Abstract
Three experiments were conducted to determine the effect of different dietary net energy (NE) and AMEn ratios (NE:AMEn) on performance, egg quality, and heat production (HP) in laying hens. In experiment 1, 62 Hy-Line Brown hens were fed 2 treatments with 31 replicates from 44 to 54 wk of age. In experiment 2, 600 hens of the same strain were fed 3 treatments from 22 to 42 wk of age with 10 replicates. Both used a completely randomized design. Diets were based on corn, wheat, wheat bran, barley, soybean meal, canola meal, meat and bone meal, and canola oil. In both experiments, the NE:AMEn ratio of diets was increased with higher oil inclusion compared with T1 controls. The AMEn (kcal/kg), NE (kcal/kg), ether extract (g/kg), and CP (g/kg), respectively, on a DM basis in experiment 1 was T1: 3,011, 2,288, 42, 202 and T2: 3,023, 2,374, 81, 203; and in experiment 2, T1: 3,026, 2,324, 25, 187; T2: 2,949, 2,315, 61, 185; and T3: 3,026, 2,397, 73, 181. Increasing the ratio of NE:AMEn decreased feed intake (P < 0.001) and increased egg mass (P < 0.05) in experiment 2 and increased egg weight (P < 0.01), decreased feed conversion ratio (P < 0.01), increased egg albumen % (P < 0.001), and decreased yolk % (P < 0.05) and shell % (P < 0.05) compared with T1 controls in both experiments. Haugh units and yolk color scores were increased with high NE:AMEn in both experiments (P < 0.001; P < 0.01). Experiment 3 was conducted in calorimetry chambers to measure HP in birds fed experiment 2 diets. Increasing the NE:AMEn increased total retained energy (RE), RE as fat, and RE in the body (kcal/kg BW0.75/D) and NE:AME. The results indicate that using oil to increase the NE:AMEn results in improved performance and egg quality and more efficient energy utilization.
Key words: net energy, energy efficiency, performance, egg production, laying hens
Introduction
Feed accounts for 65 to 75% of egg production expenses with energy representing at least 60% of feed costs. The formulation of layer feed on a net energy (NE) basis may be more accurate than using AMEn as it takes into account the energy lost as heat. Heat increment (HI) is the heat produced by an animal in excess of that associated with basal (or fasting) metabolism. The HI depends on environmental temperature, time, and the amount of feed consumed and varies throughout the day (Zhou and Yamamoto, 1997, Koh and Macleod, 1999). In chickens, the relative efficiency of energy utilization for fat, carbohydrate, and protein was determined to be 113, 100, and 78%, respectively (DeGroote, 1974). Prediction equations for determining NE values of ingredients for broilers have been reported by Wu et al. (2019) and for layers by Barzegar et al. (2019a). For both classes of birds, the equations adjust AME or AMEn to NE based on the level of protein and ether extract (EE) in ingredients. The equations reported for layers are different than those for broilers in that both protein and fat concentrations have a greater effect on the adjustment of AME or AMEn to NE. Virtually all poultry feed is formulated on an AMEn basis where measured AME values are corrected to zero nitrogen (N) retention even though productive layers and broilers retain 40 to 50% of N consumed (Cozannet et al., 2010). Such a correction reduces the value of high protein ingredients such as soybean meal. The NE prediction equations account for both N and energy lost as heat to derive energy for maintenance, body weight gain, and egg production. An NE system has been applied with economic benefit in the swine industry (Just, 1982, Noblet et al., 1994).
The ratio of NE to AME is important to consider as AME accounts for only 74 to 78% of the variation in NE (Pirgozliev et al., 1999). It was reported by Carré et al. (2014) that NE:AME ratios for digestible lipids, starch, and CP were 85, 79, and 68%, respectively, in broilers. Similarly, Wu et al. (2019) obtained NE:AME ratios of 85, 79, and 52 for lipids, starch, and CP, respectively, in growing broilers. Experimental data of Noblet et al. (2010) also confirmed that the NE:AME ratio varies with the chemical composition of diets and nutrients (fat > starch > protein = fiber). These results indicate that the AME system underestimates the energy value of dietary fat and overestimates the energy value of high-protein ingredients. Thus an NE system may better at predicting the performance of broiler and also laying hens. However, limited data exist for the implementation of a NE formulation system in laying hens (Chudy et al., 2003, Sakomura, 2004, Sakomura et al., 2005).
The objectives of the experiments reported herein were (1) to examine the effects on performance and egg quality of laying hens fed diets varying in NE:AMEn formulated using NE values predicted for ingredients and (2) to compare the predicted energy partition values of the diets to those measured using calorimeter chambers.
Materials and methods
Birds and Diets
The experiments were approved by the Animal Ethics Committee of the University of New England (UNE) and animals handled by following the Australian code of practice for the care and use of animals for scientific purposes (NHMRC, 2013).
Experiment 1
Sixty-two Hy-Line Brown hens at 95% hen day production (HDP), 44 wk of age, were used for an 11-wk experimental period. Their initial HDP was 73% at 21 wk of age. Birds were sourced from the Glenwarrie Partnership, Tamworth, NSW, Australia. The hens were housed one bird per cage in an open-sided shed at the University of New England, Australia, with 16 h light per day in the winter season. Cages were fitted with individual feeders and nipple drinkers. A completely randomized design was employed with 2 dietary treatments and 31 replicates per treatment. Ingredient composition of experimental diets is shown in Table 1 and nutrient composition in Table 2. Diets were formulated with corn, wheat, wheat bran, SBM, cold-pressed canola meal, meat, and bone meal and canola oil sourced from the local market and analyzed for nutrient content by near-infrared reflectance spectroscopy (Evonik Amino NIR). The ingredients calculated AMEn (kcal/kg) and NE (kcal/kg) on an as-is basis were 3,329 and 2,643 for corn; 3,255 and 2,541 for wheat; 2,260 and 1,723 for wheat bran; 2,298 and 1,448 for SBM; 2,550 and 1,907 for cold-pressed canola meal; 1,973 and 1,236 for meat and bone meal; 8,500 and 8,604 for canola oil, respectively. Xylanase (Econase XT25, AB Vista, Sydney) provided 600 BXU/kg feed. In addition, phytase (Axtra PHY TPT 10000, Dupont, Melbourne, VIC) added separately to provide 500 FTU/kg feed. Enzymes levels are shown in Table 1. Manufacturer's recommended nutrient matrix values were used for phytase. Two diets with high and low NE:AMEn ratios were formulated. Diets in both experiments 1 and 2 were formulated to the digestible amino acid levels, AMEn, calcium, available phosphorus, sodium, chloride, linoleic acid, and choline based on expected intake and daily nutrient intake recommendations for Hy-Line Brown hens (Hy-Line, 2016) (Table 1). The birds were fed the experimental diets for a 1 wk adaptation period before production data were collected. Ingredient AMEn and NE values were calculated based on the developed prediction equation in laying hens using AMEn, CP, and EE content of ingredients (Barzegar et al., 2019a, Barzegar et al., 2019b).
Table 1.
Ingredients composition of diets (g/kg; as-is basis).
| Diet | Experiment 1 |
Experiments 2 and 3 |
|||
|---|---|---|---|---|---|
| 1 | 2 | 1 | 2 | 3 | |
| Ingredient | |||||
| Corn | 150 | 150 | 0 | 0 | 0 |
| Wheat | 446 | 221 | 616 | 434 | 419 |
| Barley | 0 | 0 | 100 | 116 | 114 |
| Wheat bran | 50 | 240 | 20 | 120 | 120 |
| Soybean meal | 131 | 137 | 100 | 54 | 59 |
| Canola meal-cold pressed | 100 | 100 | 50 | 150 | 150 |
| Meat and bone meal | 11 | 0 | 0 | 0 | 0 |
| Canola oil | 4.5 | 40.6 | 3.3 | 19.2 | 31.8 |
| Limestone | 98.7 | 101.1 | 95.1 | 94.4 | 94.4 |
| Dicalcium phosphate | 1.8 | 3.8 | 2.0 | 0.6 | 0.7 |
| Salt | 1.2 | 1.4 | 2.0 | 1.8 | 1.8 |
| Choline 60% | 0.4 | 0.4 | 0.4 | 0.6 | 0.6 |
| UNE vitamin & mineral premix1 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Na bicarbonate | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Pigment-Jabiru Red (10%) | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 |
| Pigment-Jabiru Yellow (10%) | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 |
| Xylanase (Econase XT 25)2 | 0.05 | 0.05 | 0 | 0 | 0 |
| Phytase (Axtra TPT 10000)3 | 0.05 | 0.05 | 0.10 | 0.10 | 0.10 |
| Xylanase (Axtra XB) | 0 | 0 | 0.08 | 0.08 | 0.08 |
| L-lysine HCl 78.4 | 0.9 | 0.5 | 2.7 | 2.2 | 2.2 |
| D,L-methionine | 1.3 | 1.4 | 2.0 | 1.6 | 1.6 |
| L-threonine | 0.2 | 0.2 | 1.2 | 0.9 | 0.9 |
| L-isoleucine | 0 | 0 | 1.0 | 1.0 | 1.0 |
| L-valine | 0 | 0 | 0.8 | 0.5 | 0.5 |
UNE laying hens premix supplied per tonne: 10.0 MIU Vit A, 3.0 MIU Vit D, 20.0 g Vit E, 3.0 g Vit K, 35.0 g nicotinic acid, 12 g pantothenic acid, 1 g folic acid, 6 g riboflavin, 0.02 g cyanocobalamin, 0.10 g biotin, 5.0 g pyridoxine, 2.0 g thiamine, 8.0 g copper, 0.20 g cobalt, 0.50 g molybdenum, 1.0 g iodine, 0.30 g selenium, 60.0 g iron, 60.0 g zinc, 90.0 g manganese, 20.0 g Oxicap E2 (antioxidant).
No matrix values were used for xylanase in any formulation.
Matrix values for phytase (Axtra TPT 10,000, 500 FTU) were: 2,866% P avail, 2,844% Ca, 720,000 kcal/kg AMEn, 240% lysine, 72% methionine, 210% methionine + cystine, 214% threonine, 174% isoleucine, 64% tryptophan, 212% valine, and 204% arginine with amino acids on a digestibility basis.
Table 2.
Nutrient composition of experimental diets (g/kg, DM).
| Diet | Experiment 1 |
Experiments 2 and 3 |
|||
|---|---|---|---|---|---|
| 1 | 2 | 1 | 2 | 3 | |
| Nutrients measured | |||||
| DM % | 92 | 91 | 90 | 90 | 90 |
| CP | 202 | 203 | 187 | 185 | 181 |
| EE | 42 | 81 | 25 | 61 | 73 |
| Starch | 408 | 312 | 449 | 370 | 365 |
| Crude fiber | 50 | 70 | 33 | 61 | 48 |
| Calcium | 48 | 44 | 45 | 47 | 43 |
| Phosphorus | 5.7 | 6.3 | 4.7 | 6.2 | 5.7 |
| Sodium | 1.6 | 1.5 | 1.7 | 2.4 | 1.9 |
| Lysine | 14.4 | 10.9 | 9.6 | 10.8 | 9.5 |
| Methionine + cystine | 8.6 | 8.3 | 8.9 | 8.5 | 8.3 |
| Threonine | 8.7 | 7.4 | 6.9 | 7.6 | 6.9 |
| Isoleucine | 9.2 | 7.9 | 7.8 | 8.4 | 7.7 |
| Arginine | 13.6 | 11.9 | 9.1 | 10.2 | 9.4 |
| Valine | 10.6 | 9.2 | 8.7 | 9.3 | 8.4 |
| Tryptophan | 2.5 | 2.3 | 2.1 | 2.2 | 2.2 |
| Nutrients calculated | |||||
| AMEn (kcal/kg, DM) | 3,011 | 3,023 | 3,026 | 2,949 | 3,026 |
| NE (kcal/kg, DM) | 2,288 | 2,374 | 2,324 | 2,315 | 2,397 |
| NE:AMEn | 0.760 | 0.785 | 0.768 | 0.785 | 0.792 |
| Dig arginine | 10.7 | 11.2 | 8.8 | 9.3 | 9.3 |
| Dig lysine | 9.0 | 8.8 | 8.4 | 8.4 | 8.4 |
| Dig methionine | 4.3 | 4.4 | 4.5 | 4.2 | 4.2 |
| Dig methionine + cystine | 7.7 | 7.6 | 7.2 | 7.2 | 7.2 |
| Dig tryptophan | 2.1 | 2.2 | 1.7 | 1.9 | 1.9 |
| Dig isoleucine | 6.9 | 6.7 | 6.6 | 6.6 | 6.6 |
| Dig threonine | 6.3 | 6.3 | 5.9 | 5.9 | 5.9 |
| Dig valine | 8.1 | 8.0 | 7.4 | 7.4 | 7.4 |
| Calcium | 46 | 46 | 42 | 42 | 42 |
| Phosphorus, available | 4.0 | 3.8 | 3.5 | 3.5 | 3.5 |
Experiment 2
Six hundred Hy-Line Brown pullets obtained from Glenwarrie Farm in Tamworth at 16 wk of age were housed in the same cage facility as in experiment 1 and maintained with 16 h light per day in winter and spring seasons. The birds were fed their respective diets for an adaptation period beginning at 21 wk of age and data collection began at 22 wk of age when birds were at 78% HDP. The experiment lasted for a 20-wk period. A completely randomized design was used with 3 dietary treatments, 10 replicates per treatment, and each replicate containing 10 cages of 2 birds per cage. Diets were formulated with wheat, barley, wheat bran, SBM, cold-pressed canola meal, and canola oil. The ingredients were analyzed for nutrient content by near-infrared reflectance spectroscopy (Evonik Amino NIR) before formulation. Fatty acid profile of treatment diets was assayed by gas chromatography per accepted methods (Davi, 2000). The calculated AMEn (kcal/kg) and NE (kcal/kg) on an as-is basis were wheat, 3,303 and 2,562; barley, 2,999 and 2,342; wheat bran, 2,109 and 1,601; SBM, 2,368 and 1,530; cold-pressed canola meal, 2,394 and 1,747; canola oil, 8,500, and 8,604 for canola oil, respectively. Xylanase (Axtra XB, beta-xylanase 12,200 U and beta-glucanase 1,520 U Dupont, Melbourne, VIC) and phytase (Axtra PHY TPT 10000, Dupont, Melbourne, VIC) were added according to Table 1. Manufacturer's matrix values were used for phytase to provide 500 FTU per kg of diet. Treatment 1 (T1) was a normal commercial control diet formulated to have AMEn and NE levels of 3,026 and 2,324 (kcal/kg, DM). Treatment 2 (T2) was formulated to have the same NE (2,315 kcal/kg, DM) but lower AMEn compared with T1. Treatment 3 (T3) was formulated to have the same AMEn but higher NE (2,397 kcal/kg, DM) compared with T1. The NE:AMEn was ranked T3 > T2 > T1. Pigments (Jabiru red and yellow) were added at 0.04 and 0.03 g/kg to all diets of both experiments 1 and 2. Birds were fed ad libitum with free access to water. The performance and egg quality of laying hens fed different diets in both experiments 1 and 2 are shown in Table 3.
Table 3.
Performance and egg quality of laying hens fed different diets.
| Diet | Experiment 1 |
Experiment 2 |
P-value (diet) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | SEM | P-value (diet) | 1 | 2 | 3 | SEM | ||
| Performance parameters1 | |||||||||
| Initial BW (g/hen)2 | 2,123 | 2,084 | 24 | 0.417 | 1,935 | 1,912 | 1,940 | 5 | 0.078 |
| Final BW (g/hen) | 2,159 | 2,121 | 28 | 0.513 | 2,270a,b | 2,211c | 2,253b,c | 8 | 0.012 |
| BWT change (%) | 1.5 | 1.8 | 0.3 | 0.751 | 17.4 | 15.7 | 16.1 | 0.3 | 0.081 |
| Feed intake (g/hen/D as is) | 123.1 | 120.5 | 1.3 | 0.321 | 118.5a,b | 117.0b,c | 115.7c | 0.3 | 0.001 |
| HDP, % | 95.9 | 94.9 | 0.5 | 0.323 | 95.8 | 95.6 | 95.5 | 0.2 | 0.827 |
| Egg weight, g | 60.5b | 63.4a | 0.5 | 0.002 | 59.4b,c | 59.3c | 60.3a | 0.1 | 0.004 |
| Egg mass, g/D | 58.0 | 60.1 | 0.5 | 0.0504 | 56.9b,c | 56.7c | 57.6a,b | 0.1 | 0.030 |
| FCR, (g/g) | 2.124a | 2.007b | 0.021 | 0.004 | 2.082a,b | 2.065b | 2.010c | 0.008 | <0.001 |
| Egg quality parameters3 | |||||||||
| External | |||||||||
| Egg weight, g | 60.2b | 63.4a | 0.2 | <0.001 | 60.0b,c | 59.8c | 60.8a | 0.1 | <0.001 |
| Shell color reflectivity (%) | 18.3b | 19.0a | 0.1 | 0.003 | 18.2 | 18.0 | 18.2 | 0.1 | 0.460 |
| Breaking strength, N | 41.5 | 42.0 | 0.2 | 0.337 | 47.2 | 46.9 | 47.0 | 0.2 | 0.820 |
| Deformation, (μm) | 255 | 257 | 1 | 0.261 | 290 | 285 | 289 | 1 | 0.052 |
| Shell thickness (mm) | 0.418 | 0.419 | 0.001 | 0.355 | 0.410 | 0.412 | 0.409 | 0.001 | 0.180 |
| Yolk Weight, g | 16.1 | 16.3 | 0.1 | 0.102 | 14.7a | 14.4b,c | 14.3c | 0.1 | <0.001 |
| Yolk % | 26.7a | 25.7b | 0.1 | <0.001 | 24.5a | 24.0b | 23.5c | 0.1 | <0.001 |
| Albumen weight, g | 38.2b | 41.0a | 0.1 | <0.001 | 39.5c | 39.7b,c | 40.7a | 0.1 | <0.001 |
| Albumen % | 63.5b | 64.7a | 0.1 | <0.001 | 65.9c | 66.3b | 66.9a | 0.1 | <0.001 |
| Yolk:Albumen % | 42.2a | 39.8b | 0.2 | <0.001 | 37.2a | 36.3b | 35.2c | 0.1 | <0.001 |
| Shell weight, g | 5.9b | 6.1a | 0.1 | <0.001 | 5.8 | 5.8 | 5.8 | 0.1 | 0.297 |
| Shell % | 9.74a | 9.61b | 0.02 | 0.003 | 9.63b,c | 9.68a,b | 9.57c | 0.02 | 0.037 |
| Internal | |||||||||
| Haugh unit | 90.2b | 92.9a | 0.3 | <0.001 | 98.4b | 97.5c | 98.5a,b | 0.2 | 0.012 |
| Yolk color score | 11.4b | 11.7a | 0.1 | <0.001 | 10.8c | 10.9b,c | 11.0a,b | 0.1 | <0.001 |
a–cMeans within rows with different superscripts are different at different P-values.
Data are means of 31 hens per 2 dietary treatment in experiment 1 and 200 hens of 10 replicates per 3 dietary treatments in experiment 2. (P < 0.05) by one-way ANOVA.
Abbreviations: Initial BW, average body weight at the beginning of experiment (g/hen); Final BW, average body weight at the end of the experiment (g/hen); BWT change, body weight change as difference of initial and final body weight divided by initial body weight) (%); HDP, average hen day production (%); Egg weight, average egg weight (g) for total experimental period; Egg mass as average egg weight × average HDP (g of egg/bird/D); FCR (g/g), feed conversion ratio as total feed intake (g/hen/D, as is) divided by total egg mass (g); yolk, albumen and shell percentage calculated as correspondent parameters values ratio to the egg weight (%).
From the analysis of variance with diet and age effects; the age effect was significant for all the egg quality parameters in experiments 1 and 2 (P < 0.05). The age had no effect on albumen weight in experiment 1 (P > 0.05). Diet by age interactions was not significant (P > 0.05).
Experiment 3
In experiment 3 (indirect calorimetric measurement), 99 hens were sourced from Glenwarrie Partnership, Tamworth, NSW, Australia, at the same time as those used in experiment 2 and housed in the same shed 3 per cage. A total of 45 birds (15 cages) were selected randomly at the age of 35 wk and again at 44 wk and placed in calorimetry chambers 3 per cage to measure AME, heat production (HP), NE, and NE:AME of different diets. During the interim period, hens were fed a diet containing a mixture (33.3%) of each of the 3 experimental diets.
Measurements and Analysis
Bird Performance and Diet Measurements
Body weight was measured at the beginning and at the end of both experiments. Feed intake, egg production, and egg weight were measured for each cage. Eggs were collected daily and total number and weight recorded. Feed intake measured weekly. Approximately 2 g of diet samples were dried in crucibles in a drying oven at 105°C for 16 h to determine DM. Samples of ingredients and feeds were finely ground to ensure homogeneity. Ingredient samples were analyzed for CP, EE, crude fiber, ash, neutral detergent fiber, and acid detergent fiber (AOAC, 2016). The fatty acid profile of all diets was measured using the AOAC Official Method 996.06 (Davi, 2000) (Table 4). The CP was measured as nitrogen (N) using a LECO model FP-2000 N analyzer in triplicate using 0.15-g samples of ingredients and diets. LECO was calibrated by pure reference EDTA.
Table 4.
Fatty acid profile of diets (expressed as g/kg of diet, as is).
| Diet | Experiment 1 |
Experiments 2 and 3 |
|||
|---|---|---|---|---|---|
| 1 | 2 | 1 | 2 | 3 | |
| Myristic (14:0) | 0.07 | 0.07 | 0.03 | 0.06 | 0.06 |
| C15:0 | 0.03 | 0.04 | 0.02 | 0.03 | 0.04 |
| Palmitic (16:0) | 3.85 | 5.81 | 2.79 | 4.73 | 5.47 |
| Palmitoleic (9c-16:1) | 0.15 | 0.18 | 0.05 | 0.15 | 0.17 |
| Margaric (17:0) | 0.06 | 0.07 | 0.03 | 0.06 | 0.06 |
| Stearic (18:0) | 0.94 | 1.43 | 0.45 | 1.05 | 1.25 |
| Oleic (9c-18:1) | 15.39 | 34.73 | 6.95 | 24.68 | 30.10 |
| Linoleic (18:2 n6) | 12.39 | 21.13 | 9.31 | 17.21 | 19.99 |
| Linolenic (18:3 n3) | 2.23 | 4.79 | 1.32 | 4.04 | 4.80 |
| Arachidic (20:0) | 0.17 | 0.36 | 0.07 | 0.27 | 0.33 |
| Behenoic (22:0) | 0.11 | 0.21 | 0.07 | 0.18 | 0.21 |
| Erucic [22:1 n9] | 0.01 | 0.02 | 0.01 | 0.02 | 0.04 |
| Lignoceric (24:0) | 0.08 | 0.13 | 0.03 | 0.11 | 0.12 |
Eggshell and Egg Internal Quality Measurements
External and internal egg quality was measured on freshly laid eggs collected on 2 consecutive day determined 5 and 10 times fortnightly for experiments 1 and 2, respectively. Egg external and internal quality measurements were performed following reported procedures (Samiullah et al., 2016) using TSS (Technical Services and Supplies, Dunnington, York, UK) equipment. Shell color reflectivity (%) was measured using a hand-held Konica Minolta spectrophotometer (CM-2600d Ramsey, NJ) calibrated with a white reference tile. The top (wide) part of each egg was measured. As this is a reflectance measurement, lower values indicate a darker shell color. Shell breaking strength (N) and shell deformation (μm) was measured by quasistatic compression using TSS QC-SPA (50 N load cell) equipment. Individual eggs were placed horizontally in the egg holder before being compressed by the shell breaking strength machine to record the maximum compression force to break the egg and expressed as Newton. The eggshell was washed and dried overnight. Eggshell thickness (including inner and outer shell membranes) was measured at 3 different points around the equator of each egg using a custom-built micrometer, based on a Mitutoyo Dial Comparator Gauge Model 2,109–10 (Kawasaki, Japan). An average of 3 thickness measurements of an egg was taken. The dried eggshell weight was determined using a digital Quintix 513-1S balance (Sartorius Lab Instruments GmbH & Co. KG Goettingen, Germany). Yolk, albumen, and shell percentage calculated as correspondent parameters values ratio to the egg weight (%).
For egg internal quality, Haugh units (HU) and yolk color were measured. The egg was cracked carefully and the eggshell separated thoroughly. Albumen height was measured using a digital micrometer measuring 1 cm apart from yolk perimeter. Haugh units were calculated using the formula with the records of albumen height and egg weight: HU = 100 × log10 (H − 1.7 W0.37 + 7.56), where HU = Haugh unit, H = height of the albumen (mm) and W = egg weight (g). The yolk was separated from the albumen by rolling them down to the yolk color reader as a yolk score. Before the yolk weight was determined, the chalazae and any adhering albumen were removed and then the yolk weight measured by a digital scale. Haugh unit and yolk color were measured using the TSS QCEQCM equipment. The yolk color scoring system used in the TSS QCE-QCM is based on the 1 to 15 scale of the DSM (previously Roche) yolk color fan scoring system (DSM, 2008).
Indirect Calorimetric Measurement
The birds for experiment 3 were moved to closed-circuit calorimetry chambers, acclimated for 4 D followed by 3 D HP measurements. The design, gas exchanges, and HP measurements for closed-circuit calorimetric chambers are described by Swick et al. (2013) and Wu et al. (2019).
Calculations
Egg mass was calculated as the product of average egg weight and hen day production. Feed conversion ratio (FCR) was calculated as the ratio of feed intake to egg mass. Albumen weight was calculated by subtracting the weight of yolk and shell from the whole egg weight. Shell, albumen, and yolk percentage was calculated as their percentage of the egg weight. The AME of the diets was determined by the total collection method as previously described by Bourdillon et al. (1990). The AME values were converted to AMEn values using a GE of 8.22 kcal per gram of N as the correction factor (Hill et al., 1958). Heat production (kcal) calculated as 3.866 × oxygen consumed (L) + 1.200 × CO2 expired (L) (Brouwer, 1965). The respiratory quotient (RQ) of each 3-D run was calculated as the ratio of liters of CO2 expired to liters of O2 consumed. Heat increment was calculated by subtracting fasting HP from total HP. A value of 88 kcal/kg BW0.75 (370 kJ/kg BW0.75) per bird per day was used as fasting heat production (Wu et al., 2016). The net energy value was calculated as AME intake minus HI (per bird per day) divided by feed consumed on a DM basis. Total retained energy (RE) was calculated as ME intake minus HP. Total RE in the egg (kcal) was calculated as −19.7 + 1.81 × egg weight (Sibbald, 1979). The RE body was calculated as a total RE minus RE egg. Energy balance data as AME intake, HP, RE and its partition between body and egg production were expressed as kcal per kg BW0.75 per bird per day. Energy values of diets were expressed per kg DM, and energy utilization data were expressed as a percentage (%). Total N retained was calculated as N intake minus N in excreta. Nitrogen balance values were expressed as g/bird/D.
Statistical Analysis
A completely randomized design was used for each parameter in each experiment. Performance, egg quality parameters, energy balance, energy values, energy utilization, and nitrogen balance data were checked for normality using the Shapiro–Wilk test. Then the data were subjected to a one-way ANOVA analysis using PROC GLM and Tukey's multiple-range test of SAS 9.2 (SAS, 2010) to separate means (P < 0.05) when appropriate. The model included the effects of diet and age in experiments 1 and 2 for egg quality parameters and in experiment 3 for calorimetry measurements. Egg quality parameters were measured every 2 wks and averaged with age in the model. Energy balance parameters (at 35 and 44 wks of age) were also averaged with age in the model. Diet by age interactions was not significant, and as such, interactions were dropped from the model.
Results
Hen Performance
Initial body weight measured at the beginning of both experiments 1 and 2 were not different (P > 0.05) as shown in Table 3. Dietary treatment had no effect on body weight change of laying hens in experiments 1 and 2 (P > 0.05). Although feed intake (g/hen/D, as is) remained unchanged by feeding different diets in experiment 1 (P > 0.05), the T2 diet (high NE:AMEn) in experiment 1 and also both T2 diet (medium NE:AMEn) and T3 diet (high NE:AMEn) in experiment 2 decreased FCR (P < 0.01) compared with control (T1). Diets had no effect on %HDP in both experiment 1 and 2 (P > 0.05). Birds fed diets high in NE:AMEn had increased egg weight in both experiments (P < 0.01). Treatment had no effect on higher egg mass values in experiment 1 (P > 0.05). Egg mass was elevated in T2 and T3 as compared with T1 control in experiment 2 (P < 0.05).
Egg Quality Parameters
Age affected all the egg quality parameters in experiments 1 and 2 (P < 0.01), although it had no effect on albumen weight in experiment 1 (P > 0.05) as shown in Table 3. The weight of eggs used for egg measurements was increased with higher NE:AMEn presumably because of increased oil content of diets (P < 0.001). Darker shell color (lower shell color reflectivity) was observed in birds fed the lower NE:AMEn diet (commercial diet) in experiment 1 (P < 0.01). Dietary treatment had no effect on breaking strength, deformation, or shell thickness in either experiment (P > 0.05). Increased level of dietary NE:AMEn resulted in higher albumen (P < 0.001), less yolk (P < 0.001), and less shell (P < 0.05) when expressed as a percentage of egg weight compared with T1 control diets in either experiment. High NE:AMEn diets increased HU (P < 0.001) and yolk color scores of eggs (P < 0.001) in both experiment 1 and HU (P < 0.05) and yolk color of eggs (P < 0.001) in experiment 2.
Energy Partitions of Diets From Indirect Calorimetry Measurements in Experiment 3
Increasing the level of dietary NE:AMEn increased AME intake (P < 0.05) with the same HP and HI (P > 0.05) as shown in Table 5. Feeding hens the same measured NE:AMEn ratio (0.764 in T1 and T2) was unable to change total RE, RE as fat, and RE in body (kcal/kg BW0.75/D) (P > 0.05), but feeding the T3 diet with a high measured NE:AMEn ratio (0.775) increased total RE (P < 0.05), RE as fat (P < 0.05), and RE in body (P < 0.01). Diets with high NE:AMEn ratios (T3) increased the AME (P < 0.001), AMEn (P < 0.001), and NE (P < 0.01) values. The AME:GE changed by feeding different treatments (P < 0.05), but NE:AMEn and NE:AME were the same for all diets (P > 0.05). Different dietary NE:AMEn ratios had no effect on nitrogen balance (P > 0.05).
Table 5.
Effect of diet composition on energy balance, energy values, energy utilization, and N balance in laying hens in experiment 3.
| Diet | 1 | 2 | 3 | SEM | P-value (Diet)1 |
|---|---|---|---|---|---|
| Energy balance, kcal/kg BW0.75/D2 | |||||
| AME intake3 | 169c | 172b,c | 186a,b | 3 | 0.040 |
| HP | 133 | 134 | 135 | 1 | 0.795 |
| HI | 45 | 45 | 46 | 1 | 0.795 |
| RE | |||||
| Total | 36c | 38b,c | 51a | 2 | 0.012 |
| As protein | 25 | 25 | 24 | 1 | 0.913 |
| As fat | 10c | 14b,c | 27a | 3 | 0.021 |
| REegg | 53 | 54 | 52 | 1 | 0.215 |
| REbody | -17c | -15b,c | -1a | 2 | 0.009 |
| RQ | 1.037a | 0.986b,c | 0.982c | 0.006 | <0.001 |
| Energy values (kcal/kg DM) | |||||
| AME | 2,968c | 2,992b,c | 3,129a | 17 | <0.001 |
| AMEn | 2,856c | 2,894b,c | 3,035a | 18 | <0.001 |
| NE | 2,182c | 2,211b,c | 2,352a | 23 | 0.003 |
| Energy utilization | |||||
| AME:GE | 0.759a | 0.718c | 0.732b,c | 0.004 | <0.001 |
| NE:AMEn | 0.764 | 0.764 | 0.775 | 0.005 | 0.759 |
| NE:AME | 0.735 | 0.739 | 0.752 | 0.005 | 0.444 |
| Nitrogen balance (g/b/D) | |||||
| Intake | 2.97 | 3.03 | 2.88 | 0.06 | 0.634 |
| Excreta | 1.72 | 1.82 | 1.68 | 0.04 | 0.279 |
| Retained | 1.25 | 1.21 | 1.20 | 0.05 | 0.918 |
a–cMeans within rows with different superscripts are different at different P-values.
From the analysis of variance with diet and age effects; the age effect was significant for RE egg and RQ (P < 0.05). Diet by age interactions was not significant (P > 0.05).
Each value represents the mean of 2 replicates (at 35 and 44 wk of age) for each treatment (diet) (n = 10) during 3 D respiratory measurements (3 laying hens per calorimetry chambers).
Abbreviations: GE, gross energy; AME as [(FI × GEf) - (E × GEe)]/FI (kcal/kg DM of diet); AMEn as = [AME - [8.22 × (Ni - Ne)]]/FI (kcal/kg DM of diet); where GEf and GEe are the gross energy of feed and excreta (kcal/g DM); FI = feed intake (g DM/D/hen); E = excreta output (g DM/D/hen); 8.22 as nitrogen correction factor (kcal/g); HI, heat increment as HP - FHP (kcal/kg BW0.75/D); HP, heat production (kcal) as 3.866 × oxygen consumed (L) + 1.200 × CO2 expired (L) (Brouwer, 1965); Respiratory quotient (RQ); Net energy (NE) values expressed based on DM of the feed (total collection method); NE calculated as fasting heat production + RE. Total retained energy (RE) calculated as ME intake - HP; RE as protein calculated as total retained N × 6.25 × 5.7; RE as fat calculated as total RE - RE as protein; Total retained energy in egg (RE egg; kcal) calculated as -19.7 + 1.81 × egg weight (Sibbald, 1979); RE body calculated as total RE—RE egg. Total N retained calculated as N intake - N in excreta (g/b/D). Retained N in egg calculated as 1.936 (N% in the egg) × egg mass (Miranda et al., 2015).
Discussion
As the NE:AME ratio increases, HI decreases. Research has confirmed that the NE:AME of EE (fats and oils) is higher than protein (Pirgozliev and Rose, 1999, Carré et al., 2002, Carré et al., 2014, Wu et al., 2019). In work reported by Barzegar et al. (2019a), the NE:AME of EE, starch, and CP was reported to be 104, 78, and 49%, respectively. Recent research has confirmed that NE and NE:AME is positively related to EE (with greater contribution) and negatively related to CP (less contribution) both in broilers (Wu et al., 2019) and laying hens (Barzegar et al., 2019a). However, in the current study, the main difference in the dietary composition (experiment 3) only attributes to various amounts of fat levels in diets which were probably unable to affect HP, HI, and NE:AME.
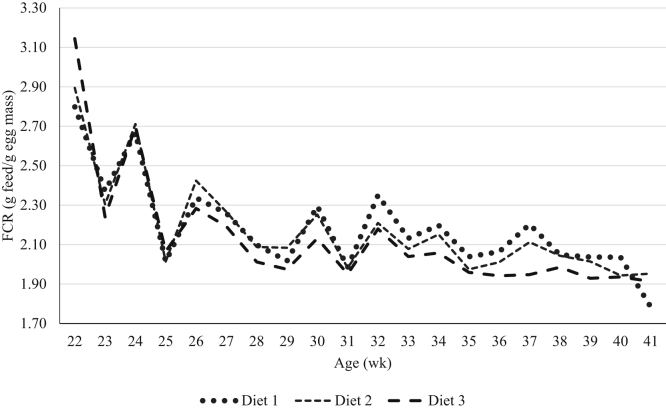
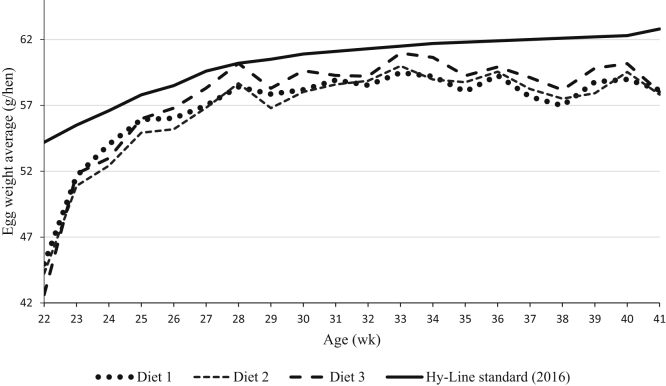
Dietary fat and energy have been shown to reduce feed intake and reduce FCR in laying hens of different ages (Wu et al., 2005a, Wu et al., 2005b). In the current study, in both experiments 1 and 2, FCR and feed intake were decreased and egg mass increased as a result of increasing the NE:AMEn because of higher level of oil in the formulation dietary treatments contained higher NE:AMEn improved the weekly FCR in experiment 2 (Figure 1). Lower feed intake and higher egg production as a result of increased levels of dietary energy and fat have been reported by others (Sell et al., 1987, Pérez-Bonilla et al., 2012). In addition to energy, dietary EE may decrease gastrointestinal nutrient transition time thereby enhancing digestion and absorption of other energy-yielding nutrients resulting in “extra-caloric” metabolic effect of fats (Mateos and Sell, 1980, Mateos and Sell, 1981). Grobas et al. (1999b) observed that an increased level of saturated fatty acid content in diets improved FCR and egg production in laying hens when linoleic acid content was maintained at a constant level. Seasonal variations and temperatures affect feed intake. In the current study, feed intake was higher in experiment 1 (cooler season) compared with experiment 2 (warmer season). Supplemental dietary fat increases the early egg size in laying hens (Grobas et al., 1999a). In addition, the extraordinary egg size is not favorable for the egg industry as it increases the eggshell quality problem and broken eggs. Therefore the poultry nutritionist objective is focused on reducing eggshell problem in particular in the late production cycle. In the current study, birds fed diets with higher EE (higher NE:AMEn ratio) had increased egg size although still less than average egg weight values by Hy-Line (2016) as shown in Figure 2. The increased level of dietary NE:AMEn is associated with higher inclusion of EE with both unsaturated and saturated fatty acids. Linoleic acid availability is a necessity for lipoprotein synthesis in the developing ova (March et al., 1990). The hens fed high NE:AMEn diets in both experiments 1 and 2 with high levels of linoleic acid as shown in Table 4 laid larger eggs with greater egg mass and albumen weight compared with their counterparts fed less linoleic acid. The minimum required linoleic acid for brown laying hens was reported to be 11.5 g/kg of diet to maintain their production (Grobas et al., 1999a). It has been reported that when linoleic acid is greater than requirement, egg weight increases (Pérez-Bonilla et al., 2012).
Figure 1.
Effect of dietary treatment on weekly FCR in experiment 2.
Figure 2.
Different dietary treatments effect on weekly egg weight variations in experiment 2.
The most important internal egg quality parameter is albumen viscosity which is measured by albumen height and HU. Laying hens egg quality and FCR may both be influenced by similar biological processes. Greenhalgh et al. (2017) observed that poor FCR laying hens had heavier yolks; however, the improved FCR birds had greater albumen weight, albumen height, and HU. This study researchers stated that probably the metabolic rates and nutrient utilization are different between high and low FCR birds where poor FCR birds tend to retain most part of dietary lipids as cholesterol resulting in heavier egg yolk and yolk:albumen ratio. Eggs with the heaviest yolks and the largest yolk:albumen ratio possibly contain the highest amount of cholesterol (Hussein et al., 1993). In the current study, the higher HU and albumen weight were observed in laying hens with improved FCR, whereas the laying hens with poor FCR showed the higher yolk weight and yolk:albumen ratio.
The high NE:AMEn diet with more fat inclusion diets showed better yolk color in both experiments 1 and 2. Yolk color is mainly determined by xanthophyll, the pigments responsible for yolk color which are known as fat-soluble nutrients. Higher fat inclusion provides a better situation for these pigments absorption in hens guts (Lázaro et al., 2003, Pirgozliev et al., 2010).
Eggshell brown color is important for retailers and consumers. However, there is no correlation between eggshell color and other external or internal egg quality parameters (Yang et al., 2009). The birds fed diet 2 in experiment 1 of the present study produced bigger eggs with the lighter shell color as they deposited the brown eggshell pigments on the bigger shell surface. Odabaşi et al. (2007) reported that egg size as the main factor which affects eggshell color brownness negatively when laying hens get older and laid bigger eggs.
In experiment, 3 it was observed that hens fed diets with different dietary compositions retained a constant amount of energy in the egg (REegg). However, hens fed diets with lower levels of NE:AMEn (T1 and T2) retained less RE in the body to maintain egg production. Farrell (1975) confirmed that regardless of energy balance status, the laying hens prioritize to retain dietary energy to meet protein-synthesis demands for egg production or body maintenance. The AME:GE decreased in parallel with an increased level of fat in dietary treatments in experiment 3. Wiseman et al. (1986) observed that the higher inclusion rate of fat decreased the dietary energy as the contribution of added dietary fat to AMEn of diet was curvilinear in laying hens. The highest AMEn value of using different levels of specific fat was observed when it was included as 3% of laying hen diets (Mateos et al., 1980).
Nitrogen intake was the same for the birds which were fed dietary treatments containing almost the same amount of CP with balanced levels of essential amino acids in experiment 3. Those birds also retained the same amount of N probably to maintain their egg production and body requirements. Different studies confirmed that N intake were correlated with protein intake while decreased dietary CP content reduced the N in excreta (Summers, 1993, Meluzzi et al., 2001, Ishibashi and Yonemochi, 2003).
The NE:AME ratios are different for dietary nutrients with higher values for fat compared with starch and CP. As in experiment 2, the diets in experiment 3 contained approximately the same amount of CP; therefore, the increased dietary NE and NE:AMEn are mainly attributed to the added EE which is known to spare protein and amino acids toward productive purposes and nutrient utilization improvement through extra caloric effect of supplemental fat. In the laying hen diets the extra caloric value of digested fat increased 15% compared with dietary carbohydrates (van der Klis and Fledderus, 2007, van der Klis et al., 2010).
From the total RE in the body, the protein retains in the body (REp) more efficient compared with the fat (REf). Lopez et al. (2008) calculated REp:RE ratio higher than REf:RE (62 vs. 38%) in laying hens when fed commercial diets. In the current study, those REp:RE and REf:RE ratios for T1 (normal commercial diet) were 71 and 29%, whereas T3 (higher EE inclusion) had lower REp:RE and improved REf:RE efficiency (48 vs. 52%).
The use of nutrients for energy affects the RQ values. The lowest RQ values were observed with increased dietary EE level (high NE:AMEn diet). This is likely a result of fat being used as the main source of energy. As in the study of Barzegar et al. (2019a), the lowest values RQ values observed in laying hens fed high-fat diets while the highest values were in birds fed the high starch diets. MacLeod (1990) also reported the lower RQ values in growing broilers fed diets containing both high fat and protein compared with higher RQ values when carbohydrates were supplied as the main ingredients of the diets.
Conclusion
Three experiments were conducted in the current study to examine the performance and egg quality of layers fed diets with different NE:AMEn ratios formulated using predicted NE values. Calorimeter chambers were used to compare predicted vs. actual measured NE values of diets. The results demonstrated that diets with a high NE:AMEn (NE-based diets) were beneficial in terms of improved nutrient utilization and lower mobilization of energy from body reserves for egg production. High NE:AMEn diets increased egg mass and decreased FCR and also enhanced the egg quality external and internal parameters of hens at different ages. The predicted vs. measured NE and NE:AMEn of diets formulated using predicted NE values of ingredients, followed trends from low to high with the high oil diet measuring highest values as expected. The results show that formulation on an NE basis may offer improvements in performance and egg quality. The relative value of ingredients will change with NE formulation and this that may offer economic incentives for its use.
Acknowledgments
Authors acknowledge Poultry CRC and the Australian Egg Corporation Limited for supporting this study.
Conflict of interest statement: The authors did not provide a conflict of interest statement.
References
- AOAC . 20th ed. AOAC International; Rockville, MD: 2016. Official Methods of Analysis. [Google Scholar]
- Barzegar S., Wu S., Noblet J., Choct M., Swick R. Energy efficiency and net energy prediction of feed in laying hens. Poult. Sci. 2019;98:5746–5758. doi: 10.3382/ps/pez362. [DOI] [PubMed] [Google Scholar]
- Barzegar S., Wu S., Noblet J., Choct M., Swick R. Metabolizable energy of corn, soybean meal and wheat for laying hens. Poult. Sci. 2019;98:5876–5882. doi: 10.3382/ps/pez333. [DOI] [PubMed] [Google Scholar]
- Bourdillon A., Carré B., Conan L., Francesch M., Fuentes M., Huyghebaert G., Janssen W., Leclercq B., Lessire M., McNab J. European reference method of in vivo determination of metabolisable energy in poultry: reproducibility, effect of age, comparison with predicted values. Br. Poult. Sci. 1990;31:567–576. doi: 10.1080/00071669008417288. [DOI] [PubMed] [Google Scholar]
- Brouwer E. Report of Sub-committee on Constants and Factors. Proc. Symp. Energy. Metab. 1965;3:441–443. Academic Press. London, UK. [Google Scholar]
- Carré B., Lessire M., Juin H. Development of the net energy system for broilers. Proc. East. Nutr. Conf. 2002;38:140–149. [Google Scholar]
- Carré B., Lessire M., Juin H. Prediction of the net energy value of broiler diets. Animal. 2014;8:1395–1401. doi: 10.1017/S175173111400130X. [DOI] [PubMed] [Google Scholar]
- Chudy A., Souffrant W., Kuhla S., Peters H. Energy and protein (AA) metabolism of high productive laying hens in dependence on exogenous factors. Publ. Eur. Assoc. Anim. Prod. 2003;109:339–344. [Google Scholar]
- Davi E. Official Methods of Analysis. 17th ed. AOAC International; Gaithersburg, MD: 2000. Fat (total, saturated, and unsaturated) in foods: method 996 06. [Google Scholar]
- Cozannet P., Lessire M., Gady C., Metayer J., Primot Y., Skiba F., Noblet J. Energy value of wheat dried distillers grains with solubles in roosters, broilers, layers, and turkeys. Poult. Sci. 2010;89:2230–2241. doi: 10.3382/ps.2010-00833. [DOI] [PubMed] [Google Scholar]
- De Groote G. A comparison of a new net energy system with the metabolisable energy system in broiler diet formulation, performance and profitability. Br. Poult. Sci. 1974;15:75–79. [Google Scholar]
- DSM . DSM; Basel, Switzerland: 2008. Yolk Color Fan. [Google Scholar]
- Farrell D. A comparison of the energy metabolism of two breeds of hens and their gross using respiration calorimetry. Br. Poult. Sci. 1975;16:103–113. doi: 10.1080/00071667508416168. [DOI] [PubMed] [Google Scholar]
- Greenhalgh S., Akter Y., Nolan B., O’shea C. Investigation of variation in feed efficiency and egg quality in laying hens. Proc. Aust. Poult. Sci. Symp. 2017;28:97–100. [Google Scholar]
- Grobas S., Mendez J., De Blas C., Mateos G. Influence of dietary energy, supplemental fat and linoleic acid concentration on performance of laying hens at two ages. Br. Poult. Sci. 1999;40:681–687. doi: 10.1080/00071669987089. [DOI] [PubMed] [Google Scholar]
- Grobas S., Mendez J., De Blas C., Mateos G. Laying hen productivity as affected by energy, supplemental fat, and linoleic acid concentration of the diet. Poult. Sci. 1999;78:1542–1551. doi: 10.1093/ps/78.11.1542. [DOI] [PubMed] [Google Scholar]
- Hill F., Anderson D.L. Comparison of metabolizable energy and productive energy determinations with growing chicks. J. Nutr. 1958;64:587–603. doi: 10.1093/jn/64.4.587. [DOI] [PubMed] [Google Scholar]
- Hussein S., Harms R., Janky D. Research note: effect of age on the yolk to albumen ratio in chicken eggs. Poult. Sci. 1993;72:594–597. doi: 10.3382/ps.0720594. [DOI] [PubMed] [Google Scholar]
- Hy-Line Hy-Line Brown Commercial Layers 2016 - Management Guide. 2016. http://www.specialisedbreeders.com.au/wp-content/uploads/2016/04/BRN-COM-AUS.pdf Accessed Feb. 2020.
- Ishibashi T., Yonemochi C. Amino acid nutrition in egg production industry. Anim. Sci. J. 2003;74:457–469. [Google Scholar]
- Just A. The net energy value of balanced diets for growing pigs. Livest. Prod. Sci. 1982;8:541–555. [Google Scholar]
- Koh K., Macleod M. Effects of ambient temperature on heat increment of feeding and energy retention in growing broilers maintained at different food intakes. Br. Poult. Sci. 1999;40:511–516. doi: 10.1080/00071669987287. [DOI] [PubMed] [Google Scholar]
- Lázaro R., Garcia M., Aranibar M., Mateos G. Effect of enzyme addition to wheat-, barley-and rye-based diets on nutrient digestibility and performance of laying hens. Br. Poult. Sci. 2003;44:256–265. doi: 10.1080/0007166031000085616. [DOI] [PubMed] [Google Scholar]
- Lopez G., Leeson S. Aspects of energy metabolism and energy partitioning in broiler chickens. In: France E.K.J., editor. Mathematical Modelling in Animal Nutrition. CABI; Wallingford, UK: 2008. pp. 339–352. [Google Scholar]
- MacLeod M. Energy and nitrogen intake, expenditure and retention at 20 in growing fowl given diets with a wide range of energy and protein contents. Br. J. Nutr. 1990;64:625–637. doi: 10.1079/bjn19900066. [DOI] [PubMed] [Google Scholar]
- March B., Macmillan C. Linoleic acid as a mediator of egg size. Poult. Sci. 1990;69:634–639. [Google Scholar]
- Mateos G., Sell J. Influence of graded levels of fat on utilization of pure carbohydrate by the laying hen. J. Nutr. 1980;110:1894–1903. doi: 10.1093/jn/110.9.1894. [DOI] [PubMed] [Google Scholar]
- Mateos G., Sell J. Nature of the extrametabolic effect of supplemental fat used in semipurified diets for laying hens. Poult. Sci. 1981;60:1925–1930. [Google Scholar]
- Meluzzi A., Sirri F., Tallarico N., Franchini A. Nitrogen retention and performance of brown laying hens on diets with different protein content and constant concentration of amino acids and energy. Br. Poult. Sci. 2001;42:213–217. doi: 10.1080/00071660120048474. [DOI] [PubMed] [Google Scholar]
- Miranda J.M., Anton X., Redondo-Valbuena C., Roca-Saavedra P., Rodriguez J.A., Lamas A., Franco C.M., Cepeda A. Egg and egg-derived foods: effects on human health and use as functional foods. Nutrients. 2015;7:706–729. doi: 10.3390/nu7010706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NHMRC . Australian Government Publ. Service; 2013. Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. [Google Scholar]
- Noblet J., Fortune H., Shi X., Dubois S. Prediction of net energy value of feeds for growing pigs. J. Anim. Sci. 1994;72:344–354. doi: 10.2527/1994.722344x. [DOI] [PubMed] [Google Scholar]
- Noblet J., Van Milgen J., Dubois S. Utilisation of metabolisable energy of feeds in pigs and poultry: interest of net energy systems? Proc. Aust. Poult. Sci. Symp. 2010;21:26–35. [Google Scholar]
- Odabaşi A., Miles R., Balaban M., Portier K. Changes in brown eggshell color as the hen ages. Poult. Sci. 2007;86:356–363. doi: 10.1093/ps/86.2.356. [DOI] [PubMed] [Google Scholar]
- Pérez-Bonilla A., Novoa S., García J., Mohiti-Asli M., Frikha M., Mateos G. Effects of energy concentration of the diet on productive performance and egg quality of brown egg-laying hens differing in initial body weight. Poult. Sci. 2012;91:3156–3166. doi: 10.3382/ps.2012-02526. [DOI] [PubMed] [Google Scholar]
- Pirgozliev V., Bedford M., Acamovic T. Effect of dietary xylanase on energy, amino acid and mineral metabolism, and egg production and quality in laying hens. Br. Poult. Sci. 2010;51:639–647. doi: 10.1080/00071668.2010.514325. [DOI] [PubMed] [Google Scholar]
- Pirgozliev V., Rose S.P. Net energy systems for poultry feeds: a quantitative review. World Poult. Sci. J. 1999;55:23–36. [Google Scholar]
- Sakomura N., Resende K., Fernandes J., Rabelo C., Longo F., Neme R. Net energy requirement models for broiler breeders, laying hens and broilers. Proc. Eur. Symp. Poult. Nutr. 2005;15:459–461. Balatonfiired: Hungary. [Google Scholar]
- Sakomura N.K. Modeling energy utilization in broiler breeders, laying hens and broilers. Revista Brasileira de Ciência Avícola. 2004;6:1–11. [Google Scholar]
- Samiullah S., Omar A.S., Roberts J., Chousalkar K. Effect of production system and flock age on eggshell and egg internal quality measurements. Poult. Sci. 2016;96:246–258. doi: 10.3382/ps/pew289. [DOI] [PubMed] [Google Scholar]
- SAS . statisticsSAS Institute; Cary, NC (EUA): 2010. SAS User's Guide 9.2. [Google Scholar]
- Sell J., Angel C., Escribano F. Influence of supplemental fat on weights of eggs and yolks during early egg production. Poult. Sci. 1987;66:1807–1812. doi: 10.3382/ps.0661807. [DOI] [PubMed] [Google Scholar]
- Sibbald I. The gross energy of avian eggs. Poult. Sci. 1979;58:404–409. [Google Scholar]
- Summers J. Reducing nitrogen excretion of the laying hen by feeding lower crude rotein diets. Poult. Sci. 1993;72:1473–1478. doi: 10.3382/ps.0721473. [DOI] [PubMed] [Google Scholar]
- Swick R.A., Wu S.-B., Zuo J., Rodgers N., Barekatain M.R., Choct M. Implications and development of a net energy system for broilers. Anim. Prod. Sci. 2013;53:1231–1237. [Google Scholar]
- van der Klis J., Fledderus J. Evaluation of raw materials for poultry: what’s up? Proc. Eur. Symp. Poult. Nutr. 2007;16:123–130. Strasbourg, France. [Google Scholar]
- van der Klis J., Kwakernaak C., Jansman A., Blok M. Energy in poultry diets: adjusted AME or net energy? Proc. Aust. Poult. Sci. Symp. 2010;21:44–49. [Google Scholar]
- Wiseman J., Cole D., Perry F., Vernon B., Cooke B. Apparent metabolisable energy values of fats for broiler chicks. Br. Poult. Sci. 1986;27:561–576. doi: 10.1080/00071668608416915. [DOI] [PubMed] [Google Scholar]
- Wu G., Bryant M., Voitle R., Roland Sr D. Effect of dietary energy on performance and egg composition of Bovans White and Dekalb White hens during phase I. Poult. Sci. 2005;84:1610–1615. doi: 10.1093/ps/84.10.1610. [DOI] [PubMed] [Google Scholar]
- Wu G., Bryant M., Voitle R., Roland Sr D. Effects of β-mannanase in corn-soy diets on commercial leghorns in second-cycle hens. Poult. Sci. 2005;84:894–897. doi: 10.1093/ps/84.6.894. [DOI] [PubMed] [Google Scholar]
- Wu S.-B., Swick R.A., Noblet J., Rodgers N., Cadogan D., Choct M. Net energy prediction and energy efficiency of feed for broiler chickens. Poult. Sci. 2019;98:1222–1234. doi: 10.3382/ps/pey442. [DOI] [PubMed] [Google Scholar]
- Wu S., Huaming Y., Zhibin B., Xiaogang Y., Yumin Z. Heat production estimated from fasting layer hens at peak lay. World Poult. Congr. 2016;25:197. Beijing, China. [Google Scholar]
- Yang H., Wang Z., Lu J. Study on the relationship between eggshell colors and egg quality as well as shell ultrastructure in Yangzhou chicken. Afr. J. Biotech. 2009;8:2898–2902. [Google Scholar]
- Zhou W., Yamamoto S. Effects of environmental temperature and heat production due to food intake on abdominal temperature, shank skin temperature and respiration rate of broilers. Br. Poult. Sci. 1997;38:107–114. doi: 10.1080/00071669708417949. [DOI] [PubMed] [Google Scholar]