Abstract
Maternal betaine was reported to regulate offspring hepatic cholesterol metabolism in mammals. However, it is unclear whether and how feeding betaine to laying hens affects hepatic cholesterol metabolism in offspring chickens. Rugao yellow-feathered laying hens (n = 120) were fed basal or 0.5% betaine-supplemented diet for 28 D before the eggs were collected for incubation. Maternal betaine significantly decreased the hepatic cholesterol content (P < 0.05) in offspring chickens. Accordingly, the cholesterol biosynthetic enzymes, sterol regulator element-binding protein 2 (SREBP2) and 3-hydroxy-3-methylglutaryl coenzyme A reductase, were decreased, while cholesterol-7alpha-hydroxylase (CYP7A1), which converts cholesterol to bile acids, was increased at both mRNA and protein levels in betaine-treated offspring chickens. Hepatic mRNA and protein expression of low-density lipoprotein receptor was significantly (P < 0.05) increased, while the mRNA abundance of cholesterol acyltransferase 1 (ACAT1) that mediates cholesterol esterification was significantly (P < 0.05) decreased in the betaine group. Meanwhile, hepatic protein contents of DNA methyltransferases 1 and betaine homocysteine methyltransferase were increased (P < 0.05), which was associated with modifications of CpG methylation on affected cholesterol metabolic genes. Furthermore, the level of CpG methylation on gene promoters was increased (P < 0.05) for sterol regulator element-binding protein 2 and abundance of cholesterol acyltransferase 1 yet decreased (P < 0.05) for cholesterol-7alpha-hydroxylase. These results indicate that maternal betaine supplementation significantly decreases hepatic cholesterol deposition through epigenetic regulation of cholesterol metabolic genes in offspring juvenile chickens.
Key words: betaine, cholesterol, chicken, liver, DNA methylation
Introduction
Cholesterol is essential to cell viability and functions. Cholesterol not only constitutes cell membranes but also serves as a precursor for the biosynthesis of steroid hormones, oxysterols, and bile acids, all of which play critical roles in health and disease (Sozen and Ozer, 2017, Benito-Vicente et al., 2018, Gluba-Brzozka et al., 2019). The liver is the main site for cholesterol metabolism and thus serves as a control center for the maintenance of cholesterol homeostasis in the body. Cholesterol and its metabolites are implicated in the pathogenesis of various human diseases, including atherosclerosis, cancer, neurodegenerative diseases, and non-alcoholic fatty liver disease (Wu et al., 2013, Coisne et al., 2016, Van den Berg et al., 2018, Ou et al., 2019). In the chicken, cholesterol metabolism in the brain and muscle was associated with aggressive behavior and meat quality, respectively (Attia et al., 2017, Idriss et al., 2017). Importantly, cholesterol level in the liver is an indicator of chicken fatty liver syndrome. Fatty liver syndrome is a common metabolic disease, which seriously affects the health and production efficiency of laying hens and causes great economic losses to the poultry industry (Polin and Wolford, 1977). Therefore, controlling hepatic cholesterol metabolism will be a prophylactic strategy to benefit the chicken health and related industry.
The homeostasis of cholesterol metabolism in the liver depends on the dynamic balance of cholesterol biosynthesis and transformation (Trapani et al., 2012, Faust and Kovacs, 2014). Sterol regulatory element binding protein 2 (SREBP2) is the key transcription factor for the activation of cholesterol biosynthesis genes such as 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMGCR) (Khesht and Hassanabadi, 2012, Sharpe and Brown, 2013). Cholesterol can be catabolized through its transformation into excretable bile acids by cholesterol-7alpha-hydroxylase (CYP7A1) (Monte et al., 2009). A number of studies have demonstrated that SREBP2 and CYP7A1 are the key factors to regulate cholesterol metabolism in the offspring in response to maternal nutrition intervention (Goharkhay et al., 2008, Sohi et al., 2011). For instance, maternal intake of grape procyanidins during the perinatal period elevated cholesterol in offspring rats was associated with upregulation of SREBP2 (Del Bas et al., 2015). Prenatal low-protein diet exposure decreased hepatic cholesterol content in weaning piglets due to persistent elevation of CYP7A1 (Cong et al., 2012). In the chicken, it is interesting to explore whether SREBP2 and CYP7A1 expressions in the progeny of laying hens are vulnerable to maternal nutrition status.
Betaine is the trimethyl derivative of glycine that can be obtained directly from diet or indirectly from dietary choline via oxidation (Lever and Slow, 2010). Betaine can activate DNA methylation through one carbon metabolism and thus regulates the transcription of cholesterol metabolic genes (Yang et al., 2017, Hu et al., 2018). Recent study provided the evidence that maternal betaine exposure enhances hepatic cholesterol accumulation along with hypermethylation of CYP7A1 gene promoter (Zhao et al., 2019). Notably, dietary betaine supplementation in hens triggers the hypomethylation of SREBP2 and HMGCR genes promoter in offspring chickens, contributing to elevated hypothalamic cholesterol content (Idriss et al., 2018). We previously reported that in ovo injection of betaine alleviates corticosterone-induced hepatic cholesterol deposition through epigenetic genes regulation in juvenile chickens (Hu et al., 2017). However, it remains unknown whether epigenetic modifications were enrolled in the regulation of hepatic cholesterol metabolic gene transcripts in the offspring chickens when the hens were fed with betaine.
Therefore, the objectives of the present study were to investigate whether maternal dietary betaine supplementation can modulate hepatic cholesterol metabolism in juvenile chickens, and how such effects, if any, are related to DNA methylation on the promoter of SREBP2 and CYP7A1 genes.
Materials and methods
Ethics Statement
The experimental protocol was approved by the Animal Ethics Committee of Nanjing Agricultural University, with the project number 31672512. The sampling procedures complied with the “Guidelines on Ethical Treatment of Experimental Animals'’ (2006) No. 398 set by the Ministry of Science and Technology, China.
Animals and Treatment
One hundred twenty Rugao yellow-feathered laying hens obtained from the Poultry Institute of Yangzhou, Jiangsu, China, were randomly divided into control and betaine groups at 38 wk of age. Each group contains 60 hens in 20 cages (3 hens per cage). Hens were fed either basal (control) or betaine-supplemented (betaine) diet for 4 wk (Table 1). Betaine hydrochloride (98% purity; SKYSTONE FEED CO., LTD, Jiangsu, China) was added to the basal diet at the level of 0.5%. The dose was chosen based on previous studies (Matthews et al., 1997, Dar et al., 2019). Initial body weight and the laying performance were recorded. Chickens were artificially inseminated at the 18th and 24th day after dietary treatments. Two hundred fertilized eggs laid on the last 4 D (25th–28th) of the dietary treatment from each group were collected. Chicks were hatched inside the incubator and were left to dry completely (up to 12 h) before transferred to brood cages. One-day-old chicks were individually weighed, wing labeled, and raised according to the standard recommended by the breeder. Chicks were subjected to continuous illumination and the temperature was controlled in the range of 35°C∼37°C during the first week and reduced approximately 3°C per week until 21°C. The relative humidity was maintained at 40∼60%, and the lighting, ventilation, as well as the feeding and management procedures complied with the Feeding Management Regulations of Rugao Chickens. All the chicks were fed with the same diet (Table 1). Growth performance was recorded weekly from hatching to 56 D of age. At 57 D of age, 30 male chickens were selected from each group (15 in each group), weighed, and killed by rapid decapitation which is considered to be acceptable for euthanasia of birds according to American Veterinary Medical Association (AVMA) Guidelines for the Euthanasia of Animals: 2013 Edition. Blood samples were taken and plasma was separated and stored at −20°C. Liver (without the gall bladder) samples were rapidly frozen in liquid nitrogen and kept at −80°C for further analysis.
Table 1.
Ingredients and nutrient composition of the diets for hens and offspring chickens.
| Hens | Starter (1–21 D) | Grower (22–57 D) | |
|---|---|---|---|
| Ingredients, g/kg | |||
| Corn | 580.0 | 566.0 | 546.0 |
| Soybean meal | 256.0 | 303.3 | 315.3 |
| Soybean oil | 30.0 | 20.0 | 30.0 |
| Corn protein meal | 0.0 | 50.0 | 50.0 |
| Dicalcium phosphate | 30.3 | 13.0 | 11.5 |
| Shell powder | 67.0 | 0.0 | 0.0 |
| Premix1 | 10.0 | 10.0 | 10.0 |
| Salt | 3.0 | 3.3 | 3.3 |
| DL-methionine | 1.8 | 12.0 | 12.0 |
| Choline chloride | 1.6 | 3.0 | 3.0 |
| Limestone | 20.3 | 13.4 | 13.4 |
| Antioxidants | 0.0 | 1.0 | 1.0 |
| Metabolizable energy, MJ/kg | 12.62 | 13.46 | 13.58 |
| Nutrient composition (%) | |||
| Crude protein | 16.96 | 21.22 | 19.71 |
| Calcium | 3.20 | 0.99 | 0.91 |
| Phosphorous | 0.59 | 0.67 | 0.61 |
| Lysine | 1.65 | 1.43 | 1.30 |
| Methionine | 0.62 | 0.50 | 0.47 |
Premix provided per kilogram of diet: trans-retinyl acetate, 10,000 IU; cholecalciferol, 5000 IU; all-rac-α-tocopherol acetate, 20 mg; menadione, 1.5 mg; thiamin, 1 mg; riboflavin, 6 mg; nicotinamide, 40 mg; choline chloride, 350 mg; calcium pantothenate, 10 mg; pyridoxine HCl, 2 mg; biotin, 0.04 mg; folic acid, 1 mg; cobalamin, 0.012 mg; Fe (ferrous sulfate), 60 mg; Cu (copper sulfate), 5 mg; Mn (manganese sulfate), 100 mg; Zn (zinc oxide), 65 mg; I (calcium iodate), 0.8 mg; Se (sodium selenite), 0.3 mg; zinc bacitracin, 30 mg; dl-methionine, 1 g.
Determination of Cholesterol Content in the Liver and Plasma
Cholesterol in the plasma and liver was measured by using respective commercial cholesterol assay kit (E1005 and E1015) purchased from Applygen Technologies Inc., China, following the manufacturer's instructions.
Total RNA Isolation and Real-Time PCR
Total RNA was isolated from 30 mg liver sample using 1 mL of TRIzol reagent (Invitrogen) and then treated with RNase-free DNase and reverse-transcribed to cDNA using reverse transcription kit (Vazyme Biotech Co., Ltd). Two microliters of diluted cDNA (1:10, vol/vol) was used for real-time PCR which was performed with a CFX RT-PCR detection system (Bio-Rad). All primers were listed in Table 2. Relative mRNA content was normalized to 18S rRNA. Data were analyzed using the method of 2−△△CT.
Table 2.
Nucleotide sequences of specific primers.
| Target genes | GenBank accession | Primer sequences (5′ to 3′) | PCR products (bp) |
|---|---|---|---|
| SREBP1 | AY029224 | F: CTACCGCTCATCCATCAACG R: CTGCTTCAGCTTCTGGTTGC |
145 |
| SREBP2 | XM_416222 | F: CCCAGAACAGCAAGCAAGG R: GCGAGGACAGGAAAGAGAGTG |
108 |
| HMGCR | NM_204485.1 | F: TTGGATAGAGGGAAGAGGGAAG R: CCATAGCAGAACCCACCAGA |
137 |
| CYP7A1 | AB109636.1 | F: CATTCTGTTGCCAGGTGATGTT R: GCTCTCTCTGTTTCCCGCTTT |
106 |
| CYP27A1 | XM422056.4 | F: AGGACTTTCGTCTGGCTCT R: CTCCGCATCGGGTATTT |
185 |
| ABCA1 | NM_204145.2 | F: TCCTCTGGCTTAGACTTGA R: CTCGTAGTTGTATTCGGTAA |
130 |
| APOA1 | NM_205525.4 | F: GTGACCCTCGCTGTGCTCTT R: CACTCAGCGTGTCCAGGTTGT |
217 |
| ACAT1 | NM_001277779.1 | F: TAATGGTTGCTGGTGG R: GCTTTGCTCTTGGTGT |
232 |
| LDLR | NM_204452.1 | F: CCACCATTTGGCAGAGGAA R: ACCGCAGTCAGACCAGAAGAG |
86 |
| RN18S | DQ018752 | F: ATAACGAACGAGACTCTGGCA R: CGGACATCTAAGGGCATCACA |
136 |
| MeDIP | |||
| SREBP2 promoter | F: GCTCTTGGGAGTTGTC R: AGGTTGTTCCAGTTGG |
224 | |
| CYP7A1 promoter | F: GAAAGATGTCAGAGCAG R: ACTATGGCAGAAGAGG |
160 | |
| ACAT1 promoter | F: TGTCAGTCCCCGCTCT R: CCTGGTAGGCATTTCTT |
190 |
Total Protein Extraction and Western Blotting
Total protein was extracted from 50 mg ground liver sample as previously described (Hu et al., 2018). The protein concentration was determined according to the manufacturer's instructions of Pierce BCA Protein Assay kit (Rockford, IL). Forty or 60 μg of protein was used for electrophoresis on a 7.5 or 10% SDS-PAGE gel. Western blot analysis for HMGCR (BS6625, Bioworld Technology, diluted 1:1000), SREBP2 (14508-1-AP, Proteintech, diluted 1:1000), CYP7A1 (AB79847, Abcam, UK, diluted 1:1000), CYP27A1 (BS2192, Bioworld Technology, diluted 1:500), BHMT (15965-1-AP, Proteintech, diluted 1:1000), MAT2B (15952-1-AP, Proteintech, diluted 1:1000), GNMT (18790-1-AP, Proteintech, diluted 1:1000), AHCYL1 (10658-3-AP, Proteintech, diluted 1:1000), DNMT1 (24206-1-AP, Proteintech, diluted 1:1000) was carried out according to the recommended protocols provided by the manufacturers. The Tublin α (BS1699, Bioworld, diluted 1:10,000) was used as loading control in the Western blot analysis. Images were captured by VersaDoc 4000 MP system (Bio-Rad) and the band density was analyzed with Quantity One software (Bio-Rad).
Methylated DNA Immunoprecipitation (MeDIP) Analysis
MeDIP analysis was performed according to previous publication (Hu et al., 2018). Briefly, high-quality genomic DNA was isolated from liver tissues and sonicated to produce small fragments ranging from 300 to 1,000 bp. Two micrograms of fragmented DNA was heat-denatured to produce single-stranded DNA, and a portion of the denatured DNA was stored as input DNA. The immunoprecipitation was performed overnight at 4°C with 2 μg antibody against 5-methyl cytosine (ab10805, Abcam, UK). Pretreated protein A/G agarose beads (40 μL, 50% slurry, sc-2003, Santa Cruz) were used to capture the antibody/DNA complexes. The beads bound to immune complexes were washed to eliminate nonspecific binding and resuspended in 250 μL digestion buffer containing proteinase K. Finally, the MeDIP DNA was purified. A small aliquot of MeDIP DNA and control input DNA was used to amplify the proximal promoter sequence of chicken cholesterol metabolism genes by real-time PCR with specific primers listed in Table 1. Data were normalized to the input and presented as the fold change relative to the average value of control group.
Bisulfite Sequencing Analysis of Promoter CpG Methylation
Bisulfite sequencing analysis was performed according to previous publication (Abobaker et al., 2017). Briefly, high-quality genomic DNA was isolated from chicken liver tissues. Bisulfite conversion of 1 μg genomic DNA was performed with the EZ DNA Methylation-GOLD Kit (ZYMO RESEARCH, CA) according to the manufacturer's protocol. CpG islands within the promoter regions of SREBP2, CYP7A1, and ACAT1 genes were respectively identified with Methyl Primer Express Software v1.0 according to the following criteria: 1) 300 bp minimum length; 2) > 50% CG content; 3) > 0.60 observed/expected ratio of CpG dinucleotides. Two regions of CpG islands of SREBP2 and one region of CpG islands of CYP7A1 and ACAT1 genes were amplified. Genesky Biotechnologies Inc., Shanghai, China, was contracted to design the primers (Table 2) and conduct the bisulfite sequencing analysis including PCR amplification, library construction, sequencing, and data analysis. After PCR amplification (HotStar Taq polymerase kit, TAKARA, Tokyo, Japan) of target CpG regions, library construction was conducted by using Nextera XT library preparation technology according to manufacturer's protocol (Illumina, San Diego, CA) and the products were sequenced on Illumina MiSeq Benchtop Sequencer (CA). FASTQ files were generated per sample, per read. The Phred quality score was higher than Q30. FASTQ sequences were aligned to in silico–converted reference sequences of the chicken genome, by the method of bisulfite amplicon sequencing (Masser et al., 2013). All samples achieved a mean coverage of >600 ×. Each tested CpG site was numbered according to its relative distance (in bp) from translation start codon (ATG). Methylation level at each CpG site was calculated as the percentage of the methylated cytosines over the total tested cytosines. The average methylation level was calculated using methylation levels of all measured CpG sites within the gene fragment.
Statistics
The results were presented as means ± SEM. Comparisons were performed using independent-samples t-test with SPSS 20.0 for windows. For bisulfite sequencing analysis, chi-square test was used to examine differences in haplotype and methylation status. The differences were considered statistically significant when P < 0.05.
Results
Body Weight, Liver Weight, Serum Cholesterol and HDL-C Concentration, Hepatic Cholesterol Content
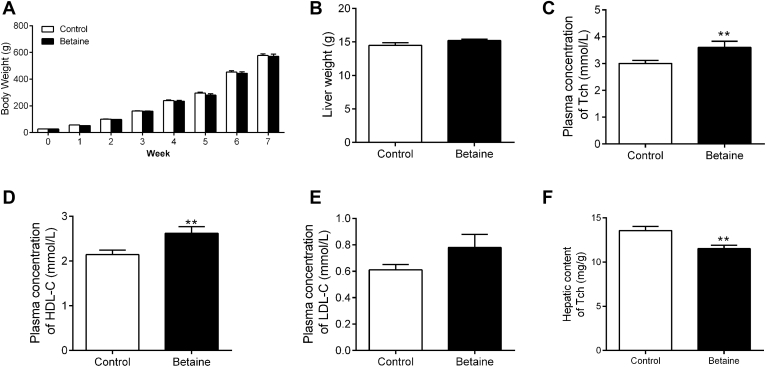
Maternal betaine did not significantly affect the offspring chicken body weight (Figure 1A) or the liver weight (Figure 1B). However, the offspring of betaine-supplemented chickens had significantly lower (P < 0.05) cholesterol concentration in the plasma (Figure 1C) and liver (Figure 1F). Also, maternal betaine supplementation significantly decreased (P < 0.05) the HDL-C content in the plasma, as compared with the control group (Figure 1D). No significant alteration (P > 0.05) was detected for LDL-C concentration in the plasma (Figure 1E).
Figure 1.
Effect of maternal betaine supplementation on body weight, liver weight, plasma cholesterol concentration, and hepatic total cholesterol content in offspring juvenile chickens. (A) Chicken body weight; (B) liver weight; (C) plasma concentration of total cholesterol; (D) plasma concentration of HDL-C; (E) plasma concentration of LDL-C; (F) hepatic content of total cholesterol. Values are means ± SEM, *P < 0.05, compared with control (n = 8).
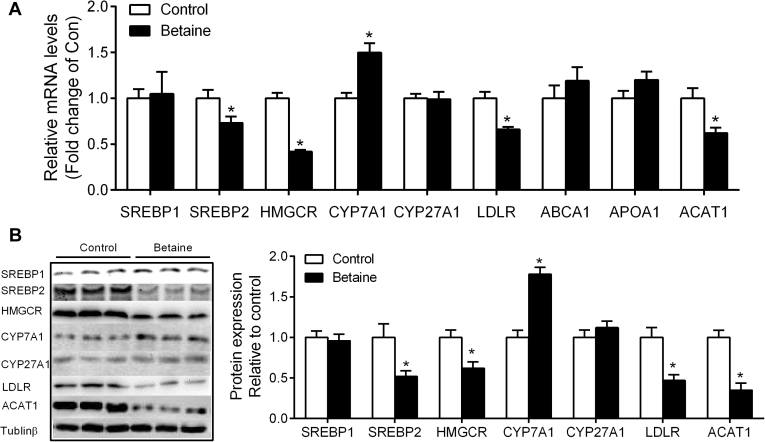
Hepatic Expression of Genes Involved in Cholesterol Metabolism
Maternal betaine significantly increased (P < 0.05) hepatic expression of cholesterol biosynthesis genes, such as SREBP2 and HMGCR, at the level of both mRNA (Figure 2A) and protein (Figure 2B). The cholesterol catabolic gene CYP7A1 was significantly increased at both mRNA and protein level in the offspring of betaine-treated chickens (Figure 2A, 2B). Hepatic mRNA and protein expression of acetyl-CoA acetyltransferase 1 (ACAT1) (Figure 2A, 2B) that esterifies cholesterol for storage was significantly decreased (P < 0.05) in the offspring of betaine-treated chickens. Hepatic mRNA and protein expression of low-density lipoprotein receptor (LDLR), which mediates the endocytosis of cholesterol-rich LDL, was also significantly decreased (P < 0.05) in the offspring of betaine-treated chickens (Figure 2A, 2B). No significant alterations (P > 0.05) were detected for other cholesterol metabolic genes, including SREBP1, cholesterol-27 alpha-hydroxylase (CYP27A1), ATP-binding cassette sub-family A member 1 (ABCA1), and apolipoprotein A1 (APOA1) at the mRNA level in the liver.
Figure 2.
Effect of maternal betaine supplementation on hepatic expression of cholesterol metabolic genes in offspring juvenile chickens. (A) Hepatic mRNA abundance of genes involved in cholesterol metabolism; (B) protein expression of cholesterol metabolic genes. Values are means ± SEM, *P < 0.05, compared with control (n = 8).
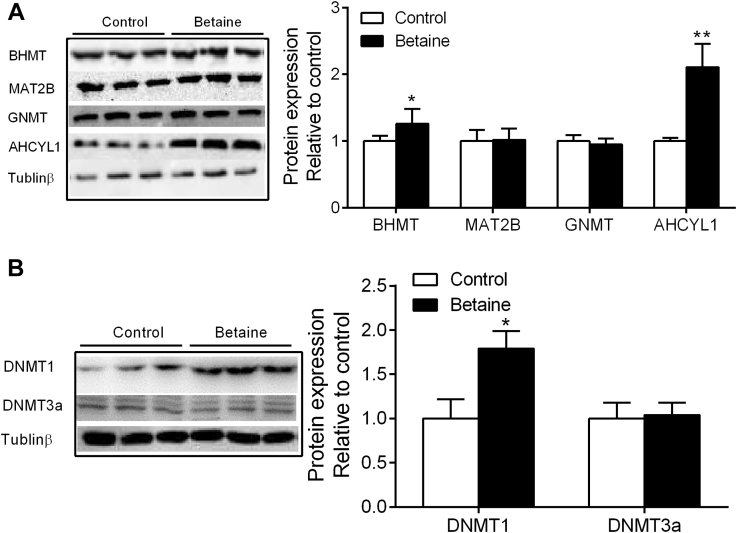
Hepatic Protein Content of Methionine Cycle and Methyl Transfer Genes
Among the 4 enzymes involved in methionine cycle, maternal betaine supplementation significantly increased (P < 0.05) the hepatic protein content of betaine homocysteine methyltransferase (BHMT) and adenosylhomocysteinase-like 1 (AHCYL1) (Figure 3A). Moreover, the protein content of DNA methyltransferase 1 (DNMT1) (P < 0.05) was significantly increased in the liver of betaine-treated offspring chickens (Figure 3B). No significant alterations (P > 0.05) were detected for hepatic protein content of methionine adenosyltransferase 2B (MAT2B), glycine N-methyltransferase (GNMT), and DNA methyltransferase 3a (DNMT3a) in the offspring of betaine-treated chickens.
Figure 3.
Effect of maternal betaine supplementation on hepatic protein content of enzymes involved in carbon metabolism methyl transfer. (A) Protein expression of BHMT, MAT2B, GNMT, and AHCYL1; (B) protein expression of DNMT1 and DNMT3a. Values are means ± SEM, ∗P < 0.05, ∗∗P < 0.01, compared with control (n = 8).
CpG Methylation of SREBP2, CYP7A1, and ACAT1 Gene Promoters in the Liver
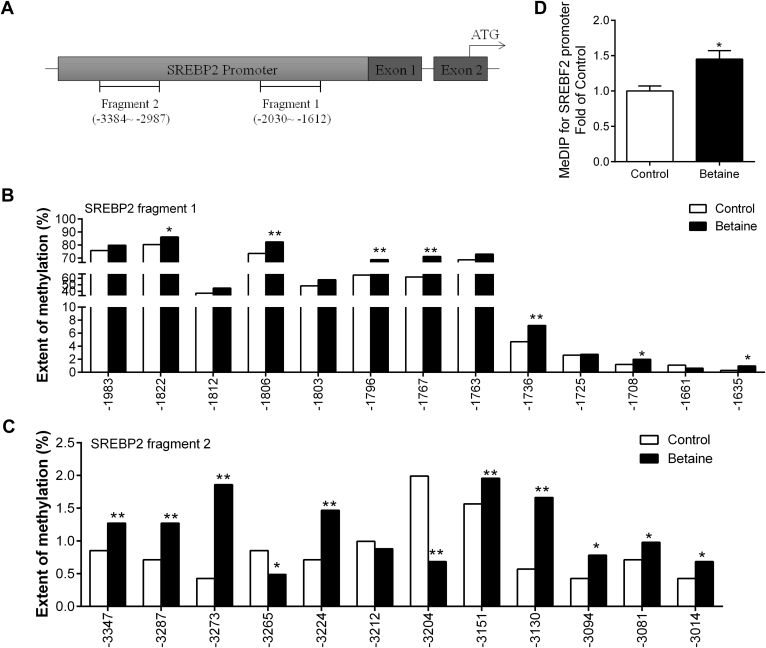
Bisulfite sequencing PCR analysis for the genomic DNA isolated from the liver revealed that the CpG methylation status of the promoter regions for SREBP2, CYP7A1, and ACAT1 genes is modulated in response to maternal betaine feeding.
For SREBP2 gene promoter, 2 regions of CpG islands were analyzed (Figure 4A). Fragment 1 spans from −2030 to −1612 upstream of the translation start codon ATG, containing 13 CpG sites, whereas fragment 2 spans from −3384 to −2987, which contains 12 CpG sites. 7 CpG sites on fragment 1 were significantly (P < 0.05) hypermethylated and 9 CpG sites on fragment 2 were significantly (P < 0.05) hypermethylated in the betaine group compared to the control group, whereas 2 CpG sites on fragment 2 were hypomethylated (Figure 4B, 4C). Concurrently, MeDIP results confirmed that SREBP2 promoter was significantly hypermethylated (P < 0.05) in the offspring of betaine-treated chickens (Figure 4D).
Figure 4.
Methylation status of SREBP2 gene promoter in the liver. (A) Schematic diagram showing the promoter sequences of chicken SREBP2 gene. CpG sites, determined in this study, on 5′-flanking promoter regions of SREBP2 localized between −3384∼−2987 and −2030∼−1612 about the translation start codon (ATG) are underlined; (B) methylation rate on SREBP2 promoter from −2030 to −1612 bp for the chicken liver; (C) methylation rate on SREBP2 promoter from −3384 to −2987 bp for the chicken liver; (D) methylation status on the promoter of SREBP2. ∗P < 0.05, ∗∗P < 0.01, compared with control.
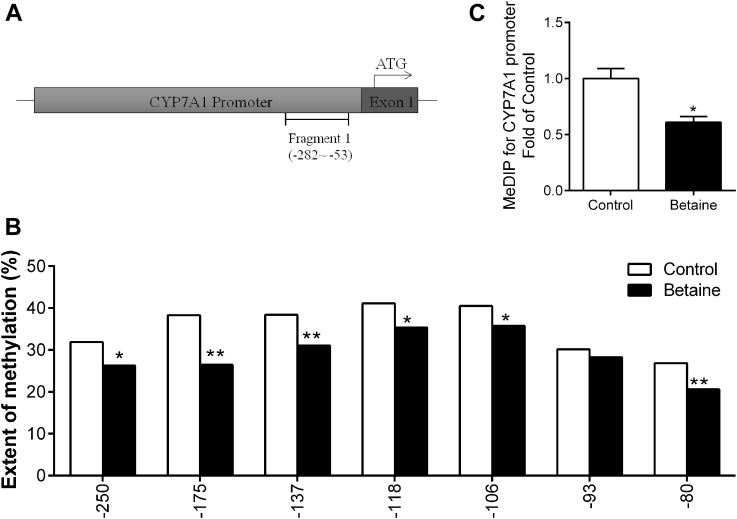
For CYP7A1 gene promoter, 1 fragment was analyzed (Figure 5A). This fragment spans from −282 to −53 upstream of the translation start codon ATG, containing 7 CpG sites. Six of 7 CpG sites detected on CYP7A1 gene promoter were hypomethylated (P < 0.05) in the betaine group (Figure 5B). MeDIP results showed that CYP7A1 promoter was significantly hypomethylated (P < 0.05) in the offspring of betaine-treated chickens (Figure 5C).
Figure 5.
Methylation status of CYP7A1 gene promoter in the liver. (A) Schematic diagram showing the promoter sequences of chicken CYP7A1 gene. CpG sites, determined in this study, on 5′-flanking promoter regions of CYP7A1 localized between −282∼−53 about the translation start codon (ATG) are underlined; (B) methylation rate on CYP7A1 promoter from −282∼−53 bp for the chicken liver; (C) methylation status on the promoter of CYP7A1. ∗P < 0.05, ∗∗P < 0.01, compared with control.
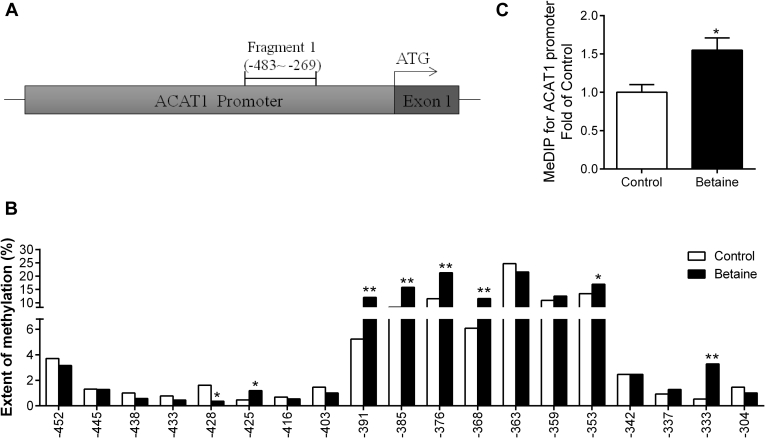
For ACAT1 gene promoter, 1 fragment was analyzed (Figure 6A). This fragment spans from −483 to −269 upstream of the translation start codon ATG, containing 19 CpG sites. 7 of 19 CpG sites detected on ACAT1 gene promoter were hypermethylated (P < 0.05) and 1 of 19 CpG sites was hypomethylated in the betaine group (Figure 6B). MeDIP results showed that ACAT1 gene promoter was significantly hypermethylated (P < 0.05) in the betaine group (Figure 4C).
Figure 6.
Methylation status of ACAT1 gene promoter in the liver. (A) Schematic diagram showing the promoter sequences of chicken ACAT1 gene. CpG sites, determined in this study, on 5′-flanking promoter regions of ACAT1 localized between −483∼−269 about the translation start codon (ATG) are underlined; (B) methylation rate on ACAT1 promoter from −483∼−269 bp for the chicken liver; (C) methylation status on the promoter of ACAT1. ∗P < 0.05, ∗∗P < 0.01, compared with control.
In general, the average methylation rate in all detected fragments respectively for SREBP2, CYP7A1, and ACAT1 genes promoters was inversely related to their mRNA abundances.
Discussion
It has been reported that gestational dietary betaine supplementation increased plasma betaine content in neonatal pig offspring (Jia et al., 2015). It is noted that mammals differ from birds in mother–fetus interaction. Maternal factors influence the fetal development via placenta in mammals, while hens deposit nutrients and regulatory signals in the egg. However, unfortunately, our attempts to detect the betaine content in eggs and offspring liver and plasma samples with HPLC failed, as it was hard to separate betaine from choline that is highly abundant in chicken eggs, as well as the liver and plasma samples. There is no doubt that the observed effects are induced by maternal betaine supplementation, but we cannot differentiate whether these are the direct effects of betaine enriched in the eggs or the indirect consequences of other nutritional or bioactive components that may be changed by betaine supplementation. We could only say that maternal betaine supplementation induced the observed effects, but whether these effects attribute to betaine enriched in the eggs remains a speculation.
Previously, it was reported that betaine supplementation can affect hepatic cholesterol metabolism in mammals (Matthews et al., 2001, Albuquerque et al., 2017, Zhao et al., 2019). In the chicken, the information on the hepatic metabolic responses of cholesterol to betaine is scarce. Previous study reported that prenatal betaine increased hepatic cholesterol content in newly hatched chicks (Hu et al., 2015). In ovo injection of betaine did not affect hepatic cholesterol content but prevented corticosterone-induced increase in hepatic cholesterol content (Hu et al., 2017). In the present study, we found that plasma and hepatic total cholesterol concentrations were significantly decreased in offspring of betaine-treated chickens, which was consistent with previous publication that dietary betaine supplementation reduced hepatic cholesterol content via improvement of bile acid metabolism in blunt-snout bream (Wang et al., 2019). It appears that the effects of betaine on hepatic cholesterol profiles are dependent on the dietary formulations, species, and the dose of supplementation.
Previous study reported betaine administration can attenuate chronic alcohol-induced hepatic cholesterol accumulation (Yang et al., 2017), which was associated with downregulated mRNA expression of SREBP2 and HMGCR. Consistent with these results, hepatic SREBP2 and HMGCR mRNA and protein expression were markedly reduced in the betaine group compared with the controls. A previous study reported that maternal betaine enhanced hepatic cholesterol accumulation through suppressing CYP7A1 expression (Zhao et al., 2019). However, in this study, maternal betaine supplementation decreased hepatic cholesterol accumulation, in association with upregulated mRNA expression of CYP7A1. The inverse results between mammals and birds may be attributed to different nutrients delivery via mother–fetus circle, the placenta in mammals while the eggs in hens. Taken together, the downregulation of SREBP2 and HMGCR indicates suppressed cholesterol biosynthesis in the liver, whereas the upregulation of CYP7A1 implicates enhanced cholesterol conversion or transformation to bile acids. The combined effects of suppressed cholesterol biosynthesis and enhanced cholesterol catabolism may contribute to lower cholesterol content in the liver of betaine-treated offspring chickens.
We previously reported that supplementation of betaine could affect cholesterol metabolism in offspring by affecting carbon metabolism and DNA methylation (Hu et al., 2015, Idriss et al., 2017, Zhao et al., 2019). In this study, maternal betaine supplementation significantly enhanced methionine metabolism and methyl transfer genes expression of BHMT, AHCYL, and DNMT1 at protein levels in the offspring chicken liver, which agrees with a previous finding that maternal betaine supplementation causes BHMT and DNMT1 upregulation in the hypothalamus of offspring cockerels (Idriss et al., 2017). It is worth noting that, one carbon metabolism is essential for providing methyl group to the methylation of DNA, RNA, and histone methylation, thus playing a critical role in the epigenetic gene regulation. DNA methylation has been regarded as a major mechanism accounting for epigenetic gene regulation of betaine (Day and Kempson, 2016, Figueroa-Soto and Valenzuela-Soto, 2018). Previously, we reported that prenatal betaine programs offspring hepatic mRNA expression of SREBF2 and CYP7A1 through DNA methylation in neonatal piglets (Cai et al., 2014). However, in the present study, the methylation status of the promoters in response to methyl donor is complex and gene specific. Here, the promoter of SREBP2 gene was found to be hypermethylated, whereas that of CYP7A1 gene was hypomethylated, being reversely linked to the transcriptional repression of SREBP2 gene and transcriptional activation of CYP7A1 gene, respectively, in the liver of betaine-treated chicks. Nevertheless, in the present study, transcriptional regulation of gene expression is complex and mismatches between the methylation level of the promoter and the mRNA abundance of the gene were also observed. For instance, HMGCR and LDLR mRNA was significantly downregulated yet no alteration was detected in the methylation status of its promoter which contains very few CpG sites. Future in-depth studies are needed to unravel the mechanisms of betaine's effect on HMGCR and LDLR gene expression.
Conclusion
In conclusion, we demonstrate that maternal betaine supplementation decreased cholesterol content in the liver of offspring juvenile chickens through epigenetic regulation of cholesterol metabolic genes. Obviously, more in-depth investigations are needed to address whether other regulatory mechanisms are involved in betaine-induced cholesterol metabolic genes expression in the offspring juvenile chicken.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31972638 and 31672512), the Fundamental Research Funds for the Central Universities (KYZ201212), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), and Jiangsu Collaborative Innovation Centre of Meat Production and Processing, Quality and Safety Control.
Conflict of Interest Statement: The authors did not provide a conflict of interest statement.
References
- Abobaker H., Hu Y., Hou Z., Sun Q., Idriss A.A., Omer N.A., Zong Y., Zhao R. Dietary betaine supplementation increases adrenal expression of steroidogenic acute regulatory protein and yolk deposition of corticosterone in laying hens. Poult. Sci. 2017;96:4389–4398. doi: 10.3382/ps/pex241. [DOI] [PubMed] [Google Scholar]
- Albuquerque A., Neves J.A., Redondeiro M., Laranjo M., Felix M.R., Freitas A., Tirapicos J.L., Martins J.M. Long term betaine supplementation regulates genes involved in lipid and cholesterol metabolism of two muscles from an obese pig breed. Meat Sci. 2017;124:25–33. doi: 10.1016/j.meatsci.2016.10.012. [DOI] [PubMed] [Google Scholar]
- Attia Y.A., Al-Harthi M.A., Korish M.A., Shiboob M.M. Fatty acid and cholesterol profiles, hypocholesterolemic, atherogenic, and thrombogenic indices of broiler meat in the retail market. Lipids Health Dis. 2017;16:40. doi: 10.1186/s12944-017-0423-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito-Vicente A., Uribe K.B., Jebari S., Galicia-Garcia U., Ostolaza H., Martin C. Familial hypercholesterolemia: the Most Frequent cholesterol metabolism Disorder caused disease. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19113426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D., Jia Y., Lu J., Yuan M., Sui S., Song H., Zhao R. Maternal dietary betaine supplementation modifies hepatic expression of cholesterol metabolic genes via epigenetic mechanisms in newborn piglets. Br. J. Nutr. 2014;112:1459–1468. doi: 10.1017/S0007114514002402. [DOI] [PubMed] [Google Scholar]
- Coisne C., Tilloy S., Monflier E., Wils D., Fenart L., Gosselet F. Cyclodextrins as Emerging Therapeutic Tools in the treatment of cholesterol-associated Vascular and neurodegenerative diseases. Molecules. 2016;21:1748. doi: 10.3390/molecules21121748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong R., Jia Y., Li R., Ni Y., Yang X., Sun Q., Parvizi N., Zhao R. Maternal low-protein diet causes epigenetic deregulation of HMGCR and CYP7alpha1 in the liver of weaning piglets. J. Nutr. Biochem. 2012;23:1647–1654. doi: 10.1016/j.jnutbio.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Dar S.A., Srivastava P.P., Varghese T., Gupta S., Krishna G., Nuzaiba P.M., Leya T. Expression of growth and Hunger related genes and Physio-Biochemical responses in Labeo rohita (Hamilton, 1822) fed with Lysine and betaine. Cell Physiol. Biochem. 2019;53:851–864. doi: 10.33594/000000177. [DOI] [PubMed] [Google Scholar]
- Day C.R., Kempson S.A. Betaine chemistry, roles, and potential use in liver disease. Biochim. Biophys. Acta. 2016;1860:1098–1106. doi: 10.1016/j.bbagen.2016.02.001. [DOI] [PubMed] [Google Scholar]
- Del Bas J.M., Crescenti A., Arola-Arnal A., Oms-Oliu G., Arola L., Caimari A. Intake of grape procyanidins during gestation and lactation impairs reverse cholesterol transport and increases atherogenic risk indexes in adult offspring. J. Nutr. Biochem. 2015;26:1670–1677. doi: 10.1016/j.jnutbio.2015.08.015. [DOI] [PubMed] [Google Scholar]
- Faust P.L., Kovacs W.J. Cholesterol biosynthesis and ER stress in peroxisome deficiency. Biochimie. 2014;98:75–85. doi: 10.1016/j.biochi.2013.10.019. [DOI] [PubMed] [Google Scholar]
- Figueroa-Soto C.G., Valenzuela-Soto E.M. Glycine betaine rather than acting only as an osmolyte also plays a role as regulator in cellular metabolism. Biochimie. 2018;147:89–97. doi: 10.1016/j.biochi.2018.01.002. [DOI] [PubMed] [Google Scholar]
- Gluba-Brzozka A., Franczyk B., Rysz J. Cholesterol Disturbances and the role of proper nutrition in CKD Patients. Nutrients. 2019;11 doi: 10.3390/nu11112820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goharkhay N., Tamayo E.H., Yin H., Hankins G.D., Saade G.R., Longo M. Maternal hypercholesterolemia leads to activation of endogenous cholesterol synthesis in the offspring. Am. J. Obstet. Gynecol. 2008;199:273.e1–273.e6. doi: 10.1016/j.ajog.2008.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Sun Q., Hu Y., Hou Z., Zong Y., Omer N.A., Abobaker H., Zhao R. Corticosterone-induced Lipogenesis activation and Lipophagy inhibition in chicken liver are alleviated by maternal betaine supplementation. J. Nutr. 2018;148:316–325. doi: 10.1093/jn/nxx073. [DOI] [PubMed] [Google Scholar]
- Hu Y., Sun Q., Li X., Wang M., Cai D., Li X., Zhao R. In Ovo injection of betaine affects hepatic cholesterol metabolism through epigenetic gene regulation in newly hatched chicks. PLoS One. 2015;10:e0122643. doi: 10.1371/journal.pone.0122643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Sun Q., Zong Y., Liu J., Idriss A.A., Omer N.A., Zhao R. Prenatal betaine exposure alleviates corticosterone-induced inhibition of CYP27A1 expression in the liver of juvenile chickens associated with its promoter DNA methylation. Gen. Comp. Endocrinol. 2017;246:241–248. doi: 10.1016/j.ygcen.2016.12.014. [DOI] [PubMed] [Google Scholar]
- Idriss A.A., Hu Y., Sun Q., Jia L., Jia Y., Omer N.A., Abobaker H., Zhao R. Prenatal betaine exposure modulates hypothalamic expression of cholesterol metabolic genes in cockerels through modifications of DNA methylation. Poult. Sci. 2017;96:1715–1724. doi: 10.3382/ps/pew437. [DOI] [PubMed] [Google Scholar]
- Idriss A.A., Hu Y., Hou Z., Hu Y., Sun Q., Omer N.A., Abobaker H., Ni Y., Zhao R. Dietary betaine supplementation in hens modulates hypothalamic expression of cholesterol metabolic genes in F1 cockerels through modification of DNA methylation. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 2018;217:14–20. doi: 10.1016/j.cbpb.2017.12.001. [DOI] [PubMed] [Google Scholar]
- Jia Y., Song H., Gao G., Cai D., Yang X., Zhao R. Maternal betaine supplementation during gestation enhances expression of mtDNA-Encoded genes through D-Loop DNA hypomethylation in the Skeletal muscle of newborn piglets. J. Agric. Food Chem. 2015;63:10152–10160. doi: 10.1021/acs.jafc.5b04418. [DOI] [PubMed] [Google Scholar]
- Khesht F.A., Hassanabadi A. Effects of sterol regulatory element-binding protein (SREBP) in chickens. Lipids Health Dis. 2012;11:20. doi: 10.1186/1476-511X-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever M., Slow S. The clinical significance of betaine, an osmolyte with a key role in methyl group metabolism. Clin. Biochem. 2010;43:732–744. doi: 10.1016/j.clinbiochem.2010.03.009. [DOI] [PubMed] [Google Scholar]
- Masser D.R., Berg A.S., Freeman W.M. Focused, high accuracy 5-methylcytosine quantitation with base resolution by benchtop next-generation sequencing. Epigenetics Chromatin. 2013;6:33. doi: 10.1186/1756-8935-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews J.O., Southern L.L., Higbie A.D., Persica M.A., Bidner T.D. Effects of betaine on growth, carcass characteristics, pork quality, and plasma metabolites of finishing pigs. J. Anim. Sci. 2001;79:722–728. doi: 10.2527/2001.793722x. [DOI] [PubMed] [Google Scholar]
- Matthews J.O., Ward T.L., Southern L.L. Interactive effects of betaine and monensin in uninfected and Eimeria acervulina-infected chicks. Poult. Sci. 1997;76:1014–1019. doi: 10.1093/ps/76.7.1014. [DOI] [PubMed] [Google Scholar]
- Monte M.J., Marin J.J., Antelo A., Vazquez-Tato J. Bile acids: chemistry, physiology, and pathophysiology. World J. Gastroenterol. 2009;15:804–816. doi: 10.3748/wjg.15.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou X., Gao J.H., He L.H., Yu X.H., Wang G., Zou J., Zhao Z.W., Zhang D.W., Zhou Z.J., Tang C.K. Angiopoietin-1 aggravates atherosclerosis by inhibiting cholesterol efflux and promoting inflammatory response. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2019;1865:158535. doi: 10.1016/j.bbalip.2019.158535. [DOI] [PubMed] [Google Scholar]
- Polin D., Wolford J.H. Role of estrogen as a cause of fatty liver hemorrhagic syndrome. J. Nutr. 1977;107:873–886. doi: 10.1093/jn/107.5.873. [DOI] [PubMed] [Google Scholar]
- Sharpe L.J., Brown A.J. Controlling cholesterol synthesis beyond 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) J. Biol. Chem. 2013;288:18707–18715. doi: 10.1074/jbc.R113.479808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohi G., Marchand K., Revesz A., Arany E., Hardy D.B. Maternal protein restriction elevates cholesterol in adult rat offspring due to repressive changes in histone modifications at the cholesterol 7alpha-hydroxylase promoter. Mol. Endocrinol. 2011;25:785–798. doi: 10.1210/me.2010-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sozen E., Ozer N.K. Impact of high cholesterol and endoplasmic reticulum stress on metabolic diseases: an updated mini-review. Redox Biol. 2017;12:456–461. doi: 10.1016/j.redox.2017.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapani L., Segatto M., Pallottini V. Regulation and deregulation of cholesterol homeostasis: the liver as a metabolic "power station. World J. Hepatol. 2012;4:184–190. doi: 10.4254/wjh.v4.i6.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berg E.H., Gruppen E.G., Ebtehaj S., Bakker S.J.L., Tietge U.J.F., Dullaart R.P.F. Cholesterol efflux capacity is impaired in subjects with an elevated Fatty Liver Index, a proxy of non-alcoholic fatty liver disease. Atherosclerosis. 2018;277:21–27. doi: 10.1016/j.atherosclerosis.2018.07.028. [DOI] [PubMed] [Google Scholar]
- Wang F., Xu J., Jakovlic I., Wang W.M., Zhao Y.H. Dietary betaine reduces liver lipid accumulation via improvement of bile acid and trimethylamine-N-oxide metabolism in blunt-snout bream. Food Funct. 2019;10:6675–6689. doi: 10.1039/c9fo01853k. [DOI] [PubMed] [Google Scholar]
- Wu Q., Ishikawa T., Sirianni R., Tang H., McDonald J.G., Yuhanna I.S., Thompson B., Girard L., Mineo C., Brekken R.A., Umetani M., Euhus D.M., Xie Y., Shaul P.W. 27-Hydroxycholesterol promotes cell-autonomous, ER-positive breast cancer growth. Cell Rep. 2013;5:637–645. doi: 10.1016/j.celrep.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Huang L., Gao J., Wen S., Tai Y., Chen M., Huang Z., Liu R., Tang C., Li J. Betaine attenuates chronic alcoholinduced fatty liver by broadly regulating hepatic lipid metabolism. Mol. Med. Rep. 2017;16:5225–5234. doi: 10.3892/mmr.2017.7295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao N., Yang S., Feng Y., Sun B., Zhao R. Enhanced hepatic cholesterol accumulation induced by maternal betaine exposure is associated with hypermethylation of CYP7A1 gene promoter. Endocrine. 2019;64:544–551. doi: 10.1007/s12020-019-01906-z. [DOI] [PubMed] [Google Scholar]