Abstract
This study was designed to determine effects of eggshell temperature (EST) pattern in week 2 and week 3 of incubation on tibia development of broiler chickens at slaughter age. A total of 468 Ross 308 eggs were incubated at an EST of 37.8°C from incubation day (E) 0 to E7. Thereafter, a 2 × 2 factorial arrangement with 2 EST (37.8°C and 38.9°C) from E8 to E14 and 2 EST (36.7°C and 37.8°C) from E15 till hatch was applied. After hatching, chickens were reared until slaughter age with the 4 EST treatments and 8 replicates per treatment. At day 41 and 42, one male chicken per replicate per day was selected, and hock burn and food pad dermatitis were scored. Rotated tibia, tibia dyschondroplasia, epiphyseal plate abnormalities, bacterial chondronecrosis with osteomyelitis, and epiphysiolysis were assessed. Tibia weight, length, thickness, head thickness, and robusticity index were determined. X-ray analyses (osseous volume, pore volume, total volume, volume fraction, mineral content, and mineral density) and a 3-point bending test (ultimate strength, yield strength, stiffness, energy to fracture, and elastic modulus) were performed. A high EST (38.9°C) in week 2 of incubation, followed by a normal EST (37.8°C) in week 3 resulted in higher mineral content (P = 0.001), mineral density (P = 0.002), ultimate strength (P = 0.04), yield strength (P = 0.03), and stiffness (P = 0.05) compared with the other 3 EST groups (week 2 × week 3 interaction). A high EST (38.9°C) in week 2 of incubation, regardless of the EST in week 3, resulted in a higher tibia weight (P < 0.001), thickness (P = 0.05), osseous volume (P < 0.001), and total volume (P < 0.001) than a normal EST (37.8°C). It can be concluded that 1.1°C higher EST than normal in week 2 of incubation appears to stimulate tibia morphological, biophysical, and mechanical characteristics of broiler chickens at slaughter age. Additionally, a 1.1°C lower EST in week 3 of incubation appears to have negative effects on tibia characteristics, particularly in interaction with the EST in week 2 of incubation.
Key words: eggshell temperature, incubation, tibia, leg health, broiler chickens
Introduction
Leg health has been demonstrated to be suboptimal in fast-growing broiler chickens (Bessei, 2006, Sherlock et al., 2010). The underlying reason might be related to a developmental imbalance between a high growth rate and immaturity of bones and joints (Kestin et al., 1992, Kestin et al., 2001). Suboptimal leg health can cause pain and may negatively affect natural locomotion-related behaviors of broiler chickens, such as accessing water and feed, especially in the last weeks of their life (Bessei, 2006, Gocsik et al., 2017). Moreover, leg problems can cause financial losses because of higher mortality, lower slaughter revenues, and increased rejections at slaughter plants (Kestin et al., 1999, Mench, 2004).
One factor that might play a role in bone development is incubation temperature. An optimal temperature for embryo development throughout incubation has been determined as 37.8°C (Lourens et al., 2005). A lower or higher temperature than this optimum might influence the quality of hatchlings (Wilson, 1991, French, 1997, Tona et al., 2005) and embryonic bone development as well. Bone development and growth plate differentiation begins during incubation and incubation temperature has been demonstrated to affect bone development and later life leg health of broiler chickens (Hammond et al., 2007, Oviedo-Rondón et al., 2008a, Oviedo-Rondón et al., 2008b, Oviedo-Rondón et al., 2009, Shim and Pesti, 2011, Van der Pol et al., 2014). Effects of incubation temperature on embryonic bone development and later life leg health might be related to the speed of bone mineralization during incubation. Embryonic bone development starts with cartilage formation in the first week of incubation (Nakane and Tsudzuki, 1999, Atalgin and Kürtül, 2009), which is followed by a rapid increase in mineralization from the second week of incubation onward (Oviedo-Rondón et al., 2008b). This is because of growth plate differentiation (Ballock and O'keefe, 2003, Hammond et al., 2007), which reaches the highest level just before hatch till a few days posthatch (Applegate and Lilburn, 2002, Yalçin et al., 2007, Oviedo-Rondón et al., 2008b).
A temperature of 38.0°C throughout incubation resulted in longer tibia in broiler chickens at slaughter age compared with incubation temperatures of 36.0°C and 37.0°C (Oviedo-Rondón et al., 2008b). Yalçin et al. (2007) concluded that both low (36.9°C) or high (39.6°C) incubation temperatures from incubation day (E) 10 until E18 resulted in lower tibia weights at slaughter age compared with a control temperature (37.8°C). Oviedo-Rondón et al. (2009) found comparable results at slaughter age when applying a high eggshell temperature (EST, 38.9°C) during the last 4 D of incubation compared with an EST of 36.9°C. Van der Pol et al. (2014) observed that a high EST (38.6°C) throughout incubation appears to result in lower tibia, femur, and metatarsus lengths at hatch compared with an EST of 36.9°C and 37.8°C. Oznurlu et al. (2016) demonstrated that a 1.0°C higher incubation temperature (38.8°C vs. 37.8°C) from E10 onward negatively affected tibia characteristics, including growth plate development, at different sampling days before and at hatch.
These different results among studies might be related to the moment, duration, and level of the incubation temperature that was applied. Moreover, studies differed in using incubation temperature or EST, which might result in differences in actual embryo temperature (Meijerhof and van Beek, 1993).
It has been suggested that a higher incubation temperature (41.0°C) than normal (37.8°C) can stimulate bone development in general and the ossification process (E8 to E14) in particular (Aygun and Narinc, 2016). Contrary to the potential beneficial effects of a higher incubation temperature in the second week of incubation, a higher temperature (39.0°C) than normal (37.8°C) in the last week of incubation has been shown to negatively affect chicken development in general and leg bone health in later life in particular (Oviedo-Rondón et al., 2009). Based on these findings, it can be hypothesized that a combination of a higher temperature than normal in the second week of incubation combined with a lower temperature in the last week of incubation might result in most optimal bone development and later life leg health.
The objectives of this study were to investigate effects of a combination of EST in week 2 (37.8°C or 38.9°C) and EST in week 3 (36.7°C or 37.8°C) of incubation on 1) tibia characteristics, 2) locomotion during rearing, and 3) leg disorders at slaughter age in fast-growing broiler chickens.
Materials and methods
Experimental Design
The experiment was setup as a 2 × 2 factorial arrangement with 2 EST (37.8°C or 38.9°C) from E8 to E14 and 2 EST (36.7°C or 37.8°C) from E15 till hatch. From E0 to E7 of incubation, EST was maintained at 37.8°C for all eggs. After hatching, chickens were reared in a completely randomized block design until slaughter age and leg bone measurements were performed after slaughtering. All procedures in this study were approved by the Governmental Commission on Animal Experiments, The Hague, The Netherlands; approval number: 2016.W-0087.001.
Animals, Incubation, Rearing, and Housing Management
A total of 468 Ross 308 eggs from a 44-week-old breeder flock were obtained from a commercial hatchery (Lagerwey, Lunteren, The Netherlands). All eggs were selected on weight within 3 weight classes: 62.0 to 62.9 g (n = 156), 63.0 to 63.9 g (n = 156), and 64.0 to 64.9 g (n = 156) and stored at 20.0°C for 48 h. Thereafter, all eggs were transported from the hatchery to the research facility of Wageningen University and Research (Wageningen, The Netherlands). At the research facility, all eggs were placed in 1 incubator with a capacity of 4,800 eggs (HatchTech Incubation Technology, B.V., Veenendaal, The Netherlands) for the first 7 D of incubation. Eggs were equally divided over the 4 treatment groups (n = 117/treatment group) and placed on 8 plastic trays (58 or 59 eggs per tray). These trays were placed in the middle of the incubator. Each tray contained 4 EST sensors (NTC Thermistors: type DC 95; Thermometrics, Somerset, UK) which were attached to 4 individual eggs, divided over different trays within the incubator or climate respiration chambers (CRC). All sensors were placed at the equator of the chosen eggs by using a heat conducting paste (Dow Corning 340 Heat Sink Compound, Dow Corning, MI) and a small piece of tape (2 × 2 cm). The incubator temperature was continuously adjusted based on the median temperature of the 4 EST sensors to maintain an EST of 37.8°C. Throughout incubation, variation in EST was less than 0.1°C. Relative humidity of incubators and CRCs was maintained between 50 and 65% throughout incubation. Eggs were turned every 30 min at an angle of 90° and not exposed to light during incubation.
At E8, all eggs were candled, and fertile eggs were divided over the same 2 HatchTech incubators, and 2 small CRC. The CRC had a capacity of 550 eggs. Eggs of the different weight classes were divided equally over the 2 incubators and the 2 CRCs. One incubator and 1 CRC were set at an EST of 37.8°C, and the other ones were set at an EST of 38.9°C. Within each incubator and CRC, 5 EST sensors (Pt-100, Sensor Data BV, Rijswijk, The Netherlands) were attached to 5 randomly chosen eggs, as described above.
At E15, all eggs were candled again, and infertile eggs or eggs lacking a vital embryo were removed, and a break-out analysis was performed (data not included in this article). All eggs containing a vital embryo were redistributed over the same 2 incubators and 2 CRCs, after which 1 incubator and 1 CRC were set at an EST of 36.7°C, and the other incubator and CRC were set at an EST of 37.8°C. Eggshell temperature was maintained based on the median temperature of the 4 EST sensors as described above. The combination of EST in week 2 and week 3 resulted in a 2 × 2 factorial arrangement with 4 treatments in total: EST 37.8 × 36.7°C; EST 37.8 × 37.8°C; EST 38.9 × 36.7°C; EST 38.9 × 37.8°C (week 2 × week 3, respectively).
At E18, all eggs were candled again, and eggs containing a vital embryo were transferred from the trays to hatching baskets. Within 12 h after emergence from the eggshell, chickens were collected, individually weighed, and numbered by using a paw-ring and transferred to a separate room, where they were placed in specially designed baskets with ad libitum access to feed and water till all chickens had hatched (HatchCare system, Hatchtech BV, Veenendaal, the Netherlands).
After all chickens had hatched, 40 male and 40 female first-grade chickens per treatment were feather-sexed and transferred to 2 adjacent houses, each having 16 floor pens (2 × 1 m; 10 chickens per pen), containing saw dust as litter. Chickens were vaccinated against infectious bronchitis (eye drop; MSD Animal Health, Boxmeer, The Netherlands) at day 0 and against Newcastle disease (Clone 30; eye drop, MSD Animal Health, Boxmeer, The Netherlands) at day 11. Each pen contained 5 male and 5 female chickens from the same treatment. Both houses were divided into 4 blocks, and the 4 treatments were randomly divided within each block. Both houses were identical and climate controlled with continuous light from day 0 till day 3 and 16 h of light and 8 h of darkness thereafter. The chickens were raised from day 1 to 42 with ad libitum access to feed and water. All chickens obtained the same pelleted available starter diet (MEbroiler = 2,850 kcal/kg; CP = 220 g/kg; apparent dLys = 11.81 g/kg) from day 0 until 10 of age, a grower diet (MEbroiler = 2,952 kcal/kg; CP = 209.7 g/kg; apparent dLys = 12.52 g/kg) from day 10 until 28 of age, and a finisher diet (MEbroiler = 2,999 kcal/kg; CP = 199.8 g/kg; apparent dLys = 11.14 g/kg) from day 28 until 42 of age (Reseach Diet Service, Wijk bij Duurstede, the Netherlands; diets optimized according to CVB, 2012).
Data Collection, Sampling, and Measurements
At day 28, 35, and 39, the gait score of all individual broiler chickens (n = 320) was measured, using the gait scoring method, described by Kestin et al. (1992) with a range from 0 (normal locomotion) to 5 (unable to stand). At day 41 and 42, one male chicken per pen per day was selected randomly to determine leg bone characteristics (8 chickens per treatment per slaughtering day, 64 chickens in total). After weighing the chicken, food pad dermatitis (FPD) and hock burns (HB) were scored. Hock burns was scored as 0 (not affected), 1 (color changes), 2 (minor lesions), or 3 (severe lesions). Food pad dermatitis was scored as 0 (no lesions), 1 (mild lesion), or 2 (severe lesion). Thereafter, chickens were stunned, cut, and bleeded. The left leg of each chicken was assessed by a veterinarian on rotated tibia (RT), tibia dyschondroplasia (TD), bacterial chondronecrosis with osteomyelitis (BCO), epiphyseal plate abnormalities (EPA), and epiphysiolysis (EPI). All abnormalities were scored in the range of 0 (no abnormalities), 1 (minor abnormality), or 2 (severe abnormality).
Right legs were deboned and tibias were obtained, packed, and frozen at −20°C. After thawing, tibia weight, proximal length, lateral cortex thickness, and femoral and metatarsal side proximal head thickness were measured, using a digital caliper. Robusticity index was calculated using the formula of Riesenfeld (1972):
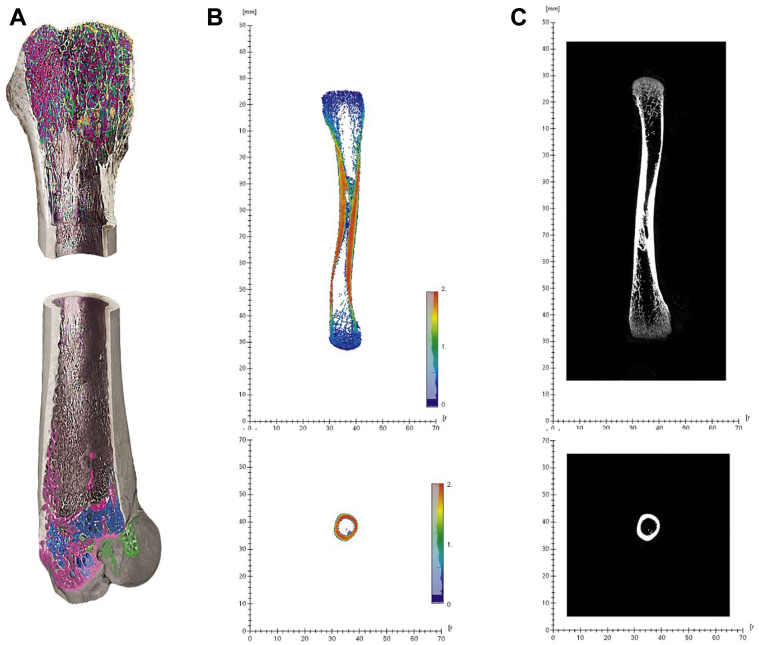
Tibia osseous volume, tibia pore volume, tibia total volume (osseous volume + pore volume), tibia volume fraction (osseous volume/total volume), tibia mineral content, and tibia mineral density were analyzed on individual tibia, using a GE Phoenix 3D X-ray microfocus CT scanner (General Electric Company, Boston, MA) (method described by Bouxsein et al., 2010) (Figure 1).
Figure 1.
Illustration of scanned bone by 3D Micro-CT X-ray and visualised in Avizo 3D viewer software. (A) Three-dimensional tibia inner view scanned by 3D Micro-CT X-ray scanner. Different colors represent different densities of bone materials and pores. (B) Two-dimensional colored tibia layer view (side) and the layer of middle point of the bone (top). Color scale represent the mineralization areas of bone from blue (less mineralization, 0) to red (more mineralization, 2). (C) Two dimensional black and white (gray scale) tibia layer view (side) and the layer of middle point of the bone (top). Shades of gray represent the mineralization areas of bone from dark gray (less mineralization) to white (more mineralization).
The same tibias, used for X-ray measures, were subjected to a 3-point bending test (method described by Jungmann et al., 2007), using an Instron electromechanical universal testing machine (Instron, Norwood, MA). Ultimate stress (maximal load of breaking point) data were used as the tibia ultimate strength; yield point (reached yield load just before the angle has changed on slope) data were used as the tibia yield strength; the slope of the selected linear part of the curve data was used as the tibia stiffness; the area under the curve of selected region data was used as the tibia energy to fracture. Elastic modulus (GPa) (Novitskaya et al., 2011) was calculated using the following formula of Turner and Burr (1993):
where, E is the elastic modulus (GPa), N is the maximal load (N), S is the span between bending fixtures (mm), T is the tibia thickness (mm), L is the tibia length (mm), and δ is the maximum deflection (mm) at the midpoint of the bone.
Statistical Analysis
All statistical analyses were performed in SAS (Version 9.4, July 2013, SAS Institute Inc., Cary, NC). Body weight, all tibia morphological, biophysical, and mechanical characteristics, and gait score were subjected to mixed model analysis, using the PROC MIXED procedure.
The statistical model used was
where, Y = dependent variable, μ = overall mean, ESTwk 2 = eggshell temperature of week 2 (37.8°C or 38.9°C), ESTwk 3 = eggshell temperature of week 3 (36.7°C or 37.8°C), ESTwk 2 × ESTwk 3 = interaction between eggshell temperature of week 2 and week 3, house = rearing house (1, 2), Ɛ = residual error.
Pen was used as the experimental unit for all analyses. Block in the house was used as a random effect. Body weight at slaughter age was added to the model as a covariable for tibia characteristics. Model assumptions were approved at both means and residuals. Non-normal distributed data were transformed using a log-transformation before analyses. Results are provided as LSmeans ± SEM. When multiple comparisons were performed, the level of significance was corrected, using Bonferroni.
Leg disorders (RT, TD, HB, FPD, EPA, BCO, and EPI) were subjected to generalized linear mixed model analysis, using the PROC GLIMMIX procedure, using the same model. Variables of HB, RT, and TD were analyzed at binary level (present or not); variables of FPD, EPA, BCO, and EPI were analyzed at multinomial level. Effects were considered to be significant at P ≤ 0.05.
Tibia morphological, biophysical, and mechanical characteristics were subjected to correlation analysis, using the PROC CORR procedure, to investigate whether or not a relationship among these characteristics exists.
Results
General Hatching Data
Hatchability of fertile eggs was on average 96.7% and not affected by EST in week 2 or week 3 of incubation. Furthermore, BW (45.9 g on average) and yolk free body mass (40.2 g on average) were not affected by EST in week 2 or week 3 of incubation.
Tibia Morphological Characteristics
No interaction effects between EST in week 2 and week 3 were found on tibia morphological characteristics. Main effects were found of EST in week 2 on tibia weight, lateral cortex thickness, metatarsal tibia head thickness, and robusticity index (Table 1). Chickens of the high EST (38.9°C) in week 2 had a higher tibia weight (Δ = 0.68 g; P < 0.001), lateral cortex thickness (Δ = 0.04 cm; P = 0.05), metatarsal side proximal tibia head thickness (Δ = 0.05 cm; P = 0.05), and lower robusticity index (Δ = 0.04 cm/g; P < 0.001) compared with the normal EST (37.8°C) in week 2. No main effects of EST in week 3 were found on tibia morphological parameters.
Table 1.
Effects of eggshell temperature in week 2 and week 3 of incubation on tibia morphological characteristics at day 41 or 42 of age in male broiler chickens.
| Variable | Tibia weight (g) | Proximal tibia length (cm) | Lateral tibia cortex thickness (cm) | Femoral side proximal tibia head thickness (cm) | Metatarsal side proximal tibia head thickness (cm) | Robusticity index (cm/g) |
|---|---|---|---|---|---|---|
| EST week 2 | ||||||
| 37.8°C | 16.71b | 13.55 | 1.25b | 3.22 | 3.02b | 0.82a |
| 38.9°C | 17.39a | 13.60 | 1.29a | 3.25 | 3.07a | 0.78b |
| SEM | 0.11 | 0.12 | 0.02 | 0.01 | 0.02 | 0.01 |
| EST week 3 | ||||||
| 36.7°C | 17.08 | 13.58 | 1.26 | 3.21 | 3.03 | 0.79 |
| 37.8°C | 17.03 | 13.57 | 1.28 | 3.25 | 3.06 | 0.80 |
| SEM | 0.11 | 0.12 | 0.02 | 0.01 | 0.02 | 0.01 |
| EST week 2 × week 3 | ||||||
| 37.8 × 36.7°C | 16.85 | 13.70 | 1.26 | 3.23 | 3.03 | 0.82 |
| 37.8 × 37.8°C | 16.58 | 13.40 | 1.24 | 3.21 | 3.02 | 0.81 |
| 38.9 × 36.7°C | 17.31 | 13.46 | 1.28 | 3.21 | 3.04 | 0.77 |
| 38.9 × 37.8°C | 17.47 | 13.74 | 1.31 | 3.29 | 3.09 | 0.79 |
| SEM | 0.15 | 0.17 | 0.02 | 0.02 | 0.02 | 0.01 |
| P-values | ||||||
| EST week 2 | <0.001 | 0.76 | 0.05 | 0.06 | 0.05 | <0.001 |
| EST week 3 | 0.71 | 0.96 | 0.57 | 0.11 | 0.25 | 0.54 |
| EST week 2 × week 3 | 0.16 | 0.10 | 0.26 | 0.06 | 0.15 | 0.16 |
a, bValues within a column and factor lacking a common superscript differ (P ≤ 0.05).
Abbreviations: EST, eggshell temperature.
Tibia Biophysical Characteristics
Interaction effects between EST treatments in week 2 and week 3 were found on bone mineral content and tibia mineral density. A normal EST (37.8°C) in week 2, followed by a normal EST (37.8°C) in week 3 resulted in a lower tibia mineral content at slaughter age (Δ = 1.1–1.7 g; P = 0.001) compared with other 3 treatment groups. The 37.8 × 37.8°C treatment group (week 2 and 3, respectively) had a lower bone mineral density (Δ = 0.02–0.05 g/cm2; P = 0.002) than the 37.8 × 36.7°C and 38.9 × 37.8°C treatment groups, with the 38.9 × 36.7°C treatment group in between and not different from the other 3 groups.
Main effects of EST in week 2 were found on osseous volume, pore volume, and total volume. Chickens of the high EST (38.9°C) treatment group in week 2 had a higher osseous volume (Δ = 5.0 cm3; P < 0.001), pore volume (Δ = 0.7 cm3; P = 0.05), and total volume (Δ = 5.9 cm3; P < 0.001) compared with the normal EST (37.8°C) treatment group. Chickens incubated at a normal EST (37.8°C) in week 3 had a higher volume fraction (Δ = 2%; P = 0.008) compared with the lower EST (36.7°C) group (Table 2).
Table 2.
Effects of eggshell temperature in week 2 and week 3 of incubation on tibia biophysical characteristics at day 41 or 42 of age in male broiler chickens.
| Variable | Tibia osseous volume (cm3) | Tibia pore volume (cm3) | Tibia total volume (cm3) | Tibia volume fraction (BV/TV, %) | Tibia mineral content (g) | Tibia mineral density (g/cm2) |
|---|---|---|---|---|---|---|
| EST week 2 | ||||||
| 37.8°C | 25.6b | 5.1b | 30.8b | 85 | 16.0 | 0.29 |
| 38.9°C | 30.6a | 5.8a | 36.5a | 85 | 16.7 | 0.31 |
| SEM | 0.5 | 0.2 | 0.7 | 0.6 | 0.2 | 0.01 |
| EST week 3 | ||||||
| 36.7°C | 27.9 | 5.6 | 33.6 | 84b | 16.5 | 0.30 |
| 37.8°C | 28.2 | 5.4 | 33.7 | 86a | 16.2 | 0.30 |
| SEM | 0.5 | 0.2 | 0.7 | 0.6 | 0.2 | 0.01 |
| EST week2 × week3 | ||||||
| 37.8 × 36.7°C | 25.7 | 5.5 | 31.4 | 83 | 16.6a | 0.30a |
| 37.8 × 37.8°C | 25.4 | 4.7 | 30.2 | 87 | 15.3b | 0.27b |
| 38.9 × 36.7°C | 30.2 | 5.6 | 35.8 | 85 | 16.4a | 0.29a,b |
| 38.9 × 37.8°C | 31.0 | 6.0 | 37.1 | 86 | 17.0a | 0.32a |
| SEM | 0.7 | 0.3 | 0.9 | 0.8 | 0.3 | 0.01 |
| P-values | ||||||
| EST week 2 | <0.001 | 0.05 | <0.001 | 0.74 | 0.006 | 0.03 |
| EST week 3 | 0.72 | 0.61 | 0.94 | 0.008 | 0.18 | 0.91 |
| EST week 2 × week 3 | 0.45 | 0.12 | 0.17 | 0.14 | 0.001 | 0.002 |
a-b Values within a column and factor lacking a common superscript differ (P ≤ 0.05).
Abbreviations: EST, eggshell temperature.
Tibia Mechanical Characteristics
Interaction effects between EST in week 2 and week 3 were found on ultimate strength, yield strength, and stiffness (Table 3). A high EST (38.9°C) in week 2 followed by a normal EST (37.8°C) in week 3 resulted in a higher bone ultimate strength (Δ = 23.2–29.0 N; P = 0.04), yield strength (Δ = 21.3–26.5 N; P = 0.03), and stiffness (Δ = 22.9–29.0 N/mm; P = 0.05) at slaughter age than the other 3 EST treatment groups. Main effects were found for EST in week 2 on energy to fracture and elastic modulus. Chickens of the high EST (38.9°C) treatment group had a higher energy to fracture (Δ = 16.4 N-mm; P < 0.001) and elastic modulus (Δ = 0.4 GPa; P = 0.02) compared with the normal EST (37.8°C) treatment group in week 2. Looking at the main effects in week 3, chickens incubated at a normal EST (37.8°C) in week 3 had a higher energy to fracture (Δ = 12.9 N-mm; P = 0.03) than chickens incubated at a lower EST (36.7°C) (Table 3).
Table 3.
Effects of eggshell temperature in week 2 and week 3 of incubation on tibia mechanical characteristics at day 41 or 42 of age in male broiler chickens.
| Variable | Ultimate strength (N) | Yield strength (N) | Stiffness (N/mm) | Energy to fracture (N-mm) | Elastic modulus (GPa) |
|---|---|---|---|---|---|
| EST week 2 | |||||
| 37.8°C | 292.7 | 254.9 | 264.7 | 281.3b | 12.8b |
| 38.9°C | 309.0 | 267.1 | 280.7 | 297.7a | 13.2a |
| SEM | 3.5 | 3.5 | 3.5 | 3.5 | 0.2 |
| EST week 3 | |||||
| 36.7°C | 294.5 | 256.5 | 266.1 | 283.2b | 13.1 |
| 37.8°C | 307.2 | 265.5 | 279.1 | 296.1a | 13.0 |
| SEM | 3.5 | 3.5 | 3.5 | 3.5 | 0.2 |
| EST week 2 × week 3 | |||||
| 37.8 × 36.7°C | 291.6b | 257.5b | 263.1b | 280.1 | 12.8 |
| 37.8 × 37.8°C | 293.8b | 252.3b | 266.2b | 283.2 | 12.9 |
| 38.9 × 36.7°C | 297.4b | 255.5b | 269.2b | 286.3 | 13.4 |
| 38.9 × 37.8°C | 320.6a | 278.8a | 292.1a | 309.0 | 13.1 |
| SEM | 4.9 | 4.8 | 5.0 | 4.9 | 0.2 |
| P-values | |||||
| EST week 2 | 0.003 | 0.002 | 0.003 | <0.001 | 0.02 |
| EST week 3 | 0.02 | 0.01 | 0.02 | 0.03 | 0.84 |
| EST week 2 × week 3 | 0.04 | 0.03 | 0.05 | 0.07 | 0.28 |
a, bValues within a column and factor lacking a common superscript differ (P ≤ 0.05).
Abbreviations: EST, eggshell temperature.
Relationship Between Tibia Morphological, Biophysical, and Mechanical Characteristics
Correlation analysis between tibial morphological, biophysical, and mechanical characteristics showed the strongest relationships between tibia weight, length, thickness, ultimate strength, stiffness, and volume (Table 4). Tibia weight was positively correlated to length (r = 0.262; P = 0.03), thickness (r = 0.306; P = 0.01), ultimate strength (r = 0.425; P = 0.005), stiffness (r = 0.404; P = 0.009), and osseous volume (r = 0.548; P < 0.001). Tibia length was positively correlated to tibia thickness (r = 0.669; P < 0.001), ultimate strength (r = 0.535; P < 0.001), and stiffness (r = 0.584; P < 0.001). Tibia thickness was positively correlated to ultimate strength (r = 0.658; P < 0.001) and stiffness (r = 0.651; P < 0.001). Ultimate strength was positively correlated to stiffness (r = 0.995; P < 0.001) and osseous volume (r = 0.395; P = 0.02). Stiffness was positively correlated to osseous volume (r = 0.389; P = 0.005).
Table 4.
Spearman correlation coefficients between tibia morphological, biophysical, and mechanical characteristics1.
| Variable | Weight (g) | Length (cm) | Thickness (cm) | Ultimate strength (N) | Stiffness (N/mm) |
|---|---|---|---|---|---|
| Weight (g) | - | ||||
| Length (cm) | 0.262** | - | |||
| Thickness (cm) | 0.306** | 0.669* | - | ||
| Ultimate strength (N) | 0.425* | 0.535* | 0.658* | - | |
| Stiffness (N/mm) | 0.404* | 0.584* | 0.651* | 0.995* | - |
| Osseous volume (cm3) | 0.548* | 0.039 | 0.081 | 0.395** | 0.389* |
Significant coefficients are indicated with asterisks, *P < 0.01 and **P < 0.05; n = 64.
Body Weight, Locomotion-Related Observations, and Leg Disorders
No interaction between EST in week 2 and week 3 of incubation (P = 0.38) or a main effect of EST in week 2 (P = 0.23) or week 3 (P = 0.90) were found on BW of selected chickens at slaughter age (Table 5). Main effects were found of EST in week 2 on gait score at day 39 (Table 5). Chickens of the high EST (38.9°C) in week 2 of incubation had a lower (better) gait score compared with the normal EST (37.8°C). No interaction effects between EST in week 2 and week 3 nor a main effect of EST in week 2 or week 3 were found on gait score at day 28 or 35 (Table 5) or FDP, HB, TD, BCO, EPA, and EPI at slaughter age (Table 6).
Table 5.
Effects of eggshell temperature in week 2 and week 3 of incubation on body weight (BW) at day 41 or 42 of age, gait score1 at day 28, 35, and 39 of age in male broiler chickens.
| Variable | BW (g)2 | Gait score at day 28 | Gait score at day 35 | Gait score at day 39 |
|---|---|---|---|---|
| EST week 2 | ||||
| 37.8°C | 3,469 | 1.9 | 2.1 | 2.4b |
| 38.9°C | 3,558 | 1.8 | 2.1 | 2.3a |
| SEM | 51 | 0.02 | 0.03 | 0.04 |
| EST week 3 | ||||
| 36.7°C | 3,508 | 1.7 | 2.1 | 2.3 |
| 37.8°C | 3,518 | 1.8 | 2.0 | 2.2 |
| SEM | 51 | 0.02 | 0.02 | 0.04 |
| EST week 2 × week 3 | ||||
| 37.8 × 36.7°C | 3,496 | 1.9 | 2.0 | 2.4 |
| 37.8 × 37.8°C | 3,442 | 2.0 | 2.1 | 2.3 |
| 38.9 × 36.7°C | 3,521 | 1.8 | 2.1 | 2.3 |
| 38.9 × 37.8°C | 3,594 | 1.9 | 2.1 | 2.3 |
| SEM | 72 | 0.03 | 0.02 | 0.04 |
| P-values | ||||
| EST week 2 | 0.23 | 0.12 | 0.10 | 0.04 |
| EST week 3 | 0.90 | 0.63 | 0.12 | 0.81 |
| EST week 2 × week 3 | 0.38 | 0.30 | 0.32 | 0.31 |
Abbreviations: EST, eggshell temperature.
Method of Kestin et al. (1992), scored within a range of 0 (normal locomotion) to 5 (unable to stand).
BW of male broiler chickens selected for slaughtering to obtain tibia.
Table 6.
Effects of eggshell temperature in week 2 and week 3 of incubation on leg disorders2 at day 41 or 42 of age in male broiler chickens1.
| Variable | Rotated tibia | Food pad dermatitis | Hock burn | Tibia dyschondroplasia | Epiphyseal plate abnormalities | Epiphysiolysis |
|---|---|---|---|---|---|---|
| EST week 2 | ||||||
| 37.8°C | 0.06 | 0.25 | 0.28 | 0 | 0.81 | 0.34 |
| 38.9°C | 0 | 0.25 | 0.15 | 0.06 | 0.87 | 0.42 |
| SEM | 0.01 | 0.04 | 0.08 | 0.01 | 0.09 | 0.1 |
| EST week 3 | ||||||
| 36.7°C | 0 | 0.31 | 0.12 | 0.06 | 1.06 | 0.37 |
| 37.8°C | 0 | 0.34 | 0.25 | 0.03 | 0.68 | 0.46 |
| SEM | 0 | 0.05 | 0.03 | 0.01 | 0.19 | 0.08 |
| EST week 2 × week 3 | ||||||
| 37.8 × 37.8°C | 0 | 0.5 | 0.31 | 0 | 0.68 | 0.43 |
| 37.8 × 36.7°C | 0.12 | 0 | 0.25 | 0 | 0.93 | 0.25 |
| 38.9 × 36.7°C | 0 | 0.31 | 0.12 | 0.06 | 1.06 | 0.37 |
| 38.9 × 37.8°C | 0 | 0.18 | 0.18 | 0.06 | 0.68 | 0.5 |
| SEM | 0.02 | 0.06 | 0.05 | 0.02 | 0.21 | 0.08 |
| P-values | ||||||
| EST week 2 | 1.00 | 0.98 | 0.24 | 0.87 | 0.26 | 0.45 |
| EST week 3 | 1.00 | 0.98 | 0.54 | 0.94 | 0.51 | 0.24 |
| EST week 2 × week 3 | 1.00 | 0.98 | 0.91 | 0.89 | 0.07 | 0.81 |
Abbreviations: BCO, bacterial chondronecrosis with osteomyelitis; EPA, epiphyseal plate abnormalities; EPI, epiphysiolysis; EST, eggshell temperature; FPD, food pad dermatitis; HB, hock burns; RT, rotated tibia; TD, tibia dyschondroplasia.
All leg disorders were analyzed as frequencies to obtain P values, but shown as means. HB was scored as 0 (not affected), 1 (color changes), 2 (minor lesions), or 3 (severe lesions). FPD was scored as 0 (no lesions), 1 (mild lesion), or 2 (severe lesion). RT, TD, BCO, EPA, and EPI were scored in the range of 0 (no abnormalities), 1 (minor abnormality), or 2 (severe abnormality).
Bacterial chondronecrosis with osteomyelitis is not displayed in the table, because no observation was recorded in any of groups.
Discussion
Tibia morphological, biophysical, and mechanical characteristics, as assessed in the current experiment, are considered as the most important indicators of bone quality and are interconnected to each other (Leblanc et al., 1986, Rath et al., 2000, Onyango et al., 2003, Krupski and Tatara, 2007, Shim et al., 2012, Charuta et al., 2013). Calcium mobilization, mineralization, and ossification of tibia of the chicken embryo begin between E7 and E8 of incubation (Holder, 1978, Blom and Lilja, 2005, Oviedo-Rondón et al., 2008a, Van der Pol et al., 2019). Fast growing broiler chickens have been shown to have lower tibia mineralization compared with slow growing broilers and other birds and mammals, mostly because of fast growth rate, and consequently they have lower bone volume, mineral content, and mineral density (Lilburn, 1994, Velleman, 2000, Bonser and Casinos, 2003). Tibia mineralization and ossification can be affected by environmental factors (such as incubation temperature) from E7 onward. The rate of mineralization in the tibia has been shown to be affected by incubation temperature, because of interferences of thyroid hormones, insulin-like growth factor 1, and growth hormone (Rommel et al., 2001, Robson et al., 2002), which all play critical roles in growth plate chondrocyte differentiation (Shao et al., 2006). Moreover, several studies have shown that melatonin can promote the osteoblast differentiation and bone formation and thus play a role in regulating bone growth (Roth et al., 1999, Koyama et al., 2002). Melatonin levels, in turn, can also be affected by the temperature during incubation (Faluhelyi et al., 2009). Rate of osteoblast division in the tibia has been positively affected by higher incubation temperature compared with normal (Robson et al., 2002). Van der Eerden et al. (2003) also indicated that biochemical mechanisms that control endochondral ossification of long bones can be affected by incubation temperatures, mainly during the plateau stage of embryonic development, from the second week of incubation until hatch.
Effects of Incubation Temperature in Week 2
In the current study, most of the tibia morphological characteristics were positively affected by a higher temperature in the second week of incubation. These results are in agreement with previous research, which indicated that bone mineralization and ossification are stimulated by a higher temperature during the second week of incubation (Rommel et al., 2001, Robson et al., 2002, Shao et al., 2006, Faluhelyi et al., 2009). This is probably because of stimulation of mineralization, growth plate formation, and chondrocyte differentiation by a higher incubation temperature (Rommel et al., 2001, Robson et al., 2002, Shao et al., 2006, Faluhelyi et al., 2009).
One of the first studies on incubation temperature and bone morphological characteristics showed that an increase in incubation temperature from 35°C to 40°C in steps of 1°C, during the first 10 D of incubation resulted in higher tibia weight and length at E10 (Brookes and May, 1972). Particularly, tibia weight appeared to be stimulated by a higher EST in week 2 of incubation, as reflected in a lower robusticity index, which suggests a stronger bone structure (Reisenfeld, 1972). Aygun and Narinc (2016) showed that a high temperature (41°C) for 3 h per day during the second week of incubation stimulated tibia morphological development, resulting in longer and thicker tibia.
With respect to leg disorders and gait score, fast-growing broiler chickens in general show low locomotion activities and high (worse) gait scores (Corr et al., 2003, Bessei, 2006, Kittelsen et al., 2017). Chickens reared at higher stocking densities may have poorer gait scores and higher prevalence of leg disorders (Bradshaw et al., 2002) because of less opportunities of locomotion. In the current study, chickens of the high EST (38.9°C) in week 2 had lower (better) gait scores at day 39 than chicken incubated at the normal EST (37.8°C) in week 2, which might be explained by the better tibia characteristics found in the same treatment group. However, no effect was found on the prevalence of leg disorders, which might be because of the noncommercial setup of the experiment with small pens and low stocking density (Oviedo-Rondón et al., 2009), and it is known that the gait score is not always correlated with leg abnormalities (Sandilands et al., 2011, Fernandes et al., 2012).
Effects of Incubation Temperature in Week 3
Regarding the third week of incubation, in the current study, lowering the temperature by 1.1°C than normal in week 3 of incubation did not affect morphological tibia characteristics at all. Only a negative effect of a lower EST during week 3 of incubation was found on tibia bone fraction and energy to fracture.
In the last week of incubation, embryos become very sensitive to incubation temperature because of high metabolism and consequently high heat production (Hulet, 2007, Willemsen et al., 2011). During this week, bone development is also known to reach the highest growth rate compared to previous weeks of incubation. Literature related to temperature in the last week of incubation and bone development demonstrates similar results. Yalçin et al. (2007) concluded that both low (36.9°C) or high (39.6°C) incubator temperatures from incubation day E10 until E18 resulted in lower tibia weights at slaughter age compared with a control temperature (37.8°C). Oviedo-Rondón et al. (2009) found comparable results at slaughter age when applying a high EST (38.9°C) during the last 4 D of incubation compared with an EST of 36.9°C.
Based on the results of the current study, it appears that a lower EST (36.7°C) in the last week of incubation does not have a positive effect on bone development at slaughter age. Maintaining the EST at 37.8°C during the last week of incubation may result in a more optimal bone development compared with a lower EST (36.7°C).
Interactions on Effects of Incubation Temperature Between Week 2 and Week 3
In the current study, an interaction between EST in week 2 and week 3 of incubation was found for a number of tibia characteristics. However, not all results were in the same direction, which was possibly related to developmental and physiological differences between cortical and trabecular parts of the tibia during the second and third week of incubation (Shao et al., 2006, Wineland et al., 2006). For tibia morphological characteristics, a tendency for an interaction was found for tibia proximal length and femoral side head thickness, indicating that an incubation temperature of 38.9°C in week 2 of incubation stimulated tibia dimensions, particularly when EST in week 3 was normal (37.8°C). This is comparable with the interaction effects for mechanical characteristics, in which ultimate strength, yield strength, and stiffness showed the highest values in the 38.9 × 37.8°C group. Regarding tibia biophysical characteristics, an interaction was also found between incubation temperature in week 2 and week 3 in tibia mineral content and tibia mineral density, and again the 38.9 × 37.8°C group showed the highest values. Interactions as seen in the current study might be explained by the stimulating effect of a higher EST on mineralization and ossification rate. With a higher EST in week 2 combined with a normal EST in week 3, the highest mineralization and ossification rate can be expected, resulting in higher mineral content, density, thickness, and breaking strength. All these parameters are strongly related to each other (Applegate and Lilburn, 2002, Yair et al., 2012). Based on the lowest values of the 37.8 × 37.8°C treatment group, it can be suggested that higher EST (38.9°C) in week 2 of incubation is highly important for bone mineralization and ossification.
In conclusion, a temperature of 38.9°C during the second week of incubation stimulates morphological, biophysical, and mechanical tibia characteristics of fast-growing broiler chickens at slaughter age compared with a temperature of 37.8°C. Incubation temperature in the third week appears to interact with the incubation temperature in the second week, resulting in a most advanced tibia development after incubation at 38.9°C in week 2, followed by 37.8°C in week 3 of incubation. However, this stimulated bone development because of incubation temperatures did not affect leg disorders in later life.
Acknowledgments
This experiment was the part of the “Healthy Bones” project, financed by a public-private partnership (project number BO-47001-011). The financial support of the Ministry of Agriculture, Nature, and Food Quality (The Netherlands), Aviagen (UK), Darling Ingredients Inc., ForFarmers, Hubbard, Marel Stork Poultry Processing BV, Nepluvi, and Nutreco (The Netherlands) is gratefully acknowledged. The authors would like to thank Lagerwey hatchery (Lunteren, The Netherlands) for providing eggs; Remco Hamoen for his precious experience in 3D micro CT X-ray scanner. Stefan Veenstra, Henny Reimert, Carla van der Pol, Marcel Heetkamp, Jan Wijnen, Ilona van der Anker-Hensen, Bjorge Laurenssen, and the animal caretakers are acknowledged for their help during the experiment.
References
- Applegate T.J., Lilburn M.S. Growth of the femur and tibia of a commercial broiler line. Poult. Sci. 2002;81:1289–1294. doi: 10.1093/ps/81.9.1289. [DOI] [PubMed] [Google Scholar]
- Atalgin S.H., Kürtül I. A morphological study of skeletal development in turkey during the pre-hatching stage. Anat. Histol. Embryol. 2009;38:23–30. doi: 10.1111/j.1439-0264.2008.00887.x. [DOI] [PubMed] [Google Scholar]
- Aygun A., Narinc D. AIP Conference Proceedings (Vol. 1726, No. 1:020015) AIP Publishing; Melville, NY: 2016. The effects of thermal manipulations during embryogenesis of broiler chicks on growth of embryo and skeletal traits. [Google Scholar]
- Ballock R.T., O'keefe R.J. The biology of the growth plate. J. Bone Joint Surg. Am. 2003;85:715–726. [PubMed] [Google Scholar]
- Bessei W. Welfare of broilers: a review. World Poult. Sci. J. 2006;62:455–466. [Google Scholar]
- Blom J., Lilja C. A comparative study of embryonic development of some bird species with different patterns of postnatal growth. Zoology. 2005;108:81–95. doi: 10.1016/j.zool.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Bonser R.H.C., Casinos A. Regional variation in cortical bone properties from broiler fowl-a first look. Br. Poult. Sci. 2003;44:350–354. doi: 10.1080/0007166031000085571. [DOI] [PubMed] [Google Scholar]
- Bradshaw R.H., D Kirkden R., Broom D.M. A review of the aetiology and pathology of leg weakness in broilers in relation to welfare. Avian Poult. Biol. Rev. 2002;13:45–103. [Google Scholar]
- Brookes M.U., May K.U. The influence of temperature on bone growth in the chick. J. Anat. 1972;111:351–363. [PMC free article] [PubMed] [Google Scholar]
- Bouxsein M.L., Boyd S.K., Christiansen B.A., Guldberg R.E., Jepsen K.J., Müller R. Guidelines for assessment of bone microstructure in rodents using micro–computed tomography. J. Bone Miner. Res. 2010;25:1468–1486. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- Charuta A., Dzierzęcka M., Komosa M., Kalinowski L., Pierzchala M. Age-and sex-related differences of morphometric, densitometric and geometric parameters of tibiotarsal bone in Ross broiler chickens. Folia Biol. 2013;61:211–220. doi: 10.3409/fb61_3-4.211. [DOI] [PubMed] [Google Scholar]
- Corr S.A., Gentle M.J., McCorquodale C.C., Bennett D. The effect of morphology on walking ability in the modern broiler: a gait analysis study. Anim. Welf. 2003;12:159–171. [Google Scholar]
- Faluhelyi N., Matkovits A., Párniczky A., Csernus V. The in vitro and in ovo effects of environmental illumination and temperature on the melatonin secretion from the embryonic chicken pineal gland. Ann. N. Y. Acad. Sci. 2009;1163:383–385. doi: 10.1111/j.1749-6632.2009.04427.x. [DOI] [PubMed] [Google Scholar]
- Fernandes B.C.D.S., Martins M.R.F.B., Mendes A.A., A Paz I.C.D.L., Komiyama C.M., Milbradt E.L., Martins B.B. Locomotion problems of broiler chickens and its relationship with the gait score. Rev. Bras. Zootec. 2012;41:1951–1955. [Google Scholar]
- French N.A. Modelling incubation temperature: the effects of incubator design, embryonic development, and egg size. Poult. Sci. 1997;76:124–133. doi: 10.1093/ps/76.1.124. [DOI] [PubMed] [Google Scholar]
- Gocsik É., Silvera A.M., Hansson H., Saatkamp H.W., Blokhuis H.J. Exploring the economic potential of reducing broiler lameness. Br. Poult. Sci. 2017;58:337–347. doi: 10.1080/00071668.2017.1304530. [DOI] [PubMed] [Google Scholar]
- Jungmann R., Schitter G., Fantner G.E., Lauer M.E., Hansma P.K., Thurner P.J. Curran Associates; 2007. Real-time microdamage and strain detection during micromechanical testing of single trabeculae. In Exp. Appl. Mech.: SEM Annual Conference and Exposition 2007 (3 Vols) p. 11. [Google Scholar]
- Hammond C.L., Simbi B.H., Stickland N.C. In ovo temperature manipulation influences embryonic motility and growth of limb tissues in the chick (Gallus gallus) J. Exp. Biol. 2007;210:2667–2675. doi: 10.1242/jeb.005751. [DOI] [PubMed] [Google Scholar]
- Holder N. The onset of osteogenesis in the developing chick limb. Development. 1978;44:15–29. [PubMed] [Google Scholar]
- Hulet R.M. Symposium: managing the embryo for performance managing incubation: where are we and why? Poult. Sci. 2007;86:1017–1019. doi: 10.1093/ps/86.5.1017. [DOI] [PubMed] [Google Scholar]
- Kestin S.C., Knowles T.G., Tinch A.F., Gregory N.G. The prevalence of leg weakness in broiler chickens and its relationship with genotype. Vet. Rec. 1992;131:190–194. doi: 10.1136/vr.131.9.190. [DOI] [PubMed] [Google Scholar]
- Kestin S.C., Su G., Sørensen P. Different commercial broiler crosses have different susceptibilities to leg weakness. Poult. Sci. 1999;78:1085–1090. doi: 10.1093/ps/78.8.1085. [DOI] [PubMed] [Google Scholar]
- Kestin S.C., Gordon S., Su G., Sørensen P. Relationships in broiler chickens between lameness, live weight, growth rate and age. Vet. Rec. 2001;148:195–197. doi: 10.1136/vr.148.7.195. [DOI] [PubMed] [Google Scholar]
- Kittelsen K.E., David B., Moe R.O., Poulsen H.D., Young J.F., Granquist E.G. Associations among gait score, production data, abattoir registrations, and postmortem tibia measurements in broiler chickens. Poult. Sci. 2017;96:1033–1040. doi: 10.3382/ps/pew433. [DOI] [PubMed] [Google Scholar]
- Koyama H., Nakade O., Takada Y., Kaku T., W Lau K.H. Melatonin at pharmacologic doses increases bone mass by suppressing resorption through down-regulation of the RANKL-mediated osteoclast formation and activation. J. Bone Miner. Res. 2002;17:1219–1229. doi: 10.1359/jbmr.2002.17.7.1219. [DOI] [PubMed] [Google Scholar]
- Krupski W., Tatara M.R. Interrelationships between densitometric, morphometric, and mechanical properties of the tibia in turkeys. Bull. Vet. Inst. Pulawy. 2007;51:621. [Google Scholar]
- Leblanc B., Wyers M., Cohn-Bendit F., Legall J.M., Thibault E., Florent J.M. Histology and histomorphometry of the tibia growth in two turkey strains. Poult. Sci. 1986;65:1787–1795. doi: 10.3382/ps.0651787. [DOI] [PubMed] [Google Scholar]
- Lilburn M.S. Skeletal growth of commercial poultry species. Poult. Sci. 1994;73:897–903. doi: 10.3382/ps.0730897. [DOI] [PubMed] [Google Scholar]
- Lourens A., van den Brand H., Meijerhof R., Kemp B. Effect of eggshell temperature during incubation on embryo development, hatchability, and posthatch development. Poult. Sci. 2005;84:914–920. doi: 10.1093/ps/84.6.914. [DOI] [PubMed] [Google Scholar]
- Meijerhof R., van Beek G. Mathematical modelling of temperature and moisture loss of hatching eggs. J. Theor. Biol. 1993;165:27–41. [Google Scholar]
- Mench J. Lameness. In: Weeks C.A., Butterworth A., editors. Measuring and Auditing Broiler Welfare. CABI; Wallingford, UK: 2004. pp. 3–17. [Google Scholar]
- Nakane Y., Tsudzuki M. Development of the skeleton in Japanese quail embryos. Dev. Growth Differ. 1999;41:523–534. doi: 10.1046/j.1440-169x.1999.00454.x. [DOI] [PubMed] [Google Scholar]
- Novitskaya E., Chen P.Y., Hamed E., Li J., Lubarda V.A., Jasiuk I., McKittrick J. Recent advances on the measurement and calculation of the elastic moduli of cortical and trabecular bone: a review. Theor. Appl. Mech. 2011;38:209–297. [Google Scholar]
- Onyango E.M., Hester P.Y., Stroshine R., Adeola O. Bone densitometry as an indicator of percentage tibia ash in broiler chicks fed varying dietary calcium and phosphorus levels. Poult. Sci. 2003;82:1787–1791. doi: 10.1093/ps/82.11.1787. [DOI] [PubMed] [Google Scholar]
- Oviedo-Rondón E.O., Small J., Wineland M.J., Christensen V.L., Grimes J.L., Funderburk S.V.L., Ort D.T., Mann K.M. Effects of incubator temperature and oxygen concentration during the plateau stage of oxygen consumption on turkey embryo long bone development. Poult. Sci. 2008;87:1484–1492. doi: 10.3382/ps.2007-00470. [DOI] [PubMed] [Google Scholar]
- Oviedo-Rondón E.O., Small J., Wineland M.J., Christensen V.L., Mozdziak P.S., Koci M.D., Funderburk S.V.L., Ort D.T., Mann K.M. Broiler embryo bone development is influenced by incubator temperature, oxygen concentration and eggshell conductance at the plateau stage in oxygen consumption. Br. Poult. Sci. 2008;49:666–676. doi: 10.1080/00071660802433149. [DOI] [PubMed] [Google Scholar]
- Oviedo-Rondón E.O., Wineland M.J., Small J., Cutchin H., McElroy A., Barri A., Martin S. Effect of incubation temperatures and chick transportation conditions on bone development and leg health. J. Appl. Poult. Res. 2009;18:671–678. [Google Scholar]
- Oznurlu Y., Sur E., Ozaydin T., Celik I., Uluisik D. Histological and histochemical evaluations on the effects of high incubation temperature on the embryonic development of tibial growth plate in broiler chickens. Microsc. Res. Tech. 2016;79:106–110. doi: 10.1002/jemt.22611. [DOI] [PubMed] [Google Scholar]
- Rath N.C., Huff G.R., Huff W.E., Balog J.M. Factors regulating bone maturity and strength in poultry. Poult. Sci. 2000;79:1024–1032. doi: 10.1093/ps/79.7.1024. [DOI] [PubMed] [Google Scholar]
- Riesenfeld A. Metatarsal robusticity in bipedal rats. Am. J. Phys. Anthropol. 1972;36:229–233. doi: 10.1002/ajpa.1330360211. [DOI] [PubMed] [Google Scholar]
- Robson H., Siebler T., Shalet S.M., Williams G.R. Interactions between GH, IGF-I, glucocorticoids, and thyroid hormones during skeletal growth. Pediatr. Res. 2002;52:137. doi: 10.1203/00006450-200208000-00003. [DOI] [PubMed] [Google Scholar]
- Rommel C., Bodine S.C., Clarke B.A., Rossman R., Nunez L., Stitt T.N., Yancopoulos G.D., Glass D.J. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI (3) K/Akt/mTOR and PI (3) K/Akt/GSK3 pathways. Nat. Cell Biol. 2001;3:1009. doi: 10.1038/ncb1101-1009. [DOI] [PubMed] [Google Scholar]
- Roth J.A., Kim B.G., Lin W.L., Cho M.I. Melatonin promotes osteoblast differentiation and bone formation. J. Biol. Chem. 1999;274:22041–22047. doi: 10.1074/jbc.274.31.22041. [DOI] [PubMed] [Google Scholar]
- Sandilands V., Brocklehurst S., Sparks N., Baker L., McGovern R., Thorp B., Pearson D. Assessing leg health in chickens using a force plate and gait scoring: how many birds is enough? Vet. Rec. 2011;168:77. doi: 10.1136/vr.c5978. [DOI] [PubMed] [Google Scholar]
- Shao Y.Y., Wang L., Ballock R.T. Thyroid hormone and the growth plate. Rev. Endocr. Metab. Disord. 2006;7:265–271. doi: 10.1007/s11154-006-9012-2. [DOI] [PubMed] [Google Scholar]
- Sherlock L., Demmers T.G.M., Goodship A.E., McCarthy I.D., Wathes C.M. The relationship between physical activity and leg health in the broiler chicken. Br. Poult. Sci. 2010;51:22–30. doi: 10.1080/00071660903460637. [DOI] [PubMed] [Google Scholar]
- Shim M.Y., Karnuah A.B., Mitchell A.D., Anthony N.B., Pesti G.M., Aggrey S.E. The effects of growth rate on leg morphology and tibia breaking strength, mineral density, mineral content, and bone ash in broilers. Poult. Sci. 2012;91:1790–1795. doi: 10.3382/ps.2011-01968. [DOI] [PubMed] [Google Scholar]
- Shim M.Y., Pesti G.M. Effects of incubation temperature on the bone development of broilers. Poult. Sci. 2011;90:1867–1877. doi: 10.3382/ps.2010-01242. [DOI] [PubMed] [Google Scholar]
- Tona K., Bruggeman V., Onagbesan O., Bamelis F., Gbeassor M., Mertens K., Decuypere E. Day-old chick quality: relationship to hatching egg quality, adequate incubation practice and prediction of broiler performance. Avian Poult. Biol. Rev. 2005;16:19. [Google Scholar]
- Turner C.H., Burr D.B. Basic biomechanical measurements of bone: a tutorial. Bone. 1993;14:595–608. doi: 10.1016/8756-3282(93)90081-k. [DOI] [PubMed] [Google Scholar]
- Van der Eerden B.C.J., Karperien M., Wit J.M. Systemic and local regulation of the growth plate. Endocr. Rev. 2003;24:782–801. doi: 10.1210/er.2002-0033. [DOI] [PubMed] [Google Scholar]
- Van der Pol C.W., van Roovert-Reijrink I.A.M., Maatjens C.M., van den Anker I., Kemp B., van den Brand H. Effect of eggshell temperature throughout incubation on broiler hatchling leg bone development. Poult. Sci. 2014;93:2878–2883. doi: 10.3382/ps.2014-04210. [DOI] [PubMed] [Google Scholar]
- Van der Pol C.W., van Roovert-Reijrink I.A.M., Maatjens C.M., Gussekloo S.W., Kranenbarg S., Wijnen J., Pieters R.P., Schipper H., Kemp B., van den Brand H. Light-dark rhythms during incubation of broiler chicken embryos and their effects on embryonic and post hatch leg bone development. PLoS One. 2019;14:0210886. doi: 10.1371/journal.pone.0210886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velleman S.G. The role of the extracellular matrix in skeletal development. Poult. Sci. 2000;79:985–989. doi: 10.1093/ps/79.7.985. [DOI] [PubMed] [Google Scholar]
- Willemsen H., Li Y., Willems E., Franssens L., Wang Y., Decuypere E., Everaert N. Intermittent thermal manipulations of broiler embryos during late incubation and their immediate effect on the embryonic development and hatching process. Poult. Sci. 2011;90:1302–1312. doi: 10.3382/ps.2011-01390. [DOI] [PubMed] [Google Scholar]
- Wilson H.R. Physiological requirements of the developing embryo: Temperature and turning. Chapter 9. In: Tullett S.G., editor. Avian Incubation. Butterworth-Heinemann; London, UK: 1991. pp. 145–156. [Google Scholar]
- Wineland M.W., Christensen V.L., Yildrum I., Fairchild B.D., Mann K.M., Ort D.T. Incubator temperature and oxygen concentration at the plateau stage in oxygen consumption affects intestinal maturation of broiler chicks. Int. J. Poult. Sci. 2006;5:229–240. [Google Scholar]
- Yair R., Uni Z., Shahar R. Bone characteristics of late-term embryonic and hatchling broilers: bone development under extreme growth rate. Poult. Sci. 2012;91:2614–2620. doi: 10.3382/ps.2012-02244. [DOI] [PubMed] [Google Scholar]
- Yalçin S., Malayoǧlu H.B., Baka M., Genin O., Pines M. Effect of temperature during the incubation period on tibial growth plate chondrocyte differentiation and the incidence of tibial dyschondroplasia. Poult. Sci. 2007;86:1772–1783. doi: 10.1093/ps/86.8.1772. [DOI] [PubMed] [Google Scholar]