Abstract
The present study investigated the changes in morphology, enzyme activities in the pancreas and mucosa, and nutrient transporter gene expression in the duodenum and jejunum in male and female pigeons during the incubation and chick-rearing periods. Forty-two pairs of White King pigeons with 2 fertile eggs per pair were randomly divided into 7 groups by different breeding stages. The crypt depth of the duodenum and jejunum reached the peak at day 1 (R1) and day 7 (R7) of chick rearing, respectively. The jejunum surface area increased to a maximum value at R1. Amylase activity in the pancreas decreased to the lowest value at R1, whereas trypsin and lipase activities peaked at 17 D of incubation (I17) and R7, respectively. In male pigeons, mucosal Na+-K+-ATPase activity in the duodenum and jejunum was the highest at R15 and it was at I17 in female pigeons. Jejunum sucrose activity in female pigeons was higher at I4 than that at I17 (P < 0.05). The gene expression of FAT/CD36 and I-FABP in the duodenum gradually increased and then declined in the late chick-rearing period. SGLT1 in the jejunum decreased to a lower level at I17 and R25 in male pigeons (P < 0.05). GLUT2 expression in female duodenum and male jejunum decreased to a lower value at I17 compared with that at R15 (P < 0.05). In the late of incubation (from I10 to I17), expression of duodenum CAT1, B0AT1, and PepT1 and jejunum CAT1, ASCT1, and PepT1 in female pigeons was significantly reduced (P < 0.05), whereas opposite results were found in male jejunum CAT1 and duodenum ASCT1. In conclusion, variations of intestinal morphology, activities of pancreatic and mucosal enzymes, and gene expression of nutrient transporters during incubation and chick-rearing periods, underlying potential changes of digestive and absorptive function and intestinal adaptation with sexual effects, may represent a complicated response to stimuli of different breeding stages.
Key words: pigeon, enzyme, nutrient transporter
Introduction
Meat-type pigeons have become an increasingly popular choice for farmers in China. However, the pattern of pigeon husbandry has made less progress compared with other traditional poultry species in recent years. Information on the nutritional standards for meat-type pigeon has not been supplied by the National Research Council or National Standardization Technical Committees in China. Knowledge of the nutrient requirements and feeding strategy of pigeons is highly limited. As an altricial species, parental pigeons feed their newly hatched baby squabs with crop milk for nearly 28 D. Interestingly, both male and female pigeons can produce this cheese-like substance, which is full of protein and lipid in the first week of secretion (Gillespie et al., 2013, Hu et al., 2016), and both of them take care of the offspring by turns (Lea et al., 1986). Considering the different nutrient requirement in breeding and nonbreeding stages, it could be irrational to use only one formulated compound diet all the time, but a free choice feeding system (whole grains of corn, pea, or wheat and concentrate feed) depending on the sensing ability of energy status in pigeons itself may lead to uneven growth and malnutrition in squabs (Xie et al., 2016). Therefore, it is necessary to understand the physiological adaptation of adult pigeons during different breeding stages.
In mammals, pregnancy and lactation considerably elevate the needs of nutrients with increased dietary intake. During special stages, extrareproductive organs, such as the intestine (Burdett et al., 1978, Burdett and Reek, 1979), pancreas (Rolls et al., 1979), adipose tissue (Sinnett-Smith et al., 1982, Eknaes et al., 2006), and liver (Kuhla et al., 2010) will undergo morphological and metabolic changes. Utilization of carbohydrates, proteins, and lipids is mobilized by these organs in favor of the development of the fetus, placenta, and maternal tissues. However, few studies have focused on the organ and metabolic changes in breeding birds, except during the migration and molting processes (Christians and Williams, 1999, Landys-Ciannelli et al., 2003, Vézina and Williams, 2003). In our previous study, the body and organs of parental pigeons, shown to be a nutrient reserve, fluctuated in weight during incubation and chick-rearing period, and serum biochemical profiles and enzyme expression in the liver of breeding pigeons also changed significantly (Wan et al., 2018; Xie et al., 2018). Dietary intake of parent pigeons was found to increase significantly when feeding squabs (Xie et al., 2016); therefore, will the pigeon intestine vary in its morphology or digestive and absorption function, similar to mammals, for dealing with these changes? Birds are oviparous animals of incubation behavior. There may be a different metabolic adaptation between birds and mammals and between male and female pigeons because of their differences in heredity, behaviors, or hormone secretion during the breeding cycle.
A variety of nutrients was absorbed in the upper intestine, and the lower intestine appears to act as part of a large functional reserve of absorptive capacity (Noguchi et al., 1977). We hypothesized that digestion and transport of nutrients in pigeon can be affected by different stages of the breeding cycle. Therefore, the objective of the present study was to determine the changes in morphology, mucosal enzyme activities, and gene expression of nutrient transporters of duodenum and jejunum in parental pigeons during incubation and chick-rearing period. The pancreatic enzyme activities of the pigeons were also examined.
Materials and methods
All procedures used in this study were approved by the Animal Care Advisory Committee of Huaiyin Normal University (ethics approval number E-502/2019).
Birds and Housing
A total of 84 (42 males and 42 females, 60 wk of age) adult White King pigeons were obtained from a commercial pigeon farm (Kunpeng Pigeon Co., Ltd., Xuzhou, China). The mean body weight before the experiment was 580 ± 20 g, and the birds were allocated to breeding pairs when sexually mature. All chosen pigeons had the same oviposition interval (lay eggs on the same day). Each pair of parent pigeons was housed in a man-made aviary equipped with a nest and perch. The whole study consisted of 50 D: a 7-D acclimation and a 43-D experimental period, including an 18-D incubation period and a 25-D chick-rearing period. As described previously (Xie et al., 2017, Xie et al., 2018), plastic eggs were placed into cages only after the second egg was laid to avoid egg breakage and maintain the broodiness of the parent pigeon. Baby pigeons hatched from the incubator were reared by parents after 18 D of incubation. Parent pigeons were fed with a compound diet of 55% corn, 24.5% soybean meal (44.2% crude protein), 11% wheat, 1.2% dicalcium phosphate, 2% limestone, 0.25% salt, 0.5% vitamin and mineral premix, 2% soybean oil, 3.42% zeolite powder, 0.07% lysine, and 0.06% methionine (16.67% crude protein, 12.00 MJ/kg of metabolizable energy, 1.13% calcium, 0.34% available P, 0.89% lysine, and 0.31% methionine). The nutrient levels were recommended by pigeon producers in southern China and Xie et al. (2016). During the study, caged birds were housed under a 16L:8D lighting cycle and received pellet feed, sand, and water ad libitum. The mean daily temperature and relative humidity were 23°C ± 4°C and 54 ± 12% relative humidity, respectively.
Sample Preparation
The parent pigeons were randomly allocated into 7 groups distinguished by different breeding stages, which included the incubation period (4 [I4], 10 [I10], and 17 D [I17]) and the chick-rearing period (1 [R1], 7 [R7], 15 [R15], and 25 D [R25]). All pigeons from each group were euthanized by cervical dislocation after a 12-h fasting. Pancreas tissues were separated and quickly frozen in liquid nitrogen and stored at −80°C for the digestive enzyme analysis. One centimeter length of intestinal tubes from the duodenum (from the pylorus to distal parts of the duodenal loop) and jejunum (from the distal part of the duodenal loop to Meckel's diverticulum) were excised and immediately perfused with 10% buffered formalin several times to avoid villus autolysis and fixed finally. The midsegments of the duodenum and jejunum were minced for the detection of transporter genes. The mucosal surface was removed from the duodenum and jejunum by gentle scraping with a glass slide on ice, which was collected for measuring digestive enzymes. Both intestinal segments and mucosa were frozen as aliquots in liquid nitrogen and then stored at −80°C. After sampling, the eggs or baby squabs were transferred to the pigeon farm to be cared for by other adult pigeons.
Intestinal Tract Histomophology
The fixed tissues were washed in running water overnight, dehydrated in ethanol, cleared in xylene, and embedded in paraffin. Four discontinuous 5-μm-thick sections per intestinal site were made, stained with hematoxylin and eosin, and finally sealed by neutral resins. Villus height and crypt depth and width were measured using a phase contrast microscope (Nikon, Japan) and analyzed with ImageJ 1.46 software (National Institutes of Health, USA). Villus height was measured from the tip of the villi to the villus crypt junction and width was at half height (Dong et al. 2012a). Each replicate per segment recorded data from at least 30 villi or crypts. The villus surface area was calculated by the formula [(2π) × (villus width/2) × (villus height)] (Sakamoto et al. 2000).
Determination of Enzymatic Activities
The sampling of the pancreas tissue and intestinal mucosa was conducted as per the procedures described by Hashemipour et al. (2013) and Jang et al. (2007) with some modifications. Briefly, the pancreas was thawed and homogenized in 10 volume of ice-cold PBS (pH 7.0) using a homogenizer. The homogenates were centrifuged at 16,000 × g for 20 min. The supernatant was divided into aliquots for measuring activities of amylase, lipase, and protease. The mucosal surface was homogenized with mannitol buffer (1:6, vol/vol) containing 2% Triton X-100 and centrifuged at 10,000 × g for 10 min. The supernatant was used for measurement of the activities of Na+-K+-ATP enzyme, aminopeptidase-N, maltase, and sucrase.
Amylase activity (EC 3.2.1.1) was determined according to Bernfeld (1955). One amylase unit was defined as the amount of amylase liberated from soluble starch at 1 μmol of glucose per minute at 37°C. Trypsin activity (EC 3.4.21.4) was analyzed using the modified method of Lainé et al. (1993). One unit of enzyme activity was defined as trypsin hydrolysis of 1 μmol of benzoyl-DL-arginine p-nitroanilide per minute. Lipase activity (EC 3.1.1.3.) was assayed according to the method of Tietz and Fiereck (1966). The lipase activity unit was defined as the amount of enzyme consumed to liberate 1 μmol of free fatty acids from the olive oil emulsion per minute. The protein concentrations were determined using the Coomassie Brilliant Blue G-250 reagent with BSA as a standard, and enzyme activities were expressed as units per gram of protein.
The activities of Na+-K+-ATPase (EC 3.6.1.3), maltase (EC 3.2.1.20), and sucrase (EC 3.2.1.48) were measured by a microplate reader using detection kits (Nanjing Jiancheng Bioengineering Institute, Najing, China). Na+-K+-ATPase activity was expressed as nanomoles of Pi per minute. One unit of maltase or sucrase was defined as the activity hydrolyzing 1 μmol of maltose or sucrase per minute at 37°C and pH 6.0. Aminopeptidase-N (EC 3.4.11.2) activity was measured by using an ELISA kit (RapidBio Research Lab, USA) at 450 nm. One unit of aminopeptidase-N was defined as 1 pg of standard antibody per milliliter solution measured at 37°C. All enzyme activities were also expressed as units per milligram of protein.
RNA Isolation and Real-Time Quantitative PCR
Total RNA was isolated from the intestinal tissue using the TRIzol method. Briefly, frozen samples were ground to a fine powder with liquid nitrogen, and 0.1 g of tissue powder was immediately transferred into 1.0 mL TRIzol reagent. An additional 200 μL of chloroform was added to remove protein. After precipitation in isopropanol and washing in 75% ethanol, the isolated RNA was resuspended using RNase-free water (diethypyrocarbonate-treated H2O). Genomic DNA was eliminated using RNase-free DNase (TaKaRa, Dalian, China). Total RNA quality was confirmed by both native RNA electrophoresis and determining the 260 nm/280 nm absorbance ratio. cDNA was synthesized by moloney murine leukemia virus reverse transcriptase at 42°C for 60 min with oligo dT-Adaptor primer.
The mRNA abundances of fatty acid translocase (FAT/CD36), intestinal fatty acid binding protein (I-FABP), sodium-dependent glucose transporter 1 (SGLT1), glucose transporter 2 (GLUT2), Na+-dependent cationic amino acid transporter (CAT1), Na+-dependent neutral amino acid transporter (B0AT1), excitatory amino acid transporter 3 (EAAT3), and oligopeptide transporter (PepT1) were detected by real-time quantitative PCR. Real-time quantitative PCR was performed using SYBR Premix Ex Taq (TaKaRa, Dalian, China) in a C1000 Touch Thermal Cycler equipped with a CFX96 Real-Time PCR Detection System (Bio-Rad, Hercules, CA) and evaluated with CFX Manager 3.1 software (Bio-Rad, Hercules, CA). The PCR program was 95°C for 30 s, followed by 39 cycles of 95°C for 5 s and 60°C for 30 s. Each sample was analyzed in triplicate. Melting curve analysis was used to verify amplification specificity. The relative expression quantity was calculated using the 2−ΔΔCt method (Livak and Schmittgen, 2001). The primers for the nutrient transporters and β-actin are shown in Table 1.
Table 1.
Primers used in the present study1.
| Target gene | Nucleotide sequence (5’→3′)2 | Accession no. | Size (bp) |
|---|---|---|---|
| FAT/CD36 | F: CAGAGGTATTAGAGCAAGGAGCAC R: ACACAGCAGGAGCAGCAACAAC |
JN020936 | 222 |
| I-FABP | F: AGAGAAAGTTAGGAGCCCACGA R: ACCAGTAAGTTCAGTCCCATCAG |
JF835078 | 161 |
| SGLT1 | F: GCTTATGTTGGCTGGAAGGGCATT R: CACTTTGAGCTGACTGCACCACA |
XM_021289572 | 86 |
| GLUT2 | F: CCTCTGGACTCGTGCCCATGTAT R: GGCTGATGAGGATACCCGTGACA |
XM_021300552 | 108 |
| CAT1 | F: CTTCGCCGTGGTGATA R: CCGAGGACGAGGATGT |
XM_005501421 | 108 |
| B0AT1 | F: TGGCATAGCAGCAATGTCGG R: CTTGGAAGGAGTTGAAGAAATACC |
XM_005509991 | 95 |
| ASCT1 | F: TCGTTCAATGAGGCGACTATG R: CGAAGATGTATTTTCCCAGGCT |
XM_013367937 | 142 |
| EAAT3 | F: ACAGGTGTTGCTGCTTTG | XM_005507165 | 171 |
| R: GGTGCTGCCCACTCTAT | |||
| PepT1 | F: GGTCCCAGTTGTAGATGCTGTGACT | XM_021290570 | 105 |
| R: GCCAAAGACGCAAGGAGCATACCA | |||
| β-actin | F: TCAGGGTGTGATGGTTGGTAT R: TCATTGTAGAAAGTGTGGTGCC |
XM_005504502 | 159 |
ASCT1, Na+-dependent amino acid transporter; B0AT1, Na+-dependent neutral amino acid transporter; CAT1, Na+-dependent cationic amino acid transporter; EAAT3, excitatory amino acid transporter 3; FAT/CD36, fatty acid translocase; GLUT2, glucose transporter 2; I-FABP, intestinal fatty acid binding protein; PepT1, oligopeptide transporter 1; SGLT1, sodium-dependent glucose transporter 1.
F, forward; R, reverse.
Statistical Analysis
All data were presented as means ± SE. Data were statistically evaluated using SPSS 17.0 (SPSS Inc., Chicago, IL) and analyzed using the GLM procedure. The model included the main effects of sex, stage, and their interactions. Means were separately analyzed according to post-hoc Tukey's honestly significant difference test. All of the statements of significance were based on P < 0.05.
Results
Small Intestinal Morphology
As shown in Table 2, the crypt depth of the duodenum was the highest at R1, whereas it was at R7 in the jejunum. Crypt depth was higher in male pigeons than in female pigeons (P < 0.05). A significant interaction of stage × sex for crypt depth in the duodenum and jejunum was also observed. The jejunum surface area of female pigeons increased to a maximum value at R1 and then dropped to the lowest level at R25. There was a significant interaction of stage × sex for villus height and surface area of the duodenum (P < 0.05), but the effect of stage or sex on these parameters was not significant (P > 0.05).
Table 2.
Villus height, crypt depth, and surface area of male and female pigeons during the incubation and chick-rearing period.
| Item1 | Villus height (μm) |
Crypt depth (μm) |
Surface area (mm2) |
|||
|---|---|---|---|---|---|---|
| Duodenum | Jejunum | Duodenum | Jejunum | Duodenum | Jejunum | |
| Stage2 | ||||||
| I4 | 1,828 ± 57 | 1,240 ± 42 | 111 ± 2.32b,c | 101 ± 1.62b | 2.97 ± 0.18 | 1.94 ± 0.09a,b |
| I10 | 1,679 ± 64 | 1,167 ± 42 | 114 ± 3.78a,b,c | 108 ± 1.68a,b | 2.72 ± 0.21 | 1.76 ± 0.09a,b |
| I17 | 1,656 ± 97 | 1,288 ± 67 | 120 ± 4.13a,b | 105 ± 2.50a,b | 2.29 ± 0.32 | 2.04 ± 0.15a,b |
| R1 | 1,560 ± 73 | 1,250 ± 72 | 126 ± 3.72a | 105 ± 2.27a,b | 2.60 ± 0.21 | 2.31 ± 0.16a |
| R7 | 1,783 ± 69 | 1,110 ± 42 | 119 ± 2.70a,b,c | 113 ± 1.68a | 2.81 ± 0.22 | 1.61 ± 0.09a,b |
| R15 | 1,699 ± 58 | 1,221 ± 36 | 107 ± 3.20b,c | 109 ± 1.57a,b | 2.81 ± 0.17 | 1.62 ± 0.08a,b |
| R25 | 1,711 ± 55 | 1,162 ± 50 | 105 ± 2.47c | 101 ± 1.92b | 2.34 ± 0.17 | 1.42 ± 0.11b |
| Sex | ||||||
| Male | 1,690 ± 32 | 1,179 ± 27 | 120 ± 1.64a | 109 ± 0.99a | 2.68 ± 0.11 | 1.74 ± 0.05 |
| Female | 1,715 ± 41 | 1,232 ± 28 | 109 ± 1.83b | 103 ± 1.06b | 2.49 ± 0.14 | 1.88 ± 0.06 |
| P-value | ||||||
| Stage | 0.129 | 0.167 | <0.001 | <0.001 | 0.276 | <0.001 |
| Sex | 0.643 | 0.168 | <0.001 | <0.001 | 0.118 | 0.098 |
| Stage × Sex | <0.001 | 0.417 | <0.001 | <0.001 | 0.006 | <0.001 |
a–cMean values within the same row not sharing a common superscript letter are significantly different (P < 0.05).
Data are shown as means ± SEM; n = 6.
The stages included day 4 (I4), 10 (I10), and 17 (I17) of the incubation period and day 1 (R1), 7 (R7), 15 (R15), and 25 (R25) of the chick-rearing period.
Enzyme Activities in the Pancreas
Pancreatic enzyme activities varied significantly with breeding stages and with interaction of stage × sex for amylase, trypsin, and lipase (P < 0.05) (Table 3). Amylase activity decreased to the lowest value at R1 (P < 0.05) and then recovered at the level of incubation period. Trypsin activity peaked at I17 and gradually decreased to the lowest level at R25. Lipase activity was higher at R7 than that during the late of chick rearing (P < 0.05). Trypsin activity in females was higher than in the male pigeon (P < 0.05), but lipase activity in females was lower (P < 0.05).
Table 3.
Effect of different breeding stages on pancreatic enzyme activities of male and female pigeons.
| Item1 | Amylase (U/mg protein) | Trypsin (U/mg protein) | Lipase (U/mg protein) |
|---|---|---|---|
| Stage2 | |||
| I4 | 1,121 ± 105a | 2,677 ± 374a,b | 32.43 ± 6.14a,b |
| I10 | 1,059 ± 133a,b | 2,164 ± 150b | 46.91 ± 6.34a |
| I17 | 1,077 ± 99a,b | 3,052 ± 632a | 30.97 ± 6.14a,b |
| R1 | 684 ± 103b | 2,983 ± 140a | 46.79 ± 6.63a,b |
| R7 | 807 ± 115a,b | 2,630 ± 168a,b | 47.75 ± 5.29a |
| R15 | 994 ± 103a,b | 2,624 ± 159a,b | 25.59 ± 5.83b |
| R25 | 1,068 ± 109a,b | 2,265 ± 252b | 26.12 ± 5.83b |
| Sex | |||
| Male | 1,032 ± 58 | 2,300 ± 133b | 44.26 ± 3.22a |
| Female | 913 ± 58 | 2,956 ± 202a | 29.04 ± 3.24b |
| P value | |||
| Stage | 0.044 | 0.002 | <0.001 |
| Sex | 0.159 | 0.016 | <0.001 |
| Stage × Sex | <0.001 | <0.001 | <0.001 |
a, bMean values within the same row not sharing a common superscript letter are significantly different (P < 0.05).
Data are shown as means ± SEM; n = 6.
The stages included day 4 (I4), 10 (I10), and 17 (I17) of the incubation period and day 1 (R1), 7 (R7), 15 (R15), and 25 (R25) of the chick-rearing period.
Enzyme Activities in Intestinal Mucosa
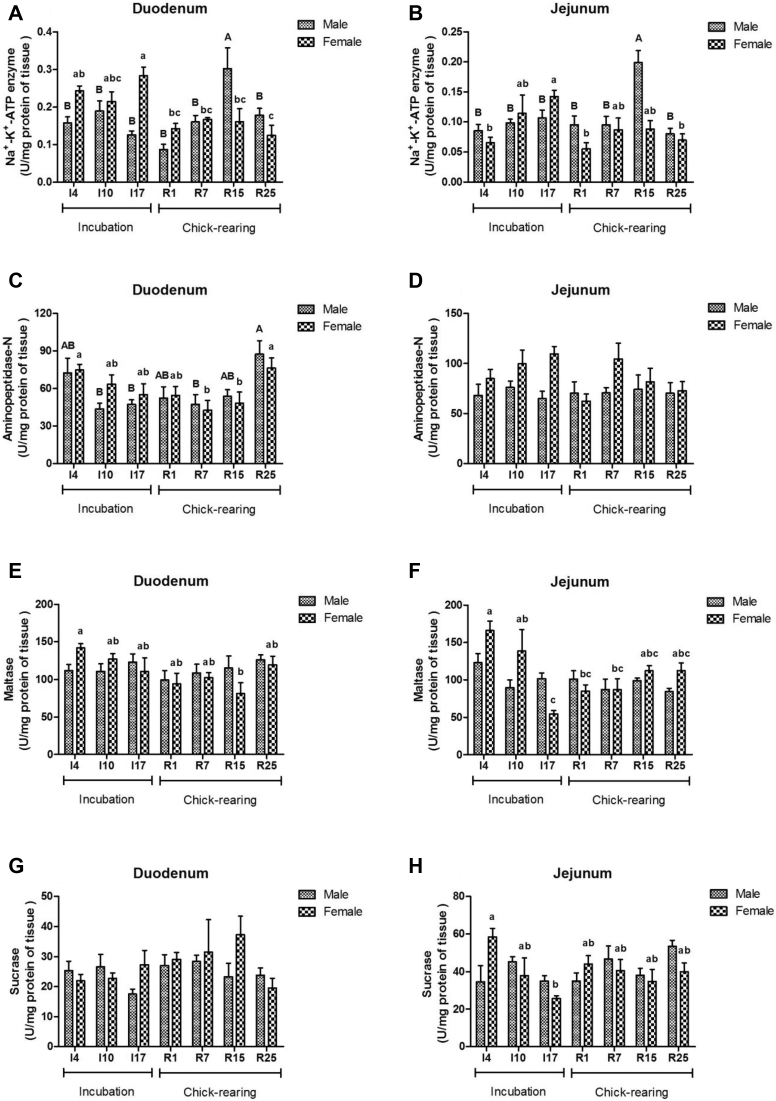
As shown in Table 4, brush border enzyme activities varied significantly with breeding stage, except sucrose in the duodenum and aminopeptidase-N in the jejunum (P < 0.05). An interaction between stage and sex was observed in Na+-K+-ATPase activity of duodenum and disaccharidase activities of the jejunum (P < 0.05). Na+-K+-ATPase activity of the duodenum and jejunum in male pigeons reached to the peak at R15, whereas it was at I17 in female pigeons (Figures 1A, 1B). Aminopeptidase-N activity of the duodenum in both male and female pigeons was higher in the late chick-rearing period (R25), but no significance was found in the jejunum (P > 0.05) (Figures 1C, 1D). The activity of maltase of the duodenum and jejunum in females was just higher in the early incubation period (I4) (P < 0.05) (Figures 1E, 1F). Sucrose activity was higher at I4 than that at I17 in the female pigeon jejunum (Figure 1H).
Table 4.
P values for the effects of stage, sex, and their interaction in activities of mucosal enzymes in thr duodenum and jejunum in male and female pigeons during the incubation and chick-rearing period.
| Item |
P value |
|||||
|---|---|---|---|---|---|---|
| Duodenum |
Jejunum |
|||||
| Stage | Sex | Stage × sex | Stage | Sex | Stage × sex | |
| Na+-K+-ATPase | <0.001 | 0.298 | <0.001 | 0.037 | 0.354 | 0.417 |
| Aminopeptidase-N | <0.001 | 0.701 | 0.709 | 0.350 | 0.002 | 0.149 |
| Maltase | 0.046 | 0.673 | 0.160 | <0.001 | 0.149 | 0.005 |
| Sucrose | 0.247 | 0.272 | 0.253 | 0.016 | 0.729 | 0.016 |
Figure 1.
Activities of mucosal enzymes in the duodenum (Na+-K+-ATPase [A], aminopeptidase-N [C], maltase [E], and sucrose [G]) and jejunum (Na+-K+-ATPase [B], aminopeptidase-N [D], maltase [F], and sucrose [H]) of male and female pigeons during incubation and chick-rearing periods. The stages included incubation period: I4, I10, and I17 and chick-rearing period: R1, R7, R15 and R25. Values are shown as means ± SE (n = 6 males and females). Bars with the different capital letters (A, B) or lowercase letters (a–c) are significantly different (P < 0.05).
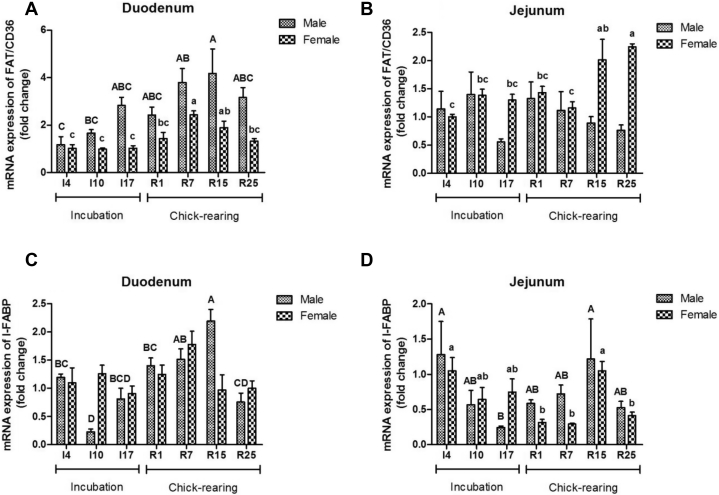
Figure 2.
The mRNA expression of fatty acid translocase (FATC/D36) (A and B) and intestinal fatty acid binding protein (I-FABP) (C and D) in the duodenum and jejunum of male and female parent pigeons during incubation and chick-rearing periods. The stages included incubation period: I4, I10, and I17; chick-rearing period: R1, R7, R15, and R25. Values are means ± SE (n = 6 males and females). Bars with the different capital letters (A–D) or lowercase letters (a–c) are significantly different (P < 0.05).
mRNA Expressions for Fatty Acid Transporters in Pigeon Small Intestine
Breeding stages had a significant effect on gene expressions of FAT/CD36 and I-FABP in both the male and female pigeon intestine (P < 0.05) (Table 5). As shown in Figure 2, mRNA abundances of FAT/CD36 and I-FABP of the duodenum showed a gradual increase during the chick-rearing period with the peak values at R15 in male pigeons, whereas the expression of the duodenum peaked at R7 in female pigeons (Figures 2A, 2C). Jejunum FAT/CD36 expression in females was higher in the late chick-rearing period (P < 0.05) (Figure 2B), but it showed no significant change in males (P > 0.05) (Figure 2B). I-FABP gene expression in the jejunum showed almost the same changing pattern in both male pigeons and female pigeons, and it increased significantly at I4 and R15 (P < 0.05) (Figure 2D).
Table 5.
P values for the effects of stage, sex, and their interaction in gene expression of nutrient transporters in the duodenum and jejunum in male and female pigeons during the incubation and chick-rearing period.
| Item |
P value |
|||||
|---|---|---|---|---|---|---|
| Duodenum |
Jejunum |
|||||
| Stage | Sex | Stage × sex | Stage | Sex | Stage × sex | |
| FAT/CD36 | <0.001 | <0.001 | 0.196 | 0.046 | <0.001 | 0.001 |
| I-FABP | <0.001 | 0.809 | <0.001 | 0.002 | 0.442 | 0.405 |
| SGLT1 | 0.062 | 0.326 | 0.957 | 0.036 | <0.001 | 0.003 |
| GLUT2 | 0.004 | 0.515 | 0.034 | 0.041 | 0.001 | 0.002 |
| CAT1 | <0.001 | 0.233 | <0.001 | <0.001 | <0.001 | 0.007 |
| B0AT1 | 0.032 | 0.168 | 0.285 | 0.003 | 0.006 | 0.070 |
| ASCT1 | 0.001 | 0.026 | 0.002 | <0.001 | 0.383 | 0.034 |
| EAAT3 | 0.006 | 0.022 | 0.327 | 0.249 | 0.089 | 0.014 |
| PepT1 | 0.002 | 0.150 | 0.934 | 0.014 | 0.005 | 0.025 |
ASCT1, Na+-dependent amino acid transporter; B0AT1, Na+-dependent neutral amino acid transporter; CAT1, Na+-dependent cationic amino acid transporter; EAAT3, excitatory amino acid transporter 3; FAT/CD36, fatty acid translocase; GLUT2, glucose transporter 2; I-FABP, intestinal fatty acid binding protein; PepT1, oligopeptide transporter 1; SGLT1, sodium-dependent glucose transporter 1.
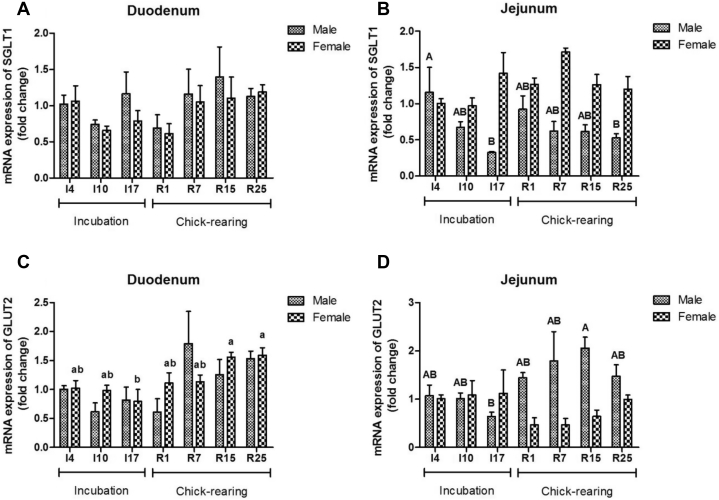
mRNA Expressions for Glucose Transporters in Pigeon Small Intestine
In the jejunum, gene expressions of SGLT1 and GLUT2 changed significantly with stage, sex, and their interaction (P < 0.05) (Table 5). SGLT1 expression in the duodenum was not influenced during incubation and chick-rearing period (P > 0.05) (Table 5), but in the jejunum, it decreased to a lower level at I17 and R25 in male pigeons (P < 0.05) (Figure 3B). The mRNA abundance of GLUT2 in the female duodenum and female jejunum decreased to a lower value at I17 compared with that at R15 (P < 0.05) (Figures 3C, 3D). Different breeding stages showed no significant effect on GLUT2 gene expression in the duodenum of male pigeons and in the jejunum of female pigeons (P > 0.05) (Figures 3C, 3D).
Figure 3.
The mRNA expression of sodium glucose transporter (SGLT1) (A and B), glucose transporter (GLUT2) (C and D) in the duodenum and jejunum of male and female parent pigeons during incubation and chick-rearing periods. The stages included incubation period: I4, I10, and I17; chick-rearing period: R1, R7, R15, and R25. Values are means ± SE (n = 6 males and females). Bars with the different capital letters (A, B) or lowercase letters (a, b) are significantly different (P < 0.05).
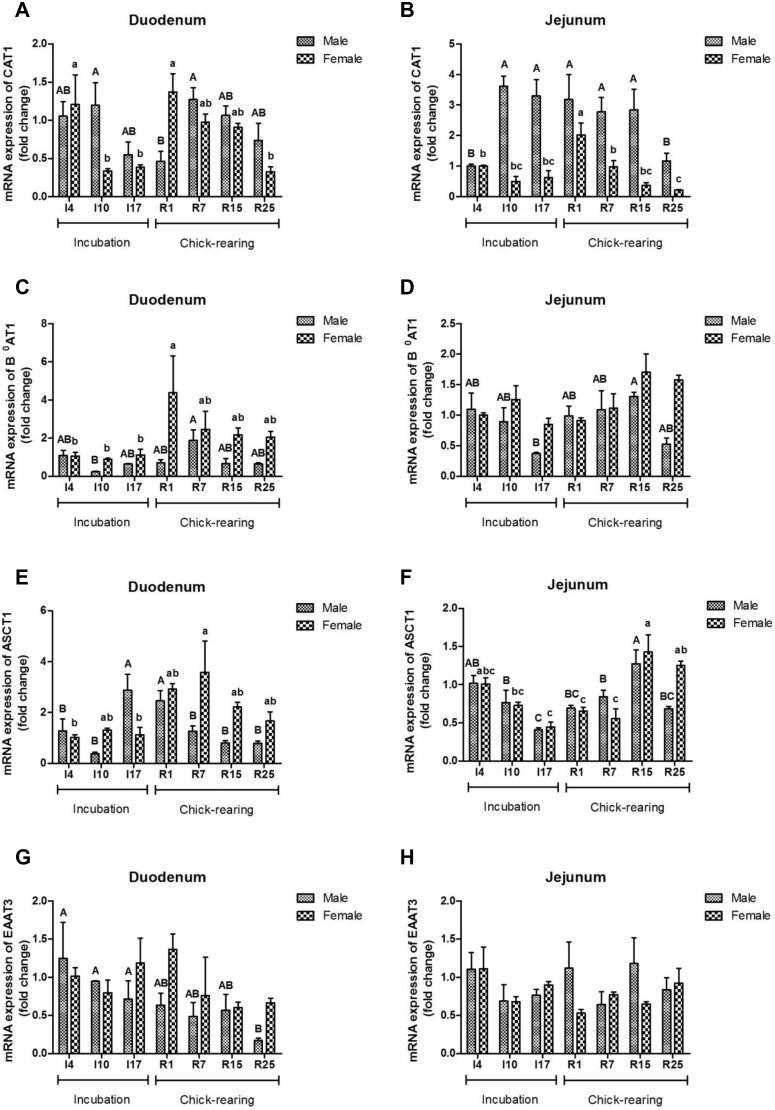
mRNA Expressions for Amino Acid Transporters in Pigeon Small Intestine
mRNA expression of duodenum CAT1 was lower at R1 than that at R7 in male pigeons (P < 0.05), and it attained lower levels from I10 to I17 in female pigeons (Figure 4A). In the male jejunum, a 2 to 3 times increase was detected in CAT1 gene expression from I10 to R15, whereas in the female jejunum, it peaked at R1 (Figure 4B) and then gradually decreased until the late chick-rearing period. Duodenum B0AT1 gene expression increased to the highest value at R7 in males and at R1 in females, but it showed a significantly higher value at R15 in the male jejunum (P < 0.05) (Figures 4C, 4D). The gene expression of duodenum ASCT1 increased significantly from I17 to R1 in male pigeons (P < 0.05), wheras it reached a peak value at R7 in female pigeons, and in the jejunum, the lower level at I17 was found both in males and females compared with that at R15 (P < 0.05) (Figures 4E, 4F). EAAT3 gene expression in the male duodenum showed a steady decrease until R25 (Figure 4G), but in the duodenum of females and the jejunum of both males and females, no significant changes were observed (P > 0.05) (Figures 4G, 4H). A significant interaction of stage and sex for gene expression of duodenum CAT1 and ASCT1 and 4 amino acid transporters tested in the jejunum was observed (P < 0.05) (Table 5).
Figure 4.
The mRNA expression of amino acid transporter genes, CAT1 (A and B), B0AT1 (C and D), ASCT2 (E and F), and EAAT3 (G and H) in duodenum and jejunum of male and female parent pigeons during incubation and chick-rearing periods. CAT1, Na+-dependent cationic amino acid transporter 1; B0AT1, Na+-dependent neutral amino acid transporter 1; ASCT1, alanine/serine/cysteine/threonine transporter 1; EAAT3, excitatory amino acid transporter 3. The stages included incubation period: I4, I10, and I17; chick-rearing period: R1, R7, R15, and R25. Values are means ± SEM (n = 6 males and females). Bars with the different capital letters (A–C) or lowercase letters (a–c) are significantly different (P < 0.05).
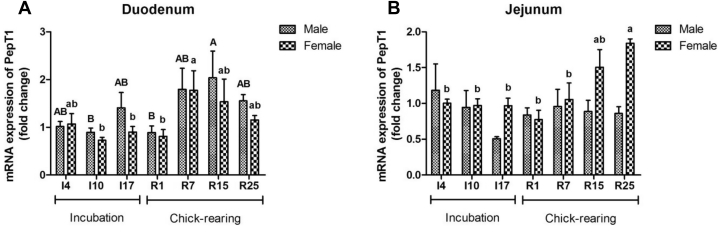
mRNA Expression for PepT1 in Pigeon Small Intestine
Gene expressions of PepT1 varied significantly with stage (P < 0.05) (Table 5). In duodenum of male pigeons, the expression reached a maximum value at R15, whereas it was at R7 in female pigeons (Figure 5A). However, the level of jejunum PepT1 gradually increased significantly in the late chick-rearing period in female pigeons (P < 0.05) (Figure 5B).
Figure 5.
The mRNA expression of oligopeptide transporter (PepT1) (A and B) in duodenum and jejunum of male and female parent pigeons during incubation and chick-rearing periods. The stages included incubation period: I4, I10, and I17; chick-rearing period: R1, R7, R15, and R25. Values are means ± SEM (n = 6 males and females). Bars with the different capital letters (A, B) or lowercase letters (a, b) are significantly different (P < 0.05).
Discussion
Villus morphological changes can reflect nutrients' absorptive capacity. In rodents, the small intestine was found to increase in weight and length with a hyperplasia of the mucosal epithelium during pregnancy and lactation (Fell et al., 1963, Cripps and Williams, 1975). These changes may have occurred in response to the increased dietary intake in the early studies. Dietary feed intake of parent pigeons also has a sharp increase during the chick-rearing period (Xie et al., 2016). In the present study, the villus height of the duodenum and jejunum was stable, and only the surface area of jejunum villus increased in the early chick-rearing periods (R1), which showed a potential enhanced absorptive capacity. The crypt is associated with villus renewal for its production site of stem cells, and deeper crypts indicate higher cell proliferation (Dong et al., 2012a). Crypt depth in male pigeons was higher than that in female pigeons, which showed the sexual difference in villus renewal most likely existed. Although the villus height of lactating animals was greater than that in virgin animals, the turnover times of cells in the mucosal epithelium decreased significantly, which resulted in a larger proportion of immature cells in the epithelium (Harding and Cairnie, 1975). Therefore, it seems that changes in intestinal morphology are not only an adaptation to increased food intake but also to other metabolic demands or even stresses during breeding stages.
Carbohydrates are direct energy sources to the bird. Egg incubation, crop milk production, feeding squabs, and self-maintenance are all accompanied by energy consumption for adult pigeons. Incubation-reduced activity levels lead to a 17–26% lower energy metabolism than during chick rearing in swallows (Hirundinidae) (Westerterp and Bryant, 1984). Our results showed that pancreatic amylase activity gradually decreased from I4 to R1 but then recovered to the beginning level in the late chick-rearing period. Pigeon serum glucose concentration was stable from egg incubation to chick rearing (Xie et al., 2018), but hepatic glycogen content was reported to decrease significantly during the incubation period and then recovered when feeding nestlings (Wan et al., 2018). In addition, our present study indicated that the activities of maltase and sucrose in mucosa also maintained a relatively lower level from I17 to R1. Thus, it is speculated that the utilization of carbohydrates in parent pigeons may be depressed during the incubation period. High concentrations of protein (9–13%) and lipid (9–11%) but extreme deficiency of carbohydrates was found in crop milk in the first 5 D after egg hatch (Shetty et al., 1992), but pure pigeon milk only constituted half of the crop content at 12 D after squab hatching, which is gradually replaced by whole feed (Vandeputte-poma, 1980). It is speculated that the relatively higher level of activities of trypsin and lipase from I17 to R7 probably satisfied the need for crop milk production. Rolls et al. (1979) reported that pancreatic trypsin and α-chymotrypsin would be enhanced with the need for lactation. With the developmental maturity of squab's digestive functions, parental pigeons simply transfer what they eat to squabs, and they do not need to digest the food.
Inconsistent results have been reported on mucosal enzyme activities during pregnancy and lactation in mammals in the past decades. Some experiments showed that the activities of digestive enzymes (dipeptidase) and metabolic enzymes (NAD-dependent isocitric dehydrogenase, glucose-6-phosphate) increased during pregnancy and lactation in the rat (Rolls, 1975, Palmer and Rolls, 1980), but no differences in specific metabolic enzymes (ATPase, lactate dehydrogenase, and alkaline phosphates) between pregnancy and lactation were found (Burdett et al., 1978), and sucrose activity in mucosal was even lower in lactation than in pregnancy (Burdett and Reek, 1979). The present study showed that the activities of different enzymes varied under the breeding stages. Although pure crop milk was clearly needed at R1 once the eggs hatched, expected enhanced activities of mucosal enzymes were not observed in our study. The higher crypt depth of the duodenum and jejunum in parental pigeons during the early chick-rearing period was found in the present study; therefore, an increased proportion of immature cells in the epithelium could be a reason for the lower specific activities of at least some enzymes (de Both and Plaisier, 1974, Willing and Kessel, 2009). Intestinal adaptation in different breeding stages was speculated to be regulated by specific hormones (Baksheev and Fuller, 2000), such as glucocorticoids, cortisol, and triiodothyronine, which were already found to affect the digestive enzymes in animals (Chapple et al., 1989; Khangembam et al., 2017). Our previous study showed that the prolactin concentration rapidly reached the peak value in both sexes on the first day of chick-rearing, but the estradiol concentration in female pigeons remained low until the late chick-rearing period (Xie et al., 2018). Whether digestive enzymes in pigeons are influenced by these hormones warrants further investigation.
Nutrient transporters can be representatives of intestine development in pigeons (Dong et al., 2012b, Xie et al., 2012, Xie et al., 2013, Zhang et al., 2017). FAT/CD36 and fatty acid–binding protein (FABP) are important components of the long-chain fatty acid transport system in various types of cells (Veerkamp and Maatman, 1995, Febbraio et al., 2001). The expression of these genes may be correlated with the need for crop milk formation. In our study, mRNA levels of FAT/CD36 and I-FABP in the duodenum continued to increase from the late incubation period to the mid chick-rearing period. Fatty acids used in lipid biosynthesis of crop milk were reported to originate from both exogenous supply and de novo synthesis, but fatty acid transport genes may play a more important role in total lipid accumulation in pigeon milk compared with genes related to fatty acid synthesis (Xie et al., 2017). However, further research was still needed to explore whether the difference existed in the uptake of fatty acid in different intestinal segments in pigeons.
SGLT1 is responsible for glucose entry into mucosal epithelial cells from the intestinal lumen with the help of Na+-K+-ATPase, but the GLUT2 pathway of intestinal sugar absorption is present in species from insects to humans, providing a major route at high sugar concentrations (Kellett et al., 2008). The period from late incubation to early chick rearing seemed a critical transition for physiological adaptation in pigeons, and hepatic glycogen content declined significantly with lower activities found in hepatic glycolysis and gluconeogenic key enzymes (Wan et al., 2018). Similarly, jejunum SGLT1 and GLUT2 in males and duodenum GLUT2 in females in our present results also maintained a lower level at I17. It is suggested that glucose absorption in the intestine may partly keep pace with glucose metabolism in the liver, which maintains the blood sugar levels stable in pigeons during the whole breeding stages (Xie et al., 2018). However, similar to fatty acid transporters, the effects of intestine segments and sexes also existed in SGLT1 and GLUT2 expression.
Transporters for amino acids and oligopeptides are capable of sensing target flux in the enteric cavity (Groneberg et al., 2001, Hyde et al., 2003), modulating substrate movement across apical, and brush border membranes of enterocytes (Zeng et al., 2011). CAT1 transports lysine, arginine, ornithine, and histidine. Lysine, arginine, and histidine are all essential amino acids for pigeon squabs, which should be provided via dietary protein to form crop milk; therefore, it is reasonable for CAT1 gene expression to sustain a high level after R1 in 2 intestine segments, but its expression was depressed from I10 to R1 in pigeon duodenum, which may be affected by the physiological stress from egg hatching.
As the main apical membrane neutral amino acid transporters, B0AT1 (for broad neutral amino acids-preferring) and ASCT1 (for Ala-, Ser-, Cys-preferring) play very important roles in the transport of amino acids across the intestine (Howard et al., 2004, Pramod et al., 2013). The expression of these genes can be influenced by many factors, such as the tissue microenvironment, amino acid concentrations, hormones, and cell factors (Pinilla et al., 2001, Weiss et al., 2005, Ducroc et al., 2010, Tümer et al., 2013). Our results showed that relatively larger increases in the B0AT1 and ASCT1 genes indicated that neutral amino acids transportation may be more efficient in the duodenum than that in the jejunum. In addition, the maximum mRNA levels of 2 genes were observed before R7 in the duodenum, but in the jejunum, it occurred after R7, which showed an intestinal segment effect.
EAAT3 gene expression in the duodenum of female pigeons and the jejunum of male and female pigeons showed no significant change in the present study. EAAT3 contributes to the cellular uptake of glutamate and aspartate, and both of them are nonessential amino acids. Zhang et al. (2017) analyzed the amino acid profile of crop milk and found that concentrations of glutamate and aspartate were stable from day 1 to day 14 after hatching. Therefore, it can be speculated that normal uptake and self-synthesis of these 2 amino acids can satisfy the needs of pigeons during the whole breeding period.
The uptake of dipeptides or tripeptides into enterocytes by PepT1 is considered to mainly occur in the proximal small intestine of poultry (Gilbert et al., 2007, Dong et al., 2012b). PepT1 gene expression was also regulated by dietary protein, development period, and hormones (Chen et al., 2005, Lu and Klaassen, 2006, de Oliveira et al., 2009). In the current study, relatively lower gene expression of PepT1 was observed until the middle of the chick-rearing period, and whether the serious fluctuation of prolactin or other physiological stresses can depress PepT1 expression still needs further research.
The mechanism of trade-off between parental effort and self-maintenance for birds is highly complicated (Matysioková and Remeš, 2014) and may be influenced by heredity, environment, hormones, and healthy conditions. The authors assumed that the pronounced fluctuation of hormones, energy consumed, and changed behaviors would probably lead to physiology stress to parental pigeons in the first several days after egg hatching. Similar to the findings of early studies in mammals, our results showed that adaptation of the intestine and pancreas must be a complex response to the various signals, and crop milk production and concentrations of nutrients in the intestinal lumen may not be the only explanation for intestinal function changes during the whole breeding stage in pigeons. The issue needs to be further clarified.
In conclusion, crypt depth, surface area, activities of pancreatic and mucosal enzymes, and gene expression of nutrient transporters varied significantly during incubation and chick-rearing period with sexual effect. Activities and expressions of enzymes and genes involved in the utilization of carbohydrates in pigeons were depressed during the short interval before and after egg hatching (from I17 to R1), whereas expressions of genes related to the transport of fatty acids and essential amino acids were enhanced during the chick-rearing period. Our results indicated that the response of the intestine and pancreas in the pigeons to the stimuli of different breeding stages must be complex and could be adjusted to biparental care, hormone changes, or physiological stresses.
Acknowledgments
The authors thank all the members in the school for their generous technical suggestions. The research was supported by National Natural Funds of China (No. 31501974), Natural Science Foundation of Jiangsu Province, China (BK20150462) and China Postdoctoral Science Foundation, China (2017M621839).
Conflict of Interest Statement: The authors did not provide a conflict of interest statement.
References
- Baksheev L., Fuller P.J. Humoral factors in intestinal adaptation. Trends Endocrinol. Metab. 2000;11:401–405. doi: 10.1016/s1043-2760(00)00307-6. [DOI] [PubMed] [Google Scholar]
- Bernfeld P. Amylases, α and β. In: Coowıck S.P., Kaplan N.O., editors. Methods in Enzymology. Acad. Press; New York: 1955. pp. 149–158. [Google Scholar]
- Burdett K., Green F., Reek C. Enzyme changes in rat small intestine during pregnancy and lactation. Histochem. J. 1978;10:343–347. doi: 10.1007/BF01007564. [DOI] [PubMed] [Google Scholar]
- Burdett K., Reek C. Adaptation of the small intestine during pregnancy and lactation in the rat. Biochem. J. 1979;184:245–251. doi: 10.1042/bj1840245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapple R.P., Cuaron J.A., Easter R.A. Effect of glucocorticoids and limiting nursing on the carbohydrate digestive capacity and growth rate of piglets. J. Anim. Sci. 1989;67:2956–2973. doi: 10.2527/jas1989.67112956x. [DOI] [PubMed] [Google Scholar]
- Chen H., Pan Y.X., Wong E.A., Webb K.E., Jr. Dietary protein level and stage of development affect expression of an intestinal peptide transporter (cPepT1) in chickens. J. Nutr. 2005;135:193–198. doi: 10.1093/jn/135.2.193. [DOI] [PubMed] [Google Scholar]
- Christians J.K., Williams T.D. Organ mass dynamics in relation to yolk precursor production and egg formation in European starlings Sturnus vulgaris. Physiol. Biochem. Zool. 1999;72:455–461. doi: 10.1086/316683. [DOI] [PubMed] [Google Scholar]
- Cripps A.W., Williams V.J. The effect of pregnancy and lactation on food intake, gastrointestinal anatomy and the absorptive capacity of the small intestine in the albino rat. Br. J. Nutr. 1975;33:17–32. doi: 10.1079/bjn19750005. [DOI] [PubMed] [Google Scholar]
- de Both N.J., Plaisier H. The influence of changing cell kinetics on functional differentiation in the small intestine of the rat. A study of enzymes involved in carbohydrate metabolism. J. Histochem. Cytochem. 1974;22:352–360. doi: 10.1177/22.5.352. [DOI] [PubMed] [Google Scholar]
- de Oliveira J.E., Druyan S., Uni Z., Ashwell C.M., Ferket P.R. Prehatch intestinal maturation of turkey embryos demonstrated through gene expression patterns. Poult. Sci. 2009;88:2600–2609. doi: 10.3382/ps.2008-00548. [DOI] [PubMed] [Google Scholar]
- Dong X.Y., Wang Y.M., Dai L., Azzam M.M.M., Wang C., Zou X.T. Posthatch development of intestinal morphology and digestive enzyme activities in domestic pigeons (Columba livia) Poult. Sci. 2012;91:1886–1892. doi: 10.3382/ps.2011-02091. [DOI] [PubMed] [Google Scholar]
- Dong X.Y., Wang Y.M., Yuan C., Zou X.T. The ontogeny of nutrient transporter and digestive enzyme gene expression in domestic pigeon (Columba livia) intestine and yolk sac membrane during pre- and posthatch development. Poult. Sci. 2012;91:1974–1982. doi: 10.3382/ps.2012-02164. [DOI] [PubMed] [Google Scholar]
- Ducroc R., Sakar Y., Fanjul C., Barber A., André B., Lostao M.P. Luminal leptin inhibits l-glutamine transport in rat small intestine: involvement of ASCT2 and B0AT1. Am. J. Physiol Gastrointest. Liver Physiol. 2010;299:G179–G185. doi: 10.1152/ajpgi.00048.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eknaes M., Kolstad K., Volden H., Hove K. Changes in body reserves and milk quality throughout lactation in dairy goats. Small Rumin. Res. 2006;63:1–11. [Google Scholar]
- Febbraio M., Hajjar D.P., Silverstein R.L. CD36: a class B scavenger receptor involved in angiogenesis, atherosclerosis, inflammation, and lipid metabolism. J. Clin. Invest. 2001;108:785–791. doi: 10.1172/JCI14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell B.F., Smith K.A., Campbell R.M. Hypertrophic and hyperplastic changes in the alimentary canal of the lactating rat. J. Pathol. Bacteriol. 1963;85:179–188. doi: 10.1002/path.1700850117. [DOI] [PubMed] [Google Scholar]
- Gilbert E.R., Li H., Emmerson D.A., Webb K.E., Jr., Wong E.A. Developmental regulation of nutrient transporter and enzyme mRNA abundance in the small intestine of broilers. Poult. Sci. 2007;86:1739–1753. doi: 10.1093/ps/86.8.1739. [DOI] [PubMed] [Google Scholar]
- Gillespie M.J., Crowley T.M., Haring V.R., Wilson S.L., Harper J.A., Payne J.S., Green D., Monaghan P., Donald J.A., Nicholas K.R., Moore R.J. Transcriptome analysis of pigeon milk production - role of cornification and triglyceride synthesis genes. BMC Genomics. 2013;14:169–181. doi: 10.1186/1471-2164-14-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groneberg D.A., Döring F., Eynott P.R., Fischer A., Daniel H. Intestinal peptide transport: ex vivo uptake studies and localization of peptide carrier PEPT1. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;281:G697–G704. doi: 10.1152/ajpgi.2001.281.3.G697. [DOI] [PubMed] [Google Scholar]
- Harding J.D., Cairnie A.B. Changes in intestinal cell kinetics in the small intestine of lactating mice. Cell Tissue Kinet. 1975;8:135–144. doi: 10.1111/j.1365-2184.1975.tb01215.x. [DOI] [PubMed] [Google Scholar]
- Hashemipour H., Kermanshahi H., Golian A., Veldkamp T. Effect of thymol and carvacrol feed supplementation on performance, antioxidant enzyme activities, fatty acid composition, digestive enzyme activities, and immune response in broiler chickens. Poult. Sci. 2013;92:2059–2069. doi: 10.3382/ps.2012-02685. [DOI] [PubMed] [Google Scholar]
- Howard A., Goodlad R.A., Walters J.R., Ford D., Hirst B.H. Increased expression of specific intestinal amino acid and peptide transporter mRNA in rats fed by TPN is reversed by GLP-2. J. Nutr. 2004;134:2957–2964. doi: 10.1093/jn/134.11.2957. [DOI] [PubMed] [Google Scholar]
- Hu X.C., Gao C.Q., Wang X.H., Yan H.C., Chen Z.S., Wang X.Q. Crop milk protein is synthesised following activation of the IRS1/Akt/TOR signalling pathway in the domestic pigeon (Columba livia) Br. Poult. Sci. 2016;57:855–862. doi: 10.1080/00071668.2016.1219694. [DOI] [PubMed] [Google Scholar]
- Hyde R., Taylor P.M., Hundal H.S. Amino acid transporters: roles in amino acid sensing and signalling in animal cells. Biochem. J. 2003;373:1–18. doi: 10.1042/bj20030405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang I.S., Ko Y.H., Kang S.Y., Lee C.Y. Effect of a commercial essential oil on growth performance, digestive enzyme activity and intestinal microflora population in broiler chickens. Anim. Feed Sci. Tech. 2007;134:304–315. [Google Scholar]
- Kellett G.L., Brot-Laroche E., Mace O.J., Leturque A. Sugar absorption in the intestine: the role of GLUT2. Ann. Rev. Nutr. 2008;28:35–54. doi: 10.1146/annurev.nutr.28.061807.155518. [DOI] [PubMed] [Google Scholar]
- Khangembam B.K., Ninawe A.S., Chakrabarti R. Effect of cortisol and triiodothyronine bath treatments on the digestive enzyme profile and growth of Catla catla larvae during ontogenic development. Aquacult. Res. 2017;48:2173–2185. [Google Scholar]
- Kuhla B., Kucia M., Görs S., Albrecht D., Langhammer M., Kuhla S., Metges C.C. Effect of a high-protein diet on food intake and liver metabolism during pregnancy, lactation and after weaning in mice. Proteomics. 2010;10:2573–2588. doi: 10.1002/pmic.200900789. [DOI] [PubMed] [Google Scholar]
- Lainé J., Beattie M., LeBel D. Simultaneous kinetic determinations of lipase, chymotrypsin, trypsin, elastase, and amylase on the same microtiter plate. Pancreas. 1993;8:383–386. doi: 10.1097/00006676-199305000-00016. [DOI] [PubMed] [Google Scholar]
- Landys-Ciannelli M.M., Piersma T., Jukema J. Strategic size changes of internal organs and muscle tissue in the Bar-tailed Godwit during fat storage on a spring stopover site. Funct. Ecol. 2003;17:151–159. [Google Scholar]
- Lea R.W., Vowles D.M., Dick H.R. Factors affecting prolactin secretion during the breeding cycle of the ring dove (Streptopelia risoria) and its possible role in incubation. J. Endocrinol. 1986;110:447–458. doi: 10.1677/joe.0.1100447. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu H., Klaassen C. Tissue distribution and thyroid hormone regulation of Pept1 and Pept2 mRNA in rodents. Peptides. 2006;27:850–857. doi: 10.1016/j.peptides.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Matysioková B., Remeš V. The importance of having a partner: male help releases females from time limitation during incubation in birds. Front. Zool. 2014;11:24. doi: 10.1186/1742-9994-11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T., Tokunaga Y., Ichikawa H., Muranishi S., Sezaki H. Regional capacities of gastrointestinal absorption and lymphatic transport for lipid-soluble dyes in rats. Chem. Pharm. Bull. 1977;25:413–419. doi: 10.1248/cpb.25.413. [DOI] [PubMed] [Google Scholar]
- Palmer M.F., Rolls B.A. Activities of some metabolic enzymes in the small intestinal mucosa during pregnancy and lactation in the rat. Reproduction. 1980;60:231–236. doi: 10.1530/jrf.0.0600231. [DOI] [PubMed] [Google Scholar]
- Pinilla J., Barber A., Lostao M.P. Active transport of alanine by the neutral amino-acid exchanger ASCT1. Can. J. Physiol. Pharmacol. 2001;79:1023–1029. [PubMed] [Google Scholar]
- Pramod A.B., Foster J., Carvelli L., Henry L.K. SLC6 transporters: structure, function, regulation, disease association and therapeutics. Mol. Aspects Med. 2013;34:197–219. doi: 10.1016/j.mam.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls B.A. Dipeptidase activity in the small intestinal mucosa during pregnancy and lactation in the rat. Br. J. Nutr. 1975;33:1–9. doi: 10.1079/bjn19750003. [DOI] [PubMed] [Google Scholar]
- Rolls B.A., Henschel M.J., Palmer M.F. The effects of pregnancy and lactation on the activities of trypsin and α-chymotrypsin in the rat pancreas. Br. J. Nutr. 1979;41:573–578. doi: 10.1079/bjn19790072. [DOI] [PubMed] [Google Scholar]
- Sakamoto K., Hirose H., Onizuka A., Hayashi M., Futamura N., Kawamura Y., Ezaki T. Quantitative study of changes in intestinal morphology and mucus gel on total parenteral nutrition in rats. J. Surg. Res. 2000;94:99–106. doi: 10.1006/jsre.2000.5937. [DOI] [PubMed] [Google Scholar]
- Shetty S., Bharathi L., Shenoy K.B., Hegde S.N. Biochemical properties of pigeon milk and its effect on growth. J. Comp. Physiol. B. 1992;162:632–636. [Google Scholar]
- Sinnett-Smith P.A., Vernon R.G., Mayer R.J. Enzyme and protein turnover in adipose tissue in pregnancy and lactation. Biochim. Biophys. Acta. 1982;714:58–64. doi: 10.1016/0304-4165(82)90126-x. [DOI] [PubMed] [Google Scholar]
- Tietz N.W., Fiereck E.A. A specific method for serum lipase determination. Clin. Chim. Acta. 1966;13:352–355. doi: 10.1016/0009-8981(66)90215-4. [DOI] [PubMed] [Google Scholar]
- Tümer E., Bröer A., Balkrishna S., Jülich T., Bröer S. Enterocyte-specific regulation of the apical nutrient transporter SLC6A19 (B0AT1) by transcriptional and epigenetic networks. J. Biol. Chem. 2013;288:33813–33823. doi: 10.1074/jbc.M113.482760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandeputte-poma J. Feeding, growth and metabolism of the pigeon, Columba livia domestica: duration and role of crop milk feeding. J. Comp. Physiol. 1980;135:97–99. [Google Scholar]
- Veerkamp J.H., Maatman R. Cytoplasmic fatty acid-binding proteins: their structure and genes. Prog. Lipid Res. 1995;34:17–52. doi: 10.1016/0163-7827(94)00005-7. [DOI] [PubMed] [Google Scholar]
- Vézina F., Williams T.D. Plasticity in body composition in breeding birds: what drives the metabolic costs of egg productions? Physiol. Biochem. Zool. 2003;76:716–730. doi: 10.1086/376425. [DOI] [PubMed] [Google Scholar]
- Wan X.P., Xie P., Bu Z., Zou X.T. Changes in hepatic glucose and lipid metabolism-related parameters in domestic pigeon (Columba livia) during incubation and chick rearing. J. Anim. Physiol. Anim. Nutr. 2018;102:e558–e568. doi: 10.1111/jpn.12796. [DOI] [PubMed] [Google Scholar]
- Westerterp K.R., Bryant D.M. Energetics of free existence in swallows and martins (hirundinidae) during breeding: a comparative study using doubly labeled water. Oecologia. 1984;62:376–381. doi: 10.1007/BF00384270. [DOI] [PubMed] [Google Scholar]
- Weiss M.D., Rossignol C., Sumners C., Anderson K.J. A pH-dependent increase in neuronal glutamate efflux in vitro: possible involvement of ASCT1. Brain Res. 2005;1056:105–112. doi: 10.1016/j.brainres.2005.07.045. [DOI] [PubMed] [Google Scholar]
- Willing B.P., Kessel A.G.V. Intestinal microbiota differentially affect brush border enzyme activity and gene expression in the neonatal gnotobiotic pig. J. Anim. Physiol. Anim. Nutr. 2009;93:586–595. doi: 10.1111/j.1439-0396.2008.00841.x. [DOI] [PubMed] [Google Scholar]
- Xie P., Zhang A.T., Wang C., Azzam M.M., Zou X.T. Molecular cloning, characterization, and expression analysis of fatty acid translocase (FAT/CD36) in the pigeon (Columba livia domestica) Poult. Sci. 2012;91:1670–1679. doi: 10.3382/ps.2011-02097. [DOI] [PubMed] [Google Scholar]
- Xie P., Liu L.L., Wang C., Zou X.T. Molecular cloning, characterization, and mRNA expression of intestinal fatty acid binding protein (I-FABP) in Columba livia. J. Poult. Sci. 2013;50:9–19. [Google Scholar]
- Xie P., Jiang X.Y., Bu Z., Fu S.Y., Zhang S.Y., Tang Q.P. Free choice feeding of whole grains in meat-type pigeons: 1. effect on performance, carcass traits, and organ development. Br. Poult. Sci. 2016;57:699–706. doi: 10.1080/00071668.2016.1206191. [DOI] [PubMed] [Google Scholar]
- Xie P., Wang X.P., Bu Z., Zou X.T. Differential expression of fatty acid transporters and fatty acid synthesis-related genes in crop tissues of male and female pigeons (Columba livia domestica) during incubation and chick rearing. Br. Poult. Sci. 2017;58:594–602. doi: 10.1080/00071668.2017.1357798. [DOI] [PubMed] [Google Scholar]
- Xie P., Wan X.P., Bu Z., Diao E.J., Gong D.Q., Zou X.T. Changes in hormone profiles, growth factors and mRNA expression of the related receptors in crop tissue, relative organ weight, and serum biochemical parameters in the domestic pigeon (Columba livia) during incubation and chick-rearing periods under artificial farming conditions. Poult. Sci. 2018;97:2189–2202. doi: 10.3382/ps/pey061. [DOI] [PubMed] [Google Scholar]
- Zeng P.L., Li X.G., Wang X.Q., Zhang D.X., Shu G., Luo Q.B. The relationship between gene expression of cationic and neutral amino acid transporters in the small intestine of chick embryos and chick breed, development, sex, and egg amino acid concentration. Poult. Sci. 2011;90:2548–2556. doi: 10.3382/ps.2011-01458. [DOI] [PubMed] [Google Scholar]
- Zhang X.Y., Zhang N.N., Wan X.P., Li L.L., Zou X.T. Gene expression of amino acid transporter in pigeon (Columbia livia) intestine during post-hatch development and its correlation with amino acid in pigeon milk. Poult. Sci. 2017;96:1120–1131. doi: 10.3382/ps/pew320. [DOI] [PubMed] [Google Scholar]