Abstract
This study investigated the effects of pterostilbene (PT) supplementation on growth performance, hepatic injury, and antioxidant variables in a broiler chicken model with diquat (DQ)-induced oxidative stress. There were 192 one-day-old male Ross 308 broiler chicks randomly allocated to one of two treatment groups: 1) broilers fed a basal diet and 2) broilers fed a diet supplemented with 400 mg/kg PT. At 20 D of age, half of the broilers in each group were intraperitoneally injected with DQ (20 mg per kg BW), whereas the other half were injected with an equivalent amount of sterile saline. Diquat induced a rapid loss of BW (P < 0.001) 24 h post-injection, but dietary PT supplementation improved the BW change of broilers (P = 0.014). Compared with unchallenged controls, the livers of DQ-treated broilers were in severe cellular damage and oxidative stress, with the presence of higher plasma transaminase activities (P < 0.05), a greater number of apoptotic hepatocytes (P < 0.001), and an increased malondialdehyde content (P = 0.007). Pterostilbene supplementation prevented the increases in plasma aspartate aminotransferase activity (P = 0.001), the percentage of hepatocyte apoptosis (P < 0.001), and the hepatic malondialdehyde accumulation (P = 0.011) of the DQ-treated broilers. Regarding the hepatic antioxidant function, PT significantly increased total antioxidant capacity (P = 0.007), superoxide dismutase activity (P = 0.016), reduced glutathione content (P = 0.011), and the ratio of reduced glutathione to oxidized glutathione (P = 0.003), whereas it reduced the concentration of oxidized glutathione (P = 0.017). Pterostilbene also boosted the expression levels of nuclear factor erythroid 2–related factor 2 (P = 0.010), heme oxygenase 1 (P = 0.037), superoxide dismutase 1 (P = 0.014), and the glutamate–cysteine ligase catalytic subunit (P = 0.001), irrespective of DQ challenge. In addition, PT alleviated DQ-induced adenosine triphosphate depletion (P = 0.010). In conclusion, PT attenuates DQ-induced hepatic injury and oxidative stress of broilers presumably by restoring hepatic antioxidant function.
Key words: pterostilbene, liver injury, oxidative stress, diquat, broiler
Introduction
Intensive modern farming significantly boosts livestock productivity and economic benefit; however, it also increases animals' risk of exposure to oxidative stress (Miller et al., 1993, Frankič et al., 2009). Factors including nutritional, physiological/pathological, and environmental causes can induce oxidative stress, which represents an imbalance between the generation of reactive oxygen species (ROS) and the scavenging capacity of antioxidants, leading to DNA damage, lipid peroxidation, and degradation of cellular proteins (Jones, 2006). The liver is the primary site of biosynthesis, metabolism, clearance, and host defense, and as such, it is extremely vulnerable to damage by ROS (Sanchez-Valle et al., 2012). Moreover, the disturbance of hepatic function has been implicated as a conjoint pathological mechanism underlying several diseases. This contributes to high morbidity and mortality on poultry farms (Avanzo et al., 2001, Lin et al., 2004, Salami et al., 2015). Therefore, it is imperative to establish appropriate nutrition strategies to reduce the risk of oxidative damage and liver disorders in broilers.
Pterostilbene (PT) is a naturally occurring antioxidant found primarily in blueberries and Pterocarpus marsupium heartwood. It has recently gained attention for its health benefits in acute and chronic diseases associated with oxidative stress (Acharya and Ghaskadbi, 2013, McFadden, 2013, Cheng et al., 2016, Liu et al., 2017). As a dimethyl ether analog of resveratrol, PT has comparable or even stronger antioxidant and anti-inflammatory properties than its parent compound (Choo et al., 2014). Importantly, PT displays longer elimination half-life, slower clearance, and higher bioavailability in vivo owing to the presence of 2 methoxy groups that cause it to exhibit increased lipophilicity and oral absorption (Athar et al., 2007, Perecko et al., 2010, Yeo et al., 2013). In this context, PT is increasingly recognized as a key player in the recovery of hepatic injury (Lee et al., 2013, El-Sayed et al., 2015), and it may constitute an attractive candidate to attenuate oxidative stress in poultry production. To the best of our knowledge, the application of PT in the diets of broilers has not been reported to date. Therefore, the present study investigated the effects of diets supplemented with PT on the growth performance and hepatic antioxidant function of broiler chicks under conditions of oxidative stress.
Materials and methods
Experimental Design, Diets, and Management
The protocols used in the animal experiments were approved by the Institutional Animal Care and Use Committee of Nanjing Agricultural University. To evaluate the hepatoprotective and antioxidant roles of PT in broiler chickens, an established model of oxidative stress–associated liver injury caused by diquat (DQ) was used in this study. Diquat is widely used as a potent chemical agent to induce oxidative stress, primarily targeting the liver (Burk et al., 1995, Osbum et al., 2006). In the metabolic process within the hepatocytes, a small amount of DQ can trigger an oxidation/reduction cycle that receives electrons from reduced form of nicotinamide adenine dinucleotide phosphate, forms a kind of highly unstable free radicals, and donates electrons to molecular oxygen, eventually resulting in the overproduction of superoxide anions (Fu et al., 1999). Substantial studies have successfully established animal models of oxidative stress with DQ, such as in piglets, chicken embryos, and rodents (Bauman et al., 1991, Awad et al., 1994, Gallagher et al., 1995, Rogers et al., 2006, Yuan et al., 2017, Zhou et al., 2017, Wang et al., 2019, Li et al., 2019). We used 192 one-day-old male Ross 308 broiler chicks and randomly divided them into 4 groups, which were designed as a 2 × 2 factorial arrangement that included a diet factor (fed either a basal diet (CON) or a diet supplemented with 400 mg PT [No. 537-42-8; BOC Sciences, Shirley, NY] per kg of diet from 1 to 21 D of age) and a stress factor (injected with either DQ [No. 45422; Sigma-Aldrich, St Louis, MO] solution [1.0 mg/mL; dissolved in 0.86% sterile saline]) at a dose of 20 mg per kg of BW or an equivalent amount of sterile saline at 20 D of age. Each of these groups contained 6 replicates (one replicate per cage) with 8 birds per replicate. The doses of PT and DQ were determined in accordance with the findings of independent prestudies by our colleagues (unpublished). Similarly, the added levels of resveratrol, the parent compound of PT, were varied from 400 to 1,000 mg/kg in the diets of broilers for the prevention and/or treatment of heat stress (Zhang et al., 2017a), transport stress (Zhang et al., 2017b), and acute liver injury caused by aflatoxin B1 (Sridhar et al., 2015). All the birds were kept in three-level wired battery cages placed in a room environmentally maintained between 32°C to 34°C. When the chicks were at the age of 1 to 7 D, the temperature of the room was gradually reduced to 26°C at the rate of 3°C to 4°C per week and kept constant thereafter. Light was manipulated to create a light cycle at a rate of 23 h light and 1 h darkness during the entire study period. Feed and fresh water were available ad libitum for 3 wk. Formulated as per the National Research Council (1994) parameters, the basal diet met the nutritional requirements of the birds. Table 1 shows the diet's composition and nutrient levels. When the chicks were 20 and 21 D of age, BW and feed intake were recorded, respectively, on a cage basis. This was carried out to calculate ADG, ADFI, and feed conversion ratio (FCR) before DQ challenge (from 1–20 D of age) and to record the change in BW after DQ challenge (from 20–21 D of age).
Table 1.
Composition and calculated nutrient levels of the basal diet (g/kg, as fed basis unless otherwise stated).
| Items | Content |
|---|---|
| Ingredients | |
| Maize | 576.10 |
| Soybean meal | 310.00 |
| Maize gluten meal | 32.90 |
| Soybean oil | 31.10 |
| Limestone | 12.00 |
| Dicalcium phosphate | 20.00 |
| L-Lysine (78%) | 3.40 |
| DL-Methionine (98%) | 1.50 |
| Sodium chloride | 3.00 |
| Premix1 | 10.00 |
| Calculated nutrient levels2 | |
| Metabolizable energy (MJ/kg) | 12.56 |
| Crude protein | 210.98 |
| Calcium | 9.97 |
| Available phosphorus | 4.57 |
| Lysine | 11.98 |
| Methionine | 4.97 |
| Methionine + cystine | 8.49 |
Premix provided per kilogram of diet: vitamin A (transretinyl acetate), 10,000 IU; vitamin D3 (cholecalciferol), 3,000 IU; vitamin E (all-rac-α-tocopherol), 30 IU; menadione, 1.3 mg; thiamin, 2.2 mg; riboflavin, 8 mg; nicotinamide, 40 mg; choline chloride, 600 mg; calcium pantothenate, 10 mg; pyridoxine·HCl, 4 mg; biotin, 0.04 mg; folic acid, 1 mg; vitamin B12 (cobalamin), 0.013 mg; Fe (from ferrous sulfate), 80 mg; Cu (from copper sulfate), 8.0 mg; Mn (from manganese sulfate), 110 mg; Zn (from zinc oxide), 60 mg; I (from calcium iodate), 1.1 mg; Se (from sodium selenite), 0.3 mg.
All nutrient contents, except metabolizable energy, were analyzed values.
Sample Collection
Six chickens from each treatment group (one bird from each cage) were randomly selected for sampling at 24 h after injection, for DQ could then lead to hepatic oxidative stress based on the findings reported by other researchers (Bauman et al., 1991, Gallagher et al., 1995). Heparinized blood samples were taken from the wing vein and centrifuged at 4,000 × g for 15 min at 4°C to produce plasma samples, which were stored at −80°C until analysis. After euthanasia via cervical dislocation, the birds were decapitated, immediately eviscerated, and the liver extracted. Liver samples from the left lobe were taken and fixed in 4% paraformaldehyde for histological observation. The remainder of the liver was snap-frozen in liquid nitrogen and stored at −80°C for further analysis.
Determination of Plasma Aminotransferase Activities
Standard spectrophotometric procedures using commercial kits (Nanjing Jiancheng Institute of Bioengineering, Nanjing, Jiangsu, China) were used to determine the activities of alanine aminotransferase (ALT; No. C009-2-1) and aspartate aminotransferase (AST; No. C010-2-1) in the plasma of broilers.
Hepatic Apoptotic Analysis
Samples from the left lobe of the liver were obtained, fixed in 4% paraformaldehyde, and embedded in paraffin blocks. The samples were divided into 5-μm sections for subsequent apoptotic analysis, which was carried out with one-step terminal deoxynucleotidyl transferase–mediated dUTP nick-end labeling (TUNEL; No. A113-03; Vazyme Biotech Co., Ltd., Nanjing, Jiangsu, China) staining. Tissue sections were permeabilized with 20 μg/mL proteinase K at room temperature for 20 min. After washing with phosphate buffer solution twice, the TUNEL mixed reagents were added to the sections and incubated in dark conditions at 37°C for 60 min. We used 4′-6-diamidino-2-phenylindole (DAPI; No. E607303; Beyotime Institute of Biotechnology, Haimen, Jiangsu, China) to label the nuclei. The TUNEL-positive cells were visualized using a fluorescent microscope (Nikon Eclipse C1; Nikon, Tokyo, Japan) and were defined by a red color with the DAPI label.
Analysis of Hepatic Antioxidant Capacity
Reduced glutathione (GSH; No. GSH-2-W) and oxidized glutathione (GSSG; No. GSSG-2-W) contents in the liver of broilers were examined using commercial assay kits (Suzhou Comin Biotechnology Co., Ltd., Suzhou, Jiangsu, China). The liver tissue homogenate (20% weight/volume) was mixed with 5% metaphosphoric acid and then centrifuged at 8,000 × g at 4°C for 10 min. The supernatants were used for GSH and GSSG analysis. The concentration of GSH was spectrophotometrically measured at 412 nm by the 5,5′-dithio-bis-(2-nitrobenzoic acid) (DTNB) method (Tietze, 1969). For GSSG, the thiol-masking agent 2-vinylpyridine was added to the supernatant to remove GSH. Then, the action of glutathione reductase in the presence of NADPH reduced GSSG to GSH, which was quantified with DTNB at 412 nm. We found the color change during the reaction and the reaction rate to be proportional to the concentrations of GSH and GSSG. The concentrations of total glutathione (T-GSH) equal the concentrations of GSH plus twice concentrations of GSSG.
Colorimetric kits for total antioxidant capacity (T-AOC; No. A015-1-2), superoxide dismutase (SOD; No. A001-1-2), and glutathione peroxidase (GSH-Px; No. A005-1-2) activities, along with malondialdehyde (MDA; No. A003-1-2) concentration, were purchased from Nanjing Jiancheng Institute of Bioengineering (Nanjing, Jiangsu, China). Using the method of Benzie and Strain (1996), we determined the activity of T-AOC at 520 nm. The amount of T-AOC that elevated the absorbance by 0.01 per minute was considered as one unit. A xanthine and xanthine oxidase procedure was used to measure SOD activity at 550 nm (Sun et al., 1988). The amount of enzyme that caused 50% inhibition of nitrite production was considered as one unit of SOD. The reaction of GSH with DTNB indicated GSH-Px activity. We also monitored the change of absorbance at 412 nm using a spectrophotometer (Hafeman et al., 1974). The amount of enzyme required to deplete 1 μmol of GSH per minute was considered one unit of GSH-Px activity. The concentration of MDA in the liver homogenate was assayed by thiobarbituric acid chromometry (Placer et al., 1966).
Measurement of Hepatic Adenosine Triphosphate Content
The level of adenosine triphosphate (ATP) in the liver of broilers was measured with an ATP content assay kit (No. A095-1-1; Nanjing Jiancheng Institute of Bioengineering). The liver samples were cut into pieces, crushed, and mixed with boiling double-distilled water to a final concentration of 10% (weight/volume). The liver homogenate was further boiled for 10 min and then centrifuged at 3,000 × g for 15 min. After that, the concentration of ATP in the supernatant was assayed by a spectrophotometric method based on the colorimetric reaction with phosphomolybdic acid and expressed as μmol/g wet weight.
Total RNA Isolation and Gene Expression Analysis
Extraction of total RNA and its reverse transcription were performed as per our previous reports (Zhang et al., 2017c). Retrieving the snap-frozen liver samples, we isolated its total RNA using TRIzol Reagent (No. 9109; TaKaRa Biotechnology, Dalian, Liaoning, China), per the manufacturer's manual. Extracted RNA was dissolved in 50 μL ultrapure water. We used a NanoDrop ND-1000UV spectrophotometer (NanoDrop Technologies, Wilmington, DE) to measure the purity and concentration of total RNA at 260 and 280 nm. Electrophoresis on a 1.5% agarose gel stained Ultra GelRed (No. GR501-01; Vazyme Biotech Co., Ltd.) was used to verify the RNA integrity. Then, we reverse transcribed 1 μg of total RNA into complementary DNA using the PrimeScript RT Reagent Kit (No. RR036 A; TaKaRa Biotechnology). Real-time polymerase chain reaction (PCR) was performed using a QuantStudio 5 Real-time PCR System (Applied Biosystems, Life Technologies, Foster City, CA) using the ChamQTM SYBR qPCR Master Mix Kit (No. Q311-02; Vazyme Biotech Co., Ltd.), as per the manufacturer's guidelines. The PCR process involved a prerun at 95°C for 30 s, 40 cycles of denaturation at 95°C for 5 s, and a 60°C annealing step for 30 s. For the melting curve conditions, we conducted 1 cycle of denaturation at 95°C for 10 s and then increased the temperature from 65 to 95°C with a change rate at 0.5 C/s. Supplemental Table 1 presents details of the primer sequences for the target and reference genes nuclear factor erythroid 2–related factor 2 (NRF2), heme oxygenase 1 (HO1), NAD(P)H quinone dehydrogenase 1 (NQO1), superoxide dismutase 1 (SOD1), superoxide dismutase 2 (SOD2), glutathione S-transferase alpha 2 (GSTA2), glutathione S-transferase alpha 3 (GSTA3), glutamate-cysteine ligase catalytic subunit (GCLC), glutamate-cysteine ligase modifier subunit (GCLM), sirtuin 1 (SIRT1), peroxisome proliferators-activated receptor gamma coactivator 1 alpha (PGC1α), nuclear respiratory factor 1 (NRF1), transcription factor A, mitochondrial (TFAM), B cell lymphoma/leukemia 2 (BCL2), caspase 3 (CASP3), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and beta actin (ACTB) used in this study. Relative expression levels of the target genes were normalized to the reference gene and then calculated in accordance with the 2−ΔΔCt method (Livak and Schmittgen, 2001).
Statistical Analysis
Before DQ challenge, the Student t test of SPSS statistical software (version 22.0 for Windows, SPSS Inc., Chicago, IL) was used to determine whether dietary PT supplementation had significant effects on the ADG, ADFI, and FCR of broilers from 1 to 20 D of age. After DQ challenge, the significance and interaction of the main effects (stress and diet) were measured by two-way analysis of variance (ANOVA) via the general linear model procedure of SPSS statistical software. A P-value less than 0.05 was considered as statistically significant. After the one-way ANOVA test, Tukey's multiple comparison test was used to differentiate significantly different treatments based on the interaction of the main effects (P < 0.05). Data are presented as mean values with pooled SEMs.
Results
Growth Performance
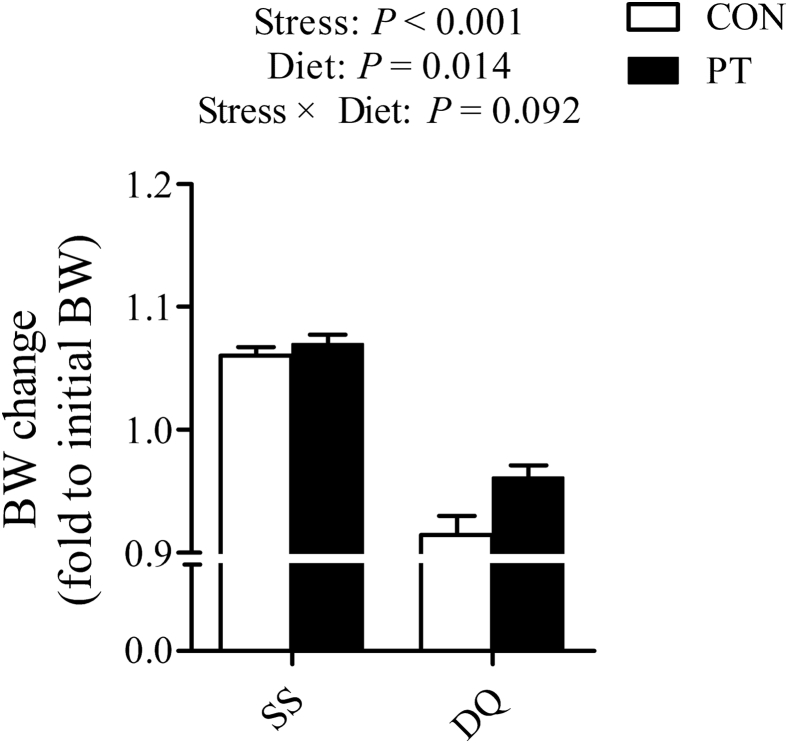
No difference was observed in the ADG, ADFI, or FCR between the CON and PT groups before DQ injection (Table 2). During the period of DQ challenge, the fold change in BW (P < 0.001) of DQ-treated broilers was dramatically lower than that of the saline-treated controls (Figure 1). In contrast, PT improved the BW change (P = 0.014) of broilers during 0 to 24 h after the challenge.
Table 2.
Effect of dietary pterostilbene supplementation on the growth performance of broilers from 1 to 20 D of age.
| Items1 | CON | PT | SEM | P-value |
|---|---|---|---|---|
| ADG (g/day) | 32.23 | 32.68 | 0.45 | 0.629 |
| ADFI (g/day) | 44.79 | 43.63 | 0.49 | 0.249 |
| FCR2 (g:g) | 1.39 | 1.34 | 0.02 | 0.071 |
Abbreviations: CON, broilers received a basal diet; PT, broilers received a pterostilbene-supplemented diet; FCR, feed conversion ratio.
Mean values with pooled SEMs, n = 12.
Feed conversion ratio was calculated by dividing the average daily feed intake by its average daily gain.
Figure 1.
Effect of dietary pterostilbene supplementation on the BW change of the sterile saline- and diquat-treated broilers from 20 to 21 D of age. BW change induced by diquat or sterile saline injection was calculated by dividing the final BW by its initial BW. Values are means with their SEs represented by vertical bars (n = 6). Abbreviations: CON, broilers received a basal diet from 1 to 21 D of age; DQ, broilers were injected with diquat solution at 20 D of age; PT, broilers received a pterostilbene-supplemented diet from 1 to 21 D of age; SS, birds were injected with sterile saline at 20 D of age.
Relative Liver Weight and Plasma Transaminase Activities
Diquat challenge induced an increase in the relative weight of the liver (P = 0.001), corresponding to higher activities of ALT (P = 0.012) and AST (P = 0.019) in the plasma (Table 3). Conversely, broilers that consumed a PT-supplemented diet showed a significant decrease in the activity of plasma AST (P = 0.006), compared with those fed with a basal diet. In addition, the PT diet alleviated the increased plasma AST activity (P = 0.001) in the DQ-challenged broilers compared with the unchallenged controls.
Table 3.
Effect of dietary pterostilbene supplementation on relative liver weight and plasma transaminase activities of 21-day-old broilers challenged with diquat.
| Items1 | SS |
DQ |
SEM | Stress effect |
Diet effect |
P-value |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | PT | CON | PT | SS | DQ | CON | PT | Stress | Diet | S × D | ||
| Relative liver weight2 (g/100 g BW) | 2.53 | 2.55 | 2.89 | 2.69 | 0.04 | 2.54 | 2.79 | 2.71 | 2.62 | 0.001 | 0.218 | 0.117 |
| ALT (U/L) | 8.33 | 9.48 | 12.98 | 10.12 | 0.57 | 8.90 | 11.55 | 10.65 | 9.80 | 0.012 | 0.386 | 0.050 |
| AST (U/L) | 32.15b | 33.97b | 48.48a | 30.84b | 1.91 | 33.06 | 39.66 | 40.32 | 32.40 | 0.019 | 0.006 | 0.001 |
a,bMeans within a row with different superscripts are different at P < 0.05.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CON, birds received a basal diet from 1 to 21 D of age; DQ, birds were injected with diquat solution at 20 D of age; PT, birds received a pterostilbene-supplemented diet from 1 to 21 D of age; SS, birds were injected with sterile saline at 20 D of age; S × D, the interaction of stress and diet effects.
Mean values with pooled SEMs, n = 6.
Relative liver weight was calculated as follows: (liver weight/final BW) × 100.
Hepatic Apoptotic Levels
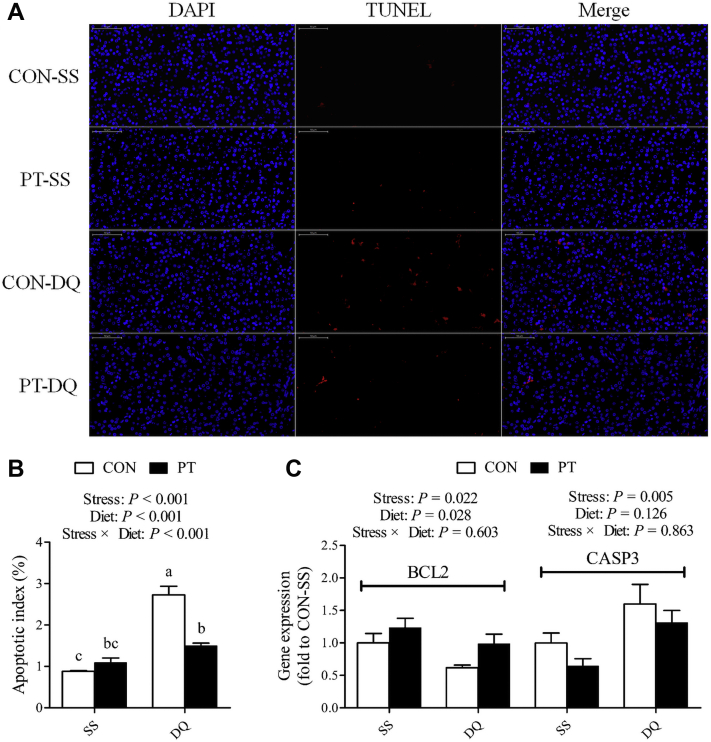
Compared with the untreated controls, the broilers' exposure to DQ showed a sharp rise in the percentage of hepatocyte apoptosis (P < 0.001; Figures 2A and 2B). Diquat challenge significantly decreased the mRNA abundance of BCL2 (P = 0.022; Figure 2C) but increased the mRNA abundance of CASP3 (P = 0.005; Figure 2C). However, PT supplementation exerted an opposite effect on the hepatic apoptotic percentage (P < 0.001), whereas it effectively attenuated the DQ-induced higher apoptotic index in the liver of broilers (P < 0.001). In addition, the BCL2 expression was up-regulated by PT treatment (P = 0.028).
Figure 2.
Effect of dietary pterostilbene supplementation on hepatic apoptotic status of 21-day-old broilers challenged with diquat. (A) Representative micrographs of TUNEL staining carried out on paraformaldehyde-fixed sections from the liver samples (400 ×magnification). (B) The percentage of hepatocyte apoptosis. (C) Real-time polymerase chain reaction analysis of hepatic mRNA expression of BCL2 and CASP3. Values are means with their SEs represented by vertical bars (n = 6). a-cMean values within a row with unlike letters were significantly different (P < 0.05) among groups. Abbreviations: BCL2, B cell lymphoma/leukemia 2; CASP3, caspase 3; CON, broilers received a basal diet from 1 to 21 D of age; DQ, broilers were injected with diquat solution at 20 D of age; PT, broilers received a pterostilbene-supplemented diet from 1 to 21 D of age; SS, birds were injected with sterile saline at 20 D of age; TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling.
Hepatic Antioxidant Capacity
Administration of DQ dramatically increased the activities of SOD (P = 0.006) and GSH-Px (P < 0.001) and the content of MDA (P = 0.007), whereas it decreased T-GSH (P = 0.004) and GSH (P = 0.002) concentrations as well as the ratio of GSH to GSSG (P = 0.010) in the liver (Table 4). The T-AOC (P = 0.007) and SOD (P = 0.016) activities; T-GSH (P = 0.037) and GSH (P = 0.011) contents; and GSH:GSSG ratio (P = 0.003) were all robustly improved because of supplementation with PT. Moreover, feeding a PT-supplemented diet to broilers obviously decreased the concentrations of GSSG (P = 0.017) and MDA (P = 0.012) in the liver compared with those fed with a basal diet.
Table 4.
Effect of dietary pterostilbene supplementation on hepatic antioxidant ability and lipid peroxidation of 21-day-old broilers challenged with diquat.
| Items1 | SS |
DQ |
SEM | Stress effect |
Diet effect |
P-value |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | PT | CON | PT | SS | DQ | CON | PT | Stress | Diet | S × D | ||
| T-AOC (U/mg protein) | 1.75a,b | 1.83a | 1.40b | 1.92a | 0.06 | 1.79 | 1.66 | 1.57 | 1.88 | 0.213 | 0.007 | 0.041 |
| SOD (U/mg protein) | 188.95 | 203.52 | 206.21 | 222.52 | 3.70 | 196.24 | 214.36 | 197.58 | 213.02 | 0.006 | 0.016 | 0.884 |
| GSH-Px (U/mg protein) | 19.14 | 21.13 | 24.71 | 23.18 | 0.60 | 20.14 | 23.95 | 21.93 | 22.16 | <0.001 | 0.800 | 0.063 |
| T-GSH (nmol/100 mg wet weight) | 129.29 | 138.74 | 91.73 | 120.93 | 5.45 | 134.01 | 106.33 | 110.51 | 129.83 | 0.004 | 0.037 | 0.266 |
| GSH (nmol/100 mg wet weight) | 116.72 | 128.85 | 75.15 | 109.91 | 5.72 | 122.78 | 92.53 | 95.93 | 119.38 | 0.002 | 0.011 | 0.194 |
| GSSG (nmol/100 mg wet weight) | 6.29 | 4.94 | 8.29 | 5.51 | 0.45 | 5.61 | 6.90 | 7.29 | 5.23 | 0.121 | 0.017 | 0.374 |
| GSH:GSSG (nmol:nmol) | 19.11 | 27.94 | 10.53 | 20.79 | 1.83 | 23.53 | 15.66 | 14.82 | 24.37 | 0.010 | 0.003 | 0.800 |
| MDA (nmol/mg protein) | 0.74b | 0.75b | 1.39a | 0.77b | 0.08 | 0.75 | 1.08 | 1.07 | 0.76 | 0.007 | 0.012 | 0.011 |
a,bMeans within a row with different superscripts are different at P < 0.05.
Abbreviations: CON, birds received a basal diet from 1 to 21 D of age; DQ, birds were injected with diquat solution at 20 D of age; PT, birds received a pterostilbene-supplemented diet from 1 to 21 D of age; SS, birds were injected with sterile saline at 20 D of age; S × D, the interaction of stress and diet effects; GSH, reduced glutathione; GSH-Px, glutathione peroxidase; GSH:GSSG, the ratio of reduced glutathione to oxidized glutathione; GSSG, oxidized glutathione; MDA, malondialdehyde; SOD, superoxide dismutase; T-AOC, total antioxidant capacity; T-GSH, total glutathione.
Mean values with pooled SEMs, n = 6.
Table 5 summarized the expression levels of genes involved in antioxidant defense. Compared with the unchallenged broilers, DQ significantly lowered the mRNA abundance of hepatic SOD1 (P = 0.021). In contrast, PT supplementation up-regulated the expression levels of NRF2 (P = 0.010), HO1 (P = 0.037), SOD1 (P = 0.014), GSTA2 (P = 0.018), and GCLC (P = 0.001). Neither DQ treatment nor PT supplementation affected the mRNA levels of NQO1, SOD2, GSTA3, and GCLM (P > 0.05).
Table 5.
Effect of dietary pterostilbene supplementation on the expression levels of hepatic antioxidant gene of 21-day-old broilers challenged with diquat.
| Items1,2 | SS |
DQ |
SEM | Stress effect |
Diet effect |
P-value |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | PT | CON | PT | SS | DQ | CON | PT | Stress | Diet | S × D | ||
| NRF2 | 1.00 | 1.78 | 1.51 | 2.10 | 0.14 | 1.39 | 1.81 | 1.26 | 1.94 | 0.100 | 0.010 | 0.707 |
| HO1 | 1.00 | 1.23 | 0.60 | 1.24 | 0.11 | 1.11 | 0.92 | 0.80 | 1.24 | 0.338 | 0.037 | 0.294 |
| NQO1 | 1.00 | 1.19 | 0.89 | 1.20 | 0.07 | 1.10 | 1.04 | 0.94 | 1.19 | 0.679 | 0.071 | 0.658 |
| SOD1 | 1.00 | 1.16 | 0.51 | 1.02 | 0.08 | 1.08 | 0.77 | 0.76 | 1.09 | 0.021 | 0.014 | 0.177 |
| SOD2 | 1.00 | 1.05 | 0.81 | 0.98 | 0.08 | 1.03 | 0.90 | 0.91 | 1.02 | 0.423 | 0.499 | 0.729 |
| GSTA2 | 1.00 | 1.44 | 0.85 | 1.63 | 0.13 | 1.22 | 1.24 | 0.92 | 1.54 | 0.934 | 0.018 | 0.482 |
| GSTA3 | 1.00 | 1.24 | 1.06 | 1.48 | 0.12 | 1.12 | 1.27 | 1.03 | 1.36 | 0.557 | 0.206 | 0.709 |
| GCLC | 1.00 | 2.00 | 0.74 | 1.92 | 0.18 | 1.50 | 1.33 | 0.87 | 1.96 | 0.566 | 0.001 | 0.765 |
| GCLM | 1.00 | 1.61 | 1.60 | 1.39 | 0.14 | 1.30 | 1.49 | 1.30 | 1.50 | 0.495 | 0.467 | 0.156 |
Abbreviations: CON, birds received a basal diet from 1 to 21 D of age; DQ, birds were injected with diquat solution at 20 D of age; PT, birds received a pterostilbene-supplemented diet from 1 to 21 D of age; SS, birds were injected with sterile saline at 20 D of age; S × D, the interaction of stress and diet effects; GCLC, glutamate–cysteine ligase catalytic subunit; GCLM, glutamate–cysteine ligase modifier subunit; GSTA2, glutathione S-transferase alpha 2; GSTA3, glutathione S-transferase alpha 3; HO1, heme oxygenase 1; NQO1, NAD(P)H quinone dehydrogenase 1; NRF2, nuclear factor, erythroid 2-related factor 2; SOD1, superoxide dismutase 1; SOD2, superoxide dismutase 2.
Mean values with pooled SEMs, n = 6.
Expressed in arbitrary units. The expression of each target gene for the CON-SS group was assigned a value of 1 and normalized against beta actin.
Hepatic ATP Content and Mitochondrial Function–Related Gene Expression
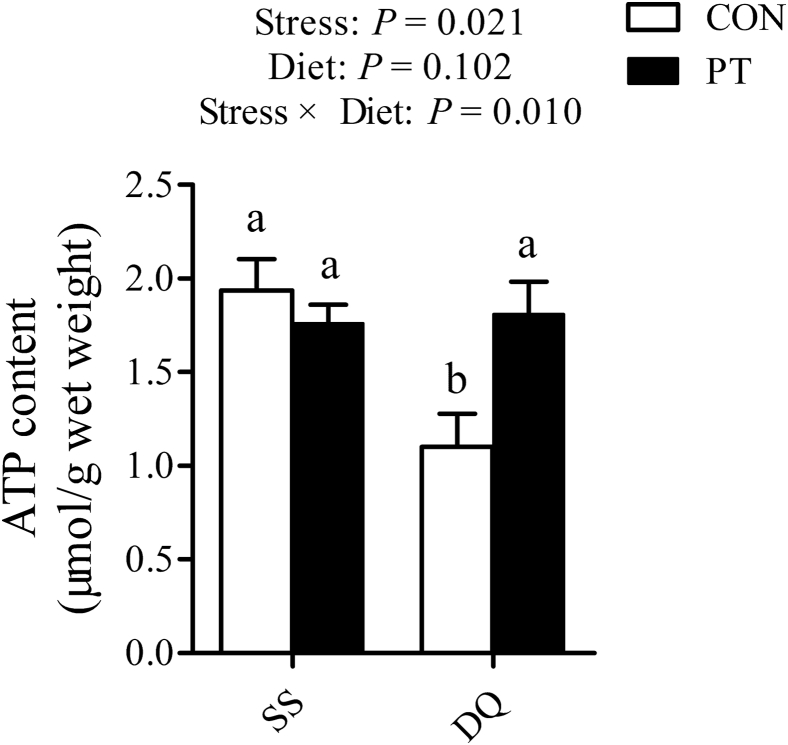
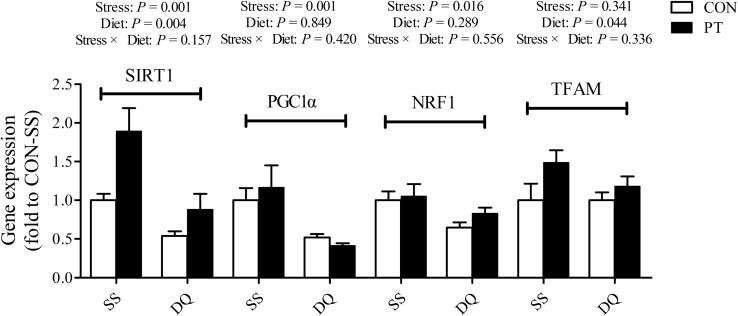
The content of ATP (P = 0.021) was significantly decreased in the liver of DQ-treated broilers compared with untreated broilers (Figure 3). Consumption of the PT-supplemented diet abolished the decrease in hepatic ATP concentration in the DQ-treated broilers (P = 0.010). In addition, the transcriptional activities of SIRT1 (P = 0.001), PGC1α (P = 0.001), and NRF1 (P = 0.016) were dramatically decreased after DQ injection (Figure 4). Conversely, the PT-fed broilers displayed significant increases in the mRNA expression of hepatic SIRT1 (P = 0.004) and TFAM (P = 0.044) when compared with those fed with a basal diet.
Figure 3.
Effect of dietary pterostilbene supplementation on hepatic ATP content of 21-day-old broilers challenged with diquat. Values are means with their SEs represented by vertical bars (n = 6). a,bMean values within a row with unlike letters were significantly different (P < 0.05) among groups. Abbreviations: ATP, adenosine triphosphate; CON, broilers received a basal diet from 1 to 21 D of age; DQ, broilers were injected with diquat solution at 20 D of age; PT, broilers received a pterostilbene-supplemented diet from 1 to 21 D of age; SS, birds were injected with sterile saline at 20 D of age.
Figure 4.
Effect of dietary pterostilbene supplementation on the expression levels of hepatic genes related to mitochondrial function of 21-day-old broilers challenged with diquat. Values are means with their SEs represented by vertical bars (n = 6). Abbreviations: CON, broilers received a basal diet from 1 to 21 D of age; DQ, broilers were injected with diquat solution at 20 D of age; NRF1, nuclear respiratory factor 1; PGC1α, peroxisome proliferators-activated receptor gamma coactivator 1 alpha; PT, broilers received a pterostilbene-supplemented diet from 1 to 21 D of age; SIRT1, sirtuin 1; SS, birds were injected with sterile saline at 20 D of age; TFAM, transcription factor A, mitochondrial.
Discussion
The multiple benefits of PT for the prevention and treatment of oxidative stress have recently drawn increased attention owing to its specific biological properties (Zhang et al., 2013, Sireesh et al., 2017, Yang et al., 2017, Yu et al., 2018); however, past studies have mainly focused on in vitro experiments at the cellular level. This study presents evidence that in vivo PT supplementation mitigates the hepatic injury and oxidative stress of broiler chicks caused by DQ challenge. Specifically, PT preserves redox balance, attenuates ATP depletion, and mitigates liver injury. These interrelated effects may cooperate to minimize the BW loss of broilers during DQ challenge.
The liver is very vulnerable to different kinds of stress including oxidative stress (Sanchez-Valle et al., 2012). Many risk factors, including toxins, environmental pollutants, and pathogenic bacteria, can disturb hepatic redox homeostasis and elicit oxidative stress–mediated damage, which in turn results in severe liver injury (Li et al., 2015). Once hepatic antioxidant responses are unable to cope with these challenges, the homeostatic systems gradually deteriorate and lead to a decline in disease resistance (Avanzo et al., 2001, Lin et al., 2004, Salami et al., 2015). The DQ-induced oxidative stress model is well known to explore the pathogenesis of liver injury and to evaluate novel hepatoprotective agents (Burk et al., 1995, Osbum et al., 2006). Therefore, we selected DQ for this study to induce hepatic tissue damage and oxidative stress and to examine the hepatoprotective roles of PT.
In this investigation, the DQ-challenged broilers exhibited a higher percentage of hepatocyte apoptosis and increased AST and ALT plasma values. Increases in plasma aminotransferases, which are recognized as the most sensitive indicators of hepatic damage, can reflect the extent of pathological severity of liver injury (Ikeda et al., 1992). Treatment with PT efficaciously counteracted the DQ-induced increase in plasma AST activity to near control value. The number of apoptotic hepatocytes and the expression level of antiapoptotic gene BCL2 also confirmed PT as a protectant. In accord with the present findings, other groups have demonstrated that PT could antagonize the deleterious effects of several hepatotoxicants, such as dimethylnitrosamine (Lee et al., 2013) and acetaminophen (El-Sayed et al., 2015).
Cellular damage caused by aberrant ROS accumulation is considered a major mediator associated with a multitude of pathological disorders. Diquat can convert molecular oxygen into superoxide anion radicals, leading to lipid peroxidation of membranes, DNA damage, and subsequent liver injury (Yuan et al. 2007). Exposure to DQ notably enhanced lipid peroxidation in the liver of DQ-injected broilers. This was due to the overproduction of ROS, with an increase in the concentration of MDA, an end product of peroxidation of polyunsaturated fatty acids, and related esters (Jain, 1984). Similar studies on rodents and pigs have also observed increased MDA levels in the plasma, liver, muscle, and intestines after DQ injection (Wang et al., 2013, Yin et al., 2015).
To counteract oxidative stress, cells are equipped with numerous antioxidant defenses, such as the GSH redox cycle and a series of enzymatic systems. Cells can be protected from oxidative damage with SOD, which converts superoxide anion to hydrogen peroxide. Then, the hydrogen peroxide is removed by GSH-Px or catalase. In the current work, the activities of SOD and GSH-Px were increased significantly in the liver of broilers after DQ challenge, but these increases seem to be inadequate to inhibit the accumulation of lipid peroxidation. GSH depletion may also constitute a key factor contributing to lipid peroxidation, as a decreased GSH content was observed in the liver of DQ-treated broilers. As a predominant endogenous antioxidant, GSH is necessary for ROS detoxification, removal of hydrogen and lipid peroxides, and repair of oxidatively damaged proteins. In addition, in this study, a significantly down-regulated mRNA abundance of SOD1 was observed in the liver of the DQ-treated broilers, indicating that the defective transcription of SOD gene may precede the decrease of its activity, possibly because cellular signaling pathways involving the antioxidant defense system are extremely sensitive to exogenous stimulus. Similarly, Gallagher et al. (1995) have shown that a 40% decline in the mRNA expression of SOD1 was observed in the liver of rats after 24 h after DQ injection. Such a defect in redox signals would eventually result in an extensive collapse in antioxidant enzymatic systems. In a study by Wang et al. (2013), the activities of SOD and GSH-Px were all significantly reduced in the plasma, muscle, and liver of weanling piglets at 14 D after DQ exposure. Thus, if the oxidative stress cannot be controlled promptly, a vicious cycle could emerge among antioxidant enzyme inactivation, GSH depletion, and excessive lipid peroxidation.
The present data revealed that supplementation with PT could improve the activity of SOD in the liver of DQ-treated broilers and provide a potential explanation for the improvement in hepatic lipid peroxidation. Pterostilbene has been found to possess beneficial effects in attenuating liver injury presumably by accessing the antioxidant defense system. Nuclear factor erythroid 2–related factor 2 is a master regulator of the antioxidant response and represents the underlying mechanism that provides a pivotal defense against DQ toxicity. Ramkumar et al. (2013) have found that PT is a potential activator of NRF2 through a reporter protein complementation imaging assay. Experiments performed by Wang et al., 2010a, Wang et al., 2010b also showed that blueberry as a source of PT could stimulate the levels of transcriptional expression of NRF2, NQO1, and HO1 and confers protection to acute hepatic injury in rats. Our findings are in accord with these observations and suggest that PT administration protects against DQ-induced liver injury by up-regulating the mRNA levels of NRF2 and its downstream HO1, SOD1, GSTA2, and GCLC.
Another possible mechanism by which PT could beneficially influence hepatic redox status may be involved in its role in preserving GSH pool. This is based on the findings of increases in T-GSH and GSH contents and GSH:GSSG ratio and a decrease in GSSG concentration. Pterostilbene can function directly as a free radical scavenger to inhibit the accumulation of endogenous superoxide, hydroxyl, and hydrogen peroxide (Acharya and Ghaskadbi, 2013). As a result, it prevents the formation of GSSG and maintains GSH in a reduced state. As mentioned earlier, PT mediates an up-regulation of the hepatic GCLC expression, which uses the catalytic activity of glutamate cysteine ligase and exploits the rate-limiting and regulatory enzyme in GSH synthesis (Jay and Dickinson, 2003). This observation may partly explain the capacity of PT to enhance the synthesis of T-GSH and to replenish GSH supplies after DQ-induced depletion.
In addition to its role in redox status, PT also affects the hepatic energy supply of the DQ-treated broilers. Substantial evidence points to detrimental effects of oxidative stress on energy generation systems (Brookes et al., 2004, Jaeschke et al., 2012). Mitochondria are not only the primary source of ROS generation but also the major targets for free radical attack. Severe damage to the bioenergetic machinery would suppress the production of ATP and trigger mitochondrial-dependent apoptosis (Brookes et al., 2004). The data presented herein substantiate this view and further reveal that the oxidative stress caused by DQ challenge leads to a sharp decline in the expression of genes related to mitochondrial function, including SIRT1, PGC1α, and NRF1. Under physiological circumstances, SIRT1 directly interacts with PGC1α and deacetylates PGC1α. They lie upstream from NRF1 and TFAM and cooperate to regulate metabolic homeostasis, mitochondrial biogenesis, and energy supply (Rodgers et al., 2005, Lagouge et al., 2006). The underexpression of SIRT1, PGC1α, and NRF1 observed in the DQ-treated broilers may be involved in the ATP depletion and the increase in hepatocyte apoptosis. However, supplementation with PT before and during DQ treatment prevented hepatic ATP loss in broilers, corresponding to the increased expression of SIRT1 and TFAM. Available literature indicates that PT is a potent activator of SIRT1 and an efficient protector against mitochondrial dysfunction (Cheng et al., 2016, Guo et al., 2016). Thus, these results, together with improvements in hepatic structure and antioxidant function, constitute plausible mechanisms of PT action to prevent the BW loss of the DQ-challenged broilers.
In conclusion, the present study provides direct proof of the protective potential of PT against the hepatic damage and oxidative stress of broilers exposure to DQ. Moreover, the study demonstrates that dietary supplementation of PT may help to improve the growth of broiler chicks experiencing oxidative stress.
Acknowledgments
This work was funded by grants from the National Natural Science Foundation of China (31802094), the Natural Science Foundation of Jiangsu Province (BK20180531), the Postdoctoral Research Foundation of China (2018M632320 and 2019T120436), and the Open Project of Shanghai Key Laboratory of Veterinary Biotechnology (klab201710). The authors thank their laboratory colleagues for their assistance.
Conflict of Interest Statement: The authors did not provide a conflict of interest statement.
Footnotes
Supplementary data associated with this article can be found in the online version https://doi.org/10.1016/j.psj.2020.01.021.
Supplementary data
References
- Acharya J.D., Ghaskadbi S.S. Protective effect of pterostilbene against free radical mediated oxidative damage. BMC Complem. Altern. Med. 2013;13:238. doi: 10.1186/1472-6882-13-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athar M., Back J.H., Tang X., Kim K.H., Kopelovich L., Bickers D.R., Kim A.L. Resveratrol: a review of preclinical studies for human cancer prevention. Toxicol. Appl. Pharmacol. 2007;224:274–283. doi: 10.1016/j.taap.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avanzo J.L., de Mendonça C.X., Jr., Pugine S.M.P., de Cerqueira Cesar M. Effect of vitamin E and selenium on resistance to oxidative stress in chicken superficial pectoralis muscle. Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 2001;129:163–173. doi: 10.1016/s1532-0456(01)00197-1. [DOI] [PubMed] [Google Scholar]
- Awad J.A., Burk R.F., Roberts L.J. Effect of selenium deficiency and glutathione-modulating agents on diquat toxicity and lipid peroxidation in rats. J. Pharmacol. Exp. Ther. 1994;270:858–864. [PubMed] [Google Scholar]
- Bauman J.W., Liu J., Liu Y.P., Klaassen C.D. Increase in metallothionein produced by chemicals that induce oxidative stress. Toxicol. Appl. Pharm. 1991;110:347–354. doi: 10.1016/s0041-008x(05)80017-1. [DOI] [PubMed] [Google Scholar]
- Benzie I.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of ''antioxidant power'': the FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Brookes P.S., Yoon Y., Robotham J.L., Anders M.W., Sheu S.S. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am. J. Physiol. Cell Physiol. 2004;287:C817–C833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- Burk R.F., Hill K.E., Awad J.A., Morrow J.D., Kato T., Cockell K.A., Reid Lyons P. Pathogenesis of diquat-induced liver necrosis in selenium-deficient rats: Assessment of the roles of lipid peroxidation and selenoprotein P. Hepatology. 1995;21:561–569. [PubMed] [Google Scholar]
- Cheng Y., Di S., Fan C., Cai L., Gao C., Jiang P., Hu W., Ma Z., Jiang S., Dong Y., Li T., Wu G., Lv J., Li T. SIRT1 activation by pterostilbene attenuates the skeletal muscle oxidative stress injury and mitochondrial dysfunction induced by ischemia reperfusion injury. Apoptosis. 2016;21:905–916. doi: 10.1007/s10495-016-1258-x. [DOI] [PubMed] [Google Scholar]
- Choo Q.Y., Yeo S.C.M., Ho P.C., Tanaka Y., Lin H.S. Pterostilbene surpassed resveratrol for anti-inflammatory application: Potency consideration and pharmacokinetics perspective. J. Funct. Foods. 2014;11:352–362. [Google Scholar]
- El-Sayed E.S.M., Mansour A.M., Nady M.E. Protective effects of pterostilbene against acetaminophen-induced hepatotoxicity in rats. J. Biochem. Mol. Tox. 2015;29:35–42. doi: 10.1002/jbt.21604. [DOI] [PubMed] [Google Scholar]
- Frankič T., Salobir K., Salobir J. The comparison of in vivo antigenotoxic and antioxidative capacity of two propylene glycol extracts of Calendula officinalis (marigold) and vitamin E in young growing pigs. J Anim Physiol. Anim. Nutr. (Berl.) 2009;93:688–694. doi: 10.1111/j.1439-0396.2008.00855.x. [DOI] [PubMed] [Google Scholar]
- Fu Y., Cheng W.H., Porres J.M., Ross D.A., Lei X.G. Knockout of cellular glutathione peroxidase gene renders mice susceptible to diquat-induced oxidative stress. Free Radic. Biol. Med. 1999;27:605–611. doi: 10.1016/s0891-5849(99)00104-5. [DOI] [PubMed] [Google Scholar]
- Gallagher E.P., Buetler T.M., Stapleton P.L., Wang C.H., Stahl D.L., Eaton D.L. The effects of diquat and ciprofibrate on mRNA expression and catalytic activities of hepatic xenobiotic metabolizing and antioxidant enzymes in rat liver. Toxicol. Appl. Pharm. 1995;134:81–91. doi: 10.1006/taap.1995.1171. [DOI] [PubMed] [Google Scholar]
- Guo Y., Zhang L., Li F., Hu C.P., Zhang Z. Restoration of Sirt1 function by pterostilbene attenuates hypoxia-reoxygenation injury in cardiomyocytes. Eur. J. Pharmacol. 2016;776:26–33. doi: 10.1016/j.ejphar.2016.02.052. [DOI] [PubMed] [Google Scholar]
- Hafeman D.G., Sunde R.A., Hoekstra W.G. Effect of dietary selenium on erythrocyte and liver glutathione peroxidase in the rat. J. Nutr. 1974;104:580–587. doi: 10.1093/jn/104.5.580. [DOI] [PubMed] [Google Scholar]
- Ikeda T., Yanaga K., Kishikawa K., Kakizoe S., Shimada M., Sugimachi K. Ischemic injury in liver transplantation: difference in injury sites between warm and cold ischemia in rats. Hepatology. 1992;16:454–461. doi: 10.1002/hep.1840160226. [DOI] [PubMed] [Google Scholar]
- Jaeschke H., McGill M.R., Ramachandran A. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: lessons learned from acetaminophen hepatotoxicity. Drug Metab. Rev. 2012;44:88–106. doi: 10.3109/03602532.2011.602688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S.K. The accumulation of malonyldialdehyde, a product of fatty acid peroxidation, can disturb aminophospholipid organization in the membrane bilayer of human erythrocytes. J. Biol. Chem. 1984;25:3391–3394. [PubMed] [Google Scholar]
- Jay F.H., Dickinson D.A. Oxidative signaling and glutathione synthesis. BioFactors. 2003;17:1–12. doi: 10.1002/biof.5520170101. [DOI] [PubMed] [Google Scholar]
- Jones D.P. Redefining oxidative stress. Antioxid. Redox Sign. 2006;8:1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- Lagouge M., Argmann C., Gerhart-Hines Z., Meziane H., Lerin C., Daussin F., Messadeq N., Milne J., Lambert P., Elliott P., Geny B., Laakso M., Puigserver P., Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Lee M.F., Liu M.L., Cheng A.C., Tsai M.L., Ho C.T., Liou W.S., Pan M.H. Pterostilbene inhibits dimethylnitrosamine-induced liver fibrosis in rats. Food Chem. 2013;138:802–807. doi: 10.1016/j.foodchem.2012.11.094. [DOI] [PubMed] [Google Scholar]
- Li K., Jiang L., Wang J., Xia L., Zhao R., Cai C., Wang P., Zhan X., Wang Y. Maternal dietary supplementation with different sources of selenium on antioxidant status and mortality of chicken embryo in a model of diquat-induced acute oxidative stress. Anim. Feed Sci. Technol. 2019;261:114369. [Google Scholar]
- Li S., Tan H.Y., Wang N., Zhang Z.J., Lao L., Wong C.W., Feng Y. The role of oxidative stress and antioxidants in liver diseases. Int. J. Mol. Sci. 2015;16:26087–26124. doi: 10.3390/ijms161125942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H., Decuypere E., Buyse J. Oxidative stress induced by corticosterone administration in broiler chickens (Gallus gallus domesticus): 1. Chronic exposure. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 2004;139:737–744. doi: 10.1016/j.cbpc.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Liu H., Zhao L., Yue L., Wang B., Li X., Guo H., Ma Y., Yao C., Gao L., Deng J., Li L., Feng D., Qu Y. Pterostilbene attenuates early brain injury following subarachnoid hemorrhage via inhibition of the NLRP3 inflammasome and Nox2-related oxidative stress. Mol. Neurobiol. 2017;54:5928–5940. doi: 10.1007/s12035-016-0108-8. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- McFadden D. A review of pterostilbene antioxidant activity and disease modification. Oxid. Med. Cell. Longev. 2013;2013:575482. doi: 10.1155/2013/575482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J.K., Brzezinska-Slebodzinska E., Madsen F.C. Oxidative stress, antioxidants, and animal function. J. Dairy Sci. 1993;76:2812–2823. doi: 10.3168/jds.S0022-0302(93)77620-1. [DOI] [PubMed] [Google Scholar]
- National Research Council. 9th rev. ed. National Academy Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Osbum W.O., Wakabayashi N., Misra V., Nilles T., Biswal S., Trush M.A., Kensler T.W. Nrf2 regulates an adaptive response protecting against oxidative damage following diquat-mediated formation of superoxide anion. Arch. Biochem. Biophys. 2006;454:7–15. doi: 10.1016/j.abb.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perecko T., Drabikova K., Rackova L., Ciz M., Podborska M., Lojek A., Harmatha J., Smidrkal J., Nosal R., Jancinova V. Molecular targets of the natural antioxidant pterostilbene: effect on protein kinase C, caspase-3 and apoptosis in human neutrophils in vitro. Neuro. Endocrinol. Lett. 2010;31:84–90. [PubMed] [Google Scholar]
- Placer Z.A., Cushman L.L., Johnson B.C. Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. Anal. Biochem. 1966;16:359–364. doi: 10.1016/0003-2697(66)90167-9. [DOI] [PubMed] [Google Scholar]
- Ramkumar K.M., Sekar T.V., Foygel K., Elango B., Paulmurugan R. Reporter protein complementation imaging assay to screen and study Nrf2 activators in cells and living animals. Anal. Chem. 2013;85:7542–7549. doi: 10.1021/ac401569j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers J.T., Lerin C., Haas W., Gygi S.P., Spiegelman B.M., Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature. 2005;434:113. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- Rogers L.K., Bates C.M., Welty S.E., Smith C.V. Diquat induces renal proximal tubule injury in glutathione reductase-deficient mice. Toxicol. Appl. Pharm. 2006;217:289–298. doi: 10.1016/j.taap.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Salami S.A., Majoka M.A., Saha S., Garber A., Gabarrou J.F. Efficacy of dietary antioxidants on broiler oxidative stress, performance and meat quality: science and market. Avian Biol. Res. 2015;8:65–78. [Google Scholar]
- Sanchez-Valle V., Chavez-Tapia N.C., Uribe M., Mendez-Sanchez N. Role of oxidative stress and molecular changes in liver fibrosis: a review. Curr. Med. Chem. 2012;19:4850–4860. doi: 10.2174/092986712803341520. [DOI] [PubMed] [Google Scholar]
- Sireesh D., Ganesh M.R., Dhamodharan U., Sakthivadivel M., Sivasubramanian S., Gunasekaran P., Ramkumar K.M. Role of pterostilbene in attenuating immune mediated devastation of pancreatic beta cells via Nrf2 signaling cascade. J. Nutr. Biochem. 2017;44:11–21. doi: 10.1016/j.jnutbio.2017.02.015. [DOI] [PubMed] [Google Scholar]
- Sridhar M., Suganthi R.U., Thammiaha V. Effect of dietary resveratrol in ameliorating aflatoxin B1-induced changes in broiler birds. J. Anim. Physiol. N. 2015;99:1094–1104. doi: 10.1111/jpn.12260. [DOI] [PubMed] [Google Scholar]
- Sun Y., Oberley L.W., Li Y. A simple method for clinical assay of superoxide dismutase. Clin. Chem. 1988;34:497–500. [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal. Biochem. 1969;27:502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Wang A.N., Cai C.J., Zeng X.F., Zhang F.R., Zhang G.L., Thacker P.A., Wang J.J., Qiao S.Y. Dietary supplementation with Lactobacillus fermentum I5007 improves the anti-oxidative activity of weanling piglets challenged with diquat. J. Appl. Microbiol. 2013;114:1582–1591. doi: 10.1111/jam.12188. [DOI] [PubMed] [Google Scholar]
- Wang C., Cao S., Zhang Q., Shen Z., Feng J., Hong Q., Lu J., Xie F., Peng Y., Hu C. Dietary tributyrin attenuates intestinal inflammation, enhances mitochondrial function, and induces mitophagy in piglets challenged with diquat. J. Agric. Food Chem. 2019;67:1409–1417. doi: 10.1021/acs.jafc.8b06208. [DOI] [PubMed] [Google Scholar]
- Wang Y.P., Cheng M.L., Zhang B.F., Mu M., Zhou M.Y., Wu J., Li C.X. Effect of blueberry on hepatic and immunological functions in mice. Hepatob. Pancreat. Dis. 2010;9:164–168. [PubMed] [Google Scholar]
- Wang Y.P., Cheng M.L., Zhang B.F., Mu M., Wu J. Effects of blueberry on hepatic fibrosis and transcription factor Nrf2 in rats. World J. Gastroentero. 2010;16:2657. doi: 10.3748/wjg.v16.i21.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Fan C., Wang B., Ma Z., Wang D., Gong B., Di S., Jiang S., Li Y., Li T., Yang Z., Luo E. Pterostilbene attenuates high glucose-induced oxidative injury in hippocampal neuronal cells by activating nuclear factor erythroid 2-related factor 2. Biochim. Biophys. Acta. Mol. Basis Dis. 2017;1863:827–837. doi: 10.1016/j.bbadis.2017.01.005. [DOI] [PubMed] [Google Scholar]
- Yeo S.C., Ho P.C., Lin H.S. Pharmacokinetics of pterostilbene in Sprague-Dawley rats: the impacts of aqueous solubility, fasting, dose escalation, and dosing route on bioavailability. Mol. Nutr. Food Res. 2013;57:1015–1025. doi: 10.1002/mnfr.201200651. [DOI] [PubMed] [Google Scholar]
- Yin J., Liu M., Ren W., Duan J., Yang G., Zhao Y., Fang R., Chen L., Li T., Yin Y. Effects of dietary supplementation with glutamate and aspartate on diquat-induced oxidative stress in piglets. PLoS One. 2015;10:e0122893. doi: 10.1371/journal.pone.0122893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W., Hu X., Wang M. Pterostilbene inhibited advanced glycation end products (AGEs)-induced oxidative stress and inflammation by regulation of RAGE/MAPK/NF-κB in RAW264.7 cells. J. Funct. Foods. 2018;40:272–279. [Google Scholar]
- Yuan D., Hussain T., Tan B., Liu Y., Ji P., Yin Y. The evaluation of antioxidant and anti-inflammatory effects of eucommia ulmoides flavones using diquat-challenged piglet models. Oxid. Med. Cell. Longev. 2017;2017:8140962. doi: 10.1155/2017/8140962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S.B., Chen D.W., Zhang K.Y., Yu B. Effects of oxidative stress on growth performance, nutrient digestibilities and activities of antioxidative enzymes of weanling pigs. Asian-austral. J. Anim. 2007;20:1600–1605. [Google Scholar]
- Zhang C., Zhao X.H., Yang L., Chen X.Y., Jiang R.S., Jin S.H., Geng Z.Y. Resveratrol alleviates heat stress-induced impairment of intestinal morphology, microflora, and barrier integrity in broilers. Poult. Sci. 2017;96:4325–4332. doi: 10.3382/ps/pex266. [DOI] [PubMed] [Google Scholar]
- Zhang C., Wang L., Zhao X.H., Chen X.Y., Yang L., Geng Z.Y. Dietary resveratrol supplementation prevents transport-stress-impaired meat quality of broilers through maintaining muscle energy metabolism and antioxidant status. Poult. Sci. 2017;96:2219–2225. doi: 10.3382/ps/pex004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Li Y., Su W., Ying Z., Zhou L., Zhang L., Wang T. Resveratrol attenuates mitochondrial dysfunction in the liver of intrauterine growth retarded suckling piglets by improving mitochondrial biogenesis and redox status. Mol. Nutr. Food Res. 2017;61:1600653. doi: 10.1002/mnfr.201600653. [DOI] [PubMed] [Google Scholar]
- Zhang L., Cui L., Zhou G., Jing H., Guo Y., Sun W. Pterostilbene, a natural small-molecular compound, promotes cytoprotective macroautophagy in vascular endothelial cells. J. Nutr. Biochem. 2013;24:903–911. doi: 10.1016/j.jnutbio.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Zhou X., He L., Wu C., Zhang Y., Wu X., Yin Y. Serine alleviates oxidative stress via supporting glutathione synthesis and methionine cycle in mice. Mol. Nutr. Food Res. 2017;61:1700262. doi: 10.1002/mnfr.201700262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.