Abstract
Purpose of review
To systematically review the available research studies that characterize the benefits, uncertainty, or weaknesses of commercially-available sleep tracking technology.
Recent findings
Sleep is a vital component of health and well-being. Research shows that tracking sleep using commercially available sleep tracking technology (e.g., wearable or smartphone-based) is increasingly popular in the general population.
Methods
Systematic literature searches were conducted using PubMed/Medline, Embase (Ovid) the Cochrane Library, PsycINFO (Ovid), CINAHL, and Web of Science Plus (which included results from Biosis Citation Index, INSPEC, and Food, Science & Technology Abstracts) (n=842).
Study Inclusion and Exclusion Criteria
Three independent reviewers reviewed eligible articles that administered a commercially-available sleep tracker to participants and reported on sleep parameters as captured by the tracker, including either sleep duration or quality. Eligible articles had to include sleep data from users for >=4 nights.
Keywords: Sleep tracking, health promotion, sleep research, mobile technology, information technology
Summary
Seven articles met criteria for review. A wearable sleep tracker (e.g., wrist-based) was utilized to track sleep in 5 of the 7 studies, a smartphone-based sleep tracker app was used to record sleep in 2 of the 7 studies. Studies in this review may be characterized in several broad categories, including studies that examined: sleep before and after a clinical procedure (e.g., surgery) (2 studies); 2) sleep and a health-related outcome (e.g., asthma symptoms (2 studies); 3) the relationship between sleep tracker data and self-reported sleep (1 study); and 4) sleep tracker data before and after major political events (1 study). Among the studies examining sleep-tracker data and health-related outcomes, sleep-tracker data was associated with health outcomes, including asthma symptoms, blood pressure, and mood.
INTRODUCTION
Sleep is a vital component of general health and well-being [1–3]. Despite its importance, national survey data from the United States (U.S.) show that only 25-50% of adults obtain the recommended 7-8 hours [4,5], and between 20-35% of adults report consistent sleep difficulties [6,7]. Global estimates of sleep show that sleep difficulties are reported by 10-40% of adults sampled, highlighting that poor sleep health is a global as well as a local issue [8]. The explosion and proliferation of technologies for sleep tracking has heightened awareness about sleep and whether sleep affects health and functional outcomes either from provided digital or derived personal insights. Although sleep tracking appears to be commonly practiced in the general population [13], an essential question remains: is sleep tracking beneficial or harmful for sleep health and health outcomes? We conducted a systematic review of the research that monitored sleep using tracking devices (e.g., (e.g. FitBit, Nike+, Jawbone, Garmin).
Sleep-tracking technologies, either in the form of a wearable sensor or smartphone-based software application (app), are powerful technologies that have become increasingly small and user-friendly [9], such that ownership of these devices has sharply increased in recent years across the globe. According to PEW Center research, 35% of individuals in the U.S. owned a smartphone in 2011 and 77% reported ownership of a smartphone device in 2017 [10]. This research also found smartphone ownership worldwide was as high as 99% in some countries in South Korea, while only 50% among developing nations. Health-behavior tracking, such as activity and sleep, are two of the most widely tracked behaviors on digital devices [12,13].
In fact, there are over 100,000 smartphone-based apps for health purposes that are available to consumers [13]. According to a geographically-representative study of adults in the U.S., approximately 26% of respondents indicated that they use their smartphone or a mobile, wearable device to track their sleep [13]. Furthermore, this study also found those who reported sleep tracking also high self-reported health.
The Centers for Disease Control (CDC) Healthy People 2020 goals include a call for technology to improve population health [9]. Although mobile technologies capable of tracking sleep are increasingly popular, there have not been reviews of the published literature to characterize the landscape of studies including commercially-available sleep trackers and their outcomes. We conducted a systematic review aiming at characterizing available evidence from studies that used commercially-available sleep trackers.
METHODS
The systematic literature search and review of the literature is based on the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines. Our objective was to review studies that included participants who wore commercially-available sleep trackers.
Eligibility Criteria
Assessment and extraction were based on the following inclusion criteria: 1) the study had to feature an epidemiological study design (i.e. randomized control trial, cohort study, etc.); 2) results of the study had to be based on a commercially-available sleep-tracking device(s), conducted via a smartphone or wearable device; 3) sleep measurements including duration or sleep quality had to be captured by the device (i.e., not self-reported); and 4) the device had to be worn for 4 days or longer.
Exclusion Criteria
We excluded articles based on the following criteria: 1) studies that asked participants to self-report their sleep on an app or other device; 2) studies that measured sleep for less than 4 days (i.e., studies whose primary aim was to validate a sleep tracking device); 3) studies that used research grade sleep measurement (e.g., actigraphy); and 4) studies that were conference proceedings, which were excluded for lacking complete data.
Search Strategy
With significant input from the review team (RR, LWM, FD, NC) a medical librarian (DV) trained in the systematic review process conducted a literature search in PubMed/Medline, Embase (Ovid) the Cochrane Library, PsycINFO (Ovid), CINAHL, and Web of Science Plus (which included results from Biosis Citation Index, INSPEC, and Food, Science & Technology Abstracts). In addition, the New York Academy of Medicine Grey Literature database was searched, and a bibliography review of key articles was conducted. The databases were not limited by year. Articles were not limited to English language.
Medical Subject Headings (MeSH), Embase’s EMTREE and additional thesauri were consulted, and keywords were used to construct a comprehensive search strategy that could be run across all databases. The various strategies included the following concepts: sleep tracking, sleep, polysomnography, behavior and devices (see Appendix for complete strategies) and the PRISMA flow diagram (Figure 1) for details regarding the screening, assessment and extraction processes in this review.
Figure 1.
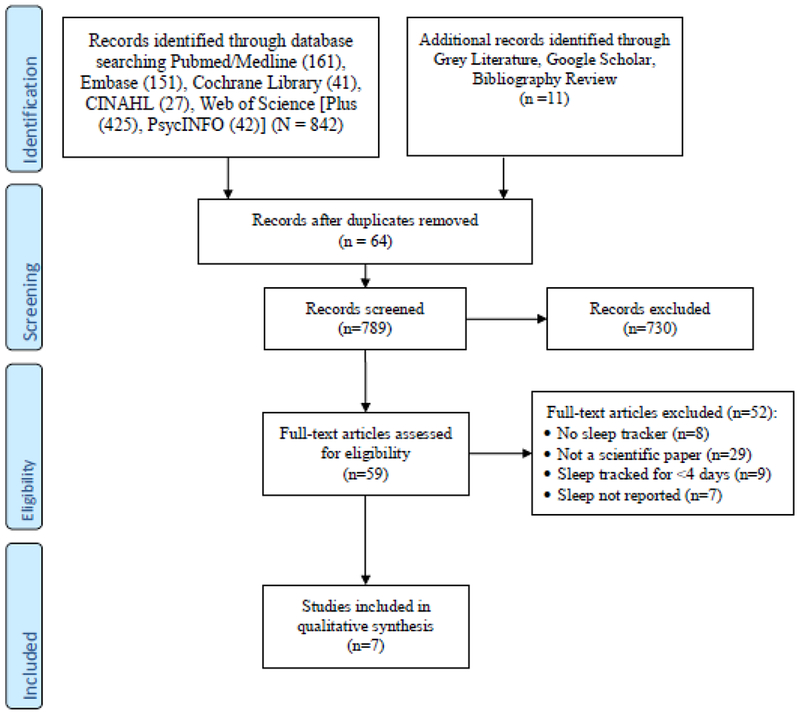
PRISMA diagram.
Data Screening, Assessment and Extraction
Bibliographic data was managed using EndNote (including removal of duplicates). Data was exported from EndNote to Covidence, a systematic review management web-based product, and all screening was conducted via Covidence. In the screening phase, two trained, experienced coders (LWM and FM) screened titles and abstracts after in-depth training. Weekly meetings were held to resolve potential problems and address any questions regarding the process. The first author (RR) resolved all screening discrepancies. Citations included for assessment and extraction were downloaded from Covidence back to EndNote for retrieval of full text. All included citations were also downloaded from EndNote to Excel for assessment and extraction.
In the full text extraction phase, three assessment questions were included before moving to extraction: 1) was sleep measured by a commercially-available sleep-tracking device (smartphone or mobile, wearable sensor); 2) was sleep measured for four (4) or more days; and 3) was sleep data collected from the sleep tracker(s)? If all three questions did not receive a YES, they were excluded from extraction with the note “did not meet inclusion criteria.” The extraction criteria included the following variables: study design, length of study, demographic variables of study participants (i.e., age, race, income, education, health conditions), number of participants, primary outcome endpoints (e.g., 3 months, 6 months, etc.), sleep-tracking device(s), how sleep was measured, time frame of sleep duration (e.g., week average, sleep duration, e.g., hours or minutes, sleep quality or awakenings), timeframe of measurement, and reason for exclusion if assessment categories were met but data was not adequate for final inclusion. LWM, FM and NC assessed and extracted based on the variables reported above. All extractions were reviewed and confirmed by RR.
Extraction Procedures
The coders extracted the demographic characteristics as provided in the published article. Demographic characteristics included age, sex, and race/ethnicity. Study characteristics were extracted, including study design (e.g., Randomized Controlled Trial), and length of follow-ups. Sleep tracker details are recorded, including manufacturer (e.g., FitBit) and classified the tracker as a smartphone-based app or wearable sensor. Sleep health parameters extracted were those data points captured by the sleep tracker over time. Sleep health data could include sleep duration, quality, or other parameters pertaining to sleep as captured by the sleep tracker.
Study Quality Evaluation and Data Analysis
The quality of the studies included in the systematic review was assessed using the Downs and Black checklist [19]. The Downs and Black Checklist is a 27-item scoring system assessing the following domains: reporting, external validity, internal validity/bias, internal validity/confounding, and power. While there is some disagreement on how to use the quality scores from the Downs and Black approach [20], we used a quality rating that determined 21 (80.8%) and higher as high quality, 11 – 20 (42.4-80.8%) moderate quality, and 10 or lower as poor quality (<42.2%) [21]. Quality ratings were determined for the studies in this systematic review independently by two reviewers (NC, LWM). Discrepancies were adjudicated through discussion until consensus with coauthors was reached. We qualitatively summarized the study characteristics, participant characteristics, and sleep-tracking characteristics of the eligible articles. In this report, we document changes in sleep health data captured by the tracking device over time.
RESULTS
Characteristics of the Study Populations
The characteristics of the study samples are shown in Table 1. Sample size ranged widely from 22 [22] individuals to 37,054 [23]. We note that individual ages were not available for two studies. Participants’ ages in the remaining five studies were as follows: one study that recruited younger adults showed an average age of 16 years [22] and another one enrolling older adults showed that 39% of the sample were 65 years of age or older. All but one study reported participants’ sex, and all study samples included more males than females, with the exception of one study where female participants exceeded the number of male participants [24]. Only three studies provided the race/ethnic breakdown of participants, including a study that recruited white (55%) and black (45%) participants [22]; another showed 24% were white and 75%, black [25]; yet, another one showed 93% were white, and 7%, other [24].
Table 1.
Sample characteristics of participants in the eligible studies (n=7).
| First Author | Year | N | Age (SD) | Males | Females | Race/Ethnicity | Study Design | Study Duration | Population | Sleep Duration Measured | Secondary Measure |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fagherazzi et al. | 2017 | 15,839 | 13658 (86.1%) | 2181 (13.5%) | - | Cross sectional | 7 | General population | Hours, minutes | Deep/Light Sleep | |
| Bian et al. | 2017 | 22 | 15.5 years (1.1) | 12 (55%) | 10 (45%) | White: (55%) Black: (32%) Other: (13%) |
Prospective observational study | 56 | Pediatric asthma patients | Hours, minutes | Quality |
| Anyz et al. | 2019 | 37,054 | - | - | - | - | Cross sectional study | 14 | General population | Hours, minutes | N/A |
| Agarwal, et al. | 2018 | 46 | <65 years: 28 (61.0%) >54 years: 18 (39.0%) |
46(100%) | 0(0) | - | Cross sectional study | 7 | Prostate cancer patients | Hours, minutes | Awakenings |
| Han et al. | 2016 | 29 | 53 years (11.0) | 16 ( 55%) | 13 (45%) | Black: 22 (76%) White: 7 (24%) |
RCT | 35 | Hemodialysis patients | Hours, minutes | Efficiency |
| Weatherall et al. | 2018 | 86 | 54.3 years (13.3) | 36 (44%) | 50 (58%) | White: 80 (93%) | Cross sectional study | 14 | Type 2 diabetes patients | Hours, minutes | N/A |
| DeMasi et al. | 2017 | 53 | 20.08 years (1.8) | 29 (57%) | 22 (43%) | - | Prospective observational study | 56 | General population | Hours, minutes | N/A |
Characteristics of Included Studies
Among the studies we examined, cross-sectional design was the most common (n=4, 57.1%) [23,24,26,27], followed by prospective observational studies (n=2, 28.6%) [22,28], and randomized controlled trial (RCT) (n=1, 14.3%) [25]. Study durations ranged from 7 days [26,27] to 56 days [22,28]. No studies had a second follow up. All studies included individuals from the general population (n=3, 42.9%). The remaining studies administered commercially available trackers to individuals with chronic conditions (n=4, 57.1%). The studies recruiting individuals with chronic conditions included asthma [22], prostate cancer [27], hemodialysis [25], and type 2 diabetes [24]. All included studies but one measured sleep duration, four studies also measured additional sleep parameters, such as ratio of deep sleep to short sleep [26], sleep quality [22], awakenings [27], and sleep efficiency [25].
Characteristics of the Sleep Trackers and Sleep Data
Characteristics of the data from sleep trackers and outcomes of the included studies are shown in Table 2. Among the included articles, 71.4% administered a wearable sleep tracker (e.g., wrist-based), and 28.6% (n=2) administered a smartphone application. Among the sleep trackers, 57.1% (n=4) were FitBit, 14.3% (n=1) were Withings, and the remaining two studies (28.6%) used smartphone-based apps ‘Sleep as Android’ and ‘Funf.’ The objectives of the studies included in this review were to compare sleep before and after a treatment procedure [25,27], examine the relationship between sleep and a health-related outcome [22,26,28], and contrast sleep duration (total sleep time) tracked by wearable versus self-reported data [24].
Table 2.
Characteristics of the sleep trackers and sleep parameters measured (n=7).
| First Author | Year | Sleep Tracker | Study Objective | Results | Take-Away | Quality Rating |
|---|---|---|---|---|---|---|
| Fagherazzi | 2017 | Wearable (Withings Pulse) | Examine sleep and health among users of a popular sleep tracker. | A larger proportion of the population slept > 6 hours (n=11,670, 73.4%), whereas fewer averaged <6 hours (n=4,169, 26.3%). A high diastolic blood pressure (>83 mm Hg) was associated with increased risk of poor deep sleep (OR 1.21, 95% CI 1.06-1.39) when compared to low diastolic blood pressure. but was not related to total sleep and deep/total ratio. Systolic blood pressure was not related to total sleep or deep sleep but was associated positively with a poor deep/total ratio (p-value<.001). | Sensed deep sleep was associated with blood pressure (diastolic and systolic), but not duration. | 53.7% |
| Bian et al. | 2017 | Wearable (FitBit Charge HR) | Examine sleep and pediatric asthma. | Average sleep quality was 0.89 (range, 0.85-0.94) was inversely associated with asthma symptoms (−.18, p-value=.02). | Sleep was inversely associated with pediatric asthma. | 57.4% |
| Anyz et al. | 2019 | App (Sleep as Android) | Compare sleep before and after major political events. | Sleep during a random, comparable night chosen from the previous year dropped 18 min on the night after ‘Brexit’ 7.02 to 6.44) (p-value<.001). Sleep during a random, comparable night chosen from the previous year among Americans dropped 38 minutes after the Trump election (7.07 to 6.29) (p-value<.001). | Sleep duration decreased significantly after major political events in the US and UK. | 46.3% |
| Agarwal, et al. | 2018 | Wearable (FitBit Charge HR) | Compare sleep before and after surgery among prostate cancer patients. | Postoperative night sleep averaged 6.0 (IQR, 317–414) whereas preoperative nights averaged 6.6 (IQR, 306–465) (p-value=.33). Nighttime awakenings on postoperative nights averaged 2.7 (IQR, 1.9–8.9) whereas preoperative nights averaged 3.3 (IQR, 1.0–9.0) (p-value =0.37). | Sleep was not different between preoperative to postoperative nights. | 64.8% |
| Han et al. | 2016 | Wearable (FitBit Flex) | Compare sleep before and after hemodialysis among patients. | Sleep among patients before a night before hemodialysis averaged 3.7 minutes, whereas nights after treatment were 6.9 minutes and nights between two 2 non hemodialysis days averaged 7.2 (p-value <.001). | Sleep was significantly lower among patients before treatment compared to after treatment. | 68.5% |
| Weatherall et al. | 2018 | Wearable (FitBit Charge) | Comparing sleep tracker-obtained data with self-reported data | Average sleep duration was 6.7 hours (SD 1.7). Self-reported data show participants had trouble falling asleep for an average of 2.3 (SD 2.7) nights in a typical week. Sleep duration was correlated with self-reported trouble sleeping (r=.28, p-value =.02). | Sleep was associated with self-reported sleep difficulty. | 57.4% |
| DeMasi et al. | 2017 | App (Funf Open Sensing Framework) | Examining sleep and well-being | Average sleep duration was 8.79 hours (1.22). Sleep duration was positively associated with mood (b = 0.072, p-value=.02). | Sleep was associated with mood. | 59.3% |
Among the studies examining sleep and health outcomes, results suggest sleep obtained from the sleep tracker were associated with several health factors. Specifically, one study found sleep was inversely associated with blood pressure [26]. Another study found an inverse relationship between sleep and asthma symptoms in a pediatric population [22]. Another study found a positive association between sleep and mood [28]. Based on the research comparing sleep with self-reported sleep, one study found sleep was associated with self-reported measures of sleep disturbance [24]. Also, among the studies that administered sleep trackers to patient populations, sleep showed much shorter sleep the night before hemodialysis compared to the night after [25]. However, another study did not find sleep to differ among prostate cancer patients the night before compared to after treatment [25]. Overall the study quality among the eligible articles was moderate and ranged from 46.3 [23]-68.5% [25], on a scale from 0 to 100%.
DISCUSSION
Smartphones and other mobile technology, such as wearable sensors, have the potential to capture various data including activity sensing that might relate to health. There have been several reviews on the capabilities of mobile technologies for such health purposes as sleep tracking [15,29]. However, as attention to and use of sleep-tracking technologies in research grows, there is a need to review the evidence on the application of sleep trackers to general audiences or clinical populations.
Among the studies selected for this review, sample size varied widely from 22 participants in a pilot study that administered sleep trackers to participants, to several thousand participants in a study that analyzed historical patterns of sleep parameters captured by sleep trackers. Interestingly, all studies had a majority of male participants with only one exception. Four studies did not include race or ethnicity of the participants. Among the studies that disclosed racial/ethnic characteristics, whites were the majority of participants (55-93% of participants). These data do suggest the importance of collecting race/ethnicity data in future studies using sleep trackers.
Results from our review suggest that mobile health tools are used in a range of functions in health research on sleep and health research with possible future applications for clinical research or population health surveillance. Three of the studies included in this review examined sleep tracking among the general population, whereas four studies included patient populations with chronic conditions, including type 2 diabetes and patients undergoing hemodialysis. It is promising that the majority of studies address patient populations with chronic illnesses as previous research suggests that there are far fewer health-related mobile technologies for chronically-ill individuals [31,32].
Although commercially available sleep-tracking devices are increasingly popular, one criticism is that few of these devices have been validated against polysomnography (PSG), the ‘gold standard’ for sleep measurement [14,15]. However, this is starting to change as an emerging literature has compared several fitness tracking devices with PSG, finding mixed results for the validity of these trackers compared to gold standard sleep measurement [16,17]. Future health researchers interested in low-cost sensors for sleep assessment in their studies may consider including sleep trackers that have been shown to be strongly correlated with gold standard measurements. Further, it would be helpful to convene a task force to recommend rigorous standardization and validation protocols to govern the acceptability and usability of currently available sleep trackers.
Among the results of studies identified in this review that administered commercially-available sleep trackers to either general audiences or clinical populations, several studies found sleep was associated with several health outcomes, including mood and blood pressure. Research found sleep was more disturbed before treatment for patients with hemodialysis but not significantly disturbed for patients with prostate cancer on preoperative nights compared to postoperative nights.
Limitations
Results from this review must be interpreted with caution, as our summary of the literature is restricted to relationships between sleep parameters, which have been criticized because of limited data evidencing their validity when compared to measures of sleep derived from PSG. Specifically, where studies have shown wearable devices are efficacious in measuring physical activity, sleep-tracking devices have demonstrated highly variable performance relative to objective measures derived from polysomnography or actigraphy [14,16,17]. Therefore, future research may consider the validity of sleep trackers before using them in rigorous health research.
Although we conducted a rigorous systematic review, several limitations should be mentioned. First, the research that provided sleep parameters captured from sleep trackers varied widely in its objective, nature, and scope. Our review was thus limited to summarizing these parameters. Our research effort originally aimed to measure and evaluate the effect of sleep tracking on user sleep over time. However, we found in the course of our review that the existing literature challenged our ability to provide adequate answers to this question. Most importantly, the studies that included sleep parameters from trackers emanated from a medical intervention, i.e., surgery for prostate cancer patients, which limited our ability to draw conclusions about sleep tracking alone as a behavioral change catalyst for the general population. Future research may consider measuring users who perform sleep tracking over time and how tracking relates to psychological factors, such as the effect of sleep on stress.
Conclusion
We conducted a systematic review of research that included sleep tracker-derived data. We summarized the literature, finding highly variable sample sizes and objectives among the published research on this topic. While a large body of evidence touts the benefits of health technologies, it is yet unclear whether these devices are efficacious for accurate sleep measurement. Furthermore, the compelling question as to whether or not sleep tracking and the behavioral feedback it affords users is beneficial for sleep or if this practice perhaps induces anxiety about sleep onset or duration. In this review, we characterize the health research that has included sleep tracking devices. Future studies may build on the findings in this review to better understand the effects of sleep tracking when these data are shared with patients. For, the compelling question as to whether sleep tracking is helpful or harmful for patients remains unanswered.
Appendix
Search Strategies for the current review.
(sleep tracker OR sleep trackers OR sleep tracking OR mobile phone OR mobile phones OR mobile apps OR mobile technology OR mobile technologies OR mobile device OR mobile devices OR iWatch OR Fitbit OR jawbone OR wearable devices) AND (sleep OR polysomnography) AND (behavior OR behaviors OR behaviour OR behaviours OR self management OR monitor OR monitoring)
(sleep tracker OR sleep trackers OR sleep tracking) AND (sleep OR polysomnography) AND (behavior OR behaviors OR behaviour OR behaviours OR self management OR monitor OR monitoring OR well being)
(sleep OR polysomnography) AND (tracker OR trackers OR tracking) AND (device OR devices OR technology OR mobile) AND (behavior OR behaviors OR behaviour OR behaviours OR self management OR monitor OR monitoring)
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Human and Animal Rights Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest
Rebecca Robbins, Azizi Seixas, Lillian Walton Masters, Nicholas Chanko, Fatou Diaby, Dorice Vieira, and Girardin Jean-Louis each declare no conflict of interest.
References
- 1.Jean-Louis G, Kripke DF, Ancoli-Israel S. Sleep and quality of well-being. Sleep. 2000;23: 1115–1121. [PubMed] [Google Scholar]
- 2.Watson NF, Badr MS, Belenky G, Bliwise DL, Buxton OM, Buysse D, et al. Joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society on the recommended amount of sleep for a healthy adult: Methodology and discussion. Sleep. 2015;38: 1161–1183. doi: 10.5665/sleep.4886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, et al. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health J Natl Sleep Found. 2015; 1: 40–43. doi: 10.1016/j.sleh.2014.12.010 [DOI] [PubMed] [Google Scholar]
- 4.Liu Y, Wheaton AG, Chapman DP, Cunningham TJ, Lu H, Croft JB. Prevalence of Healthy Sleep Duration among Adults — United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65: 137–141. doi: 10.15585/mmwr.mm6506a1 [DOI] [PubMed] [Google Scholar]
- 5.Sheehan CM, Frochen SE, Walsemann KM, Ailshire JA. Are U.S. adults reporting less sleep?: Findings from sleep duration trends in the National Health Interview Survey, 2004–2017. Sleep. 2019;42. doi: 10.1093/sleep/zsy221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foley DJ, Monjan A, Simonsick EM, Wallace RB, Blazer DG. Incidence and remission of insomnia among elderly adults: an epidemiologic study of 6,800 persons over three years. Sleep. 1999;22 Suppl 2: S366–372. [PubMed] [Google Scholar]
- 7.Morin CM, Jarrin DC. Epidemiology of Insomnia. Sleep Med Clin. 2013;8: 281–297. doi: 10.1016/j.jsmc.2013.05.002 [DOI] [PubMed] [Google Scholar]
- 8.Stranges S, Tigbe W, Gómez-Olivé FX, Thorogood M, Kandala N-B. Sleep Problems: An Emerging Global Epidemic? Findings From the INDEPTH WHO-SAGE Study Among More Than 40,000 Older Adults From 8 Countries Across Africa and Asia. Sleep. 2012;35: 1173–1181. doi: 10.5665/sleep.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Godfrey A, Conway R, Meagher D, ÓLaighin G. Direct measurement of human movement by accelerometry. Med Eng Phys. 2008;30: 1364–1386. doi: 10.1016/j.medengphy.2008.09.005 [DOI] [PubMed] [Google Scholar]
- 10.Mobile Fact Sheet. In: Pew Research Center: Internet, Science & Tech [Internet] 12 January 2017. [cited 27 Jul 2017]. Available: http://www.pewinternet.org/fact-sheet/mobile/ [Google Scholar]
- 11.Patrick K, Griswold WG, Raab F, Intille SS. Health and the mobile phone. Am J Prev Med. 2008;35: 177–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Research2guidance. Mobile Health Market Report 2013–2017: The Commercialization of mHealth Applications. Berlin; 2013. [Google Scholar]
- *13.Robbins R, Krebs P, Rapoport DM, Jean-Louis G, Duncan DT. Examining Use of Mobile Phones for Sleep Tracking Among a National Sample in the USA. Health Commun. 2018;0: 1–7. doi: 10.1080/10410236.2017.1422104 [DOI] [PubMed] [Google Scholar]; This study used a sample of geographically representative adults to examine self-reported use of sleep tracking apps. Results of the study show approximately one quarter of adults reported use of sleep tracking apps.
- 14.de Zambotti M, Claudatos S, Inkelis S, Colrain IM, Baker FC. Evaluation of a consumer fitness-tracking device to assess sleep in adults. Chronobiol Int. 2015;32: 1024–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shelgikar AV, Anderson PF, Stephens MR. Sleep tracking, wearable technology, and opportunities for research and clinical care. Chest. 2016;150: 732–743. [DOI] [PubMed] [Google Scholar]
- 16.de Zambotti M, Rosas L, Colrain IM, Baker FC. The Sleep of the Ring: Comparison of the OURA Sleep Tracker Against Polysomnography. Behav Sleep Med. 2017;0: 1–15. doi: 10.1080/15402002.2017.1300587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *17.de Zambotti M, Goldstone A, Claudatos S, Colrain IM, Baker FC. A validation study of Fitbit Charge 2™ compared with polysomnography in adults. Chronobiol Int. 2018;35: 465–476. doi: 10.1080/07420528.2017.1413578 [DOI] [PubMed] [Google Scholar]; This study compared a commercially available sleep tracker, the FitBit Charge 2, to the polysomnography, which is the gold standard for sleep measurement. Results suggested the sleep tracker overestimated sleep time, yet did not differ from PSG in measurement of wake after sleep onset (WASO) or time spent in rapid eye movement (REM) sleep.
- 18.Koh HK. A 2020 vision for healthy people. N Engl J Med. 2010;362: 1653–1656. [DOI] [PubMed] [Google Scholar]
- 19.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52: 377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kunz R, Oxman AD. The unpredictability paradox: review of empirical comparisons of randomised and non-randomised clinical trials. BMJ. 1998;317: 1185–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartling L, Brison RJ, Crumley ET, Klassen TP, Pickett W. A systematic review of interventions to prevent childhood farm injuries. Pediatrics. 2004;114: e483–e496. [DOI] [PubMed] [Google Scholar]
- 22.Bian J, Guo Y, Xie M, Parish AE, Wardlaw I, Brown R, et al. Exploring the Association Between Self-Reported Asthma Impact and Fitbit-Derived Sleep Quality and Physical Activity Measures in Adolescents. JMIR MHealth UHealth. 2017;5. doi: 10.2196/mhealth.7346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *23.Anýž J, Bakštein E, Dudysová D, Veldová K, Kliková M, Fárková E, et al. No wink of sleep: Population sleep characteristics in response to the brexit poll and the 2016 U.S. presidential election. Soc Sci Med 1982. 2019;222: 112–121. doi: 10.1016/j.socscimed.2018.12.024 [DOI] [PubMed] [Google Scholar]; This study used data from a commercially available sleep tracker to compare sleep before and after major political events in the United Kingdom and the United States. Results suggested a significant drop in sleep duration on the night after major political events in both countries.
- 24.Weatherall J, Paprocki Y, Meyer TM, Kudel I, Witt EA. Sleep Tracking and Exercise in Patients With Type 2 Diabetes Mellitus (Step-D): Pilot Study to Determine Correlations Between Fitbit Data and Patient-Reported Outcomes. JMIR MHealth UHealth. 2018;6: e131. doi: 10.2196/mhealth.8122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han M, Williams S, Mendoza M, Ye X, Zhang H, Calice-Silva V, et al. Quantifying Physical Activity Levels and Sleep in Hemodialysis Patients Using a Commercially Available Activity Tracker. Blood Purif. 2016;41: 194–204. doi: 10.1159/000441314 [DOI] [PubMed] [Google Scholar]
- 26.Fagherazzi G, El Fatouhi D, Bellicha A, El Gareh A, Affret A, Dow C, et al. An International Study on the Determinants of Poor Sleep Amongst 15,000 Users of Connected Devices. J Med Internet Res. 2017;19. doi: 10.2196/jmir.7930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agarwal DK, Viers BR, Rivera ME, Nienow DA, Frank I, Tollefson MK, et al. Physical activity monitors can be successfully implemented to assess perioperative activity in urologic surgery. mHealth. 2018;4. doi: 10.21037/mhealth.2018.09.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeMasi O, Feygin S, Dembo A, Aguilera A, Recht B. Well-Being Tracking via Smartphone-Measured Activity and Sleep: Cohort Study. JMIR MHealth UHealth. 2017;5. doi: 10.2196/mhealth.7820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Behar J, Roebuck A, Domingos JS, Gederi E, Clifford GD. A review of current sleep screening applications for smartphones. Physiol Meas. 2013;34: R29. doi: 10.1088/0967-3334/34/7/R29 [DOI] [PubMed] [Google Scholar]
- 30.Flack JM, Amaro H, Jenkins W, Kunitz S, Levy J, Mixon M, et al. Epidemiology of minority health. Health Psychol Off J Div Health Psychol Am Psychol Assoc. 1995; 14: 592–600. [DOI] [PubMed] [Google Scholar]
- 31.Jongbloed K, Parmar S, van der Kop M, Spittal PM, Lester RT. Recent Evidence for Emerging Digital Technologies to Support Global HIV Engagement in Care. Curr HIV/AIDS Rep. 2015;12: 451–461. doi: 10.1007/s11904-015-0291-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Reilly GA, Spruijt-Metz D. Current mHealth technologies for physical activity assessment and promotion. Am J Prev Med. 2013;45: 501–507. doi: 10.1016/j.amepre.2013.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]


