Abstract
Respiratory tract diseases are closely related to atmosphere pollution. Ammonia is one of the harmful pollutants in the atmosphere environment, which has a great threat to human and animal respiratory tract health, but the mechanism of causing diseases is not clear. In this study, broiler lung tissue was used as a model to study the effect of high ammonia on respiratory tract diseases through the relationship between respiratory microflora, NLRP3 inflammasome, and inflammatory factors. For this, we validated the occurrence of lung tissue inflammation under ammonia exposure and detected the lung tissue microbial constituent by 16S rDNA sequencing. Moreover, the relative expression levels of NLRP3 and caspase-1 mRNA and the content of IL-1β and IL-6 were measured. After 7-D ammonia exposure, the proportion of the phylum Proteobacteria and the genus Escherichia/Shigella in lung tissue was significantly increased, the expression levels of NLRP3 and caspase-1 mRNA were significantly increased, and the content of IL-1β in lung tissue and serum was higher than that in the control group. In conclusion, high ammonia induced lung tissue inflammation via increasing the proportion of Escherichia/Shigella, activating NLRP3 inflammasome, and promoting IL-1β release. These findings provided a reference for the prevention and control of respiratory tract diseases in humans and animals caused by ammonia pollution.
Key words: ammonia, respiratory tract flora, NLRP3 inflammasome, IL-1β, inflammation
Introduction
Ammonia is one of the harmful pollutants in the atmosphere, which has bad smell at high concentration and negative effect on human and animal health (Tao et al., 2019). Ammonia is one of the gas components of haze and plays a vital role in haze formation (Ye et al., 2011, Saraswati et al., 2019). Many studies have shown that haze pollution is associated with human diseases, including respiratory diseases, mental health problems, and lung cancer, and it also affects traffic and life (Emmanuel, 2000, Gao et al., 2017). Similarly, studies have shown that ammonia can cause respiratory diseases in humans, including chronic bronchitis, pneumonia, and asthma (Woto-Gaye et al., 1999, Lei, 2014), and also cause cardiovascular and cerebrovascular diseases (Braissant et al., 2013). Atmosphere ammonia enters the respiratory tract of humans and animals by breathing and corrodes the respiratory system in the form of NH4+, which causes respiratory cilia damage or loss. The cilia on the mucosal surface are damaged, which can not prevent bacteria from entering the respiratory system, so it leads to disease susceptibility (Ghaly and MacDonald, 2013).
In recent years, with the expansion of breeding scale, more and more attention has been paid to the harm of ammonia to livestock respiratory system, especially in broiler chickens. And, it is worth mentioning that the high ammonia in livestock and poultry houses will also endanger the breeders and managers. Livestock industry guidelines suggest maintaining ammonia concentration below 25 ppm in broiler houses (Carlile, 1984). However, ammonia concentration always exceed the standard in actual production during the cold periods, and this phenomenon is prevalent all over the world (in China, United States, Great Britain, Norway, and so on) (Wathes et al., 1997, Von Wachenfelt et al., 2002, Qi et al., 2018). Studies have shown that broilers exposed to 25-ppm ammonia for 6 wk could stimulate the mucosa of broilers (Wang et al., 2006), cause oxidative stress injury and tissue pathological damage in trachea and lung tissue (Xiong et al., 2016), and lead to the decline of disease resistance in broilers. Exposure to 50-ppm ammonia could drop the number of cilia in the trachea and lungs of broilers and increase the inflammatory factors (Ritz et al., 2004).
Therefore, to cure or prevent diseases induced by ammonia exposure, the use of antibiotics is inevitable (Li et al., 2015). It is estimated that the global consumption of antimicrobial used for chickens, pigs, and cattle will increase by 67%, from 63,151 tons in 2010 to 105,596 tons in 2030 (Van Boeckel et al., 2015). The increasing use of antibiotics in intensive animal production is linked to the emergence of antibiotic-resistant bacterial strains and transferable resistance genes (Donoghue, 2003, Bywater et al., 2005). On the one hand, a large number of drug residues and drug resistance genes in feces affect human health through water pollution and air pollution (Barbosa and Levy, 2000). On the other hand, the use of antibiotics resulted in edible meats containing residues that pose extreme health concerns for human beings (Heuer et al., 2009). According to Food and Agriculture Organization of the United Nations forecast, in recent years, the proportion of chicken in world's meat consumption remain 36 to 40%, which means that the use of antibiotics poses a huge potential risk to human health.
Studies have been found that there is a correlation between bacterial flora, inflammasome, and inflammatory injury in human beings, and numerous bacteria and their components can activate or modulate nucleotide binding oligomerization domain-like receptor family, pyrin domain containing protein 3 (NLRP3), and inflammasome activation (Kim and Jo, 2013). Earlier studies showed that gram-negative Salmonella typhimurium or Francisella tularensis activated NLRP3 inflammasome and secreted the interleukin-1β (IL-1β) and the interleukin-18 (IL-18) (Mariathasan et al., 2006). And, a Crohn's disease research showed that Escherichia coli strains induced IL-1β production through NLRP3-dependent mechanism (De la Fuente et al., 2014). Upon detecting stress, NLRP3 recruits apoptosis-associated speck-like protein containing CARD (ASC) and pro-caspase-1, which results in caspase-1 activation and processing of cytoplasmic targets, including the proinflammatory cytokines IL-1β and IL-18 (Vandanmagsar et al., 2011). The NLRP3 inflammasome activation and the downstream cytokines (Schmitz et al., 2005) play a key role in innate (Allen et al., 2009, Thomas et al., 2009) and adaptive (Ichinohe et al., 2009) immune defense against bacteria infection in vivo.
High ammonia concentration causes respiratory tract inflammation, but up to now, there are no studies on the relationship between respiratory flora, inflammasome, and inflammation injury in livestock, and the inflammatory pathway under ammonia exposure has not been reported. Therefore, in this research, broiler lung tissue was used as a model to investigate whether the occurrence of lung tissue inflammation under ammonia exposure was related to respiratory tract microflora disorder and activation of NLRP3 inflammasome for the first time, to provide a theoretical basis for the application of nondrug (probiotics, prebiotics, and so on) control methods to regulate and control respiratory tract flora and provide a reference for the prevention and control of respiratory tract diseases in humans and animals caused by air pollution, especially harmful gas pollution.
Materials and methods
Birds, Diets, and Experimental Design
The handing protocol of animals in the study was approved by the Institutional Animal Care and Use Committee of the Chinese Academy of Agricultural Sciences. A total of 160 one-day-old male broiler chickens (Huadu Co. Ltd., Hebei, China) were housed in cages in a temperature- and humidity-controlled room and had free access to feed and water. After feeding the same starter diet for 21 D, one hundred and thirty-two 22-day-old broilers with similar weight were randomly divided into 2 treatment groups with 6 replicate cages (11 broilers per cage) for each treatment, according to a completely randomized design as follows: ammonia concentration < 3 ppm control group and 35 ± 3 ppm group. All broilers received normal food and were fed daily (Table 1).
Table 1.
Ingredients and nutrient compositions of the basal diet (g/kg diet as-fed basis).
| Ingredients (g/kg) | Content (%) |
|---|---|
| Corn | 56.51 |
| Soybean meal | 35.52 |
| Soybean oil | 4.50 |
| NaCl | 0.30 |
| Limestone | 1.00 |
| Dicalcium phosphate | 1.78 |
| DL-Methionine | 0.11 |
| Premix1 | 0.28 |
| Total | 100.00 |
| Calculated nutrient levels | |
| Metabolizable energy (MJ/kg) | 12.73 |
| Crude protein (g/kg) | 20.07 |
| Available Phosphorus (g/kg) | 0.40 |
| Calcium (g/kg) | 0.90 |
| Lysine (g/kg) | 1.00 |
| Methionine (g/kg) | 0.42 |
| Methionine + cysteine (g/kg) | 0.78 |
Premix provided the following per kg of the diet: vitamin A, 10,000 IU; vitamin D3, 3400 IU; vitamin E, 16 IU; vitamin K3, 2.0 mg; vitamin B1, 2.0 mg; vitamin B2, 6.4 mg; vitamin B6, 2.0 mg; vitamin B12, 0.012 mg; pantothenic acid calcium, 10 mg; nicotinic acid, 26 mg; folic acid, 1 mg; biotin, 0.1 mg; choline, 500 mg; Zn (ZnSO4·7H2O), 40 mg; Fe (FeSO4·7H2O), 80 mg; Cu (CuSO4·5H2O), 8 mg; Mn (MnSO4·H2O), 80 mg; I (KI) 0.35 mg; Se (Na2SeO3), 0.15 mg.
Sample Collection
At the seventh and 21st D of the experiment, 3 broilers of close to average weight were selected for each replicate. Wing venous blood was collected, and serum was obtained by centrifugation of a respective blood sample at 3,000r/min for 15 min at 4°C and stored at −80°C until further analysis. The broilers were sacrificed after collection of blood samples. The left lung tissues were isolated, washed with PBS and divided into 4 parts, mixed with 3 samples of each repetition into one sample, and then stored at −80°C until further analysis.
One lung tissue was dissected and fixed with 4% paraformaldehyde solution. Routine material was taken, dehydrated, paraffin embedded, sliced (micron thick), stained with hematoxylin and eosin, observed, and photographed under optical microscope. The microflora of lung tissue was measured by Shanghai Meiji Biological Company. The contents of interleukin (IL-1β, IL-6) in serum and lung tissue were detected by using an ELISA kit. The mRNA levels of NLRP3 and caspase-1 in lung tissue were identified by RT-qPCR.
DNA Extraction and PCR Amplification DNA (16s rDNA)
Microbial DNA was extracted from lung tissue samples using the E.Z.N.A. soil DNA Kit (Omega Bio-tek, Norcross, GA) according to manufacturer's protocols. The final DNA concentration and purification were determined by using a NanoDrop 2000 UV-vis spectrophotometer (Thermo Scientific, Wilmington, DE), and DNA quality was checked by 1% agarose gel electrophoresis. The V3-V4 hypervariable regions of the bacteria 16S rDNA gene were amplified with primers 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) by thermocycler PCR system (GeneAmp 9700; ABI). The PCR reactions were conducted using the following program: 3 min of denaturation at 95°C, 29 cycles of 30 s at 95°C, 30s for annealing at 55°C, 45 s for elongation at 72°C, and a final extension at 72°C for 10 min. PCR reactions were performed in triplicate 20-μL mixture containing 4 μL of 5 × FastPfu Buffer, 2 μL of 2.5 mmol dNTPs, 0.8 μL of each primer (5 μmol), 0.4 μL of FastPfu Polymerase, and 10 ng of template DNA. The resulted PCR products were extracted from a 2% agarose gel and further purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA) and quantified using QuantiFluor-ST (Promega, San Luis Obispo, CA) according to the manufacturer's protocol.
Illumina MiSeq Sequencing
Purified amplicons were pooled in equimolar and paired-end sequenced (2 × 300) on an Illumina MiSeq platform (Illumina, San Diego, CA) according to the standard protocols by Majorbio Bio-Pharm Technology Co. Ltd. (Shanghai, China).
Total RNA Extraction and Quantitative RT-PCR
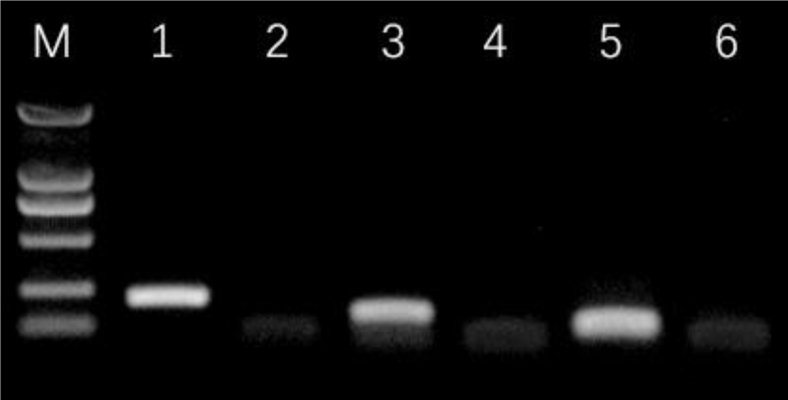
Following the manufacturer's instructions, total RNA in the samples was extracted using Trizol reagent (Invitrogen Co., Carlsbad, CA). The list of primers is given in Table 2. Then 100 μL of water without RNase was add to dissolve the RNA in water. DNasel was used to digest DNA in sample RNA at 37°C for 30 min, and RNA agarose gel electrophoresis was subsequently performed. RNA was reverse transcribed into cDNA and then detected by PCR. qRT-PCR cycling conditions were predenatured at 95°C for 5 min and 95°C for 10 s, 60°C for 30 s, 72°C for 30 s, 40 PCR cycles, and 4°C pause; electrophoresis at 120 V voltage for 20 min and photography with gel ultraviolet analyzer after electrophoresis. All experiments were carried out in triplicate. The 2−ΔΔCt method was used to evaluate the relative expression levels of genes by comparing sample expression relative to a housekeeping gene (β-actin). PCR product electrophoretogram was shown in Figure 1.
Table 2.
Primer Sequences for qRT-PCR.
| Primers | Sequences (5′ to 3′) | Bases | Product (bp) |
|---|---|---|---|
| β-actin | F:TCCACCGCAAATGCTTCTAA | 20 | 205 bp |
| R:GGGGCGTTCGCTCCA | 15 | ||
| NLRP3 | F:GCTCCTTGCGTGCTCTAAGACC | 22 | 150 bp |
| R:TTGTGCTTCCAGATGCCGTCAG | 22 | ||
| Caspase-1 | F:ACTTCGGATGGCTGGAGATGTGT | 23 | 110 bp |
| R:CAGGAGACAGTATCAGGCGTGGAA | 24 |
Figure 1.
PCR product electrophoretogram; 2,000 bp marker (M) shown as a reference. Lanes: 1, β-actin; 2, β-actin negative control; 3, NLRP3; 4, NLRP3 negative control; 5, Caspase-1; 6, Caspase-1 negative control.
Statistical Analysis
Numerical results are expressed as mean ± SEM. The significance of the difference between means was determined by ANOVA followed by Tukey's test, with P < 0.05 being considered significant.
Results
Ammonia Exposure Induce Pulmonary Injury
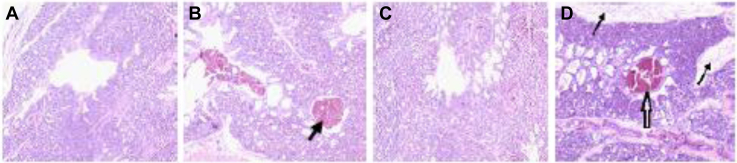
During the whole experimental period, the tissue structure of the control group was normal, and no obvious pathological changes were observed in Figures 2A and 2C. In the 35-ppm ammonia group, there were local tissue hemorrhage, a large number of red blood cells in the bronchus, focal infiltration of inflammatory cells in tissues, and connective tissue hyperplasia in some lobules in Figures 2 Band 2D.
Figure 2.
Effect of ammonia exposure on lung tissue structure. (A) Control group at 7 D; (B) 35-ppm group at 7 D; (C) control group at 21 D; (D) 35-ppm group at 21 D. Lung tissue cells were stained with hematoxylin and eosin (HE). The arrow in (B) indicates local tissue hemorrhage, the thin arrow in (D) indicates connective tissue hyperplasia, and the thick arrow in (D) indicates local tissue hemorrhage. Graphs were observed at 20×, the size unit of the photograph is 50 μm.
Ammonia Exposure Increased the Proportion of Escherichia/Shigella
16s rDNA Sequencing analysis was used to compare the microbial population of lung tissue in the control group and 35-ppm group at 7 D and 21 D of the experiment. After sequencing, an average of 51,139 (42,456-57,042) valid tags were obtained. Sequence clustering with at least 97% similarity is operational taxonomic unit.
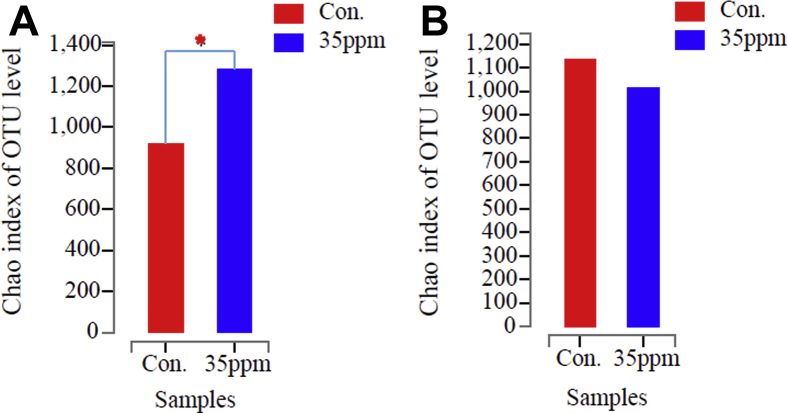
Chao1 index was used to evaluate the sequence richness of samples and to analyze the diversity in Figure 3. Welch's text was used to analyze the differences in species diversity among different groups. Chao1 index reflects microbial community richness. Under 7-D ammonia exposure, Chao1 index shows that species richness at 35-ppm ammonia concentration is significantly higher than that of control group in Figure 3A (P < 0.05). There is no significant difference in species richness between the 2 groups under 21-D ammonia exposure, but the species richness of the experimental group is lower than that of the control group, as shown in Figure 3B (P > 0.05).
Figure 3.
Chao1 index of microorganism OTU level in lung tissue under ammonia exposure. (A) Chao 1 index of control group and 35-ppm group at 7 D. (B) Chao 1 index of control group and 35-ppm group at 21 D. ∗ Indicates significant differences between the 2 groups.
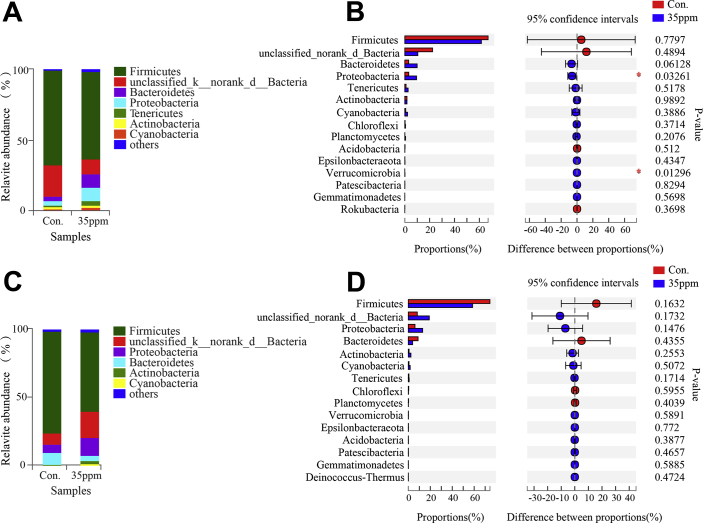
According to the community composition of bacteria at phylum level, 37 phylums were identified in the lung tissue under ammonia exposure. Under ammonia exposure, Firmicutes and unclassified bacteria were the dominant phylums in Figures 4A and 4B. And, there were significant changes in Proteobacteria and Verrucomicrobia microflora, as shown in Figure 4C, under 7-D ammonia exposure, but no significant changes in microflora under 21-D ammonia exposure, shown in Figure 4D.
Figure 4.
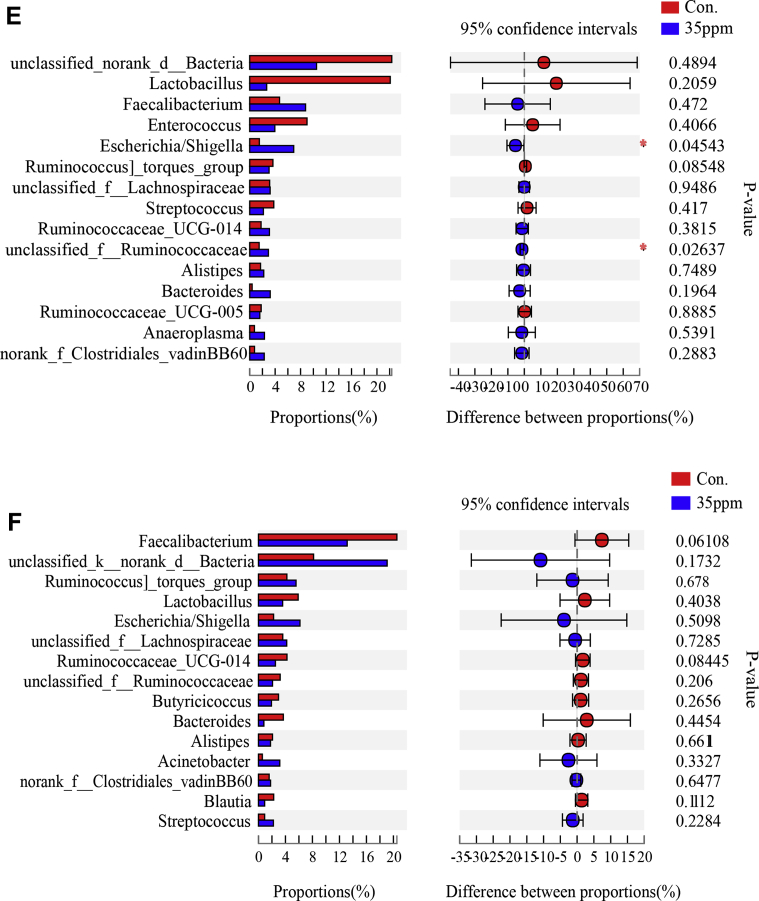
Effect of ammonia exposure on lung tissue microflora. (A) Histogram of phylum level at 7 D between control group and 35-ppm group. (B) Histogram of phylum level at 21 D between control group and 35-ppm group. (C) Difference of Student t test bar plot on phylum level at 7 D. (D) Difference of Student t test bar plot on phylum level at 21 D. (E) Difference of Student t test bar plot on genus level at 7 D. (F) Difference of Student t test bar plot on genus level at 21 D. ∗Indicates significant differences between the 2 groups.
The different genera of the first 15 species were analyzed. It was found that there were 2 difference genera under 7-D ammonia exposure (Figure 4E) Escherichia/Shigella and unclassified_f__Ruminococcaceae, while there was no difference in genera under 21-D ammonia exposure (Figure 4F).
NLRP3 Inflammasome Was Activated Under Ammonia Exposure
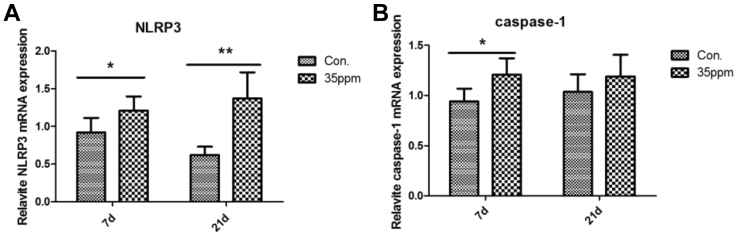
Effect of ammonia on the relative expression of NLRP3 inflammasome mRNA is shown in Figure 5. Compared with the control group, 35-ppm ammonia exposure significantly increased the expression of NLRP3 and caspase-1 mRNA at 7 D (P < 0.05). At 21-day ammonia exposure, the expression of NLRP3 mRNA significantly increased in the 35-ppm group, but the expression of caspase-1 mRNA did not show significant difference compared with the control group (P > 0.05).
Figure 5.
Effect of different ammonia concentrations on mRNA expression. (A) Relative NLRP3 mRNA expression of control group and 35-ppm group at 7 D and 21 D. (B) Relative caspase-1 mRNA expression of control group and 35-ppm group at 7 D and 21 D. The time from left to right is 7 D and 21 D, respectively. ∗∗P < 0.01 and ∗P < 0.05 indicate significant differences between the 2 groups.
IL-1β Content was Significantly Increased Under Ammonia Exposure
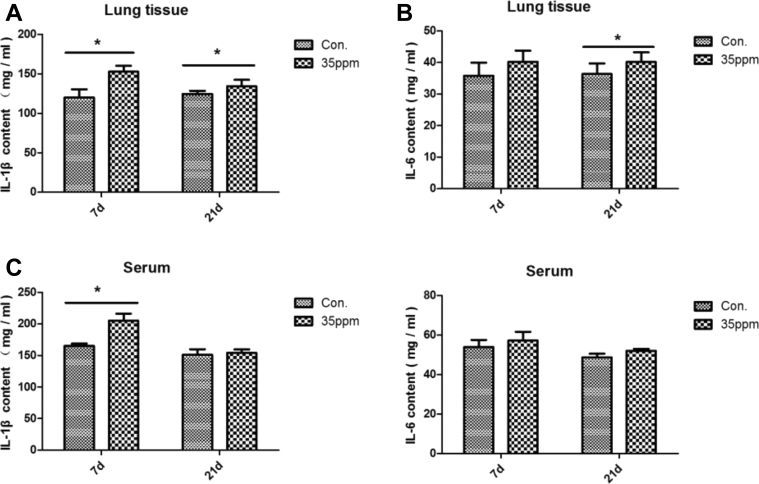
Figure 6 shows the effect of ammonia on cellular inflammatory factor in lung tissue and serum. In lung tissue, 35-ppm ammonia exposure significantly increased IL-1β content under 7-day and 21-day ammonia exposure (P < 0.05) and increased IL-6 content at 21 day (P < 0.05). But in serum, the IL-1β content in the 35-ppm group was significantly higher at 7 D (P < 0.05), while there was no significant difference at 21 D (P > 0.05), and ammonia had no significant effect on the content of IL-6 in serum.
Figure 6.
Effect of different ammonia concentrations on cellular inflammatory factor. (A) The content of IL-1β in lung tissue between control group and 35-ppm group. (B) The content of IL-6 in lung tissue between control group and 35-ppm group. (C) The content of IL-1β in serum between control group and 35-ppm group. (D) The content of IL-6 in serum between control group and 35-ppm group. The time from left to right is 7 D and 21 D, respectively. ∗P < 0.05 indicates significant differences between the 2 groups.
Discussion
Exogenous ammonia exposure has a negative impact on the respiratory system of humans and animals (Wheeler et al., 2003). Our present study found that 35-ppm ammonia exposure can induce lung tissue damage. Under the optical microscope, local tissue bleeding can be seen after ammonia exposure, and a large number of red blood cells have emerged. Our results are consistent with the previous results, and it has been found that 35-ppm ammonia can significantly affect the respiratory system of poultry, such as the injury of tracheal cilia and histopathological changes of tracheal epithelium (Nageraja et al., 1983). A study (Oyetunde et al., 1978) showed that a high ammonia level of 100 ppm would cause a large amount of mucus in poultry tracheal and the cilia exfoliated. The aforementioned results show that high ammonia exposure has a significant negative effect on respiratory tract.
Lower respiratory tract microflora is affected by changing oral cavity or upper respiratory tract microflora, but they are always in dynamic equilibrium with the host, which plays an important role in maintaining respiratory tract health of animals. This research is the first one to study the effect of ammonia exposure on bacterial flora of lung tissue using broilers as a model, and it found that on the seventh day of the experiment, the species richness of the lung tissue of broilers increased under high ammonia exposure. In terms of phylum, the proportion of Proteobacteria increased significantly under 7-D ammonia exposure. Similarly, in a survey of environmental pollution, the relative abundance of Proteobacteria was higher in participants from regions heavily polluted with PM2.5 (Li et al., 2019), which indicated that ammonia exposure and other air pollutants could cause respiratory tract flora disorder. A recently study has suggested that abnormalities of Proteobacteria are often associated with microecological disorder, and there is a view that the increase of Proteobacteria is considered as a factor for disease diagnosis and potential disease (Shin et al., 2015). Hilty et al. (Hilty et al., 2010) found that the number of Proteobacteria was abundant in asthmatic children. Huang et al. (Huang et al., 2014) reported that the increase of microbiota at exacerbation of COPD was primarily due to the Proteobacteria. This means the increase of Proteobacteria in respiratory tract may be related to respiratory tract diseases in broilers.
In terms of genus, the proportion of Escherichia/Shigella increased significantly after 7 D of high ammonia exposure in our research. Nagaraja (Nagaraja et al., 1984) found that more E. coli was found in lungs and air sacs of turkeys exposed to NH3, and it was consistent with the findings of our study. Escherichia/Shigella belongs to the phylum Proteobacteria and is generally considered to be nonpathogenic bacteria, but when stimulated by stress, Escherichia/Shigella can become pathogenic bacteria (Lutful Kabir, 2010), leading to the occurrence of diseases. In some human inflammatory diseases, microflora changes are typified by alterations in the dominant organisms, specifically reduction in beneficial Firmicutes and increase in numbers of Proteobacteria (including E. coli) (Mondot et al., 2011). Our study also found that the proportion of unclassified_f__Ruminococcaceae is significantly increased; however, it is impossible to distinguish which genus it belongs to, so it is not analyzed here. Based on the aforementioned results, we indicated that the high concentration of ammonia exposure disturbed the ecological balance of the lung tissue flora, increased the proportion of the phylum Proteobacteria and the genus Escherichia/Shigella, and damaged the lung tissue of broilers.
Recently, the inflammasome, a multimeric protein complex consisting of nucleotide binding oligomerization domain-like receptor, ASC, and caspase-1 was shown to be important in the maturation and secretion of IL-1β and related family members (Zhou et al., 2011, Wen et al., 2013). In particular, the NLRP3 inflammasome has been extensively studied and can be activated by a variety of stimuli including microbial infection (Leemans et al., 2011, Vladimer et al., 2013). Upon detecting cellular stress, NLRP3 recruits ASC and procaspase-1, which results in caspase-1 activation and processing of cytoplasmic targets, including the proinflammatory cytokines IL-1β and IL-18 (Zhou et al., 2011). The research has been found that bacteria represent a potential trigger for NLRP3 activation (Pedicino et al., 2013). And in the case of bacterial infection, pore-forming toxins and bacterial mRNA represent the major triggers of NLRP3 activation. Study has shown that live nonpathogenic and pathogenic E. coli both can elicit the NLRP3 inflammasome (Kayagaki et al., 2011, Sander et al., 2011). In our current experiment, under 7-D ammonia exposure, we found that the proportion of Escherichia/Shigella in lung tissue increased significantly, and the mRNA expression of NLRP3 and caspase-1 also increased, which means the increase of lung tissue Escherichia/Shigella can be a factor of activating NLRP3 inflammasome under ammonia exposure.
The importance and mechanisms of action of NLRP3 inflammasome activation have recently been elucidated in the respiratory tract inflammation (Guo et al., 2015, Liu et al., 2015, Sayan and Mossman, 2016). After the activation of NLRP3 inflammasome, mature caspase-1 was released, and subsequent IL-1β and IL-18 were excessively secreted (Vandanmagsar et al., 2011). However, excessive release of these cytokines, particularly IL-1β, is associated with autoimmunity and autoimmune disorders (Iwasaki and Medzhitov, 2015). In our study, we found that the mRNA expression of NLRP3 and caspase-1 were all increased under ammonia exposure, and the content of IL-1β was also increased in lung tissue. This suggested that there is activation of NLRP3 inflammasome under ammonia exposure, which could induce respiratory tract inflammation. That is to say, NLRP3 inflammasome induces inflammatory response by releasing mature caspase-1 through the activated inflammasome (Martinonet al., 2006), which leads to the division and secretion of IL-1 family cytokines (Hornung et al., 2008). In addition, previous literature found that E. coli strains induced IL-1β secretion through an NLRP3-dependent mechanism (Mariathasan et al., 2006). Combined with the aforementioned studies, we found that Escherichia/Shigella could activate NLRP3 inflammasome, while the activation of NLRP3 inflammatory pathway could promote the secretion of IL-1β, leading to inflammation of lung tissue.
In addition, we also found that compared with 7 D, there was no significant change in the flora of lung tissue under 21-D ammonia exposure, but the expression of NLRP3 was significantly increased, the content of IL-1β was increased, and the inflammatory damage of lung tissue still existed. This likely indicates that the inflammatory damage caused by the significant increase of pathogenic bacteria in the early ammonia exposure period did not recover or is the secondary effect of the significant increase of pathogenic bacteria in the early stage or both. Moreover, with the prolongation of ammonia exposure time, the lung inflammatory response and the machine of lung injury are more complicated. Therefore, no matter if under 7-D ammonia exposure or 21-D ammonia exposure, we think the lung tissue damage in broilers is caused by activating NLRP3 inflammasome via pathogenic bacteria.
In conclusion, we found that the proportion of Escherichia/Shigella in lung tissue increased under 7-D ammonia exposure, the numerous Escherichia-Shigella could activate or modulate NLRP3 inflammasome activation, and then the NLRP3 inflammasome induces expression of IL-1β and lung tissue inflammation. In other words, high ammonia exposure induces lung tissue inflammation by activating the NLRP3 inflammasome though Escherichia/Shigella. These results provided a solid scientific basis for the application of nondrug (probiotics, prebiotics, and so on) control methods to regulate and control respiratory tract flora and provide a reference for the prevention and control of respiratory tract diseases in humans and animals caused by air pollution, especially harmful gas pollution.
Acknowledgements
The study was supported by the National Key Research and Development Program of China (No. 2016YFD0500509).
Conflict of Interest Statement: The authors did not provide a conflict of interest statement.
References
- Allen I.C., Scull M.A., Moore C.B., Holl E.K., McElvania-Tekippe E., Taxman D.J., Guthrie E.H., Pickles R.J., Ting J.P.-Y. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009;30:556–565. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa T.M., Levy S.B. The impact of antibiotic use on resistance development and persistence. Drug Resist. Updates. 2000;3:303–311. doi: 10.1054/drup.2000.0167. [DOI] [PubMed] [Google Scholar]
- Braissant O., Mclin V.A., Cudalbu C. Ammonia toxicity to the brain. J. Inherit. Metab. Dis. 2013;36:595–612. doi: 10.1007/s10545-012-9546-2. [DOI] [PubMed] [Google Scholar]
- Bywater R., McConville M., Phillips I., Shryock T. The susceptibility to growth-promoting antibiotics of Enterococcus faecium isolates from pigs and chickens in Europe. J. Antimicrob. Chemother. 2005;56:538–543. doi: 10.1093/jac/dki273. [DOI] [PubMed] [Google Scholar]
- Carlile F.S. Ammonia in poultry houses: a literature review. Worlds Poult. Sci. J. 1984;40:99–113. [Google Scholar]
- De la Fuente M., Franchi L., Araya D., Díaz-Jiménez D., Olivares M., Álvarez-Lobos M., Golenbock D., González M.J., López-Kostner F., Quera R., Núñez G., Vidal R., Hermoso M.A. Escherichia coli isolates from inflammatory bowel diseases patients survive in macrophages and activate NLRP3 inflammasome. Int. J. Med. Microbiol. 2014;304:384–392. doi: 10.1016/j.ijmm.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue D.J. Antibiotic residues in poultry tissues and Eggs: human health concerns? Poul. Sci. 2003;82:618–621. doi: 10.1093/ps/82.4.618. [DOI] [PubMed] [Google Scholar]
- Emmanuel S.C. Impact to lung health of haze from forest fires: the Singapore experience. Respirology. 2000;5:175–182. doi: 10.1046/j.1440-1843.2000.00247.x. [DOI] [PubMed] [Google Scholar]
- Gao J., Woodward A., Vardoulakis S., Kovats S., Wilkinson P., Li L., Xu L., Li J., Yang J., Li J., Cao L., Liu X., Wu H., Liu Q. Haze, public health and mitigation measures in China: a review of the current evidence for further policy response. Sci. Total Environ. 2017;578:148–157. doi: 10.1016/j.scitotenv.2016.10.231. [DOI] [PubMed] [Google Scholar]
- Ghaly A.E., MacDonald K.N. Development and testing of an ammonia Removal Unit from the Exhaust gas of a manure Drying system. Am. J. Environ. Sci. 2013;9:51–61. [Google Scholar]
- Guo H., Callaway J.B., Ting J.P. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat. Med. 2015;21:677–687. doi: 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer O.E., Kruse H., Grave K., Collignon P., Karunasagar I., Angulo F.J. Human health consequences of use of antimicrobial agents in aquaculture. Clin. Infect. Dis. 2009;49:1248–1253. doi: 10.1086/605667. [DOI] [PubMed] [Google Scholar]
- Hilty M., Burke C., Pedro H., Cardenas P., Bush A., Bossley C., Davies J., Ervine A., Poulter L., Pachter L., Moffatt M.F., Cookson W.O.C. Disordered microbial communities in asthmatic airways. PLoS One. 2010;5:e8578. doi: 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung V., Bauernfeind F., Halle A., Samstad E.O., Kono H., Rock K.L., Fitzgerald K.A., Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.J., Sethi S., Murphy T., Nariya S., Boushey H.A., Lynch S.V. Airway Microbiome dynamics in exacerbations of chronic Obstructive Pulmonary disease. J. Clin. Microbiol. 2014;52:2813–2823. doi: 10.1128/JCM.00035-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinohe T., Lee H.K., Ogura Y., Flavell R., Iwasaki A. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J. Exp. Med. 2009;16:79–87. doi: 10.1084/jem.20081667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki A., Medzhitov R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 2015;16:343–353. doi: 10.1038/ni.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayagaki N., Warming S., Lamkanfi M., Walle L.V., Louie S., Dong J., Newton K., Qu Y., Liu J., Heldens S., Zhang J., Lee W.P., Roose-Girma M., Dixit V.M. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- Kim J.J., Jo E.K. NLRP3 inflammasome and host Protection against bacterial infection. J. Korean Med. Sci. 2013;28:1415–1423. doi: 10.3346/jkms.2013.28.10.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leemans J.C., Cassel S.L., Sutterwala F.S. Sensing damage by the NLRP3 inflammasome. Immunological Rev. 2011;243:152–162. doi: 10.1111/j.1600-065X.2011.01043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y. Hazards of haze and Countermeasures. Appl. Mech. Mater. 2014;507:817–820. [Google Scholar]
- Li C., Lu Q., Tang X., Zhang J., Ding Z., Zhang H. Influence of ammonia concentration on growth performance and meat quality of broilers. Scientia Agricultura Sinica. 2014;47:4516–4523. [Google Scholar]
- Li X., Sun Y., An Y., Wang R., Lin H., Liu M., Li S., Ma M., Xiao C. Air pollution during the winter period and respiratory tract microbial imbalance in a healthy young population in Northeastern China. Environ. Pollut. 2019;246:972–979. doi: 10.1016/j.envpol.2018.12.083. [DOI] [PubMed] [Google Scholar]
- Liu Z., Zhao H., Liu W., Li T., Wang Y., Zhao M. NLRP3 inflammasome activation is essential for paraquat-induced acute lung injury. Inflammation. 2015;38:433–444. doi: 10.1007/s10753-014-0048-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutful Kabir S.M. Avian Colibacillosis and Salmonellosis: a closer Look at Epidemiology, Pathogenesis, diagnosis, control and public health concerns. Int. J. Environ. Res. Public Health. 2010;7:89–114. doi: 10.3390/ijerph7010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariathasan S., Weiss D.S., Newton K., McBride J., O'Rourke K., Roose-Girma M., Lee W.P., Weinrauch Y., Monack D.M., Dixit V.M. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- Martinon F., Petrilli V., Mayor A., Tardivel A., Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- Mondot S., Kang S., Furet J.P., Aguirre de Carcer D., McSweeney C., Morrison M., Marteau P., Doré J., Leclerc M. Highlighting new phylogenetic specificities of Crohn's disease microbiota. Inflamm. Bowel Dis. 2011;17:185–192. doi: 10.1002/ibd.21436. [DOI] [PubMed] [Google Scholar]
- Nageraja K.V., Emery D.A., Jordan K.A., Newman J.A., Pomeroy B.S. Scanning electron microscopic studies of adverse effects of ammonia on tracheal tissues of turkeys. Am. J. Vet. Res. 1983;44:1530–1536. [PubMed] [Google Scholar]
- Nagaraja K.V., Emery D.A., Jordan K.A., Sivanandan V., Newman J.A., Pomeroy B.S. Effect of ammonia on the quantitative clearance of Escherichia coli from lungs, air sacs, and livers of turkeys aerosol vaccinated against Escherichia coli. Am. J. Vet. Res. 1984;45:392–395. [PubMed] [Google Scholar]
- Oyetunde O.O., Thomson R.G., Carlson H.C. Aerosol exposure of ammonia, dust and Escherichia coli in broiler chickens. Can. Vet. J. 1978;19:187–193. [PMC free article] [PubMed] [Google Scholar]
- Pedicino D., Giglio A.F., Galiffa V.A., Cialdella P., Trotta F., Graziani F., Liuzzo G. Infections, immunity and atherosclerosis: pathogenic mechanisms and unsolved questions. Int. J. Cardiol. 2013;166:572–583. doi: 10.1016/j.ijcard.2012.05.098. [DOI] [PubMed] [Google Scholar]
- Qi L., Li F., Liu X., Liu T., Zhang Y., Shi T., Jing Q., Song L., Ju Y., Ai W., Wang Y. Environmental indicator Monitoring and production performance analysis of broilers under different feeding Patterns in winter. Shandong Agric. Sci. 2018;50:138–141. [Google Scholar]
- Ritz C.W., Fairchild B.D., Lacy M.P. Implications of ammonia production and Emissions from Commercial poultry Facilities: a review. J. .Appl. Poult. Res. 2004;13:684–692. [Google Scholar]
- Sander L.E., Davis M.J., Boekschoten M.V., Amsen D., Dascher C.C., Ryffel B., Swanson J.A., Müller M., Blander J.M. Detection of prokaryotic mRNA signifies microbial viability and promotes immunity. Nature. 2011;474:385–389. doi: 10.1038/nature10072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraswati S., Sharma K., Saxen M., Mand T.K. Characteristics of gaseous and particulate ammonia and their role in the formation of secondary inorganic particulate matter at Delhi, India. Atmos. Res. 2019;218:34–49. [Google Scholar]
- Sayan M., Mossman B.T. The NLRP3 inflammasome in pathogenic particle and fibre-associated lung inflammation and diseases. Part Fibre Toxicol. 2016;13:51. doi: 10.1186/s12989-016-0162-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz N., Kurrer M., Bachmann M.F., Kopf M. Interleukin-1 is responsible for acute lung immunopathology but increases survival of respiratory influenza virus infection. J. Virol. 2005;79:6441–6448. doi: 10.1128/JVI.79.10.6441-6448.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin N., Whon T., Bae J. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015;33:496–503. doi: 10.1016/j.tibtech.2015.06.011. [DOI] [PubMed] [Google Scholar]
- Tao Z., Xu W., Zhu C., Zhang S., Shi Z., Song W., Liu H., Li H. Effects of ammonia on intestinal microflora and productive performance of laying ducks. Poult. Sci. 2019;98:1947–1959. doi: 10.3382/ps/pey578. [DOI] [PubMed] [Google Scholar]
- Thomas P.G., Dash P., Aldridge J.R., Jr., Ellebedy A.H., Reynolds C., Funk A.J., Martin W.J., Lamkanfi M., Webby R.J., Boyd K.L., Doherty P.C., Kanneganti T. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity. 2009;30:566–575. doi: 10.1016/j.immuni.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandanmagsar B., Youm Y.H., Ravussin Anthony., Galgani J.E., Stadler K., Mynatt R.L., Ravussin E., Stephens J.M., Dixit V.D. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 2011;7:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Boeckel T.P., Brower C., Gilbert M., Grenfell B.T., Levin S.A., Robinson T.P., Teillant A., Laxminarayan R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. 2015;112:5649–5654. doi: 10.1073/pnas.1503141112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vladimer G.I., Marty-Roix R., Ghosh S., Weng D., Lien E. Inflammasomes and host defenses against bacterial infections. Curr. Opin. Microbiol. 2013;16:23–31. doi: 10.1016/j.mib.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Wachenfelt E., Odén K., Gunnarsson S. Swedish University of Agricultural Sciences, Department of Agricultural Biosystems and Technology; Alnarp, Sweden: 2002. Värphöns I Lågbeläggningssystem; pp. 1–78. In Swedish. [Google Scholar]
- Wathes C.M., Holden M.R., Sneath R.W., White R.P., Phillips V.R. Concentrations and emission rates of aerial ammonia, nitrous oxide, methane, carbon dioxide, dust and endotoxin in UK broiler and layer houses. Br. Poult. Sci. 1997;38:14–28. doi: 10.1080/00071669708417936. [DOI] [PubMed] [Google Scholar]
- Wang M., Meng X., Zhao Z., Jing L. Hazard and prevention of ammonia to poultry industry. China Poult. 2006;28:27–28. [Google Scholar]
- Wen H., Miao E.A., Ting J.P. Mechanisms of NOD-like receptor-associated inflammasome activation. Immunity. 2013;39:432–441. doi: 10.1016/j.immuni.2013.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler E.F., Casey K.D., Zajaczkowski J.S., Topper P.A., Gates R.S., Xin H., Liang Y., Tanaka A. Ammonia emissions from U.S. poultry houses: Part III - broiler houses. Proc. 3rd International Conference on Air Pollution from Agricultural Operations, Research Triangle Park, NC. 2003 [Google Scholar]
- Woto-Gaye G., Mendez V., Boye I.A., Ndiaye P.D. Death fromammonia poisoning : anatomo-pathologic features. Dakar Med. 1999;44:199–201. [PubMed] [Google Scholar]
- Xiong Y., Tang X., Meng Q., Zhang H. Differential expression analysis of the broiler tracheal proteins responsible for the immune response and muscle contraction induced by high concentration of ammonia using iTRAQ-coupled 2D LC-MS/MS. Sci. China Life Sci. 2016;59:1166–1176. doi: 10.1007/s11427-016-0202-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X., Ma Z., Zhang J., Du H., Chen J., Chen H., Yang X., Gao W., Geng F. Important role of ammonia on haze formation in Shanghai. Environ. Res. Lett. 2011;6:024019. [Google Scholar]
- Zhou R., Yazdi A.S., Menu P., Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]