Abstract
The objective was to determine phytase effects on prececal amino acid (AA) digestibility and phytate (InsP6) breakdown when different oilseed meals were used in broiler chicken diets. The study included 14 diets: a corn-soybean meal (SBM) basal diet and 6 diets that contained SBM, rapeseed meal (RSM), and sunflower meal (SFM) with 2 inclusion levels at the expense of corn starch (150 and 300 g/kg SBM or SFM, or 100 and 200 g/kg RSM). Each diet was mixed with or without a phytase supplement of 1,500 FTU/kg. Diets were provided to broilers for 5 D. Digesta from the posterior half of the ileum were collected on day 21. The average essential AA digestibility, calculated by a regression approach, without and with phytase was 84 and 85% (SBM), 74 and 77% (SFM), and 66 and 73% (RSM), respectively. In the diets, phytase effects on AA digestibility were lower owing to other protein sources also present in the diet, but significant. Prececal InsP6 disappearance was significantly affected by interactions between oilseed meal, inclusion level, and phytase supplementation. Overall, prececal InsP6 disappearance was higher in SBM diets (52%) than in SFM diets (38%) and intermediate in RSM diets (43%). Across diets, phytase supplementation effects on prececal InsP6 degradation linearly increased with the InsP6 concentration of the diet up to 12 g/kg DM. The only exception from linearity was the diet with the high inclusion of SFM, which contained 15.9 g InsP6/kg DM. In the ileal content, the concentration of myo-inositol was significantly increased by phytase supplementation, and this effect was highest in the diets that contained SBM as the only oilseed meal. Concentrations of lower inositol phosphates were increased by phytase supplementation, and this effect was most remarkable for Ins(1,2,3,4)P4 and inositol tetrakisphosphates. The study showed that phytase effects on AA digestibility varied among the 3 tested oilseed meals, but these differences were not detectable in the diets containing these meals. Although phytase effects on ileal content of InsP6 and its degradation products were substantial, they were not related to the effects on AA digestibility.
Key words: phytate, energy, protein feeds, regression
Introduction
Phytases are widely used feed additives in nonruminant nutrition. The main function of phytase is the cleavage of myo-inositol 1,2,3,4,5,6-hexakis (dihydrogen phosphate) (InsP6) and its salts (phytate) to increase phosphorus (P) utilization by the animal. Phytase supplementation also affected amino acid (AA) digestibility of broiler chickens in some, but not all, studies (Ravindran et al., 1999, Rutherfurd et al., 2002, Rodehutscord et al., 2004, Kong and Adeola, 2014, Sommerfeld et al., 2018a, Sommerfeld et al., 2018b). Inconsistent effects may partly be related to different protein sources used in the diet and by differences in InsP6 storage in seeds (Erdman, 1979, Yiu et al., 1983, Adeola and Sands, 2003). In rapeseed, InsP6 is located in globoid crystals within protein storage vacuoles (Gillespie et al., 2005) and is tightly associated with proteins (Yiu et al., 1983). In soybeans, InsP6 is evenly distributed throughout the seed (Han and Wilfred, 1988). Its storage location is also different in sunflower seeds, in which InsP6 is found in crystalloids or globoids within the kernel (Erdman, 1979, Allen and Arnott, 1981, Miller et al., 1986). These differences in InsP6 location and association with proteins among types of seeds might contribute to differences in AA digestibility that have been reported for different oilseed meals (e.g., Ravindran et al., 1999, Senkoylu and Dale, 1999).
Values of AA digestibility are affected by endogenous protein secretion into the digestive tract and the different approaches used to consider basal endogenous AA losses. Often, basal endogenous AA loss is considered using estimates taken from literature but is also estimated independently using N-free diets. This presumes that basal endogenous protein loss is identical for the test feed and the N-free diet (Rutherfurd et al., 2002). Alternatively, AA digestibility is studied using the regression approach. The regression approach implies that basal endogenous losses are excluded in the way AA digestibility is calculated; thus, this is the approach with the highest accuracy (Ravindran et al., 2017). In this context, investigations of phytase effects are of specific interest because pure InsP6 or phytate administered to chickens was found to increase mucin secretion (Onyango et al., 2009). This implies that basal endogenous AA losses may be different depending on the type of diet and whether InsP6 is degraded in the digestive tract or not.
In the present study, we investigated whether the effects of phytase supplementation on AA digestibility differ among oilseed meals and diets containing different oilseed meals. We used rapeseed meal (RSM), soybean meal (SBM), and sunflower meal (SFM) and combined the measures of AA digestibility with InsP6 degradation measures. The hypotheses were that differences in phytase effects on AA digestibility exist among oilseed meals and that these differences are reflected in differences in InsP6 disappearance and formation of lower inositol phosphate isomers (InsPx) and myo-inositol.
Materials and methods
Animals and Management
The experiment was conducted at the Agriculture Experiment Station of the University of Hohenheim in accordance with German Animal Welfare Legislation following approval of the Regierungspräsidium Tübingen, Germany (approval no. HOH49-17TE). Unsexed Ross 308 broilers were obtained from a commercial hatchery (Brüterei Süd ZN der Bwe-Brüterei Weser-Ems GmbH & Co. KG, Regenstauf, Germany). The hatchlings were allocated into groups of 15 and placed in 74 floor pens (115 × 230 cm ground area, 260 cm height). Seven pens each were used for the basal diet with and without phytase, and 5 pens for each of the other diets. The temperature in the animal house was continuously reduced from 34°C at the beginning to 26°C on day 21 of the experiment. The light regimen was 24L:0D during the first 3 D and 18L:6D thereafter. For the first 15 D, the birds were kept on wood shavings. On day 16, the litter was removed from the floor and birds were then kept on perforated floors until the end of the experiment. Birds were reallocated among pens on this day to achieve a similar animal weight (8,117 g ± 267 g) in each pen. The pens were randomly allocated to treatment diets in a completely randomized block design to achieve equal distribution of treatments within the building.
Diets
Feed and water were offered for ad libitum consumption throughout the experiment. A commercial starter was provided for the first 15 D and contained (per kg) 215 g CP, 11 g Ca, 5.5 g P, 12.5 MJ ME, 110 mg monensin sodium, 10 IU endo-1,4-β-xylanase, and 750 FTU of a 6-phytase (Deutsche Tiernahrung Cremer GmbH & Co. KG, Düsseldorf, Germany). A total of 14 experimental diets was mixed (Table 1). The basal diet consisted of mainly corn starch, corn, SBM, and corn gluten meal and was formulated to meet or exceed the recommendations of the Gesellschaft für Ernährungsphysiologie (GfE, 1999). Titanium dioxide (TiO2, 5 g/kg) was used as an indigestible marker. In the other diets, corn starch was substituted for one of the oilseed meals at 2 different levels: 100 or 200 g RSM/kg (RSM1 and RSM2), 150 or 300 g SBM/kg (SBM1 and SBM2), and 150 or 300 g SFM/kg (SFM1 and SFM2). The inclusion of RSM was lower than that of the other meals because we wanted to avoid reduced feed intake of the birds at higher inclusion of RSM. The inclusion of oilseed meals at the expense of corn starch implied that differences in CP and AA content among diets originated only from the oilseed meals. One half of each diet was supplemented with 1,500 FTU phytase/kg (Natuphos E 5000 G, BASF SE, Germany) and labelled with “+”, whereas the other half remained without a phytase supplement (“−”). All diets contained the same amount of monocalcium phosphate. The results of chemical analyses of all diets and the 3 oilseed meals are provided in Tables 2 and 3. Analyzed concentrations overall confirmed the calculated values (Table 2). Analyzed phytase activity was slightly higher than intended. The diets were produced by Research Diet Services (Research Diet Services BV, Hoge Maat 10, 3961NC, Wijk bij Duurstede, Netherlands) and provided to the broilers in pelleted form from day 16 to 21.
Table 1.
Composition of the experimental diets (g/kg as fed, unless otherwise stated).
| Diet | Basal | Basal+ | RSM1 | RSM2 | RSM1+ | RSM2+ | SBM1 | SBM2 | SBM1+ | SBM2+ | SFM1 | SFM2 | SFM1+ | SFM2+ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Corn starch | 300.00 | 300.00 | 200.00 | 100.00 | 200.00 | 100.00 | 150.00 | 0.00 | 150.00 | 0.00 | 150.00 | 0.00 | 150.00 | 0.00 |
| SBM | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 150.00 | 300.00 | 150.00 | 300.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| RSM | 0.00 | 0.00 | 100.00 | 200.00 | 100.00 | 200.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| SFM | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 150.00 | 300.00 | 150.00 | 300.00 |
| Corn | 267.25 | 267.25 | 267.25 | 267.25 | 267.25 | 267.25 | 267.25 | 267.25 | 267.25 | 267.25 | 267.25 | 267.25 | 267.25 | 267.25 |
| SBM | 250.00 | 250.00 | 250.00 | 250.00 | 250.00 | 250.00 | 250.00 | 250.00 | 250.00 | 250.00 | 250.00 | 250.00 | 250.00 | 250.00 |
| Corn gluten | 75.00 | 75.00 | 75.00 | 75.00 | 75.00 | 75.00 | 75.00 | 75.00 | 75.00 | 75.00 | 75.00 | 75.00 | 75.00 | 75.00 |
| Soybean oil | 40.00 | 40.00 | 40.00 | 40.00 | 40.00 | 40.00 | 40.00 | 40.00 | 40.00 | 40.00 | 40.00 | 40.00 | 40.00 | 40.00 |
| l-Arginine·HCl | 4.70 | 4.70 | 4.70 | 4.70 | 4.70 | 4.70 | 4.70 | 4.70 | 4.70 | 4.70 | 4.70 | 4.70 | 4.70 | 4.70 |
| l-Valine | 4.40 | 4.40 | 4.40 | 4.40 | 4.40 | 4.40 | 4.40 | 4.40 | 4.40 | 4.40 | 4.40 | 4.40 | 4.40 | 4.40 |
| dl-Methionine | 3.50 | 3.50 | 3.50 | 3.50 | 3.50 | 3.50 | 3.50 | 3.50 | 3.50 | 3.50 | 3.50 | 3.50 | 3.50 | 3.50 |
| l-Lysine·HCl | 2.70 | 2.70 | 2.70 | 2.70 | 2.70 | 2.70 | 2.70 | 2.70 | 2.70 | 2.70 | 2.70 | 2.70 | 2.70 | 2.70 |
| Glycine | 3.50 | 3.50 | 3.50 | 3.50 | 3.50 | 3.50 | 3.50 | 3.50 | 3.50 | 3.50 | 3.50 | 3.50 | 3.50 | 3.50 |
| l-Threonine | 2.30 | 2.30 | 2.30 | 2.30 | 2.30 | 2.30 | 2.30 | 2.30 | 2.30 | 2.30 | 2.30 | 2.30 | 2.30 | 2.30 |
| l-Isoleucine | 1.80 | 1.80 | 1.80 | 1.80 | 1.80 | 1.80 | 1.80 | 1.80 | 1.80 | 1.80 | 1.80 | 1.80 | 1.80 | 1.80 |
| l-Tryptophan | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| MCP | 22.00 | 22.00 | 22.00 | 22.00 | 22.00 | 22.00 | 22.00 | 22.00 | 22.00 | 22.00 | 22.00 | 22.00 | 22.00 | 22.00 |
| Limestone | 8.60 | 8.60 | 8.60 | 8.60 | 8.60 | 8.60 | 8.60 | 8.60 | 8.60 | 8.60 | 8.60 | 8.60 | 8.60 | 8.60 |
| Premix1 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Titanium dioxide | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| NaCl | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 |
| Phytase2, FTU/kg feed | - | 1,500 | - | - | 1,500 | 1,500 | - | - | 1,500 | 1,500 | - | - | 1,500 | 1,500 |
-, No exogenous phytase added; +, with phytase addition.
Numbers 1 and 2 indicate the inclusion level of the respective oilseed meal.
Abbreviations: MCP, monocalcium phosphate; RSM, rapeseed meal; SBM, soybean meal; SFM, sunflower meal.
P-free vitamin/mineral premix.
Phytase added on top of the diets.
Table 2.
Analyzed concentrations of crude nutrients, amino acids, calcium, phosphorus, InsP6, and phytase activity in the experimental diets (g/kg DM, unless otherwise stated).
| Diet | Basal | Basal+ | RSM1 | RSM2 | RSM1+ | RSM2+ | SBM1 | SBM2 | SBM1+ | SBM2+ | SFM1 | SFM2 | SFM1+ | SFM2+ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dry matter | 900.0 | 899.0 | 897.0 | 897.0 | 900.0 | 900.0 | 899.0 | 897.0 | 902.0 | 898.0 | 897.0 | 898.0 | 901.0 | 904.0 |
| Organic matter | 937.0 | 938.0 | 928.0 | 918.0 | 929.0 | 918.0 | 927.0 | 916.0 | 928.0 | 917.0 | 928.0 | 918.0 | 928.0 | 920.0 |
| Crude protein | 240.0 | 239.0 | 290.0 | 343.0 | 292.0 | 342.0 | 314.0 | 384.0 | 317.0 | 387.0 | 289.0 | 341.0 | 283.0 | 335.0 |
| Ether extract | 73.0 | 75.0 | 81.0 | 88.0 | 85.0 | 89.0 | 71.0 | 71.0 | 70.0 | 80.0 | 75.0 | 78.0 | 75.0 | 78.0 |
| Crude fiber | 19.0 | 18.0 | 40.0 | 58.0 | 40.0 | 59.0 | 24.0 | 31.0 | 24.0 | 27.0 | 47.0 | 75.0 | 43.0 | 76.0 |
| GE, MJ/kg DM | 19.5 | 19.3 | 19.0 | 20.0 | 19.6 | 20.0 | 19.7 | 19.9 | 19.7 | 19.9 | 19.6 | 20.0 | 19.6 | 20.0 |
| Ala | 13.3 | 12.9 | 15.5 | 17.8 | 15.7 | 18.0 | 16.6 | 19.8 | 16.4 | 20.1 | 15.5 | 17.9 | 15.1 | 17.5 |
| Arg | 17.9 | 17.2 | 20.9 | 24.1 | 21.2 | 24.4 | 23.7 | 28.6 | 23.4 | 29.1 | 22.1 | 26.8 | 22.0 | 26.3 |
| Asx | 22.5 | 22.1 | 26.3 | 30.1 | 26.5 | 30.4 | 31.5 | 40.1 | 31.3 | 40.9 | 27.3 | 32.1 | 26.7 | 31.6 |
| Cys | 3.5 | 3.5 | 4.7 | 5.8 | 4.8 | 5.9 | 4.5 | 5.6 | 4.6 | 5.7 | 4.4 | 5.1 | 4.3 | 4.9 |
| Glx | 43.3 | 42.3 | 51.3 | 60.6 | 52.3 | 61.2 | 56.7 | 70.0 | 56.5 | 71.2 | 53.5 | 63.4 | 52.1 | 62.2 |
| Gly | 12.9 | 12.6 | 15.5 | 18.3 | 15.5 | 18.7 | 16.2 | 19.1 | 16.1 | 19.4 | 16.2 | 19.7 | 15.9 | 19.2 |
| His | 6.7 | 7.0 | 8.3 | 9.9 | 8.3 | 9.7 | 8.6 | 10.9 | 8.4 | 10.8 | 7.9 | 9.2 | 7.5 | 9.0 |
| Ile | 10.6 | 9.4 | 12.5 | 14.6 | 12.7 | 15.3 | 14.8 | 16.8 | 14.5 | 18.1 | 12.8 | 15.4 | 13.1 | 15.0 |
| Leu | 23.1 | 21.9 | 26.3 | 30.0 | 26.6 | 30.4 | 29.0 | 34.0 | 28.6 | 35.1 | 26.2 | 29.7 | 25.7 | 29.0 |
| Lys | 13.3 | 12.9 | 16.1 | 19.0 | 16.3 | 19.4 | 18.2 | 22.5 | 18.2 | 23.0 | 15.2 | 17.3 | 15.0 | 16.9 |
| Met | 7.6 | 7.5 | 8.7 | 9.7 | 8.7 | 9.8 | 8.7 | 9.6 | 8.7 | 9.7 | 9.1 | 10.2 | 9.0 | 10.1 |
| Phe | 12.0 | 11.4 | 14.0 | 16.2 | 14.2 | 16.4 | 16.1 | 19.6 | 15.9 | 20.2 | 14.4 | 17.0 | 14.2 | 16.6 |
| Pro | 15.0 | 14.5 | 18.0 | 21.1 | 18.0 | 21.5 | 18.1 | 22.2 | 18.5 | 22.4 | 17.1 | 19.1 | 16.4 | 19.2 |
| Ser | 12.6 | 12.5 | 14.9 | 17.3 | 15.0 | 17.3 | 16.4 | 20.7 | 16.4 | 20.7 | 14.9 | 17.1 | 14.4 | 16.8 |
| Thr | 11.4 | 11.1 | 13.9 | 16.3 | 14.0 | 16.5 | 14.5 | 17.4 | 14.5 | 17.6 | 13.6 | 15.5 | 13.4 | 15.5 |
| Trp | 2.6 | 2.7 | 3.3 | 4.1 | 3.4 | 4.1 | 3.6 | 4.4 | 3.6 | 4.5 | 3.3 | 4.0 | 3.3 | 4.1 |
| Tyr | 8.5 | 8.1 | 10.0 | 11.6 | 10.1 | 11.8 | 11.2 | 13.6 | 11.1 | 14.0 | 9.9 | 11.3 | 9.6 | 11.1 |
| Val | 14.2 | 13.0 | 16.7 | 19.5 | 16.8 | 20.2 | 18.4 | 20.5 | 18.1 | 21.7 | 17.0 | 19.9 | 17.3 | 19.5 |
| Phytase, FTU/kg | <60 | 1,760 | <60 | <60 | 1,930 | 2,040 | <60 | <60 | 1,770 | 1,700 | <60 | <60 | 1,930 | 1,920 |
| Calcium | 8.2 | 7.9 | 9.0 | 10.7 | 9.3 | 10.9 | 8.9 | 9.5 | 9.0 | 9.5 | 10.5 | 10.3 | 9.3 | 10.0 |
| Phosphorus | 8.7 | 8.4 | 9.8 | 11.9 | 10.3 | 12.1 | 9.9 | 11.0 | 10.0 | 11.1 | 11.3 | 12.9 | 10.6 | 12.3 |
| InsP6, μmol/g DM | 11.7 | 11.5 | 15.6 | 19.4 | 15.9 | 19.5 | 14.8 | 17.8 | 14.6 | 17.9 | 17.5 | 24.3 | 18 | 23.9 |
| InsP6-P | 2.2 | 2.1 | 2.9 | 3.6 | 3.0 | 3.6 | 2.8 | 3.3 | 2.7 | 3.3 | 3.3 | 4.5 | 3.3 | 4.4 |
Abbreviations: GE, gross energy; n.a., not analyzed; RSM1 and RSM2, rapeseed meal at 100 g/kg and 200 g/kg; SBM1 and SBM2, soybean meal at 150 g/kg and 300 g/kg; SFM1 and SFM2, sunflower meal at 150 g/kg and 300 g/kg.
+ = added phytase.
Table 3.
Chemical analyses of the used oilseed meals (g/kg DM, unless otherwise stated).
| Oilseed meal | RSM | SBM | SFM |
|---|---|---|---|
| Organic matter | 928.0 | 921.0 | 926.0 |
| Crude protein | 371.0 | 530.0 | 372.0 |
| Ether extract | 49.0 | 24.0 | 26.0 |
| Crude fiber | 154.0 | 46.0 | 213.0 |
| GE (MJ/kg DM) | 20.1 | 19.8 | 19.6 |
| Ala | 16.5 | 23.7 | 17.0 |
| Arg | 21.4 | 38.9 | 31.2 |
| Asx | 27.6 | 62.0 | 35.1 |
| Cys | 7.4 | 7.3 | 5.6 |
| Glx | 62.1 | 96.2 | 73.0 |
| Gly | 18.7 | 22.4 | 22.8 |
| His | 12.4 | 15.6 | 10.7 |
| Ile | 11.9 | 22.6 | 14.8 |
| Leu | 24.8 | 40.7 | 24.4 |
| Lys | 19.4 | 32.7 | 14.2 |
| Met | 7.0 | 7.4 | 9.1 |
| Phe | 14.4 | 27.2 | 17.4 |
| Pro | 22.1 | 27.9 | 16.3 |
| Ser | 17.3 | 28.8 | 17.4 |
| Thr | 16.7 | 21.4 | 15.0 |
| Trp | 4.9 | 6.9 | 5.2 |
| Tyr | 10.5 | 18.1 | 9.9 |
| Val | 15.7 | 23.2 | 17.8 |
| Ca | 7.8 | 3.4 | 5.5 |
| P | 10.7 | 7.8 | 13.5 |
| Glucosinolates, g/kg | 3.3 | n.a. | n.a. |
| ADFom, g/kg | 192 | n.a. | 180 |
| aNDFom, g/kg | 262 | n.a. | 192 |
| Starch, g/kg | 19 | 18 | 54 |
| Total sugar, g/kg | 99 | 105 | 74 |
| TIA, g/kg | n.a. | 3.06 | n.a. |
| Urease activity, mg N/g min 30°C | n.a. | <0.02 | n.a. |
| Reactive lysine, % | n.a. | 95 | n.a. |
| Protein digestibility index, % | n.a. | 11.2 | n.a. |
| Nitrogen solubility index, % | n.a. | 15 | n.a. |
Glucosinolates (ISO 9167-1:1992), total sugar (determined as glucose, Luff-Schoorl titrimetry), starch (heat stable α-amylase assay), TIA (NEN-EN-ISO 14902), urease activity, (NEN 3557:1995nl), reactive lysine (ANAL-10334), PDI (water soluble nitrogen after extraction using an Ultra-Turrax and centrifugation), and NSI (NEN-3517) were analyzed by NutriControl analytical solutions (Veghel, Netherlands).
Abbreviations: GE, gross energy; n.a., not analyzed; NSI, nitrogen solubility index; PDI, protein dispersibility index; RSM, rapeseed meal; SBM, soybean meal; SFM, sunflower meal; TIA, trypsin inhibitor activity.
Measurements and Sampling Procedure
Animals were weighed on day 16 and day 21, and feed consumption within this period was determined on a pen basis. On day 21, all birds were weighed, stunned using a gas mixture (35% CO2, 35% N2, and 30% O2), and then euthanized by CO2 exposure. The posterior half of the section between Meckel's diverticulum and 2 cm anterior to the ileo-ceco-colonic junction was excised. The digesta was flushed out using ice-cold deionized water, pooled on a pen basis, and immediately frozen at −20°C until being freeze-dried.
Chemical Analyses
Samples of all diets were ground to pass through a 0.5-mm sieve (Ultra Centrifugal Mill ZM 200; Retsch GmbH, Haan, Germany) or pulverized using a vibrating cup mill (PULVERISETTE 9; Fritsch GmbH, Idar-Oberstein, Germany). Digesta samples were pulverized using the same vibrating cup mill. Ground samples were analyzed for proximate nutrients and fiber fractions according to the methods of Verband Deutscher Landwirtschaftlicher Untersuchungs- und Forschungsanstalten (VDLUFA), 2012. The concentrations of Ti, P, and Ca in pulverized feed and digesta samples were analyzed using inductively coupled plasma optical emission spectrometry after wet digestion (Zeller et al., 2015a). InsP3 to InP6 isomers were analyzed in pulverized feed and digesta samples, as described by Zeller et al. (2015a) with modifications noted by Sommerfeld et al. (2018b). Using this methodology, separation of enantiomers was not possible; therefore, we were unable to distinguish between the d- and l-forms. Some InsP3 isomers could not be identified because standards were unavailable. Clear discrimination of the isomers Ins(1,2,6)P3, Ins(1,4,5)P3, and Ins(2,4,5)P3 was not possible because they coeluted. Myo-inositol in feed and digesta samples was analyzed according to the study by Sommerfeld et al. (2018a) using gas chromatography coupled with mass spectroscopy after derivatization.
The concentrations of AA were determined according to a previously described method (Rodehutscord et al., 2004) with modifications (Siegert et al., 2017). In this assay, methionine and cysteine were determined as methionine sulphone and cysteic acid. The amide residue in the side group of asparagine (Asx) and glutamine (Glx) is lost during acid hydrolysis, and aspartic acid and glutamic acid are formed (Fontaine, 2003). Hence, aspartic acid was analyzed together with Asx and glutamic acid together with Glx. Determination of tyrosine and histidine might have been affected by the oxidation process (Mason et al., 1980). Tryptophan analysis followed previously described procedures (Fatufe et al., 2005). Phytase activity of the diets was analyzed by BASF SE (Ludwigshafen, Germany) according to method ISO EN 30024.
Calculations and Statistics
The ADG, ADFI, and gain:feed ratio (G:F) were calculated on a pen basis from day 16 to day 21 and corrected for mortality. Prececal InsP6 degradation and pc digestibility of CP, AA, P, Ca, and gross energy (GE) (y) were calculated on a pen basis using the following equation:
| [1] |
Daily nutrient intake was calculated as the product of feed intake (g DM/D) and analyzed nutrient concentrations in the diets (g/kg DM). The amount of nutrient digested per day was calculated by multiplying daily intake and determined digestibility. Statistical evaluation of all traits determined for the diets was performed according to the following model:
| [2] |
where yijkl is the mean value of each treatment, μ is the mean of all treatments, phytasej is the fixed effect of phytase supplementation j (0 or 1,500 FTU/kg), levelk is the fixed effect of the inclusion level k (1 or 2) of the oilseed meal (meali, RSM, SBM, or SFM), blockl is the random block effect, and eijk is the residual error.
Effects of phytase in the basal diet were evaluated for all traits according to the following model:
| [3] |
For the single oilseed meals (not diets), pc CP and AA digestibility were calculated as the slope of linear regressions between the ingested and digested amounts. Because the differences in intake of CP and AA originated only from the respective oilseed meal, the slope of the regression reflected the pc CP and AA digestibility of this oilseed meal. The following model was applied to determine the effect of phytase supplementation on pc CP and AA digestibility of the oilseed meals (Siegert et al., 2019a):
| [4] |
where digkl is the daily amount of CP or AA pc digested in combinations of oilseed meal k and phytase supplementation l, αkl is the intercept of combinations of oilseed meal k and phytase supplementation l, ingkl is the daily amount of ingested CP or AA in combinations of oilseed meal k and phytase supplementation l, βkl is the digestibility of CP or AA in combinations of oilseed meal k and phytase supplementation l, and ekl is the residual error. The assumption of linearity between intake and digested amounts of CP and AA has been confirmed in previous studies (Rodehutscord et al., 2004, Rezvani et al., 2008, Kluth and Rodehutscord, 2009).
Regressions to calculate pc AA digestibility, comparisons among the slopes, and analysis of variance for all other traits were performed using PROC MIXED (version 9.4 of the SAS system for Windows, 2016; SAS Institute Inc., Cary, NC). Significant diet effects were determined using t tests if P ≤ 0.05. Normal distribution and homogeneity of variance were tested before statistical analysis.
Results
Performance Traits
Mortality was low (1.4%) and not related to any treatment. Phytase supplementation had no effect on performance traits when the basal diet was fed (Table 4). In the diets with different inclusion levels of the oilseed meals, ADG ranged between 66 g/D (RSM2+ and RSM2−) and 77 g/D (SBM1+) (Table 5). For ADG, the interaction between meal and level was significant (P = 0.031), indicating that increasing the inclusion rate of SBM but not for the other meals led to a decrease in ADG. The ADFI ranged between 75 g/D (SBM2+) and 84 g/D (SFM1+) and was significantly influenced by the oilseed meal and inclusion level. ADFI was highest when SFM was used and declined at the higher level of inclusion. The gain:feed ratio ranged between 0.86 (RSM2− and SFM1+) and 0.92 (SBM1+ and SBM1−) and decreased with an increase in the inclusion level of SBM, but not for the other oilseed meals, which lead to a meal × level interaction.
Table 4.
Performance traits and prececal disappearance/digestibility of nutrients in the basal diet with (+) or without (−) phytase supplementation (least square means, pooled SEM; n = 7 pens per diet).
| Trait | Phytase |
SEM | P value | |
|---|---|---|---|---|
| − | + | |||
| Performance | ||||
| ADG, g/D | 68 | 67 | 2.5 | 0.662 |
| ADFI, g/D | 87 | 84 | 2.7 | 0.202 |
| G:F, g/g | 0.79 | 0.80 | 0.02 | 0.679 |
| Prececal disappearance/digestibility, % | ||||
| Essential amino acids | ||||
| Arg | 93 | 93 | 0.2 | 0.106 |
| His | 87b | 88a | 0.5 | 0.030 |
| Ile | 89 | 88 | 0.7 | 0.450 |
| Leu | 89 | 89 | 0.6 | 0.990 |
| Lys | 91 | 91 | 0.4 | 0.245 |
| Met | 95 | 95 | 0.4 | 0.940 |
| Phe | 88a | 88a | 0.6 | 0.721 |
| Thr | 85a,b | 86a | 0.6 | 0.441 |
| Trp | 84 | 85 | 0.9 | 0.211 |
| Val | 90 | 90 | 0.6 | 0.571 |
| Nonessential amino acids | ||||
| Ala | 88 | 88 | 0.7 | 0.918 |
| Asx | 84 | 85 | 0.6 | 0.130 |
| Cys | 77 | 78 | 0.8 | 0.654 |
| Glx | 90 | 91 | 0.4 | 0.230 |
| Gly | 88 | 88 | 0.5 | 0.375 |
| Pro | 88 | 88 | 0.5 | 0.720 |
| Ser | 85 | 86 | 0.6 | 0.122 |
| Tyr | 88 | 88 | 0.7 | 0.702 |
| InsP6 | 26b | 83a | 2.1 | <0.001 |
| P | 68b | 72a | 1.7 | 0.040 |
| Ca | 55 | 51 | 0.6 | 0.191 |
| CP | 88 | 89 | 0.5 | 0.459 |
| GE | 85 | 85 | 0.5 | 0.793 |
Values in the same column not sharing the same superscript letter are significantly different (P ≤ 0.05).
Abbreviations: ADG, average daily weight gain; ADFI, average daily feed intake; Ca, calcium; CP, crude protein; GE, gross energy; G:F, gain:feed ratio; InsP6, phytate; P, phosphorus.
Table 5.
ADG, ADFI, and G:F of broiler chickens in the experimental period of 5 D (least square means, pooled SEM; n = 5 pens per diet).
| Meal | Level | Phy | ADG, g/D | ADFI, g/D | G:F, g/g |
|---|---|---|---|---|---|
| RSM | 1 | − | 69 | 80 | 0.87 |
| 2 | − | 66 | 77 | 0.86 | |
| 1 | + | 70 | 81 | 0.87 | |
| 2 | + | 66 | 76 | 0.88 | |
| SBM | 1 | − | 75 | 81 | 0.92 |
| 2 | − | 69 | 77 | 0.90 | |
| 1 | + | 77 | 83 | 0.92 | |
| 2 | + | 67 | 75 | 0.90 | |
| SFM | 1 | − | 69 | 81 | 0.86 |
| 2 | − | 70 | 81 | 0.87 | |
| 1 | + | 72 | 84 | 0.86 | |
| 2 | + | 70 | 80 | 0.88 | |
| SEM | 2.8 | 2.1 | 0.015 | ||
| 2-way interactions | |||||
| Meal × level | |||||
| RSM | 1 | 70b,c | 81 | 0.87c | |
| 2 | 66b | 76 | 0.87c | ||
| SBM | 1 | 76a | 82 | 0.92a | |
| 2 | 68b,c | 76 | 0.90b | ||
| SFM | 1 | 71b | 83 | 0.86c | |
| 2 | 70b | 80 | 0.88c | ||
| SEM | 2.4 | 1.8 | 0.013 | ||
| Main effects | |||||
| RSM | 68 | 78b | 0.87 | ||
| SBM | 72 | 79b | 0.91 | ||
| SFM | 71 | 81a | 0.87 | ||
| SEM | 2.2 | 1.6 | 0.011 | ||
| 1 | 72 | 82a | 0.88 | ||
| 2 | 68 | 78b | 0.88 | ||
| SEM | 2.2 | 1.5 | 0.011 | ||
| − | 70 | 79 | 0.88 | ||
| + | 71 | 80 | 0.88 | ||
| SEM | 2.2 | 1.5 | 0.011 | ||
| P value | |||||
| Meal | 0.014 | 0.028 | <0.001 | ||
| Phy | 0.463 | 0.610 | 0.378 | ||
| Level | <0.001 | <0.001 | 0.876 | ||
| Meal × level | 0.031 | 0.188 | 0.028 | ||
| Phy × level | 0.237 | 0.056 | 0.499 | ||
| Meal × Phy | 0.897 | 0.872 | 0.919 | ||
| Meal × Phy × level | 0.905 | 0.909 | 0.780 | ||
Values in the same column and within the same subheading not sharing the same superscript letter are significantly different (P ≤ 0.05).
Level 1 = 150 g/kg SBM, 100 g/kg RSM, 150 g/kg SFM; level 2 = 300 g/kg SBM, 200 g/kg RSM, 300 g/kg SFM.
− = without added phytase; + = added phytase.
Abbreviations: Phy, phytase; RSM, rapeseed meal; SBM, soybean meal; SFM, sunflower meal.
Prececal AA Digestibility
Phytase supplementation did not significantly affect AA digestibility of the basal diet except for His (Table 4). The AA digestibility of the other experimental diets was significantly increased by phytase supplementation by 1 or 2 percentage points (pp), independent of the oilseed meal and the level at which it was included (Table 6). The interaction meal × level was significant, except for Ile, Leu, Met, Val, and Ala. When this interaction was significant, an increase in the inclusion level of SBM either increased or did not affect pc AA digestibility. For RSM and SFM diets, an increase in the inclusion level led to a decrease or did not affect pc AA digestibility. The pc digestibility of Ile, Leu, Met, Val, and Ala was highest in SBM diets and lowest in RSM diets (Ile and Val), or it was not different between RSM and SFM diets (Leu and Ala).
Table 6.
Prececal amino acid digestibility (%) of the diets with (+) and without phytase supplementation (least square means, pooled SEM; n = 5 pens per diet).
| Meal | Level | Essential amino acids |
Nonessential amino acids |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phy | Arg | His | Ile | Leu | Lys | Met | Phe | Thr | Trp | Val | Ala | Asx | Cys | Glx | Gly | Pro | Ser | Tyr | ||
| RSM | 1 | − | 90 | 83 | 85 | 86 | 86 | 94 | 84 | 81 | 81 | 86 | 85 | 80 | 76 | 87 | 84 | 83 | 81 | 83 |
| 2 | − | 88 | 82 | 84 | 84 | 84 | 93 | 83 | 79 | 80 | 85 | 84 | 78 | 75 | 86 | 82 | 80 | 79 | 82 | |
| 1 | + | 91 | 85 | 87 | 88 | 88 | 94 | 86 | 82 | 82 | 88 | 86 | 82 | 77 | 89 | 85 | 84 | 82 | 85 | |
| 2 | + | 89 | 82 | 86 | 86 | 86 | 93 | 85 | 80 | 81 | 87 | 85 | 80 | 76 | 88 | 83 | 81 | 80 | 84 | |
| SBM | 1 | − | 91 | 85 | 88 | 88 | 90 | 94 | 87 | 84 | 83 | 89 | 86 | 83 | 74 | 89 | 86 | 85 | 83 | 87 |
| 2 | − | 91 | 86 | 88 | 88 | 90 | 94 | 87 | 85 | 85 | 89 | 87 | 84 | 76 | 89 | 86 | 86 | 85 | 88 | |
| 1 | + | 92 | 86 | 89 | 88 | 90 | 94 | 87 | 85 | 84 | 89 | 87 | 84 | 78 | 90 | 86 | 87 | 85 | 88 | |
| 2 | + | 92 | 86 | 89 | 88 | 91 | 94 | 88 | 85 | 85 | 90 | 87 | 85 | 79 | 90 | 86 | 87 | 86 | 89 | |
| SFM | 1 | − | 90 | 82 | 85 | 85 | 86 | 94 | 84 | 81 | 81 | 87 | 84 | 79 | 74 | 88 | 81 | 84 | 80 | 84 |
| 2 | − | 91 | 82 | 86 | 85 | 86 | 94 | 85 | 82 | 82 | 87 | 85 | 80 | 74 | 88 | 79 | 83 | 79 | 84 | |
| 1 | + | 92 | 83 | 87 | 87 | 88 | 94 | 86 | 83 | 82 | 89 | 86 | 82 | 77 | 89 | 83 | 85 | 82 | 86 | |
| 2 | + | 92 | 83 | 87 | 87 | 87 | 94 | 87 | 83 | 83 | 88 | 86 | 82 | 76 | 90 | 81 | 85 | 81 | 86 | |
| SEM | 0.3 | 0.5 | 0.7 | 0.7 | 0.4 | 0.4 | 0.6 | 0.6 | 0.7 | 0.6 | 0.7 | 0.5 | 0.7 | 0.4 | 0.4 | 0.5 | 0.5 | 0.6 | ||
| 2-way interactions | ||||||||||||||||||||
| Meal × level | ||||||||||||||||||||
| RSM | 1 | 90c | 84b | 86 | 87 | 87b | 94 | 85b | 82b | 81d | 87 | 86 | 81c | 77a,b | 88c | 84b | 83c | 82c | 84c | |
| 2 | 89d | 82c | 85 | 85 | 85c | 93 | 84c | 80c | 81d | 86 | 84 | 79d | 75c | 87d | 83c | 81d | 79e | 83d | ||
| SBM | 1 | 92a | 85a | 88 | 88 | 90a | 94 | 87a | 84a | 84b | 89 | 87 | 83b | 76b,c | 89a,b | 86a | 86a | 84b | 87b | |
| 2 | 92a | 86a | 89 | 88 | 90a | 94 | 88a | 85a | 85a | 89 | 87 | 84a | 78a | 90a,b | 86a | 87a | 85a | 88a | ||
| SFM | 1 | 91b | 83c | 86 | 86 | 87b | 94 | 85b | 82b | 81d | 88 | 85 | 81c | 76b,c | 89c | 82d | 85b | 81d,c | 85c | |
| 2 | 91b | 82c | 87 | 86 | 87b | 94 | 86b | 82b | 83c | 88 | 85 | 81c | 75b,c | 89b,c | 80e | 84b | 80d | 85c | ||
| SEM | 0.2 | 0.4 | 0.6 | 0.6 | 0.4 | 0.3 | 0.5 | 0.5 | 0.6 | 0.5 | 0.6 | 0.4 | 0.5 | 0.3 | 0.3 | 0.4 | 0.4 | 0.5 | ||
| Main effects | ||||||||||||||||||||
| RSM | 89 | 83 | 85c | 86b | 86 | 93b | 85 | 81 | 81 | 86c | 85b | 80 | 76 | 88 | 84 | 82 | 80 | 84 | ||
| SBM | 92 | 86 | 89a | 88a | 90 | 94a | 87 | 84 | 84 | 89a | 87a | 84 | 77 | 90 | 86 | 86 | 85 | 88 | ||
| SFM | 91 | 82 | 86b | 86b | 87 | 94a | 86 | 82 | 82 | 88b | 85b | 81 | 76 | 89 | 81 | 84 | 81 | 85 | ||
| SEM | 0.2 | 0.3 | 0.5 | 0.5 | 0.4 | 0.3 | 0.4 | 0.4 | 0.5 | 0.5 | 0.6 | 0.4 | 0.3 | 0.3 | 0.3 | 0.4 | 0.3 | 0.5 | ||
| 1 | 91 | 84 | 87 | 87 | 88 | 94 | 86 | 83 | 82 | 88 | 86 | 82 | 76 | 89 | 84 | 85 | 82 | 85 | ||
| 2 | 91 | 83 | 87 | 86 | 87 | 94 | 86 | 82 | 83 | 88 | 85 | 81 | 76 | 89 | 83 | 84 | 82 | 85 | ||
| SEM | 0.2 | 0.3 | 0.5 | 0.5 | 0.3 | 0.3 | 0.4 | 0.4 | 0.5 | 0.5 | 0.6 | 0.4 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.5 | ||
| − | 90b | 83b | 86b | 86b | 87b | 94b | 85b | 82b | 82b | 87b | 85b | 81b | 75b | 88b | 83b | 83b | 81b | 85b | ||
| + | 91a | 84a | 87a | 87a | 88a | 94a | 87a | 83a | 83a | 88a | 86a | 82a | 77a | 89a | 84a | 85a | 83a | 86a | ||
| SEM | 0.2 | 0.3 | 0.5 | 0.5 | 0.3 | 0.3 | 0.4 | 0.4 | 0.5 | 0.5 | 0.6 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.5 | ||
| P values | ||||||||||||||||||||
| Meal | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.003 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.0306 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| Level | 0.013 | 0.036 | 0.644 | 0.087 | <0.001 | 0.053 | 0.673 | 0.048 | 0.030 | 0.083 | 0.135 | 0.325 | 0.790 | 0.338 | <0.001 | 0.008 | 0.015 | 0.844 | ||
| Phy | <0.001 | 0.001 | <0.001 | <0.001 | <0.001 | 0.011 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| Meal × level | <0.001 | <0.001 | 0.079 | 0.083 | <0.001 | 0.156 | <0.001 | <0.001 | 0.010 | 0.101 | 0.065 | <0.001 | 0.005 | 0.006 | 0.001 | <0.001 | <0.001 | 0.004 | ||
| Meal × Phy | 0.383 | 0.256 | 0.108 | 0.187 | 0.201 | 0.435 | 0.185 | 0.160 | 0.430 | 0.088 | 0.234 | 0.220 | 0.136 | 0.343 | 0.131 | 0.589 | 0.602 | 0.519 | ||
| Level × Phy | 0.782 | 0.384 | 0.871 | 0.644 | 0.751 | 0.503 | 0.673 | 0.580 | 0.370 | 0.882 | 0.433 | 0.735 | 0.653 | 0.933 | 0.641 | 0.979 | 0.384 | 0.659 | ||
| Meal × level × Phy | 0.577 | 0.559 | 0.194 | 0.879 | 0.508 | 0.882 | 0.874 | 0.713 | 0.694 | 0.239 | 0.958 | 0.865 | 0.964 | 0.928 | 0.612 | 0.585 | 0.907 | 0.954 | ||
Level 1 = 150 g/kg SBM, 100 g/kg RSM, 150 g/kg SFM; level 2 = 300 g/kg SBM, 200 g/kg RSM, 300 g/kg SFM. Values in the same column within a subheading not sharing the same superscript letter are significantly different (P ≤ 0.05).
Abbreviations: Phy, phytase; RSM, rapeseed meal; SBM, soybean meal; SFM, sunflower meal.
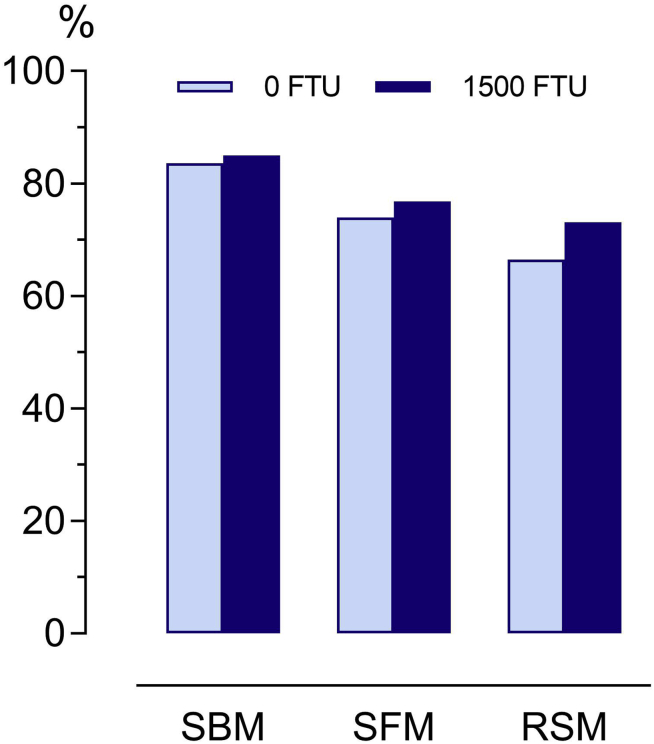
The digestibility of the single oilseed meals was determined from regression analysis (Table 7). Based on the average of all AA, pc digestibility was higher by 7 pp in RSM, 3 pp in SFM, and 1 pp in SBM upon phytase supplementation. In RSM, pc digestibility was significantly increased by phytase supplementation for Arg (6 pp), Ile (14 pp), Lys (8 pp), Pro (11 pp), and Val (11 pp) (P ≤ 0.04). In SBM and SFM, pc digestibility of Cys was increased by 8 pp (P = 0.039) and 10 pp (P = 0.037), respectively. Changes in pc digestibility of other essential AA ranged from −2 pp to +9 pp in RSM, −4 to +3 pp in SBM, and −1 to +4 pp in SFM. Numerical changes in digestibility of nonessential AA ranged between 4 pp and 8 pp in RSM, 0 pp and 2 pp in SBM, and 1 pp and 6 pp in SFM.
Table 7.
Effect of a phytase supplementation on prececal CP and amino acid digestibility (%) of RSM, SBM, and SFM in broiler chickens calculated by regression analysis.
| Phytase | RSM |
P value | SBM |
P value | SFM |
P value | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| − | + | − | + | − | + | |||||
| CP | 63 | 67 | 0.324 | 83 | 84 | 0.951 | 72 | 74 | 0.577 | |
| SE | 3.1 | 2.8 | 2.1 | 2.0 | 2.8 | 2.6 | ||||
| Essential amino acids | ||||||||||
| Arg | 73 | 79 | 0.040 | 88 | 90 | 0.337 | 86 | 88 | 0.352 | |
| SE | 2.5 | 1.9 | 1.3 | 1.2 | 1.4 | 1.3 | ||||
| His | 68 | 66 | 0.705 | 84 | 81 | 0.363 | 66 | 65 | 0.729 | |
| SE | 3.0 | 3.4 | 2.2 | 2.6 | 3.4 | 3.8 | ||||
| Ile | 66 | 80 | 0.004 | 86 | 89 | 0.227 | 80 | 84 | 0.311 | |
| SE | 0.4 | 2.4 | 2.3 | 1.6 | 2.9 | 2.2 | ||||
| Leu | 66 | 75 | 0.158 | 82 | 84 | 0.507 | 73 | 75 | 0.798 | |
| SE | 4.8 | 3.5 | 2.9 | 2.4 | 4.2 | 3.6 | ||||
| Lys | 65 | 73 | 0.030 | 88 | 90 | 0.612 | 73 | 74 | 0.955 | |
| SE | 2.7 | 2.2 | 1.5 | 1.4 | 3.3 | 3.0 | ||||
| Met | 84 | 86 | 0.518 | 89 | 85 | 0.298 | 88 | 88 | 0.855 | |
| SE | 2.7 | 2.3 | 2.6 | 2.7 | 1.9 | 1.7 | ||||
| Phe | 67 | 76 | 0.065 | 84 | 87 | 0.373 | 79 | 81 | 0.496 | |
| SE | 3.9 | 2.9 | 2.0 | 1.7 | 2.8 | 2.5 | ||||
| Thr | 60 | 67 | 0.100 | 81 | 81 | 0.949 | 70 | 74 | 0.422 | |
| SE | 3.1 | 2.6 | 2.4 | 2.2 | 3.2 | 2.7 | ||||
| Trp | 71 | 70 | 0.756 | 84 | 84 | 0.825 | 77 | 77 | 0.817 | |
| SE | 2.9 | 2.2 | 2.2 | 2.3 | 2.7 | 2.5 | ||||
| Val | 67 | 78 | 0.013 | 84 | 87 | 0.382 | 80 | 83 | 0.351 | |
| SE | 3.5 | 2.3 | 2.7 | 1.9 | 2.8 | 2.2 | ||||
| Nonessential amino acids | ||||||||||
| Ala | 65 | 73 | 0.160 | 81 | 81 | 0.945 | 73 | 74 | 0.905 | |
| SE | 4.4 | 3.5 | 2.9 | 2.7 | 3.7 | 3.4 | ||||
| Asx | 57 | 65 | 0.174 | 82 | 84 | 0.588 | 69 | 73 | 0.288 | |
| SE | 4.3 | 3.6 | 1.7 | 1.6 | 2.9 | 2.7 | ||||
| Cys | 70 | 74 | 0.154 | 73b | 81a | 0.039 | 66b | 76a | 0.037 | |
| SE | 2.5 | 2.2 | 2.7 | 2.6 | 3.2 | 3.3 | ||||
| Glx | 74 | 80 | 0.061 | 86 | 88 | 0.422 | 82 | 86 | 0.161 | |
| SE | 2.5 | 2.1 | 1.5 | 1.4 | 1.9 | 1.7 | ||||
| Gly | 66 | 72 | 0.143 | 81 | 82 | 0.848 | 62 | 66 | 0.177 | |
| SE | 3.1 | 2.5 | 2.5 | 2.3 | 2.1 | 2.0 | ||||
| Pro | 56 | 66 | 0.034 | 82 | 84 | 0.700 | 70 | 76 | 0.328 | |
| SE | 3.6 | 2.9 | 2.9 | 2.7 | 4.6 | 3.7 | ||||
| Ser | 58 | 63 | 0.363 | 83 | 84 | 0.925 | 63 | 66 | 0.510 | |
| SE | 4.2 | 3.8 | 2.3 | 2.3 | 3.8 | 3.7 | ||||
| Tyr | 64 | 72 | 0.104 | 86 | 88 | 0.460 | 75 | 77 | 0.634 | |
| SE | 4.1 | 3.1 | 2.3 | 2.0 | 3.9 | 3.3 | ||||
Values in the same row and oilseed meal not sharing a common superscript letter are significantly different (P ≤ 0.05).
Abbreviations: CP, crude protein; RSM, rapeseed meal; SBM, soybean meal; SE, standard error of the estimated slope; SFM, sunflower meal.
Prececal Digestibility of CP, InsP6, P, Ca, and GE
Phytase supplementation significantly increased pc InsP6 disappearance and pc P digestibility of the basal diet, but not pc CP and pc GE digestibility (Table 4). Phytase supplementation also significantly increased pc P digestibility of oilseed meal–containing diets by an average of 7 pp (Table 8). Phytase supplementation increased pc InsP6 disappearance to the same extent in both SBM diets; however, InsP6 disappearance was lower at high inclusion levels in RSM and SFM diets (RSM: 10 pp, P = 0.005; SFM: 12 pp, P = 0.011) and without (RSM: 16 pp, P < 0.001; SFM: 10 pp, P = 0.006) phytase supplementation. In the RSM diets, pc CP and P digestibility were decreased by increasing the inclusion level (2 pp each; P < 0.001), but no significant changes for SBM and SFM diets were found. An increase in the inclusion level led to a decrease in pc Ca digestibility in SFM diets (4 pp, P = 0.022), but not in SBM and RSM diets, leading to a meal × level and phytase × level interaction. Supplementation of phytase led to a decrease in Ca digestibility by 5 pp at the low inclusion level and reached the same level as in the diets with the high inclusion level. For pc GE digestibility, meal × level, phytase × level, and meal × phytase interactions were significant. Increases in oilseed meal inclusion decreased GE digestibility by 4 pp in SBM diets and by 9 and 8 pp in RSM and SFM diets, respectively. Supplementation of phytase led to an increase in pc GE digestibility only at the low inclusion level (2 pp). Supplementation of phytase increased pc GE digestibility only in SFM diets.
Table 8.
Prececal InsP6 disappearance and digestibility (%) of CP, P, Ca, and GE of the experimental diets (least square means, pooled SEM, n = 5 pens per diet).
| Meal | Level | Phy | Disappearance or digestibility, % |
||||
|---|---|---|---|---|---|---|---|
| CP | InsP6 | P | Ca | GE | |||
| RSM | 1 | − | 85 | 19e,f | 61 | 51 | 76 |
| 2 | − | 83 | 2g | 58 | 46 | 67 | |
| 1 | + | 86 | 80a,b | 68 | 47 | 76 | |
| 2 | + | 85 | 70c,d | 66 | 46 | 68 | |
| SBM | 1 | − | 87 | 20e,f | 65 | 55 | 78 |
| 2 | − | 88 | 22e | 66 | 57 | 75 | |
| 1 | + | 88 | 86a | 74 | 52 | 79 | |
| 2 | + | 88 | 81a,b | 77 | 57 | 74 | |
| SFM | 1 | − | 85 | 2g | 58 | 52 | 72 |
| 2 | − | 85 | 13f | 57 | 45 | 65 | |
| 1 | + | 87 | 75b,c | 63 | 45 | 76 | |
| 2 | + | 86 | 63d | 63 | 43 | 66 | |
| SEM | 0.5 | 3.5 | 1.5 | 2.3 | 0.8 | ||
| 2-way interactions | |||||||
| Meal × level | |||||||
| RSM | 1 | 86b | 49 | 64b | 49b | 76b | |
| 2 | 84c | 36 | 62c | 46b,c | 67e | ||
| SBM | 1 | 87a | 53 | 69a | 54a | 79a | |
| 2 | 88a | 52 | 71a | 57a | 75c | ||
| SFM | 1 | 86b | 38 | 61b | 48b | 74d | |
| 2 | 86b | 38 | 60b | 44c | 66f | ||
| SEM | 0.4 | 3.0 | 1.3 | 1.9 | 0.7 | ||
| Phy × level | |||||||
| 1 | − | 86 | 13 | 62 | 53a | 75b | |
| 2 | − | 85 | 12 | 60 | 49b | 69c | |
| 1 | + | 87 | 80 | 68 | 48b | 77a | |
| 2 | + | 86 | 72 | 69 | 49b | 69c | |
| SEM | 0.4 | 2.8 | 1.2 | 1.7 | 0.7 | ||
| Meal × Phy | |||||||
| RSM | − | 84 | 11 | 60 | 49 | 72b | |
| + | 86 | 75 | 67 | 47 | 72b | ||
| SBM | − | 87 | 21 | 66 | 56 | 77a | |
| + | 88 | 84 | 76 | 55 | 77a | ||
| SFM | − | 85 | 8 | 58 | 49 | 69c | |
| + | 86 | 69 | 63 | 44 | 71b | ||
| SEM | 0.4 | 2.7 | 1.3 | 1.9 | 0.7 | ||
| Main effects | |||||||
| RSM | 85 | 43 | 63 | 48 | 72 | ||
| SBM | 88 | 52 | 70 | 55 | 77 | ||
| SFM | 86 | 38 | 60 | 46 | 70 | ||
| 0.3 | 2.6 | 1.1 | 1.7 | 0.7 | |||
| 1 | 86 | 47 | 65 | 50 | 76 | ||
| 2 | 86 | 42 | 64 | 49 | 69 | ||
| SEM | 0.3 | 2.6 | 1.1 | 1.6 | 0.7 | ||
| − | 85 | 13 | 61b | 51 | 72 | ||
| + | 87 | 76 | 68a | 48 | 73 | ||
| SEM | 0.3 | 2.6 | 1.1 | 1.6 | 0.7 | ||
| Meal | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| Phy | 0.058 | <0.001 | <0.001 | 0.008 | 0.009 | ||
| Level | <0.001 | 0.003 | 0.450 | 0.202 | <0.001 | ||
| Meal × level | 0.002 | 0.002 | 0.019 | 0.016 | <0.001 | ||
| Phy × level | 0.296 | 0.016 | 0.139 | 0.033 | 0.024 | ||
| Meal × Phy | 0.749 | 0.736 | 0.084 | 0.583 | 0.007 | ||
| Meal × Phy × level | 0.919 | 0.002 | 0.981 | 0.813 | 0.102 | ||
Level 1 = 150 g/kg SBM, 100 g/kg RSM, 150 g/kg SFM; level 2 = 300 g/kg SBM, 200 g/kg RSM, 300 g/kg SFM. − = without added phytase; + = added phytase. Values in the same column and within the same subheading not sharing the same superscript letter are significantly different (P ≤ 0.05).
Abbreviations: Ca, calcium; CP, crude protein; GE, gross energy; P, phosphorus; Phy, phytase; RSM, rapeseed meal; SBM, soybean meal; SFM, sunflower meal.
Concentrations of InsPx and Myo-Inositol in Ileal Digesta
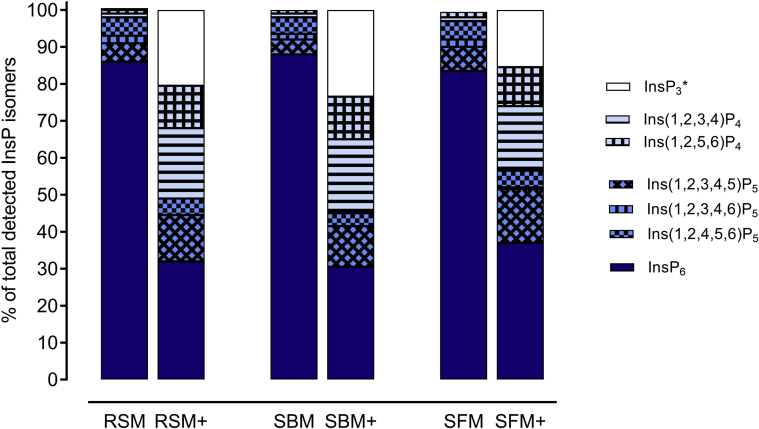
In ileal digesta, the concentrations of InsP6 and Ins(1,2,4,5,6)P5 were decreased by phytase supplementation in all diets (P < 0.001; Table 9), whereas concentrations of myo-inositol and InsP4 isomers were increased by phytase supplementation (P < 0.05). The concentration of Ins(1,2,3,4,5)P5 was significantly increased upon phytase supplementation in RSM and SFM diets, but not in SBM diets. Significant effects of oilseed meal and inclusion level were found for InsP6 and Ins(1,2,4,5,6)P5, with concentrations decreasing from SFM to RSM and SBM and higher concentrations with high meal inclusion. The proportion of InsP isomers in the sum of all detected InsP isomers is illustrated in Figure 1. The proportion of InsP6 was reduced from 84% in SFM and 88% in SBM diets to 31% in SBM and 37% in SFM diets. In contrast, the increase was particularly pronounced for InsP4 and InsP3 isomers.
Table 9.
Concentration of phytate (InsP6, InsP isomers, and myo-inositol [μmol/g DM]) in the digesta of the small intestine of broiler chickens fed the experimental diets (least square means, pooled SEM; n = 5 pens per diet).
| Meal | Level | Phy | Myo-inositol | InsP31 | Ins(…)P4 |
Ins(…)P5 |
InsP6 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| (1,2,3,4) | (1,2,5,6) | (1,2,3,4,5) | (1,2,4,5,6) | (1,2,3,4,6) | ||||||
| RSM | 1 | − | 3.3 | 0.3 | 0.4 | 0.6 | 2.5 | 2.6 | 1.0 | 46.9 |
| 2 | − | 2.9 | <LOQ | 0.3 | 0.7 | 2.8 | 3.0 | 1.1 | 50.6 | |
| 1 | + | 10.6 | 10.0 | 7.6 | 4.7 | 4.4 | 1.5 | <LOQ | 11.7 | |
| 2 | + | 10.6 | 7.2 | 8.7 | 5.2 | 6.5 | 2.1 | 0.2 | 15.8 | |
| SBM | 1 | − | 4.0 | <LOQ | 0.3 | 0.6 | 2.3 | 2.5 | 0.8 | 46.2 |
| 2 | − | 6.2 | <LOQ | 0.4 | 0.6 | 2.3 | 2.3 | 0.8 | 47.4 | |
| 1 | + | 17.0 | 9.6 | 6.6 | 4.0 | 3.1 | 1.0 | nd | 8.1 | |
| 2 | + | 19.4 | 5.3 | 6.3 | 3.4 | 3.8 | 1.2 | nd | 11.3 | |
| SFM | 1 | − | 3.2 | 0.3 | 0.7 | 0.9 | 4.1 | 3.2 | 1.0 | 53.8 |
| 2 | − | 4.4 | 0.3 | 0.8 | 1.0 | 4.6 | 3.3 | 1.1 | 53.5 | |
| 1 | + | 12.8 | 10.3 | 9.5 | 5.9 | 6.8 | 2.1 | 0.2 | 16.2 | |
| 2 | + | 11.7 | 6.1 | 8.9 | 5.0 | 8.6 | 2.6 | 0.3 | 22.9 | |
| SEM | 1.34 | 0.70 | 0.71 | 0.42 | 0.78 | 0.26 | 0.05 | 2.40 | ||
| 2-way interactions | ||||||||||
| Meal × Phy | ||||||||||
| RSM | − | 3.1c | - | 0.4c | 0.6c | 2.6d | 2.8 | 1.0 | 48.7 | |
| + | 10.6b | 8.6 | 8.2a | 5.0a | 5.5b | 1.8 | - | 13.8 | ||
| SBM | − | 5.1c | - | 0.4c | 0.6c | 2.3d | 2.4 | 0.8 | 46.8 | |
| + | 18.2a | 7.4 | 6.4b | 3.7b | 3.5c,d | 1.1 | - | 9.7 | ||
| SFM | − | 3.8c | 0.3 | 0.8c | 0.9c | 4.4b,c | 3.2 | 1.0 | 53.7 | |
| + | 12.2b | 8.2 | 9.2a | 5.4a | 7.7a | 2.3 | 0.2 | 19.5 | ||
| SEM | 1.07 | 0.49 | 0.56 | 0.32 | 0.62 | 0.23 | 0.04 | 2.02 | ||
| Main effects | ||||||||||
| RSM | 6.8 | - | 4.3 | 2.8 | 4.0 | 2.3b | - | 31.2b | ||
| SBM | 11.7 | - | 3.4 | 2.2 | 2.9 | 1.8c | - | 28.3c | ||
| SFM | 8.0 | 4.2 | 5.0 | 3.2 | 6.0 | 2.8a | 0.6 | 36.6a | ||
| SEM | 0.90 | 0.35 | 0.47 | 0.26 | 0.53 | 0.21 | 0.04 | 2.02 | ||
| 1 | 8.5 | - | 4.2 | 2.8 | 3.9b | 2.2b | - | 30.5b | ||
| 2 | 9.2 | - | 4.2 | 2.6 | 4.8a | 2.4a | - | 33.6c | ||
| SEM | 0.84 | - | 0.43 | 0.24 | 0.50 | 0.21 | - | 1.97 | ||
| − | 4.0 | - | 0.5 | 0.7 | 3.1 | 2.8a | 1.0 | 49.8a | ||
| + | 13.7 | 8.1 | 7.9 | 4.7 | 5.5 | 1.8b | - | 14.3b | ||
| SEM | 0.84 | 0.35 | 0.43 | 0.24 | 0.62 | 0.21 | 0.04 | 1.97 | ||
| P value | ||||||||||
| Meal | <0.001 | 0.235 | 0.003 | 0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| Phy | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| Level | 0.283 | <0.001 | 0.874 | 0.518 | 0.018 | 0.009 | 0.002 | <0.001 | ||
| Meal × level | 0.237 | 0.528 | 0.656 | 0.354 | 0.580 | 0.097 | 0.378 | 0.716 | ||
| Phy × level | 0.676 | 0.006 | 0.978 | 0.390 | 0.100 | 0.078 | 0.428 | 0.075 | ||
| Meal × Phy | 0.002 | 0.892 | 0.025 | 0.023 | 0.049 | 0.288 | 0.365 | 0.365 | ||
| Meal × Phy × level | 0.641 | -2 | 0.491 | 0.416 | 0.818 | 0.884 | -2 | 0.283 | ||
Level 1 = 150 g/kg SBM, 100 g/kg RSM, 150 g/kg SFM; level 2 = 300 g/kg SBM, 200 g/kg RSM, 300 g/kg SFM. − = without added phytase; + = added phytase; values in the same column and within the same subheading not sharing the same superscript letter are significantly different (P ≤ 0.05).
Abbreviations: <LOQ, not quantifiable in most samples; nd, not detectable in most samples; Phy, phytase; RSM, rapeseed meal; SBM, soybean meal; SFM, sunflower meal.
At least one of the following isomers: Ins(1,2,6)P3, Ins(1,4,5)P3, Ins(2,4,5)P3.
No P values given due to values under the limit of quantification or detection.
Figure 1.
Changes in the proportion of InsP isomers in the sum of all detected InsP isomers in the ileal digesta of 21-day-old boiler chickens by phytase supplementation (+). Birds were provided diets that contained either rapeseed meal (RSM), soybean meal (SBM), or sunflower meal (SFM) (data pooled across 2 inclusion levels). ∗At least one of the following isomers: Ins(1,2,6)P3, Ins(1,4,5)P3, Ins(2,4,5)P3.
Discussion
One hypothesis of the present study was that phytase effects on pc AA digestibility differed among oilseed meals used in the diets. This hypothesis was confirmed by calculations made for the single oilseed meals: The average increase in AA digestibility was 7 pp in RSM, whereas it was 3 pp in SFM and 1 pp in SBM. Because AA digestibility in the absence of phytase was lower in RSM and SFM than in SBM, phytase supplementation reduced the differences in AA digestibility levels among oilseed meals although they did not cease it (Figure 2). In the oilseed meal–containing diets, this difference in a phytase effect among the meals was no longer visible. While overall the phytase effect on AA digestibility of the diets was significant, an interaction of phytase with the type and level of oilseed meal was not found (Table 5). The differences found in single meals were not detectable in diets likely because the effect of type of oilseed meal was diluted by the other feed ingredients. At the high level of inclusion in the diet, the proportion of CP that originated from RSM was 22% and from SFM 33%. The remainder originated from the basal diet, and phytase did not significantly affect AA digestibility of the basal diet, except that of His (Table 4). This might be taken as an example of how important it is to distinguish between a raw material and a diet when enzyme effects on AA digestibility are studied.
Figure 2.
Effect of supplemented phytase on the mean AA digestibility of soybean meal (SBM), sunflower meal (SFM), and rapeseed meal (RSM) determined using the regression approach. The P values for individual amino acids of each oilseed meal are provided in Table 7.
One approach to explain phytase effects on AA digestibility is the cleavage of phytate-protein complexes. Such an effect should be reflected in phytase effects being specifically pronounced for the AA that are highly abundant in protein fractions associated with InsP6. This was not observed in the present study. For soybeans and soy products, an association of InsP6 with β-conglycinin and glycinin has been described (Prattley and Stanley, 1982, Nishinari et al., 2018), which are the main protein fractions of soybeans (Wagner and Sorgentini, 1996). In these protein fractions, the concentration of Cys is low, whereas those of Arg, Asx, and Glx are relatively high (Riblett et al., 2001). Cruciferin and napin are the main storage proteins in rapeseed (Aider and Barbana, 2011), and they are rich in Arg, Glx, and Leu. In sunflower seeds, the main AA are Asx, Arg, and Glx (Conde et al., 2005). In the present study, the effect of phytase supplementation on digestibility of the highly abundant AA in the respective proteins was not systematically higher than that for other AA, although InsP6 degradation was remarkably increased. This lack of congruence, together with the overall low phytase effects on AA digestibility in SBM, indicated that enzyme accessibility of protein-phytate complexes may not be the limiting factor for AA digestibility in SBM. Although effects of phytase supplementation on pc AA digestibility were stronger in RSM and SFM than those in SBM, the results imply that cleavage of protein-phytate complexes was not the main factor causing the increase in pc AA digestibility in these oilseed meals.
Another possible reason for a phytase-induced increase in pc AA digestibility is the reduction of endogenous AA loss. However, the present data obtained for the diets provided no indication that the increase in AA digestibility upon phytase supplementation was caused by lower endogenous AA losses. Endogenous proteins of broilers contain high proportions of Asx, Glx, and Thr (Kluth and Rodehutscord, 2009). The overall phytase effect on digestibility of these AA in the diets was +1.4 pp, which is similar to the average effect on all AA of +1.3 pp (Table 6). Using the data from regression analysis, digestibility of Asx, Glx, and Thr also was not higher than the average for all AA, perhaps except for that of SFM, where the increase in digestibility of these 3 AA was 1 pp higher than the average (Table 6). This led us conclude that the effects on endogenous protein secretion likewise were not the reason for AA digestibility effects of phytase. This is consistent with conclusions drawn in previous studies (Borda-Molina et al., 2019, Siegert et al., 2019b). If endogenous secretion is affected in the anterior digestive tract, reabsorption in the more distal sections might be responsible for the effects not measured in the terminal ileum.
Comparisons of AA digestibility values across studies should be conducted with caution. Assay details have large effects on the determined digestibility values. Moreover, processing details vary among cracking plants, and pc AA digestibility of oilseed meals in nonruminants is strongly influenced by processing details, such as heat treatment (Goodarzi Boroojeni et al., 2014, Bryan et al., 2017). Despite limitations in comparability among studies, the results of Senkoylu and Dale (1999) are in accordance with the results of the present study where digestibility of most AA was lower for RSM than that of SBM and intermediate for SFM. Cruciferin had lower solubility than glycinin, and cruciferin formed more insoluble aggregates than glycinin when heated (Ramlan et al., 2002). The protein solubility of oilseed meals can be used as an indicator for reduced protein and AA digestibility in broilers caused by overprocessing (Parsons, 1996). We did not measure protein solubility of the oilseed meals; however, the combination of cruciferin content and heat treatment could be a reason for the AA digestibility to be lower in RSM than in SBM in the present study. Proteins from SFM and sunflower cake have a high solubility, similar to SBM (Schingoethe and Ahrar, 1979, Salgado et al., 2011). The inclusion of high levels of SFM in broiler diets was reported to reduce DM digestibility (Rama Rao et al., 2006). The high crude fiber level of SFM and its association with reduced DM and nutrient digestibility (Rama Rao et al., 2006, de Vries, 2015) and energy digestibility in the present study perhaps were the reasons for lower AA digestibility values than those of SBM, although protein solubility might not have been reduced. Neutral detergent insoluble nitrogen (NDiN) might also have contributed to the variability of pc AA digestibility among the oilseed meals. The NDiN concentration of RSM was negatively correlated with Lys digestibility in laying hens (Rezvani et al., 2012), and crude fiber and NDiN concentrations were positively correlated (Mustafa et al., 1996). Hence, the different fiber and NDiN levels could also be causative for the differences in AA digestibility among the oilseed meals. This might explain the lower level of pc AA digestibility for RSM and SFM, despite phytase supplementation (Figure 2).
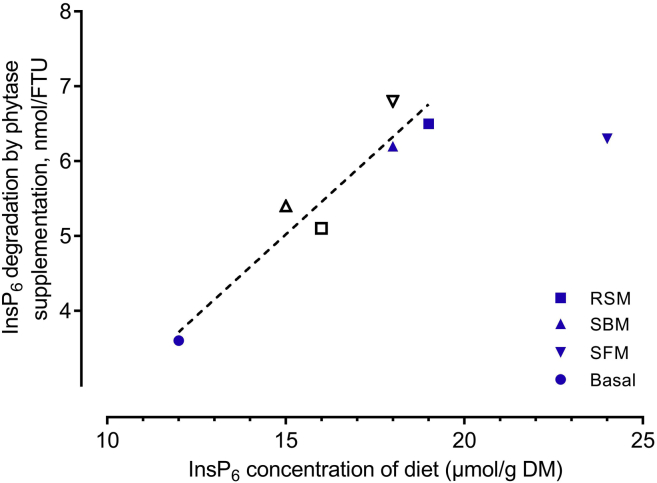
In the present study, pc InsP6 degradation was low overall when phytase was not supplemented. This can be explained by MCP and limestone contained in the diets (Rodehutscord, 2017a). However, InsP6 degradation was remarkably increased by phytase supplementation although to a different extent among the diets. When calculated across all diets, the amount of InsP6 that disappeared upon phytase supplementation showed a linear response to increasing InsP6 concentration in the diet, except for that of SFM2 (Figure 3). The calculated equation showed that the efficiency of the supplemented phytase increased by 0.44 nmol/FTU per each incremental μmol of InsP6 contained in the diet up to 19 μmol InsP6/kg DM (12.5 g InsP6/kg DM). The type of oilseed meal apparently was not a relevant determinant in this relationship, except for the high level of SFM inclusion. The reasons for deviating results of SFM2 compared to that of the other treatments are unclear. One possible reason is an upper limit of InsP6 degradation capacity. An upper level of pc InsP6 degradation was indicated in the P digestibility ring test (Rodehutscord et al., 2017b). However, the ring test was conducted using diets without phytase supplementation. Because differences in InsP6 concentration in the present study were achieved by oilseed meal inclusion, changes in the InsP6 concentration cannot be distinguished from other intrinsic factors of the oilseed meals. Other factors, such as high content of fiber fractions in SFM (Rama Rao et al., 2006, de Vries, 2015), might have been involved, as discussed before.
Figure 3.
Prececal InsP6 degradation caused by phytase supplementation (1,500 FTU/kg) to the basal diet and diets with inclusion of rapeseed meal (RSM), soybean meal (SBM), and sunflower meal (SFM) at 2 levels each (open symbols = 150 g/kg for SBM and SFM, 100 g/kg for RSM; filled symbols = 300 g/kg for SBM and SFM, 200 g/kg for RSM), on the expense of corn starch (300 g/kg, Basal). Linear regression (without SFM2): y = −1.51 + 0.44× (R2 = 0.91, RSME = 0.38).
The phytase supplementation caused an increase in P digestibility that was lower than the increase in InsP6-P disappearance in all diets (except SFM2) (Figure 4). This is a consequence of incomplete dephosphorylation of InsP6. Although some part of the InsP6 contained in the diet was completely dephosphorylated by the end of the ileum as indicated by remarkably increased myo-inositol concentrations, a greater part remained in the form of InsP4, InsP3, and likely, InsP2 and InsP1 isomers.
Figure 4.
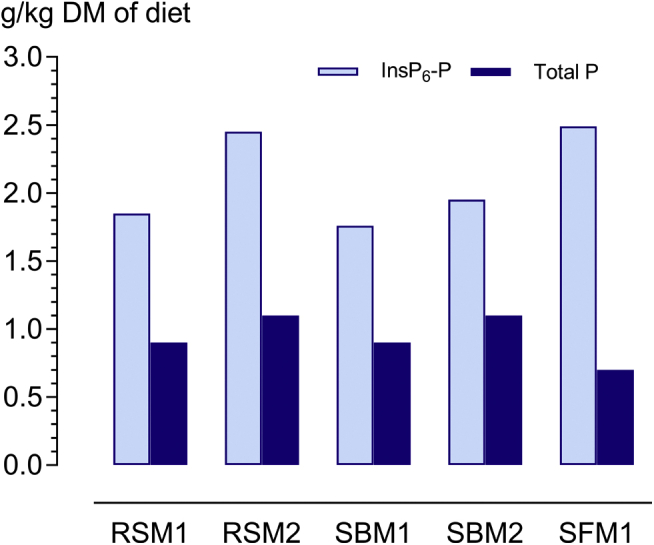
Increments in the degraded amount of InsP6-P and total P due to phytase supplementation (1,500 FTU/kg) in soybean meal-corn–based diets (SBM) or diets with rapeseed meal (RSM) and sunflower meal (SFM). Diet SFM2 is not included because probably the InsP6 content was too high as discussed in the text.
The present study does not provide evidence that the effects of phytase supplementation on pc CP and AA digestibility can be predicted from effects on pc InsP6 degradation and vice versa. Significant correlations with pc AA digestibility were determined for digesta concentrations of InsP6, Ins(1,2,5,6)P4, Ins(1,2,3,4)P4, and InsP3 (data not shown). These significant correlations were mainly caused by the separation of the data points into 2 clusters of observations with and without phytase supplementation. Within the 2 clusters, the data points were randomly scattered and no connection between pc AA digestibility and the concentration of InsP isomers was observed.
Concentrations of InsP6, Ins(1,2,4,5,6)P5, and Ins(1,2,3,4,6)P5 isomers in the digesta were significantly reduced by phytase supplementation, whereas concentrations of Ins(1,2,3,4,5)P5, InsP4 isomers, InsP3 isomers, and myo-inositol were significantly or numerically increased in all diets. The shift from higher to lower InsP isomers and myo-inositol was similar for all diets (Figure 1). The used phytase is a hybrid designed from genes of Hafnia sp., Yersinia mollaretii, and Buttiauxella gaviniae and was classified as a 6-phytase (Rychen et al., 2017). The present results confirmed this classification. The isomer Ins(1,2,3,4,5)P5 had the highest concentration among all InsP5 isomers in the digesta after phytase supplementation. Based on the description of the degradation pathway of 6-phytase by Pontoppidan et al. (2012) and the concentrations of InsP isomers in the present study, the used phytase seems to have a degradation pathway involving Ins(1,2,3,4,5)P5 and Ins(1,2,3,4)P4 as main degradation products. Ins(1,2,5,6)P4 was also increased in the ileum, which indicates a highly active alternative degradation pathway of the phytase product. This can probably be explained by the combination of genes of 3 different microorganisms. In studies that used a modified Escherichia coli 6-phytase, the Ins(1,2,5,6)P4 was the InsP4 isomer that was found in the ileum in specifically high concentrations (Zeller et al., 2015b, Sommerfeld et al., 2018b).
Prececal GE digestibility of the basal diets was significantly higher than that in all other diets, which can be explained by the high concentration of corn starch in the basal diets. Consistent with this, pc GE digestibility was lower for the diets with the higher inclusion rates of each oilseed meal, independent of phytase supplementation. In the basal diet and in the diets containing RSM and SBM, phytase did not change pc GE digestibility. This is consistent with the results of Zaefarian et al. (2013) who found no influence of 500 FTU phytase on pc GE digestibility of corn-SBM-based diets in 21-day-old broilers. In the present study, the GE digestibility was higher (within each inclusion rate) for the SBM than RSM diets and lowest for SFM diets. These findings further support the theory that the fiber fractions in the diets, especially the relatively high fiber content in SFM, considerably influenced the nutritive value of the diets and the oilseed meals.
In conclusion, the results of the present study demonstrated that the effects of phytase supplementation on pc AA digestibility and on pc InsP6 degradation can differ among oilseed meals. This suggests that phytase dosage might be optimized based on the composition of the diet. Increased pc AA digestibility seemed to be independent from basal endogenous AA losses and not related to disappearance of InsPx.
Acknowledgement
This study was funded by a research grant of BASF SE, Ludwigshafen, Germany.
Conflict of Interest Statement: Dieter Feuerstein is an employee of BASF SE. The remaining authors declare that they have no conflicts of interest.
References
- Adeola O., Sands J.S. Does supplemental dietary microbial phytase improve amino acid utilization? A perspective that it does not. J. Anim. Sci. 2003;81:E78–E85. [Google Scholar]
- Aider M., Barbana C. Canola proteins: composition, extraction, functional properties, bioactivity, applications as a food ingredient and allergenicity – a practical and critical review. Trends Food Sci. Technol. 2011;22:21–39. [Google Scholar]
- Allen R.D., Arnott H.J. Effects of exogenous enzymes on oilseed protein bodies [Yucca rupicola, Helianthus annuus] Scan. Elec. Microsc. (USA) 1981;3:561–570. [Google Scholar]
- Borda-Molina D., Zuber T., Siegert W., Camarinha-Silva A., Feuerstein D., Rodehutscord M. Effects of protease and phytase supplements on small intestinal microbiota and amino acid digestibility in broiler chickens. Poult. Sci. 2019;98:2906–2918. doi: 10.3382/ps/pez038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan D.D.S.L., MacIsaac J.L., Rathgeber B., McLean N., Anderson D.M. Meal residual oil level and heat treatment after oil extraction affects the nutritive value of expeller pressed canola meal for broiler chickens. Can. J. Anim. Sci. 2017;96:658–667. [Google Scholar]
- Conde J.M., Escobar M.d.M.Y., Pedroche Jiménez J.J., Rodríguez F.M., Rodríguez Patino J.M. Effect of enzymatic treatment of extracted sunflower proteins on solubility, amino acid composition, and surface activity. J. Agric. Food Chem. 2005;53:8038–8045. doi: 10.1021/jf051026i. [DOI] [PubMed] [Google Scholar]
- de Vries S. Fiber in poultry nutrition; bonus or burden? Proc. Eur. Symp. Poult. Nutr. 2015;20:40–47. [Google Scholar]
- Erdman J.W. Oilseed phytates: nutritional implications. J. Am. Oil Chem. Soc. 1979;56:736–741. [Google Scholar]
- Fatufe A.A., Hirche F., Rodehutscord M. Estimates of individual factors of the tryptophan requirement based on protein and tryptophan accretion responses to increasing tryptophan supply in broiler chickens 8-21 days of age. Arch. Anim. Nutr. 2005;59:181–190. doi: 10.1080/17450390500147925. [DOI] [PubMed] [Google Scholar]
- Fontaine J. Amino acid analysis of feeds. In: D'Mello J.P.F., editor. Amino Acids in Animal Nutrition. CABI Publishing; Willingford, Oxon, UK, Cambridge, MA: 2003. pp. 15–40. [Google Scholar]
- Gesellschaft für Ernährungsphysiologie (GfE) DLG-Verlag; Frankfurt am Main, Germany: 1999. Empfehlungen zur Energie- und Nährstoffversorgung der Legehennen und Masthühner (Broiler) [Google Scholar]
- Gillespie J., Rogers S.W., Deery M., Dupree P., Rogers J.C. A unique family of proteins associated with internalized membranes in protein storage vacuoles of the Brassicaceae. Plant J. 2005;41:429–441. doi: 10.1111/j.1365-313X.2004.02303.x. [DOI] [PubMed] [Google Scholar]
- Goodarzi Boroojeni F., Mader A., Knorr F., Ruhnke I., Röhe I., Hafeez A., Männer K., Zentek J. The effects of different thermal treatments and organic acid levels on nutrient digestibility in broilers. Poult. Sci. 2014;93:1159–1171. doi: 10.3382/ps.2013-03563. [DOI] [PubMed] [Google Scholar]
- Han Y.W., Wilfred A.G. Hydrolysis of phytate in soybean and cottonseed meals by Aspergillus ficuum phytase. J. Agric. Food Chem. 1988;36:259–262. [Google Scholar]
- Kluth H., Rodehutscord M. Effect of inclusion of cellulose in the diet on the inevitable endogenous amino acid losses in the ileum of broiler chicken. Poult. Sci. 2009;88:1199–1205. doi: 10.3382/ps.2008-00385. [DOI] [PubMed] [Google Scholar]
- Kong C., Adeola O. Evaluation of amino acid and energy utilization in feedstuff for swine and poultry diets. Asian-Australas. J. Anim. Sci. 2014;27:917–925. doi: 10.5713/ajas.2014.r.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason V.C., Rudemo M., Bech-Andersen S. Hydrolysate preparation for amino acid determinations in feed constituents. 6. The influence of phenol and formic acid on the recovery of amino acids from oxidized feed proteins. Z. Tierphysiol. Tierernahr. Futtermittelkd. 1980;43:35–48. [PubMed] [Google Scholar]
- Miller N., Pretorius H.E., Du Toit L.J. Phytic acid in sunflower seeds, pressed cake and protein concentrate. Food Chem. 1986;21:205–209. [Google Scholar]
- Mustafa A.F., Christensen D.A., McKinnon J.J. Chemical characterization and nutrient availability of high and low fiber canola meal. Can. J. Anim. Sci. 1996;76:579–586. [Google Scholar]
- Nishinari K., Fang Y., Nagano T., Guo S., Wang R. Soy as a food ingredient. In: Yada R.Y., editor. Proteins in Food Processing. Woodhead Publishing is an imprint of Elsevier; Duxford, UK: 2018. pp. 149–186. [Google Scholar]
- Onyango E.M., Asem E.K., Adeola O. Phytic acid increases mucin and endogenous amino acid losses from the gastrointestinal tract of chickens. Br. J. Nutr. 2009;101:836–842. doi: 10.1017/S0007114508047740. [DOI] [PubMed] [Google Scholar]
- Parsons C.M. Digestible amino acids for poultry and swine. Anim. Feed Sci. Technol. 1996;59:147–153. [Google Scholar]
- Pontoppidan K., Glitsoe V., Guggenbuhl P., Quintana A.P., Nunes C.S., Pettersson D., Sandberg A.-S. In vitro and in vivo degradation of myo-inositol hexakisphosphate by a phytase from Citrobacter braakii. Arch. Anim. Nutr. 2012;66:431–444. doi: 10.1080/1745039X.2012.735082. [DOI] [PubMed] [Google Scholar]
- Prattley C.A., Stanley D.W. Protein-phytate interactions in soybeans. I. Localization of phytate in protein bodies and globoids. J. Food Biochem. 1982;6:243–254. [Google Scholar]
- Rama Rao S.V., Raju M.V.L.N., Panda A.K., Reddy M.R. Sunflower seed meal as a substitute for soybean meal in commercial broiler chicken diets. Br. Poult. Sci. 2006;47:592–598. doi: 10.1080/00071660600963511. [DOI] [PubMed] [Google Scholar]
- Ramlan M., Maruyama N., Adachi M., Hontani N., Saka S., Kato N., Ohkawa Y., Utsumi S. Comparison of protein chemical and physicochemical properties of rapeseed cruciferin with those of soybean glycinin. J. Agric. Food Chem. 2002;50:7380–7385. doi: 10.1021/jf0202537. [DOI] [PubMed] [Google Scholar]
- Ravindran V., Cabahug S., Ravindran G., Bryden W.L. Influence of microbial phytase on apparent ileal amino acid digestibility of feedstuffs for broilers. Poult. Sci. 1999;78:699–706. doi: 10.1093/ps/78.5.699. [DOI] [PubMed] [Google Scholar]
- Ravindran V., Adeola O., Rodehutscord M., Kluth H., van der Klis J.D., van Eerden E., Helmbrecht A. Determination of ileal digestibility of amino acids in raw materials for broiler chickens – results of collaborative studies and assay recommendations. Anim. Feed Sci. Technol. 2017;225:62–72. [Google Scholar]
- Rezvani M., Kluth H., Elwert C., Rodehutscord M. Effect of ileum segment and protein sources on net disappearance of crude protein and amino acids in laying hens. Br. Poult. Sci. 2008;49:28–36. doi: 10.1080/00071660701812971. [DOI] [PubMed] [Google Scholar]
- Rezvani M., Kluth H., Bulang M., Rodehutscord M. Variation in amino acid digestibility of rapeseed meal studied in caecectomised laying hens and relationship with chemical constituents. Br. Poult. Sci. 2012;53:665–674. doi: 10.1080/00071668.2012.729130. [DOI] [PubMed] [Google Scholar]
- Riblett A.L., Herald T.J., Schmidt K.A., Tilley K.A. Characterization of β-conglycinin and glycinin soy protein fractions from four selected soybean genotypes. J. Agric. Food Chem. 2001;49:4983–4989. doi: 10.1021/jf0105081. [DOI] [PubMed] [Google Scholar]
- Rodehutscord M. Advances in understanding the role of phytate in phosphorus and calcium nutrition of poultry. In: Applegate T., editor. Achieving Sustainable Production of Poultry Meat. Burleigh Dodds Science Publishing; Cambridge, UK: 2017. pp. 165–180. [Google Scholar]
- Rodehutscord M., Adeola O., Angel R., Bikker P., Delezie E., Dozier III W.A., Umar Faruk M., Francesch M., Kwakernaak C., Narcy A., Nyachoti C.M., Olukosi O.A., Preynat A., Renouf B., Saiz del Barrio A., Schedle K., Siegert W., Steenfeldt S., van Krimpen M.M., Waititu S.M., Witzig M. Results of an international phosphorus digestibility ring test with broiler chickens. Poult. Sci. 2017;96:1679–1687. doi: 10.3382/ps/pew426. [DOI] [PubMed] [Google Scholar]
- Rodehutscord M., Kapocius M., Timmler R., Dieckmann A. Linear regression approach to study amino acid digestibility in broiler chickens. Br. Poult. Sci. 2004;45:85–92. doi: 10.1080/00071660410001668905. [DOI] [PubMed] [Google Scholar]
- Rutherfurd S.M., Chung T.K., Moughan P.J. The effect of microbial phytase on ileal phosphorus and amino acid digestibility in the broiler chicken. Br. Poult. Sci. 2002;43:598–606. doi: 10.1080/0007166022000004516. [DOI] [PubMed] [Google Scholar]
- Rychen G., Aquilina G., Azimonti G., Bampidis V., Bastos M.d.L., Bories G., Chesson A., Flachowsky G., Gropp J., Kolar B., Kouba M., López-Alonso M., López Puente S., Mantovani A., Mayo B., Ramos F., Saarela M., Villa R.E., Wallace R.J., Wester P., Brantom P., Dierick N.A., Glandorf B., Herman L., Kärenlampi S., Aguilera J., Anguita M., Cocconcelli P.S. Safety and efficacy of Natuphos® E (6-phytase) as a feed additive for avian and porcine species. EFSA J. 2017;15:5042. doi: 10.2903/j.efsa.2017.5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado P.R., Molina Ortiz S.E., Petruccelli S., Mauri A.N. Sunflower protein concentrates and isolates prepared from oil cakes have high water solubility and antioxidant capacity. J. Am. Oil Chem. Soc. 2011;88:351–360. [Google Scholar]
- Schingoethe D.J., Ahrar M. Protein solubility, amino acid composition, and biological value of regular and heat-treated soybean and sunflower meals. J. Dairy Sci. 1979;62:925–931. [Google Scholar]
- Senkoylu N., Dale N. Sunflower meal in poultry diets: a review. Worlds Poult. Sci. J. 1999;55:153–174. [Google Scholar]
- Siegert W., Boguhn J., Maurer H.P., Weiss J., Zuber T., Möhring J., Rodehutscord M. Effect of nitrogen fertilisation on the amino acid digestibility of different triticale genotypes in caecectomised laying hens. J. Sci. Food Agric. 2017;97:144–150. doi: 10.1002/jsfa.7701. [DOI] [PubMed] [Google Scholar]
- Siegert W., Ganzer C., Kluth H., Rodehutscord M. Effect of amino acid deficiency on precaecal amino acid digestibility in broiler chickens. J. Anim. Physiol. Anim. Nutr. 2019;103:723–737. doi: 10.1111/jpn.13066. [DOI] [PubMed] [Google Scholar]
- Siegert W., Zuber T., Sommerfeld V., Krieg J., Feuerstein D., Kurrle U., Rodehutscord M. Prececal amino acid digestibility and phytate degradation in broiler chickens when using different oilseed meals, phytase and protease supplements in the feed. Poult. Sci. 2019;98:5700–5713. doi: 10.3382/ps/pez355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerfeld V., Schollenberger M., Kühn I., Rodehutscord M. Interactive effects of phosphorus, calcium, and phytase supplements on products of phytate degradation in the digestive tract of broiler chickens. Poult. Sci. 2018;97:1177–1188. doi: 10.3382/ps/pex404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerfeld V., Künzel S., Schollenberger M., Kühn I., Rodehutscord M. Influence of phytase or myo-inositol supplements on performance and phytate degradation products in the crop, ileum, and blood of broiler chickens. Poult. Sci. 2018;97:920–929. doi: 10.3382/ps/pex390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verband Deutscher Landwirtschaftlicher Untersuchungs- und Forschungsanstalten (VDLUFA) VDLUFA-Verlag; Darmstadt: 2012. Handbuch der Landwirtschaftlichen Versuchs- und Untersuchungsmethodik (VDLUFA-Methodenbuch), Bd. III. Die chemische Untersuchung von Futtermitteln. [Google Scholar]
- Wagner J.R., Sorgentini D.A. Thermal and electrophoretic behavior, hydrophobicity, and some functional properties of acid-treated soy isolates. J. Agric. Food Chem. 1996;44:1881–1889. [Google Scholar]
- Yiu S.H., Altossar I., Fulcher R.G. The effects of commercial processing on the structure and microchemical organization of rapeseed. Food Struct. 1983;2:165–173. [Google Scholar]
- Zaefarian F., Romero L.F., Ravindran V. Influence of a microbial phytase on the performance and the utilisation of energy, crude protein and fatty acids of young broilers fed on phosphorus-adequate maize- and wheat-based diets. Br. Poult. Sci. 2013;54:653–660. doi: 10.1080/00071668.2013.830209. [DOI] [PubMed] [Google Scholar]
- Zeller E., Schollenberger M., Kühn I., Rodehutscord M. Hydrolysis of phytate and formation of inositol phosphate isomers without or with supplemented phytases in different segments of the digestive tract of broilers. J. Nutr. Sci. 2015;4:e1. doi: 10.1017/jns.2014.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller E., Schollenberger M., Witzig M., Shastak Y., Kühn I., Hoelzle L.E., Rodehutscord M. Interactions between supplemented mineral phosphorus and phytase on phytate hydrolysis and inositol phosphates in the small intestine of broilers. Poult. Sci. 2015;94:1018–1029. doi: 10.3382/ps/pev087. [DOI] [PubMed] [Google Scholar]