Abstract
The biosecurity status of 397 broiler farms in Central Luzon, the highest poultry meat–producing region in the Philippines, was assessed using Biocheck.Ugent. This online biosecurity assessment tool quantifies biosecurity level or compliance of surveyed farms. The system generates scores that reflect the current biosecurity status of each farm in terms of the different external and internal biosecurity measures being implemented in each farm. It was initially developed for pigs and broilers but recently is available for layers, swine, and cattle (beef, dairy, and veal).
The overall biosecurity score of broiler farms in Central Luzon was 71.2%, with average external and internal biosecurity scores of 68.5 and 77.2%, respectively. Bataan had the highest biosecurity scores (76.5%) compared with the other 6 provinces. This was also true for the external and internal biosecurity scores of the province, with mean scores of 72.1 and 80.1%, respectively. Of the 11 subcategories of external and internal biosecurity that were assessed, purchase of day-old chicks, feeds and water supply, supply of materials, cleaning and disinfection, and materials between compartments had scores higher than the global scores. Low scores were generated from transport of live animals and infrastructure and biological vectors.
The mean biosecurity score of farms with traditional/conventional type of housing was 7.8% lower than that with tunnel vent housing. Every year as the farm gets older, there was a corresponding drop of 0.2% in the biosecurity score.
Biosecurity measures are in place in broiler farms in the country. However, there were areas with low scores which need to be prioritized to improve and upgrade the farms' biosecurity status.
To date, this is the first quantitative assessment of biosecurity in broiler farms in the Philippines. High biosecurity scores may entail greater protection from disease incursion.
Key words: Biocheck.UGent, biosecurity scoring tool, biosecurity assessment
Introduction
Poultry is a progressive animal enterprise and one of the major and fastest producers of meat worldwide. In the Philippines, poultry has been a significant contributor to the country's agriculture sector (PCARRD, 2006). It is characterized by widely diverse production and marketing systems consisting of a few large integrated enterprises and a big number of smallhold farmers. There are also medium-scale producers who depend largely on large integrated farms for the supply of breeding stocks and feedstuffs (NAST, 2005). As of January 2018, the total chicken inventory was estimated at 175.77 million birds, or a 0.26% growth compared with 2017 statistics. In 2017, Central Luzon was the country's top chicken producer with 35.78% share in the total broiler inventory (PSA, 2017). However, the industry is faced by many setbacks such as high importation of poultry meat, disease incursion, shortage of supply, and high prices of raw feed ingredients (PCAARD, 2006). The first highly pathogenic avian influenza (HPAI-H5N6) outbreak in the Philippines in 2017 affected layer farms, backyard chicken, quail, and duck farms (Lee and Lao, 2018). The incursion of this disease may be due to poor biosecurity (Boklund, 2008, Niemi et al., 2009, Racicot et al., 2012). Surprisingly, HPAI was not reported in broiler chickens. Presumably this was due to a more robust implementation of biosecurity program in this type of operation. However, this needs further investigation. While it is generally agreed that enhanced compliance to biosecurity measures is the best way to minimize the risk of disease introduction (Boklund et al., 2004; Niemi et al., 2009).
In the Philippines, there is a National Standard on Code of Animal Husbandry Practices for Chicken—Broilers and Layers developed by the Bureau of Agriculture and Fishereis Standards in 2016. This standard was made to harmonize with the Association of Southeast Asian Nations Food Safety Module: Good Animal Husbandry Practices for Layers and Broilers. Details on hydgiene and sanitation are explicitly written on the Association of Southeast Asian Nations Biosecurity Management Manual for Commercial Poultry Farming. Although monitoring of commercial farms is an integral component of the national guideline, documents are very limited on the compliance of most farms. The Bureau of Agriculture and Fishereis Standards, however, issues Good Animal Husbandry Practice certificates to poultry farms on voluntary basis to ensure that the farming practices of the establishment provide greater confidence on product safety. Biosecurity measures recommended in the manual are practically sufficient.
The absence or minimal intrusion of diseases contributes greatly to food security, indicating the paramount importance of biosecurity. Poor biosecurity means exposing poultry flocks to various infectious diseases, and consequently this is associated with great economic losses (Conan et al., 2013). Regular monitoring and assessment of compliance to various biosecurity measures in poultry farms is crucial. Most of the tools used for biosecurity assessment are developed as checklists or manuals (Dewulf et al., 2018). As qualitative methods, they just indicate if a particular biosecurity measure is being complied or not or if it is present or not. This kind of descriptive assessment is useful, but a quantitative method would be very practical and of greater value. When biosecurity level is quantified, specific areas of hygiene and sanitation can be identified and improved easily.
No studies to our knowledge have been carried out to assess the current biosecurity status of broiler farms in the Philippines. This study aimed to quantify the biosecurity status of broiler farms in Central Luzon using the Biocheck.Ugent online tool.
Materials and methods
Study Design and Area
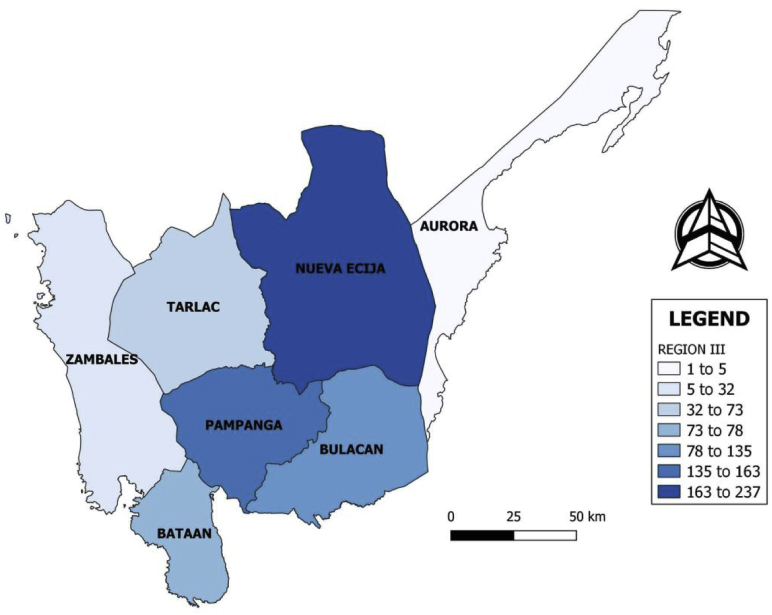
There are 723 broiler farms in Central Luzon (region 3), Philippines. This region is composed of 7 provinces namely, Aurora, Bataan, Bulacan, Nueva Ecija, Pampanga, Tarlac, and Zambales (Figure 1). The popular breeds or strains of broilers raised in the Philippines are Ross, Cobb, Hybro, Avian, Hubbard, and Starbro (PCAARD, 2006). The initial list of farms was provided by the Department of Agriculture-Bureau of Animal Industry, Provincial Veterinary Offices, and Municipal Agricultural Offices. Each farm was personally visited for geotagging and to secure consent to conduct the biosecurity assessment survey. A total of 397 (54.91%) broiler farms agreed to participate in the study. Table 1 shows the distribution of study participants. Immediately after a consent was given, personal interviews were conducted.
Figure 1.
Broiler farms in Central Luzon, Philippines.
Table 1.
Distribution of survey participants by province.
| Province | Number of broiler farms | Number of respondents |
|---|---|---|
| Aurora | 5 | 2 |
| Bataan | 78 | 56 |
| Bulacan | 135 | 54 |
| Nueva Ecija | 237 | 131 |
| Pampanga | 163 | 77 |
| Tarlac | 73 | 52 |
| Zambales | 32 | 25 |
| Total | 723 | 397 |
Data Collection
The Biocheck.UGent scoring system was used to quantify biosecurity of farms. It is a risk-based scoring system to quantify on-farm biosecurity which is credible, reproducible, and can be validated (Laanen et al., 2010, Gelaude et al., 2014, Postma et al., 2016).
The online questionnaire was translated into the local dialect by a professional translator and reproduced for the convenience of both interviewers and interviewees. This also addressed problems of internet connectivity in remote areas. The translated tool was pretested, and interviewers were trained how to administer it. While it was the goal of this research project to include all the farms in the survey, some farm owners refused to participate because HPAI was confirmed in the region. Thus, only those who consented comprised the final list of participants. Actual visits to each farm was conducted where the researcher or trained staff personally interviewed a contact person who was usually the farm supervisor, manager, or the farm owner. Trained interviewers personally conducted the interviews. All participants in one province were completed first before moving to the next province.
Validation of survey results was conducted by actual visits to each farm. Unfortunately, the survey period coincided with the occurrence of HPAI in 2 provinces of the region, specifically in Pampanga and Nueva Ecija. This made validation very difficult because of the limited access given to our research team. When given access, photographs were taken and validation of the answers were carried out. In most instances, interviews were held in the farms' offices; however, others were held outside farm gates because of the threat of disease introduction.
After the data were completed from July to December 2017, each form was encoded using the online tool, and individual reports were generated. The data were further collated in Excel format for consolidation and statistical analysis.
Quantification of Biosecurity
The Biocheck.UGent scoring system assesses general biosecurity and is based on the transmission of infectious poultry diseases. It includes 79 dichotomous or trichotomous questions that are divided into several subcategories for external and internal biosecurity. Each subcategory consists of 2 to 19 questions. The answer to every question results in a score between zero (when this measure is not implemented at all) and one (when the measure is fully implemented). Depending on the importance of a particular biosecurity measure, the score per question is multiplied by a weight factor (Laanen et al., 2013; Gelaude et al., 2014). The subcategories also have a specific weight factor equal to their determined relative importance for disease transmission as determined by a large group of poultry specialists (Laanen et al., 2013; Gelaude et al., 2014).
The final score for both internal and external biosecurity can range from zero, indicating a total absence of the described biosecurity measures, to 100, indicating a full application of the described measures. The average of the internal and external biosecurity scores provides the overall biosecurity score (Dewulf et al., 2018). This proportional result of the subcategory was then multiplied by the weight of the subcategory to obtain the subcategory score. The final score of the internal and external biosecurity was the sum of the different subcategory scores. The overall biosecurity score was the sum of the external and internal biosecurity score. Owing to the different relative weight, the external biosecurity score counts for 70% and the internal score counts for 30% in the total biosecurity score. For ease of interpretation of the results, category and subcategory scores were recalculated each time to a score of 100 and presented as a percentage in the reports (Gelaude et al., 2014). The Biocheck.UGent online tool provides a risk-based score that takes into account the relative importance of all different biosecurity measures (Dewulf, et al., 2018). Presently, Biocheck.UGent is available for the qunantitative assessment of biosecurity for poultry (broilers and layers), swine, and cattle (beef, dairy, and veal).
The generated scores per farm allow evaluation of the strong and weak points of biosecurity compliance, which can be the basis for recommendations to improve biosecurity. The automatic advice features of the system were not yet available during the survey period. The individual reports generated from the system were consolidated by province for analysis and comparison.
Data Analysis
Field editing of data entries in questionnaires was performed to check for consistency and completeness of data. Microsoft Excel (Microsoft Corp., Santa Rosa, CA) was used to encode the data. The encoded data were imported to Stata/MP 13.0 for Windows. Data cleaning was further performed to check for encoding errors, inadmissible values, incompleteness, and inconsistencies.
Medians were obtained for quantitative variables such as the external (purchase of pullets, transport of animals, feed and water supply, removal of manure and dead animals, entrance of visitors and personnel, supply of materials, infrastructure and biological vectors, and location) and internal (disease management and vaccination, cleaning and disinfection, and materials and measures between compartments) biosecurity scores. Other quantitative variables included capacity, age of farm, number of poultry houses, and age of the newest and oldest poultry houses. Frequencies and percentages were computed for qualitative variables such as farm characteristics including type of farm, type of operation, and type of housing. Graphs and maps were constructed using Microsoft Excel (2013) and MapInfo Pro (Pitney Bowes Inc., Stamford, CT).
The external and internal biosecurity scores of broiler farms were compared by province using Kruskal-Wallis test for the comparison of medians. The use of this nonparametric method was necessary because Shapiro-Wilk Test revealed that the scores were not normally distributed. Moreover, Dunn's test of multiple comparison using rank sums was used. Spearman correlation was also used to test for the correlation of internal and external biosecurity scores per province.
Likewise, the overall biosecurity scores in the various external and internal biosecurity subcategories and subtotals of broiler farms in the region were compared to the global average using t-test for one population mean. The global averages were taken from all the broiler farms that (Biocheck.Ugent).
Finally, simple and multiple linear regression analyses were performed to determine the farm characteristics that were associated with biosecurity scores of each type of farm.
A 0.05 level of significance was used in all hypothesis testing.
Results and discussion
Farm Characteristics
A total of 397 broiler farms participated in the study. Table 2 shows the characteristics of broiler farms surveyed. Most farms were contract growers (76.6%). More than half of the broiler farms were medium commercial farms (56.7%) with one of every 5 being a large commercial broiler farm (20.9%). A large commercial farm has more than 100,000 birds per harvest while a medium commercial farm has between 21,000 and 99,000 birds per harvest (PCAARD, 2006).
Table 2.
Characteristics of broiler farms in Central Luzon.
| Farm characteristic | Frequency (n = 397), no. (%) |
|---|---|
| Type of housing | |
| Tunnel vent | 232 (58.4) |
| Traditional | 160 (40.3) |
| No response | 5 (1.3) |
| Age of farm (in yr) | |
| Mean ± standard deviation (s.d.) | 9.6 ± 9.3 |
| Median | 6 |
| Range | 0 - 57 |
| Capacity | |
| Mean ± s.d. | 73,226.5 ± 85,070.9 |
| Median | 45,000 |
| Range | 1,300 - 900,000 |
| Number of workers | |
| Mean ± s.d. | 10 ± 9 |
| Median | 7 |
| Range | 1 - 77 |
Almost 3 of every 5 of these farms (58.4%) had tunnel vent type of housing while 40.3% had traditional type of housing. The participating broiler farms were 9.6 yr old, on the average, with the oldest being 57 yr. The median capacity of these farms was 45,000 chickens, and the median number of farm workers in each farm was 7.
The participating broiler farms had, on the average, 4 poultry houses. The oldest and newest poultry houses had mean age of 8.9 and 4.9 yr, respectively.
Biosecurity Scores
To our knowledge, this is the first time that a quantitative assessment of biosecurity among broiler farms was conducted in the Philippines. During the first recorded bird flu outbreak in the country, the broiler farms remained unaffected while the rest of the other species such as chicken layers, quails, and ducks had succumbed to the infection. It is very crucial to benchmark the real status of biosecurity in the Philippine broiler industry so that disease incursion can be prevented. The emergence and spread of diseases including the dreaded HPAI has caused substantial poultry-related economic losses and public health concerns in relation to a potential pandemic (Conan et al., 2013).
This study presents the scores for the external, internal, and overall biosecurity scores of broiler farms per province in Central Luzon (Figure 1, Figure 2, Figure 3). Table 3 shows the results of the test of comparison of medians using Kruskal-Wallis test.
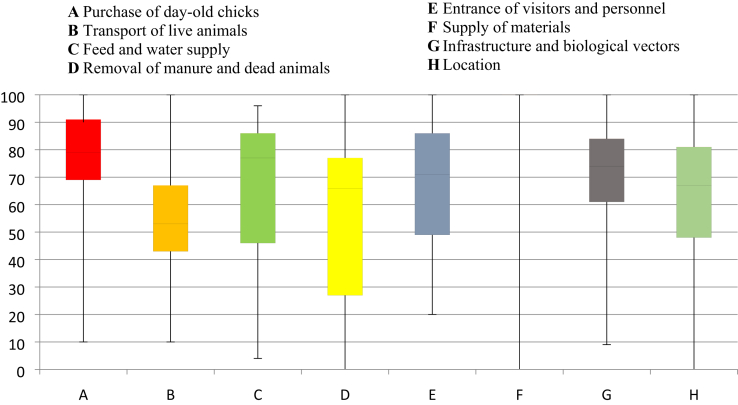
Figure 2.
Box and whiskers plot of external biosecurity scores of broiler farms.
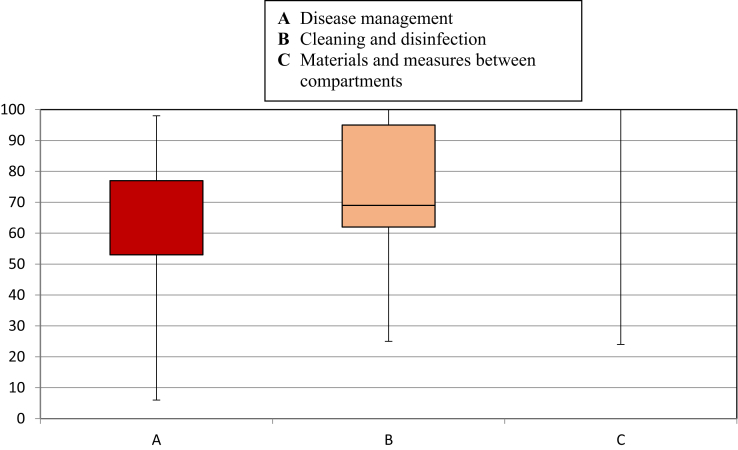
Figure 3.
Box and whiskers plot of internal biosecurity of broiler farms.
Table 3.
Comparison of median external and internal biosecurity scores of broiler farms in Central Luzon.
| Biosecurity category | Province |
Kruskal-Wallis P value1 | ||||||
|---|---|---|---|---|---|---|---|---|
| Aurora (n = 2) | Bataan (n = 56) | Bulacan (n = 54) | Nueva Ecija (n = 131) | Pampanga (n = 77) | Tarlac (n = 52) | Zambales (n = 54) | ||
| External biosecurity | ||||||||
| Purchase of day-old chicks | 90.0 | 79.0c | 79.0e | 79.0a | 79.0a,d | 79.0b,c,d,e | 79.0b | 0.024 |
| Transport of live animals | 59.0 | 60.0a,b,c,e | 53.0a,f | 53.0d,e,i | 61.0f,g,h,i | 52.5c,d,h | 50.0b,g | <0.001 |
| Feed and water supply | 39.0 | 77.0 | 67.0 | 77.0 | 77.0 | 79.0 | 69.0 | 0.128 |
| Removal of manure and dead animals | 56.5 | 77.0 | 67.0 | 63.0 | 77.0 | 63.5 | 63.5 | 0.093 |
| Entrance of visitors and personnel | 30.0 | 80.0 | 65.0 | 71.0 | 74.0 | 66.5 | 73.0 | 0.107 |
| Supply of materials | 56.0 | 100.0d,e,g | 100.0a,b,f,h | 100.0f,g,k | 100.0h,i,j,k | 100.0b,c,e,j | 100.0a,c,d,i | 0.002 |
| Infrastructure and biological vectors | 64.0 | 75.5g | 75.5a,c,e | 77.0c,d,h | 63.0e,f,g,h | 71.5b,d | 80.0a,b,f | <0.001 |
| Location | 71.0 | 67.0c | 67.0a | 63.0a,b,c | 63.0 | 81.0b | 65.0 | 0.034 |
| Subtotal | 57.0 | 73.0 | 68.5 | 70.0 | 70.0 | 67.0 | 67.5 | 0.086 |
| Internal biosecurity | ||||||||
| Disease management | 68.0 | 77.0 | 77.0 | 77.0 | 77.0 | 77.0 | 77.0 | 0.598 |
| Cleaning and disinfection | 50.5 | 87.0a,b,c,f | 66.0a,d,h | 74.0d,e,f,g | 79.0h,i,j | 62.0c,g,j | 67.0b,e,i | <0.001 |
| Materials and measures between compartments | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 0.331 |
| Subtotal | 67.5 | 82.0a,b,c,i | 76.0a,e | 79.0e,f,g,h | 77.0h,i | 77.0h,i | 73.0b,f | <0.001 |
| Total biosecurity scores | 60.0 | 76.5a,b,c,e | 72.0a,d | 73.0d | 72.0e | 70.0c | 72.5b | 0.047 |
a–kMedians in the same subcategory (row) with the same letter superscript are statistically different at P < 0.05.
Excluding Aurora in the comparison of medians.
General Biosecurity Scores
The total biosecurity scores of 7 provinces in the region are presented in Figure 2. The overall total biosecurity score of broiler farms in the region is 71.2 with a range of 14.6 (between 60.0 to 74.6). This is slightly higher than the score of broiler farms in Europe, which was 70.9 (Van Limbergen et al., 2017). This was the average of 400 conventional broiler farms from 5 different states of Europe. It is important to note here that the standard of biosecurity among Philippine broiler farms is at par with European standards. This may be the reason why the broiler industry was not affected when the country was hit with bird flu. This apparent resilience may be attributed to higher compliance with biosecurity measures. However, the score of 71.2 may not be ideal. Europe was not without disease outbreaks in their conventional poultry operations. HPAI is a highly contagious livestock disease in poultry. Outbreaks of HPAI in naïve and nonvaccinated populations have a huge veterinary impact, for example, an epidemic spread resulting in large numbers of diseased animals, high mortality, and other negative production effects (Swayne, 2008). This score still leaves farms with 28.8% chance of being infected with disease entities. The broiler industry should not take chances. Elevating its scores to higher level always entails greater protection.
Table 3 shows that Bataan garnered the highest total biosecurity score (mean = 74.6/median = 76.5) compared with the other 6 provinces. This is likewise true for the external and internal biosecurity scores of the province, with mean scores of 72.1 and 80.1, respectively. Aurora obtained the lowest external, internal, and overall biosecurity scores. The overall median biosecurity score of Bataan was statistically higher than the median scores of broiler farms in Bulacan, Zambales, Pampanga, and Tarlac (P < 0.05). It is possible that Bataan farms scored the highest because the province had municipal and provincial provisions for biosecurity and they also have a monitoring system manned by the Provincial Veterinary Office to ensure compliance. Aurora, on the other hand being a province endowed with aquatic resources, was not much into poultry.
Table 4 presents the global average scores for the different subcategories of biosecurity. The average global score was 64, with 63 and 64 for external and internal biosecurity, respectively. The overall average scores for the participating broiler farms were likewise presented. Among the broiler farms, the mean regional biosecurity score was higher than the global average of 71.2%, compared to 64%.
Table 4.
Global and regional average scores for the different subcategories of biosecurity.
| Subcategory | Global average | Broiler |
|
|---|---|---|---|
| Overall, average | t-test, P value | ||
| External biosecurity | |||
| Purchase of 1-day-old chicks | 56 | 77.6 | <0.001 |
| Transport of live animals | 61 | 55.6 | <0.001 |
| Feed and water supply | 58 | 65.6 | <0.001 |
| Removal of manure and dead animals | 59 | 59.3 | 0.847 |
| Entrance of visitors and personnel | 69 | 68.5 | 0.666 |
| Supply of materials | 56 | 89.1 | <0.001 |
| Infra and biological vectors | 77 | 71.5 | <0.001 |
| Location | 65 | 64.7 | 0.802 |
| Subtotal | 63 | 68.5 | <0.001 |
| Internal biosecurity | |||
| Disease management | 73 | 67.9 | <0.001 |
| Cleaning and disinfection | 61 | 74.2 | <0.001 |
| Materials and measures between compartments | 59 | 95.1 | <0.001 |
| Subtotal | 64 | 77.2 | <0.001 |
| Total | 64 | 71.2 | <0.001 |
For the external biosecurity category, the regional scores were above the global average for the following subcategories: 1) purchase of day-old chicks, 2) feed and water supply, and 3) supply of materials. Moreover, the broiler farms in the region can improve on the following subcategories: 1) transport of live animals and 2) infrastructure and biological vectors. This is because the mean score in these subcategories was even lower than the already low global average (Table 3).
Scores on internal biosecurity subcategories on cleaning and disinfection and materials and measures between compartments are higher than the global scores. Broiler farmers have good internal biosecurity measures. This will certainly contribute to prevent disease spread.
External Biosecurity
The biosecurity scores of farms in the 7 provinces of the region in the external and internal subcategories are 68.5 and 77.2%, respectively. The total biosecurity score for the region is 71.2%. The average external biosecurity scores of farms were higher than the internal biosecurity. Among the subcategories of external biosecurity, the overall mean score was highest in the supply of materials subcategory (mean = 89.1%), followed by purchase of day-old chicks (mean = 77.6%). The lowest mean score was obtained in the transport of live animals category.
The box and whiskers plot in Figure 2 becomes very useful because it shows how many of the surveyed farms are falling in particular range of biosecurity scores. Let us take for example subcategory A (purchase of day-old chicks). The upper whisker represents 25% of the total farms surveyed while the red box represents the second and third quartiles or the 50% of the total population, and the lower whisker represents the last quadrant (25%) of the population. We can say that 75% of the farms had biosecurity score of 70% and above while 25% had scores below 70%. This will easily identify farms that are noncompliant with specific biosecurity measures. In the event of a region-wide upscaling of biosecurity, this is a baseline information. However, the biosecurity scores of farms are widely spread apart. There is no consistency among farms in their compliance to each subcategory of biosecurity.
Each farm is equipped with necessary supplies and materials, and these were exclusively used in the farm. These materials are also cleaned and disinfected on a regular basis. About 75% of the farms in the region had scores above 70 in the purchase of day-old chick subcategory. Most farms start with clean stocks. Unfortunately, those who do not have clean stocks increase their likelihood of being infected.
Table 3 shows the median scores of the broiler farms in the 7 provinces as well as the comparison of these provincial medians using Kruskal-Wallis tests. Comparison of the provincial medians was performed except for the Province of Aurora because there were only 2 participating broiler farms from said province. At least 2 provinces had statistically different medians in 5 of the 8 external biosecurity subcategories namely: purchase of day-old chicks, transport of live animals, supply of materials, infrastructure and biological vectors, and location. Zambales had the highest score in the subcategory infrastructure and biological vectors (P < 0.05), followed by Nueva Ecija and Bataan. In terms of location, Tarlac province had the highest score followed by Bataan and Bulacan (P < 0.05). Farms from Bataan garnered the highest external biosecurity scores of 72.1 while Aurora scored lowest in this category.
Detailed Description of the Regional External Biosecurity
Purchase of Day-Old Chicks
Among broiler farms, delivery trucks were disinfected before entering 97% of broiler farms. This is a good practice because Gelaude and colleagues (2014) stated that transport vehicles or trucks' frequent movement from one farm to another can increase the risk of spreading disease. Chicks are delivered first in around 4 of every 5 (81.6%) broiler farms before they are delivered to other farms. Suppliers of chicks should deliver to only one farm at a time, unfortunately, 18.4% of the farms had suppliers delivering to multiplefarms.
Similarly, 80.7% of the participating farms did not change their supplier of chicks for the past 2 yr. Half of the broiler farms have suppliers that deliver chicks straight to the farms. For most farms (83.3%), delivery trucks do not contain chicks for other farms. For 56% of these farms, drivers of delivery vans do not get empty crates from the farm after making deliveries to other farms.
There are around 60% broiler farms which had chicks delivered in to their farms 3 to 6 times a year. While this may be the actual practice in most farms, stricter biosecurity measures should be implemented considering that a more frequent introduction and purchase of more birds will likely increase risk of disease introduction (Laanen et al., 2013).
Thirty-two percent or 127 broiler farms have various age categories for their poultry. The farms practice all-in-all-out system, but there was a substantial delay in the delivery of chicks in some farms such that 18.9% of these farms had chicks older than 7 D.
Transport of Live Animals
For at least 90% of broiler farms, transport vehicles were always cleaned on arrival to the farm. Entrance of individuals and traders to farms wherein there would be a possibility of direct contact with the animals was prohibited for 85.4% of the broiler farms. However, in only around one-third of the farms were drivers and catching teams provided with farm-specific or disposable clothes and shoes when animals were being loaded to the vehicles. This is quite a significant risk because every time truck drivers and the catching team enter the farm, the possibility of introducing disease is also high, especially when they are not wearing appropriate clothing during the process (Berndtson et al., 1996, Lister, 2008). Proper cleaning and disinfection of transport vehicles is crucial to prevent transmission of disease, especially if these vehicles will come from other farms (Rajkowski et al., 1998, Gelaude et al., 2014, Dewulf et al., 2018).
For most broiler farms (61.5%), broilers were harvested from the farm 6 to 12 times per year. An average of 61,439 ± 74,090 chickens are removed from the broiler farms every harvest. The harvesting crates have been associated with the transmission of pathogens in farms (Slader et al., 2002, Lister, 2008). This is the very reason why harvesting crates should be thoroughly cleaned and disinfected before they are used. It was observed that these crates were being reused between farms (Slader et al., 2002). These crates are usually owned by chick suppliers. It is the suppliers' responsibility to clean and disinfect these crates before delivery of chicks. Reusing these crates without cleaning and disinfection poses disease risks. However, whether the crates were cleaned and disinfected before delivery was not included in the survey questions. As these crates were owned by chick suppliers, it was assumed that they should be the ones to clean and disinfect crates. However, a question was included to ask whether delivery trucks pick up empty crates after unloading. This may contribute to a risk of disease transmission if the truck already came from a different farm. However, it was possible that crates were automatically reloaded in the trucks after unloading the chicks.
Finally, a mean of 45.8 ± 141.1 min was computed for the number of minutes needed to harvest 1,000 chickens. Harvesting usually started late in the afternoon and completed late in the evening. This was carried out per housing unit. Harvesting time in bigger farms may take longer. Theoretically, harvesting should be carried out the shortest possible time because the longer it is done, the higher the possibility of disease transmission. Others recommend to do harvesting in a few steps as possible and provide farm-specific clothing to reduce risks of transmission (Berndtson et al., 1996, McDowell et al., 2008).
Feed and Water Supply
Around 73.3% of the broiler farms had division of clean and dirty areas; of these, 88% had clear separation between the clean and dirty areas while only a little more than half of them provided access to the feed warehouses without going through the clean area. In four-fifths of the broiler farms, feed suppliers did not have access to the poultry houses while 3 quarters had well-sealed feed warehouses to protect against water, birds, and vermin.
The feed storage facilities were said to be filled up more than 35 times per year in around 69% of broiler farms. Silos are uncommon in broiler farms in the region. Feeds are usually transported in 50-kg bags/sacs. For the farms wherein feeds were being brought in a bag, around 2 of every 5 farms had multiple providers of feeds (40.5%). Finally, more than 80% of the farms had the same company supplier of feeds. This entails very frequent movement of delivery trucks, drivers, and haulers during delivery of feeds. This in itself poses greater risk of transmission because feeds can be contaminated en route to production, transportation, or storage (Lister, 2008). Feed silos give more security than feeds in warehouses against rats and other vermin (Nespeca et al., 1997, Al-Saffar et al., 2006, Charisis, 2008, van Steenwinkel et al., 2011)
It has been shown that the drinking water can easily be contaminated with pathogens (Lister, 2008). Most broiler farms (78.1%) had annual bacteriology analysis of their potable water. Among those reported to have bacteriologic analysis, water samples were taken from the source for 85%. The 21.9% of those who did not have their water tested should do so because quality of water is assured when water is tested at least annually to include a systematic cleaning of pipes (Jeffrey, 1997, Gelaude et al., 2014, Dewulf et al., 2018).
Removal of Manure and Dead Animals
Almost all (96.9%) the broiler farms reported that manure was removed after each harvest. Around 84% of broiler farms sold manure, and 95% of them know how the buyers would use the manure. The primary purpose of buyers was to use the manure as organic fertilizer for vegetable farming. A few of the farmers still practice spreading the manure in nearby areas. This can maintain the potential risk for the spread of many pathogens such as Gumboro, Avian Influenza, and Infectious Bronchitis (Lister, 2008). This also poses an opportunity for human contact and risk of transmission of pathogens, posing biosecurity threat (Nachman, 2005). Biological composting and anaerobic storage are required before using manure as organic fertilizer or spreading manure in the fields (Manuja et al., 2014). While it is recommended that the removal of manure should always be carried out via the dirty road (Pritchard et al., 2005), in most broiler farms in the region, there was no distinction between dirty and clean roads when manure is transported. As the entire farm is empty, they believed that this was no longer necessary especially when cleaning and disinfection will be carried out after removal of manure.
Biosecurity agencies in Australia, New Zealand, United States, and Canada have recognized the potential benefits of composting for both routine and emergency management of mortalities and have identified it as a preferred method of carcass disposal (Department of Agriculture, Fisheries and Forestry, 2005). Composting is particularly suitable for broiler-farm mortalities and litter (Wilkinson, 2007).
Among broiler farms, 267 or 67.3% had separate or segregated storage for poultry carcasses. Of these, 92.5% had completely closed storage to prevent access of vermin, dogs, or cats. Likewise, 91.8% regularly cleaned and disinfected the storage. Only around one-tenth of these broiler farms had cooling facility for their carcass storage. Carcasses are a potential source of infection, and they should be removed right away from animal houses and placed and stored in a well-insulated designated area. Removal of dead animals should be done at least once a day (Meroz and Samberg, 1995, Pritchard et al., 2005). Unfortunately, only 10% of farmers in the region had a cooling facility for carcass storage. This facility can be totally closed, thus removing possibility for the spread of pathogens or access to vermin. This also reduces decomposition rate of carcasses, thus reducing frequency of visits of carcass collectors and rendering companies (Vangroenweghe et al., 2009). Seventeen percent of the respondents from broiler farms said that no protective measures were followed when manipulating carcasses.
Entrance of Personnel and Visitors
Humans can serve as mechanical and biological vectors for transmission of infectious diseases in farms (Amass and Baysinger, 2006, Lister, 2008). There was an incident when visitors were implicated in the infection of a poultry farm with HPAI (Vieira et al., 2009). In this survey, farmers and their personnel adhered to access rules in 87.4% of the broiler farms.
Almost 3-quarters (74.3%) had their visitors and personnel wash and disinfect their hands before they would enter farm premises. It should be a practice to wash hands before and after a visit to a farm. The hands of animal handlers may transfer germs through direct contact with the sick animals (Lister, 2008, Vangroenweghe et al., 2009).
A little more than half of the broiler farms had their visitors and personnel wear farm-specific clothing and shoes before entering the farm premises. Although compliance is relatively high, these farms are still vulnerable to infection. As humans can serve as a mechanical vector for the transmission of infectious diseases, it is recommended to take specific biosecurity measures at the moment they enter a farm (Lister, 2008). When visitors and personnel enter farms, they should always wear clean, herd-specific clothes and footwear to avoid disease transmission through leftovers of excreta from other infected animals (Nespeca et al., 1997, Lister, 2008; Dorea et al., 2010). It is recommended to minimize the number of people or prevent unauthorized persons from going into the animal houses to ensure that external staff spend as little time as possible in and around the farm premises (Carey et al., 2005, Charisis, 2008).
In almost half of the broiler farms, 56.7% of visitors were never granted access inside the buildings, while for 10% of these farms, visitors had access inside the farm for more than 12 times per year.
Employees of 11.6% of broiler farms also kept poultry or birds at their homes, while around 5% of these farms had employees who also work for other poultry farms. Other animals raised in the farm premises can serve as vectors for the transmission of multiple infectious poultry diseases. Employing personnel with poultry pets at home should be discouraged because this entails risk of possible infection (van Steenwinkel et al., 2011, Ssematimba et al., 2013).
The number of animal caretakers for each poultry house should be limited, especially when an animal caretaker is responsible for several poultry houses at the same time. In this way, pathogens can be exchanged very easily between the different poultry populations within a farm or between the poultry populations of several farms (Kapperud et al., 1993).
A poultry-free downtime of 24–71 h is usually required for visitors and personnel before they can have access to a poultry farm (Charisis, 2008, Lister, 2008). The duration of downtime depends on the presence of other preventive biosecurity measures such as the use of farm-specific clothing and footwear, hand hygiene, or taking a shower before entering the farm.
The hygiene lock where visitors should put on company clothes and shoes is especially intended to decrease the risk of mechanical disease through persons (Evans and Sayer, 2000, Vangroenweghe et al., 2009). Furthermore, attention should be paid to the presence of a sink, as hand hygiene (cleaning and disinfecting of hands) is really essential for the on-farm biosecurity (Vangroenweghe et al., 2009).
Supplies and Equipment
A high proportion of broiler farms had their own power sprayer, jetmatic pumps, and generator. Nine of every 10 farms also exclusively use their materials and equipment. This is good practice because when equipment are shared, pathogens can also be transferred through these equipment (Pritchard et al., 2005).
However, only 15.4% of broiler farms owned feed mixers. For practical reasons, most farms do not have their own feed mixers. This entails additional cost, and most farms could not afford to own one. The constant movement of feed delivery trucks then becomes a big threat to disease introduction.
Infrastructure and Biological Vectors
Most broiler farms had good biosecurity practices in terms of infrastructure and keeping at bay the biological vectors. Biosecurity scores of broiler farms with ventilated type of housing were higher in this subcategory than those raised in traditional housing. Because of the innate characteristics of tunnel-ventilated poultry houses, intrusion of wild birds, rodents, and other vermin can be minimized. Nespeca et al. (1997) mentioned that a strong enclosure around poultry houses can minimize contact with rodents and other wild animals.
However, only less than half reported not having vermin problems. Moreover, only 12.9% implemented vermin control. Rodents play a significant role in both the mechanical and biological transmission of certain infectious germs. These species will be important for the spread of certain pathogens within a poultry farm and also for the introduction of pathogens from a neighboring farm (Amass and Baysinger, 2006, Lister, 2008). To control vermin, an efficient control program is required. This is often developed in collaboration with specialized companies (Nespeca et al., 1997, van Steenwinkel et al., 2011; Filippitzi et al., 2018; Dewulf, 2018).
Birds in the conventional type of housing are very vulnerable to diseases because migratory birds, vermin, and pet animals could not be easily prevented from getting into the farm's premises.
Location of the Farm
Sixty-three percent of the broiler farms had a creek or running water within a radius of 1 km where the farm is located. For around half of the broiler farms, the nearest poultry farm is more than 1 km away. However, for 70 broiler farms (17.6%), the nearest poultry farm is less than 500 m away. Finally, for around one-third of the farms, poultry transport vehicles were able to travel via public roads within the location of the farms. Farm location is important especially when distance between each farm and the possibility of airborne transmission is high (Sims, 2008, Gelaude et al., 2014).
The distance to these neighboring poultry farms, the presence of animal transport along the public road in the environment of the farm, and the dominant wind direction at the farm will further determine the probability of airborne disease transmission (Nespeca et al., 1997, Lister, 2008, van Steenwinkel et al., 2011). It is suggested that a minimum distance of 500 m between 2 different poultry farms (preferably more than 1 km) may significantly reduce the risk of spread of infectious diseases. This distance also applies to the location of a farm with respect to hobby poultry farms (Lister, 2008, van Steenwinkel et al., 2011).
Manure from 11.3% of broiler farms was disposed on neighboring farmlands. This will significantly increase the risk of disease transmission for the farms in the vicinity of those fields (Alexander, 2007, Charisis, 2008, Lister, 2008). In addition, the risk of infection by spreading litter on the surrounding fields will be further aggravated by the wind direction, the presence of vermin or wild birds, and the movement of personnel or equipment (Vieira et al., 2009).
Internal Biosecurity Scores
The mean scores of the broiler farms in the internal biosecurity subcategories are presented in Table 3. The highest average score was obtained for the subcategory materials and measures between compartments. Farms from Bataan garnered the highest internal biosecurity scores of 80.1. Aurora province had the lowest internal biosecurity score.
As shown in Figure 3, 75% of the farms had biosecurity scores above 50 in terms of disease management and above 60 for the cleaning and disinfection subcategories. All farms were compliant (100%) in the materials and measures between compartments subcategory. Farmers are more consistent in complying with internal biosecurity measures than external biosecurity.
A comparison of the provincial median scores (Table 3) of broiler farms in the region was also performed across provinces. The province of Aurora was not included because it has only 2 participating farms. Moreover, some provinces obtained statistically different median scores in the cleaning and disinfection subcategory.
Detailed Description of Internal Biosecurity
Disease Management and Vaccination
About 90% of the broiler farms vaccinated their flocks on regular basis. The disease status of the farm was also regularly checked. Farmers were aware of the vaccination protocol against diseases that are present in the region. When vaccines are administered correctly, less losses due to illness or mortality will be incurred (Cserep, 2008). In addition, vaccination promotes animal welfare and may aid in the eradication of certain infectious diseases (Capua and Marangon, 2006, Cserep, 2008). In addition to vaccination, it is also valuable to know the disease status of poultry farms. In this way, the health of the flock can be ascertained. A well-planned health program also facilitates implementation of appropriate intervention especially when a vaccine needs to be reintroduced (Carey et al., 2005, Al-Saffar et al., 2006).
Among broiler farms, only a quarter (25.2%) classified their poultry in different age categories. Animals of different ages may have different levels of sensitivity to certain pathogens (Filippitzi et al., 2018; Dewulf, 2018). Therefore, it is crucial to separate different age groups to avoid the transmission of pathogens between groups. The presence of microbial material from batch to batch, the overall performance of broilers in number of days to market, efficiency of feed utilization, percent liveability and consequent total weight at market age, and so on remain poor if poultry are in different age groups (Prabakaran, 2003). In addition, the work on the farm should be performed from the youngest to the oldest poultry population (Carey, 2005). Three out of 5 broiler farms had similar vaccination program for newly-delivered chicks, which is in consonance with the provincial vaccination protocol. Finally, for 65% of the broiler farms, the animal density in each poultry house was more than 42 kg/m2. The stocking density of a poultry population will notably affect the extent of a particular disease incidence (Sims, 2008, van Steenwinkel et al., 2011). When birds are housed close together, stress will be induced, their susceptibility to infectious diseases increases, and the poultry population will shed more pathogens. For this reason, the overall on-farm infection pressure will increase dramatically at long last (Gelaude et al., 2014).
The number of entrance and exit points in the farm is of prime importance because entrance and exit points also serve as entry points for disease-causing agents. Therefore, a one-way traffic flow in entrance and exit points is integral to prevent disease transmission. However, only 43% of the broiler farms strictly observed a one-entrance-one-exit policy.
Majority (96%) of the broiler farms removed dead animals daily. Only 21.8% of the farms had equipment for removing carcasses; although 89.4% claimed to manage carcasses properly, only 17.6% of these farms had equipment for removing carcasses. During necropsy, 84.4% of the farms collected samples for antibiotic sensitivity testing; of these, only 17.6% had equipment for removing carcasses.
Cleaning and Disinfection
As much as 90% of the broiler farms practice cleaning and disinfecting the farm, feeding systems, feed silos, poultry houses, and loading and unloading areas after every production cycle. However, only 31.2% of the broiler farms checked the efficacy of cleaning and disinfection through a hygienogram. After cleaning and disinfection, at least 80% of the broiler farms had sanitary transition period of more than 8 D that is strictly implemented in every production cycle.
Among broiler farms, 94.7% use disinfection baths for vehicles entering the farms; only 51.9% of which consistently adhere to the use of disinfection bath. Only a little more than half (56.2%) had farm-hygiene lock, have strict separation between the clean and dirty area (90%), and have have changing room for farm-specific clothing (85.2%). The principle of the clean and dirty road on a poultry farm means that there is a clear separation between the clean and the dirty (risky) sections of the premises (Carey et al., 2005, Ssematimba et al., 2013). Poultry transport vehicles are constantly in contact with other farms and slaughterhouses, and this creates a quite extensive risk for disease transmission (Amass and Baysinger, 2006, Gelaude et al., 2014).
Moreover, approximately one of every 2 broiler farms had house-hygiene locks; almost all of which had strict separation of clean and dirty areas, footbath or boot washer, and washing and disinfecting areas in the house-hygiene lock. The hygiene lock where visitors should put on company clothes and shoes is especially intended to decrease the risk of mechanical disease transmission through persons (Hald et al., 2000, Vangroenweghe et al., 2009).
Finally, 78.3% of the broiler farms had disinfection baths/boot washers at the farm entrance. To prevent the spread of pathogens through footwear, boot washers and disinfecting baths can be placed at the entrance of each poultry house (Nespeca et al., 1997, Vangroenweghe et al., 2009). Disinfection baths that are not properly used and maintained can be a possible transmission pathway for pathogens and therefore a waste of money (Vangroenweghe et al., 2009).
Materials and Measures Between Compartments
A large proportion (90%) of the broiler farms had protocols regarding cleaning and disinfection of materials every after-production cycle. Most of the farms were equipped with necessary materials to carry out cleaning and disinfection properly. The housing design also favors efficient cleaning and disinfection. Only one-fifth of the broiler farms had different designs for poultry houses. The old housing units may not be very accessible to newer methods and equipment for cleaning and disinfection. On the other hand, the tunnel type of housing affords ease of cleaning and disinfection. Entry of pathogens can be carried by supplies and other materials. This happens especially when the material was previously in contact with poultry or when it was manufactured or packaged at other poultry farms (Pritchard et al., 2005).
To prevent the transfer of pathogens from one company to another, it is advised to use proprietary, farm-specific materials. It is also recommended to provide this material to anyone who needs it at the farm (Lister, 2008, Gelaude et al., 2014). However, if nonproprietary material has to be introduced at the farm or to certain poultry houses, this can be done via specific hatches with disinfectant UV radiation (Filippitzi et al., 2018).
The transmission of pathogens can easily occur indirectly through all the materials used in a poultry farm (Laanen et al., 2011, Gelaude et al., 2014; Filippitzi et al., 2018). There should be specific materials for every task related to cleaning and disinfection. As much as possible, they should not be moved from one section to another (Vangroenweghe et al., 2009, Laanen et al., 2011, Gelaude et al., 2014).
Correlation of External Biosecurity and Internal Biosecurity Scores of the Broiler Farms
Moderate positive linear relationships between the external and internal biosecurity scores of the broiler farms in the provinces were obtained (range: 0.437 – 0.554). The analysis excluded Aurora province because as previously mentioned, there were only 2 participating farms in the area (Table 5).
Table 5.
Correlation of the external biosecurity and internal biosecurity scores of broiler farms according to province.
| Province1 | Spearman's rho | P value |
|---|---|---|
| Bataan | 0.484 | <0.001 |
| Bulacan | 0.554 | <0.001 |
| Nueva ecija | 0.427 | <0.001 |
| Pampanga | 0.520 | <0.001 |
| Tarlac | 0.437 | 0.001 |
| Zambales | 0.592 | 0.006 |
| Overall | 0.489 | <0.001 |
Excluding Aurora.
Farm Characteristics Associated With Biosecurity Score of Broiler Farms
The association of several farm characteristics with the biosecurity scores of the broiler farms was tested (Table 6). Using multiple linear regression, it was shown that the age of the farm and the type of poultry housing were associated with biosecurity scores. Controlling for the other variable, the mean biosecurity scores of farms with traditional/conventional type of housing was 7.8% lower than those with tunnel vent housing. Moreover, a drop of 0.2% in the biosecurity may result for every year of increase in the age of the poultry.
Table 6.
Results of linear regression on the correlates of biosecurity scores of broiler farms in Central Luzon.
| Characteristic | Crude regression coefficient (95% confidence interval) | P value | Adjusted regression coefficient (95% confidence interval) | P value |
|---|---|---|---|---|
| Type of farm1 | ||||
| Medium commercial | 4.8 (2.4, 7.1) | <0.001 | ||
| Large commercial | 7.4 (4.6, 10.2) | <0.001 | ||
| Type of operation2 | ||||
| Contract grower | 5.0 (2.7, 7.4) | <0.001 | ||
| Internal | 4.9 (−0.1, 9.9) | 0.056 | ||
| Age of poultry | −0.2 (−0.3, −0.1) | 0.002 | 0.1 (0.0, 0.2) | 0.050 |
| House type3 | ||||
| Traditional/conventional | −7.8 (−9.5, −6.1) | <0.001 | −8.6 (−10.5, −6.7) | <0.001 |
Reference group: Small Commercial.
Reference group: Independent producer.
Reference group: Tunnel vent.
Conclusion
Based from a total of 397 broiler farms surveyed in Central Luzon, the overall total biosecurity score of broiler farms is 71.2 and ranges between 60.0 and 74.6. This is higher than the global score of 64. Among the 7 provinces, Bataan had the highest biosecurity score, followed by Nueva Ecija.
Regional scores for external and internal biosecurity were 68.5 and 77.2%, respectively. These values are higher than the global scores of 63 and 64%, respectively. Comparing each of the 7 provinces, Bataan had the highest scores.
For the various subcategories, the regional scores were above the global average for the following subcategories: 1) purchase of 1-day-old chicks, 2) feed and water supply, 3) supply of materials, 4) cleaning and disinfection, and 5) materials and measures between compartments.
Moreover, the broiler farms in the region can improve on the following subcategories: 1) transport of live animals, 2) infrastructure and biological vectors, and 3) disease management. This is because the mean scores in these subcategories were even lower than the already low global average.
The mean biosecurity score of farms with traditional/conventional type of housing was 7.8% lower than that with tunnel vent housing. A drop of 0.2% in the biosecurity score would result for every year of increase in the age of the poultry. Biosecurity measures are in place in broiler farms in the country. However, subcategories of external and internal biosecurity with low scores should be prioritized for improvement. Government's participation and involvement for the strict implementation and monitoring of biosecurity measures in broiler farms in the country is crucial to prevent disease transmission.
Acknowledgment
This project was generously funded by the Bureau of Animal Industry, Department of Agriculture, Philippines.
The authors did not provide a conflict of interest statement.
References
- Alexander D.J. An overview of the epidemiology of avian influenza. Vaccine. 2007;25:5637–5644. doi: 10.1016/j.vaccine.2006.10.051. [DOI] [PubMed] [Google Scholar]
- Al-Saffar A., Al-Nasser A., Al-Haddad A., Al-Bahouh M., Mashaly M. Kuwait Institute for Scientific Research; Safat, Kuwait: 2006. Principles of Poultry Biosecurity Program; pp. 1–67. [Google Scholar]
- Amass S.F., Baysinger A. Swine disease transmission and prevention. In: Straw B.E., Zimmerman J.J., D'Allaire S., Taylor D.J., editors. Diseases of Swine. 9th ed. Blackwell Publishing Ltd.; Oxford, UK: 2006. pp. 1075–1098. [Google Scholar]
- Berndtson E., Emanuelson U., Engvall A., Danielsson-Tham L. A 1-year epidemiological study of Campylobacter in 18 Swedish chicken farms. Prev. Vet. Med. 1996;26:167–185. [Google Scholar]
- Boklund A., Alban L., Mortensen S., Houe H. Biosecurity in 116 Danish fattening swineherds: descriptive results and factors analysis. Prev. Vet. Med. 2004;66:49–62. doi: 10.1016/j.prevetmed.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Boklund A. PhD Thesis; Denmark: 2008. Exotic Disease in Swine: Evaluation of Biosecurity and Control of Strategies for Classical Swine Fever. [Google Scholar]
- Capua I., Marangon S. Control of avian influenza in poultry. Emerg. Infect. Dis. 2006;12:1319–1324. doi: 10.3201/eid1209.060430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey J.B., Jeffrey J.S., Prochaska J.F. Poultry facility biosecurity (L-5182) 2005. http://repository.tamu.edu/bitstream/handle/1969.1/87791/pdf_ 823.pdf?sequence=1 AgriLife Extension.
- Charisis N. Avian influenza biosecurity: a key for animal and human protection. Vet. Ital. 2008;44:657–669. [PubMed] [Google Scholar]
- Conan A., Goutard F.L., Khiev R., Ponsich A., Tarantola A., Sornd S., Vong S. A community-based education trial to improve backyard poultry biosecurity in rural Cambodia. Acta Tropica. 2013;125:294–302. doi: 10.1016/j.actatropica.2012.12.006. [DOI] [PubMed] [Google Scholar]
- Cserep T. Vaccines and vaccination. In: Patisson M., McMullin P.F., Bradburry J.M., Alexander D.J., editors. Poultry Diseases. 6th ed. Saunders Elsevier; China: 2008. p. 66. [Google Scholar]
- Department of Agriculture, Fisheries and Forestry . Department of Agriculture, Fisheries and Forestry; Canberra: 2005. Quads—Emergency Management Working Group. Carcass Disposal Workshop Report. [Google Scholar]
- Dewulf J., Immerseel F.V., Luyckx K., Postma M., Vabeselaere B. How to measure biosecurity and the hygiene status of farms. Page 117. In: Dewulf J., Immerseel F.V., editors. Biosecurity in Animal Production and Veterinary Medicine: From Principles to Practice. Vol. 22. Belgie; Leuven: 2018. p. 3000. (Uitgeverij Acco, Blijde Inkomststraat). [Google Scholar]
- Dorea F.C., Berghaus R., Hofacre C., Cole D.J. Survey of biosecurity protocols and practices adopted by growers on commercial poultry farms in Georgia – USA. Avian Dis. 2010;54:1007–1015. doi: 10.1637/9233-011210-Reg.1. [DOI] [PubMed] [Google Scholar]
- Evans S.J., Sayer A.R. A longitudinal study of Campylobacter infection of broiler flock in Great Britain. Prev. Vet. Med. 2000;46:209–223. doi: 10.1016/s0167-5877(00)00143-4. [DOI] [PubMed] [Google Scholar]
- Filippitzi M.E., Brinch Kruse A., Postma M., Sarrazin S., Maes D., Alban L., Nielsen L.R., Dewulf J. Review of transmission routes of 24 infectious diseases preventable by biosecurity measures and comparison of the implementation of these measures in pig herds in six European countries. Transbound. Emerg. Dis. 2018;65:381–398. doi: 10.1111/tbed.12758. [DOI] [PubMed] [Google Scholar]
- Gelaude P., Dewulf J., Laanen M., Schlepers M., Verlinden M. Biocheck.UGent: a quantitative tool to measure biosecurity at broiler farms and the relationship with technical performances and antimicrobial use. Poult. Sci. 2014;93:1–12. doi: 10.3382/ps.2014-04002. [DOI] [PubMed] [Google Scholar]
- Hald B., Madsen M., Wedderkopp A. Thermophilic Campylobacter spp. in Danish broiler production: a cross-sectional survey and a retrospective analysis of risk factors for occurrence in broiler flocks. Avian Pathol. 2000;29:123–131. doi: 10.1080/03079450094153. [DOI] [PubMed] [Google Scholar]
- Jeffrey J.S. Biosecurity for Poultry Flocks. Poultry Fact Sheet No. 26. 1997. http://animalscience.ucdavis.edu/avian/pfs26.htm
- Kapperud G., Skjerve E., Vik E., Hauge K., Lysaker A., Aalmen I., Ostroff S.M., Potter M. Epidemiology investigation of risk factors for Campylobacter colonization in Norwegian broiler flocks. Epidemiol. Infect. 1993;111:245–255. doi: 10.1017/s0950268800056958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laanen M., Callens B., Dewulf J., De Jong E., Maes D., Persoons M., Ribbens S., Strubbe M. Relationship between biosecurity and production/antimicrobial treatment characteristics in pig herds. Vet. J. 2013;198:508–512. doi: 10.1016/j.tvjl.2013.08.029. [DOI] [PubMed] [Google Scholar]
- Laanen M., Beek J., Dewulf J., Maes D., Ribben S., Vangroenweghe F. Bioveiligheid op varkensbedrijven: ontwikkeling van een online score systeem en de resultaten van de eerste 99 deelnemende bedrijven. Vlams Diergen. Tijd. 2010;79:302–306. [Google Scholar]
- Laanen M., Dewulf J., Maes D., Ribbens S., Persoons D. 2011. Link between biosecurity and production and treatment characteristics in pig herds. Voordracht: “Proceedings of the 9th International Conference on the Epidemiology and Control of Biological, Chemical and Physical Hazards in Pigs and Pork”. Maastricht, Nederland. [Google Scholar]
- Lee H., Lao A. Transmission dynamics and control strategies assessment of avian influenza A (H5N6) in the Philippines. Infect. Dis. Model. 2018;3:35–59. doi: 10.1016/j.idm.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister S.A. Biosecurity in poultry management. In: Patisson M., McMullin P.F., Bradburry J.M., Alexander D.J., editors. Poultry Diseases. 6th ed. Saunders Elsevier; Beijing, China: 2008. pp. 48–65. [Google Scholar]
- Manuja B.K., Manuja A., Singh R.K. Globalization and livestock biosecurity. Agric. Res. 2014;3:22–31. doi: 10.1007/s40003-014-0097-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell S.W.J., Menzies F.D., McBride S.H., Oza A.N., McKenna J.P., Gordon A.W., Neill S.D. Campylobacter spp. in conventional broiler flocks in Northern Ireland: epidemiology and risk factors. Prev. Vet. Med. 2008;84:261–276. doi: 10.1016/j.prevetmed.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Meroz M., Samberg Y. Disinfecting poultry production premises. Rev. Sci. Tech. OIE. 1995;14:273–291. doi: 10.20506/rst.14.2.839. [DOI] [PubMed] [Google Scholar]
- Nachman K.E., Graham J.P., Price L.B., Silbergeld E.K. Arsenic: a roadblock to potential animal waste management solutions. Environ. Health Persp. 2005;113:1123–1124. doi: 10.1289/ehp.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academy of Science and Technology . DOST Cmpd.; 2005. NAST Agriculture 2020: Industry Strategic Plan 2020 – Swine-Poultry-Corn Cluster. Bicutan, Taguig, Metro Manila, Philippines. [Google Scholar]
- Nespeca R., Vaillancourt J.P., Morgan Morrow W.E. Validation of a poultry biosecurity survey. Prev. Vet. Med. 1997;31:73–86. doi: 10.1016/s0167-5877(96)01122-1. [DOI] [PubMed] [Google Scholar]
- Niemi J.K., Lyytikäinen T., Sahlström L., Virtanen T., Lehtonen H. Selected Paper Prepared for Presentation at the Agricultural and Applied Economics Association 2009 AAE and ACCI Join Annual Meeting. 2009. Risk classification in animal disease prevention: who benefits from differentiated policy? p. 28. Milwaukee, Wisconsin. [Google Scholar]
- PCAARD . Philippine Council for Agriculture, Forestry, and Natural Resources, Research and Development, Department of Science and Technology; Los Baños, Laguna: 2006. The Philippines Recommends for Broiler Production. [Google Scholar]
- Postma M., Backhans A., Collineau L., Loesken S., Sjölund M., Belloc C., Emanuelson U., Grosse Beilage E., Stärk K.D.C., Dewulf J. The biosecurity status and its associations with production and management characteristics in farrow-to-finish pig herds. Animal. 2016;10:478–489. doi: 10.1017/S1751731115002487. [DOI] [PubMed] [Google Scholar]
- Prabakaran R.P. Food and Agriculture Organization of United Nations Rome; Rome, Italy: 2003. Good practices in planning and management of integrated commercial poultry production in South Asia. [Google Scholar]
- Pritchard G., Dennis I., Waddilove J. Biosecurity: reducing disease risks to pig breeding herds. Practice. 2005;27:230–237. [Google Scholar]
- PSA . Philippine Statistics Authority; Quezon City, Philippines: 2017. Chicken Situation Report. January-December 2017. [Google Scholar]
- Racicot M., Venne D., Durivage A., Vaillancourt J.P. Evaluation of strategies to enhance biosecurity compliance on poultry farms in Québec: Effect of audits and camera. Prev. Vet. Med. 2012;103:208–218. doi: 10.1016/j.prevetmed.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Rajkowski K.T., Eblen S., Laubauch C. Efficacy of washing and sanitizing trailers used for swine transport in reduction of Salmonella and Escherichia coli. J. Food Prot. 1998;61:31–35. doi: 10.4315/0362-028x-61.1.31. [DOI] [PubMed] [Google Scholar]
- Sims L.D. Risks associated with poultry production systems. Int. Conference: Poult. 21st Century. 2008:1–24. [Google Scholar]
- Slader J., Bolton F.J., Domingue G., Humphrey T.J., Jorgensen F., McAlpine K., Owen R.J. Impact of transport crate reuse and of catching and processing on Campylobacter and Salmonella contamination of broiler chickens. Appl. Environ. Microbiol. 2002;68:713–719. doi: 10.1128/AEM.68.2.713-719.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ssematimba A., Hagenaars T.J., de Wit J.J., Ruiterkamp F., Fabre T.H., Stegeman J.A., de Jong M.C.M. Avian influenza transmission risks: analysis of biosecurity measures and contact structure in Dutch poultry farming. Prev. Vet. Med. 2013;109:106–115. doi: 10.1016/j.prevetmed.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Swayne D.E., editor. Avian Influenza. 1st ed. Blackwell Publishing; Ames, IA: 2008. [Google Scholar]
- van Limbergen T., Dewulf J., Klinkenberg M.R., Ducatelle P., Gelaude P., Mendez J., Heinola K., Papasolomontos S., Szeleszczuk S., Maes D. Scoring biosecurity in European conventional broiler production. Poult. Sci. 2018;97:74–83. doi: 10.3382/ps/pex296. [DOI] [PubMed] [Google Scholar]
- van Steenwinkel S., Ribbens S., Ducheyne E., Goossens E., Dewulf J. Assessing biosecurity practices, movements and densities of poultry sites across Belgium, resulting in different farm riskgroups for infectious disease introduction and spread. Prev. Vet. Med. 2011;98:259–270. doi: 10.1016/j.prevetmed.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Vangroenweghe F., Ribbens S., Vandersmissen T., Beek J., Dewulf J., Maes D., Castryck F. Hygiene protocol–Hygiene lock (In Dutch) In: Vangroenweghe F., editor. Keeping Pigs Healthy. 1st ed. DCL Print & Signs; Zelzate, Belgium: 2009. pp. 115–116. [Google Scholar]
- Vieira A.R., Hofacre C.L., Smith J.A., Cole D D. Human contacts and potential pathways of disease introduction on Georgia poultry farms. Avian Dis. 2009;53:55–62. doi: 10.1637/8364-051608-Reg.1. [DOI] [PubMed] [Google Scholar]
- Wilkinson K.G. The biosecurity of on-farm composting. J. Appl. Microbiol. 2007;102:609–618. doi: 10.1111/j.1365-2672.2006.03274.x. [DOI] [PubMed] [Google Scholar]