Abstract
With the majority of conventional cage (CC) laying facilities transitioning into cage-free (CF) systems in the near future, it is important to characterize biological markers of health in layers housed in commercial housings for sustainable production. The objectives of this study were to compare i) blood markers, that is heterophil:lymphocyte (H:L) ratios and susceptibility to avian pathogenic Escherichia coli (APEC) and ii) lung and ceca microbiome between hens at different maturity stages in commercial CC and CF farms. Laying hens at 3 maturity stages were randomly sampled (N = 20 per maturity and per farm). Blood was tested for H:L ratios and APEC killing ability using microscopy and in vitro assay, respectively. Microbiomes were assessed using 16S rRNA sequencing and QIIME2 analysis. Data show H:L ratios did not differ between maturities in both farms. Avian pathogenic Escherichia coli killing was only different in CC hens, where χ7122 level was higher (P < 0.05) in peak compared with early lay. In both farms, microbiome diversity was consistently different (P < 0.05) in both ceca and lung of early lay compared with peak and late lay. In the ceca and lung, relative abundances of the 3 predominant phyla (Bacteroidetes, Firmicutes, and Proteobacteria) did not significantly change with maturity in both farms. Potential pathogens Campylobacter and Staphylococcus reached greater (P < 0.05) abundances in CC lungs in early lay and in CF lungs in late lay, respectively. Overall, this study showed no differences in the stress marker H:L but identified some differences in resistance to APEC and microbiome composition across maturity stages in CC and CF. The lung and gut microbiomes were highly similar, with both serving as potential reservoirs for Campylobacter and Staphylococcus. Future studies on controllable environments for CF and CC are needed to develop adequate strategies for each housing and maturity stage to reduce pathogens and optimize disease-resistance.
Key words: layer, cage-free, conventional cage, microbiome, stress marker, APEC
Introduction
In the United States., approximately 81.6% of egg-laying chickens are housed in conventional cages (CC), whereas Only 13.3% of egg-laying chickens are housed in cage-free (CF) aviaries (USDA, 2019). However, because several retailers and restaurants have pledged to source only CF eggs in the near future (e.g., 2025), over half of U.S. hen housing is expected to switch to CF systems (Starmer, 2017). Compared with CC, CF system faces some challenges such as increased bacterial infections, poor air quality, and reduced production performance (Reviewed by Lay et al., 2011)
Exposure to stressors reduces performance in food producing animals (Gebregeziabhear and Ameha, 2015). Additionally, the host microbiota at mucosal surfaces have a major impact on health and performance in poultry (Shang et al., 2018). The chicken gut and lungs are 2 of the primary barriers between pathogens and the host (Bingula et al., 2017). Factors such as age may influence the composition of these commensal communities (Awad et al., 2016, Kers et al., 2018, Ngunjiri et al., 2019). The overgrowth of pathogenic bacteria in these sites is detrimental to both chicken health and consumers. Thus, identifying critical laying stages more susceptible to increased abundances of potentially virulent bacteria is imperative.
The egg-laying cycle of hens in poultry production can be categorized by 3 different maturity stages, that is, early lay (∼18 wk-old), peak lay (∼25 wk-old), and late lay (65–75 wk-old). One way to increase food production is to understand and improve animal welfare by developing management strategies targeted for specific maturity stages of the animals. To our knowledge, differences in biological markers for stress and health and microbiome at multiple points during egg production have not yet been investigated. The objectives of this study were to characterize a biological stress marker, potential resistance to avian pathogenic Escherichia coli (APEC), and the gut and lung microbiome of laying hens across laying stages, for example early, peak, and late lay in CC and CF farms.
Materials and methods
Ethics Statement
All animal handling and treatment methods used in this study were approved by the Iowa State University Institutional Animal Care and Use Committee number 4-17-8502-G. Efforts were made by investigators to minimize suffering in laying hens during sample collection.
Animals
Chickens from 2 commercial farms (CC and CF) in Iowa were sampled between May and September of 2017 across 6 sampling trips total (3 CC, 3 CF) (Table 1). Conventional cage facility had a nominal capacity of 200,000 hens, 6 to 8 hens per cage at a stocking density of 67 to 80 in2/hen. Manure was collected on a belt below each cage tier and was removed twice per wk. Conversely, the CF aviary house had a nominal capacity of 50,000 hens, where hens had free access to litter (mix of some bedding material, mostly feces) floor with multitier colonies for feeding, drinking, perching, and laying eggs. All hens were given feed and water ad libitum and were raised to up 90 wk (i.e., single cycle) before depopulation. Both farms provided data on flock productivity, including body weight, weekly average rate of lay, and mortality (Table 1). Samples were collected from the 3 laying stages: i) early lay (17–23 wk), ii) peak lay (25–39 wk), and iii) late lay (64–88 wk) from the CC and CF commercial farms. We randomly sampled 20 animals for each maturity group (N = 20 × 3 maturities × 2 farms; N = 120 total).
Table 1.
Flock production information of laying hens in conventional cage (CC) and cage-free (CF) farms.
| Production stage | Sampling dates1 | # Of hens2 | Age3 | Weekly mortality4 | Cumulative mortality5 | Rate of egg production6 | Case weight7 | Body weight8 |
|---|---|---|---|---|---|---|---|---|
| CC Early lay | 5/17 & 9/17 | 20 | 17–21 | 0.03 ± 2.98E-05a | 0.04 ± 8.94E-05a | 3.17 ± 0.03a | 14.69 ± 0.01a | 1.25 ± 0.04a |
| CC Peak lay | 5/17 & 6/17 | 20 | 25–39 | 0.11 ± 5.71E-05b | 1.18 ± 1.36E-03b | 93.33 ± 0.01b | 20.61 ± 0.33b | 1.48 ± 0.01b |
| CC Late lay | 5/17 | 20 | 64–74 | 0.16 ± 1.20E-04c | 5.78 ± 2.73E-03c | 77.10 ± 0.02c | 23.24 ± 0.08c | 1.50 ± 0.01b |
| CF Early lay | 6/17 & 8/17 | 20 | 17–23 | 0.06 ± 8.64E-05a | 0.45 ± 1.05E-03a | 2.99 ± 0.01a | 16.33 ± 0.44a | 1.37 ± 0.10a |
| CF Peak lay | 6/17 & 9/17 | 20 | 28–34 | 0.09 ± 6.56E-05b | 2.23 ± 9.11E-04b | 90.70 ± 0.02b | 21.60 ± 0.28b | 1.60 ± 0.01b |
| CF Late lay | 6/17, 8/17 & 9/17 | 20 | 65–88 | 0.28 ± 2.06E-04c | 9.74 ± 1.42E-03c | 42.00 ± 0.20c | 23.01 ± 0.13c | 1.54 ± 0.03b |
a-cSignificant differences were only assessed between maturity stages within each environment. Data represented as mean ± SEM. Statistical differences between production stages were calculated using Student t test with different letters indicating significant (P < 0.05) differences.
Dates the commercial hens were sampled in month/year. Barn temperatures for 6/17, 8/17, and 9/17 were recorded at 25.8°C, 26.7°C, and 26.1°C, respectively.
Total number of hens sampled within each production stage.
Weeks of age.
Percentage of deaths the week before sampling.
Percentage of cumulative deaths to date.
Percentage of hens laying ≥1 egg per day.
Weight in kilograms of 30 dozen eggs.
Weight in kilograms of hens.
Heterophil to Lymphocyte Ratio
The heterophil:lymphocyte (H:L) ratio is a well-established stress marker used in poultry (Gross and Siegel, 1983). To calculate these ratios, blood was collected from the wing vein using heparinized needles and syringes. Approximately 3 μL of fresh blood were aliquoted onto a standard microscope slide (one slide per hen, N = 120), briefly air dried, fixed, and stained using a Neat Stain Hematology Staining Kit (25,034, Polysciences, Inc., Warrington, PA). For each slide, approximately 100 leukocytes (lymphocytes, monocytes, heterophils, basophils, or eosinophils) were enumerated via light microscopy based on phenotypic characteristics (De Macchi et al., 2013). The H:L ratio was then calculated by averaging heterophils and lymphocytes identified in slides from each laying stage.
Blood Bactericidal Ability Assay
Heparinized blood from the wing vein was pooled (N = 5 hens, 4 pools per maturity group from each farm) into CO2-independent media (18045088, Gibco, Waltham, MA) at a 1:20 ratio. Avian pathogenic Escherichia coli strains, including APEC-O1 (Johnson et al.,2007), APEC-O2 (Johnson et al., 2005), and χ7122 (O78:K80) (Mellata et al., 2010) were mixed into fresh blood medium (20 μL bacterial inoculum containing 102 CFU: 200 μL blood) and co-incubated at 37°C for 30 min, diluted in PBS, and plated for enumeration on MacConkey agar (212123, BD Difco, Franklin Lake, NJ). A nonpathogenic E. coli strain (MG1655) was used as a serum-sensitive control (Barbieri et al., 2013)
Microbiome Sample Collection and DNA Isolation
Middle lung tissue and right and left ceca contents (pooled within hen) were aseptically collected from individual hens, immediately placed and transported on dry ice, and stored at −80°C. Total DNA was isolated using the DNeasy PowerSoil Kit (12,888, Qiagen, Germantown, MD) using the following modifications: 0.25 g ceca contents and 0.025 g lung tissue used as input, and samples were homogenized for 20 min using bead-containing tubes. A NanoDrop 2,000 spectrophotometer was used to assess DNA quality postextraction (260–280 nm ratios). Concentrations were determined using a Qubit fluorometer (double-stranded DNA broad range kit, Q32850, Thermo Fisher Scientific, Waltham, MA), adjusted to 50 ng/μL in nuclease-free water and shipped on dry ice to GeneSeek Neogen, Inc. (Lincoln, Nebraska).
Microbiome 16S rRNA Sequencing
For 16S rRNA sequencing of 120 (N = 20 × 3 laying stages × 2 farms) lung and 120 ceca samples, DNA libraries were prepared using the MiSeq V3 kit (Cat # MS-102-3003, Illumina, San Diego, CA) following all manufacturer's instructions with 300 × 300 paired-end sequencing with 600 cycles (Illumina). Samples were individually barcoded for multiplexing and sequenced for V4 of the 16S rRNA gene (515F: GTGYCAGCMGCCGCGGTAA, 806R: GGACTACHVGGGTWTCTAAT) as previously reported (Caporaso et al., 2011) with all samples multiplexed on a single run. Both primers were previously used for chickens (Zhao et al., 2013) and produce diversity measures comparable to full-length 16S rRNA sequences (Youssef et al.,2009).
Microbiome Diversity and Abundances Analyses
Raw FastQ files were obtained from GeneSeek, and 16S rRNA analyses were performed using Quantitative Insights into Microbial Ecology (QIIME2, version 2017.12; Caporaso et al., 2010). More specifically, paired-end sequences were merged using the join paired-end function using default parameters. The split libraries command was used to sparse apart individual samples with the criteria that 97% of sequences have Phred >25, and all other default parameters were used. Sequences from samples were assigned to taxonomy using the pick open sourced reference operational taxonomic units using default parameters in QIIME, which utilizes uclust and GreenGenes (versions 13.8) reference database (DeSantis et al., 2006, Edgar, 2010). Alpha (Chao1, Simpson, and Shannon) and beta diversity (Bray–Curtis Distance) were calculated using the core diversity analyses command with default parameters QIIME algorithm (Lozupone and Knight, 2005).
Statistical Analyses
All statistical analyses were performed in GraphPad Prism software (San Diego, CA). Comparisons between H:L ratios, blood bactericidal ability, microbiota alpha and beta diversity, phyla relative abundances, and bacterial abundances were performed using an ANOVA followed by Tukey's test for multiple means comparison between laying stages. A P value ≤ 0.05 was considered statistically significant. Because we did not set up the CC and CF environments and sampled commercial farms from different locations, no pairwise comparisons were made between CC and CF hens given the range of variable factors (diet, vaccination, environment, etc.; reviewed in Kers et al., 2018), which could confound any statistical conclusions on the environment effect.
Results
Flock Production Information
Data on flock productivity included weekly and cumulative mortality, rate of egg production, case weight, and body weight (Table 1). In both CF and CC, weekly mortality, cumulative mortality, and case weight significantly (P < 0.05) increased over laying stages. Additionally, egg production was significantly (P < 0.05) higher at peak lay, with 90.70% (CF) and 93.33% (CC) of hens laying ≥ 1 egg/D during this stage. Although body weight significantly (P < 0.05) increased from early to peak lay, no differences were identified between peak and late lay in both farms.
Maturity Stage Did Not Impact Heterophil to Lymphocyte Ratio
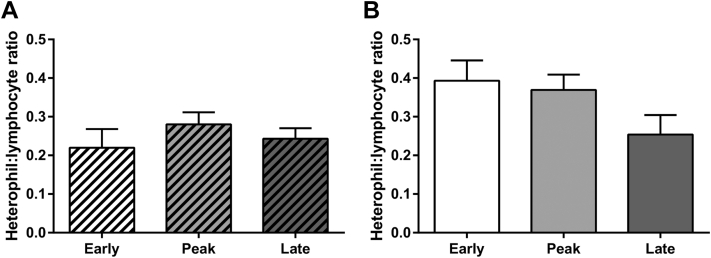
The H:L ratio was assessed as a marker for stress. In Figure 1, no significant differences were detected in H:L ratio between different laying stages of hens in both CC (Figure 1A) and CF (Figure 1B) farms.
Figure 1.
Heterophil to lymphocyte (H:L) ratios. The H:L ratios in whole blood were calculated from (A) conventional cage (CC, dashed column) and (B) cage-free (CF) hens during early (white), peak (light gray), and late (dark gray) laying stages of production. Ratios were calculated by counting 100 immune cells per hen. Bars represent mean ± SEM from 20 hens. Statistical comparisons were calculated using one-way ANOVA and Tukey's test for multiple comparisons between laying stages.
Maturity Stage Impacts Blood Bactericidal Ability in CC But Not in CF Hens
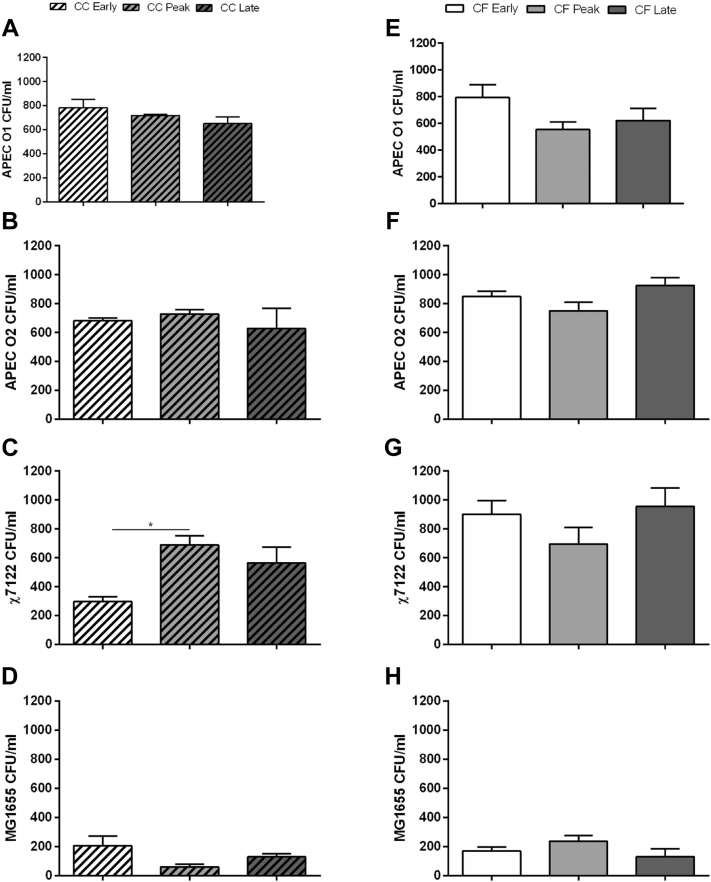
Data from in vitro APEC blood killing ability are summarized in Figure 2. In CC group, although killing of APEC O1 (Figure 2A) and APEC O2 (Figure 2B) were not different in blood between laying stages, χ7122 (Figure 2C) was most effectively killed by blood from early lay compared with peak lay hens (P < 0.05). However, no significant differences in bactericidal responses were identified between laying stages of CF hens (Figures 2E–2G). The killing ability of the nonpathogenic control E. coli MG1655 was the same between laying groups in both CC (Figure 2D) and CF (Figure 2H).
Figure 2.
Whole blood bactericidal assay. Heparinized whole blood was collected from the wing vein of hens in conventional (CC, dashed column; A–D) and cage-free (CF; E–H) environment at early (white), peak (light gray), and late (dark gray) lay and pooled (n = 5 hens) within barn into CO2 independent media. The strains APEC O1 (A and E), APEC O2 (B and F), χ7122 (C and G), and MG1655 (D and H) were mixed with the whole blood solution. Values represent the average of data from 4 independent pools, assayed in triplicate. Statistical differences were calculated using one-way ANOVA and Tukey's test for multiple comparisons between maturity stages. ∗, P < 0.05. APEC, avian pathogenic Escherichia coli.
Microbiome Diversity Analysis Identified Differences in Evenness in the Ceca and Lung
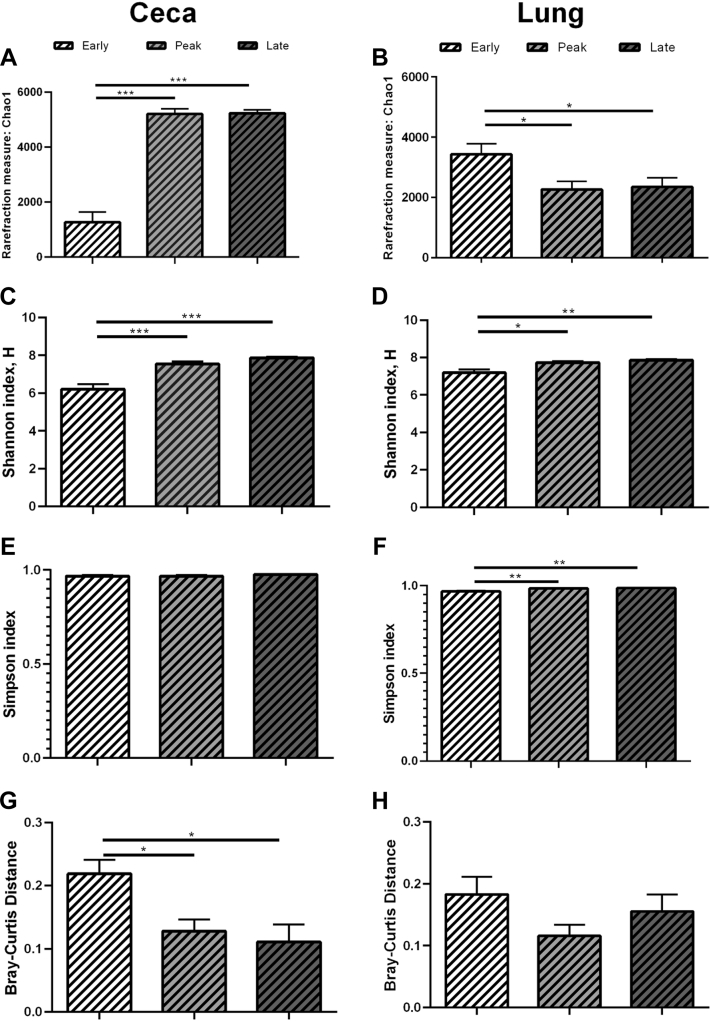
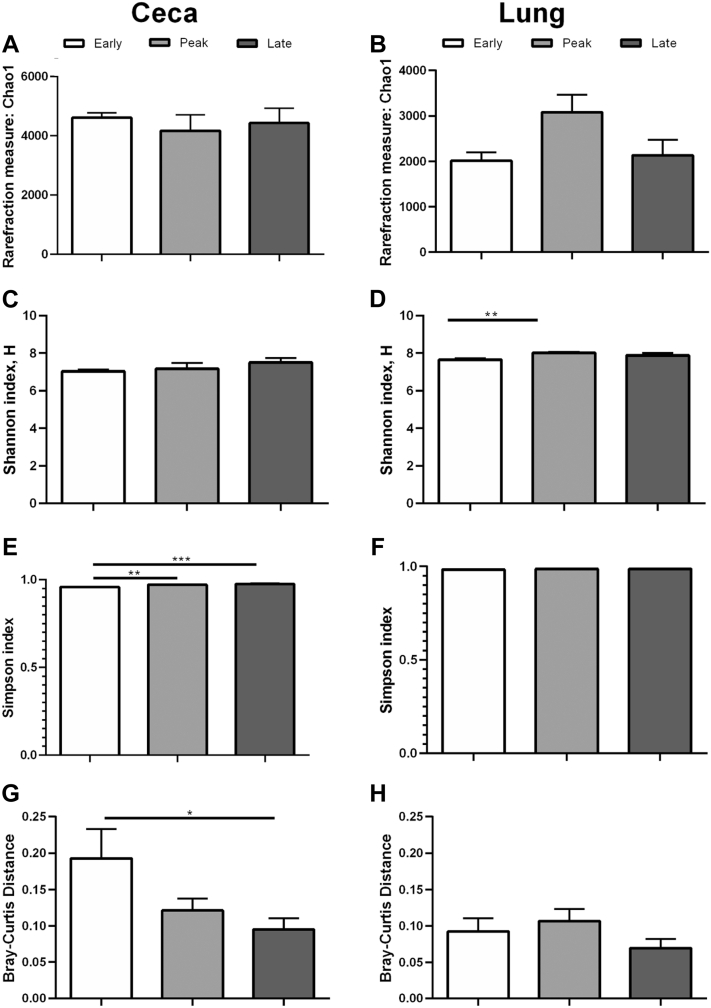
The 16S rRNA analyses were completed for the microbiome from ceca and lung of hens in CC and CF farms from early, peak, and late lay stages. The 16S rRNA data set is available in the NCBI Sequence Read Archive repository with accession BioProject ID PRJNA447997. Alpha diversity was measured as richness (Chao1) and diversity (Simpson and Shannon) and are found in Figure 3. In CC, richness in the ceca microbiome was lowest (P < 0.001) in early lay compared with the other laying stages (Figure 3A), and this relationship was the opposite in the lung (Figure 3B) with early lay having higher (P < 0.05) richness compared with the other laying stages. Using the Shannon index (i.e., greater weight to more-prevalent taxa), diversity was lowest in the ceca (Figure 3C, P < 0.001) and lung (Figure 3D, P < 0.05) of early lay hens compared with peak and late laying stages. However, according to the Simpson index (i.e., all taxa are assumed to be represented equally), only the lung microbiome exhibited significant differences, with early lay hens having less diversity (Figure 3F, P < 0.01) vs. peak and late laying stages. In the ceca, beta diversity was higher (Figure 3G, P < 0.05) during early lay compared with peak and late laying stages, indicating more unique species were identified. In CF, diversity of the ceca and lung microbiomes did not differ significantly in most cases. However, Shannon diversity index was significantly lower (P < 0.05) in the lung during early lay compared with peak lay (Figure 4D). Simpson diversity index was significantly lower in the ceca during early lay compared with both other groups (Figure 4E). Bray–Curtis distance (beta diversity) was higher (P < 0.05) in hens during early lay compared with late lay in the ceca (Figure 4G).
Figure 3.
Microbiome diversity in CC hens. Diversity was measured as Chao1, Shannon, and Simpson in the ceca and lung of hens in a conventional cage (CC, dashed column) environment at early (white), peak (light gray), and late (dark gray) lay. (A) Chao1 in the ceca, (B) Chao1 in the lung, (C) Shannon in the ceca, (D) Shannon in the lung, (E) Simpson in the ceca, (F) Simpson in the lung, (G) Brey–Curtis distance beta diversity in the ceca, and (H) Bray–Curtis distance beta diversity in the lung. Bars represent the interquartile range from 20 hens/group. Statistical significance (P < 0.05) was determined using a one-way ANOVA with Tukey's method for multiple testing correction. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.0001.
Figure 4.
Microbiome diversity in CF hens. Diversity was measured as Chao1, Shannon, and Simpson in the ceca and lung of hens in a cage-free (CF) environment at early (white), peak (light gray), and late (dark gray) lay. (A) Chao1 in the ceca, (B) Chao1 in the lung, (C) Shannon in the ceca, (D) Shannon in the lung, (E) Simpson in the ceca, (F) Simpson in the lung, (G) Brey–Curtis distance beta diversity in the ceca, and (H) Brey–Curtis distance beta diversity in the lung. Bars represent the interquartile range from 20 hens/group. Statistical significance (P < 0.05) was determined using a one-way ANOVA with Tukey's method for multiple testing correction. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001.
Abundances of Bacterial Taxa Detected
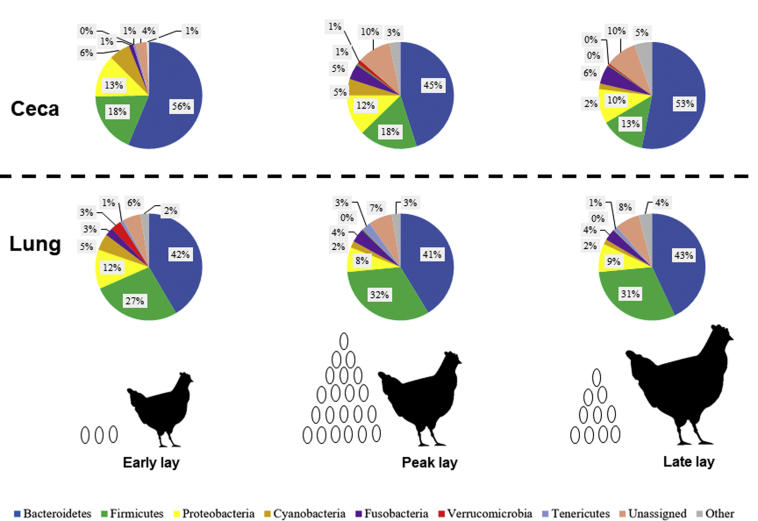
Abundances of bacterial phyla in CC and CF hens are described in Figures 5 and 6, respectively. The 3 major phyla in both ceca and lung across laying stages were Bacteroidetes, Firmicutes, and Proteobacteria. Across laying stages, the ceca microbiome had higher relative abundances of Bacteroidetes and lower relative abundances of Firmicutes, compared with the lung. In the CC ceca, the relative abundance increases in Fusobacteria from 1, 5, and 6% in early, peak, and late lays, respectively, and was statistically lower (P < 0.05) during early lay compared with peak and late laying stages (Figure 5). Additionally, the relative abundance of unassigned sequences increased from 4 to 10% from early to peak and late laying stages, respectively, and was statistically lower (P < 0.05) during early lay compared with peak and late laying stages. In the CC lung, Verrucomicrobia relative abundance was higher in early lay (3%) compared with lower abundances at peak and late laying stages (<0.05%; P < 0.05). Otherwise, no significant differences were seen in the CC lung across laying stages.
Figure 5.
Pie charts of relative microbial abundances in the ceca and lung across production stages in CC hens. Relative microbial abundances were based on 16S rRNA sequencing of ceca (top) and lung (bottom) in hens from a conventional cage (CC) environment during early, peak, and late stages of production. Each color represents a particular phylum. Each pie chart represents the average (n = 20) relative abundances of phyla.
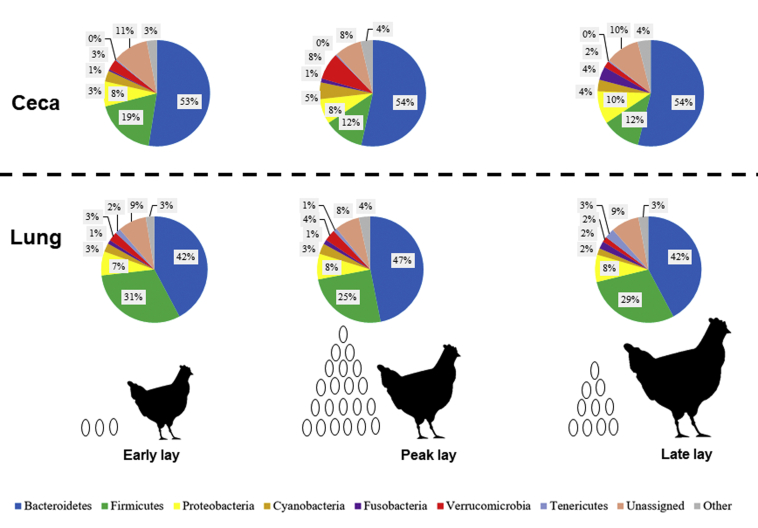
Figure 6.
Pie charts of relative microbial abundances in the ceca and lung across production stages in CF hens. Relative microbial abundances were based on 16S rRNA sequencing of ceca (top) and lung (bottom) in hens from a cage-free (CF) environment during early, peak, and late stages of production. Each color represents a particular phylum. Each pie chart represents the average (n = 20) relative abundances of phyla.
In the CF ceca (Figure 6), the relative abundance of Verrucomicrobia increased from early (3%) to peak (8%) lay, though abundance of this taxon decreased at late (2%) lay (P < 0.05). There was a significant (P < 0.05) decrease in Firmicutes from early (19%) to peak and late (both 12%) laying stages. Additionally, the relative abundance of Fusobacteria increased significantly (P < 0.05) from 1 to 4% from early and peak to late lay, respectively. In the CF lung (Figure 6), relative abundances for Bacteroidetes were significantly higher (P < 0.05) at peak lay (47%) compared with both early and late laying stages (both 42%). However, the inverse was seen for Firmicutes as levels were significantly lower (P < 0.05) at peak lay (25%) vs. both early (31%) and late lay (29%).
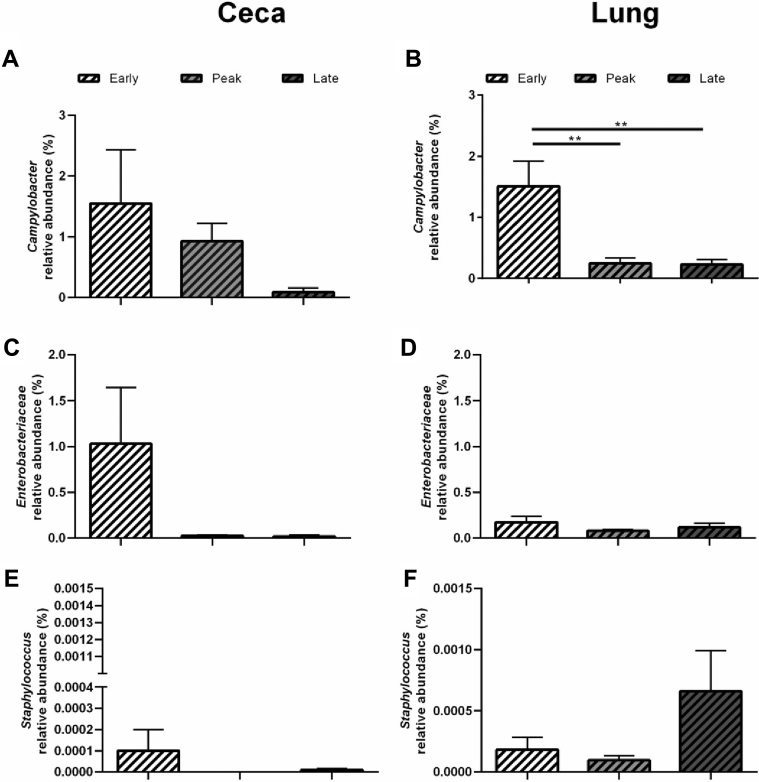
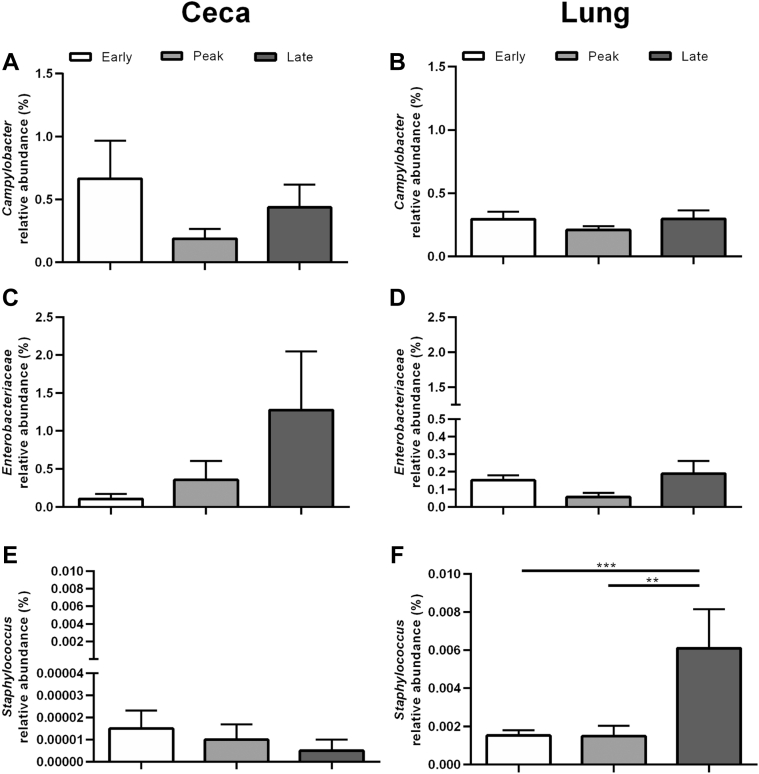
Looking specifically at potential-foodborne pathogens (i.e., Campylobacter, Enterobacteriaceae, and Staphylococcus), Campylobacter was highest during early lay (P < 0.05) in the lung compared with peak and late laying stages in CC hens (Figure 7B). Conversely, Staphylococcus levels were significantly (P < 0.05) greater in the lung at late lay compared with other laying stages in CF hens (Figure 8F). No other differences between laying stages were seen with Campylobacter, Enterobacteriaceae, nor Staphylococcus in either CC (Figure 7) or CF (Figure 8) layer hens.
Figure 7.
Relative abundances of Campylobacter, Enterobacteriaceae, and Staphylococcus in the ceca and lung microbiomes of CC hens. Bars represent the mean ± SEM relative abundance of sequences from 20 hens in a conventional cage (CC, dashed column) environment at early (white), peak (light gray), and late (dark gray) lay. Data for the ceca is displayed on the left side and lung on the right side. The relative abundances are shown for (A) Campylobacter in the ceca and (B) lung, (C) Enterobacteriaceae in the ceca and (D) lung, (E) Staphylococcus in the ceca and (F) lung. Statistical significance was determined using a one-way ANOVA with Tukey for multiple testing correction. ∗∗, P < 0.01.
Figure 8.
Relative abundances of Campylobacter, Enterobacteriaceae, and Staphylococcus in the ceca and lung microbiomes of CF hens. Bars represent the mean ± SEM relative abundance of sequences from 20 hens in a cage free (CF) environment at early (white), peak (light gray), and late (dark gray) lay. Data for the ceca is displayed on the left side and lung on the right side. The relative abundances are shown for (A) Campylobacter in the ceca and (B) lung, (C) Enterobacteriaceae in the ceca and (D) lung, (E) Staphylococcus in the ceca and (F) lung. Statistical significance was determined using a one-way ANOVA with Tukey for multiple testing correction. ∗∗, P < 0.01. ∗∗∗, P < 0.001.
Discussion
In this study, we compared different characteristics, for example, H:L ratio, resistance to APEC, ceca, and lung microbiomes, between laying stages of hens from CC and CF commercial farms. Previous studies have shown that early exposures of chickens to stress (i.e., in commercial hatcheries) have prolonged effects later in life and that chickens negatively respond to stress at different maturities (Ericsson et al., 2016, Hedlund et al., 2019). Likewise, age and body site drive major shifts in the chicken gut and lung microbiota (Awad et al., 2016, Ngunjiri et al., 2019). However, changes in biological markers of stress and disease susceptibility as well as shifts in the mature hen microbiome between early, peak, and late laying stages have not been previously investigated. Expression of natural behavior, which maximizes mobility and opportunities for social interactions, has largely factored into the shift from CC to CF settings (Brambell, 1965). Modern CF aviary houses have large hen capacities, as there were 50,000 hens in each barn in the current study. However, mortality in CF environments is a concern, supported by data in the current study showing the flock had a cumulative mortality of approximately 10% by late lay. This mortality rate is similar to that reported for CF flocks in Great Britain at 8.55% mortality (Weeks et al.,2012). Notably, this is about 1.7 times higher than that of CC birds (6%) (Table 1). Modern CC production typically consists of 6 to 8 hens/battery cage in a multilevel design where eggs and manure are collected continuously via conveyer belts. This design improves egg production rates, supported by this study where >93% of CC hens laid ≥1 egg per day. In our study, the weekly mortality rate increased with age of the bird, which could be partially attibuted to bone fractures, considered a major cause of mortality of laying hens in caged environments (McCoy et al., 1996). However, in our study, we were not given information on specific causes of mortality from the farm records to confirm such speculations.
More frequent social encounters can exacerbate negative, stress-induced behaviors such as cannibalism and feather-pecking (Newberry, 2004) in high-density environments (Cloutier et al.,2002). In chickens, stress can have a profound and lasting effect on behavior, especially when experienced at puberty and early life (Ericsson et al. 2016). However, how stress levels and its consequences vary during different stages of egg production in the CC and CF farms have not been investigated. In this study, we did not identify any significant difference in stress levels as measured by H:L ratio between maturity stages, and all H:L ratios were in the range of 0.2 (CC) and 0.5 (CF), which are both below that characteristic of high degree of stress (≤0.8) (Gross and Siegel, 1993). These data suggest that maturity stage did not affect stress levels in either farms, or management practices in these farms are efficient in controlling the changes between laying stages. Other biological marker of stress, such as circulating glucocorticoids, were not investigated in this study; however, because these markers, for example corticosterone levels, were positively correlated with H:L ratios (Maxwell, 1993), H:L ratios are still a good indicator of stress in chickens. Future studies should still use additional means of quantifying biological stress to more holistically study stress in layer hens (Scanes, 2016).
Susceptibility to infection by APEC and other microbes is commonly observed during conditions of high stress (Collingwood et al., 2014). Avian pathogenic E. coli is a diverse group of opportunistic E. coli pathogens (most commonly O1, O2, and O78), which often cause lethal respiratory and systemic diseases in the commercial farms. This diversity makes it difficult to broadly immunize against APEC (Schouler et al., 2012; Mellata, 2013), although recent attempts using prophylactics have shown preliminary success (Van Goor et al., 2017, Redweik et al., 2020). Because a correlation between blood phagocyte immune response in vitro with in vivo disease susceptibility was reported in humans (Guirado et al., 2015), we used blood killing ability against 3 APEC serotypes (O1, O2, O78) to evaluate whether susceptibility of chickens to APEC infections would be different depending on laying stages. In the current study, we only found differences between laying stages in CC hens, where χ7122 killing had worse survival in peak lay vs. early lay whole blood. Because we observed no differences in H:L ratios between laying stages in CC nor CF hens, the difference in χ7122 killing is independent of this stress biomarker. Thus, other factors not accounted for in this study could had influenced the blood killing ability. Future studies using in vivo challenge to incorporate all facets of host immunity are needed to verify the sensitivity to APEC.
Infectious diseases are a concern for hens raised in high stocking density environment, especially in the CF environment. Increased opportunities for social interactions combined with a constant exposure to litter mainly composed of feces in CF houses result in an increased risk for disease and mortality (Fossum et al., 2009). Furthermore, loss of microbial diversity at mucosal surfaces is linked to infectious and noninfectious disease susceptibility (Mosca et al., 2016). Previous studies on other animals have shown an impact of age on both lung (Glendinning et al.,2017) and gut (Lourenco et al., 2019) in sheep and calves, respectively. In chickens, a strong correlation between age and composition of the gut microbiome was determined when tested from hatch to 28 D of age (Ballou et al., 2016). Here, we characterized the gut and lung microbiomes of CC and CF hens at early, peak, and late laying stages to identify differences in composition based on niche site (i.e., ceca vs. lung) and maturity.
A recent study, identified a possible interaction between gut and lung microbiota, including potential pathogens in chickens (Ngunjiri et al., 2019). In our study, the composition of the microbiota at the phyla level, Bacteroidetes, Firmicutes, and Proteobacteria comprised at least 74% relative abundance in both the ceca and lung of both CC and CF hens. However, we found few differences at the phyla level between the gut and lung. The similarity of the microbial relative abundances between the lung and ceca microbiomes may be because of the population density in both CC (200,000) and CF (50,000) chickens/barn, allowing extensive inhalation of fecal material within the poultry environment (Radon et al., 2002). In CF, relative abundances of Verrucomicrobia were particularly high in the ceca during peak lay. Verrucomicrobia is commonly found in soil and has been suggested as a marker for a healthy gut microbiome (Janssen et al., 2002, Belzer and De Vos, 2012). Additionally, different relative abundances of Bacteroidetes and Firmicutes were observed in the CF lung between maturities with significantly higher Bacteroidetes and lower Firmicutes during peak lay compared with both early and late lay. This change of diversity observed between maturity stages in CF hens has been implicated as a signature of gut health and shifts with age (Ley et al., 2006). However, these differences between laying stages were not as distinct in CC, suggesting that CC hens may be less susceptible to microbiome changes across laying stages than CC hens, but this needs to be confirmed by experiments with controlled factors, which may have affected microbiota of hens raised in CC and CF farms.
Maturity stage has an impact on the colonization and shedding of particular pathogens; in a longitudinal study, prevalence of hens shedding Salmonella was highest during onset of lay (Gole et al., 2014). Thus, we investigated the differences of common foodborne pathogens in both the gut and lung microbiomes. We identified higher levels of Staphylococcus in the lung of CF hens during late lay compared with other laying stages. Although late lay hens also experienced highest mortality rates compared with early and peak lay hens, we do not have data from the CF farm to suggest Staphylococcus abundance is related to these mortalities. Outbreaks of Staphylococcus, a Gram-positive foodborne pathogen, were found by the Foodborne Disease Outbreak Surveillance System to be commonly associated with meat and poultry dishes (Bennett et al., 2013). Additionally, Campylobacter is a major foodborne pathogen for humans primarily transmitted via consumption of contaminated poultry products (Skarp et al., 2016). Interestingly, in CC hens at early lay, microbiome richness was inversely associated with Campylobacter abundance in the lungs. This suggests certain microbes that could reduce Campylobacter abundance may have been missing. Certain probiotic taxa like lactobacilli are posited to reduce Campylobacter colonization (Ding et al., 2005, Bereswill et al., 2011, Kaakoush et al., 2014). Conversely, Campylobacter could be supported by other microbes, as our study and others (Sofka et al., 2015, Sakaridis et al., 2018) found Proteobacteria to be positively associated with Campylobacter colonization, though only our study shows this phenomenon occurs in the lung. Altogether, these differences in Staphylococcus and Campylobacter lung abundances between laying stages in CF and CC hens, respectively, have implications for foodborne disease. Lung colonization of foodborne bacterial pathogens in chickens may be of concern to consumers. During evisceration, the lungs are removed, but aerosolization of bacteria during this process could consequently serve as a source for human foodborne pathogens. Importantly, this study sampled layer birds, which are not used for meat production. Future studies should investigate these trends in broiler breeds to determine pathogen abundance in the lungs. Although no significant differences in Enterobacteriaceae levels were found between maturities in both CC and CF, 16S is not able to distinguish between Enterobacteriaceae species because of the high genetic similarities within the Enterobactericeae family (Saputra et al., 2015). Complementary methods should be used to verify differences in the level of Enterobacteriaceae species such as E. coli and Salmonella (Ngunjiri et al., 2019).
While this study provides important data on the gut and lung microbiota and potential susceptibility to APEC at different laying stages in hens from CC and CF commercial farms, it has limitations that must be acknowledged. Cage-free and CC farms of this study are located in separate locations in Iowa and managed by different teams. Additionally, details regarding vaccination record, diet, antimicrobials, and other factors, which all can affect the chicken microbiome and health (Kers, et al., 2018), were proprietary. Thus, statistical comparison between CC and CF birds and speculation on the environment effect were omitted.
Conclusions
Overall, this study identified no differences in stress marker across stages of production in either CC or CF hens. Additionally, only laying stage-related differences in resistance against one APEC strain were seen in CC hens, suggesting CF management systems established are more consistent in minimizing variability in APEC resistance between laying stages. In addition, this was the first to compare the lung and gut microbiome of chickens in different stages of production. Surprisingly, the gut and lung microbiome appear to be relatively similar in composition, which may be because of the frequent interaction with fecal material in the commercial farming environment. Finally, environment and laying stage influenced the abundance of Campylobacter (CC) and Staphylococcus (CF) in the lungs, which may be a source of poultry product contamination. Future studies will seek to experimentally study specific host and environmental factors to enable comparisons between CC and CF hens.
Acknowledgments
This research was funded by the Iowa State University (ISU) Egg Industry Center Funding to AVG, HX, and MM; ISU start-up funding and USDA-NIFA Hatch project IOW03902 to MM. The authors thank the commercial poultry facilities in Iowa for their kind donation of hens along with access to their facilities. They thank Allison Brost and Bienvenido Cortes (Iowa State University, Ames, IA) for assistance in sampling.
Conflict of Interest Statement: The authors did not provide a conflict of interest statement.
References
- Awad W.A., Mann E., Dzieciol M., Hess C., Schmitz-Esser S., Wagner M., Hess M. Age-related differences in the Luminal and Mucosa-associated gut microbiome of broiler chickens and shifts associated with Campylobacter jejuni infection. Front. Cell. Infect. Microbiol. 2016;6:154. doi: 10.3389/fcimb.2016.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballou A.L., Ali R.A., Mendoza M.A., Ellis J.C., Hassan H.M., Croom W.J. Development of the Chick microbiome: how early exposure influences future microbial diversity. Front. Vet. Sci. 2016;3:2. doi: 10.3389/fvets.2016.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri N.L., De Oliveira A.L., Tejkowski T.M., Pavanelo D.B., Rocha D.A., Matter L.B. Genotypes and pathogenicity of cellulitis isolates reveal traits that modulate APEC virulence. PLoS One. 2013;8:e72322. doi: 10.1371/journal.pone.0072322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belzer C., De Vos W.M. Microbes inside--from diversity to function: the case of Akkermansia. ISME J. 2012;6:1449–1458. doi: 10.1038/ismej.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett S.D., Walsh K.A., Gould L.H. Foodborne disease outbreaks caused by Bacillus cereus, Clostridium perfringens, and Staphylococcus aureus--United States, 1998-2008. Clin. Infect. Dis. 2013;57:425–433. doi: 10.1093/cid/cit244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bereswill S., Plickert R., Fischer A., Kühl A.A., Loddenkemper C., Batra A. What you eat is what you get: novel Campylobacter models in the quadrangle relationship between nutrition, obesity, microbiota and susceptibility to infection. Eur. J. Microbiol. Immunol. 2011;1:237–248. doi: 10.1556/EuJMI.1.2011.3.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingula R., Filaire M., Radosevic-Robin N., Bey M., Berthon J.Y., Bernalier-Donadille A., Vasson M.P., Filaire E. Desired Turbulence? Gut-lung Axis, immunity, and lung Cancer. J. Oncol. 2017;2017:5035371. doi: 10.1155/2017/5035371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambell F.W.R. Her Majesty's Stationary Office; London: 1965. Report of the Technical Committee to Enquire into the Welfare of Animals Kept under Intensive Livestock Husbandry Systems. [Google Scholar]
- Caporaso J.G., Lauber C.L., Walters W.A., Berg-Lyons D., Lozupone C.A., Turnbaugh P.J. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. U.S.A. 2011;108(Suppl 1):4516–4522. doi: 10.1073/pnas.1000080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloutier S., Newberry R.C., Honda K., Alldredge J.R. Cannibalistic behaviour spread by social learning. Anim. Behav. 2002;63:1153–1162. [Google Scholar]
- Collingwood C., Kemmett K., Williams N., Wigley P. 2014. Is the concept of avian pathogenic Escherichia coli as a single pathotype fundamentally flawed? Front. Vet. Sci. 2014;1:5. doi: 10.3389/fvets.2014.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Macchi B.M., Miranda F.J., De Souza F.S., De Carvalho E.C., Albernaz A.P., Do Nascimento J.L. Chickens treated with a nitric oxide inhibitor became more resistant to Plasmodium gallinaceum infection due to reduced anemia, thrombocytopenia and inflammation. Vet. Res. 2013;44:8–15. doi: 10.1186/1297-9716-44-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantis T.Z., Hugenholtz P., Larsen N., Rojas M., Brodie E.L., Keller K. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 2006;72:5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding W., Wang H., Griffiths M.W. Probiotics down-regulate flaA sigma28 promoter in Campylobacter jejuni. J. Food Prot. 2005;68:2295–2300. doi: 10.4315/0362-028x-68.11.2295. [DOI] [PubMed] [Google Scholar]
- Edgar R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- Ericsson M., Henriksen R., Bélteky J., Sundman A.-S., Shionoya K., Jensen P. Long- term and Transgenerational effects of stress experienced during different life Phases in chickens (Gallus gallus) PLoS One. 2016;11:e0153879. doi: 10.1371/journal.pone.0153879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossum O., Jansson D.S., Etterlin P.E., Vågsholm I. Causes of mortality in laying hens in different housing systems in 2001 to 2004. Acta Vet. Scand. 2009;51:3. doi: 10.1186/1751-0147-51-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebregeziabhear E., Ameha N. The effect of stress on productivity of animals: a review. J. Biol. 2015;5:2224–3208. [Google Scholar]
- Glendinning L., McLachlan G., Vervelde L. Age-related differences in the respiratory microbiota of chickens. PLoS One. 2017;12:e0188455. doi: 10.1371/journal.pone.0188455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gole V.C., Caraguel C.G., Sexton M., Fowler C., Chousalkar K.K. Shedding of Salmonella in single age caged commercial layer flock at an early stage of lay. Int. J. Food Microbiol. 2014;189:61–66. doi: 10.1016/j.ijfoodmicro.2014.07.030. [DOI] [PubMed] [Google Scholar]
- Gross W.B., Siegel H.S. Evaluation of the heterophil/lymphocyte ratio as a measure of stress in chickens. Avian Dis. 1983;27:972–979. [PubMed] [Google Scholar]
- Gross W.B., Siegel P.B. General principles of stress and welfare. In: Grandin T., editor. Livestock, Handling and Transport. CAB International; Wallingford, UK: 1993. pp. 21–34. [Google Scholar]
- Guirado E., Mbawuike U., Keiser T.L., Arcos J., Azad A.K., Wang S.H. Characterization of host and microbial determinants in individuals with latent tuberculosis infection using a human granuloma model. M. Bio. 2015;6:e02537–e025314. doi: 10.1128/mBio.02537-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund L., Whittle R., Jensen P. Effects of commercial hatchery processing on short- and long-term stress responses in laying hens. Sci. Rep. 2019;9:2367. doi: 10.1038/s41598-019-38817-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen P.H., Yates P.S., Grinton B.E., Taylor P.M., Sait M. Improved culturability of soil bacteria and isolation in pure culture of novel members of the divisions Acidobacteria, Actinobacteria, Proteobacteria, and Verrucomicrobia. Appl. Environ. Microbiol. 2002;68:2391–2396. doi: 10.1128/AEM.68.5.2391-2396.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson T.J., Kariyawasam S., Wannemuehler Y., Mangiamele P., Johnson S.J., Doetkott C. The genome sequence of avian pathogenic Escherichia coli strain O1:K1:H7 shares strong similarities with human extraintestinal pathogenic E. coli genomes. J. Bacteriol. 2007;189:3228–3236. doi: 10.1128/JB.01726-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson T.J., Siek K.E., Johnson S.J., Nolan L.K. DNA sequence and comparative genomics of pAPEC-O2-R, an avian pathogenic Escherichia coli transmissible R plasmid. Antimicrob. Agents Chemother. 2005;49:4681–4688. doi: 10.1128/AAC.49.11.4681-4688.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaakoush N.O., Sodhi N., Chenu J.W., Cox J.M., Riordan S.M., Mitchell H.M. The interplay between Campylobacter and Helicobacter species and other gastrointestinal microbiota of commercial broiler chickens. Gut Pathog. 2014;6:18. doi: 10.1186/1757-4749-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kers J.G., Velkers F.C., Fischer E.A.J., Hermes G.D.A., Stegeman J.A., Smidt H. Host and environmental factors affecting the intestinal microbiota in chickens. Front. Microbiol. 2018;9:235. doi: 10.3389/fmicb.2018.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lay D.C., Fulton R.M., Hester P.Y., Karcher D.M., Kjaer J.B., Mench J.A. Hen welfare in different housing systems. Poult. Sci. 2011;90:278–294. doi: 10.3382/ps.2010-00962. [DOI] [PubMed] [Google Scholar]
- Ley R.E., Turnbaugh P.J., Klein S., Gordon J.I. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- Lourenco J.M., Rothrock M.J., Jr., Fluharty F.L., Callaway T.R. The Successional changes in the gut microbiome of Pasture-raised chickens fed Soy-containing and Soy-free diets. Front. Sustain. Food Sys. 2019;3:35. [Google Scholar]
- Lozupone C., Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005;71:8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell M.H. Avian blood leucocyte response to stress. World Poult. Sci. J. 1993;49:34–43. [Google Scholar]
- McCoy M.A., Reilly G.A., Kilpatrick D.J. Density and breaking strength of bones of mortalities among caged layers. Res. Vet. Sci. 1996;60:185–186. doi: 10.1016/s0034-5288(96)90017-x. [DOI] [PubMed] [Google Scholar]
- Mellata M. Human and avian extraintestinal pathogenic Escherichia coli: infections, zoonotic risks, and antibiotic resistance trends. Foodborne Pathog. Dis. 2013;10:916–932. doi: 10.1089/fpd.2013.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellata M., Ameiss K., Mo H., Curtiss R. Characterization of the contribution to virulence of three large plasmids of avian pathogenic Escherichia coli chi7122 (O78:K80:H9) Infect. Immun. 2010;78:1528–1541. doi: 10.1128/IAI.00981-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosca A., Leclerc M., Hugot J.P. Gut microbiota diversity and human diseases: should We Reintroduce Key Predators in our Ecosystem? Front. Microbiol. 2016;7:455. doi: 10.3389/fmicb.2016.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newberry R.C. Cannibalism. In: Perry G.C., editor. Welfare of the Laying Hen. CABI Publishing; Wallingford, UK: 2004. pp. 239–258. [Google Scholar]
- Ngunjiri J.M., Taylor K.J.M., Abundo M.C., Jang H., Elaish M., Mahesh K.C., Ghorbani A., Wijeratne S., Weber B.P., Johnson T.J., Lee C.-W. Farm stage, bird age, and body site dominantly affect the quantity, taxonomic composition, and dynamics of respiratory and gut microbiota of commercial layer chickens. Appl. Environ. Microbiol. 2019;85:e03137–e031318. doi: 10.1128/AEM.03137-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radon K., Danuser B., Iversen M., Monso E., Weber C., Hartung J., Donham K., Palmgren U., Nowak D. Air contaminants in different European farming environments. Ann. Agric. Environ. Med. 2002;9:41–48. [PubMed] [Google Scholar]
- Redweik G., Stromberg Z., Van Goor A., Mellata M. Protection against avian pathogenic Escherichia coli and Salmonella Kentucky exhibited in chickens given both probiotics and live Salmonella vaccine. Poult. Sci. 2020;99:752–762. doi: 10.1016/j.psj.2019.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaridis I., Ellis R.J., Cawthraw S.A., Van Vliet A.H.M., Stekel D.J., Penell J. Investigating the association between the caecal microbiomes of broilers and Campylobacter burden. Front. Microbiol. 2018;9:927. doi: 10.3389/fmicb.2018.00927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saputra D., Rasmussen S., Larsen M.V., Haddad N., Sperotto M.M., Aarestrup F.M., Lund O., Sicheritz-Pontén T. Reads2Type: a web application for rapid microbial taxonomy identification. BMC Bioinf. 2015;16:398. doi: 10.1186/s12859-015-0829-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanes C.G. Biology of stress in poultry with emphasis on glucocorticoids and the heterophil to lymphocyte ratio. Poult. Sci. 2016;95:2208–2215. doi: 10.3382/ps/pew137. [DOI] [PubMed] [Google Scholar]
- Schouler C., Schaeffer B., Bree A., Mora A., Dahbi G., Biet F., Oswald E., Mainil J., Blanco J., Moulin-Schouleur M. Diagnostic strategy for identifying avian pathogenic Escherichia coli based on four patterns of virulence genes. J. Clin. Microbiol. 2012;50:1673–1678. doi: 10.1128/JCM.05057-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y., Kumar S., Oakley B., Kim W.K. Chicken gut microbiota: Importance and detection Technology. Front. Vet. Sci. 2018;5:254. doi: 10.3389/fvets.2018.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarp C.P., Hanninen M.L., Rautelin H.I. Campylobacteriosis: the role of poultry meat. Clin. Microbiol. Infect. 2016;22:103–109. doi: 10.1016/j.cmi.2015.11.019. [DOI] [PubMed] [Google Scholar]
- Sofka D., Pfeifer A., Gleiss B., Paulsen P., Hilbert F. Changes within the intestinal flora of broilers by colonisation with Campylobacter jejuni. Berl. Munch Tierarztl. Wochenschr. 2015;128:104–110. [PubMed] [Google Scholar]
- Starmer E. A momentous change is underway in the egg case. USDA AMS. 21 Feb 2017. https://www.usda.gov/media/blog/2016/06/27/momentous-change-underway- egg-case
- United States Department of Agriculture . National Agricultural Statistics Service (USDA-NASS); Washington, D.C.: 2019. National Agricultural Statistics Service (USDA-NASS). Chicken and Eggs; pp. 1948–9064. [Google Scholar]
- Weeks C.A., Brown S.N., Richards G.J., Wilkins L.J., Knowles T.G. Levels of mortality in hens by end of lay on farm and in transit to slaughter in Great Britain. Vet. Record. 2012;170:647. doi: 10.1136/vr.100728. [DOI] [PubMed] [Google Scholar]
- Van Goor A., Stromberg Z.R., Mellata M. A recombinant multi-antigen vaccine with broad protection potential against avian pathogenic Escherichia coli. PLoS One. 2017;12:e0183929. doi: 10.1371/journal.pone.0183929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef N., Sheik C.S., Krumholz L.R., Najar F.Z., Roe B.A., Elshahed M.S. Comparison of species richness estimates obtained using nearly complete fragments and simulated pyrosequencing-generated fragments in 16S rRNA gene-based environmental surveys. Appl. Environ. Microbiol. 2009;75:5227–5236. doi: 10.1128/AEM.00592-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Wang G., Siegel P., He C., Wang H., Zhao W. Quantitative genetic background of the host influences gut microbiomes in chickens. Sci. Rep. 2013;3:1163. doi: 10.1038/srep01163. [DOI] [PMC free article] [PubMed] [Google Scholar]