Abstract
Reproduction trait is one of the most important economic traits in poultry industry. This study was aimed to investigate the mRNA expression levels, single nucleotide polymorphisms (SNP) of POMC gene, and the association with reproduction traits in chickens. Five SNP (g.958 G > A, g.1374 G > C, g.1393 G > A, g.1817 C > T, and g.1918G > A) were detected in introns of POMC gene in 317 Zhenning yellow chickens. Association analysis revealed that g.958 G > A and g.1817 C > T showed significantly associations with fertilization rate, hatching rate of hatching eggs, and hatching rate of fertilized eggs in chickens. Simultaneously, g.1374 G > C and g.1918G > A were both associated with egg weight at 300 D of age (P < 0.05). The SNP of g.958 G > A, g.1393 G > A, and g.1817 C > T were all associated with E2 hormone levels (P < 0.05). The result of mRNA expression levels in different tissues showed that POMC mRNA expression level in the pituitary was higher than those in the other tissues and varied in different genotypes. In conclusion, the results in this study provided new evidences that polymorphisms of the POMC gene have potential effects on reproduction traits in chickens. The 5 SNP detected in this study could be potential markers for improving reproduction traits in chickens.
Key words: POMC, polymorphisms, expression, reproduction traits, chicken
Introduction
Reproduction trait is one of the most important economic traits in poultry industry. Studies reported that the reproduction traits were determined not only by environmental factors but also by the genetic components (Xu et al., 2011). In recent years, it was an effective way to improve the important economic traits such as reproduction traits by candidate genes in chicken breeding. It was well known that the hypothalamic–pituitary–gonadal axis played a crucial role in the regulation of egg production (Kuo et al., 2005). Many studies had demonstrated genes in the hypothalamic–pituitary–gonadal axis were significantly associated with reproduction traits in chickens (Cui et al., 2006).
Proopiomelanocortin (POMC) was a polypeptide precursor of several peptide hormones in the pituitary gland such as adrenocorticotropic hormone which stimulates the adrenal cortex to release glucocorticoids (Baker, 1980, Bertagna, 1994). Studies had found that glucocorticoids played an important role in mediating stress, health, survival, and reproduction (Hing et al., 2014). Takeuchi et al. (1999) firstly cloned the chicken POMC gene which had 3 introns. Gerets et al. (2000) found complete POMC transcription could only be detected in the hypothalamus and pituitary, and the expression of the pituitary was higher than that of the hypothalamus. Little, however, is known concerning the polymorphism of avian POMC gene. The function of the POMC gene has not been fully explored. In recent years, more and more studies have found that introns which had no coding function in genes play important biological functions in the regulation of gene expression as well as exons (Casas et al., 2005, Fang et al., 2008). In addition, Sharp et al. (1984) and El Halawani and Rozenboim, (1993) reported that reproductive-related hormones such as prolactin (PRL) was absolutely necessary for poultry spawning and hatching behavior. Li et al. (2011)found that different levels of reproductive hormones such as PRL might regulate the development of ovarian follicular and reduce hen egg-laying performance. Studies above suggested that reproductive hormones played a stimulatory role in different performance traits especially reproduction traits in chickens. Hence, it is particularly important to explore the association between POMC gene polymorphism and hormone levels.
The objective of this study was to investigate the mRNA expression and polymorphisms of the POMC gene and elucidate the association between single nucleotide polymorphisms (SNP) and reproduction traits in chickens. In addition, the association of its polymorphisms with the levels of main reproductive hormones was analyzed for the further study of the function of POMC gene.
Materials and methods
The study was carried out in accordance with the Chinese Animal Welfare Guidelines and approved by the Animal Welfare Committee of the College of Animal Science of Zhejiang University.
Animals and Sample Collection
A total of 317 Zhenning yellow chickens (hens) were selected as experimental animals from Zhenning Poultry Co., Ltd. (Ningbo, China). All birds had free access to feed and water and were kept in single cages to facilitate the statistics of the number of eggs. Blood samples (2 mL) were collected from the wing vein in the blood vessels with 20% EDTA anticoagulant and stored at −20°C used for extraction of DNA for each chicken. Six of the 317 chickens were randomly selected and used for mRNA expression analysis, and 20 different tissues including heart, liver, spleen, lung, kidney, pectoral muscle, leg muscle, muscular stomach, glandular stomach, abdominal fat, ovary, hypothalamus, pituitary, brain, large and white follicle , small and yellow follicle, primary follicular membrane, first order granular film, secondary follicular membrane, and secondary granular film were separately isolated and frozen immediately in liquid nitrogen and stored at −80°C. What's more, 45 individuals of the 317 chickens were selected randomly to extract pituitary tissues, which was used to determine the POMC mRNA expression level in the pituitary tissue of the different genotypes among 5 SNP sites. In addition, 55 chickens of the 317 chickens were randomly selected to measure the reproductive hormone levels.
Egg weight at 300 D of age (EW300) was calculated according to the records of 300 ± 3 D of age. Egg production at 300 D of age (E300) was measured individually during the whole experiment. For the next generation, the fertilization rate (FR), hatching rate of hatching eggs (HEHR), and hatching rate of fertilized eggs (FEHR) were calculated according to the records of eggs which were collected for 1 wk. All the reproduction traits in this study were measured according to the Standards of The Poultry Production Performance Terms and Measurement Statistics Method (NY/T823-2004).
Isolation of Genomic DNA, PCR Amplification, and DNA Sequencing
Genomic DNA was extracted from frozen blood samples using the TIANGEN blood genomic DNA extraction kit (TIANGEN, Beijing, China). The primer pairs (Table1) based on the chicken POMC gene sequences (GenBank NO.NC_006090.5) were designed using Primer Premier 5.0 software (Premier Biosoft International, Palo Alto, CA). PCR was performed in a total volume of 50 μL, which included 25 μL of 2 × Taq PCR MasterMix, 2 μL of each primer, 2 μL genomic DNA, and double-distilled water. The reaction conditions were as follows: 94°C for 5 min, followed by 30 cycles of 94°C for 30 s, 55°C for 45 s, 72°C for 1 min, and a final extension at 72°C for 10 min.
Table 1.
Primer sequences used for amplification of POMC gene.
| Primers | Sequences (5′-3′) | Tm(°C) | Product size (bp) |
|---|---|---|---|
| POMC1-F | TCTCCAAGGGCCATCCAGAG | 59.5 | 1,748 |
| POMC1-R | TCCCTGATTTCCTGGTTTGTC | 55.6 | |
| POMC2-F | AGGCGGAGTGATACCTTGAGC | 59.5 | 984 |
| POMC2-R | CTTCCCTTCCTCTCGTTCCA | 57.4 | |
| POMC3-F | GGAGAGCATCCGCAAGTACG | 59.5 | |
| POMC3-R | TTGGGAGTAACCTATGCTGAAGT | 56.0 | 1,275 |
| POMC4-F | GCTGCCAACCTCCATACCTAATAC | 59.6 | |
| POMC4-R | TGTCATGTAATCCGGGTTTAGG | 55.8 | 1,472 |
Owing to the complexity of POMC gene, fragments were divided into 4 segments in the sequencing process and primers were designed respectively.
The PCR products were sequenced by direct sequencing method in the Qingkezixi Biotechnology co., LTD (Hangzhou, China). The sequencing results were analyzed using the DNAStar software. The discovery of the SNP was conducted using the Seqman software.
RNA Extraction and cDNA Synthesis
Total RNA was extracted using the TRIzol reagent (TIANGEN, Beijing, china). The quality and concentration of the obtained total RNA were checked by the NanoDrop (Thermo Scientific). High-quality RNA samples were then reversely transcribed into first strand cDNA immediately using the PrimeScript RT reagent Kit (Takara). Chicken cDNA samples were stored in −20°C and then used for quantitative PCR (qPCR) analysis.
Real-Time Quantitative PCR
The primers based on the chicken POMC gene sequences (GenBank NO.NC_006090.5) for qPCR were shown in Table 2, and chicken β-actin gene was used as the internal control. The qPCR assays were performed using the Takara SYBR Premix Ex Taq II kits (Takara Bio,China) on a 96-well Applied Biosystems 7,900 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA). The 10 μL reaction mix included 1.0 μL of cDNA template, 5 μL of SYBR PremixExTaq II, 0.4 μL of forward and reverse primers, 0.2 μL of Rox Reference Dye II, and 3 μL of sterile water. Detection of each sample was performed simultaneously 3 times. The procedure for qPCR was as follows: predenaturation at 95°C for 30 s,followed by 40 cycles of 95°C for 5 s, and 60°C for 30 s. The mRNA expression levels were normalized to the chicken β-actin gene and calculated using the 2−ΔΔCt method.
Table 2.
Primers used for real-time quantitative PCR of POMC gene.
| Primers | Sequences (5′-3′) | Tm (°C) | Product size (bp) |
|---|---|---|---|
| POMC-F | GGAGAACAGCAAGTGCCAGGAC | 59 | 53 |
| POMC-R | CACACGCCAAAACACCAGCC | 59 | |
| β-Actin-F | TGCTGTGTTCCCATCTATCG | 60 | 150 |
| β-Actin-R | TTGGTGACAATACCGTGTT | 60 |
Determination of Reproductive Hormone Levels
Serum was isolated by centrifugation of blood at 3,000 × g for 10 min and stored at −20°C until analyses. Levels of follicle-stimulating hormone, estradiol (E2), luteinizing hormone, and PRL were analyzed using the ELISA kits (Zeyu Biological Technology Co., Ltd., Jiangsu, China) according to the recommendations of the manufacturer.
Statistical Analysis
The Excel program was used to calculate genotype frequency, allelic frequencies, heterozygosity, effective allele numbers, and polymorphism information content. Hardy–Weinberg equilibrium was analyzed by the chi-square tests with a significance level of 0.05. Associations between SNP of the POMC gene and reproduction traits and reproductive hormone levels were analyzed using a general linear model procedure in SPSS 21.0.The model was as below:
where Yij is the phenotypic value of traits or reproductive hormone levels, μ is the overall mean, Gi is the fixed effect of genotype, and eij is random error.
Results
Identification of SNP in the POMC Gene
In this study, 5 mutation sites (g.958 G > A, g.1374 G > C, g.1393 G > A, g.1817 C > T, and g.1918G > A) were identified in introns of the POMC gene, whereas g.958 G > A was located in intron1, and g.1374 G > C, g.1393 G > A, g.1817 C > T, and g.1918G > A were in intron 2. Genotypes and allele frequencies and estimated values of population (heterozygosity, effective allele numbers, polymorphism information content) were shown in Table 3. The AA genotype frequencies of 4 SNP (g.958 G > A, g.1374 G > C, g.1393 G > A, and g.1918G > A) were higher than other genotypes. The frequency of AA genotype in g.1817 C > T SNP was lower than other genotypes. The chi-square test showed that all SNP were in agreement with the Hardy–Weinberg equilibrium (P > 0.05).
Table 3.
Genotypes and allele frequencies and diversity parameters of SNP in POMC gene.
| SNP | Genotype frequencies(n) |
Allelic frequencies |
P-value4 | Genetic polymorphism |
|||||
|---|---|---|---|---|---|---|---|---|---|
| AA | AB | BB | A | B | PIC1 | He2 | Ne3 | ||
| g.958 G > A | 0.57 (181) | 0.35 (112) | 0.08 (24) | 0.75 | 0.25 | 0.60 | 0.31 | 0.38 | 1.61 |
| g.1374 G > C | 0.62 (197) | 0.30 (96) | 0.08 (24) | 0.77 | 0.23 | 0.29 | 0.29 | 0.35 | 1.54 |
| g.1393 G > A | 0.75 (240) | 0.20 (62) | 0.05 (15) | 0.85 | 0.15 | 0.25 | 0.22 | 0.25 | 1.33 |
| g.1817 C > T | 0.08 (190) | 0.30 (102) | 0.62 (25) | 0.23 | 0.77 | 0.22 | 0.29 | 0.36 | 1.55 |
| g.1918G > A | 0.61 (194) | 0.31 (97) | 0.08 (26) | 0.77 | 0.23 | 0.29 | 0.29 | 0.36 | 1.56 |
Abbreviation: SNP, single nucleotide polymorphism.
Numbers in parentheses indicated the number of individuals.
PIC: polymorphism information content.
He: gene heterozygosity.
Ne: effective number of alleles.
The test of Hardy–Weinberg equilibrium P-value> 0.05 suggested the population conforms to Hardy–Weinberg equilibrium.
Association Analysis Between POMC SNP and Reproduction Traits in Chickens
The analysis of association between genotypes and reproduction traits in chickens was shown in Table 4. There was no significant difference in the E300 among all SNP in chicken POMC gene (P > 0.05). The SNP of g.958 G > A and g.1817 C > T were associated with the FR, HEHR, and FEHR (P < 0.05). Furthermore, in the SNP g.958 G > A, birds with GG genotype had a greater FR and FEHR than those with AA genotype (P < 0.05). The HEHR of birds with GG genotype was higher than those of which with GA (P < 0.01) and AA genotype (P < 0.05). At the SNP of g.1817 C > T, the HEHR and FEHR of birds with TT genotype were both higher than those with CC genotype (P < 0.05). For the FR, birds with CT and TT genotype had both higher values than those with CC genotype (P < 0.05). At the g.1374 G > C site, significant difference in EW300 was observed between GG and GC genotype (P < 0.05). At the g.1918G > A site, significant difference in EW 300 was also observed between GG and AA genotype (P < 0.05).
Table 4.
Association analysis of SNP in POMC gene with reproduction traits (mean ± SE).
| SNP | Genotypes (n) | 1EW300 (g) | 2E300 (n) | 3FR (%) | 4HEHR (%) | 5FEHR (%) |
|---|---|---|---|---|---|---|
| g.958 G > A | GG (181) | 53.37 ± 0.403 | 86.93 ± 0.227 | 95.99 ± 0.95a | 88.35 ± 1.52A,a | 91.80 ± 1.42a |
| GA (112) | 53.26 ± 0.563 | 86.96 ± 0.295 | 90.85 ± 1.90b | 79.84 ± 2.71B,b | 86.09 ± 2.53b | |
| AA (24) | 53.75 ± 1.250 | 87.17 ± 0.726 | 90.28 ± 3.61a,b | 77.08 ± 5.57b | 86.81 ± 5.40a,b | |
| g.1374 G > C | GG (197) | 52.87 ± 0.393b | 87.04 ± 0.220 | 92.51 ± 1.29 | 83.04 ± 1.83 | 88.79 ± 1.72 |
| GC (96) | 54.43 ± 0.605a | 86.66 ± 0.325 | 95.75 ± 1.32 | 86.20 ± 2.41 | 89.58 ± 2.20 | |
| CC (24) | 53.13 ± 1.077a,b | 87.50 ± 0.626 | 95.83 ± 2.30 | 89.58 ± 3.45 | 93.75 ± 2.98 | |
| g.1393 G > A | GG (240) | 53.46 ± 0.362 | 87.00 ± 0.200 | 94.17 ± 0.97 | 85.17 ± 1.53 | 90.17 ± 1.40 |
| GA (62) | 53.62 ± 0.745 | 86.98 ± 0.398 | 93.28 ± 2.24 | 84.27 ± 3.34 | 88.98 ± 3.11 | |
| AA (15) | 53.15 ± 1.447 | 86.13 ± 0.861 | 88.89 ± 7.03 | 74.44 ± 7.61 | 78.89 ± 7.88 | |
| g.1817 C > T | CC (26) | 52.50 ± 1.047 | 86.42 ± 0.800 | 85.90 ± 5.82b | 74.36 ± 6.62b | 80.77 ± 6.65b |
| CT (96) | 53.44 ± 0.594 | 86.66 ± 0.302 | 94.62 ± 1.38a | 84.37 ± 2.38a,b | 88.98 ± 2.08a,b | |
| TT (195) | 52.44 ± 0.403 | 87.18 ± 0.217 | 94.36 ± 1.06a | 85.90 ± 1.68a | 90.77 ± 1.57a | |
| g.1918G > A | GG (194) | 53.09 ± 0.413b | 86.89 ± 0.228 | 94.76 ± 1.11 | 85.44 ± 1.77 | 89.48 ± 1.66 |
| GA (97) | 53.30 ± 0.553a,b | 86.88 ± 0.320 | 92.35 ± 1.63 | 83.08 ± 2.44 | 89.78 ± 2.14 | |
| AA (26) | 55.58 ± 1.050a | 87.77 ± 0.465 | 91.35 ± 4.30 | 82.69 ± 5.17 | 87.50 ± 5.13 |
a,b,A,BMeans of different genotypes at the same locus with the different uppercase letters were extremely significant (P < 0.01), and the different lowercase letters was significant (P < 0.05); The difference between the same letters was not significant (P > 0.05).
Means without superscript was not significant (P > 0.05).
Numbers in parentheses indicated the number of individuals.
Abbreviation: SNP, single nucleotide polymorphism.
EW300 = egg weight at 300d of age.
E300 = egg production at 300d of age.
FR = fertilization rate.
HEHR = hatching rate of hatching eggs.
FEHR = hatching rate of fertilized eggs.
Association Analysis Between POMC SNP and Reproductive Hormone Levels in Chickens
As shown in Table 5, there were significant differences in E2 level (P < 0.05) among g.958 G > A, g.1393 G > A, and g.1817 C > T. There was, however, no significant difference in the follicle-stimulating hormone, luteinizing hormone, and PRL levels across all polymorphic sites (P > 0.05).
Table 5.
Association analysis of SNP in POMC gene with reproductive hormone levels in chickens (mean ± SE).
| SNP | Genotypes | FSH(U/L) | LH (ng/L) | PRL (ng/L) | E2 (pmol/L) |
|---|---|---|---|---|---|
| g.958 G > A | GG (32) | 13.32 ± 1.47 | 47.63 ± 1.51 | 151.56 ± 21.43 | 78.81 ± 3.18a |
| GA (21) | 10.69 ± 0.92 | 44.57 ± 1.41 | 106.18 ± 11.39 | 65.73 ± 5.41b | |
| AA (2) | 9.48 ± 1.72 | 36.48 ± 3.60 | 94.70 ± 20.35 | 78.78 ± 3.04a,b | |
| g.1374 G > C | GG (34) | 12.70 ± 1.28 | 45.23 ± 1.37 | 141.87 ± 19.67 | 74.46 ± 3.70 |
| GC (17) | 11.65 ± 1.60 | 47.52 ± 1.84 | 112.25 ± 16.76 | 73.88 ± 5.06 | |
| CC (4) | 9.96 ± 1.50 | 46.82 ± 5.33 | 134.32 ± 40.06 | 68.01 ± 13.05 | |
| g.1393 G > A | GG (37) | 11.56 ± 1.03 | 45.46 ± 1.34 | 125.40 ± 13.88 | 69.97 ± 3.71b |
| GA (16) | 13.18 ± 2.08 | 47.28 ± 2.05 | 139.49 ± 32.02 | 79.18 ± 3.85a,b | |
| AA (2) | 15.50 ± 6.18 | 47.28 ± 0.14 | 198.66 ± 113.27 | 101.97 ± 7.18a | |
| g.1817 C > T | CC (7) | 9.42 ± 0.62 | 49.44 ± 3.84 | 77.99 ± 9.62 | 58.16 ± 7.04b |
| CT (12) | 14.68 ± 2.32 | 47.40 ± 2.18 | 154.79 ± 30.85 | 69.79 ± 6.31a,b | |
| TT (36) | 11.88 ± 1.17 | 44.95 ± 1.28 | 135.15 ± 17.44 | 78.20 ± 3.41a | |
| g.1918G > A | GG (39) | 12.80 ± 1.23 | 46.51 ± 1.39 | 140.20 ± 17.57 | 74.32 ± 3.37 |
| GA (12) | 10.62 ± 0.99 | 43.69 ± 1.29 | 101.43 ± 13.60 | 67.12 ± 6.75 | |
| AA (4) | 10.69 ± 3.72 | 48.69 ± 1.85 | 146.02 ± 59.96 | 88.93 ± 4.84 |
a,b,A,BMeans of different genotypes at the same locus with the different uppercase letters were extremely significant (P < 0.01), and the different lowercase letters was significant (P < 0.05); The difference between the same letters was not significant (P > 0.05).
Means without superscript was not significant (P > 0.05).
Abbreviations: E2, estradiol; FSH, follicle-stimulating hormone; LH, luteinizing hormone; PRL, prolactin; SNP, single nucleotide polymorphism.
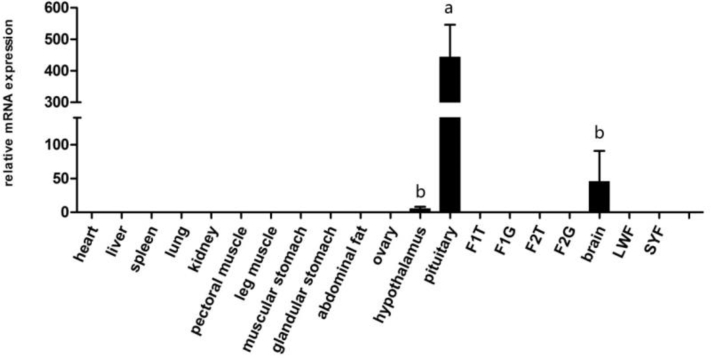
Relative Expression Levels of Chicken POMC mRNA in 20 Tissues
The POMC gene expression in different tissues was investigated (Figure1). The results showed that the expression level of the POMC gene mRNA varied in different tissues. Chicken POMC gene was expressed only in the pituitary, brain, and hypothalamus, while no expression in other tissues. The highest mRNA expression level was observed in the pituitary.
Figure 1.
Relative expression levels of the POMC gene in different tissues of chickens. Relative mRNA expression levels were normalized with β-actin mRNA. Data represent mean ± SD. Bars with different lowercase letters were significantly different (P < 0.05). The difference between the same letters was not significant (P > 0.05). Means without lowercase was not significant (P > 0.05). LWF, large and white follicle; SYF, small and yellow follicle.
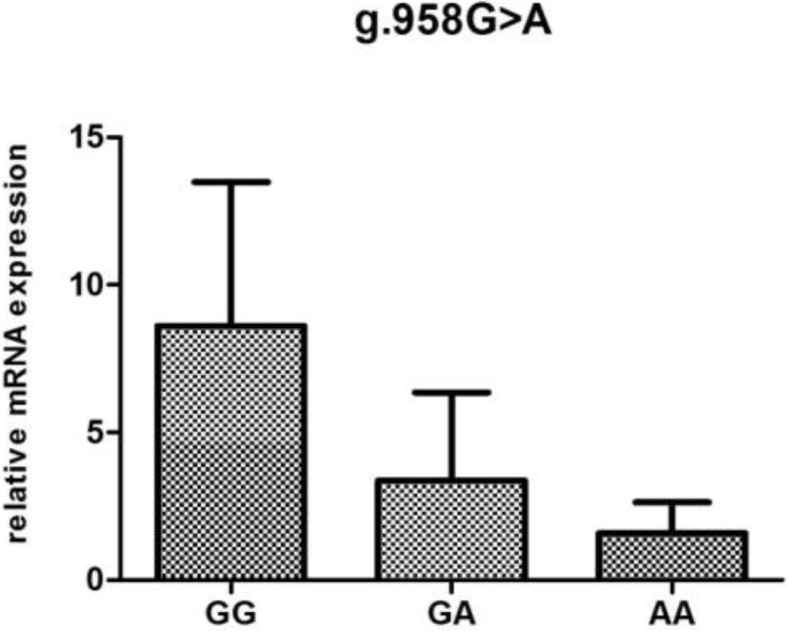
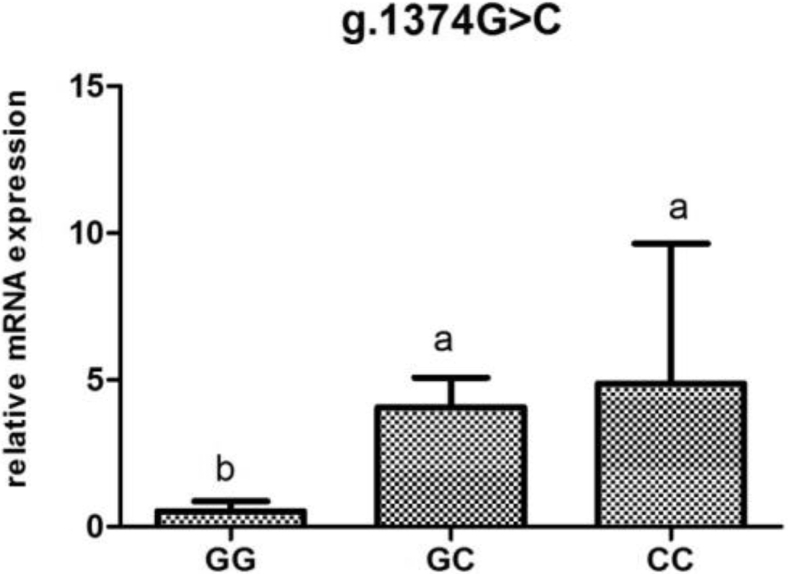
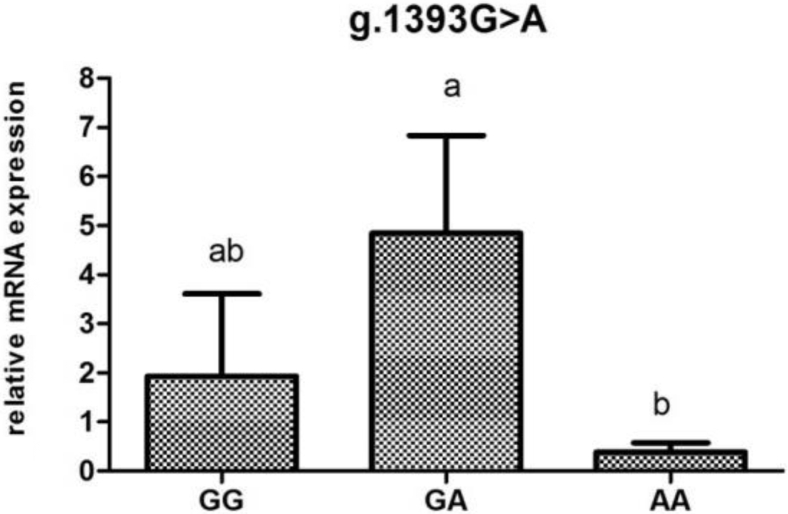
POMC mRNA Expression Analysis According to Genotype
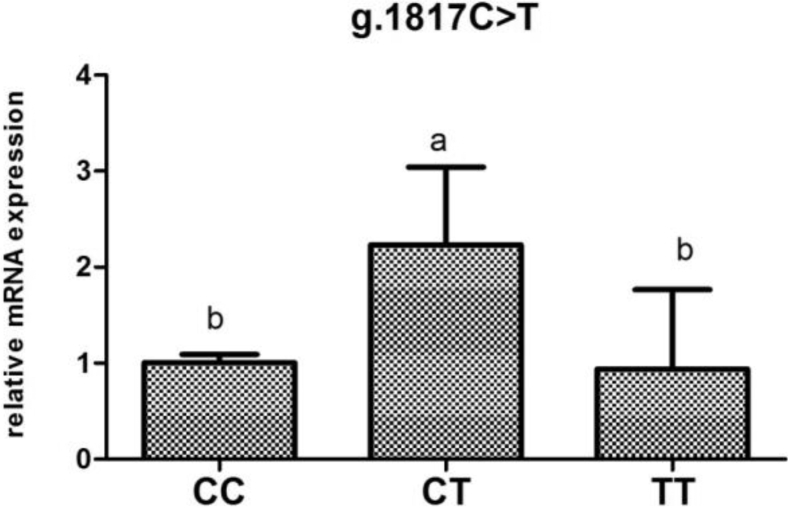
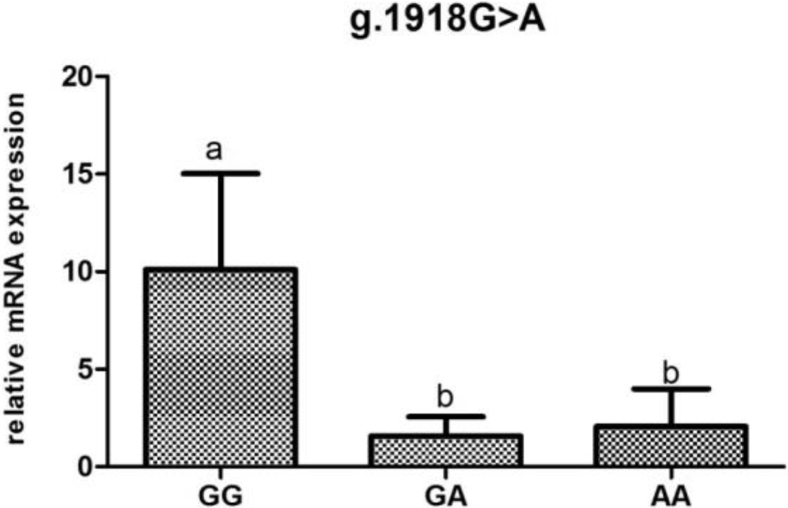
To investigate the association of mRNA levels with reproductive traits and reproductive hormones, the POMC mRNA expression were determined according to genotypes at different loci (Figure 2, Figure 3, Figure 4, Figure 5, Figure 6).The POMC gene mRNA expression in the pituitary in g.1374 G > C was significantly higher in the GC and CC genotype than that in the GG genotype (P < 0.05). In the SNP of g.1393 G > A, the POMC gene mRNA expression level in the pituitary in the GA genotype was higher than that in the AA genotype (P < 0.05). In the g.1817 C > T, the individuals with CT genotype had higher mRNA expression level than those with CC and TT genotype (P < 0.05). Meanwhile, for the g.1918G > A, the relative mRNA expression level in the GG genotype was higher than in the GA and AA genotype (P < 0.05).
Figure 2.
Relative expression of the POMC gene in the pituitary tissue according to genotype in g.958 G > A in chickens. Relative mRNA expression levels were normalized with β-actin mRNA. Data represent mean ± SD. Bars with different lowercase letters were significantly different (P < 0.05). The difference between the same letters was not significant (P > 0.05). Means without lowercase was not significant (P > 0.05).
Figure 3.
Relative expression of the POMC gene in the pituitary tissue according to genotype in g.1374 G > C in chickens. Relative mRNA expression levels were normalized with β-actin mRNA. Data represent mean ± SD. Bars with different lowercase letters were significantly different (P < 0.05). The difference between the same letters was not significant (P > 0.05). Means without lowercase was not significant (P > 0.05).
Figure 4.
Relative expression of the POMC gene in the pituitary tissue according to genotype in g.1393 G > A in chickens. Relative mRNA expression levels were normalized with β-actin mRNA. Data represent mean ± SD. Bars with different lowercase letters were significantly different (P < 0.05). The difference between the same letters was not significant (P > 0.05). Means without lowercase was not significant (P > 0.05).
Figure 5.
Relative expression of the POMC gene in the pituitary tissue according to genotype in g.1817 C > Tin chickens. Relative mRNA expression levels were normalized with β-actin mRNA. Data represent mean ± SD. Bars with different lowercase letters were significantly different (P < 0.05). The difference between the same letters was not significant (P > 0.05). Means without lowercase was not significant (P > 0.05).
Figure 6.
Relative expression of the POMC gene in the pituitary tissue according to genotype in g.1918G > A in chickens. Relative mRNA expression levels were normalized with β-actin mRNA. Data represent mean ± SD. Bars with different lowercase letters were significantly different (P < 0.05).The difference between the same letters was not significant (P > 0.05). Means without lowercase was not significant (P > 0.05).
Discussion
The studies about identification of chicken genes and their relationship to economic traits are increasing as the selection of alleles for genes that improve economics contributes to the choice of poultry (Jin et al., 2016, Wang et al., 2017, Wang et al., 2018). The previous study had detected 2 silent SNP mutations in chicken POMC gene and reported that the c.C495 T mutation was significantly associated with the chicken production traits (P < 0.05) (Bai et al., 2012).Seong and Kong, (2015) demonstrated that the POMC polymorphism significantly influenced the longissimus dorsi muscle area and marbling scores in chickens. There was, however, few researches about associations of the POMC gene polymorphisms with reproduction traits in chickens. In the previous study conducted by our group, several mutations in the exons of chicken POMC gene were identified, which were significantly associated with reproduction traits in chickens (unpublished data). Further analysis conducted in this study allowed us to detect several mutations in the introns of chicken POMC gene.
In the current study, 5 SNP were detected and SNP with very low frequency were discarded in genotype quality control and data filtering (Table 3). Association analysis revealed that g.958 G > A and g.1817 C > T showed significantly associations with FR, HEHR, and FEHR in chickens. Simultaneously, g.1374 G > C and g.1918G > A were both significantly associated with EW300 (P < 0.05) (Table 4).Moreover, we evaluated the association analysis between POMC SNP and reproductive hormones in chickens. The analysis revealed g.958 G > A, g.1393 G > A, and g.1817 C > T were all significantly associated with E2 hormone level (P < 0.05;Table 5). Wang et al. (2013) reported the exon 3 of POMC gene was associated with growth traits in Hu sheep. Xu et al. (2016) demonstrated that 2 mutation sites had significant correlation with body length of Guanling cattle (P < 0.05).Few studies reported the association between POMC gene polymorphism and reproductive traits in chickens. The current study is the first to demonstrate the association between POMC gene intron polymorphisms and chicken reproduction traits.
The function of chicken POMC gene in reproduction was further confirmed by analyzing the mRNA expression pattern in the study. We found levels of expression in the pituitary of chickens were higher than those in the other tissues. This result was found to be consistent with those of previous studies to some extent (Civelli et al., 1982, Berghman et al., 1998, Gerets et al., 2000). However, Noy et al. (2017)found POMC mRNA was further detected in heart, with additional very low expression in other organs. In addition, the POMC gene expression also had been detected in the gonads (Chen et al., 1986, Facchinetti et al., 1988, Gallinelli et al., 1995). This difference indicated that POMC gene might function with different levels as species or phase vary. Furthermore, we calculated mRNA expression level according to the genotype in SNP. For the g.958 G > A, individuals carrying GG genotype had the highest relative gene expression level, and those with GA and AA genotype were lower. In addition, the former had the highest values of FR, HEHR, FEHR, and the highest level of E2 hormone. For the g.1374 G > C, the mRNA expression level and EW300 of individuals with GC genotype and CC genotype were significant higher than those of individuals with GG genotype (P < 0.05). However, in the g.1918G > A, chickens with the highest expression level in AA genotype had the lowest values of EW300. These results indicated that different genotypes might have a diverse influence on the chicken. Together with the results of previous studies and the outcome of association analysis in the present investigation, we suggest that the expression of this gene might have an important impact on reproduction traits in chickens.
Moreover, the results from the association analysis implied that 5 polymorphic sites detected in this study could be used as genetic markers notably affecting the reproduction traits in chickens. Along with our previous studies, we herein provided important information on association of key candidate genes with reproduction traits and reproductive hormones in chickens.
In conclusion, our study provided evidences that polymorphisms of POMC gene have potential effects on reproduction traits of chickens. In addition, more studies on the polymorphisms and function of chicken POMC gene are required to improve chicken reproduction traits.
Acknowledgments
The research was supported by the Fundamental Research Funds for the Central Universities (No. 2019XZZX006-01) and the Major Science and Technology Projects of Zhejiang Province: New Variety Breeding of Livestock and Poultry (No. 2016C02054-15).
Conflict of Interest Statement: The authors did not provide any conflict of interest statement.
References
- Bai Y., Sun G., Kang X., Han R., Tian Y., Li H., Wei Y., Zhu S. Polymorphisms of the pro-opiomelanocortin and agouti-related protein genes and their association with chicken production traits. Mol. Biol. Rep. 2012;39:7533–7539. doi: 10.1007/s11033-012-1587-y. [DOI] [PubMed] [Google Scholar]
- Baker B. The evolution of ACTH, MSH, and LPH structure, function, and development. Horm.Evol. 1980;2:643–722. [Google Scholar]
- Bertagna X. Proopiomelanocortin-derived peptides. Endocrinol.Metab.Clin.North. Am. 1994;23:467–485. [PubMed] [Google Scholar]
- Berghman L.R., Devreese B., Verhaert P., Gerets H., Arckens L., Broeck J.V., Beeumen J.V., Vaudry H., Vandesande F. The molecular characterisation of chicken pituitary N-terminal pro-opiomelanocortin (POMC) Mol. Cel. Endoc. 1998;142:119. doi: 10.1016/s0303-7207(98)00112-9. [DOI] [PubMed] [Google Scholar]
- Casas E., White S.N., Riley D.G., Smith T.P., Brenneman R.A., Olson T.A., Johnson D.D., Coleman S.W., Bennett G.L., Chase C.J. Assessment of single nucleotide polymorphisms in genes residing on chromosomes 14 and 29 for association with carcass composition traits in Bosindicus cattle. J. Anim. Sci. 2005;83:13–19. doi: 10.2527/2005.83113x. [DOI] [PubMed] [Google Scholar]
- Chen C.L., Chang C.C., Krieger D.T., Bardin C.W. Expression and regulation of proopiomelanocortin-like gene in the ovary and placenta: comparison with the testis. Endocrinology. 1986;118:2382–2389. doi: 10.1210/endo-118-6-2382. [DOI] [PubMed] [Google Scholar]
- Civelli O., Birnberg N., Herbert E. Detection and quantitation of pro-opiomelanocortin mRNA in pituitary and brain tissues from different species. J. Biol. Chem. 1982;257:6783–6787. [PubMed] [Google Scholar]
- Cui J.X., Du H.L., Liang Y., Deng X.M., Li N., Zhang X.Q. Association of polymorphisms in the promoter region of chicken prolactin with egg production. Poult. Sci. 2006;85:26–31. doi: 10.1093/ps/85.1.26. [DOI] [PubMed] [Google Scholar]
- El Halawani M.E., Rozenboim I. The Ontogeny and control of Incubation Behavior in Turkeys. Poult. Sci. 1993;72:906–911. [Google Scholar]
- Facchinetti F., Storchi A.R., Petraglia F., Volpe A., Genazzani A.R. Expression of proopiomelanocortin-related peptides in human follicular fluid. Peptides. 1988;9:1089–1092. doi: 10.1016/0196-9781(88)90094-0. [DOI] [PubMed] [Google Scholar]
- Fang H., Wang A., Gao B., Sun H. The regulatory effect of the first intron and 3'-regulatory region of ovalbumin gene on transgene expression. Chin. J. Biotechnol. 2008;24:333–338. [PubMed] [Google Scholar]
- Gallinelli A., Garuti G., Matteo M.L., Genazzani A.R., Facchinetti F. Expression of pro-opiomelanocortin gene in human ovarian tissue. Hum. Reprod. 1995;10:1085–1089. doi: 10.1093/oxfordjournals.humrep.a136099. [DOI] [PubMed] [Google Scholar]
- Gerets H.H., Peeters K., Arckens L., Vandesande F., Berghman L.R. Sequence and distribution of pro-opiomelanocortin in the pituitary and the brain of the chicken (Gallus gallus) J. Comp. Neurol. 2000;417:250–262. [PubMed] [Google Scholar]
- Hing S., Narayan E., Thompson R.C., Godfrey S. A review of factors influencing the stress response in Australian marsupials. Conserv Physiol. 2014;2:u27. doi: 10.1093/conphys/cou027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S., Moujahid E.M., Duan Z., Zheng J., Qu L., Xu G., Yang N., Chen S. Association of AMPK subunit gene polymorphisms with growth, feed intake, and feed efficiency in meat-type chickens. Poult. Sci. 2016;95:1492–1497. doi: 10.3382/ps/pew081. [DOI] [PubMed] [Google Scholar]
- Kuo Y.M., Shiue Y.L., Chen C.F., Tang P.C., Lee Y.P. Proteomic analysis of hypothalamic proteins of high and low egg production strains of chickens. Theriogenology. 2005;64:1490–1502. doi: 10.1016/j.theriogenology.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Li W.L., Liu Y., Yu Y.C., Huang Y.M., Liang S.D., Shi Z.D. Prolactin plays a stimulatory role in ovarian follicular development and egg laying in chicken hens. Domest. Anim. Endocrinol. 2011;41:57–66. doi: 10.1016/j.domaniend.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Noy E.B., Scott M.K., Grommen S.V.H., Robert K.A., Groef B.D. Molecular cloning and tissue distribution of Crh and Pomc mRNA in the fat-tailed dunnart( Sminthopsiscrassicaudata ), an Australian marsupial. Gene. 2017;627:26–31. doi: 10.1016/j.gene.2017.06.004. [DOI] [PubMed] [Google Scholar]
- Seong J., Kong H.S. Association between polymorphisms of the CRH and POMC genes with economic traits in Korean cattle (Hanwoo) GMR. 2015;14:10415–10421. doi: 10.4238/2015.September.8.2. [DOI] [PubMed] [Google Scholar]
- Sharp P.J., MacNamee M.C., Talbot R.T., Sterling R.J., Hall T.R. Aspects of the neuroendocrine control of ovulation and broodiness in the domestic hen. J. Exp. Zool. 1984;232:475–483. doi: 10.1002/jez.1402320314. [DOI] [PubMed] [Google Scholar]
- Takeuchi S., Teshigawara K., Takahashi S. Molecular cloning and characterization of the chicken pro-opiomelanocortin (POMC) gene. BiochimBiophysActa. 1999;1450:452–459. doi: 10.1016/s0167-4889(99)00046-4. [DOI] [PubMed] [Google Scholar]
- Wang Y., Guo F., Qu H., Luo C., Wang J., Shu D. Associations between variants of bone morphogenetic protein 7 gene and growth traits in chickens. Br. Poult. Sci. 2018;59:264–269. doi: 10.1080/00071668.2018.1454586. [DOI] [PubMed] [Google Scholar]
- Wang Y.C., Han R.L., Li Z.J., Geng J., Tian Y.D., Jiang R.R., Wu J.P., Kang X.T. Polymorphisms of Flanking region of the ASB15 gene and their associations with performance traits in chicken. Anim. Biotechnol. 2017;28:53–60. doi: 10.1080/10495398.2016.1200986. [DOI] [PubMed] [Google Scholar]
- Wang C.L., Meng C.L., Cao S.X., Zhang J., Meng C.H., Wang H.L., Fang Y.F., Zhu D.D., Mao D.G. Single nuclear polymorphisms in exon 3 of POMC gene and the association with growth traits in Hu sheep and East Friesian x Hu crossbred sheep. Hereditas (Beijing) 2013;35:1095–1100. doi: 10.3724/sp.j.1005.2013.01095. [DOI] [PubMed] [Google Scholar]
- Xu H., Zeng H., Luo C., Zhang D., Wang Q., Sun L., Yang L., Zhou M., Nie Q., Zhang X. Genetic effects of polymorphisms in candidate genes and the QTL region on chicken age at first egg. BMC Genet. 2011;12:33. doi: 10.1186/1471-2156-12-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L.X., Zhu L.L., Zhang L., Liu J., He G.Z. Polymorphism of POMC gene in Guanling cattle and its association with growth traits. Sw. J. Agric. Sci. (China) 2016;29:451–454. [Google Scholar]