Abstract
The objective of this study was to determine the effects of cadmium (Cd) on histological changes, lipid metabolism, and oxidative and endoplasmic reticulum (ER) stress in the liver of layers. A total of 480 hens at 38 wk of age were randomly assigned in 5 groups that were fed a basal diet or basal diet supplemented with CdCl2 2.5H2O at 7.5, 15, 30, and 60 mg Cd/kg feed for 9 wk. The results showed that accumulation of Cd was the greatest in the kidney, followed by the liver, pancreas, and lung. Diet contaminated with 30 mg Cd/kg induced antioxidant defenses accompanied by the increase of the activities of antioxidant enzymes in the liver, while dietary supplementation with 60 mg Cd/kg decreased the antioxidant levels significantly (P < 0.05). Immunofluorescence assay showed Cd induced reactive oxygen species production and endoplasmic reticulum stress in hepatocytes. Exposure to 60 mg Cd/kg significantly upregulated the expression of cytochrome C, caspase 3, caspase 9, caspase 7, Grp78, and Chop (P < 0.05). Histopathology and quantitative real-time PCR results presented periportal fibrosis, bile duct hyperplasia, and periportal inflammatory cell infiltration in the liver accompanied by upregulating the expression of tumor necrosis factor-α, IL-6 and IL-10 in the 30- or 60-mg Cd/kg groups. Oil Red O staining and RT-qPCR results showed dietary supplementation with 7.5, 15, and 30 mg Cd/kg promoted the synthesis of lipid droplets and upregulated the expression of fatty acid synthase, while dietary supplementation with 60 mg Cd/kg attenuated the synthesis of lipid droplets and downregulated the expression of acyl-CoA oxidase 1, carnitine palmitoyltransferase-1, and perixisome proliferation-activated receptor α (P < 0.05). Besides, the expression of vitellogenin (VTG) II and microsomal triglyceride transfer protein were upregulated in the 7.5-mg Cd/kg group, and the expressions of apolipoprotein B, vitellogenin II, and apolipoprotein very-low-density lipoprotein-II were downregulated in the 30- and/or 60-mg Cd/kg groups (P < 0.05). Conclusively, although low-dose Cd exposure promoted the synthesis of lipids and lipoproteins in the liver, the increase of Cd exposure could trigger liver injury through inducing oxidative and endoplasmic reticulum stress and negatively affect lipid metabolism and yolk formation in laying hens.
Key words: cadmium, liver, endoplasmic reticulum stress, lipid metabolism, laying hens
Introduction
Environmental pollution has increased over the past decades, leading to potential risks to all biological systems, including poultry sector (Alagawany et al., 2018). Cadmium (Cd), as a toxic heavy metal, is ubiquitous in a natural environment with a wide variety of adverse effects. Human activities contribute to the wide spread of Cd occurrence, including mining residues, the combustion of fossil fuels, electroplating, and manufacture of Nickel–Cd batteries (Martelli et al., 2006). It is for these reasons that Cd is found in almost everything we eat, drink, and breathe (Thompson and Bannigan, 2008). Continuous exposure to low levels of Cd leads to its deposition in different tissues day after day, causing toxic effects on various organs (Khafaga et al., 2019). Its chemical similarity to some nutritive metals enables it to mimic and displace the metals in various biological structures (Ghosh and Thomas, 1995). The emerging evidence has found that Cd may cause serious health problems even at low concentrations because of its high accumulation tendency (Järup and Åkesson, 2009). Cd pollution in animal production has been a problem for several countries, and in some situations, the concentration of Cd in manure and animal feed can reach up to even 130 mg/kg (Li et al., 2010). Chronic exposure to Cd can induce hepatotoxicity, renal dysfunction, and neurotoxicity (Branca et al., 2018, Rong et al., 2018).
The liver is one of the primary sites of Cd accumulation and a main target organ affected by Cd toxicity. The effects of Cd intoxication on the redox status of the liver have been documented in most studies (El-Sokkary et al., 2010). Reactive oxygen species (ROS) are usually closely related to Cd toxicology, both in intact animals (Yamano et al., 2000) and in a variety of cell culture systems (Hassoun and Stohs, 1996, He et al., 2008). It has been suggested that Cd can enhance the production of ROS through depleting glutathione (GSH) and protein-bound sulfhydryl groups, resulting in increased lipid peroxidation (LPO) and DNA damages (Hassoun and Stohs, 1996, Waisberg et al., 2003). Birds subchronically exposed to Cd show disorder of the oxidative and antioxidative balance (Li et al., 2013). In addition, oxidative stress can trigger several types of injuries through different signaling pathways, such as inflammation (Wu et al., 2014) and endoplasmic reticulum (ER) stress (Jin et al., 2016). Endoplasmic reticulum is a critical organelle responsible for cell survival and normal function, in which most secreted and transmembrane proteins are synthesized, folded, assembled, and modified (Galluzzi et al., 2014). A variety of environmental stimuli can induce ER stress by disturbing the ER homeostasis (Zhang et al., 2013, Wang et al., 2016). Many studies have demonstrated that Cd exposure can cause oxidative stress and ER stress in various organs (Jin et al., 2016).
In avian species, the liver gives the most important contribution to lipogenesis and accounts for about 95% of the de novo fatty acid synthesis. In addition, the liver is also a vital organ for the intermediary metabolism of lipids and energy (Huang et al., 2013), in which several key enzymes, such as acyl-CoA oxidase 1 (ACOX1), carnitine palmitoyltransferase-1 (CPT1), fatty acid synthase (FASN), and perixisome proliferation-activated receptor α (PPARα), play a central role in the normal process of lipid metabolism (Oaxaca-Castillo et al., 2007, Schlaepfer et al., 2014, Pawlak et al., 2015). Besides, in laying hens, the ovum vitellogenin (VTG), very-low-density lipoprotein (VLDL), apolipoprotein B (apoB), and the microsomal triglyceride transfer protein (MTP) play a critical role in the formation of yolk protein (Schneider et al., 1990, Shen et al., 1993). Lipids of the eggs not only act as a significant source of fat to human diet but also are important for chick embryo development (Noble and Cocchi, 1990). Recent studies have revealed that high dose of Cd exposure could affect the normal processes of lipid metabolism by regulating the lipid synthesis and fatty acids β-oxidation (Wu et al., 2017). Nevertheless, it is not clear whether Cd exposure will interfere with the liver lipid metabolism and yolk lipoprotein synthesis in laying hens.
Based on these studies, the objective of this study was to evaluate the toxic effects of Cd on Cd residues in organs, serum biochemistry, serum and liver antioxidant enzymes activities, liver histopathology, and hepatic lipid metabolism, oxidative stress, and ER stress of laying hens.
Materials and methods
The present study was approved by the Animal Care and Welfare Committee and the Scientific Ethical Committee of the Zhejiang University (No. ZJU2013105002), Hangzhou, China.
Birds, Diets, and Management
A total 480 Hy-Line Brown laying hens at 38 wk of age were obtained from a commercial poultry layer farm in Jiande, China. After 3 D of acclimation, hens were randomly assigned in 5 treatments, each of which included 6 replicates of 16 laying hens. The protocol of treatments was as follows: 1) basal diet (control); 2) basal diet +7.5 mg Cd/kg; 3) basal diet +15 mg Cd/kg; 4) basal diet +30 mg Cd/kg; and 5) basal diet +60 mg Cd/kg. During the whole experimental period (10 wk, including a 1-wk adaptation period and a 9-wk experimental stage), diet and water were provided ad libitum and birds were kept in a naturally ventilated poultry house with temperature between 23°C and 26°C, the relative humidity between 65 and 75%, and illumination at 16 h/D. All the animals were treated humanely with care in accordance with the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Science and published by the National Institute of Health. The composition of basal diet is presented in Table 1.
Table 1.
Ingredient compositions and nutrient levels of basal diet for hens (air-dry basis).
| Basal ingredients | Value | Nutrient level2 | Value |
|---|---|---|---|
| Corn, % | 65 | Metabolism energy, MJ/kg | 2.65 |
| Soybean meal (42.0% CP), % | 21 | Crude protein, % | 15.73 |
| Hydrolyzed feather meal, % | 1 | Ether extract, % | 6.32 |
| Fish meal, % | 1 | Lysine, % | 0.78 |
| Limestone, % | 7 | Methionine, % | 0.34 |
| Premix1, % | 5 | Cysteine, % | 0.32 |
| Total phosphorus, % | 0.61 | ||
| Total, % | 100 | Calcium, % | 3.45 |
The premix provided the following per kilogram of diet: vitamin A, 7,000 IU; vitamin D3, 2,500 IU; vitamin E, 49.5 mg; vitamin K3, 1 mg; vitamin B1, 1.5 mg; vitamin B2, 4 mg; vitamin B6, 2 mg; vitamin B12, 0.02 mg; niacin, 30 mg; folic acid, 0.55 mg; pantothenic acid, 10 mg; biotin, 0.16 mg; chloride choline, 500 mg; sodium chloride, 2,500 mg; Cu, 20 mg; Fe, 70 mg; Mn, 100 mg; Zn, 70 mg; I, 0.4 mg; Se, 0.5 mg.
Estimated from Chinese feed database provided with tables of feed composition and nutritive values in China (2015 26th edn.).
The actual concentrations of dietary Cd were detected by graphite furnace atomic absorption spectrometry as described by MOHC (2015). Briefly, for Cd analysis, 1.5 g of feed sample (12 samples per treatment) was weighed. The sample was transferred to a microwave digestion vessel and digested with mixed solution of 5 mL of HNO3 and 1 mL of H2O2 in a microwave oven (MDS-2000, CEM Corp., Matthews, NC). The acid was evaporated to dryness. The sample was rinsed 3 times with 1 mL of 1% HNO3 and then transferred to a 10-mL volumetric flask and brought to final volume with 1% HNO3. Twenty microliters of samples and configured Cd standard solutions were introduced into the graphite tube using a sample dispenser, and the absorbance was measured. In this experimental method, the limit of detection and limit of quantification were 0.001 mg/kg and 0.003 mg/kg, respectively. The results showed that the actual concentrations of Cd in the 5 groups of feed samples were 0.47 ± 0.03, 7.58 ± 0.18, 15.56 ± 0.35, 30.55 ± 0.21, and 60.67 ± 0.29 Cd/kg, respectively. CdCl2 was purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China; purity ≥ 99%).
Collection of Samples and Measurements
At the end of the experimental period, 12 birds per treatment (2 birds in each replicate) were randomly selected. After the hens were made to fast for 12 h (water was offered ad libitum), the blood samples were collected in 1.5-mL Eppendorf tubes by puncture of the wing vein. These tubes were centrifuged at 3,000 × g for 15 min to separate serum and stored at −80°C for biochemical and serum antioxidants analysis. Birds were euthanized by cervical dislocation. Samples of the liver, kidney, spleen, lung, heart, pancreas, ovary, magnum, and shell gland were dissected and kept at −20°C for analyzing the residues of Cd. The residues of Cd in various organs were detected by graphite furnace atomic absorption spectrometry in accordance with the method for the detection of Cd residues in feed. Three portions of the liver were snap frozen in liquid nitrogen and stored at −80°C for antioxidant, ROS, oil red O staining, and molecular analysis. Another portion of the liver was selected and sampled in the 4% paraformaldehyde for the histopathological analysis.
Serum Biochemical Analysis
Serum contents of total protein, albumin (ALB), and total bile acid (TBA), as well as activities of aspartate aminotransferase (GOT), alanine aminotransferase (GPT), and alkaline phosphatase (ALP), were determined in serum samples using the commercially available assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) as per the instructions of the manufacturer.
Serum and Liver Antioxidant Enzyme Assays
Liver tissue samples were cut into small pieces (about 0.1 g), and then, ice cold physiological saline was added to it at a ratio of 1:9 to prepare 10% tissue homogenate. The homogenate was centrifuged at 3,000 rpm for 10 min at 4°C. The supernatant was collected and stored at −80°C for the following analysis. The activities of catalase (CAT), glutathione peroxide (GSH-Px), total superoxide dismutase (T-SOD), and total antioxidant capacity (T-AOC) and the content of GSH and malondialdehyde (MDA) in the serum and hepatic supernatants were assayed using commercially available assay kits (Nanjing Jiancheng Bioengineering Institute) as per the instructions of the manufacturer.
Histopathological Analysis
The liver tissues fixed with 4% paraformaldehyde were trimmed and embedded in paraffin wax. The paraffin-embedded tissues were cut into 5- to 6-μm-thick sections using a microtome (RM2016, Leica Microsystems, Wetzlar, Germany), then stained with hematoxylin and eosin (H&E) for histopathological observation by optical microscopy at a final magnification of ×400 (Nikon Eclipse 80i, Nikon, Tokyo, Japan).
Reactive Oxygen Species and Lipid Assays
Dihydroethidium (DHE) oxidative fluorescence dye was used to evaluate in situ production of ROS (Wang et al., 2014). Dihydroethidium stock solution was prepared by dissolving Dihydroethidium (D7008, Sigma Aldrich, St. Louis, MO) in dimethyl sulfoxide at a concentration of 5 mmol and then diluted in PBS to a final concentration of 5 μmol before use. Fresh liver tissues (10 mm) of laying hens, 5 in each group, were embedded in Tissue-Tek OCT compound (Sakura, Tokyo, Japan) and made into 8- to 10-μm-thick frozen sections. Dihydroethidium working solution (200 μL) was topically applied to the liver sections, and the slides were subsequently incubated at 37°C for 30 min. Excess Dihydroethidium was rinsed off 3 times with PBS, and the images were immediately captured using a fluorescence microscope (Nikon Eclipse Ti-SR, Nikon) at excitation and emission wavelengths of 520 and 610 nm, respectively. All the aforementioned procedures were carried out with protection from light. Image-pro plus 6.0 (IPP) was used to analyze fluorescence optical density and calculate ROS levels.
Liver frozen sections (about 8-μm thick) were prepared on the 3-Aminopropyl-Triethoxysilane-coated glass slides. The slides were air-dried and fixed in 10% ice cold formalin for 10 min and then washed 3 times with distilled water before staining with oil red O reagent (Sigma-Aldrich) for 10 min. After oil red O staining, the slides were rinsed with 60% isopropanol and restained by hematoxylin. Sections were observed by optical microscopy at a final magnification of ×400 (Nikon Eclipse 80i, Nikon).
Total RNA Extraction and Quantitative Real-Time PCR
Total RNA was extracted from liver samples using TRIzol (Invitrogen, Carlsbad, CA). The concentration and purity of RNA samples were detected and assessed using nucleic acid concentration analyzer NanoDrop 2,000 (Thermo Fisher, Waltham, MA). Complementary DNA (cDNA) was synthesized using PrimeScript RT reagent Kit with gDNA Erase (RR047 A, Takara, Dalian, China) following the manufacturer's recommended protocol. Quantitative real-time PCR (qRT-PCR) was performed in triplicate in the CFX96 Touch Real-Time PCR detection system (Bio-Rad Laboratories, Hercules, CA) using TB Green Premix Ex Taq (RR420 A, Takara) according to the manufacturer’s instructions, and the expression of target genes was normalized to that of β-actin. The primer sequences for qRT-PCR are presented in Table 2. The relative expression of each gene was calculated by the 2−ΔΔCt method (Livak and Schmittgen, 2001).
Table 2.
Primer used for quantitative real-time PCR.
| Target gene | Primer | Primer sequence (5′-3′) | Accession No. |
|---|---|---|---|
| β-Actin | Forward | TCCCTGGAGAAGAGCTATGAA | NM_205518.1 |
| Reverse | CAGGACTCCATACCCAAGAAAG | ||
| IL-10 | Forward | CCAGGGACGATGAACTTAACA | NM_001004414.2 |
| Reverse | GATGGCTTTGCTCCTCTTCT | ||
| IL-1β | Forward | CTCACAGTCCTTCGACATCTTC | XM_015297469.1 |
| Reverse | CGGTACATACGAGATGGAAACC | ||
| IL-6 | Forward | GATCCGGCAGATGGTGATAAA | NM_204628.1 |
| Reverse | GAGGATTGTGCCCGAACTAAA | ||
| TNF-α | Forward | GACAGCCTATGCCAACAAGTA | AY765397.1 |
| Reverse | TCCACATCTTTCAGAGCATCAA | ||
| Cyt C | Forward | GGAGATATTGAGAAGGGCAAGAA | XM_015281453.2 |
| Reverse | ATCATCTTTGTTCCTGGGATGT | ||
| Chop | Forward | GCACAGCCCATTTCTGTTTC | XM_015273173.2 |
| Reverse | TGCCATCCCATTCTGCTAAG | ||
| Grp78 | Forward | GTTACTGTGCCAGCCTACTT | NM_205491.1 |
| Reverse | CCGCTTCGCTTTCTCTACTT | ||
| Caspase 9 | Forward | GACCTGCTAACCATGCTACTT | XM_424580.6 |
| Reverse | TTCCACTGAATCCTCCAATCC | ||
| Caspase 3 | Forward | TCCCTGGTTCCAAAGGAATG | XM_015276122.2 |
| Reverse | AGTAGCCTGGAGCAGTAGAA | ||
| Caspase 7 | Forward | TGCAAAGCCAGACAGAAGTAG | XM_025151846.1 |
| Reverse | GGTCCATCGGTGCCATAAAT | ||
| ACOX1 | Forward | ACTGAGCTGTGTCTCTTGTATG | XM_015295164.2 |
| Reverse | GCTTCAGGTGTTTGTGGAAAG | ||
| CPT1 | Forward | GAGAAGAGTGCAGTGAGAAGAG | XM_015286798.2 |
| Reverse | CCAGCCACAGAAGTAGAGTAAG | ||
| FASN | Forward | CCTGGAGATGTGGAGTATGTTG | NM_205155.3 |
| Reverse | TCAAGGAGCCATCGTGTAAAG | ||
| PPARα | Forward | GATGCTGCGTGAAGTGAAATG | XM_025150258.1 |
| Reverse | CTGGTGAAAGGGTGTCTGTTAT | ||
| ApoB | Forward | ATTCCTGACTTGAAGATACCAGAG | NM_001044633.1 |
| Reverse | GTTCGCAGATGCTGTAGTATTATG | ||
| MTP | Forward | AGGAGAGGGAAAGCAGAAATG | NM_001109784.2 |
| Reverse | ATTTCCCATTACCCGCAGTAG | ||
| VTG II | Forward | TCATCTGCCTCCTCTCCTAATC | NM_001031276.1 |
| Reverse | ACTGCTCCTACTACTGCTACTT | ||
| ApoVLDL II | Forward | CTCACTAAACTGGCGGAACA | M25774.1 |
| Reverse | GCCAAGTCATTCAGGAGGAA |
Statistical Analysis
Data of the experiment were statistically analyzed by one-way ANOVA using SPSS 20.0 (SPSS Inc., Chicago, IL). Tukey post hoc test was used to compare the significant differences (P < 0.05) between means.
Results
Cadmium Residues in Organs
The Cd residues in the tissues of laying hens fed diet contaminated with CdCl2 are given in Table 3. We detected the Cd residues in the tissues of the heart, liver, spleen, lung, kidney, pancreas, ovary, shell gland, and magnum. With the increase in supplementation of dietary Cd, the Cd residues in these tissues increased linearly (P < 0.05). The highest amount of residues were found in the kidney (0.156, 0.195, 0.272, and 0.572 mg Cd/kg), followed by the liver (0.133, 0.182, 0.252, and 0.541 mg Cd/kg), pancreas (0.098, 0.132, 0.185, and 0.446 mg Cd/kg), and lung (0.085, 0.120, 0.189, and 0.426 mg Cd/kg) from the 7.58- to 60.67-mg Cd/kg groups.
Table 3.
Effects of cadmium chloride on cadmium deposition in organs.
| Item | Dietary Cd dosage, mg/kg |
P-value | SEM | ||||
|---|---|---|---|---|---|---|---|
| 0.47 | 7.58 | 15.56 | 30.55 | 60.67 | |||
| Heart, mg Cd/kg wet wt | 0.004e | 0.054d | 0.097c | 0.178b | 0.323a | <0.001 | 0.007 |
| Liver, mg Cd/kg wet wt | 0.016e | 0.133d | 0.182c | 0.252b | 0.541a | <0.001 | 0.01 |
| Spleen, mg Cd/kg wet wt | 0.002e | 0.042d | 0.083c | 0.158b | 0.263a | <0.001 | 0.008 |
| Lung, mg Cd/kg wet wt | 0.010e | 0.085d | 0.120c | 0.189b | 0.426a | <0.001 | 0.008 |
| Kidney, mg Cd/kg wet wt | 0.026e | 0.156d | 0.195c | 0.272b | 0.572a | <0.001 | 0.008 |
| Pancreas, mg Cd/kg wet wt | 0.014e | 0.098d | 0.132c | 0.185b | 0.446a | <0.001 | 0.012 |
| Ovary, mg Cd/kg wet wt | 0.013e | 0.083d | 0.107c | 0.144b | 0.297a | <0.001 | 0.004 |
| Shell gland, mg Cd/kg wet wt | 0.012e | 0.070d | 0.097c | 0.124b | 0.208a | <0.001 | 0.004 |
| Magnum, mg Cd/kg wet wt | 0.005d | 0.010c | 0.015b,c | 0.017b | 0.025a | <0.001 | 0.002 |
a-eMeans within a column with different superscripts are significantly different (P < 0.05).
Values are means and SEM of 12 hens (2 hens per replicate).
Serum Biochemistry
The effects of dietary Cd on the serum biochemical changes are shown in Table 4. When compared with the control group, the level of GOT was significantly increased in the 15.56-, 30.55-, and 60.67-mg Cd/kg groups (P < 0.05). The level of TBA was significantly increased in the 60.67-mg Cd/kg group. On the contrary, the level of ALB was significantly decreased in the 60.67-mg Cd/kg group (P < 0.05). The activity of ALP was significantly decreased in the 30.55- and 60.67-mg Cd/kg groups (P < 0.05).
Table 4.
Effects of cadmium chloride on serum biochemistry of laying hens.
| Item | Dietary Cd dosage, mg/kg |
P-value | SEM | ||||
|---|---|---|---|---|---|---|---|
| 0.47 | 7.58 | 15.56 | 30.55 | 60.67 | |||
| ALB, g/L | 20.85a | 20.33a | 21.36a | 21.23a | 15.11b | 0.001 | 1.131 |
| TP, g/L | 52.45 | 50.62 | 55.49 | 52.26 | 51.83 | 0.817 | 4.107 |
| GOT, IU/L | 24.20d | 26.55c,d | 31.59b,c | 33.99b | 42.70a | <0.001 | 1.963 |
| GPT, IU/L | 0.92 | 1.00 | 1.02 | 1.40 | 1.79 | 0.078 | 0.304 |
| ALP (King Unit2/100 mL) | 21.8a,b | 24.99a | 14.34b,c | 10.83c | 6.73c | <0.001 | 2.480 |
| TBA μmol/L | 8.44b | 8.08b | 8.76b | 12.33a,b | 15.87a | 0.017 | 2.110 |
a-cMeans within a column with different superscripts are significantly different (P < 0.05).
Values are represented as the mean and SEM (n = 8).
Abbreviations: ALB, albumin; ALP, alkaline phosphatase; GOT, aspartate aminotransferase; GPT, alanine aminotransferase; TBA, total bile acid; TP, total protein.
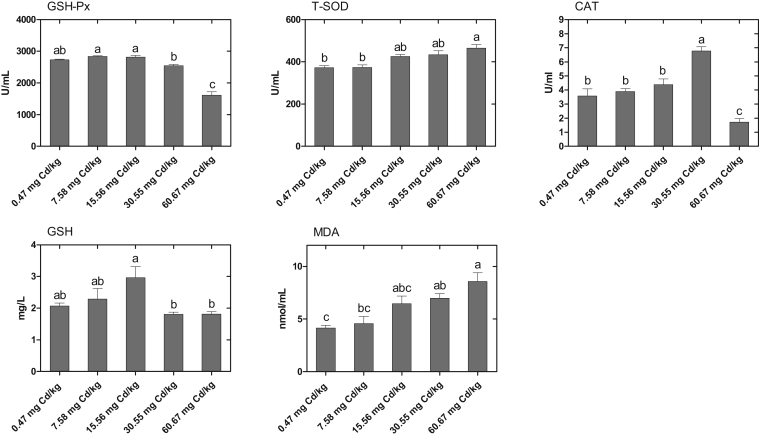
Serum Antioxidant Parameters
The effects of Cd on the antioxidant parameters in the serum of laying hens are summarized in the Figure 1. When compared with the control group, the activity of CAT was significantly increased in the 30.55-mg Cd/kg group and then significantly decreased in the 60.67-mg Cd/kg group (P < 0.05). The activity of GSH-Px was significantly decreased in the 60.67-mg Cd/kg group (P < 0.05). In contrast, the activity of T-SOD in the 60.67-mg Cd/kg group was significantly increased (P < 0.05). The level of MDA was significantly increased in the 30.55- and 60.67-mg Cd/kg groups (P < 0.05).
Figure 1.
Effects of cadmium chloride on antioxidant parameters in the serum of laying hens. Values are represented as the mean ± SE (n = 8). a-cColumns with different superscript letters are significantly different (P < 0.05). Abbreviations: CAT, catalase; GSH-Px, glutathione peroxide; GSH, glutathione; T-SOD, total superoxide dismutase; MDA, malondialdehyde.
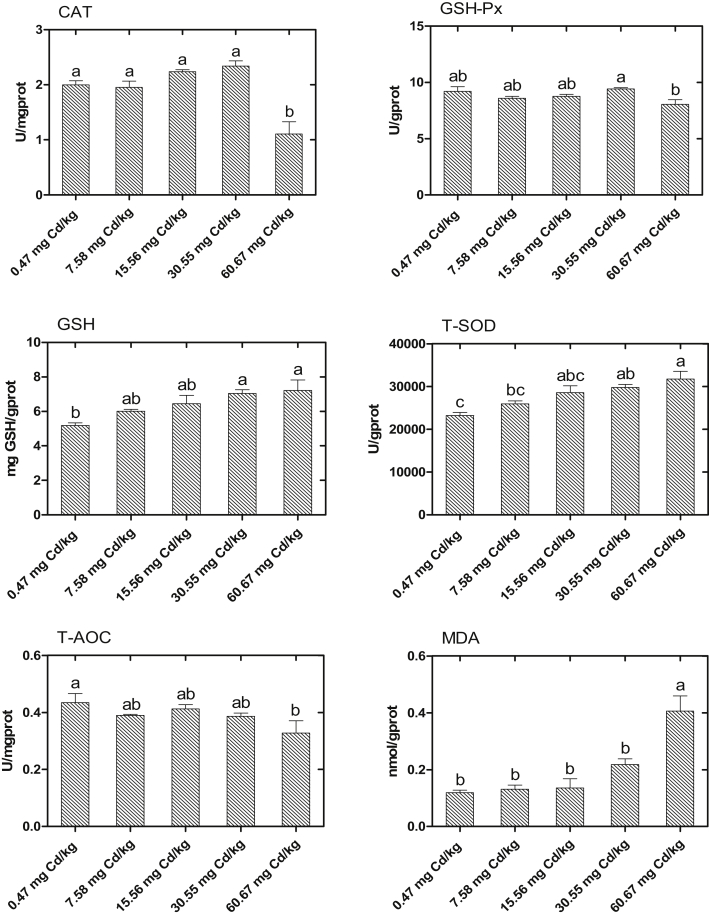
Hepatic Antioxidant Parameters
The effects of Cd on the liver antioxidant indices of laying hens are shown in Figure 2. Compared with the control group, the activity of T-SOD and the concentration of GSH presented increase with the increase of dietary Cd and were significantly increased in the 30.55- and 60.67-mg Cd/kg groups (P < 0.05). The activities of CAT and T-AOC were significantly decreased, and the concentration of MDA was significantly increased in the 60.67-mg Cd/kg group as compared with the control group (P < 0.05).
Figure 2.
Effects of cadmium chloride on liver antioxidant indices of laying hens. Values are represented as the mean ± SE (n = 8). a-cColumns with different superscript letters are significantly different (P < 0.05). Abbreviations: CAT, catalase; GSH-Px, glutathione peroxide; GSH, glutathione; T-SOD, total superoxide dismutase; MDA, malondialdehyde; T-AOC, total antioxidant capacity.
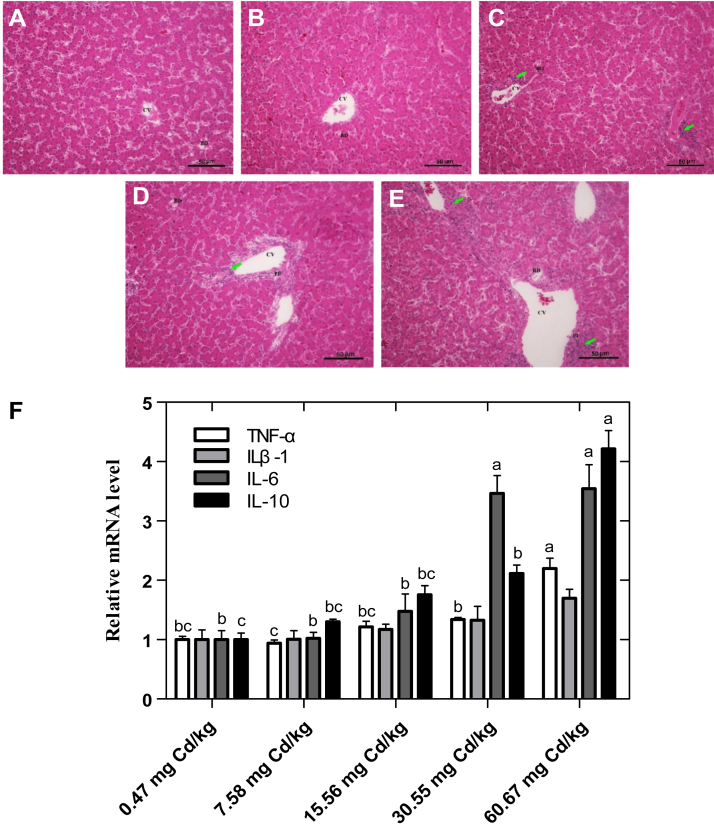
Histopathological Variations and Gene Expression of Inflammatory Cytokines
The results of the histopathological changes and the gene expression of proinflammatory cytokines in the liver are shown in Figure 3. The photomicrographs of the control (0.47 mg Cd/kg) and 7.58-mg Cd/kg groups showed apparently normal histoarchitecture of the liver. However, histological analysis of the liver sections revealed a significant damage in the liver tissues of laying hens with the increase of dietary Cd supplementation (30.55 and 60.67 mg Cd/kg). Liver tissues from these treatments had periportal fibrosis, bile duct hyperplasia, and periportal inflammatory cell infiltration. Correspondingly, we assessed the mRNA expression levels of inflammatory cytokines in the liver tissues (Figure 3F) and showed that the expression of the proinflammatory factor of tumor necrosis factor (TNF)-α increased significantly in the 60.67-mg Cd/kg group. The expression of IL-6, as well as the anti-inflammatory factor of IL-10, presented significant increase in the 30.55- and 60.67-mg Cd/kg groups (P < 0.05).
Figure 3.
Hepatic histomorphology and histopathology and mRNA expression of inflammatory cytokines from various groups of experimental laying hens. The liver sections were stained with hematoxylin and eosin (100 magnification). (A) Control (0.47 mg Cd/kg); (B) 7.58 mg Cd/kg; (C) 15.56 mg Cd/kg; (D) 30.55 mg Cd/kg; (E) 60.67 mg Cd/kg. Scale bar = 50 μm. Green arrow = periportal infiltration by inflammatory cells. (F–I) Method of 2−ΔΔCt was applied for calculation of relative gene expression with β-actin as the endogenous control and the average ΔCt value of 0.47 μmol Cd group as the calibrator to normalize the signal. Values were expressed as mean ± SE (n = 6). a-dColumns with different superscript letters are significantly different (P < 0.05). Abbreviations: PF, periportal fibrosis, BD, bile duct, CV, central vein.
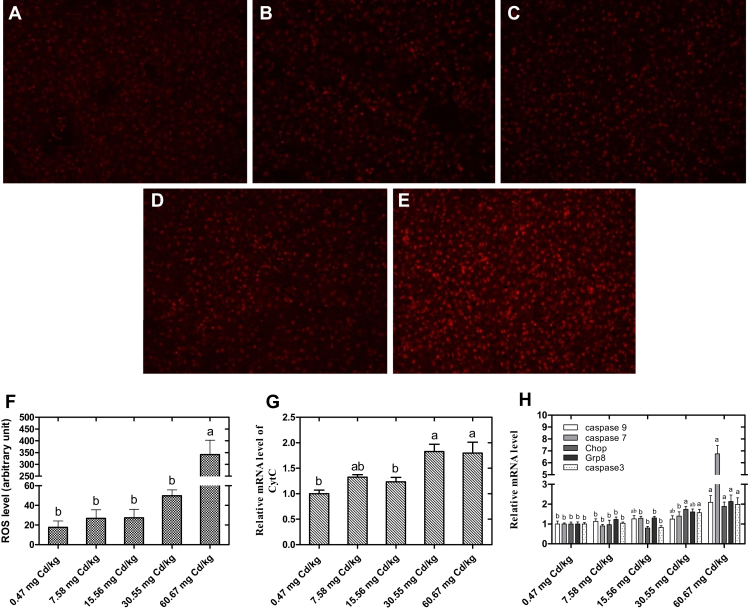
Endoplasmic Reticulum Stress
An immunofluorescence assay was used to measure intracellular ROS in the liver tissues of laying hens exposed to Cd. The result showed that the ROS level was significantly increased in the 60.67-mg Cd/kg group (Figures 4A–4F). Correspondingly, the mRNA expression levels of Cytochrome C (Cyt C) (Figure 4G) and ER stress–related genes (Figure 4H) were detected and showed that with the increase of dietary Cd supplementation, the expressions of Cyt C and ER stress–related genes caspase 3, caspase 7, caspase 9, Chop, and Grp8 were significantly upregulated in the groups that had relatively high dose of Cd addition (30.55 or/and 60.67 mg Cd/kg) (P < 0.05) as compared with the control group.
Figure 4.
Effects of cadmium chloride on the endoplasmic reticulum (ER) stress of liver in laying hens. (A–E) ROS fluorescence intensity in the liver tissues (400 magnification). (A) Control (0.47 mg Cd/kg); (B) 7.58 mg Cd/kg; (C) 15.56 mg Cd/kg; (D) 30.55 mg Cd/kg; (E) 60.67 mg Cd/kg. (F) Densitometric analysis of dihydroethidium (DHE) fluorescence in each experimental group. (G, H) Method of 2−ΔΔCt was applied for calculation of relative gene expression with β-actin as the endogenous control and the average ΔCt value of 0.47 μmol Cd group as the calibrator to normalize the signal. Values were expressed as mean ± SE (n = 6). a-cColumns with different superscript letters are significantly different (P < 0.05). Abbreviation: ROS, reactive oxygen species.
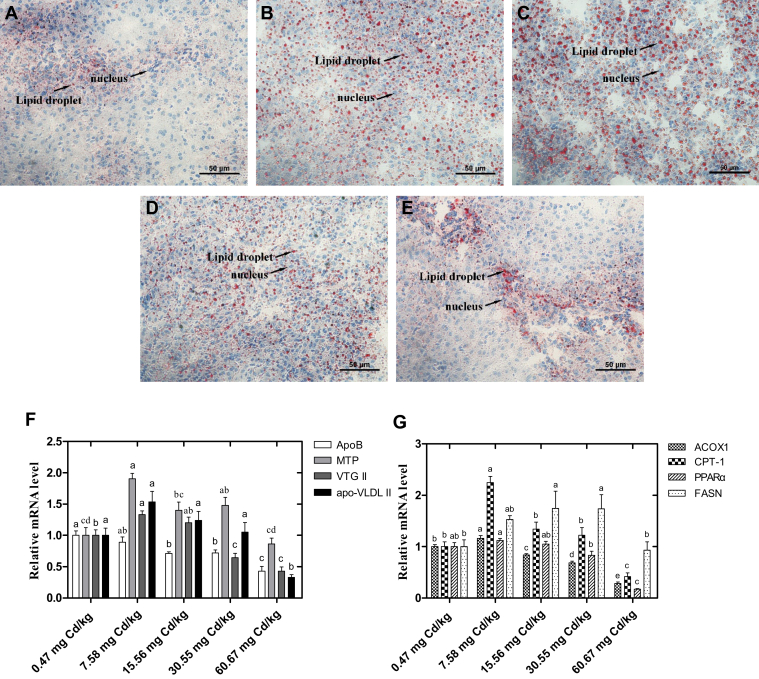
Lipid Deposition and Metabolism
The liver sections of laying hens stained with oil red O after 60-day Cd exposure are shown in Figure 5. With the increase of dietary Cd content, lipid deposition in the liver was increased in the 7.58-,15.56- and 30.55-mg Cd/kg groups and then attenuated with the continued increase of Cd addition. Besides, we detected the transcript levels of genes involved in hepatic lipid metabolism (Figures 5F and 5G). The results showed that when compared with the control group, the mRNA expression level of apoB was decreased with the increase of dietary Cd, and with significantly lower values in the 15.56-, 30.55-, and 60.67-mg Cd/kg groups (P < 0.05). The expression of MTP was significantly increased in the 7.58- and 30.55-mg Cd/kg groups (P < 0.05). Similarly, the expression of fatty acid synthase was significantly increased in the 15.56- and 30.55-mg Cd/kg groups (P < 0.05). Low dose of Cd exposure (7.58 mg Cd/kg) increased the expression of ACOX1, CPT1 and yolk precursor protein VTG II significantly (P < 0.05). But with the increase of dietary Cd addition, the expression of VTG II, apolipoprotein(ado)-VLDL II, ACOX1, CPT1, and PPARα presented significant decrease in the 60.67-mg Cd/kg group (P < 0.05).
Figure 5.
Effects of Cd on the lipid deposition and metabolism in the liver of laying hens. The liver sections were stained with oil red O (400 magnification, scale bar = 50 μm). (A) Control (0.47 mg Cd/kg); (B) 7.58 mg Cd/kg; (C) 15.56 mg Cd/kg; (D) 30.55 mg Cd/kg; (E) 60.67 mg Cd/kg. (F, G) The transcript levels of genes involved in hepatic lipid metabolism. a-eColumns with different superscript letters are significantly different (P < 0.05). Method of 2−ΔΔCt was applied for calculation of relative gene expression with β-actin as the endogenous control and the average ΔCt value of 0.47 μmol Cd group as the calibrator to normalize the signal. Values were expressed as mean ± SE (n = 6). a-eColumns with different superscript letters are significantly different (P < 0.05). Abbreviations: ApoB, apolipoprotein B; MTP, microsomal triglyceride transfer protein; VTG II, vitellogenin II; apo-VLDL II, apolipoprotein very-low-density lipoprotein II; ACOX1, acyl-CoAoxidase 1; CPT-1, carnitine palmitoyltransferase-1; PPARα, peroxisome proliferator activated receptor α; FASN, fatty acid synthase.
Discussion
Cd accumulation in avian tissues and eggs has been reported in several studies (Leach et al., 1979). Many studies reported that Cd is mainly accumulated in the kidney and liver and exhibits time- and dose-dependent patterns (Wang et al., 2017). It was consistent with our results that the concentration of Cd in the liver and kidney was higher than that in other tissues and that it was significantly increased with the increase of supplementation of dietary Cd. Our results also showed that the concentration of Cd in the kidney was higher than that in the liver. The possible explanation is that, in the liver, Cd2+ induces the synthesis of metallothionein (MT) and forms MT–Cd complex, which is stored in the liver and transferred to other organs (Chan et al., 1993), mainly the kidneys, a major organ from which Cd is excreted from the organism (Hoet et al., 2012). In addition, Scheuhammer (1987) reported that the concentration ratio of Cd in the liver and kidney < 1 indicated a chronic exposure to relatively low doses of Cd. In the present study, the concentration ratios of Cd in the liver and kidney were <1, suggesting chronic exposure. Following the tissues of liver and kidney, we found that the lung and pancreas had a high concentration of Cd accumulation. The International Agency for Research on Cancer reported that inhalation exposure to Cd could lead to respiratory injury and lung cancer (ATSDR, 2012). Besides, because the lung is a highly vascularized organ (Nai et al., 2017), dietary Cd can have a high rate of accumulation in this organ also. These results suggest that Cd can accumulate in and result in toxicity to various organs, and the accumulation of Cd is the greatest in the kidney, followed by the liver, pancreas, and lung in laying hens.
The liver is one of the most susceptible organs after acute or chronic exposure to Cd. The assay for the activities of blood levels of GPT, GOT, and ALP as important biomarkers of the liver function is of clinical relevance. These enzymes are located in the liver cells and released into the plasma in response to the liver cell injury or damage. (Imafidon et al., 2018, Ozer et al., 2008). In the present study, after the Cd administration, the activity of GOT was significantly elevated in the 15.56-, 30.55-, and 60.67-mg Cd/kg groups. The alteration of GOT may be due to the Cd exposure disrupting the hepatic cells as a result of necrosis or a consequence of altering the cell membrane permeability (Ozer et al., 2008). Cd can mimic and displace some nutritive metals and produce structural perturbations in enzymes (Casalino et al., 2002). Treviño et al. (2015) reported that Cd can substitute Zn or interact with nucleophilic ligands essential for the enzymatic activity to produce an inhibition on the activity of ALP. Similarly, our results showed that with the increased supplementation of dietary Cd (30.55 and 60.67 mg Cd/kg), the activity of ALP decreased significantly as compared with the control group. In addition, the level of serum TBA increased significantly in the 60.67-mg Cd/kg group. Serum TBA can also be considered to be used as an alternative marker to evaluate the liver function. Because bile acid metabolism mostly occurs in hepatocytes, accumulation of bile acid usually predicts impairment in hepatocyte uptake, synthesis, or secretion function (Lalisang, 2012). The suppressive effects of Cd were also presented on ALB secretion, and the addition of Cd (60.67 mg Cd/kg) significantly reduced the levels of serum ALB. Serum ALB is an important prognostic factor in advancing the liver disease (Pugh et al., 1973), as well as the major extracellular source of reduced sulfhydryl groups, which are potent scavengers of ROS and reactive nitrogen species (Quinlan et al., 1998). Therefore, in the present study, these results indicated that Cd exposure is associated with the liver dysfunction of laying hens.
Oxidative stress is defined as the presence of metabolic and radical substances or so-called reactive (oxygen, nitrogen, or chlorine) species (Elnesr et al., 2019). These substances can modify lipids, proteins, or nucleic acids, resulting in cell death (Halliwell and Whiteman, 2004). Induction of oxidative stress is considered as an important mechanism of Cd toxicity. Cd induced oxidative stress in various cells and organs of the body by disrupting the balance of oxidant/antioxidant system is well documented (Valko et al., 2005). Furthermore, Cd is known to induce the production of superoxide anions, nitric oxide, hydroxyl radicals, and hydrogen peroxide (Rodríguez-Serrano et al., 2006, Badisa et al., 2007). The components of endogenous antioxidant defense system, including GSH, GSH-Px, SOD, and CAT, play an important role in the free radical scavenging and maintaining the intracellular redox balance (Stohs and Bagchi, 1995, Koizumi et al., 1996). In the present study, measurement of antioxidants released appeared to be essential. Our results showed that the activities of CAT and T-SOD presented significant increase in the serum or liver of the 30.55-mg Cd/kg group. The possible explanation may be that the chronic Cd administration motivates the antioxidant defense. Similarly, the increased level of GSH in the liver of the 30.55-mg Cd/kg group may also be attributed to the stimulation of the antioxidant defense. These results are consistent with the report by Shaikh et al. (1999) that chronic Cd exposure resulted in a gradual rise in hepatic antioxidant defense. Therefore, the increased activities of T-SOD and CAT and the level of GSH in 30.55-mg Cd/kg group with the simultaneous unchanged MDA concentration allow us to conclude that the liver antioxidant defense system is still effectively protected from the action of Cd-induced free radicals. However, the activities of CAT, GSH-Px, and T-AOC in the serum or liver were significantly decreased in the 60.67-mg Cd/kg group. In addition, dietary supplementation of Cd at 60.67 mg/kg induced a significant increase of MDA, an indicator of lipid peroxidation (Janero, 1990), concentration in the liver. These results demonstrated that diets contaminated with high dose of Cd (60.67 mg Cd/kg) can decrease these antioxidant levels, resulting in oxidative stress in laying hens.
Cd, as a transition element, cannot generate ROS directly. However, Cd could induce the accumulation of ROS by modulating the activities of various enzymes, such as nitric oxide synthase (Zhong et al., 2015), which leads to oxidative damage that in turn leads to pathological changes of cellular structure and functions (Huo et al., 2017). In the present study, many severe pathological changes and inflammatory responses with the increases dosage of Cd addition were reflected in the liver pathological slices, such as periportal fibrosis, bile duct hyperplasia, and inflammatory cells infiltrated around the hepatic central vein. In animals, the induction of the expression of cytokines by the environmental chemicals was considered as an inflammatory response (Ji et al., 2010). Therefore, we examined the mRNA expression levels of proinflammatory and anti-inflammatory cytokines (TNF-α, IL-6, IL-1β, and IL-10) in the liver tissues and showed that with the increased dose of dietary Cd exposure, the mRNA expression levels of TNF-α and IL-6 presented an increasing trend and were significantly increased in the relative high Cd addition groups (30.55 or 60.67 mg Cd/kg), which is consistent with the results of pathological observation. Tumor necrosis factor-α, a cytokine secreted by activated macrophages, is a necessary and sufficient mediator of local and systemic inflammation (Xie et al., 2017), which, together with IL-1β, plays an important role in the onset of inflammatory processes that promote the expression and release of other cytokines (Låg et al., 2010). IL-6, a traditional marker of inflammation, is reported to act as both proinflammatory and anti-inflammatory cytokines in different liver injury models (short- and long-term exposure) (Jin et al., 2006). Besides, with the increase of dietary Cd addition, the expression of anti-inflammatory cytokine IL-10 increased significantly; the possible reason may be that Cd triggers the inflammatory defense system. These results clearly demonstrated that dietary Cd exposure induced the hepatic inflammation response characterized by the histopathological damage in the relative high dose of Cd exposure.
Many studies have demonstrated that Cd toxicity seems to be crucially mediated by the induction of cell damage in various organs as a consequence of the production of ROS (Oh and Lim, 2006). Similarly, in the present study, we found that the accumulation level of ROS in the liver was elevated significantly in the group supplemented with high dose of Cd (60.67 mg Cd/kg). Overproduction of ROS can subsequently lead to antioxidant defenses, lipid peroxidation, and even oxidative damage in organisms, which has been proven by our results. Furthermore, the relationship between oxidative stress and cell apoptosis has been well documented by many previous studies (Faverney et al., 2001, Oh and Lim, 2006). The initiation of apoptosis is mediated by the translocation of proapoptotic protein Bad to mitochondria followed by the downregulation of antiapoptotic protein Bcl-2, which results in the reduction of membrane potential and Cyt C release and subsequently leading to caspase activation. In accordance with these reports, we observed that the relative high dose of Cd treatment (30.55 and/or 60.67 mg Cd/kg) upregulated the mRNA expression of Cyt C, as well as the caspase 9 and caspase 3. In addition, ER stress is a crucial event downstream of oxidative stress in the process of Cd-induced apoptosis (Kitamura and Hiramatsu, 2010), which is also a feature of acute and chronic liver diseases (Yoshiuchi et al., 2009). The key signaling pathways activated by ER stress are termed the unfold protein response, which is driven by 3 major pathways—ATF6 pathway, IRE1α pathway, and PERK-eIF2α pathway (Maiers and Malhi, 2019). Our data showed the expressions of ER chaperones Grp78, Chop, and caspase 7 were upregulated upon Cd treatment (30.55 and/or 60.67 mg Cd/kg). Caspase 7 is known to be essential for ER stress–induced apoptosis (Nakagawa et al., 2000). Chop is also one of the central transcriptional regulators involved in apoptosis caused by ER stress (Ji et al., 2005). Consequently, it can be concluded that Cd could induce the oxidative- and ER-stress mediated apoptosis in liver cells of laying hens.
The liver is an important organ in lipometabolism. Lipid acts as a major energy source, the stores of which support a series of physiological, reproductive, and developmental processes. In avian species, the liver gives the most important contribution to lipogenesis and accounts for about 95% of the de novo fatty acid synthesis (Huang et al., 2013). Lipid droplets are ubiquitous and considered as dynamic cellular organelles (Wilfling et al., 2014) and not only play an important role in lipid storage but also respond to ER stress, protein glycosylation, and pathogen infection (Fei et al., 2009, Herker and Ott, 2011, Krahmer et al., 2013). In the present study, oil red O staining showed the deposition of lipid droplets increased in the relative low dose of Cd addition groups and then decreased with the continuous increase of dietary Cd supplementation. Similarly, many studies have found that Cd can trigger lipid accumulation by increasing lipid synthesis (Chen et al., 2013). Correspondingly, we observed that the expression of fatty acid synthase, one of the key enzymes for de novo fatty acid synthesis, increased significantly with the increase of dietary Cd addition (15.56 and 30.55 mg Cd/kg). The expression of the ACOX1 and CPT1, the main regulatory enzymes of fatty acids oxidation (Oaxaca-Castillo et al., 2007, Schlaepfer et al., 2014), presented significant increase only in the 7.58-mg Cd/kg group. In addition, PPARα is a transcriptional regulator of genes involved in mitochondrial β-oxidation and fatty acid transport and its deficiency will lead to exaggerated lipid accumulation in the liver (Pawlak et al., 2015). The expression of PPARα, ACOX1, and CPT1 decreased significantly in the 60.67-mg Cd/kg group. These results demonstrated that Cd can regulate the accumulation of lipids in the liver by regulating the expression of key enzymes in the fatty acids synthesis and β-oxidation process. Besides, in laying hens, the yolk formation depends on the lipids synthesized in the liver. The amount of lipoprotein produced by the liver directly affects the egg production (Shivaprasad and Jaap, 1977). Accordingly, we evaluated the expression levels of VTG II, apo-VLDL II, ApoB, and MTP, which play a critical role in the formation of yolk protein (Schneider et al., 1990, Shen et al., 1993), and found that the expression of VTG II and MTP increased significantly in the 7.58-mg Cd/kg group. However, with the increase of dietary Cd supplementation (30.55 and/or 60.67 mg Cd/kg), the expression levels of ApoB, VTG II, and apo-VLDL II decreased significantly. In laying hens, the major yolk components of the ovum are VTG and VLDL (Shen et al., 1993). The VLDL, which contains large amounts of apo-VLDL II and apoB, mainly functions in the transport of triacylglycerols, phospholipids, and cholesterol (Schneider et al., 1990, Shen et al., 1993). These triglyceride-rich lipoproteins are synthesized and transported from the liver primarily to the growing oocytes (Schneider et al., 1990). Microsomal triglyceride transfer protein is mainly responsible for the assembly of the triglyceride-rich lipoproteins in the liver (Wetterau et al., 1992). Therefore, these results indicated that Cd may affect the egg production and yolk quality by regulating the synthesis of lipoproteins.
In conclusion, dietary Cd can accumulate in various organs of laying hens, among which the kidney and liver are the main target organs. Cd can lead to liver dysfunction and injury, induce oxidative and ER stress in the liver of laying hens, regulate the lipid synthesis and metabolism, and ultimately may affect the egg production and yolk quality. We found that low dose of Cd exposure could promote the synthesis of lipids, as well as the lipoproteins related to yolk formation; however, with the increased dose of Cd exposure, these effects become negative.
Acknowledgments
This research was supported by the Modern Argo-industry Technology Research System of China (CARS-40-K10).
Conflict of Interest Statement: The authors did not provide a conflict of interest statement.
References
- Alagawany M., Abd El-Hack M.E., Farag M.R., Elnesr S.S., El-Kholy M.S., Saadeldin I.M., Swelum A.A. Dietary supplementation of Yucca schidigera extract enhances productive and reproductive performances, blood profile, immune function, and antioxidant status in laying Japanese quails exposed to lead in the diet. Poult. Sci. 2018;97:3126–3137. doi: 10.3382/ps/pey186. [DOI] [PubMed] [Google Scholar]
- ATSDR . Agency for Toxic Substances and Disease Registry; Atlanta, GA: 2012. Toxicological Profile for Cadmium. [PubMed] [Google Scholar]
- Badisa V.L., Latinwo L.M., Odewumi C.O., Ikediobi C.O., Badisa R.B., Ayuk-Takem L.T., Nwoga J., West J. Mechanism of DNA damage by cadmium and interplay of antioxidant enzymes and agents. Environ. Toxicol. 2007;22:144–151. doi: 10.1002/tox.20248. [DOI] [PubMed] [Google Scholar]
- Branca J.J.V., Morucci G., Pacini A. Cadmium-induced neurotoxicity: still much ado. Neural Regen. Res. 2018;13:1879–1882. doi: 10.4103/1673-5374.239434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casalino E., Calzaretti G., Sblano C., Landriscina C. Molecular inhibitory mechanisms of antioxidant enzymes in rat liver and kidney by cadmium. Toxicology. 2002;179:37–50. doi: 10.1016/s0300-483x(02)00245-7. [DOI] [PubMed] [Google Scholar]
- Chan H.M., Zhu L.F., Zhong R., Grand D., Goyer R.A., Cherian M.G. Nephrotoxicity in rats following liver transplantation from cadmium-exposed rats. Toxicol. Appl. Pharmacol. 1993;123:89–96. doi: 10.1006/taap.1993.1225. [DOI] [PubMed] [Google Scholar]
- Chen Q.L., Gong Y., Luo Z., Zheng J.L., Zhu Q.L. Differential effect of waterborne cadmium exposure on lipidmetabolism in liver and muscle of yellow catfish Pelteobagrus fulvidraco. Aquat. Toxicol. 2013;142-143:380–386. doi: 10.1016/j.aquatox.2013.09.011. [DOI] [PubMed] [Google Scholar]
- Elnesr S.S., Elwan H.A.M., Xu Q.Q., Xie C., Dong X.Y., Zou X.T. Effects of in ovo injection of sulfur-containing amino acids on heat shock protein 70, corticosterone hormone, antioxidant indices, and lipid profile of newly hatched broiler chicks exposed to heat stress during incubation. Poult. Sci. 2019;98:2290–2298. doi: 10.3382/ps/pey609. [DOI] [PubMed] [Google Scholar]
- El-Sokkary G.H., Nafady A.A., Shabash E.H. Melatonin administration ameliorates cadmium-induced oxidative stress and morphological changes in the liver of rat. Ecotoxicol. Environ. Saf. 2010;73:456–463. doi: 10.1016/j.ecoenv.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Faverney C.R., Devaux A., Lafaurie M., Girard J.P., Bailly B., Rahmani R. Cadmium induces apoptosis and genotoxicity in rainbow trout hepatocytes through generation of reactive oxygene species. Aquat. Toxicol. 2001;53:65–76. doi: 10.1016/s0166-445x(00)00154-5. [DOI] [PubMed] [Google Scholar]
- Fei W., Wang H., Fu X., Bielby C., Yang H. Conditions of endoplasmic reticulum stress stimulate lipid droplet formation in Saccharomyces cerevisiae. Biochem. J. 2009;424:61–67. doi: 10.1042/BJ20090785. [DOI] [PubMed] [Google Scholar]
- Galluzzi L., Bravo-San Pedro J.M., Kroemer G. Organelle-specific initiation of cell death. Nat. Cell Biol. 2014;16:728–736. doi: 10.1038/ncb3005. [DOI] [PubMed] [Google Scholar]
- Ghosh P., Thomas P. Binding of metals to red Drum vitellogenin and Incorporation into oocytes. Mar. Environ. Res. 1995;39:161–168. [Google Scholar]
- Halliwell B., Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br. J. Pharmacol. 2004;142:231–255. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassoun E.A., Stohs S.J. Cadmium-induced production of superoxide anion and nitric oxide, DNA single strand breaks and lactate dehydrogenase leakage in J774A.1 cell cultures. Toxicol. 1996;112:219–226. doi: 10.1016/0300-483x(96)03404-x. [DOI] [PubMed] [Google Scholar]
- He X., Chen M.G., Ma Q. Activation of Nrf2 in defense against cadmium-induced oxidative stress. Chem. Res. Toxicol. 2008;21:1375–1383. doi: 10.1021/tx800019a. [DOI] [PubMed] [Google Scholar]
- Herker E., Ott M. Unique ties between hepatitis C virus replication and intracellular lipids. Trends Endocrinol. Metab. 2011;22:241–248. doi: 10.1016/j.tem.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoet P., Haufroid V., Deumer G., Dumont X., Lison D., Hantson P. Acute kidney injury following acute liver failure: potential role of systemic cadmium mobilization? Intensive Care Med. 2012;38:467–473. doi: 10.1007/s00134-011-2449-0. [DOI] [PubMed] [Google Scholar]
- Huang J.B., Zhang Y., Zhou Y.B., Zhang Z.Z., Xie Z.W., Zhang J.S., Wan X.C. Green tea polyphenols alleviate obesity in broiler chickens through the regulation of lipid-metabolism-related genes and transcription factor expression. J. Agric. Food Chem. 2013;61:8565–8572. doi: 10.1021/jf402004x. [DOI] [PubMed] [Google Scholar]
- Huo J.F., Dong A.G., Wang Y.H., Lee S.C., Ma C.G., Wang L. Cadmium induces histopathological injuries and ultrastructural changes in the liver of freshwater turtle (Chinemys reevesii) Chemosphere. 2017;186:459–465. doi: 10.1016/j.chemosphere.2017.08.029. [DOI] [PubMed] [Google Scholar]
- Imafidon C.E., Olukiran O.S., Ogundipe D.J., Eluwole A.O., Adekunle I.A., Oke G.O. Acetonic extract of Vernonia amygdalina (Del.) attenuates Cd-induced liver injury: potential application in adjuvant heavy metal therapy. Toxicol. Rep. 2018;5:324–332. doi: 10.1016/j.toxrep.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janero D.R. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic. Biol. Med. 1990;9:515–540. doi: 10.1016/0891-5849(90)90131-2. [DOI] [PubMed] [Google Scholar]
- Ji Y.L., Wang H., Liu P., Wang Q., Zhao X.F., Meng X.H., Yu T., Zhang H., Zhang C., Zhang Y., Xu D.X. Pubertal cadmium exposure impairs testicular development and spermatogenesis via disrupting testicular testosterone synthesis in adult mice. J. Reprod. Toxicol. 2010;29:176–183. doi: 10.1016/j.reprotox.2009.10.014. [DOI] [PubMed] [Google Scholar]
- Ji C., Mehrian-Shai R., Chan C., Hsu Y.H., Kaplowitz N. Role of CHOP in hepatic apoptosis in the murine model of intragastric ethanol feeding. Alcohol Clin. Exp. Res. 2005;29:1496–1503. doi: 10.1097/01.alc.0000174691.03751.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X., Zimmers T.A., Perez E.A., Pierce R.H., Zhang Z., Koniaris L.G. The paradoxical effects of short- and long-term interleukin-6 exposure on liver injury and repair. Hepatology. 2006;43:474–484. doi: 10.1002/hep.21087. [DOI] [PubMed] [Google Scholar]
- Jin Y.X., Zhang S.B., Tao R.H., Huang J., He X.Z., Qu L.Y., Fu Z.W. Oral exposure of mice to cadmium (II), Chromium (VI) and their Mixture induce oxidative- and endoplasmic reticulum-stress mediated apoptosis in the livers. Environ. Toxicol. 2016;31:693–705. doi: 10.1002/tox.22082. [DOI] [PubMed] [Google Scholar]
- Järup L., Åkesson A. Current status of cadmium as an environmental health problem. Toxicol. Appl. Pharmacol. 2009;238:201–208. doi: 10.1016/j.taap.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Kitamura M., Hiramatsu N. The oxidative stress: endoplasmic reticulum stress axis in cadmium toxicity. Biometals. 2010;23:941–950. doi: 10.1007/s10534-010-9296-2. [DOI] [PubMed] [Google Scholar]
- Khafaga A.F., Abd El-Hack M.E., Taha A.E., Elnesr S.S., Alagawany M. The potential modulatory role of herbal additives against Cd toxicity in human, animal, and poultry: a review. Environ. Sci. Pollut. Res. Int. 2019;26:4588–4604. doi: 10.1007/s11356-018-4037-0. [DOI] [PubMed] [Google Scholar]
- Koizumi T., Shirakura G., Kumagai H., Tatsumoto H., Suzuki K.T. Mechanism of cadmium-induced cytotoxicity in rat hepatocytes: cadmium-induced active oxygen-related permeability changes of the plasma membrane. Toxicology. 1996;14:125–134. doi: 10.1016/s0300-483x(96)03477-4. [DOI] [PubMed] [Google Scholar]
- Krahmer N., Hilger M., Kory N., Wilfling F., Stoehr G., Mann M., Farese R.V., Jr., Walther T.C. Protein correlation profiles identify lipid droplet proteins with high confidence. Mol. Cell Proteomics. 2013;12:1115–1126. doi: 10.1074/mcp.M112.020230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Låg M., Rodionov D., Ørevik J., Bakke O., Schwarze P.E., Refsnes M. Cadmium-induced inflammatory responses in cells relevant for lung toxicity: expression and release of cytokines in fibroblasts, epithelial cells and macrophages. Toxicol. Lett. 2010;193:252–260. doi: 10.1016/j.toxlet.2010.01.015. [DOI] [PubMed] [Google Scholar]
- Lalisang T.J. Serum bile acid: an alternative liver function marker in the Obstructive Jaundice Patient. Acta Med. Indones. 2012;44:233–238. [PubMed] [Google Scholar]
- Leach R.M., Wang K.W., Bake D.E. Cadmium and the food chain: the effect of dietary cadmium on tissue composition in chicks and laying hens. J. Nutr. 1979;109:437–443. doi: 10.1093/jn/109.3.437. [DOI] [PubMed] [Google Scholar]
- Li J.H., Jiang C.Y., Li S., Xu S.W. Cadmium induced hepatotoxicity in chickens (Gallus domesticus) and ameliorative effect by selenium. Ecotoxicol Environ. Saf. 2013;96:103–109. doi: 10.1016/j.ecoenv.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Li Y.X., Xiong X., Lin C.Y., Zhan F.S., Wei L., Wei H. Cadmium in animal production and its potential hazard on Beijing and Fuxin farmlands. J. Hazard Mater. 2010;177:475–480. doi: 10.1016/j.jhazmat.2009.12.057. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-Delta Delta C(T) method. Method. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Martelli A., Rousselet E., Dycke C., Bouron A., Moulis J.M. Cadmium toxicity in animal cells by interference with essential metals. Biochimie. 2006;88:1807–1814. doi: 10.1016/j.biochi.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Maiers J.L., Malhi H. Endoplasmic reticulum stress in metabolic liver diseases and hepatic fibrosis. Semin. Liver Dis. 2019;39:235–248. doi: 10.1055/s-0039-1681032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOHC . China Standards Press; Beijing: 2015. National Food Cadmium Standard: Determination of Cadmium in Foods (GB/T 5009.15-2015) [Google Scholar]
- Nai G.A., Marin F.F., Queiroz L.M.M., Estrella M.P.S. Respiratory tract cadmium-induced injuries—poisoning via intake and water pH could influence their genesis? An experimental study in rats. Comp. Clin. Pathol. 2017;26:997–1002. [Google Scholar]
- Nakagawa T., Zhu H., Morishima N., Li E., Xu J., Yankner B.A., Yuan J. Caspase-12 mediates endoplasmic-reticulumspecific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- Noble R.C., Cocchi M. Lipid metabolism and the neonatal chicken. Prog. Lipid Res. 1990;29:107–140. doi: 10.1016/0163-7827(90)90014-c. [DOI] [PubMed] [Google Scholar]
- Oaxaca-Castillo D., Andreoletti P., Vluggens A., Yu S., van Veldhoven P.P., Reddy J.K., Cherkaoui-Malki M. Biochemical characterization of two functional human liver acyl CoA oxidase isoforms 1a and 1b encoded by a single gene. Biochem. Biophys. Res. Commun. 2007;360:314–319. doi: 10.1016/j.bbrc.2007.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S.H., Lim S.C. A rapid and transient ROS generation by cadmium triggers apoptosis via caspase-dependent pathway in HepG2 cells and this is inhibited through N-acetylcysteine-mediated catalase upregulation. Toxicol. Appl. Pharmacol. 2006;212:212–223. doi: 10.1016/j.taap.2005.07.018. [DOI] [PubMed] [Google Scholar]
- Ozer J., Ratner M., Shaw M., Bailey W., Schomaker S. The current state of serum biomarkers of hepatotoxicity. Toxicology. 2008;245:194–205. doi: 10.1016/j.tox.2007.11.021. [DOI] [PubMed] [Google Scholar]
- Pawlak M., Lefebvre P., Staels B. Molecular mechanism of PPARα action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J. Hepatology. 2015;62:720–733. doi: 10.1016/j.jhep.2014.10.039. [DOI] [PubMed] [Google Scholar]
- Pugh R.N., Murray-Lyon I.M., Dawson J.L., Pietroni M.C., Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br. J. Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- Quinlan G.J., Margarson M.P., Mumby S., Evans T.W., Gutteridge J.M. Administration of albumin to patients with sepsis syndrome: a possible beneficial role in plasma thiol repletion. Clin. Sci. (Lond). 1998;95:459–465. [PubMed] [Google Scholar]
- Rodríguez-Serrano M., Romero-Puertas M.C., Zabalza A., Corpas F.J., Gómez M., Del Río L.A., Sandalio L.M. Cadmium effect on oxidative metabolism of pea (Pisum sativum L.) roots. Imaging of reactive oxygen species and nitric oxide accumulation in vivo. Plant Cell Environ. 2006;29:1532–1544. doi: 10.1111/j.1365-3040.2006.01531.x. [DOI] [PubMed] [Google Scholar]
- Rong F., Hu P.C., Wang Y., Lin H.Y., Su K., Feng X.S., Wei L., Yang F. Betulinic acid protects mice from cadmium chloride-induced toxicity by inhibiting cadmium-induced apoptosis in kidney and liver. Toxicol. Lett. 2018;299:56–66. doi: 10.1016/j.toxlet.2018.09.003. [DOI] [PubMed] [Google Scholar]
- Scheuhammer A.M. The chronic toxicity of aluminium, cadmium, mercury, and lead in birds: a review. Environ. Pollut. 1987;46:263–295. doi: 10.1016/0269-7491(87)90173-4. [DOI] [PubMed] [Google Scholar]
- Schneider W.J., Carroll R., Severson D.L., Nimpf J. Apolipoprotein VLDL-lI inhibits lipolysis of triglyceride-rich lipoproteins in the laying hen. J. Lipid Res. 1990;31:507–513. [PubMed] [Google Scholar]
- Schlaepfer I.R., Rider L., Rodrigues L.U., Gijón M.A., Pac C.T., Romero L., Cimic A., Sirintrapun S.J., Glodé L.M., Eckel R.H., Cramer S.D. Lipid catabolism via CPT1 as a therapeutic target for prostate cancer. Mol. Cancer Ther. 2014;13:2361–2371. doi: 10.1158/1535-7163.MCT-14-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X.Y., Steyrer E., Retzek H., Sanders E.J., Schneider W.J. Chicken oocyte growth: receptor-mediated yolk deposition. Cell Tissue Res. 1993;272:459–471. doi: 10.1007/BF00318552. [DOI] [PubMed] [Google Scholar]
- Shivaprasad H.L., Jaap R.G. Egg and yolk production as influenced by liver weight, liver lipid and plasma lipid in three strains of small bodied chickens. Poult. Sci. 1977;56:1384–1390. [Google Scholar]
- Shaikh A., Vu T.T., Zaman K. Oxidative stress a mechanism of chronic cadmium induced hepatotoxicity and renal toxicity and protection by antioxidants. Toxicol. Appl. Pharmacol. 1999;154:256–263. doi: 10.1006/taap.1998.8586. [DOI] [PubMed] [Google Scholar]
- Stohs S.J., Bagchi D. Oxidative mechanism in the toxicity of metal ions. Free Radic. Biol. Med. 1995;18:321–336. doi: 10.1016/0891-5849(94)00159-h. [DOI] [PubMed] [Google Scholar]
- Thompson J., Bannigan J. Cadmium: toxic effects on the reproductive system and the embryo. Reprod. Toxicol. 2008;25:304–315. doi: 10.1016/j.reprotox.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Treviño S., Andrade-García A., Herrera Camacho I., León-Chavez B.A., Aguilar-Alonso P., Flores G., Brambila E. Chronic cadmium exposure lead to inhibition of serum and hepatic alkaline phosphatase activity in Wistar rats. J. Biochem. Mol. Toxicol. 2015;29:587–594. doi: 10.1002/jbt.21732. [DOI] [PubMed] [Google Scholar]
- Valko M., Morris H., Cronin M.T.D. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005;12:1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- Wang L., Cheng X.F., Li H., Qiu F., Yang N., Wang B., Lu H.C., Wu H.W., Shen Y., Wang Y.Q., Jing H. Quercetin reduces oxidative stress and inhibits activation of c-Jun N-terminal kinase/activator protein-1 signaling in an experimental mouse model of abdominal aortic aneurysm. Mol. Med. Rep. 2014;9:435–442. doi: 10.3892/mmr.2013.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.C., Jiang L., Li Y.F., Luo X.G., He J. Excessive selenium supplementation induced oxidative stress and endoplasmic reticulum stress in chicken spleen. Biol. Trace Elem. Res. 2016;172:481–487. doi: 10.1007/s12011-015-0596-9. [DOI] [PubMed] [Google Scholar]
- Wang L., Zhang S., Wang Z., Xu M., Yuan L., Cui J., Liu S. A protective role of Heme-regulated eIF2a kinase in cadmium-induced liver and kidney injuries. Chemosphere. 2017;185:284–289. doi: 10.1016/j.chemosphere.2017.07.018. [DOI] [PubMed] [Google Scholar]
- Waisberg M., Joseph P., Hale B., Beyersmann D. Molecular and cellular mechanisms of cadmium carcinogenesis. Toxicology. 2003;192:95–117. doi: 10.1016/s0300-483x(03)00305-6. [DOI] [PubMed] [Google Scholar]
- Wetterau J.R., Aggerbeck L.P., Bouma M.E., Eisenberg C., Munck A., Hermier M., Schmitz J., Gay G., Rader D.J., Gregg R.E. Absence of microsomal triglyceride transfer protein in individuals with abetalipoproteinemia. Science. 1992;258:999–1001. doi: 10.1126/science.1439810. [DOI] [PubMed] [Google Scholar]
- Wilfling F., Haas J.T., Walther T.C., Farese R.V., Jr. Lipid droplet Biogenesis. Curr. Opin. Cell Biol. 2014;29:39–45. doi: 10.1016/j.ceb.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q., Yao H.D., Tan S.R., Zhang Z.W., Zhu Y.H., Xu S. Possible correlation of selenoprotein W with inflammation factors in chicken skeletal muscles. Biol. Trace Elem. Res. 2014;161:167–172. doi: 10.1007/s12011-014-0092-7. [DOI] [PubMed] [Google Scholar]
- Wu C., Zhang Y.H., Chai L.H., Wang H.Y. Histological changes, lipid metabolism and oxidative stress in the liver of Bufo gargarizans exposed to cadmium concentrations. Chemosphere. 2017;179:337–346. doi: 10.1016/j.chemosphere.2017.03.131. [DOI] [PubMed] [Google Scholar]
- Xie W., Ge M., Li G., Zhang L., Tang Z., Li R., Zhang R. Astragalus polysaccharide protect against cadmium-induced cytotoxicity through the MDA5/NF-κB pathway in chicken peripheral blood lymphocytes. Molecules. 2017;22:1610. doi: 10.3390/molecules22101610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamano T., DeCicco L.A., Rikans L.E. Attenuation of cadmium-induced liver injury in senescent male fischer 344 rats: role of Kupffer cells and inflammatory cytokines. Toxicol. Appl. Pharmacol. 2000;162:68–75. doi: 10.1006/taap.1999.8833. [DOI] [PubMed] [Google Scholar]
- Yoshiuchi K., Kaneto H., Matsuoka T.A., Kasami R., Kohno K., Iwawaki T., Nakatani Y., Yamasaki Y., Shimomura I., Matsuhisa M. Pioglitazone reduces ER stress in the liver: direct monitoring of in vivo ER stress using ER stress-activated indicator transgenic mice. Endocr. J. 2009;56:1103–1111. doi: 10.1507/endocrj.k09e-140. [DOI] [PubMed] [Google Scholar]
- Zhang S., Jiang C., Liu H., Guan Z., Zeng Q., Zhang C., Lei R., Xia T., Gao H., Yang L., Chen Y., Wu X., Zhang X., Cui Y., Yu L., Wang Z., Wang A. Fluoride-elicited developmental testicular toxicity in rats: Roles of endoplasmic reticulum stress and inflammatory response. Toxicol. Appl. Pharmacol. 2013;271:206–215. doi: 10.1016/j.taap.2013.04.033. [DOI] [PubMed] [Google Scholar]
- Zhong L.Y., Wang L.M., Xu L.R., Liu Q.L., Jiang L.L., Zhi Y.E., Lu W., Zhou P. The role of nitric oxide synthase in an Early Phase Cd-induced acute cytotoxicity in MCF-7 cells. Biol. Trace Elem. Res. 2015;164:130–138. doi: 10.1007/s12011-014-0187-1. [DOI] [PubMed] [Google Scholar]