Abstract
Body weight–related traits and feeding behavior traits are important in poultry breeding and production. To investigate the heritability of feeding behavior and their genetic correlation with body weight–related traits in Pekin ducks, 5,594 Pekin ducks were selected. The information about body weight–related traits and feeding behavior from 3 to 6 wk of age were recorded by automatic electronic feeders. The heritability estimates for body weight, residual feed intake, and feeding behavior traits are relatively high (ranging from 0.29 to 0.65). We observed that total feed intake, meal feed intake, body weight at the age of 3 wk, and daily body weight gain had strong positive genetic correlations with body weight at the age of 6 wk. Moreover, body weight at the age of 3 wk also showed a positive genetic correlation with the feed conversion ratio (0.33). Total feeding time, daily feed intake, and feeding rate had significant positive phenotypic correlations with feed efficiency. However, the average interval between meals, the number of daily visits, and the number of meals all had a low genetic or phenotypic relationship with body weight and feed efficiency. In conclusion, our study revealed that body weight, residual feed intake, and feeding behavior traits were all highly heritable traits, and the selection for certain feeding behaviors could improve feed efficiency in Pekin ducks. This study is the first report about genetic parameter estimates about feeding behaviors in ducks based on large datasets and provides solid data for genetic study in ducks.
Key words: Pekin duck, body weight, feeding behavior, genetic parameter, selection response
Introduction
Pekin ducks are widely raised in the world, especially in Asia. And they are famous for the delicious taste of Beijing roast duck around the world, which requires a high skin/fat ratio or body fat ratio (Zhu et al., 2019a). It is also reported that Pekin duck has become the predominant breed used for meat in the global duck industry and can offer an efficient way for dissecting artificial selection mechanisms in farm animals (Zhou et al., 2018). In recent years, the breeding aim of Pekin ducks usually focuses on feed efficiency and fat deposition. Feed intake, feed conversion ratio (FCR), and residual feed intake (RFI) are the main factors that have to be taken into consideration when evaluating feed efficiency in Pekin ducks. Some studies have concluded that the direct selection for FCR and RFI can improve feed efficiency greatly in meat ducks (Kennedy et al., 1993, Pingel, 1999, Larzul et al., 2004, Basso et al., 2012). As for fat deposition, Chen et al. (2010) found that body fat in Pekin ducks can degrade into a variety of volatile components, such as hexanal and dimethyl trisulphide, while heating at a high temperature and the interaction among them lead to the special taste of Beijing roast duck. Body weight has a strong positive genetic correlation with fat traits (Li et al., 2006). Great breeding progress has been made since artificial selection on various traits in ducks.
Feeding behavior is also an important trait in Pekin ducks. On the one hand, feeding behavior can reflect the feeding habits and physiological status of ducks. On the other hand, feeding behavior also shows a strong correlation with FCR in modern broiler lines (Howie et al., 2011). Feeding behavior also differs significantly between the groups of low and high RFI level in Pekin ducks (Drouilhet et al., 2016). Although feeding behavior has great potential to realize indirect selection for feed efficiency in Pekin ducks, collecting information about feeding behavior is a time consuming and complex process in previous breeding programs. Therefore, the genetics of feeding behavior remains unknown in Pekin ducks. With the widespread use of electronic feeders, individual feeding behavior can be recorded precisely and automatically nowadays, which enables us to gain insight into the genetic correlations among feeding behavior, body weight, and feed efficiency. We developed an automatic feeding behavior recording machine and has applied in the large-scale measuring in Pekin duck breeding (Zhu et al., 2017). Investigation of feeding behavior and their genetic association with the breeding traits (such as growth rate, feed efficiency) would be critically useful for developing novel selection methods.
The objectives of this study were to provide estimated genetic parameters of feeding behavior traits and their genetic correlation with performance traits and to evaluate the genetic progress of measured traits over the selection.
Materials and methods
Ducks and Housing
All experimental ducks had been selected for high feed efficiency and moderate body weight while recording all duck feeding behavior for 3 generations in Beijing Golden Star Duck Inc. And pedigrees for these ducks could trace back 5 generations, which consisted of 25,120 individuals. To improve the growth rate and fat deposition in the early growth stage while avoiding the excessive weight, the ducks were selected at the age of 6 wk. All ducklings were recorded with banded wings after birth, and they can access freely to water and feed, whereas the standard pellets were used as the same as our previous study (Lin et al., 2018).
Collection of Feeding Behavior Information
With the development of radio frequency identification) technology, we used electronic tags as individual identifiers to record the feeding behavior of each duck in this study. The details of feeding behavior recording were the same as our previous research (Zhu et al., 2017). During the recording process, the feeding visits with no feed intake or invalid individual identification would be moved when conducting the analysis. In this study, we calculated the intervals between feeding visits to estimate the meal criterion. The meal criterion was estimated using the method reported by Howie et al. (2009). For each duck, any 2 feeding visits with an interval less than the meal criterion would be combined into one meal visit. As the variation coefficients of feeding behavior traits, such as total feed intake, feeding time, and meal times, for each duck during the test period were all less than 5%, we summarized the average values of these traits based on the meal criterion for the purpose of simplifying the further analysis in bivariate models. The recording behavior information included total feed time (TFT), daily feed intake (DFI), meal feed intake (MFI), visit duration, number of visits per day (NV), whereas the derived traits included meal duration (MD), average interval between meals (AIBM), daily feeding rate (DFR), number of meals per day (NM).
Measurements of Body Weight and Feed Efficiency
The body weight of Pekin ducks at the age of 3 wk (BW21) and 6 wk (BW42) were both measured manually in this study. Based on the total feed consumption and body weight gain (BWG) during the test period, the FCR and DFI could be determined, respectively. In addition, we computed the RFI over the whole study using a linear regression model (Koch et al., 1963). The regression equation was constructed as follows:
By the values of RFI, we divided all ducks into 3 groups with different levels of RFI. The high-RFI group and low-RFI group consisted of ducks with the highest 10% level or lowest 10% level of RFI. The middle-RFI group consisted of ducks with RFI level ranging from 45 to 55% according to the corresponding distribution. There were 559 ducks in each group, and they could be used for further analysis of RFI levels.
Statistical Analysis
The normal distribution test of the traits was conducted using the Shapiro–Wilk method implemented in R statistical software (version 3.5.3; https://mirrors.tuna.tsinghua.edu.cn/CRAN/, TUNA Team, Tsinghua University, Beijing, China), and all traits in this study met the hypothesis of normal distribution. The descriptive statistics were calculated by the SUMMARY procedure, and the impact of different sexes or RFI levels were analyzed using one-way ANOVA. Then, the genetic parameters for the body weight–related traits and feeding behavior traits were estimated using the AI-REML module algorithm (Center for Quantitative Genetics and Genomics, University of Aarhus, Tjele, Denmark) (Jensen, 1997) of derivative free multivariate (Madsen et al., 2013). The animal model was performed as follows:
where Yij was the recorded phenotypic value; μ was the population mean; Li was the fixed effect of the Pekin ducks (sex and generation); aij was the random direct genetic effect of ducks, and eij was the random residual effect. The same animal model was used to estimate heritability of all traits, and 2-trait analyses were conducted to estimate the genetic correlations among these traits, whereas the phenotypic correlations were calculated using simple Pearson correlations.
The estimated breeding values of different traits were also obtained using animal model combined with the best linear unbiased prediction, which were calculated based on the mixed model equations. The general form of the model was shown as follows:
where y, b, a, and e were the vectors of observations, fixed effects of all fixed factors, random effects of all random factors, and random residual effects, respectively; X and Z were the design matrix for b and a, respectively; A was the additive genetic relationship matrix based on the pedigree of the population; I was an identity matrix; σa2 and σe2 were the additive genetic variances and residual variances for different traits, respectively.
Results
Description of Phenotypic Data
The information about body weight, feed efficiency, and feeding behavior was collected from 5,594 ducks (2,744 males and 2,850 females) in this study. The statistical summary of phenotypic data was provided in Table 1. Throughout the study, the average FCR was 2.61, whereas the mean for RFI was 0, with a standard derivation of 0.46 kg. In addition, the average total feeding time was 220.41 min during the study, the mean of daily meal times was 8.10 min, the mean of MD was 84.86 s, and the mean of DFI was 0.28 kg. The meal criteria of different generations were also estimated, which referred to be 1,023 s, 1,043 s, and 944 s, respectively. The intervals between feeding visits were all adjusted by loge transformation, and their distributions are shown in supplementary data (Supplementary Figure 1).
Table 1.
Summary of phenotypic values for the traits measured.
| Categories | All (n = 5,594) |
|||
|---|---|---|---|---|
| Mean | SD1 | Min | Max | |
| General traits | ||||
| Average body weight at day 21, kg | 1.26 | 0.19 | 0.90 | 1.69 |
| Average body weight at day 42, kg | 3.32 | 0.22 | 2.90 | 4.25 |
| Body weight gain, kg | 2.06 | 0.24 | 1.24 | 2.94 |
| Daily body weight gain, kg/D | 0.10 | 0.01 | 0.07 | 0.15 |
| Feed intake, kg | 5.36 | 0.65 | 3.14 | 7.26 |
| Residual feed intake, kg | 0.00 | 0.46 | −1.68 | 1.41 |
| Feed conversion ratio | 2.61 | 0.24 | 1.80 | 3.29 |
| Feeding behavior traits | ||||
| Total feeding time, min | 220.41 | 48.40 | 103.58 | 481.63 |
| Daily feed intake, kg/D | 0.28 | 0.03 | 0.17 | 0.37 |
| Meal feed intake, g | 35.10 | 6.96 | 18.79 | 68.77 |
| Visit duration, s | 71.70 | 17.56 | 33.58 | 175.48 |
| Meal duration, s | 84.86 | 22.32 | 37.74 | 226.85 |
| Average interval between meals, min | 179.27 | 36.20 | 91.90 | 360.93 |
| Daily feeding rate, g/min | 25.67 | 5.18 | 11.65 | 48.19 |
| Number of meals per day | 9.59 | 2.03 | 4.24 | 21.24 |
| Number of visits per day | 8.10 | 1.55 | 3.81 | 15.17 |
SD, standard deviation.
Estimation of Genetic Parameters and Phenotypic Correlation
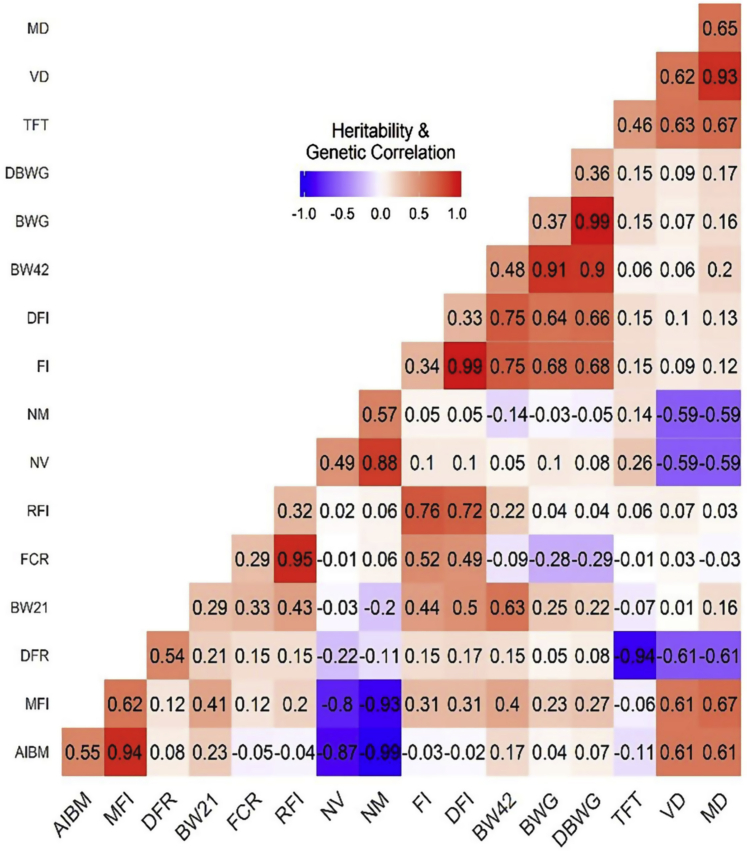
Estimated genetic parameters for traits measured are summarized in Figure 1. The diagonal of Figure 1 was the heritability of different traits, whereas the lower triangle showed the genetic correlations between these traits. Meal duration had the highest estimated heritability (0.65) among the traits measured, followed by the heritability of visit duration, MFI, NM, AIBM, and DFR (ranging from 0.54 to 0.62), whereas FCR had the lowest heritability (0.29).
Figure 1.
Genetic parameters estimated. Numbers in the diagonal means the heritability for each trait. Numbers under the diagonal means the genetic correlation between traits. Colors ranged from purple to red represents the values of heritability and genetic correlation from negative (−1) to positive (1). Abbreviations: AIBM, average interval between meals; BW21, average body weight at day 21; BW42, average body weight at day 42; BWG, body weight gain; DBWG, daily body weight gain; DFI, daily feed intake; DFR, daily feeding rate; FI, feed intake; FCR, feed conversion ratio; MD, meal duration; MFI, meal feed intake; NM, number of meals per day; NV, number of visits per day; RFI, residual feed intake; TFT, total feed time; VD, visit duration.
A moderately positive genetic correlation (0.33) was discovered between BW21 and FCR, whereas BW42 showed strongly positive genetic correlations with FI, MFI, and BWG (ranging from 0.40 to 0.91). In the meantime, AIBM, NV, NM, and MD had low genetic correlations with growth rate or feed efficiency–related traits.
To eliminate the impact of sexes, phenotypic correlations were estimated in male or female ducks separately (Table 2). The phenotypic correlations between any 2 traits were similar in male and female ducks. Significantly positive phenotypic correlations were found between BW42, BW21, daily BWG, DFI, and MFI. In addition, the positive phenotypic correlations between TFT, DFI, DFR, and feed efficiency traits were significant. The body weight showed no significant phenotypic correlation with AIBM, NV, and NM.
Table 2.
Phenotypic correlations between body weight, feed efficiency, and feeding behavior.1
| Traits | BW21 | BW42 | FI | FCR | BWG | RFI | DBWG | AIBM | VD | MD | NV | NM | TFT | DFI | MFI | DFR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BW21 | 0.45** | 0.00 | 0.22** | −0.20** | 0.18** | 0.13** | 0.09 | 0.07 | 0.10* | −0.01 | −0.07 | −0.09 | 0.29** | 0.24** | 0.11** | |
| BW42 | 0.45** | 0.60** | −0.04 | 0.78** | 0.17** | 0.68** | −0.09 | 0.04 | 0.05 | 0.09 | 0.07 | 0.15** | 0.62** | 0.24** | 0.16** | |
| FI | −0.06 | 0.48** | 0.60** | 0.66** | 0.79** | 0.23** | −0.25** | −0.05 | −0.07 | 0.16** | 0.20** | 0.28** | 0.74** | 0.16** | 0.23** | |
| FCR | 0.26** | −0.08 | 0.63** | −0.20** | 0.96** | −0.40** | −0.15** | −0.06 | −0.08 | 0.09 | 0.12** | 0.12** | 0.46** | 0.11** | 0.19** | |
| BWG | −0.34** | 0.68** | 0.55** | −0.29** | 0.06 | 0.66** | −0.16** | 0.00 | −0.01 | 0.11** | 0.13** | 0.23** | 0.48** | 0.10* | 0.10* | |
| RFI | 0.18** | 0.12** | 0.82** | 0.96** | −0.03 | −0.23** | −0.20** | −0.06 | −0.09 | 0.12** | 0.16** | 0.18** | 0.60** | 0.14** | 0.22** | |
| DBWG | 0.12** | 0.63** | −0.01 | −0.52** | 0.56** | −0.38** | −0.15** | 0.08 | 0.12** | 0.20** | 0.15** | 0.11** | 0.62** | 0.17** | 0.01 | |
| AIBM | 0.06 | −0.08* | −0.23** | −0.13** | −0.14** | −0.18** | −0.10 | 0.51** | 0.55** | −0.83** | −0.96** | −0.25** | −0.28** | 0.85** | 0.12** | |
| VD | 0.14** | 0.01 | −0.08 | 0.00 | −0.10* | −0.02 | 0.07 | 0.51** | 0.92** | −0.50** | −0.50** | 0.56** | 0.03 | 0.53** | −0.58** | |
| MD | 0.19** | 0.06 | −0.12** | −0.05 | −0.09 | −0.08 | 0.18** | 0.53** | 0.90** | −0.34** | −0.52** | 0.60** | 0.05 | 0.58** | −0.62** | |
| NV | 0.02 | 0.13** | 0.12** | 0.03 | 0.12** | 0.06 | 0.23** | −0.80** | −0.47** | −0.26** | 0.87** | 0.33** | 0.27** | −0.70** | −0.23** | |
| NM | −0.06 | 0.07 | 0.19** | 0.10 | 0.13** | 0.14** | 0.11* | −0.96** | −0.49** | −0.49** | 0.86** | 0.26** | 0.25** | −0.84** | −0.16** | |
| TFT | −0.03 | 0.11* | 0.26** | 0.17** | 0.14** | 0.21** | 0.06 | −0.28** | 0.53** | 0.59** | 0.39** | 0.30** | 0.22** | −0.14** | −0.82** | |
| DFI | 0.37** | 0.58** | 0.62** | 0.44** | 0.30** | 0.54** | 0.54** | −0.24** | 0.08* | 0.14** | 0.27** | 0.21** | 0.23** | 0.26** | 0.17** | |
| MFI | 0.24** | 0.20** | 0.10 | 0.09 | 0.02 | 0.11* | 0.17** | 0.87** | 0.54** | 0.59** | −0.67** | −0.86** | −0.16** | 0.27** | 0.22** | |
| DFR | −0.01 | 0.11** | 0.23** | 0.14** | 0.13** | 0.19** | −0.06 | 0.16** | −0.57** | −0.63** | −0.31** | −0.20** | −0.84** | 0.07 | 0.21** |
*Significant levels at P < 0.05
**P < 0.01.
Abbreviations: AIBM, average interval between meals; BWG, body weight gain; BW21, average body weight at day 21; BW42, average body weight at day 42 DBWG, daily body weight gain; DFI, daily feed intake; DFR, daily feeding rate; FI, feed intake; MFI,meal feed intake; MD, meal duration; NM, number of meals per day; NV, number of visits per day; RFI, residual feed intake; TFT, total feed time; VD, visit duration.
The upper triangle showed the phenotypic correlations in male ducks, and the lower triangle showed the phenotypic correlations in female ducks.
Comparison Between Male and Female Ducks
The results of one-way ANOVA for different sexes are provided in Table 3. The BW42, BWG, FI, MFI, and AIBM in male ducks were all significantly higher than those in female ducks. At the same time, the RFI levels, FCR, and daily feeding times of male ducks were significantly lower than those of female ducks. However, the TFT and MD were almost the same between male and female ducks.
Table 3.
Comparison of performance traits in male and female ducks.
| Categories | Male pekin duck |
Female pekin duck |
||
|---|---|---|---|---|
| Mean | SD1 | Mean | SD | |
| General Traits | ||||
| Average body weight at day 21, kg | 1.25B | 0.18 | 1.27A | 0.20 |
| Average body weight at day 42, kg | 3.41A | 0.22 | 3.24B | 0.18 |
| Body weight gain, kg | 2.15A | 0.23 | 1.97B | 0.22 |
| Daily body weight gain, kg/D | 0.11A | 0.01 | 0.10B | 0.01 |
| Feed intake, kg | 5.43A | 0.66 | 5.29B | 0.64 |
| Residual feed intake, kg | -0.10B | 0.45 | 0.10A | 0.44 |
| Feed conversion ratio | 2.53B | 0.22 | 2.69A | 0.23 |
| Feeding behavior traits | ||||
| Total feeding time, min | 221.35a | 49.17 | 219.41a | 47.57 |
| Daily feed intake, kg/D | 0.27a | 0.03 | 0.28a | 0.03 |
| Meal feed intake, g | 35.49A | 7.10 | 34.68B | 6.78 |
| Visit duration, s | 72.61a | 18.04 | 70.72b | 16.98 |
| Meal duration, s | 85.59a | 22.53 | 84.08a | 22.08 |
| Average interval between meals, min | 181.97A | 37.27 | 176.37B | 34.80 |
| Daily feeding rate, g/min | 25.74a | 5.25 | 25.59a | 5.11 |
| Number of meals per day | 7.99B | 1.53 | 8.22A | 1.56 |
| Number of visits per day | 9.42B | 1.95 | 9.77A | 2.10 |
A,B,a,bDifferent letters in the same row mean significant differences between different groups. Capital: P < 0.01; Lowercase: P < 0.05.
SD, standard deviation.
Correlation Between Growth-Related Traits and RFI Levels
The correlations between growth-related traits and RFI levels are presented in Table 4. The ducks within the high-RFI group tended to have higher FI, TFT, FCR, MFI, DFR, NM, and NV than ducks in the low-RFI group. Whereas the MD and AIBM seemed to be lower in the high-RFI group than those in the low-RFI group. Besides, there was no significant difference in BWG between the low-RFI group and the high-RFI group.
Table 4.
Comparison of performance traits between low, middle, and high RFI levels.1
| Categories | Low RFI(n = 559) |
Middle RFI(n = 559) |
High RFI(n = 559) |
|||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD2 | Mean | SD | |
| General traits | ||||||
| Average body weight at day 21, kg | 1.25a | 0.21 | 1.26a | 0.21 | 1.27a | 0.13 |
| Average body weight at day 42, kg | 3.35A | 0.25 | 3.30B | 0.21 | 3.36A | 0.21 |
| Body weight gain, kg | 2.10A | 0.24 | 2.04B | 0.25 | 2.09A | 0.19 |
| Daily body weight gain, kg/D | 0.11A | 0.01 | 0.10B | 0.01 | 0.10B | 0.01 |
| Feed intake, kg | 4.58C | 0.51 | 5.34B | 0.48 | 6.18A | 0.41 |
| Residual feed intake, kg | −0.86C | 0.26 | 0.02B | 0.03 | 0.76A | 0.17 |
| Feed conversion ratio | 2.18C | 0.13 | 2.63B | 0.09 | 2.96A | 0.12 |
| Feeding behavior traits | ||||||
| Total feeding time, min | 198.72B | 52.19 | 225.28A | 48.49 | 231.51A | 39.66 |
| Daily feed intake, kg/D | 0.25C | 0.03 | 0.28B | 0.02 | 0.30A | 0.02 |
| Meal feed intake, g | 33.93B | 7.24 | 34.00B | 6.39 | 36.63A | 6.35 |
| Visit duration, s | 72.32a | 21.74 | 70.59a | 17.31 | 69.54a | 14.87 |
| Meal duration, s | 87.68a | 27.13 | 83.32a | 21.84 | 81.69b | 18.29 |
| Average interval between meals, min | 195.25A | 43.84 | 170.16B | 30.22 | 170.05B | 28.54 |
| Daily feeding rate, g/min | 24.20B | 5.08 | 25.38B | 5.11 | 27.54A | 4.61 |
| Number of meals per day | 7.51B | 1.60 | 8.49A | 1.54 | 8.45A | 1.39 |
| Number of visits per day | 9.13B | 2.09 | 10.04A | 2.19 | 9.94A | 1.83 |
A,B,a,b,CDifferent letters in the same row mean significant differences between different groups. Capital: P < 0.01; Lowercase: P < 0.05.
RFI, Residual Feed Intake.
SD, standard deviation.
Selection Response
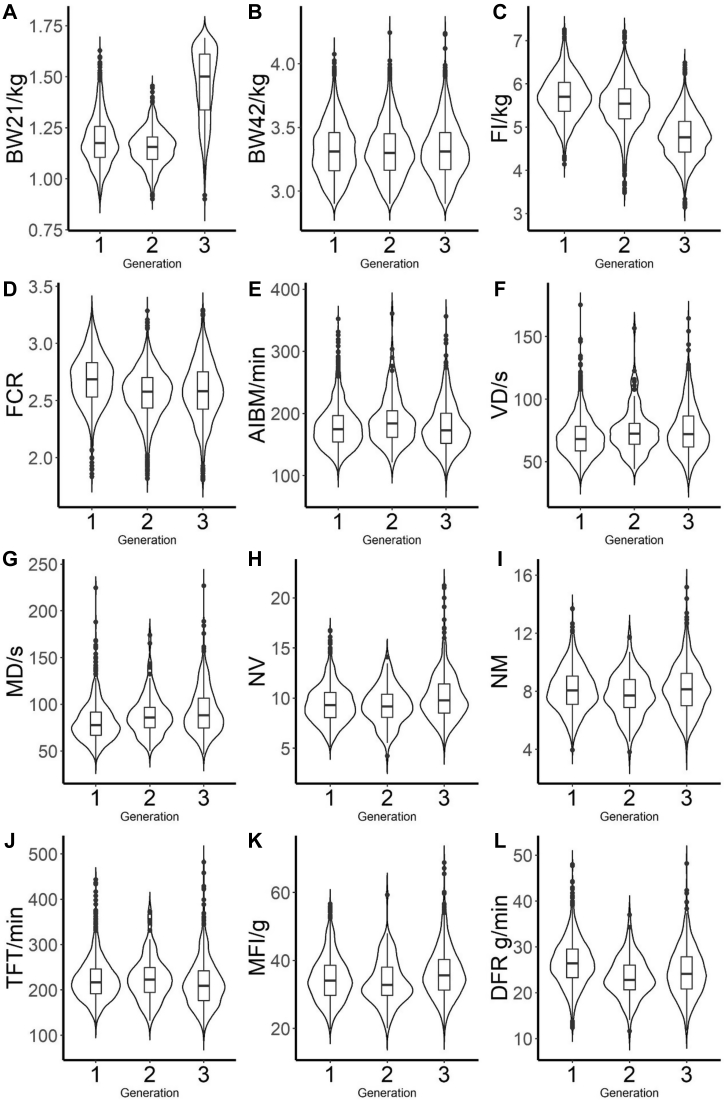
We compared phenotypic values and estimated breeding values for each trait over generations. The generational average phenotypic values of the main traits measured are illustrated in Figure 2. After the selection, the phenotypic value of BW21 was much higher than that of the initial population, whereas the improvements of BW42 were not noticeable. The phenotypic values of FCR and FI continued to decrease over a generation. As for feeding behavior traits, the phenotypic values of TFT and DFR tend to decrease slightly, and the phenotypic value of MD kept on increasing among the generations. However, the phenotypic values of AIBM and MFI were consistent over generations.
Figure 2.
Phenotypic improvements of the main traits over generations for (A) average body weight at day 21 (BW21); (B) average body weight at day 42 (BW42); (C) feed intake (FI); (D) feed conversion ratio (FCR); (E) average interval between meals (AIBM); (F) visit duration (VD); (G) meal duration (MD); (H) number of visits per day (NV); (I) number of meals per day (NM); (J) total feed time (TFT); (K) meal feed intake (MFI); and (L) daily feeding rate (DFR). Violin plot shows the overall distribution of phenotypic value, and box plot shows the median of different traits over generations. Most recent 3 generations data were used to compare phenotypic improvements.
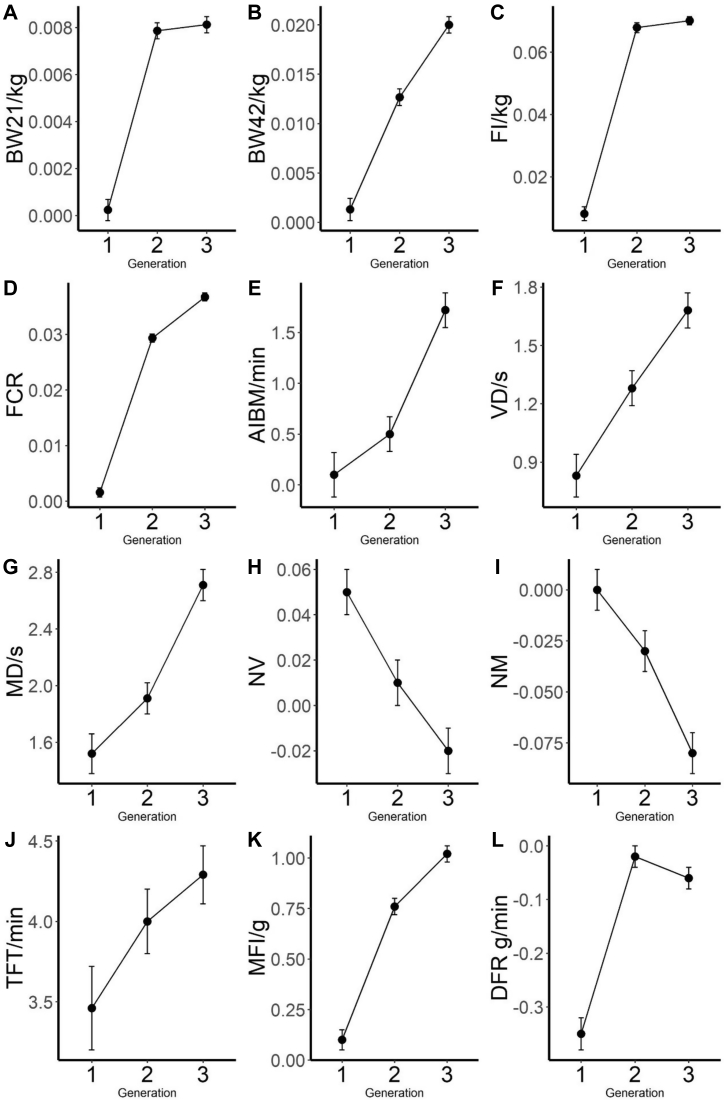
Generational average estimated breeding values (EBV) of the main traits measured are given in Figure 3. The EBV of the most measured traits shared a common process of constant increasing with little fluctuation, whereas the EBV of NV and NM show a decline across the generations. The EBV of BW21 increased by 8 g during the selection, and improvements of BW42 and FI were 20 g and 40 g, respectively. The EBV in AIBM, TFT, MD, and MFI were also changed over generations, which increased by one unit spanning the generations, as these traits are correlated with selected growth and feed efficiency traits.
Figure 3.
Genetic improvements of the main traits over generations for (A) average body weight at day 21 (BW21); (B) average body weight at day 42 (BW42); (C) feed intake (FI); (D) feed conversion ratio (FCR); (E) average interval between meals (AIBM); (F) visit duration (VD); (G) meal duration (MD); (H) number of visits per day (NV); (I) number of meals per day (NM); (J) total feed time (TFT); (K) meal feed intake (MFI); and (L) daily feeding rate (DFR). X-axis denotes the generations, Y-axis denotes the estimated breeding values, and vertical error bar denotes the standard error of corresponding traits.
Discussion
Heritability Estimates
As given in Figure 1, the estimated heritabilit of BW21 (0.29) and BW42 (0.48) were relatively high; thus, they could be improved by direct phenotypic selection. Previous studies showed that the heritability of body weight at the age of 6 wk lay between 0.32 and 0.64 (Li et al., 2006, Deng et al., 2019). And Xu et al. (2011) estimated the heritability of body weight at the age of 7 wk is 0.53 in Pekin ducks. Our findings were consistent with these studies. In the meantime, our study revealed that the heritability of FCR and RFI were 0.29 and 0.32, respectively, which agreed well with the previous research studies (Zhang et al., 2017, Zhu et al., 2019b).
Moreover, with the full application of electronic feeders, it was the first time for us to achieve a complete estimation of genetic parameters for feeding behavior traits in Pekin ducks. Our study revealed that the heritability of feeding behavior–related traits were all high, which ranged from 0.33 to 0.65. In a previous study on modern broiler lines, Howie et al. (2011) reported the heritability of feeding behavior traits to lie in the range of 0.30–0.55 in broilers. So, it can be inferred that feeding behavior traits are highly heritable traits in Pekin ducks and broiler lines. We could also conclude that feeding habits can pass on from parents to offspring and significant progress can be obtained through direct selection on the phenotypic value of feeding behavior traits in Pekin ducks. Meanwhile, the estimations of heritability in our study showed a bit higher than that in previous research studies, which might be due to the fact that we used average values of feeding behavior traits combined with animal model and the animal genetic effect might be mixed with permanent environmental effect to make the genetic variance a little overestimated. And further research will suggest the influence of details in repeated records on feeding behavior traits in Pekin ducks.
Phenotypic and Genetic Correlations
Phenotypic and genetic correlations between body weight and feed efficiency have been studied intensively in ducks. It was reported that the phenotypic and genetic correlations between FCR and BW42 are both negative (Zhang et al., 2017). Similar results were also obtained from the research studies on meat ducks and laying ducks (Pingel, 2011, Basso et al., 2012). In our study, the phenotypic and genetic associations between FI, FCR, and BW42 were consistent with the previous studies, and RFI showed robust positive phenotypic and genetic correlations with FCR and FI. Whereas, both BW42 and BWG had low phenotypic and genetic correlations with RFI. Therefore, it could be inferred that the selection of high feed efficiency might be achieved by selecting the ducks with low-RFI levels, which would have little effect on the increase of body weight.
The phenotypic and genetic associations between body weight and daily feeding times were very low (Table 2). According to our results, it seemed that the selection for high body weight at the age of 6 wk would have a massive influence on the feed consumption per meal, but the daily feeding frequencies were stable over the generations (Figures 2 and 3).
The Contrast Between Male and Female Ducks
Generally speaking, livestock of different genders often have significant differences in performance traits. The BWG and FCR of male broiler are usually superior to those of the female broiler (Salim et al., 2012). The male ducks tend to have higher feed consumption than the female ducks (Erdem et al., 2015). In our study, the male ducks had higher BWG than the female ducks, but the RFI in male ducks was much lower than in female ducks, which implied that the male ducks tended to have higher feed efficiency and grow faster than the female ducks.
This study was also the first to compare the feeding behavior between male Pekin ducks and female Pekin ducks in detail. We found that the male ducks had lower daily feeding frequencies than female ducks, but the feeding time per meal in male ducks was much longer than female ducks. So, it is evident that the male ducks might tend to extend the feeding time per meal to reduce daily feeding frequencies, which differed from the female ducks significantly.
Impacts of RFI Levels
Body weight and feeding behavior are closely associated with certain species, and significant differences in feeding behavior can be found among different genetic backgrounds. Previous studies about beef cattle report that the FI, DFR, and daily feeding frequencies in the low-RFI group are significantly lower than the high-RFI group, whereas the MD in the low-RFI group is much higher than the high-RFI group. However, no noticeable difference in body weight is detected among the cattle with different RFI levels (Bingham et al., 2009, Kelly et al., 2010). Swennen et al. (2007) stated that there is no significant difference between the low- and high-RFI groups in male chickens. Drouilhet et al. (2016) discovered that the mule ducks from the high-RFI group tend to have higher DFI, MFI, DFR, and AIBM than the low-RFI group, whereas the body weight in the high-RFI group is significantly lower than the low-RFI group. In our study, we found the Pekin ducks in the high-RFI group seemed to have higher FI and TFT, which suggested that the RFI might be closely associated with feed efficiency. In the meantime, the FCR and daily feeding times of the high-RFI group were significantly higher than the low-RFI group, which indicated that the ducks in the low-RFI group tend to have high feed efficiency combined with low daily feeding times. Besides, the results of DFR and AIBM among the RFI levels in our study differed from those of the previous study (Drouilhet et al., 2016), and they might be due to the differences of species and estimation for meal criteria.
Improvements in the Selection Process
Generally, the EBV of selected traits illustrate genetic improvements directly. As the estimated heritability of BW42 was relatively high, genetic improvement of BW42 could be achieved by direct phenotypic selection. Owing to the strong positive phenotypic and genetic correlations between BW21 and BW42, the breeding value of BW21 was also improved over the selection. At the same time, the EBV of FCR showed a slight increase in this study, which might be explained by the positive phenotypic and genetic associations between FCR and BW21. On the other hand, as some body weight traits had been selected across generations and the information used as selection criteria in the animal model with best linear unbiased prediction, the estimation of breeding values for these traits might be a little biased. And our future research will focus on multivariable model that combines the target traits with extra traits to show more accurate genetic trends of the corresponding traits.
As for feeding behavior, the phenotypic and EBV of MFI tended to increase across the generations, which were consistent with the positive phenotypic and genetic correlations between MFI and BW42. The phenotypic values of DFR and TFT both decreased during the test periods, and they were corresponding to the decline of total feed consumption. Besides, the EBV of AIBM and MD increased slightly after the selection, and they could be attributed to the strong phenotypic and genetic correlations among feeding behavior traits.
In conclusion, we estimated the genetic parameters and selection response of body weight–related traits and feeding behavior traits in this study. The heritability of RFI, BW42, and feeding behavior traits were all high. We also found that TFT, DFI, and daily feeding frequencies increased significantly with the increasing RFI levels. According to the results, we may achieve the indirect selection of RFI by feeding behavior traits in the breeding program, and they also provide new insight into the improvements of feed efficiency in Pekin ducks.
Acknowledgments
We acknowledged that Mr. Guocheng Li helped us to collect information during the experiment. The work was supported by the National Waterfowl-Industry Technology Research System (CARS-42-09), the National Scientific Supporting Projects of China (2015BAD03B06), the Leading Technology of Modern Agricultural Science and Technology City (Z181100002418008), Primary Research & Development Plan of Jiangsu Province (BE2017349), and the Program for Changjiang Scholar and Innovation Research Team in University (IRT1191).
Ethics Statement: The present study was carried out in agreement with the guidelines on live animals research approved by the Beijing Administration Committee of Laboratory Animals, under the leadership of the Beijing Association for Science and Technology (permit number: SYXK 2007–0023). All efforts were made to minimize animal suffering during the study.
Conflict of Interest Statement: The authors did not provide a conflict of interest statement.
Footnotes
Supplementary data associated with this article can be found in the online version at http://doi.org/10.1016/j.psj.2019.11.070.
Supplementary data
Supplementary Figure 1.
The distribution of log-transformed visits intervals over different generations. X-axis denotes the loge transformed visits intervals, and Y-axis denotes the corresponding frequencies. The labels of A, B, and C denote the first generation, the second generation, and the third generation, respectively.
References
- Basso B., Bordas A., Dubos F., Morganx P., Marie-Etancelin C. Feed efficiency in the laying duck: appropriate measurements and genetic parameters1. Poult. Sci. 2012;91:1065–1073. doi: 10.3382/ps.2011-02008. [DOI] [PubMed] [Google Scholar]
- Bingham G., Friend T., Lancaster P., Carstens G. Relationship between feeding behavior and residual feed intake in growing Brangus heifers. J. Anim. Sci. 2009;87:2685–2689. doi: 10.2527/jas.2009-1851. [DOI] [PubMed] [Google Scholar]
- Chen G.J., Song H.L., Ma C.W. Aroma-active compounds of Beijing roast duck. Flavour. Frag. J. 2010;24:186–191. [Google Scholar]
- Drouilhet L., Monteville R., Molette C., Lague M., Cornuez A., Canario L., Ricard E., Gilbert H. Impact of selection for residual feed intake on production traits and behavior of mule ducks. Poult. Sci. 2016;95:1999–2010. doi: 10.3382/ps/pew185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng M.-T., Zhu F., Yang Y.-Z., Yang F.-X., Hao J.-p., Chen S.-R., Hou Z.-C. Genome-wide association study reveals novel loci associated with body size and carcass yields in Pekin ducks. BMC Genomics. 2019;20:1. doi: 10.1186/s12864-018-5379-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdem E., Onbaşilar E.E., Hacan Ö.G. Effects of 16L: 8D photoperiod on growth performance, carcass characteristics, meat composition, and blood parameters of Pekin ducks. Turk. J. Vet. Anim. Sci. 2015;39:568–575. [Google Scholar]
- Howie J., Avendano S., Tolkamp B., Kyriazakis I. Genetic parameters of feeding behavior traits and their relationship with live performance traits in modern broiler lines. Poult. Sci. 2011;90:1197–1205. doi: 10.3382/ps.2010-01313. [DOI] [PubMed] [Google Scholar]
- Howie J.A., Tolkamp B.J. A novel flexible method to split feeding behaviour into bouts. Appl. Anim. Behav. Sci. 2009;116:101–109. [Google Scholar]
- Jensen J. Residual maximum likelihood estimation of (co) variance components in multivariate mixed linear models using average information. J. Soc. Agric. Statis. 1997;49:215–236. [Google Scholar]
- Kelly A., McGee M., Crews D., Jr., Fahey A., Wylie A., Kenny D. Effect of divergence in residual feed intake on feeding behavior, blood metabolic variables, and body composition traits in growing beef heifers. J. Anim. Sci. 2010;88:109–123. doi: 10.2527/jas.2009-2196. [DOI] [PubMed] [Google Scholar]
- Kennedy B., Van der Werf J., Meuwissen T. Genetic and statistical properties of residual feed intake. J. Anim. Sci. 1993;71:3239–3250. doi: 10.2527/1993.71123239x. [DOI] [PubMed] [Google Scholar]
- Koch R.M., Swiger L.A., Chambers D., Gregory K.E. Efficiency of feed use in beef cattle. J. Anim. Sci. 1963;22:486–494. [Google Scholar]
- Larzul C., Guy G., Bernadet M. Feed efficiency, growth and carcass traits in female mule ducks. Arch. Geflugelkd. 2004;68:265–268. [Google Scholar]
- Li Z., Shuisheng H., Xiaolin L., Wei H., Ling Z., Junying Y. Estimation of genetic parameters on growth traits of peking ducks. Chin. Poult. 2006;3:005. (Chinese) [Google Scholar]
- Lin F.B., Zhu F., Hao J.P., Yang F.X., Hou Z.C. In vivo prediction of the carcass fatness using live body measurements in Pekin ducks. Poult. Sci. 2018;97:2365–2371. doi: 10.3382/ps/pey079. [DOI] [PubMed] [Google Scholar]
- Madsen P., Jensen J. Danish Inst. Agric. Sci., Res. Centre, Centre; Foulum, Denmark: 2013. A User’s Guide to DMU. [Google Scholar]
- Pingel H. Influence of breeding and management on the efficiency of duck production. Lohmann Inform. 1999;22:7–13. [Google Scholar]
- Pingel H. Results of selection for breast muscle percentage and feed conversion ratio in pekin ducks. Biotechno. Anim. Husb. 2011;27:769–776. [Google Scholar]
- Salim H., Lee H., Jo C., Lee S., Lee B.D. Effect of sex and dietary organic zinc on growth performance, carcass traits, tissue mineral content, and blood parameters of broiler chickens. Blol. Trace. Elem. Res. 2012;147:120–129. doi: 10.1007/s12011-011-9282-8. [DOI] [PubMed] [Google Scholar]
- Swennen Q., Verhulst P.-J., Collin A., Bordas A., Verbeke K., Vansant G., Decuypere E., Buyse J. Further investigations on the role of diet-induced thermogenesis in the regulation of feed intake in chickens: Comparison of adult cockerels of lines selected for high or low residual feed intake. Poult. Sci. 2007;86:1960–1971. doi: 10.1093/ps/86.9.1960. [DOI] [PubMed] [Google Scholar]
- Xu T., Liu X., Huang W., Hou S. Estimates of genetic parameters for body weight and carcass composition in Pekin ducks. J. Anim. Vet. Adv. 2011;10:23–28. [Google Scholar]
- Zhang Y., Guo Z.B., Xie M., Zhang Z., Hou S. Genetic parameters for residual feed intake in a random population of Pekin duck. Asian Austral. J. Anim. 2017;30:167. doi: 10.5713/ajas.15.0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Li M., Cheng H., Fan W., Yuan Z., Gao Q., Xu Y., Guo Z., Zhang Y., Hu J., Liu H., Liu D., Chen W., Zheng Z., Jiang Y., Wen Z., Liu Y., Chen H., Xie M., Zhang Q., Huang W., Wang W., Hou S., Jiang Y. An intercross population study reveals genes associated with body size and plumage color in ducks. Nat. Commun. 2018;9:2648. doi: 10.1038/s41467-018-04868-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F., Gao Y.H., Lin F.B., Hao J.P., Yang F.X., Hou Z.C. Systematic analysis of feeding behaviors and their effects on feed efficiency in Pekin ducks. J. Anim. Sci. Biotechno. 2017;8:81. doi: 10.1186/s40104-017-0212-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F., Cui Q.-Q., Yang Y.-z., Hao J.-P., Yang F.-X., Hou Z.-C. Genome-wide association study of the level of blood components in Pekin ducks. Genomics. 2019;2:17. doi: 10.1016/j.ygeno.2019.02.017. [DOI] [PubMed] [Google Scholar]
- Zhu F., Cheng S.-R., Yang Y.-z., Hao J.-P., Yang F.-X., Hou Z.-C. Genome-Wide association study of growth and feeding traits in pekin ducks. Front. Genet. 2019;10:702. doi: 10.3389/fgene.2019.00702. [DOI] [PMC free article] [PubMed] [Google Scholar]