2010;21:331–344. doi: 10.1016/j.cytogfr.2010.09.002. [DOI] [PubMed] [Google Scholar]
- SAS Institute. SAS Inst. Inc.; Cary, NC: 2010. SAS/STAT User’s Guide. Version 9.2 for Windows. [Google Scholar]
- Shi Q.X., Wang W., Chen M.H., Zhang H.F., Xu S.W. Ammonia induces Treg/Th1 imbalance with triggered NF-κB pathway leading to chicken respiratory inflammation response. Sci. Total Environ. 2019;659:354–362. doi: 10.1016/j.scitotenv.2018.12.375. [DOI] [PubMed] [Google Scholar]
- Sinkora J., Rehakova Z., Sinkora M., Cukrowska B., Tlaskalova-Hogenova H. Early development of immune system in pigs. Vet. Immunol. Immunopathol. 2002;87:301–306. doi: 10.1016/s0165-2427(02)00056-9. [DOI] [PubMed] [Google Scholar]
- Skowrońska M., Albrecht J. Oxidative and nitrosative stress in ammonia neurotoxicity. Neurochem. Int. 2013;5:731–737. doi: 10.1016/j.neuint.2012.10.013. [DOI] [PubMed] [Google Scholar]
- Swiergiel A.H., Dunn A.J. Feeding, exploratory, anxiety and depression related behaviors are not altered interleukin-6-deficient male mice. Behav. Brain Res. 2006;171:94–108. doi: 10.1016/j.bbr.2006.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Kishimoto T. Targeting interleukin-6: all the way to treat autoimmune and inflammatory diseases. Int. J. Biol. Sci. 2012;8:1227–1236. doi: 10.7150/ijbs.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verschuere S., Bracke K.R., Demoor T., Plantinga M., Verbrugghe P., Ferdinande L., Lambrecht B.N., Brusselle G.G.G., Cuvelier C.A. Cigarette smoking alters epithelial apoptosis and immune composition in murine GALT. Lab. Invest. 2011;91:1056–1067. doi: 10.1038/labinvest.2011.74. [DOI] [PubMed] [Google Scholar]
- Wang D.X., Zhang Y.M., Chi Q.R., Hu X.Y., Li S.P., Li S. Ammonia exposure induced abnormal expression of cytokines and heat shock proteins via glucose metabolism disorders in chicken neutrophils. Environ. Sci. Pollut. Res. Int. 2019;26:10529–10536. doi: 10.1007/s11356-019-04516-4. [DOI] [PubMed] [Google Scholar]
- Webel D.M., Mahan D.C., Johnson R.W., Baker D.H. Pretreatment of young pigs with vitamin E attenuates the elevation in plasma interleukin-6 and cortisol caused by a challenge dose of lipopolysaccharide. J. Nutr. 1998;128:1657–1660. doi: 10.1093/jn/128.10.1657. [DOI] [PubMed] [Google Scholar]
- Wei F.X., Hu X.F., Xu B., Zhang M.H., Li S.Y., Sun Q.Y., Lin P. Ammonia concentration and relative humidity in poultry houses affect the immune response of broilers. Genet. Mol. Res. 2015;14:3160–3169. doi: 10.4238/2015.April.10.27. [DOI] [PubMed] [Google Scholar]
- Wong S., Pinkney J. Role of cytokines in regulating feeding behaviour. Curr. Drug Targets. 2004;5:251–263. doi: 10.2174/1389450043490532. [DOI] [PubMed] [Google Scholar]
- Xing Z., Gauldie J., Cox G., Baumann H., Jordana M., Lei X.F., Achong M.K. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J. Clin. Invest. 1998;101:311–320. doi: 10.1172/JCI1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y., Tang X.F., Meng Q.S., Zhang H.F. Differential expression analysis of the broiler tracheal proteins responsible for the immune response and muscle contraction induced by high concentration of ammonia using iTRAQ-coupled 2D LC-MS/MS. Sci. China Life Sci. 2016;59:1166–1176. doi: 10.1007/s11427-016-0202-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahav S. Ammonia affects performance and thermo regulation of male broiler chickens. Anim. Res. 2004;53:289–293. [Google Scholar]
- Yang X.Y., Cui J., Chen X.F., Wu F.Y., Chen B.J. Analysis of the effect of ammonia on the intestinal flora of mice by Illumina-HiSeq high-throughput sequencing technology. Chin. J. Vet. Med. 2019;39:948–955. [Google Scholar]
- Yi B., Chen L., Sa R.N., Zhong R.Q., Xing H., Zhang H.F. Transcriptome profile analysis of breast muscle tissues from high or low levels of atmospheric ammonia exposed broilers (Gallus gallus) PLoS One. 2016;11:e0162631. doi: 10.1371/journal.pone.0162631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.Z., Li C., Tang X.F., Lu Q.P., Sa R.N., Zhang H.F. High concentrations of atmospheric ammonia induce alterations in the hepatic proteome of broilers (Gallus gallus): an iTRAQ-based quantitative proteomic analysis. PLoS One. 2015;10:e0123596. doi: 10.1371/journal.pone.0123596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.Z., Li C., Tang X.F., Lu Q.P., Sa R.N., Zhang H.F. Proteome changes in the small intestinal mucosa of broilers (Gallus gallus) induced by high concentrations of atmospheric ammonia. Proteome Sci. 2015;13:9. doi: 10.1186/s12953-015-0067-4. [DOI] [PMC free article] [PubMed] [Google Scholar]