Abstract
This study was performed to investigate the effects of lignocellulose supplementation (LS) on performance parameters, egg quality, aerobic bacterial load of eggshell, serum biochemical parameters, and jejunal histomorphological traits of laying hens between 18 and 38 wk of age. A total of 640 pullets at 16 wk of age were allotted to 4 treatment groups as 0 kg (control, CONT), 0.5 kg, 1 kg, and 2 kg LS per ton of feed. Body weight (BW), daily feed intake, egg production (EP), egg weight (EW), and efficiency of feed utilization (EF) were determined as the mean of each 3-wk period between 18 and 38 wk of age. Laying hens in the 1 kg LS group had a higher BW mean (1632.1 g, P < 0.001). The highest mean value of EP and EW were observed in 1 kg LS group (81.8% and 57.3 g, respectively), whereas the lowest values were found in the 2 kg LS group (78.6% and 54.4 g, respectively, P < 0.001). The mean of EF was the lowest in the 1 kg LS group (2.72, P < 0.001). There was a decline in eggshell breaking strength and eggshell thickness in the 2 kg LS, when compared with the 0.5 and 1 kg LS groups (P < 0.001). The total aerobic bacterial load of the eggshell was the lowest in the 1 kg LS group (4.7 log10 cfu/mL). The level of aspartate amino transferase and alanine amino transferase showed an increment in both the CONT and 2 kg LS groups (P < 0.001). The high level of LS (2 kg per ton of feed) caused a decline in the levels of IgY, IgA and IgM, when compared to the 0.5 and 1 kg LS groups (P < 0.001). Laying hens in 0.5 and 1 kg LS groups had longer villus height (1335.9 μm) in the jejunum than the others (P < 0.001). These findings showed that the 1 kg LS per ton of feed improved EP and EW, eggshell quality, immunoglobulin levels and intestinal morphology, and decreased the total aerobic bacterial load.
Key words: lignocellulose, laying performance, villus growth, immunoglobulin, hepatic enzyme
Introduction
The formulation and composition of diet is critical to egg production (EP) and eggshell quality and therefore profitability, because feed composition represents approximately 65 to 70% of the total cost of production (Bell and Weaver, 2002, Godfray et al., 2010). Over the years, many adjustments to the feed composition of laying hens have been made depending on various factors, including production goal, strain, age, egg weight (EW), production system, etc. (Sakomura et al., 2019). Recently, some alternative dietary ingredients have been tested to decrease the EP cost without adverse effects on egg laying performance.
One alternative that may very well have a positive effect on feed intake (FI), growth and development, gut structure and digestibility, and the overall health in poultry is fiber (Mateos et al., 2012, He et al., 2015, Jiménez–Moreno et al., 2016, Kheravii et al., 2017). It is already known that the potential effects of dietary fiber are related to the physicochemical characteristics and structure of the fiber source (Raninen et al., 2011, Yokhana et al., 2016). Fiber sources, such as sunflower hulls, pea hulls, rice hulls, soy hulls, and sugar beet pulp, have been investigated for numerous parameters, including growth performance, FI, intestinal development, nutrient digestibility, and immune response in broilers (Sadeghi et al., 2015, Sozcu, 2019) and layer pullets (Guzmán et al., 2015a, Guzmán et al., 2015b, Hussein et al., 2017, Röhe et al., 2019a, Röhe et al., 2019b).
Many studies have investigated the effects of fiber inclusion with various sources, for example, cereal straw, sugar beet pulp, oat hulls, and other special products, including varying contents of soluble and insoluble fibers, in the diets of layer pullets during the growing phase (Guzmán et al., 2015a, Guzmán et al., 2015b, Yokhana et al., 2016, Hussein et al., 2017) rather than during the EP stage. Guzmán et al. (2015b) reported that supplementing the diet with a moderate amount of fiber supplementation with 2 or 4% of cereal straw, sugar beet pulp, and sunflower hulls improved growth performance of young pullets from 1 to 5 wk of age compared with pullets fed a control diet that was contained basically cereals and soybean meal. They reported an increment for FI by 3.6% and average daily gain by 4.1% during experimental period.
Recently, there has been particular interest in lignocellulose that made from wood and could be used as a high-quality fiber source in poultry nutrition. It has a content of approximately 55% of crude fiber with cellulose and hemicellulose and 25 to 30% of aromatic polymers, such as lignin. The content of lignocellulose could be changed depending on the source (Jørgensen et al., 2007, Harmsen et al., 2010, Bogusławska-Tryk et al., 2015). Besides, cellulose and lignin as components of lignocellulose are insoluble, and nonfermentable fiber fractions could affect gut health status and performance parameters (Hetland and Svihus, 2001; Krás et al., 2013) by increasing beneficial microflora population, especially Bifidobacterium and Lactobacillus (Cao et al., 2003; Shakouri et al., 2006; Saki et al., 2010). A recent study investigated the effects of lignocellulose supplementation (LS) in broiler nutrition exhibited positive effects broiler growth performance via changes in hepatic enzyme activities, immunoglobulin levels, villus growth in jejunum, and microflora in cecum (Sozcu, 2019). Therefore, this study was designed to evaluate the effects of LS as an insoluble fiber source in laying hen nutrition. Therefore, some important issues including laying performance, egg quality parameters, aerobic bacterial load of eggshell, serum biochemical parameters (hepatic enzyme and serum immunoglobulin levels), and jejunal histomorphological traits were measured between 18 and 38 wk of age.
Material and methods
Ethical Approval
The care and use of animals were approved by the ethics committee of Uludag University and practiced in accordance with the laws and regulations of Turkey (License Number 2015-13/08).
Birds, Experimental Design, Housing, and Diets
A total of 640 beak-trimmed pullets (Super Nick, White egg layers, 16 wk of age) were used for this experiment between 18 and 38 wk of age. A total of 640 hens were placed into the cages as 4 dietary treatments and with 4 replicates (n = 40 hens/cage) in each dietary treatments. At 16 wk of age, all pullets were individually weighed with a precision of ±0.1 g digital scale and then randomly allocated into cages. The layer hens were kept in an enrichment cage system which was designed according to the optimum standards of the EU Directive 1999/74/EC for laying hens kept in cage systems. In cage system, each hen was provided a total floor area of 750 cm2. The galvanized wire cage system was equipped with nipple drinkers (8 nipples per cage), through type galvanized feeders with a space of 12 cm of feeder per hen, an egg belt, a manure belt, nail rasps with an amount of 8 rasps per cage, perches with a space of 18 cm per hen, a nesting area that was surrounded by an orange curtain with an floor area of 102 cm2 per cage, and a scratching pad area with green artificial turf with a floor area of 5.92 cm2 per cage.
The lighting program was gradually increased by 1 h per wk from 12 h L:12 h D at 16 wk of age to 16 h L: 8 h D from 20 wk to the end of the experimental period. Standard commercial layer diets were used between 18 and 24 wk of age as the layer 1 diet (18.76% CP and 2785 ME kcal/kg), and then between 25 and 38 wk of age, the layer 2 diet was used (17.35% CP and 2815 ME kcal/kg) in the experiment (Table 1). The crude protein level of diets was analyzed with conventional methods as Kjehdahl method according to the AOAC procedures (2000). The metabolizable energy value was calculated according to the equations explained by Larbier and Leclercq (1994). Calcium and phosphorus contents of the diets were determined using flame atomic absorption spectrophotometry and the calorimetric method, respectively (AOAC, 2000). Feed and water were offered ad libitum to all hens during the experimental period. The treatment groups were assigned according to the lignocellulose supplement amount as CONT (no supplementation of lignocellulose): 0.5 kg, 1 kg, and 2 kg lignocellulose supplementation per ton of feed. The lignocellulose contained with an amount of 90% lignin that was processed at low temperature (Lignochar, Global Nutritech Biotechnology LLC, Richmond, VA). The nutrient content of the lignocellulose was analyzed in a special laboratory (Table 2).
Table 1.
The composition and nutrient level of laying hen diets between 18 and 38 wk of age.
| Item | Diet |
|
|---|---|---|
| Layer 1 | Layer 2 | |
| Ingredient (%) | ||
| Corn, grain | 62.86 | 65.32 |
| Soybean meal, 48% | 24.32 | 22.32 |
| Dicalcium phosphate | 1.52 | 1.45 |
| Limestone | 10.60 | 10.05 |
| NaCl | 0.35 | 0.35 |
| Premix1 | 0.30 | 0.30 |
| DL-Methionine | 0.21 | 0.18 |
| L-Lysine-HCL | 0.02 | 0.03 |
| Calculated chemical analysis | ||
| ME, kcal/kg | 2,785 | 2,815 |
| Available phosphorus | 0.42 | 0.37 |
| Chemical analysis | ||
| Dry matter | 90.6 | 91.1 |
| Crude ash | 7.2 | 7.2 |
| Crude fiber | 2.9 | 3.1 |
| Crude protein | 18.76 | 17.35 |
| Calcium | 4.25 | 3.94 |
| Phosphorus | 0.73 | 0.71 |
Layer 1 diet was used between 18 and 24 wk of age, and layer 2 diet was used between 25 and 38 wk of age.
Ingredients in 1 kg of premix: Vitamin A 8.000 IU; Vitamin D3 2.000 IU; Vitamin B2 4 mg; Vitamin B12 10 mg; Vitamin E 15 mg; Vitamin K3 2 mg; Vitamin B1 3 mg; Niacin 30 mg; Cal-D-pantothenic acid 10 mg; Vitamin B6 5 mg; Folic acid 1 mg; D-biotin 0.05 mg; Vitamin C 50 mg; Choline Chloride 300 mg; Mn 60 mg; Zn 50 mg; Fe 60 mg; Cu 5 mg; Co 0.5 mg; Iodine 2 mg; Se 0.15 mg.
Table 2.
The chemical composition of lignocellulose.
| Determined chemical analysis | DM basis (%) |
|---|---|
| Ash | 12.87 |
| Dry matter | 97.8 |
| Crude fat | 0.07 |
| Crude protein | 4.2 |
| Soluble protein | 0.5 |
| Acid detergent fibers | 92.8 |
| Neural detergent fibers | 93.8 |
| Lignin | 92.6 |
Determined chemical analysis were made in Cumberland Valley Analytical Services, Hagerstown, USA.
Measurements and Data Collection
The experiment started at 18 wk of age and ended at 38 wk of age. The hens were individually weighed weekly from 18 wk of age to 38 wk of age. Feed intake and EW were recorded weekly. All eggs were collected daily from each group, and the hens were monitored daily until the end of the experiment. Egg production values and mortality were recorded daily. İn the study, EP was calculated by dividing the number of daily eggs by the number of hens on the same day. The efficiency of feed utilization (EF) was calculated as a ratio between weekly FI and multiplication weekly EP and EW. Data for BW, FI, EP, EW, EF were calculated and given as the mean of each 3-wk period between 18 and 38 wk of age.
Egg Quality Parameters
A total of 25 eggs from each treatment group were randomly sampled and weighed at 38 wk of age to measure egg quality and content. The measurements were performed 24 h after eggs were laid. The eggshell breaking strength (kg/cm2) was measured by an eggshell force reader machine (Egg Force Reader, Orka Food Technology, Ramat HaSharon, Israel). After that, the eggs were weighed and broken to separate yolk and albumen. The chalazae were carefully removed from the yolk, and then, the yolk weight was measured with a ±0.01 g precision. The eggshells were carefully washed and dried for 24 h in a drying oven at 105°C (Nüve FN-500, Ankara, Turkey) and then weighed with a precision of 0.01 g. Albumen weight was calculated by subtracting yolk and shell weights from total EW. The ratio of eggshell, albumen, and yolk were calculated as a percentage of EW. Eggshell thickness was measured using a caliper with a ±0.01 mm precision at 3 different points (air cell, sharp end, and equator region) of the eggshell. The eggshell thickness was given as an average of these 3 regions measurement values of the shell.
To calculate yolk index, albumen index, and Haugh unit, egg yolk diameter (YD), albumen length (AL), and albumen width (AW) were measured with digital caliper with a ±0.01 mm precision (Mitutoyo, 300 mm, Neuss, Germany). The albumen height (AH) and yolk height (YH) were measured using a tripod micrometer. Egg yolk index, albumen index, as well as Haugh unit values were calculated with the formulas given by Funk, 1948, Heiman and Carver, 1936 and Haugh (1937) as listed below:
Aerobic Bacterial Load of Eggshell
To determine the aerobic bacterial load of eggshells, 15 eggs from each treatment group were randomly collected. The eggshell surface was then aseptically swabbed with a sterile cotton swab and diluted with normal saline, according to the swab method explained by Loongyai et al. (2011). The swab samples were sent to the microbiological laboratory for further analysis. Each sample was serially diluted, and then, an amount of 1 mL of dilution was poured on the surface of plate count agar for enumeration of all aerobic bacteria on the eggshell. The plates were incubated at 37°C for 48 h. The aerobic bacteria populations of eggshells were expressed as a colony forming unit (CFU) as log10 cfu/egg.
Serum Biochemical Parameters
At 38 wk of age, 4 mL blood samples were collected in nonheparinized tubes from the jugular veins of 20 hens per treatment group. Serum was collected by the method of Calneck et al. (1992) and stored at −20°C for future analysis. The serum samples were analyzed for activity of aspartate amino transferase (AST) and alanine amino transferase (ALT) using an automatic analyzer (Roche Cobas 6000 C501, Roche Diagnostics, Regensburg, Germany).
Plasma levels of immunoglobulins (IgY, IgA, and IgM) were measured by a commercial kit (Roche Cobas 6000 E601, Roche Diagnostics, North America) according to the analysis techniques described by Carew et al. (1997).
Histomorphological Traits of Jejunum
At 38 wk of age, those hens sampled for blood analysis were euthanized by cervical dislocation to collect the jejunum samples for jejunal villus micrometry analysis (n = 20 hens from each treatment group). Small intestine samples were sectioned from the midpoint of jejunum segments (Mahmoud and Edens, 2012). Samples were trimmed to 2 cm and washed with 0.9% NaCl to remove the intestinal contents. Then, the samples were fixed in a 10% buffered formaldehyde solution and dehydrated in a graded alcohol series which were from 30, 50, 70, 80, 95 to 100% during a 24-h period. Jejunal slices were embedded in paraffin, sectioned to 5 μm thickness by a microtome (Leica, RM2155, Germany), and then put on a glass slide and stained with hematoxylin and eosin for microscopic evaluation (Gridley, 1960, Sakamoto et al., 2000).
For morphological evaluations, jejunal morphometric variables, including villus height (VH), villus width, villus apparent surface area (VASA), crypt depth (CD), and thickness of Tunica muscularis, were measured by a light microscope (Leica, DM-500, Switzerland) with a computerized image system (Leica Application Suite, LAS Version 3.7.0, Leica Microsystems, Switzerland) (Sakamoto et al., 2000). Jejunal morphometric variables were measured in 20 villi for each bird, and the ratio between villus height and crypt depth (VH/CD) was then calculated for each treatment group.
Statistical Analysis
In the study, data of laying performance, egg quality parameters, aerobic bacterial load of eggshell, serum biochemical parameters, and jejunal histomorphological traits of laying hens were analyzed using the GLM procedures of SAS statistical software (Version 9.1., SAS Institute Inc, 2003). Each replicate was considered an experimental unit for performance parameters (n = 4 cages/treatment), egg quality parameters (n = 25 eggs/treatment), aerobic bacterial load of eggshell (n = 15 eggs/treatment), serum biochemical parameters, and jejunal histomorphological traits (n = 20 hens/treatment). Significant differences among the means were compared using the Tukey test and were considered statistically different at a level of P < 0.05. Effects of the LS levels (0.5, 1 and 2 kg per ton of feed) were determined by the contrast analysis of the GLM procedure. Orthogonal polynomial contrasts were also applied to determine the linear and quadratic responses to different levels of LS.
Results
The effects of dietary LS on the performance parameters of laying hens are presented in Table 3. Mean BW at 16 wk of age and between 18 and 20 wk of age were similar among the experimental groups (P > 0.05). Between 21 and 38 wk of age (except 24–26 wk of age), hens supplemented with 1 kg of lignocellulose were quadratically heavier compared with the other treatment groups (P < 0.001). The highest mean BW was observed in hens supplemented with 1 kg of lignocellulose with a value of 1632.1 g between 18 and 38 wk of age. Conversely, mean daily feed intake (DFI) showed a quadratic change among the treatment groups (P < 0.001). At 18 to 20, 21 to 23, 27 to 29, 33 to 35, and 36 to 38 wk of age, DFI was higher in hens supplemented with 1 kg of lignocellulose. Between 18 and 38 wk of age, mean DFI was higher, with values of 98.0 ad 99.2 g in the 0.5 and 1 kg LS groups, respectively (P < 0.001).
Table 3.
Mean body weight of laying hens fed different lignocellulose levels from 18 to 38 wk of age.
| Age (wk) | Supplementation amount (kg/ton of feed) |
Pooled SEM |
P-values |
|||||
|---|---|---|---|---|---|---|---|---|
| 0 | 0.5 | 1 | 2 | Fixed | Linear | Quadratic | ||
| Mean body weight (g) | ||||||||
| 16 | 1160.3 | 1155.8 | 1162.8 | 1157.3 | 23.32 | 0.974 | 0.933 | 0.905 |
| 18–20 | 1329.5 | 1342.5 | 1356.3 | 1327.3 | 16.61 | 0.099 | 0.784 | 0.056 |
| 21–23 | 1483.0c | 1534.5b | 1598.8a | 1471.3c | 22.60 | <0.001 | 0.806 | <0.001 |
| 24–26 | 1611.3b | 1660.0a | 1682.3a | 1604.5b | 22.8 | 0.001 | 0.578 | <0.001 |
| 27–29 | 1627.5b,c | 1654.5a,b | 1676.3a | 1617.5c | 14.17 | <0.001 | 0.462 | <0.001 |
| 30–32 | 1635.0b,c | 1668.8a,b | 1687.3a | 1617.8c | 16.93 | <0.001 | 0.296 | <0.001 |
| 33–35 | 1643.8b,c | 1681.3a,b | 1704.5a | 1630.8c | 18.84 | <0.001 | 0.430 | 0.001 |
| 36–38 | 1655.0c | 1693.3b | 1719.3a | 1635.8c | 9.45 | <0.001 | 0.290 | <0.001 |
| Mean | 1569.3c | 1605.0b | 1632.1a | 1557.8c | 9.78 | <0.001 | 0.472 | <0.001 |
| Mean daily feed intake (g) | ||||||||
| 18–20 | 79.0b,c | 80.8a,b | 81.8a | 78.0c | 1.11 | 0.002 | 0.302 | <0.001 |
| 21–23 | 90.0a,b | 90.5a,b | 92.0a | 88.3b | 1.56 | 0.031 | 0.193 | 0.016 |
| 24–26 | 95.3a,b | 97.0a | 97.3a | 94.3b | 1.58 | 0.041 | 0.276 | 0.007 |
| 27–29 | 97.5a,b | 99.5a,b | 101.0a | 97.0b | 1.81 | 0.028 | 0.623 | 0.003 |
| 30–32 | 100.8a,b | 103.3a | 103.5a | 100.0b | 1.46 | 0.007 | 0.347 | 0.001 |
| 33–35 | 103.8b | 105.3a,b | 107.8a | 103.0b | 1.48 | 0.002 | 0.601 | 0.001 |
| 36–38 | 107.5b | 109.5a,b | 111.0a | 107.0b | 1.60 | 0.016 | 0.609 | 0.002 |
| Mean | 96.3b | 98.0a | 99.2a | 95.4b | 0.63 | <0.001 | 0.300 | <0.001 |
| Egg production (%) | ||||||||
| 18–20 | 21.8c | 22.5b | 23.5a | 21.5c | 0.21 | 0.001 | 0.614 | <0.001 |
| 21–23 | 70.3c | 72.6b | 73.5a | 70.3c | 0.35 | <0.001 | 0.701 | <0.001 |
| 24–26 | 90.0b | 91.8a | 92.4a | 89.5b | 0.32 | <0.001 | 0.362 | 0.001 |
| 27–29 | 92.1c | 94.4b | 95.6a | 91.8c | 0.32 | <0.001 | 0.600 | 0.001 |
| 30–32 | 92.4c | 94.5b | 95.8a | 92.0c | 0.33 | <0.001 | 0.536 | <0.001 |
| 33–35 | 93.1c | 94.7b | 95.9a | 92.6d | 0.27 | <0.001 | 0.415 | <0.001 |
| 36–38 | 93.3c | 95.2b | 96.4a | 92.7d | 0.24 | <0.001 | 0.390 | <0.001 |
| Mean | 78.0c | 80.8b | 81.8a | 78.6d | 0.18 | <0.001 | 0.494 | <0.001 |
| Mean egg weight (g) | ||||||||
| 18–20 | 45.0b,c | 45.4a,b | 45.8a | 44.6c | 0.21 | <0.001 | 0.213 | <0.001 |
| 21–23 | 50.3c | 51.0b | 51.7a | 49.9c | 0.35 | <0.001 | 0.367 | 0.001 |
| 24–26 | 54.3c | 55.6b | 56.6a | 53.9c | 0.23 | <0.001 | 0.501 | <0.001 |
| 27–29 | 57.0c | 58.3b | 58.9a | 56.1d | 0.26 | <0.001 | 0.156 | <0.001 |
| 30–32 | 58.5c | 59.9b | 61.8a | 57.4d | 0.37 | <0.001 | 0.318 | <0.001 |
| 33–35 | 60.3c | 61.8b | 62.7a | 58.9d | 0.44 | 0.001 | 0.108 | <0.001 |
| 36–38 | 61.5c | 62.9b | 63.8a | 59.9d | 0.37 | <0.001 | 0.072 | <0.001 |
| Mean | 55.3c | 56.4b | 57.3a | 54.4d | 0.27 | <0.001 | 0.193 | <0.001 |
| EF | ||||||||
| 18–20 | 8.08a,b | 7.91b | 7.60c | 8.12a | 0.12 | <0.001 | 0.596 | <0.001 |
| 21–23 | 2.55a | 2.45b,c | 2.42c | 2.52a,b | 0.04 | 0.005 | 0.811 | <0.001 |
| 24–26 | 1.95a | 1.90a,b | 1.86b | 1.95a | 0.03 | 0.003 | 0.795 | <0.001 |
| 27–29 | 1.86a,b | 1.81b | 1.79b | 1.88a | 0.03 | 0.009 | 0.310 | 0.001 |
| 30–32 | 1.87a,b | 1.82b | 1.75c | 1.89a | 0.03 | <0.001 | 0.504 | <0.001 |
| 33–35 | 1.85a | 1.80b | 1.80b | 1.89a | 0.02 | <0.001 | 0.080 | <0.001 |
| 36–38 | 1.87a,b | 1.83b | 1.80b | 1.93a | 0.04 | 0.002 | 0.107 | <0.001 |
| Mean | 2.86a | 2.79b | 2.72c | 2.88a | 0.02 | <0.001 | 0.499 | <0.001 |
a,b,c Means bearing different superscripts within the same line are significantly different.
n: 4 replicate per treatment group (40 laying hen/pen).
With the exception of 24 to 26 wk of age, EP was quadratically the highest in the 1 kg LS group. Higher EP was observed in the 0.5 and 1 kg LS groups between 24 and 26 wk of age (91.8 and 92.4%, respectively, P < 0.001). At the end of the experimental period, mean EP was the highest in the 1 kg LS group, with a value of 81.8% (P < 0.001). Egg weight was the highest in the 1 kg LS group compared with the other treatment groups (P < 0.001). Between 18 and 38 wk of age, the highest value of mean EW was observed in the 1 kg LS group, with a value of 57.3 g (P < 0.001). Feed utilization efficiency was affected by lignocellulose supplementation during the experimental period (P < 0.001). Quadratic changes in EF were observed among treatment groups. Between 18 and 38 wk of age, mean EF was found to be more efficient in the 1 kg LS group (2.72, P < 0.001).
Effects of LS on egg quality and egg content are presented in Table 4. Eggshell breaking strength was higher in the eggs obtained from hens fed the diets supplemented with 0.5 and 1 kg lignocellulose (3.32 and 3.60 kg/cm2, respectively, P < 0.001). The highest value for eggshell thickness, however, was observed in the 1 kg LS group (0.476 mm, P < 0.001). A higher yolk index value was observed for both the 0.5 and 1 kg LS groups (52.1 and 52.9%, respectively), whereas the lowest value of albumen index was found in the 2 kg LS group (P < 0.001).
Table 4.
Egg quality parameters of laying hens fed different lignocellulose levels at 38 wk of age.
| Items | Supplementation amount (kg/ton of feed) |
Pooled SEM |
P-values |
|||||
|---|---|---|---|---|---|---|---|---|
| 0 | 0.5 | 1 | 2 | Fixed | Linear | Quadratic | ||
| Egg quality parameters | ||||||||
| Eggshell breaking strength (kg/cm2) | 2.14c | 3.32a | 3.60a | 2.71b | 0.40 | <0.001 | 0.144 | <0.001 |
| Eggshell thickness (mm) | 0.417c | 0.431b | 0.476a | 0.428b | 0.01 | <0.001 | 0.126 | <0.001 |
| Yolk index (%) | 51.4a,b | 52.1a | 52.9a | 50.5b | 1.6 | 0.001 | 0.107 | <0.001 |
| Albumen index (%) | 11.0a | 11.1a | 11.4a | 10.0b | 0.9 | 0.001 | 0.004 | 0.004 |
| Haugh unit | 88.1 | 89.6 | 90.3 | 87.6 | 3.5 | 0.113 | 0.533 | 0.018 |
| Egg content | ||||||||
| Yolk ratio (%) | 23.8b | 24.4b | 25.7a | 24.3b | 1.1 | <0.001 | 0.261 | <0.001 |
| Albumen ratio (%) | 66.2a | 66.6a | 64.3b | 66.1a | 1.5 | 0.001 | 0.440 | 0.017 |
| Eggshell ratio (%) | 9.5 | 9.0 | 9.8 | 9.6 | 1.6 | 0.408 | 0.903 | 0.513 |
a,b,cMeans bearing different superscripts within the same line are significantly different.
n: 25 eggs per treatment group.
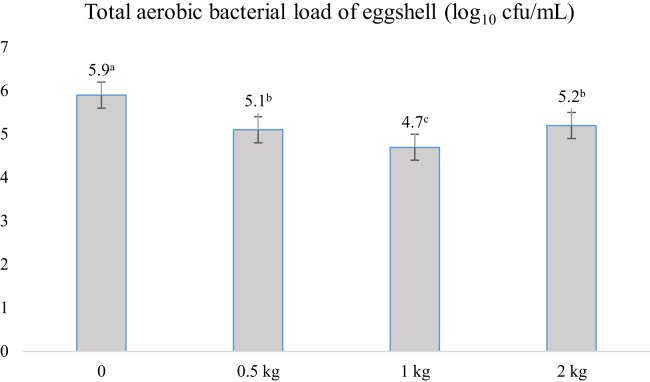
Total aerobic bacterial load of eggshells was affected by supplementation of lignocellulose at 38 wk of age (Figure 1). As the amount of lignocellulose supplementation increased, there was a significant decline of aerobic bacterial population on the surface of the eggshell (P < 0.05). The total load was the lowest for the 1 kg LS group, whereas it was the highest in the CONT group (4.7 vs. 5.9 log10 cfu/egg, respectively, P < 0.05).
Figure 1.
Total aerobic bacterial load of eggshell of laying hens fed with different lignocellulose supplementation at 38 wk of age. Bars represent mean ± SE. (P < 0.05, n: 15 samples per treatment group). a,b,cMeans bearing different superscripts are significantly different.
An increased activity of AST and ALT was observed in the CONT (169.7 and 19.7 IU/L, respectively) and the 2 kg LS group (175.5 and 20.3 IU/L respectively, P < 0.01), whereas the lowest levels of ALP and ALT were observed in the CONT group (139.7 and 11.7 IU/L, respectively, Table 5). The mean levels of serum immunoglobulins (IgY, IgA an IgM) were quadratically lower in the CONT diet (129.2, 31.0 and 8.2 mg/dL, respectively) and the 2 kg LS group (127.2, 30.6 and 8.5 mg/dL, respectively, P < 0.01).
Table 5.
Hepatic enzyme levels and serum immunoglobulins of laying hens fed different lignocellulose levels at 38 wk of age.
| Items | Supplementation amount (kg/ton of feed) |
Pooled SEM |
P-values |
|||||
|---|---|---|---|---|---|---|---|---|
| 0 | 0.5 | 1 | 2 | Fixed | Linear | Quadratic | ||
| Hepatic enzyme levels (IU/L) | ||||||||
| AST | 169.7a | 148.2b | 158.2b | 175.5a | 12.8 | <0.001 | 0.519 | <0.001 |
| ALT | 19.7a | 14.4b | 15.8b | 20.3a | 1.4 | <0.001 | 0.831 | <0.001 |
| Serum immunoglobulin levels (mg/dL) | ||||||||
| IgY | 129.2b | 132.2a | 134.4a | 127.2b | 1.9 | <0.001 | 0.068 | <0.001 |
| IgA | 31.0b | 34.2a | 35.8a | 30.6b | 1.7 | <0.001 | 0.393 | <0.001 |
| IgM | 8.2b | 9.6ab | 9.8a | 8.5bb | 1.0 | 0.001 | 0.985 | <0.001 |
a,b,cMeans bearing different superscripts within the same column are significantly different.
n: 20 samples per treatment group.
Abbreviations: AST, aspartate amino transferase; ALT, alanine amino transferase; IgY, immunoglobulin-Y; IgA, immunoglobulin-A; IgM, immunoglobulin-M.
The effects of LS on jejunal morphological traits at 38 wk of age are presented in Table 6. There was a quadratic increment in VH (P < 0.001) in hens fed lignocellulose supplemented diets. The highest mean value of VH was observed in hens fed the 0.5 and 1 kg LS groups (1290.9 and 1355.9 μm, respectively, P < 0.01). Villus width was higher in the 0.5 kg and 1 kg LS groups (124.5 and 132.7 μm, respectively, P < 0.01). The mean value of VASA was lower in the CONT and 2 kg LS groups (200105 and 216278 μm2, respectively, P < 0.01). Contrast analysis showed a quadratic reduction in CD in hens fed the 0.5 kg and 1 kg LS groups (107.3 and 108.8 μm, respectively, P < 0.01). The VH/CD showed a quadratic decline in the CONT and 2 kg LS groups (9.4 and 9.5, respectively, P < 0.001). Thickness of T. muscularis was found to be the lowest in the 1 kg LS group (164.4 μm, P < 0.001).
Table 6.
Jejunal morphological traits of laying hens fed with different lignocellulose levels at 38 wk of age.
| Items | Supplementation amount (kg/ton of feed) |
Pooled SEM |
P-values |
|||||
|---|---|---|---|---|---|---|---|---|
| 0 | 0.5 | 1 | 2 | Fixed | Linear | Quadratic | ||
| Villus height (μm) | 1068.0b | 1290.9a | 1355.9a | 1093.4b | 67.3 | <0.01 | 0.706 | <0.01 |
| Villus width(μm) | 97.8b | 124.5a | 132.7a | 103.3b | 12.1 | <0.01 | 0.939 | <0.01 |
| VASA (μm2) | 200105b | 267639a | 270669a | 216278b | 21,979 | <0.01 | 0.885 | 0.001 |
| Crypt depth (μm) | 114.8a | 107.3b | 108.8b | 115.9a | 11.7 | 0.018 | 0.263 | 0.090 |
| VH/CD | 9.4b | 12.0a | 12.5a | 9.5b | 1.1 | <0.01 | 0.635 | <0.01 |
| T-TM (μm) | 206.4b | 199.6b | 164.4c | 236.1a | 16.1 | 0.001 | 0.003 | <0.01 |
a,b,cMeans bearing different superscripts within the same column are significantly different.
n: 20 samples per treatment group.
Abbreviations: VASA, villus apparent surface area; VH/CD, villus height/crypt depth; T-LM, thickness of Tunica muscularis. Magnification 100 x, scale bar 100 μm.
Discussion
Results from this study clearly demonstrate that LS affected laying hen performance between 18 and 38 wk of age, both positively and negatively depending on the amount of LS in the laying hen diet. Lignocellulose supplementation in the amount of 1 kg per ton of feed resulted in better BW and DIF, EP, EW, and a more effective EF when compared with the other treatment groups. In contrast, the higher level of LS in the amount of 2 kg per ton of feed caused a negative effect for those parameters between 18 and 38 wk of age. A previous study reported by Incharoen and Maneechote (2013) support these findings. They concluded that the supplementation of whole rice hull as an insoluble fiber source at levels up to 6% enhanced the growth and development of layer pullets until 8 wk of age. Observed improvement of performance parameters could be attributed to properties of the insoluble fiber source that improve total tract retention of nutrients (Jimenez-Moreno et al., 2009) and stimulate gizzard activity (Hetland and Svihus, 2001), resulting in an increment in BW and more efficient use of feed.
Lignocellulose supplementation applied at the higher level of 2 kg per ton of feed resulted in a decrease in BW, DFI, and an increase in EF when compared with the 0.5 and 1 kg LS groups. The high level of lignocellulose in the layer hen diet may have reduced the digestibility of all nutrients (Annison, 1993, Jørgensen et al., 1996, Smits et al., 1997). This could in turn cause a reduction in EP and EW in the 2 kg LS group compared with the 0.5 and 1 kg LS groups. Similarly, the highest EW observed in the 1 kg LS group could be related to the highest BW and DFI of the laying hens in this group. The current study's results found that 1 kg LS in laying diet could be an optimum level for EP and EW, whereas the higher level of LS of 2 kg per ton of feed caused a harmful effect when compared with the CONT and the lower level of LS (0.5 kg per ton of feed).
Laying hens fed either the 0.5 or 1 kg LS per ton of feed produced eggs with higher breaking strength and thicker eggshells. This observation is supported by Chen and Chen (2004), who reported that inulin supplementation as a fiber source improved eggshell quality in laying hens because of an increase in calcium absorption depending on the fiber levels in the diet. They proposed that the increase in intestinal absorption of minerals in birds supplemented with dietary fiber could be related to their high binding and sequestering capabilities (Roberfroid et al., 2002). The observed decline in eggshell breaking strength and eggshell thickness in the 2 kg LS group is in agreement with Mohebbifar et al. (2011), which may be caused from the high level of lignocellulose in the diet. Contrary to the current findings, Shang et al. (2010) reported no significant effects of inulin supplementation on eggshell breaking strength and eggshell thickness in laying hens.
In the study, it is not clear why the yolk index and albumen index mean values were higher in the 0.5 and 1 kg LS groups than the CONT and 2 kg LS groups. This may be because of the functional properties and content of lignocellulose, as well as the amount that was supplemented in the diet. Contrary to our findings, Röhe et al. (2019a) found no significant differences for egg quality parameters when laying hens were fed a diet with 10% lignocellulose between 23 and 52 wk of age. There is some published research investigating the effects of LS on egg quality parameters and total aerobic bacterial load of eggshell surface in laying hens. Other articles focused on the effect of various other fiber sources on egg quality parameters, including inulin and cellulose in broiler breeders (Mohiti-Asli et al., 2012) and whole rice hull in laying hens (Incharoen and Maneechote, 2013).
The bacterial load of the eggshell surface is affected by various factors, including the diet content of laying hens (Smith et al., 2000) and the environmental conditions of the egg during and after being laid (De Reu et al., 2005, Wall et al., 2008). Diet could increase the moisture of the hen's excreta, and this could result in an increment of the microbial population of ostensibly clean eggs (Smith et al., 2000). Furthermore, because the passageway for evacuating eggs and excreta is the same, the bacterial contamination may have occurred during the laying of the egg in the cloaca. Results from this present study showed that increasing LS caused a decline in the aerobic bacterial load of the eggshell. This may be related to the population of intestinal bacteria and the subsequent bacterial load of the excreta. A study performed by Sozcu (2019) reported a significant decline for populations of Staphylococcus spp., Escherichia coli, and Enterobacter spp. in the cecum of broilers fed a diet with 2 kg lignocellulose supplementation. In this current study, the observed decline of the bacterial load of the eggshell could possibly be attributed to the antibacterial effect of lignin containing many phenolic monomers and also because of the abrasive effect of lignocellulose that restricts the adhering of pathogenic bacteria on the surface (Jiménez -Moreno et al., 2011, Bogusławska-Tryk et al., 2015).
Egg content including yolk ratio and albumen ratio showed variability among the treatment groups in this study. Similar to current findings, Incharoen and Maneechote (2013) observed a higher albumen ratio in the 6% whole rice hull supplementation, which was attributed to the functional properties of whole rice hulls as an insoluble fiber source. Conversely, Mohiti-Asli et al. (2012) observed a decline in yolk weight when broiler breeders were fed a diet supplemented with 3% inulin. These contradictory findings could be related to the type and composition of the fiber source. In the current study, lignocellulose had a content of approximately 92.8% of acid detergent fiber, 93.8% neural detergent fiber, and 92.6% of lignin (Table 2).
Current results showed that lignocellulose supplementation affected hepatic enzyme levels (AST and ALT) in laying hens at 38 wk of age. Because hepatic enzymes are released when degenerative changes in liver occur, these enzymes are accepted as indicators for the health status and functionality of the liver (Cheesborough, 1991, Johnston, 1999). Lower levels of AST and ALT in both the 0.5 and 1 kg LS groups could be proof of a hepatoprotective effect of lignocellulose in laying hens. A similar effect for lignocellulose in broiler nutrition was reported by Sozcu (2019).
Lignocellulose supplementation caused a fluctuation in immunoglobulin levels in laying hens depending on the amount of LS in the diet. Results showed that LS in the amount of 0.5 and 1 kg per ton of feed provided an immune-stimulating effect with an increase in levels of IgY, IgA, and IgM, when compared with the CONT and 2 kg LS group. It is widely known that IgY and IgA have a preventative effect against pathogenic bacteria (Burke et al., 1988, Muir et al., 2000, Ohashi et al., 2014). This is consistent with the lower bacterial load of the eggshells in this group.
Current results showed that LS in the amount of 0.5 and 1 kg per ton of feed stimulated the mucosal development of jejunum by increasing VH, villus width, VASA, and VH/CD ratio, while decreasing CD and thinning the T. muscularis. Similar to the current findings, Röhe et al. (2020) reported that when laying hens were fed a diluted diet supplemented with 10% lignocellulose, this resulted in a larger colorectal villus area. Villus size, including height and width, is especially significant for the absorptive capacity of the intestine (Iji et al., 2001). Therefore, a higher ratio between VH and CD in both the 0.5 and 1 kg LS groups could be accepted as an indicator for an increase in the digestion and absorption of nutrients. Likewise, a higher BW and more efficient feed utilization were observed in these groups when compared with either the CONT or 2 kg LS groups. These results are in accordance with the findings of Makivic et al., 2019, Sozcu, 2019 and Röhe et al. (2020), who reported that diets supplemented with insoluble fiber sources resulted in an improvement in digestive tract development and, subsequently, growth performance in broilers and laying hens. Thickening of the T. muscularis, the intestinal wall that is a passageway for nutrients, negatively affects the digestive process and absorption of nutrients (Teirlynck et al., 2009). Lower mean values for the thickness of this wall in both the 0.5 and 1 kg LS groups could be an accepted indicator for more efficient nutrient absorption and the subsequent improvement of performance parameters.
In conclusion, this study found that the LS as an insoluble fiber source caused significant positive changes in laying hens. Furthermore, based on the results of this study, LS in the amount of 1 kg per ton of feed is the optimum level for egg production as well as for the hepatoprotective effect and immune-stimulating effect. This inclusion rate was also optimal for decreasing eggshell bacterial load and increasing villus enlargement in the jejunum of laying hens. Therefore, lignocellulose can effectively be used in the feed of laying hens to improve animal performance and health.
Acknowledgments
The authors would like to thank Global Nutritech Biotechnology LLC (Richmond, VA, USA) for their financial support.
Conflicts of Interest Statement: The authors did not provide any conflict of interest statement.
References
- Annison G. The role of wheat non-starch polysaccharides in broiler nutrition. Aust. J. Agric. Res. 1993;44:405–422. [Google Scholar]
- AOAC – Association of Official Analytical Chemists . 17th ed. Association of Official Analytical Chemists; Gaithersburg, Maryland, USA: 2000. Official Methods of Analysis. [Google Scholar]
- Bell D.D., Weaver W.D., Jr. 5th ed. Kluwer Academic Publishers; Norwell, MA: 2002. Commercial Chicken Meat and Egg Production. [Google Scholar]
- Bogusławska-Tryk M., Szymeczko R., Piotrowska A., Burlikowska K., Śliżewska K. Ileal and cecal microbial population and short-chain fatty acid profile in broiler chickens fed diet supplemented with lignocellulose. Pakistan Vet. J. 2015;35:212–216. [Google Scholar]
- Burke D.S., Nisalak A., Johnson D.E., Scott R.M. A prospective study of dengue infections in Bangkok. Am. J. Trop. Med. Hyg. 1988;38:172–180. doi: 10.4269/ajtmh.1988.38.172. [DOI] [PubMed] [Google Scholar]
- Calneck B.W., Barnes H.J., Beard C.W., Reid M.W., Yoder H.W., Jr. 9th ed. Wolfe Publishing Ltd.; Gloucestershire, England: 1992. Diseases of Poultry. [Google Scholar]
- Cao B., Zhang X., Guo Y., Karasawa Y., Kumao T. Effects of dietary cellulose levels on growth, nitrogen utilization, retention time of diets in digestive tract and caecal microflora of chickens. Asian Australas. J. Anim. Sci. 2003;16:863–866. [Google Scholar]
- Carew L.B., Evarts K.G., Alster F.A. Growth and plasma thyroid hormone concentrations of chicks fed diets deficient in essential amino acids. Poult. Sci. 1997;76:1398–1404. doi: 10.1093/ps/76.10.1398. [DOI] [PubMed] [Google Scholar]
- Cheesborough M. 2nd ed. Tropical health technology and Butterworth scientific limited Cambridge; Edinburgh, England: 1991. Medical Laboratory Manual for Tropical Countries. [Google Scholar]
- Chen Y.C., Chen T.C. Mineral utilization in layers as influenced by dietary oligofructose and inulin. Int. J. Poult. Sci. 2004;3:442–445. [Google Scholar]
- De Reu K., Grijspeerdt K., Heyndrickx M., Zoons J., De Baere K., Uyttendaele M., Debevere J., Herman L. Bacterial eggshell contamination in conventional cages, furnished cages and aviary housing systems for laying hens. Br. Poult. Sci. 2005;46:149–155. doi: 10.1080/00071660500065359. [DOI] [PubMed] [Google Scholar]
- Funk E.M. The relation of yolk index determined in natural position to the yolk index as determined after separating the yolk from the albumen. Poult. Sci. 1948;27:367. [Google Scholar]
- Godfray H.C.J., Beddington J.R., Crute I.R., Haddad L., Lawrence D., Muir J.F., Pretty J., Robinson S., Thomas S.M., Toulmin C. Food security: the challenge of feeding 9 billion people. Science. 2010;327:812–818. doi: 10.1126/science.1185383. [DOI] [PubMed] [Google Scholar]
- Gridley M.F. MacGraw-Hill Book Company; New York, USA: 1960. Manual of Histologic and Special Staining Technique. [Google Scholar]
- Guzmán P., Saldaǹa B., Kimiaeitalab M.V., García J., Mateos G.G. Inclusion of fiber in diets for brown-egg laying pullets: effects on growth performance and digestive tract traits from hatching to 17 weeks of age. Poult. Sci. 2015;94:2722–2733. doi: 10.3382/ps/pev288. [DOI] [PubMed] [Google Scholar]
- Guzmán P., Saldaǹa B., Mandalawi H.A., Pèrez-Bonilla A., Lázaro R., Mateos G.G. Productive performance of brown-egg laying pullets from hatching to 5 weeks of age as affected by fiber inclusion, feed form, and energy concentration of the diet. Poult. Sci. 2015;94:249–261. doi: 10.3382/ps/peu072. [DOI] [PubMed] [Google Scholar]
- Haugh R.R. The Haugh unit for measuring egg quality. U.S. Egg Poult. Mag. 1937;43:552–555. [Google Scholar]
- Harmsen P.F.H., Huijgen W.J.J., Bermúdez López L.M., Bakker R.R.C. Wageningen University & Research Centre; Wageningen: 2010. Literature Review of Physical and Chemical Pretreatment Processes for Lignocellulosic Biomass.ftp://ftp.ecn.nl/pub/www/library/report/2010/e10013.pdf [Google Scholar]
- He L.W., Meng Q.X., Li D.Y., Zhang Y.W., Ren L.P. Influence of feeding alternative fiber sources on the gastrointestinal fermentation, digestive enzyme activities and mucosa morphology of growing Greylag geese. Poult. Sci. 2015;94:2464–2471. doi: 10.3382/ps/pev237. [DOI] [PubMed] [Google Scholar]
- Heiman V., Carver J.S. Albumen index as a physical measurement of observed egg quality. Poult. Sci. 1936;15:141–148. [Google Scholar]
- Hetland H., Svihus B. Effect of oat hulls on performance, gut capacity and feed passage time in broiler chickens. Br. Poult. Sci. 2001;42:354–361. doi: 10.1080/00071660120055331. [DOI] [PubMed] [Google Scholar]
- Hussein S.M., Yokhana S., Frankel T.L. Supplementing the feeds of layer pullets, at different ages with two different fiber sources improves immune function. Poult. Sci. 2017;96:2718–2727. doi: 10.3382/ps/pex051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iji P.A., Saki A.A., Tivey D.R. Intestinal development and body growth of broiler chicks on diets supplemented with non-starch polysaccharides. Anim. Feed Sci. Technol. 2001;89:175–188. [Google Scholar]
- Incharoen T., Maneechote P. The effects of dietary whole rice hull as insoluble fiber on the flock uniformity of pullets and on the egg performance and intestinal mucosa of laying hens. Am. J. Agr. Biol. Sci. 2013;8:323–329. [Google Scholar]
- Jiménez–Moreno E., de Coca-Sinova A., González-Alvarado J.M., Mateos G.G. Inclusion of insoluble fiber sources in mash or pellet diets for young broilers. 1. Effects on growth performance and water intake. Poult. Sci. 2016;95:41–52. doi: 10.3382/ps/pev309. [DOI] [PubMed] [Google Scholar]
- Jiménez -Moreno E., Romero C., Berrocoso J.D., Frikha M., Mateos G.G. Effects of the inclusion of oat hulls or sugar beet pulp in the diet on gizzard characteristics, apparent ileal digestibility of nutrients, and microbial count in the ceca in 36- day-old broilers reared on floor. Poult. Sci. 2011;90(Suppl. 1):153. (Abstr.) [Google Scholar]
- Jiménez-Moreno E., González-Alvarado J.M., de Coca-Sinova A., Lázaro R., Mateos G.G. Effects of source of fibre on the development and pH of the gastrointestinal tract of broilers. Anim. Feed Sci. Technol. 2009;154:93–101. [Google Scholar]
- Johnston D.E. Special considerations in interpreting liver function tests. Am. Fam. Physician. 1999;59:2223–2230. [PubMed] [Google Scholar]
- Jørgensen H., Kristensen J.B., Felby C. Enzymatic conversion of lignocellulose into fermentable sugars: challenges and opportunities. Biofuels Bioprod. Bioref. 2007;1:119–134. [Google Scholar]
- Jørgensen H., Zhao X.Q., Knudsen K.E.B., Eggum B.O. The influence of dietary fibre source and level on the development of the gastrointestinal tract, digestibility and energy metabolism in broiler chickens. Br. J. Nutr. 1996;75:379–395. doi: 10.1079/bjn19960141. [DOI] [PubMed] [Google Scholar]
- Kheravii S.K., Swick R.A., Choct M., Wu S.B. Article navigation coarse particle inclusion and lignocellulose-rich fiber addition in feed benefit performance and health of broiler chickens. Poult. Sci. 2017;96:3272–3281. doi: 10.3382/ps/pex123. [DOI] [PubMed] [Google Scholar]
- Krás R.V., Kessler A.M., Ribeiro A.M.L., Henn J.D., dos Santos I.I., Halfen D.P., Bockor L. Effect of dietary fiber and genetic strain on the performance and energy balance of broiler chickens. Braz. J. Poult. Sci. 2013;15:15–20. [Google Scholar]
- Larbier M., Leclercq B. Nottingham University Press; Nottingham: 1994. Nutrition and Feeding of Poultry: Intake of Food and Water; pp. 7–14. [Google Scholar]
- Loongyai W., Wiriya B., Sangsawang N. Detection of Salmonella and Escherichia coli in egg shell and egg content from different housing systems for laying hens. Int. J. Poult. Sci. 2011;10:93–97. [Google Scholar]
- Mahmoud K.Z., Edens F.W. Breeder age affects small intestinal development of broiler chicks with immediate or delayed access to feed. Br. Poult. Sci. 2012;53:32–41. doi: 10.1080/00071668.2011.652596. [DOI] [PubMed] [Google Scholar]
- Makivic L., Glisic M., Boskovic M., Djordjevic J., Markovic R., Baltic M., Sefer D. Performances, ileal and cecal microbial populations and histological characteristics in broilers fed diets supplemented with lignocellulose. Kafkas Universitesi Veteriner Fakültesi Dergisi. 2019;25:83–91. [Google Scholar]
- Mateos G.G., Jiménez-Moreno E., Serrano M.P., Lázaro R.P. Poultry response to high levels of dietary fiber sources varying in physical and chemical characteristics. J. Appl. Poult. Res. 2012;21:156–174. [Google Scholar]
- Mohebbifar A., Afsari M., Torki M. Egg quality characteristics and productive performance of laying hens fed olive pulp included diets supplemented with enzymes. Glob. Veter. 2011;6:409–416. [Google Scholar]
- Mohiti-Asli M., Shivazad M., Zaghari M., Rezaian M., Aminzadeh S., Mateos G.G. Effects of feeding regimen, fiber inclusion, and crude protein content of the diet on performance and egg quality and hatchability of eggs of broiler breeder hens. Poult. Sci. 2012;91:3097–3106. doi: 10.3382/ps.2012-02282. [DOI] [PubMed] [Google Scholar]
- Muir W.I., Bryden W.L., Husband A.J. Investigation of the site of precursors for IgA producing cells in the chicken intestine. Immun. Cell Biol. 2000;78:294–296. doi: 10.1046/j.1440-1711.2000.00915.x. [DOI] [PubMed] [Google Scholar]
- Ohashi Y., Hiraguchi M., Ushida K. The composition of intestinal bacteria affects the level of luminal IgA. Biosci. Biotechnol. Biochem. 2014;70:3031–3035. doi: 10.1271/bbb.60164. [DOI] [PubMed] [Google Scholar]
- Raninen K., Lappi J., Mykkanen H., Poutanen K. Dietary fiber type reflects physiological functionality: Comparison of grain fiber, inulin, and polydextrose. Nutr. Rev. 2011;69:9–21. doi: 10.1111/j.1753-4887.2010.00358.x. [DOI] [PubMed] [Google Scholar]
- Roberfroid M.B., Cumps J., Devogelaer J.P. Dietary chicory inulin increases whole-body bone mineral density in growing male rats. J. Nutr. 2002;132:3599–3602. doi: 10.1093/jn/132.12.3599. [DOI] [PubMed] [Google Scholar]
- Röhe I., Julia U., Dijkslag A., te Paske J., Zentek J. Impact of an energy- and nutrient-reduced diet containing 10% lignocellulose on animal performance, body composition and egg quality of dual purpose laying hens. Arch. Anim. Nutr. 2019;73:1–17. doi: 10.1080/1745039X.2018.1551950. [DOI] [PubMed] [Google Scholar]
- Röhe I., Vahjen W., Metzger F., Zentek J. Effect of a “diluted” diet containing 10% lignocellulose on the gastrointestinal tract, intestinal microbiota, and excreta characteristics of dual purpose laying hens. Poult. Sci. 2020;99:310–319. doi: 10.3382/ps/pez492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi A., Toghyani M., Gheisari A. Effect of various fiber types and choice feeding of fiber on performance, gut development, humoral immunity, and fiber preference in broiler chicks. Poult. Sci. 2015;94:2734–2743. doi: 10.3382/ps/pev292. [DOI] [PubMed] [Google Scholar]
- Sakamoto K., Hirose H., Onizuka A., Hayashi M., Futamura N., Kawamura Y., Ezaki T. Quantitative study of changes in intestinal morphology and mucus gel on total parenteral nutrition in rats. J. Surg. Res. 2000;94:99–106. doi: 10.1006/jsre.2000.5937. [DOI] [PubMed] [Google Scholar]
- Saki A.A., Hemati Matin H.R., Tabatabai M.M., Zamani P., Naseri Harsini R. Microflora population, intestinal condition and performance of broilers in response to various rates of pectin and cellulose in the diet. Arch. Geflügelk. 2010;74:183–188. [Google Scholar]
- Sakomura N.K., Reis M.D.P., Ferreira N.T., Gous R. Modeling egg production as a means of optimizing dietary nutrient contents for laying hens. Anim. Front. 2019;9:45–51. doi: 10.1093/af/vfz010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakouri M.D., Kermanshahi H., Mohsenzadeh M. Effect of different non starch polysaccharides in semi purified diets on performance and intestinal microflora of young broiler chickens. Int. J. Poult. Sci. 2006;5:557–561. [Google Scholar]
- SAS Institute Inc . SAS Institute Inc.; Cary, NC: 2003. SAS/STAT User’s Guide, version 9.1. [Google Scholar]
- Shang H.M., Hu T.M., Lu Y.J., Wu H.X. Effects of inulin on performance, egg quality, gut microflora and serum and yolk cholesterol in laying hens. Br. Poult. Sci. 2010;51:791–796. doi: 10.1080/00071668.2010.531005. [DOI] [PubMed] [Google Scholar]
- Smith A., Rose S.P., Wells R.G., Pirgozliev V. The effect of changing the excreta moisture of caged laying hens on the excreta and microbial contamination of their egg shells. Br. Poult. Sci. 2000;41:168–173. doi: 10.1080/713654903. [DOI] [PubMed] [Google Scholar]
- Smits C.H., Veldman A., Verstegen M.W.A., Beynen A.C. Dietary carboxymethylcellulose with high instead of low viscosity reduces macronutrient digestion in broiler chickens. J. Nutr. 1997;127:483–487. doi: 10.1093/jn/127.3.483. [DOI] [PubMed] [Google Scholar]
- Sozcu A. Growth performance, pH value of gizzard, hepatic enzyme activity, immunologic indicators, intestinal histomorphology, and cecal microflora of broilers fed diets supplemented with processed lignocellulose. Poult. Sci. 2019;98:6880–6887. doi: 10.3382/ps/pez449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teirlynck E., Bjerrum L., Eeckhaut V., Huygebaert G., Pasmans F., Haesebrouck F., Dewulf J., Ducatelle R., Van Immerseel F. The cereal type in feed influences gut wall morphology and intestinal immune cell infiltration in broiler chickens. Br. J. Nutr. 2009;102:1453–1461. doi: 10.1017/S0007114509990407. [DOI] [PubMed] [Google Scholar]
- Wall H., Tauson T., Sørgjerd S. Bacterial contamination of eggshells in furnished and conventional cages. J. Appl. Poult. Res. 2008;17:11–16. [Google Scholar]
- Yokhana S.J., Parkinson G., Frankel T.L. Effect of insoluble fiber supplementation applied at different ages on digestive organ weight and digestive enzymes of layer-strain poultry. Poult. Sci. 2016;95:550–559. doi: 10.3382/ps/pev336. [DOI] [PMC free article] [PubMed] [Google Scholar]