Abstract
The effects of storage temperature (4°C, 25°C, and 35°C) on sensory quality, physicochemical properties, texture, molecular forces, flavor, and microbial indexes of preserved eggs were studied. The results showed that the sensory quality, weight loss rate, pH, and color of preserved eggs were significantly different at different storage temperatures (P < 0.05). Compared with high temperature and normal temperature storage, low temperature storage reduced weight loss rate by 55.15 and 64.1%, respectively, improved the sensory score (P < 0.05), inhibited the reduction of pH and the increase of total volatile base nitrogen (P < 0.05), and decreased the change of color (P < 0.05). During storage, there was no difference in the springiness of preserved egg white stored at different temperatures (P > 0.05). Hardness and chewiness at 3 different temperatures increased first and then decreased, and low temperature significantly inhibited the progress of these changes to a certain extent (P < 0.05). The content of ionic bond in egg white first decreased and then increased, and content of disulfide bond increased first and then decreased. Content of ionic bond in yolk decreased all the time, and high temperature could promote this change. Whatever the temperature was, the content of free amino acids in preserved egg white and yolk increased first and then decreased, and the total content of amino acids stored at different temperatures was significantly different (P < 0.05). The content of free fatty acids in yolk decreased. At the end of storage, no microorganisms were detected in 3 temperatures during the storage period of 84 D. The results showed that low temperature storage is more conducive for preservation of preserved eggs.
Key words: preserved egg, storage, temperature, quality
Introduction
The preserved egg, also called Pidan, is a traditional characteristic egg product in China. Ancient books of traditional Chinese medicine recorded preserved eggs possessed the effect of clearing away heat and reducing fire, soothing the liver to improve eyesight. Modern scientific research studies also proved the anti-inflammatory activity of the preserved egg (Zhang et al., 2018, Zhang et al., 2019). Combined with its unique flavor, taste, and nutritional value, preserved eggs are popular with consumers (Zhao et al., 2016a). The processing of preserved eggs has a long history. Preserved eggs were originally prepared by wrapping fresh eggs in the mixture of mud, plant lime, soda ash, quicklime, and lead oxide (Wang and Fung, 1996), which was troublesome and unsanitary. At present, enterprises use sodium hydroxide instead of soda ash and quicklime, heavy metal compounds containing copper sulfate, zinc sulfate, iron sulfate, and other metal compounds instead of lead oxide and soak preserved eggs with the method of feed liquid (Chang et al., 1999, Ganesan and Benjakul, 2010). When the preserved eggs are pickled, microorganisms, temperature, humidity, oxygen, and other factors in the environment might lead to a series of quality problems such as drying shrinkage, darkening the color, and mildew taste during storage without packaging, which result in a shorter shelf life, thus affecting the sales of the produce at home and abroad.
At present, the storage methods adopted by most domestic enterprises are not effective. For example, traditional preserved eggs usually use the method of “mud wrapped bran,” which can prevent the damage and extend the shelf life, but it is easy to affect the appearance, pollute the environment (Yin et al., 2012), and the cost of packaging and transportation is relatively high. Nowadays, more preserved eggs are sold directly without packaging. The moisture and flavor substances of preserved eggs are easy to be lost and eventually dried up, finally be deteriorated (Ma et al., 2015). In addition, liquid paraffin is still used for preservation of preserved eggs, but the cost of paraffin coating is high and oil stain is produced, which is not conducive to sale. At the same time, the academic research on the storage of preserved eggs is mainly focused on the coating preservation of preserved eggs (Hu and Meihu, 2012, Yin et al., 2012, Ma et al., 2015). Studies on the effect of temperature on storage of preserved eggs are rarely reported in the literature. The storage temperature is the main factor affecting the quality of food; the biochemical reaction, enzyme activity, microbial growth and reproduction, moisture content and activity are all without exception restricted by temperature (Quan and Benjakul, 2017), so the significance of storage and preservation of preserved eggs is obvious. In this article, the sensory quality, weight loss rate, pH, color, total volatile base nitrogen, texture, intermolecular force, free fatty acids, free amino acids, and microorganisms of preserved eggs stored at different temperatures were determined periodically to monitor the quality changes of preserved eggs and to find the suitable storage temperature for preserved eggs. This research also can provide a reference for future research on preservation of preserved eggs.
Materials and methods
Materials
Fresh duck eggs were obtained from a farm in Nanchang County, Jiangxi Province, China. Standard compounds were purchased from Shanghai Institute of Metrology and Testing Technology. The other reagents used were of analytical grade and purchased from Xilong Scientific Co., Ltd.
Pickling and Storage of Preserved Eggs
A certain amount of water is boiled and then cooled. Pickling solutions was prepared, which contained 4.5% (m/v) NaOH and 4.0% (m/v) NaCl, then cooled to room temperature, followed by adding 0.4% (m/v) CuSO4 which is completely dissolved in a small amount of water in advance (Tu, et al., 2013), and stirred well. The fresh duck eggs purchased from the farm were washed, graded, and then placed in the pickling barrel. The aforementioned pickling liquid was added to the pickling barrel. The eggs were pickled at 25°C for 40 D.
After pickling of the preserved eggs, 600 elastic eggs were picked out, next divided into 3 equal portions, and then stored at 4°C, 25°C, and 35°C, respectively. The humidity was controlled at 40% ± 5%. The elastic eggs were taken out on days 0, 14, 28, 42, 56, 70, and 84 D, and the indexes were determined.
Sensory Evaluation
According to Chinese standard GB/T 5009.47-2003 and GB 2749-2015 (China, 2015), the sensory evaluation (Table 1) of preserved egg was set. Six food professional researchers scored the samples, with a full score of 15 and a corruption score of 0.
Table 1.
Sensory evaluation.
| Index | 3 points | 2 points | 1 points | 0 points |
|---|---|---|---|---|
| Color | Brown transparent | Yellow transparent | Dark brown opaque | Brown-black opacity |
| Appearance | Full and complete form | slightly defective | Large defect | Severe defect or liquefaction |
| Texture | Elasticity | Hardness increases | Hardness is very high | Shrinkage |
| Flavor | Rich fragrance | The fragrance fades | No fragrance | Mildew taste heavier |
| Taste | Normal, odorless | Taste light | A slight odor | Heavy odor |
Determination of Moisture and Weight Loss Rate
The water content was determined by direct measurement of Chinese standard GB 5009.3-2016 (China, 2016c). The experiment was performed in triplicate. The weight loss rate is the degree of water loss in preserved eggs after a certain period of time. The weight of 10 preserved eggs was measured regularly, and the formula for calculating weight loss rate is as follows:
where M1 is the weight of preserved eggs before storage, g; M2 is the weight of preserved eggs after storage, g.
Determination of pH
As per the analysis method of Chinese standard GB/T 5009.47-2003 (China, 2003), 5 preserved eggs were washed and shelled, then the preserved egg white or yolk was added to water in a ratio of 2:1 to form a homogenate by using a homogenizer. About 15.00 g homogenate (equivalent to 10.00 g sample) was weighed, mixed with water, diluted to 150 mL, filtered with double-layer gauze, and measured the pH value. The experiment was performed in triplicate.
Determination of the Color
The color of the preserved egg white/yolk was measured using colorimeter (NS810, Shenzhen 3nh Technology Co., Ltd., China). The preserved egg white was cut into cubes with a height of 1 cm. A white A4 paper was placed at the bottom of the test. The surface color of the egg yolk was measured. The data were expressed as L∗ (brightness), a∗ (red/green), and b∗ (yellow/blue). The experiment was repeated 10 times.
Determination of Texture Characteristics
Texture profile analysis was performed using a TEE 32 texture analyzer (Stable Micro Systems, Surrey, UK). The tip part of the preserved egg white was cut into cubes with a width and height of 1 cm. The hardness, springiness, and chewiness of the preserved egg white were measured by using P/36R probe. Texture profile analysis model was selected. The measured parameters are as follows: the pretest speed was 5.0 mm/s, the test speed was 2.0 mm/s, and the post-test speed was 2.0 mm/s; the compression ratio 60%, the recovery time 5s, and the trigger force 5g. The experiment was repeated 6 times.
Selective Protein Solubility of Preserved Eggs
Selective protein solubility was determined as per the method described by Pérez-Mateos et al. (Pérez-Mateos et al., 1997), with a slight modification. Samples were successively solubilized in 4 solvents: 0.6 mol/L NaCl (S1), 0.6 mol/L NaCl + 1.5 mol/L urea (S2), 0.6 mol/L NaCl + 8 mol/L urea (S3), and 0.6 mol/L NaCl + 8 mol/L urea + 0.5 mol/L β-mercaptoethanol (S4). The protein content dissolved in each combination solvent represents the destructive force, that is, the proportion of the intermolecular interaction of the gel is maintained.
The evenly crushed 0.6 g sample was accurately weighed and placed in a 15-mL centrifuge tube; 5.4 mL S1 was added and treated by using a homogenizer (Ultra Turrax homogenizer, IKA T18 digital, IKA Works Guangzhou Co., Ltd., China) for 2 min (12,000 r/min). The mixture was centrifuged at 10,000 r/min for 20 min, and carefully the supernatant and precipitation were separated. A total of 5.4 mL of S2 (0.6 mol/L NaCl + 1.5 mol/L urea) was added to the precipitate part, and the operation of S1 was repeated, followed by S3 and S4. The protein content of the supernatant was determined by the bicinchoninic acid method. Part S4 was dialyzed overnight with S1 to remove the interference of β-mercaptoethanol on protein determination. The experiment was performed in triplicate.
Determination of Free Fatty Acids
Fat Extraction
Ten grams of yolk homogenate was taken in a triangular bottle, 140 mL CM solution (V trichloromethane: V methanol = 2:1) was added, and was shaken well. The mixture was extracted for 3 h and then was filtered. After filtration, NaCl solution was added. After the mixture was stratified, the lower layer of the extract was collected, dried with anhydrous Na2SO4, and concentrated using a rotary evaporation in a water bath at 40°C to get fat.
Extraction and Methylation of Free Fatty Acids
Hundred milligrams of fat that had been extracted was taken in a beaker to which 15 mL acetone–methanol solution (V acetone: V methanol = 2:1) was added. Then, about 200 mg resin was added. The mixture was allowed to stand after being shaken well, and the mixture was washed with acetone–methanol solution and then blow-dried with N2 to remove resin; finally, free fatty acid was obtained. After that, 5 mL sodium hydroxide–methanol solution was added and evaporated in a water bath at 60°C for 10–15 min. Then, 5 mL of the methylation reagent was added from the upper part of the condensing tube using a pipette and was evaporated in a water bath at 60°C for 30 min. After cooling, 2 mL n-hexane and 2 mL saturated saline were added. The mixture was shaken, and the upper layer (n-hexane layer) was collected, filtered through the organic phase membrane, and filled in a gas bottle for measurement.
Gas Chromatographic Conditions
Chromatographic column CP-Sil88 for FAME (100m∗0.25 mm, 0.2 ms) (6890N, Agilent Technologies, Inc., Santa Clara, CA): the injection port temperature was 230°C, the temperature of the detector was 240°C, and the temperature was programmed to rise to 45°C from the initial temperature, which was maintained for 4 min; 13 C/min to 175°C, which was maintained for 27 min; and 4°C to 215°C, which was maintained at 35 min. The carrier gas was N2, column flow rate was 1.8 mL/min, injection time was 1 min, and injection volume was 1.0 μL, and there was no shunt injection.
Determination of Free Amino Acids
The egg whites were separated from the yolks and mashed separately. Five grams of the egg white (yolk) was weighed and 20 mL ultrapure water was added. This mixture was homogenized in an ice bath and was filled in a 50-mL beaker. Twenty milliliter of sulfosalicylic acid was added, shaken well, and stored in a refrigerator at 4°C for 17 h. Then, the mixture was filtered using a medium-speed filter paper. The pH was adjusted to higher than 6.0 with NaOH solution; the volume was made up to 50 mL using a volumetric flask, and finally, free amino acid content in the mixture was measured using an amino acid analyzer (L-8900, Hitachi, Ltd., Japan).
Determination of Total Volatile Base Nitrogen
As per the analysis method of Chinese GB 5009.228-2016 (China, 2016d), the trace diffusion method was adopted. The experiment was performed in triplicate.
Determination of Microbial Indicators
The total number of bacterial colonies was determined according to Chinese standard GB 4789.2-2016 (China, 2016a). Coliform group count for food microbiology inspection was determined according to Chinese standard GB 4789.3-2016 (China, 2016b).
Statistical Analysis
All the data were expressed as mean and standard deviation. Statistical analysis was performed using SPSS 25. Significance test was carried out by repeated measurement of variance analysis. The significance level was P < 0.05. The experimental results were plotted by using Origin 2018 software.
Results and discussion
Effects of Different Storage Temperatures on Sensory Quality of Preserved Eggs
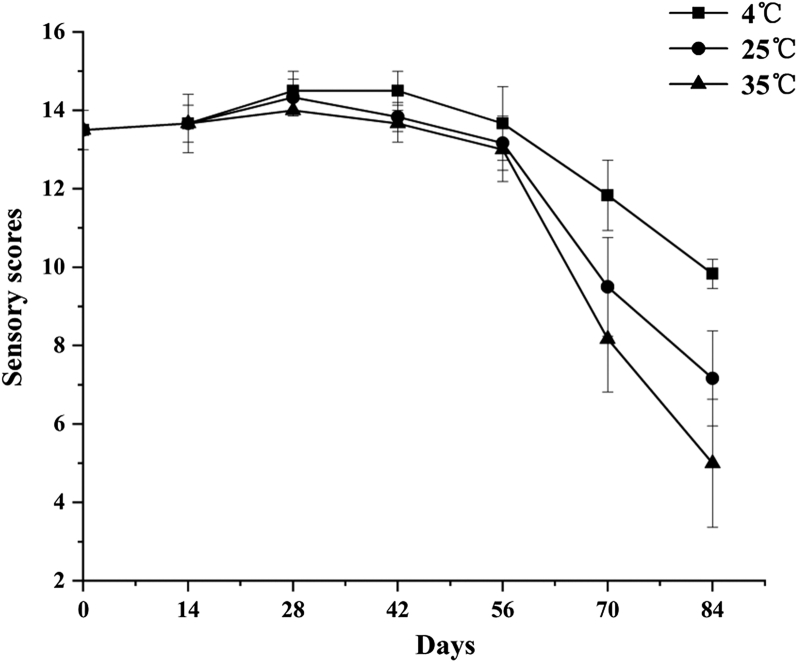
As shown in Figure 1, the sensory scores of preserved eggs at different storage temperatures were significantly different (P < 0.05). During the whole storage process, the sensory scores of the low-temperature group were always the highest, whereas those of the high-temperature group were the lowest at the end of storage. In the first 56 D of the storage period, there was no significant difference in sensory scores among the treatment groups (P > 0.05), but there was significant difference in sensory scores between the treatment groups at 70 D and 84 D (P < 0.05). The final sensory scores at 4°C, 25°C and 35°C were 9.83, 7.17, and 5, respectively at 84 D. The experimental selection was fresh and selected runny preserved eggs with high sensory score; the preserved eggs have a little alkaline taste when they are out of the pickling container, so the taste score is not high. With the extension of storage time, the alkali taste gradually disappeared, and the taste score increased. However, the color score gradually decreased, and the color of the low-temperature group became bright and yellow. At the later stage of storage, all 3 groups of preserved egg whites showed darker color, greater hardness, even drying shrinkage, and mildew taste. The deepening of the color is mainly due to the Maillard reaction that leads to accumulation of pigments (Ganasen and Benjakul, 2011a). The decrease in alkaline taste is related to the decrease of free alkalinity of preserved eggs, and the decrease in free alkalinity is related to the loss of volatile alkaline nitrogen–containing substances (Ganasen and Benjakul, 2011b). The hardness of the protein becomes more and even shrinkage is related to the loss of water dispersion, and storage at different temperatures will also affect the rate of water loss (Ma et al., 2015). The mildew taste is related to protein degradation, changes in free amino acids and free fatty acids; fatty acids are easily oxidized during storage, producing a mildew taste and deteriorating amino acids (Dong et al., 2017).
Figure 1.
Effects of storage temperatures on sensory quality of preserved eggs.
Effects of Different Storage Temperatures on Weight Loss Rate and Moisture of Preserved Eggs
The weight loss rate is an important index for the quality change of eggs during storage. The main reason for the loss of egg weight is that the inner structure of the duck eggshell became loose during the process of soaking in alkali solution, which exposed the pores of the eggshell surface and promoted the gas emission of gas generated by biochemical reactions inside the eggs (Ruan, 2014). The weight loss rate of preserved eggs is related to the porosity and the thickness of eggshell, and the chemical composition of the egg white and yolk inside the egg also plays an important role (Ruan, 2014).
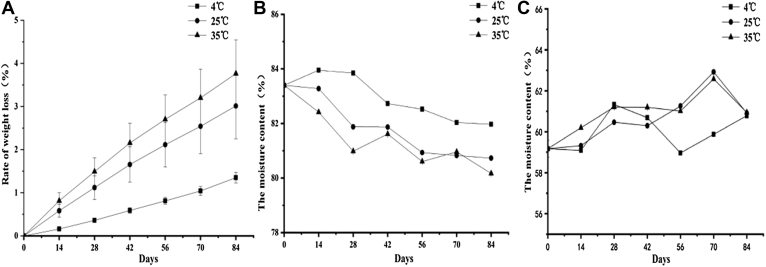
As shown in Figure 2, the weight loss rate of preserved eggs increased with the extension of storage time (P < 0.05), and the higher the temperature was, the greater the weight loss rate was (P < 0.05), which was similar to the research results of the study carried out by Ma Lei et al. (Ma et al., 2015). Increasing storage temperature can promote the decomposition of proteins in the egg white and yolk and produce a series of volatile alkaline nitrogen–containing substances. Meanwhile, internal water vapor is easier to volatilize, so the higher the temperature, the greater the weight loss rate. At the same time, it can also be considered that the weight loss rate of preserved eggs may be related to the specific surface area of the egg itself. The larger the specific surface area, the more volatile substances from the eggshell.
Figure 2.
Effects of different storage temperatures on the preserved egg rate of weight loss (A) and the moisture of egg white (B) and yolk (C).
Water is the main component of egg products. The moisture in egg products has a great influence on the color, flavor and texture of egg products (Miranda et al., 2014). As shown in Figure 2, the moisture of preserved eggs white decreased with the storage time prolonged, and there was a significant difference between different temperatures (P < 0.05), and the higher the temperature, the faster the moisture loss; whereas the moisture of the egg yolk showed a "rising and falling" cycle state. Coincidentally, it was found that the egg white was in the state of moisture loss, whereas the yolk was in the state of moisture increase during storage at 25°C for the first 28 D, and more egg white dehydration corresponded to more increase in yolk moisture. Similarly, the same rule was observed from 28 to 42 D. It can be inferred that during the storage process, the cause of egg white dehydration is not only the escape of water through the eggshell but also the infiltration of some water into the yolk. The dehydration of preserved egg whites leads to different trends of moisture in preserved egg yolks. Similarly, water in the egg yolk can also enter into the egg white, and further research is needed on why such water migration occurred. The higher the storage temperature, the faster the loss of water may be due to the faster evaporation rate of water in the higher temperature preserved eggs, which was similar to the result of weight loss rate.
Effect of Storage Temperature on pH Value of the Preserved Egg White and Yolk
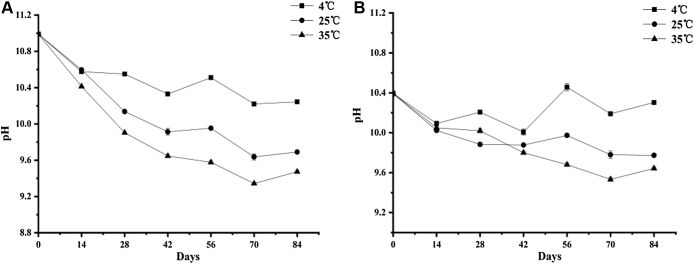
As can be observed from Figure 3, the pH values of the preserved egg white and yolk just out of the pickling container in this study were 10.99 and 10.39, respectively; after storage for 84 D, the pH values of the egg white and yolk were 10.24 and 9.69, 9.47 and 10.3, and 9.77 and 9.64 at 4°C, 25°C, and 35°C, respectively. The results showed that the pH values of the preserved egg white and egg yolk at different storage temperatures were significantly different (P < 0.05). During storage, the pH values of the preserved egg white generally showed downward trend and decreased significantly in the early stage (P < 0.05), whereas the later changes were slow. The pH of preserved egg yolks stored at 35°C decreased first and then increased during the whole storage period, and the decrease was greater than that at 4°C and 25°C (P < 0.05). It can be concluded that the higher the storage temperature, the faster the pH value of the preserved egg white decreased (P < 0.05).
Figure 3.
Effects of storage temperatures on pH value of preserved egg white (A) and yolk (B).
The pH value of the preserved egg yolk just out of the pickling container was lower than that of the egg white, which is related to the sequence of alkali infiltration in the process of pickled penetration. During the pickling process, the alkali solution must enter the egg white first and then penetrate into the egg yolk. It is more difficult for the egg yolk to have a contact with the alkali solution than the egg white. In addition, the fresh duck egg yolk is acidic (pH = 6.50), and the egg white is alkaline (pH = 8.94) (Xu et al., 2017), which made the pH of the egg white increase more easily than that of yolk under the action of alkali solution. One of the reasons why the pH of the preserved egg white and yolk decreased with time might be that a series of biochemical reactions will occur in the preserved eggs during the pickling process, producing substances such as alcohols, aromatics, and heterocyclic compounds (Chen et al., 2015, Zhang et al., 2015), which contain some volatile basic nitrogenous substances, then dissipated to the outside through the pores of the eggshell, which eventually leads to a decrease in pH. This kind of speculation also appeared in previous studies (Ganasen and Benjakul, 2011a, Ganasen and Benjakul, 2011c). Compared with low temperature, the reason why the pH value of the preserved egg white and yolk decreased faster under high temperature storage may be that high temperature can accelerate the rate of free alkaline volatile substances passing through the pores of the eggshell.
Interestingly, it was found that pH change curve of the preserved egg white and yolk in each storage period basically maintained the same change trend. It can be inferred that the change of preserved egg white and yolk pH was not independent of each other and did not interfere with each other but was related to each other. It can be speculated that the basic ions inside preserved eggs were in a state of dynamic migration between the egg white and yolk all the time.
Effects of Different Storage Temperatures on the Color of the Preserved Egg White and Yolk
Color is an important sensory index of preserved eggs. At present, there are 2 common reasons for the formation of color of preserved eggs: one is that H2S and NH3 produced by strong alkali–acting proteins react with metal compounds and proteins to form various pigment substances; and the other is that the Maillard reaction occurs inside the eggs to produce brown substances (Shao et al., 2017). Table 2 showed that during storage, the L∗ and b∗ values of the preserved egg white that was stored at high temperature and normal temperature were characterized by downward trend (P < 0.05), that is, the color of the egg white gradually deepened and darkened, and the higher the storage temperature, the faster the value decreased (P < 0.05). However, the L∗ and b∗ values of the preserved egg white that was stored at high temperature increased first and then decreased (P < 0.05) and the phenomenon of "yellowing" appeared. On the other hand, during storage, the L∗ value of the preserved egg yolk showed a downward trend (P < 0.05), whereas the a∗ and b∗ values showed an upward trend (P < 0.05), and the higher the temperature, the greater the change.
Table 2.
Effect of storage temperature on color of preserved eggs.
| Days | Protein color value |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| L∗ |
a∗ |
b∗ |
|||||||
| 4°C | 25°C | 35°C | 4°C | 25°C | 35°C | 4°C | 25°C | 35°C | |
| 0 D | 33.95 ± 1.3a | 33.95 ± 1.3a | 33.95 ± 1.3a | 7.77 ± 1.5a | 7.77 ± 1.45b,c | 7.77 ± 1.45c | 18.42 ± 2.2c | 18.42 ± 2.2a | 18.42 ± 2.20a |
| 14 D | 35.24 ± 0.9a | 32.84 ± 1.4a | 32.58 ± 0.8a | 6.48 ± 2.2a | 6.72 ± 1.56c | 9.0 ± 2.28b,c | 18.6 ± 1.7c | 15.42 ± 1.3a | 6.03 ± 1.65c |
| 28 D | 37.79 ± 1.9a,b | 31.79 ± 1.5a | 32.15 ± 0.9a | 7.76 ± 0.9a | 9.63 ± 1.75b,c | 11.84 ± 0.99a,b | 20.6 ± 1.6c | 11.66 ± 1.83b | 8.85 ± 1.1b |
| 42 D | 43.7 ± 1.7c | 28.35 ± 0.7b | 25.4 ± 0.5b | 6.64 ± 0.9a | 10.06 ± 1.69b | 13.22 ± 1.44a | 25.58 ± 2.1b | 12.07 ± 1.33b | 5.87 ± 0.79c |
| 56 D | 48.25 ± 0.9d | 25.86 ± 1.0c | 25.47 ± 0.4b | 6.73 ± 1.4a | 15.98 ± 1.9a | 6.88 ± 1.6c | 26.97 ± 1.21a,b | 11.83 ± 3.31b | −0.74 ± 0.77e |
| 70 D | 49.36 ± 0.8d | 24.31 ± 1.1c | 22.41 ± 0.9c | 7.5 ± 0.5a | 9.82 ± 2.3b | 8.11 ± 1.47b,c | 27.77 ± 1.01a | 1.13 ± 1.59d | −0.23 ± 1.14d |
| 84 D | 36.67 ± 0.6b | 22.97 ± 0.8d | 20.97 ± 0.7d | 10.21 ± 2.6b | 9.53 ± 0.94b | 10.53 ± 1.66b | 21.03 ± 2.5c | 5.21 ± 1.85c | 4.06 ± 1.47c |
| Days | The outer color value of egg yolk |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| L∗ |
a∗ |
b∗ |
|||||||
| 4°C | 25°C | 35°C | 4°C | 25°C | 35°C | 4°C | 25°C | 35°C | |
| 0 D | 41.46 ± 0.66a | 41.46 ± 0.66a | 41.46 ± 0.66a | −5.34 ± 0.47e | −5.34 ± 0.47e | −5.34 ± 0.47f | −1.69 ± 0.62d | −1.69 ± 0.62e | −1.69 ± 0.62f |
| 14 D | 40.23 ± 0.82a | 40.17 ± 0.90a | 39.54 ± 0.56b | −4.49 ± 0.5d,e | −4.51 ± 0.24e | −4.46 ± 0.31e | −1.36 ± 0.99d | −1.16 ± 0.73e | −0.72 ± 0.90e,f |
| 28 D | 38.6 ± 0.93b | 38.49 ± 1.06c | 37.54 ± 0.86c | −4.74 ± 0.63d,e | −3.37 ± 0.23d | −2.82 ± 0.42d | 0.01 ± 1.22d | 3.39 ± 0.32d | 0.18 ± 0.53e |
| 42 D | 36.94 ± 0.70c | 36.21 ± 0.74d | 35.63 ± 0.99d | −3.67 ± 0.73c,d | −3.26 ± 0.77d | −2.96 ± 0.62d | 2.48 ± 1.24c | 3.83 ± 0.63c,d | 1.64 ± 0.38d |
| 56 D | 36.06 ± 1.03c,d | 33.16 ± 0.46e | 33.60 ± 0.65e | −3.28 ± 0.53c | −1.85 ± 0.68c | 0.37 ± 0.28c | 4.66 ± 0.91b | 4.48 ± 0.22c | 4.59 ± 0.43c |
| 70 D | 34.36 ± 0.86e | 32.93 ± 1.33e,f | 32.30 ± 0.71f | −0.96 ± 0.78a | −0.38 ± 0.71b | 1.06 ± 0.36b | 6.85 ± 0.92a | 6.53 ± 1.04b | 7.61 ± 0.64b |
| 84 D | 35.72 ± 0.30d | 31.45 ± 0.18f | 29.75 ± 0.88g | −1.81 ± 0.39b | 1.47 ± 0.37a | 2.35 ± 0.48a | 7.89 ± 0.83a | 8.79 ± 0.53a | 11.19 ± 1.28a |
a-fDifferent superscript letters in the same column indicate significant difference (P < 0.05).
Under the condition of normal temperature and high temperature, the L∗ value of the egg white and yolk showed a decreasing trend, and the decreasing trend of high temperature was more obvious. The possible reason was that the Maillard reaction occurred during storage, producing brown or even black melanoids, which reduced the L∗ value. High temperature could promote and low temperature could significantly slow down the Maillard reaction (Bakry et al., 2019). In addition, the color change of the preserved egg white is also related to the decrease of moisture content in the egg white. The loss of moisture will lead to the accumulation of pigment content. High temperature accelerates the loss of moisture in preserved eggs, which will further lead to the increase of preserved egg white pigment (Ganasen and Benjakul, 2011a). The content of free amino acids is also related to Maillard reactions, and the content of free amino acids participating in Maillard reactions decreases, the reaction products also decrease (Ganesan and Benjakul, 2010). Therefore, under the condition of low temperature, L∗ value decreased more slowly. It can also be found that the change in the trend of a∗ and b∗ values of the egg white and yolk is different, which indicated that the biochemical reactions affecting the color change in the egg white and yolk were different. It might be that lipids (such as triglyceride or lecithin) in the egg yolk participated in the formation of volatile flavor substances in the Maillard reaction and then affected the color change of the egg yolk (Farmer and Mottram, 2010).
Effects of Different Storage Temperatures on Texture Characteristics of Preserved Eggs
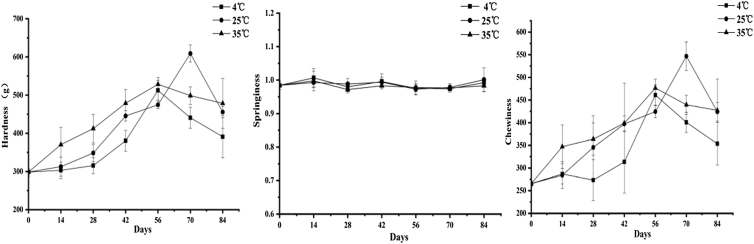
Texture properties of the preserved egg white at different storage temperatures were measured at different storage times, including hardness, springiness, and chewiness. These parameters can reflect the changes in texture properties of food gels. The results were shown in Figure 4.
Figure 4.
Effects of storage temperatures on protein structure properties of preserved eggs.
The hardness values of the preserved egg white at different storage temperatures were increased first and then decreased with the prolongation of storage time (P < 0.05), and the hardness values under low temperature storage conditions were significantly lower than normal temperature and high temperature storage (P < 0.05). The reason for the increase in hardness might be that the egg white was dehydrated gradually during storage; and the increasing of disulfide bonds made the proteins structure stable and the binding rigid (Dombkowski et al., 2014, Liu et al., 2016). At low temperature, the egg white lost less water and the disulfide bond content was not increased as much as at normal temperature and high temperature (P < 0.05) hence, the hardness is lower.
Springiness is an important parameter to characterize the binding state of the internal structure of protein gels (Xiong et al., 2015), and high springiness indicates that the gel has a superior ability to maintain a complete network structure (Juana Fernandez-Lopez1 et al., 2006). The results showed that the springiness of the preserved egg white remained at about 1.0 during the storage period. There was no significant change in the springiness of the preserved egg white with the extension of storage time under 3 storage temperatures (P > 0.05) and no significant difference between diverse temperatures (P > 0.05). The reason for this phenomenon might be that the moisture content of the preserved egg white during the storage process still remained more than 80% and has not been seriously decreased. Meanwhile, there was no significant change in the net charge number on the protein surface owing to the influence of external factors. Therefore, the network structure of egg white gel would not change significantly, and the springiness would not change significantly (Qun et al., 2014).
The chewiness is a comprehensive index of sensory texture of food, which is directly related to food taste and closely related to hardness, springiness, and cohesiveness. As can be seen from the Figure 4, the change trend of chewiness and hardness was approximately the same, both of which increased first and then decreased (P < 0.05), which was related to the change rule of egg white gel hardness.
Effects of Different Storage Temperatures on the Selective Protein Solubility of the Preserved Egg
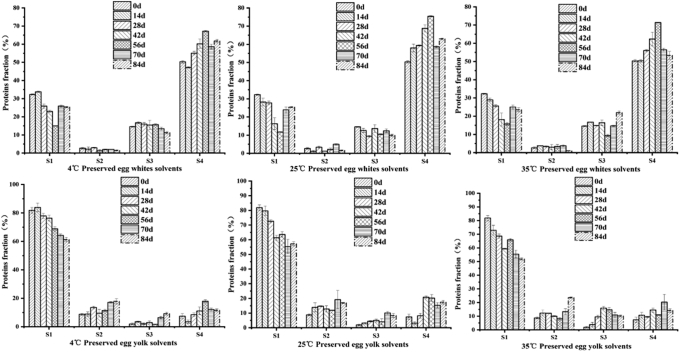
Preserved egg whites and egg yolks are special gels formed by strong alkali, and the texture of the preserved egg white and egg yolk gel changed greatly during storage. To explore the reason, different denaturants are used to treat preserved egg white and egg yolk samples: 0.6 M NaCl (ion bond), 1.5 M urea (hydrogen bond), 8M urea (hydrogen bond, hydrophobic interaction), 0.5 M β-mercaptoethanol (disulfide bond) (Pérez-Mateos et al., 1997). The continuous solubility of proteins in denaturants with different ratios represents the corresponding proportion of forces, thereby analyzing the role of different forces in the storage of preserved eggs.
As shown in Figure 5, the distribution of preserved egg white proteins and egg yolk proteins at different temperatures in different solvents was significantly different, and it also changed with the prolongation of storage time (P < 0.05). The results showed that proteins of the preserved egg white were mainly dissolved in S1, S3, and S4, accounting for about 20, 15, and 60%, respectively, indicating that the main forces were ionic bond, hydrophobic bond, and disulfide bond. The remaining S2 accounted for about 5%, indicating that there were few hydrogen bonds. The proteins of the preserved egg yolk were mainly dissolved in S1, S2, and S4, accounting for 65, 10, and 10%, respectively, indicating that the main forces were ionic bond, hydrogen bond, and disulfide bond. In addition, no insoluble precipitates were observed after proteins were treated with S4, indicating that there were no other forms of covalent bonds in the proteins; conversely, some undissolved precipitates were observed in the egg yolk after the S4 treatment, indicating the presence of chemical bonds in the egg yolk that were not easily destroyed by β-mercaptoethanol.
Figure 5.
Protein fraction (%) of preserved egg gels in different solutions: 0.6 M sodium chloride (S1); 0.6 M sodium chloride + 1.5 M urea (S2); 0.6 M sodium chloride + 8 M urea (S3); and 0.6 M sodium chloride + 8 M urea + 0.5 M β-mercaptoethanol (S4).
With the prolongation of storage time, the solubility of preserved egg white gel at 3 storage temperatures showed a trend of first decreasing and then increasing in S1 (P < 0.05), the solubility in S4 increased first and then decreased (P < 0.05), and there was no obvious change trend in S2 and S3 parts. This indicated that the proportion of ionic and disulfide bonds in the preserved egg white gel presented a "complementary" dynamic balance during storage. Compared with normal temperature and high temperature, the difference of S1 at low temperature was significant (P < 0.05). The decrease of ionic bond contents might be related to the bonding and aggregation of protein molecules under the conditions of decreased pH (Yang et al., 2019), 3 types of ionic bonds, electrostatic attractions within zwitterions, “salt bridges,” and “water ionic bonds” (Zhao et al., 2016b). The free alkali ions in preserved egg gel were flowing and decreasing in preserved egg whites and yolks after preserved eggs were taken out of the pickling container, resulting in an unstable state of total electric charge inside the preserved egg. The forming condition of an ionic bond is that the electrostatic repulsion and electrostatic attraction must be balanced, and the unstable state of the net charge may destroy the balance of electrostatic repulsion and electrostatic attraction, thus further influencing the stability of ionic bond content, and the decrease of pH may be related to the decrease of ion bond content. The decrease in pH leads to a decrease in the net negative charge on the surface of the protein molecule, further reducing the electrostatic repulsion, which is beneficial to the stability of the protein molecule (Zhao et al., 2016a). Low temperature can reduce the loss of free alkali ions to a certain extent; hence, the rate of S1 decrease at low temperature was slower than that at normal temperature and high temperature. Because of the large amounts of sulfhydryl (SH) group and disulfide bond in proteins of preserved egg whites and the alkaline environment, the whole preserved egg white was negatively charged, and the repulsion between charges prompted the protein molecules to expand and expose their internal groups; hence, it can provide good conditions for SH oxidation and SH-SS exchange reaction (Ganesan et al., 2014, Zhao et al., 2016b). At the same time, a decrease in pH during storage will reduce the electrostatic interaction and promote the formation of disulfide bonds (Felix et al., 2017). Therefore, part S4 was in a rising state during the first 8 wk, and the cross-linking between protein molecules or polypeptide chains through disulfide bonds would promote the protein structure to be more stable, which may also be one of the reasons for the hardness enhancement of preserved egg whites.
The solubility of the egg yolk gel in S1 at the 3 storage temperatures during the storage period decreased overall, the main reason may be similar to the decrease of egg white gel in S1, and the low temperature storage also significantly reduced the degree of this change (P < 0.05). A high proportion of ionic bonds indicated that noncovalent bonds dominate, and electrostatic repulsion may occur between negatively charged groups and water ionic bonds. The higher pH value also leads to greater electrostatic repulsion, which will hinder the interaction between protein molecules in the system and affect the formation of gel structure (Chen, et al., 2015). Treatment S2 and S3 showed a slow upward trend compared with 0 D. The infiltration of a large amount of alkali solution during the pickling process of the preserved eggs, coupled with water migration, led to the difficulty in the stable existence of hydrogen bond and hydrophobic effect. However, during storage, the environment of a large amount of OH− aqueous solution gradually changed as the free alkali solution was gradually lost; hence, the hydrogen bond and hydrophobic interaction gradually increased.
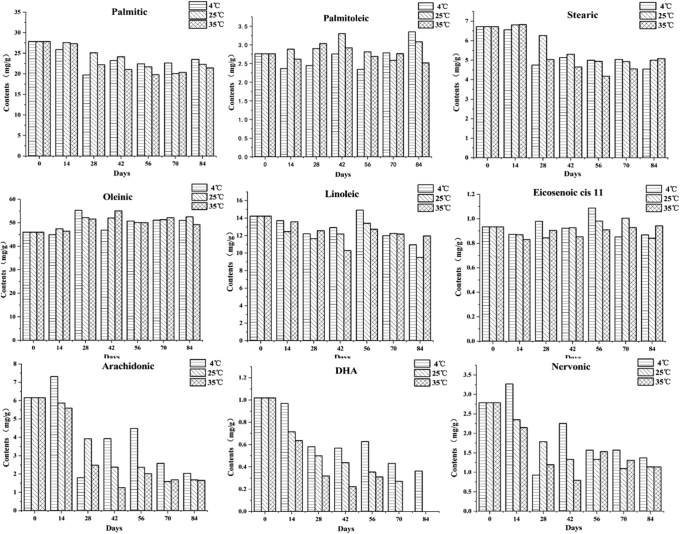
Effects of Different Storage Temperatures on Free Fatty Acid Content in the Preserved Egg Yolk
Free fatty acids are precursors of flavor to make a certain contribution to the unique flavor of food. Similarly, the formation of special flavor of preserved eggs is also inseparable from the role of free fatty acids (Zhao et al., 2014). During the storage process, as seen in Figure 6, a total of 9 free fatty acids were detected, including palmitic acid, palmitoleic acid, stearic acid, oleinic acid, linoleic acid, cis-11-eicosenoic acid, arachidonic acid, docosahexaenoic acid (DHA), and nervonic acid. Except for arachidonic acid, DHA, and nervonic acid, the changes of other fatty acids during storage were small, indicating that there was no significant degradation during storage. Research studies (Yu et al., 2019) have found that fat degradation was mainly caused by endogenous lipase. For example, triglycerides or phospholipids in lipids can be hydrolyzed into free fatty acids, and phospholipids hydrolysis was the main factor for the production of free fatty acids (He et al., 2012, Li et al., 2019), and the hydrolysis process was mainly produced by lipase (Plagemann et al., 2011). On the other hand, fat contains acidic lipase (Alami et al., 2017); alkaline conditions can inhibit the activity of acidic lipase, At the same time, during the pickling period of up to 40 days, the lipase activity gradually weakened, and its ability to degrade triglycerides and phospholipids also gradually weakened (Deng, 2013). In addition, the content of triglycerides and phospholipids in preserved eggs was also limited, The 40-D pickling cycle has caused the triglyceride and phospholipid content to be consumed more, and the content of free fatty acids that can be decomposed during storage also gradually decreased (Deng, 2013). Based on the 2 aforementioned factors, the production of free fatty acids during storage was severely affected. Inhibition might be the reason why free fatty acids in preserved eggs no longer increase. Second, owing to the action of oxygen in the external environment, free fatty acids, especially unsaturated fatty acids in food, are easily oxidatively degraded without light and catalysts (Nielsen et al., 2017, Xie et al., 2018). The decrease of arachidonic acid, DHA, and nervonic acid also fits this idea. These reasons were the main cause of the change of free fatty acids. With the exception of DHA, temperature did not significantly change the content of the other 8 fatty acids at the end of storage. The possible reason was that temperature can affect the activity of lipase and the hydrolysis of phospholipids, but the alkaline conditions reduced the activity of lipase in preserved eggs that were stored at 3 different temperatures. Therefore, the activity of the internal acidic lipases could hardly be significantly affected by different temperatures, which eventually resulted in little difference between most free fatty acids.
Figure 6.
Effects of storage temperatures on free fatty acid content of preserved egg yolk.
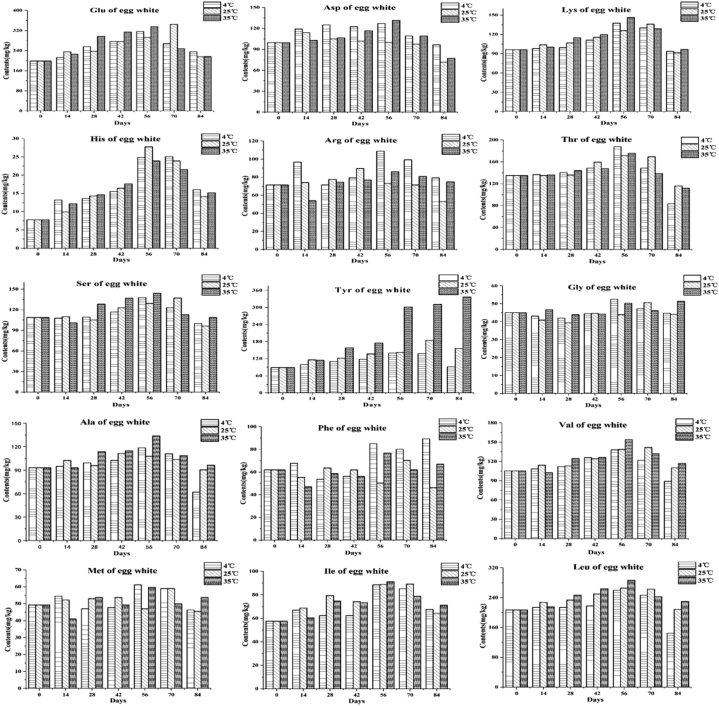
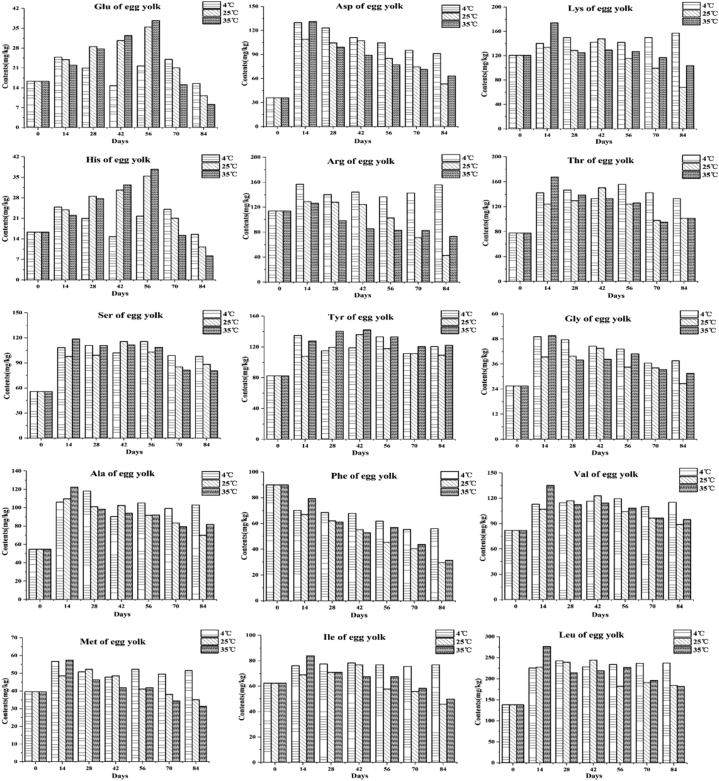
Effects of Different Storage Temperatures on Free Amino Acids in Preserved Eggs
Amino acids are not only an important source of food flavor but also can produce a series of volatile substances through decarboxylation, deamination, transamination, Maillard reaction with reducing sugar, thus affecting the overall flavor of food (Liu, 2013). During the storage period of preserved eggs, as seen in Figure 7, 15 kinds of free amino acids were detected in the egg white and yolk, and the overall trend of free amino acids content in the egg white and yolk at 3 storage temperatures showed a trend of first rising and then declining. Among them, content of free amino acids in egg whites changed from 1,425.81 mg/kg to 1,338.58 mg/kg, 1,423.24 mg/kg, and 1,724.38 mg/kg after storage at 4°C, 25°C, and 35°C, respectively. The free amino acid content of egg yolks changed from 1,122.12 mg/kg to 1,685.48 mg/kg, 1,138.55 mg/kg, and 1,219.59 mg/kg after storage at 4°C, 25°C, and 35°C, which indicated that different storage temperatures had significant effects on the free amino acid content of preserved eggs (P < 0.05).
Figure 7.
Effects of storage temperatures on free amino acid of preserved egg white and yolk.
During storage, proteins, polypeptides, and proteases degrade to produce free amino acids. Therefore, the content of free amino acids presented an increasing trend. Meanwhile, free amino acids will undergo decarboxylation, deamination, and Maillard reaction with reducing sugar to decrease their contents (Poulsen et al., 2013). It has been found that polypeptides were produced by proteinase acting on proteins and then were further decomposed into amino acid by the peptidase (Zhou et al., 2017). However, the action of alkali inside the preserved egg can inhibit the activity of protease or peptidase, which indirectly affects the content of amino acids. Therefore, the change of free amino acids is a complicated process. The increase of free amino acids content in preserved eggs may be attributed to the fact that the preserved eggs were still affected by alkali, even though they were removed from the alkaline pickling solution, that the proteins continued to be degraded into amino acids (Chen et al., 2015). The possible reason for the decline in the later stage is that, with the prolongation of storage time, polypeptides and amino acids were decomposed into ammonia and amines (Figure 8), and the rate was faster than that of polypeptides into amino acids.
Figure 8.
Effects of different storage temperatures on volatile salt nitrogen in preserved egg white (A) and yolk (B).
The content of free amino acids in preserved eggs was significantly affected by different storage temperatures. Figure 7 shows that high temperature storage increased the content of free amino acids in egg whites, further may also promote the Maillard reaction, and the final reaction products increase and the color darkens. On the one hand, at the end of storage, the content of free amino acids in yolk stored at low temperature is higher than that stored at high temperatures. The possible reason was that amino acids were more easily degraded to amines at high temperature than those stored at low temperature (Kadidlova et al., 2010). At the same time, the Maillard reaction can be slowed down at low temperature; hence, the consumption of free amino acids was less (Bakry et al., 2019), which was related to the color change. On the other hand, the content of free amino acids in preserved egg whites stored at low temperature is lower than that stored at high temperature. Although low temperature can slowe down the degradation of amino acids to amines and other substances to some extent, the phenomenon of "yellowing" occurred in preserved egg whites stored at low temperature, which may be related to this result.
Effects of Different Storage Temperatures on Total Volatile Basic Nitrogen in the Preserved Egg White and Yolk
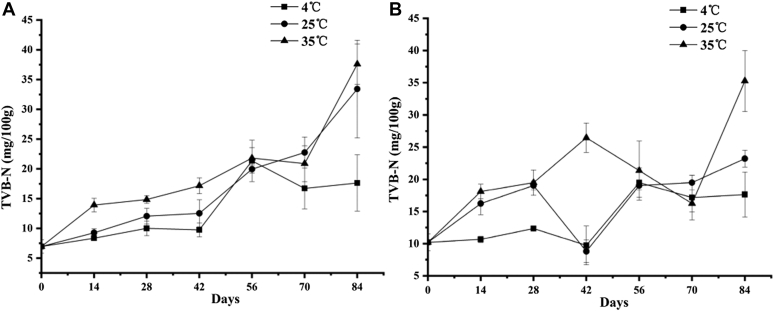
The content of total volatile basic nitrogen (TVB-N) in high-protein foods represents the rate at which amino acids are destroyed and is negatively related to their freshness (negative correlation). As can be seen from Figure 8, the TVB-N values of the preserved egg white and egg yolk increased significantly during storage (P < 0.05). Within 84 D of storage, the TVB-N value of the preserved egg white increased from 6.96 to 17.64 (4°C), 33.43 (25°C), and 37.6 (35°C), and those of the yolk increased from 10.21 to 17.64 (4°C), 23.21 (25°C), and 35.28 (35°C). The TVB-N value of freshly pickled preserved egg yolks was higher than that of preserved egg whites. The possible reason was that the strong alkali could degrade the preserved egg white and yolk (Chen et al., 2015, Yang et al., 2019). It was also reported that amino acids in preserved egg whites decreased by 16.065 g/mg and by 42.066 mg/g in preserved egg yolks during pickling (Deng, 2013). The more amino acids in the egg yolk were reduced, and the more basic nitrogen-containing substances such as ammonia and amines were produced, thus the higher TVB-N value (Wu et al., 2016). The TVB-N values of preserved egg whites stored at different temperatures all showed an upward trend, whereas the trend of egg yolks increased first, then decreased, and then increased, and the changes were more complicated. Regardless of the egg yolk or egg white, the TVB-N value was the lowest when stored at 4°C. The increase of TVB-N value is due to the continued decomposition of proteins during storage (Yang et al., 2017), and the low temperature can still inhibit this change to a certain extent.
Effect of Storage Temperature on Microbiological Indexes of Preserved Egg White and Yolk
The total number of bacterial colonies and the number of coliforms in preserved eggs were regularly measured by GB/T 4789-2016. The results showed that neither of them was detected during storage. The possible reason was that compared with the fresh duck eggs before pickling, the preserved egg shells with metal ions possessed a denser structure, which could better isolate from the outside and prevent the invasion of spoilage microorganisms. Second, during storage, the pH of preserved eggs remained higher than 9.4, which exceeded the highest tolerable pH of bacteria, yeasts, and most fungi (Tucker and Featherstone, 2010). Excessive pH could affect the uptake of microbial enzymes and cellular nutrients, thus affecting the normal metabolism of microorganisms. Therefore, during the whole storage period, preserved eggs were not infected by microorganisms.
Conclusions
During the 12-wk storage period, the sensory quality of the preserved eggs gradually decreased. It was manifested that as the storage time prolongs, the internal moisture of the preserved eggs was gradually lost to the outside of the shell, the proteinaceous substances were degraded, and the free amino acid content was slowly increased. At the same time, volatile basic nitrogen-containing substances were also generated, which caused the pH value to decrease. It further affected the internal Maillard reaction, which eventually results in deeper brown eggs; on the other hand, the reduction of ionic bonds, the increase of disulfide bonds, and the loss of moisture cause the hardness and chewiness of the preserved eggs to increase. During storage, free amino acids in the egg white/yolk mostly increased first and then decreased, which indicated that preserved eggs stored for a certain period of time were more easily digested and absorbed and have a higher nutritional value. However, the total amount of free fatty acids decreased during storage time. Saturated fatty acids were oxidatively degraded. Compared with normal temperature and high temperature storage, low temperature can significantly inhibit the occurrence of this series of changes. Finally, during storage, the sensory score of low temperature storage was better than normal temperature and high temperature storage. Therefore, it was concluded that low temperature storage is more conducive to the preservation of preserved eggs.
Acknowledgments
We gratefully acknowledge the financial support provided by the Program of the National Natural Science Foundation of China (Grant Nos. 31760439 and 31871832).
Conflict of Interest Statement: The authors declare no conflicts of interest.
Contributor Information
Yonggang Tu, Email: tygzy1212@aliyun.com.
Yan Zhao, Email: tygzy1212@aliyun.com.
References
- Alami N.H., Nasihah L., Umar R.L.A., Kuswytasari N.D., Zulaika E., Shovitri M. Lipase Production in Lipolytic Yeast from Wonorejo Mangrove Area. In: Murkovic M., Risuleo C., Prasetyo E.N., Shovitri M., Nyanhongo G.S., editors. Proceeding of International Biology Conference 2016: Biodiversity and Biotechnology for Human Welfare. AIP Conference Proceedings; Surabaya, Indonesia: 2017. [Google Scholar]
- Bakry A.M., Ma C., Xiong S., Yin T., Zhang B., Huang Q. Chitosan-glucose Maillard reaction products and their preservative effects on fresh grass carp (Ctenopharyngodon idellus) fillets during cold storage. J. Sci. Food Agric. 2019;99:2158–2164. doi: 10.1002/jsfa.9408. [DOI] [PubMed] [Google Scholar]
- Chang H.M., Tsai C.F., Li C.F. Changes of amino acid composition and lysinoalanine formation in alkali-pickled duck eggs. J. Agric. Food Chem. 1999;47:1495–1500. doi: 10.1021/jf980951k. [DOI] [PubMed] [Google Scholar]
- Chen Z., Li J., Tu Y., Yan Z., Luo X., Wang J., Wang M. Changes in gel characteristics of egg white under strong alkali treatment. Food Hydrocolloids. 2015;45:1–8. [Google Scholar]
- China. The Standard Press of PR China; Beijing, China: 2003. Chinese Standard GB/T 5009.47-2003 in Method for Analysis of Hygienic Standard of Egg and Egg products. [Google Scholar]
- China. The Standard Press of PR China; Beijing,China: 2015. Chinese Standard GB2749-2015 in Eggs and Egg products. [Google Scholar]
- China. The Standard Press of PR China; Beijing ,China: 2016. Chinese Standard GB4789.2-2016 in Determination of Total Number of Bacterial Colonies in Food Microbiology test. [Google Scholar]
- China. The Standard Press of PR China; Beijing,China: 2016. Chinese Standard GB4789.3-2016 in Food Microbiology Test for Coliform count. [Google Scholar]
- China. The Standard Press of PR China; Beijing,China: 2016. Chinese Standard GB5009.3-2016 in Determination of Moisture in foods. [Google Scholar]
- China. The Standard Press of PR China; Beijing, China: 2016. Chinese Standard GB5009.228-2016 in Determination of Volatile Base Nitrogen in food. [Google Scholar]
- Deng Wenhui. NanChang University; Nanchang, China: 2013. Changes of Amino Acids and Fatty Acids in Preserved Egg Processing and the Role of Forming Preserved Egg Flavor Master. [Google Scholar]
- Dombkowski A.A., Kazi Zakia S., Craig D.B. Protein disulfide engineering. FEBS Lett. 2014;588:206–212. doi: 10.1016/j.febslet.2013.11.024. [DOI] [PubMed] [Google Scholar]
- Dong H., Li X., Xi J., Zhou Y., Song J. Free amino acid content and composition correlations in flue-cured tobacco from different planting areas and different flavor types. Tob. Sci. Technol. 2017;50:15–22. [Google Scholar]
- Farmer L.J., Mottram D.S. Interaction of lipid in the maillard reaction between cysteine and ribose: the effect of a triglyceride and three phospholipids on the volatile products. J. Sci. Food Agric. 2010;53:505–525. [Google Scholar]
- Felix M., Romero A., Rustad T., Guerrero A. Physicochemical, microstructure and bioactive characterization of gels made from crayfish protein. Food Hydrocolloids. 2017;63:429–436. [Google Scholar]
- Ganesan P., Benjakul S. Influence of different cations on chemical composition and microstructure of pidan white and yolk during pickling and aging. Int. J. Food Prop. 2010;13:1150–1160. [Google Scholar]
- Ganasen P., Benjakul S. Chemical composition, physical properties and microstructure of pidan white as affected by different divalent and monovalent cations. J. Food Biochem. 2011;35:1528–1537. [Google Scholar]
- Ganasen P., Benjakul S. Effects of green Tea and Chinese Tea on the composition and physical properties of pidan white. J. Food Process. Preserv. 2011;35:907–916. [Google Scholar]
- Ganasen P., Benjakul S. Physical properties and microstructure of pidan yolk as affected by different divalent and monovalent cations. J. Food Biochem. 2011;43:77–85. [Google Scholar]
- Ganesan P., Benjakul S., Baharin B.S. Effect of different cations in pickling solution on FTIR characteristics of pidan white and yolk in Comparison to the fresh duck egg. Sains Malays. 2014;43:1883–1887. [Google Scholar]
- He Z., Huang Y., Li H., Qin G., Wang T., Yang J. Effect of high-pressure treatment on the fatty acid composition of intramuscular lipid in pork. Meat Sci. 2012;90:170–175. doi: 10.1016/j.meatsci.2011.06.022. [DOI] [PubMed] [Google Scholar]
- Hu Jie, Meihu M. Preservative effect of different coatings on preserved eggs. Food Sci. (Chinese) 2012;33:274–277. [Google Scholar]
- Juana Fernandez-Lopez1 A.M., Jose María Fernández-Ginés E.S.-B., Pérez-Alvarez E.S.A.J.A. Gelling and color properties of Ostrich(Struthio Camelus) egg white. J. Food Qual. 2006;29:171–183. [Google Scholar]
- Kadidlova H., Ciprysova Z., Hoza I., Pavel B. The effect of long-term storage on amino acid content of ready-to-eat entrees. Int. J. Food Sci. Technol. 2010;45:966–970. [Google Scholar]
- Li Y., Wang J., Liu C., Guo A., Han D. Effect of drying temperature on fatty acid composition in Air-dried beef during Chilled storage. Food Sci. 2019;40:14–21. [Google Scholar]
- Liu L. Study on Preparation of meat-Like process flavor substance from enzyme-hydrolyzed Wheat protein by maillard reaction. J. Chin. Cereals Oils Assoc. 2013;28:63–68. [Google Scholar]
- Liu T., Wang Y., Luo X., Li J., Reed S.A., Xiao H., Young T.S., Schultz P.G. Enhancing protein stability with extended disulfide bonds. Proc. Natl. Acad. Sci. U. S. A. 2016;113:5910. doi: 10.1073/pnas.1605363113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Lei, Yan Wenjing, Zhao Jianying, Zhang Jianhao, Wang Fang, Kaiming P. Preserved effect of nano-SiO2 and nano-TiO2 modified composite coating materials on pidan. Trans. Chin. Soc. Agric. Eng. 2015;31:269–280. [Google Scholar]
- Miranda G., Berna À., González R., Mulet A. The storage of dried Apricots: the effect of packaging and temperature on the changes of texture and moisture. J. Food Process. Preserv. 2014;38:565–572. [Google Scholar]
- Nielsen N.S., Lu H.F.S., Bruheim I., Jacobsen C. Quality changes of Antarctic krill powder during long term storage. Eur. J. Lipid Sci. Technol. 2017;119:1600085. [Google Scholar]
- Pérez-Mateos M., Lourenço H., Montero P., Borderías A.J. Rheological and biochemical characteristics of high-pressure- and heat-induced gels from Blue whiting (Micromesistius poutassou) Muscle proteins. J. Agric. Food Chem. 1997;45:44–49. [Google Scholar]
- Plagemann I., Zelena K., Krings U., Berger R.G. Volatile flavours in raw egg yolk of hens fed on different diets. J. Sci. Food. Agric. 2011;91:2061–2065. doi: 10.1002/jsfa.4420. [DOI] [PubMed] [Google Scholar]
- Poulsen M.W., Hedegaard R.V., Andersen J.M., de Courten B., Bugel S., Nielsen J., Skibsted L.H., Dragsted L.O. Advanced glycation endproducts in food and their effects on health. Food Chem. Toxicol. 2013;60:10–37. doi: 10.1016/j.fct.2013.06.052. [DOI] [PubMed] [Google Scholar]
- Quan T.H., Benjakul S. Quality, protease inhibitor and gelling property of duck egg albumen as affected by storage conditions J. Food Sci. Techn. 2017;55:513–522. doi: 10.1007/s13197-017-2960-6. -Mysore- [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qun H., Wangen Y., Yongguo J., Hongjie C., Xuemei S. Factors influencing texture properties of duck egg white protein gel. Food Sci. (Chinese) 2014;35:68–71. [Google Scholar]
- Ruan Xiaojuan. Huazhong Agricultural University; 2014. The Influence of Alkali on Egg Shell and Gel Mechanism of Duckegg White. Master. [Google Scholar]
- Shao Y., Zhao Y., Xu M., Chen Z., Wang S., Tu Y. Effects of copper ions on the characteristics of egg white gel induced by strong alkali. Poult. Sci. 2017;96:4116–4123. doi: 10.3382/ps/pex213. [DOI] [PubMed] [Google Scholar]
- Tu Y.G., Yan Z., Xu M.S., Xin L., Du H.Y. Simultaneous Determination of 20 Inorganic Elements in preserved egg prepared with different metal ions by ICP-AES. Food Analyt. Meth. 2013;6:667–676. [Google Scholar]
- Tucker G., Featherstone S. Wiley-Blackwell; West Sussex, UK: 2010. Hurdles to Microbial Growth. [Google Scholar]
- Wang J., Fung D.Y.C. Alkaline-Fermented foods: a Review with Emphasis on pidan Fermentation. Crit. Rev. Microbiol. 1996;22:101–138. doi: 10.3109/10408419609106457. [DOI] [PubMed] [Google Scholar]
- Wu X., Song X., Qiu Z., He Y. Mapping of TBARS distribution in frozen–thawed pork using NIR hyperspectral imaging. Meat Sci. 2016;113:92–96. doi: 10.1016/j.meatsci.2015.11.008. [DOI] [PubMed] [Google Scholar]
- Xie H., Zhou D., Hu X., Liu Z., Song L., Zhu B. Changes in lipid profiles of dried Clams (Mactra chinensis Philippi and Ruditapes philippinarum) during accelerated storage and Prediction of shelf life. J. Agric. Food Chem. 2018;66:7764–7774. doi: 10.1021/acs.jafc.8b03047. [DOI] [PubMed] [Google Scholar]
- Zhenjie Xiong, Sun Da-Wen, Qiong Dai, Zhong Han, Zeng Xi-An. Application of Visible hyperspectral imaging for Prediction of springiness of fresh Chicken meat. Food Analyt. Meth. 2015;8:380–391. [Google Scholar]
- Xu L., Zhao Y., Xu M., Yao Y., Nie X., Du H., Tu Y.G. Effects of salting treatment on the physicochemical properties, textural properties, and microstructures of duck eggs. PLoS One. 2017;12:e0182912. doi: 10.1371/journal.pone.0182912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q., Sun D.-W., Cheng W. Development of simplified models for nondestructive hyperspectral imaging monitoring of TVB-N contents in cured meat during drying process. J. Food Eng. 2017;192:53–60. [Google Scholar]
- Yang Y., Zhao Y., Xu M., Wu N., Yao Y., Du H., Liu H., Tu Y. Changes in physico-chemical properties, microstructure and intermolecular force of preserved egg yolk gels during pickling. Food Hydrocolloids. 2019;89:131–142. [Google Scholar]
- Yin Y., Liu Y., Zhang J., Wei S. Effect of composite conting with nano-silicon dioxide on fresh-keeping of preserved eggs. Trans. Chin. Soc. Agric. Eng. 2012;28:281–287. [Google Scholar]
- Yu Z., Fan W., Wang L., He H., Lv Y., Qi J., Lu Y., Wu W. Slowing down lipolysis significantly enhances the oral absorption of intact solid lipid nanoparticles. Biomater. Sci. 2019;7:4273–4282. doi: 10.1039/c9bm00873j. [DOI] [PubMed] [Google Scholar]
- Zhang X., Jiang A., Chen M., Ockerman H.W., Chen J. Effect of different alkali treatments on the chemical composition, physical properties, and microstructure of pidan white. J. Food Sci. Technol. 2015;52:2264–2271. doi: 10.1007/s13197-013-1201-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Zhao Y., Wu N., Yao Y., Xu M., Du H., Tu Y. The anti-inflammatory activity of peptides from simulated gastrointestinal digestion of preserved egg white in DSS-induced mouse colitis. Food Funct. 2018;9:6444–6454. doi: 10.1039/c8fo01939h. [DOI] [PubMed] [Google Scholar]
- Zhang M., Zhao Y., Yao Y., Xu M., Du H., Wu N., Tu Y. Isolation and identification of peptides from simulated gastrointestinal digestion of preserved egg white and their anti-inflammatory activity in TNF-α-induced Caco-2 cells. J. Nutrit. Biochem. 2019;63:44–53. doi: 10.1016/j.jnutbio.2018.09.019. [DOI] [PubMed] [Google Scholar]
- Zhao Yan T.Y.D.W., Jianke Li. Anges of fatty acids during the processing of preserved egg. Food Sci. (Chinese) 2014;35:69–72. [Google Scholar]
- Zhao Y., Chen Z., Li J., Xu M., Shao Y., Tu Y. Changes of microstructure characteristics and intermolecular interactions of preserved egg white gel during pickling. Food Chem. 2016;203:323–330. doi: 10.1016/j.foodchem.2016.02.044. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Chen Z., Li J., Xu M., Shao Y., Tu Y. Formation mechanism of ovalbumin gel induced by alkali. Food Hydrocolloids. 2016;61:390–398. [Google Scholar]
- Zhou Y.J., Ma Y.Q., Yao G.M., Li J.X., Wang S.J. Changes of protein composition and its relevance with textural properties during processing of fermented solid beef. J. Food Process. Preserv. 2017;41:e13224. [Google Scholar]