Abstract
Intestinal epithelial cells are major producers of antimicrobial proteins, which play an important role in innate immunity. In addition to defensins, the Ribonuclease A superfamily includes important antimicrobial proteins involved in host-defense mechanisms in vertebrates. Angiogenin-4 (Ang4), a member of this RNase superfamily, has been demonstrated to be secreted by Paneth cells in mice. We have successfully cloned and characterized a new chicken gene (chAng4), found for the first time in a nonmammalian species, from intestinal epithelial and lymphoid cells. Characterization of chAng4 revealed 99% nucleotide and 97% amino acid sequence homology to mouse Ang4. Similar functional regions were identified, suggesting a role in innate immunity and regulation of gut microbiota. Furthermore, the mRNA expression pattern of chAng4 was studied in broilers in the presence or absence of beneficial bacteria (probiotics) and organic acids. The results showed that one-day-old chickens expressed low levels of Ang4 in almost all the evaluated tissues (crop, proventriculus, duodenum, jejunum, ileum, and cecal tonsils), except in the bursa of Fabricius that presented the highest expression level. The addition of probiotics and organic acids for either 7 or 14 consecutive days demonstrated a direct effect of probiotics and organic acids on chAng4 expression; moreover, broilers receiving probiotics and organic acids for only 7 D showed higher levels of chAng4 expression compared with those treated for 14 D. Broilers without treatment had a constant high level of expression in cecal tonsils and bursa. In conclusion, we were able to identify and characterize a new antimicrobial gene in chickens (chAng4) throughout the gastrointestinal tract. chAng4 mRNA gene expression was associated with the presence of naturally occurring and supplemented (probiotic) bacteria. The encoded protein might have a potential bactericidal effect against intestinal nonpathogenic and pathogenic microbes, modulating the intestinal microbiota and the innate immunity, and thereby may help minimize the use of antibiotics in poultry feed.
Key words: angiogenin, antimicrobial protein, Ang4, Paneth cells, B cells
Introduction
Early bacterial colonization of the intestine can alter its morphology, physiology, and susceptibility to infectious diseases (Diaz-Carrasco et al., 2019). The intestinal microbiota plays a critical role in the development and maturation of the gut and its lymphoid structures, and in the function of immune system cells (Hooper et al., 2012, Maki et al., 2019). Initial interactions between commensal bacteria and the host immune system can shape microbiota composition, maintain intestinal homeostasis, and exert immune-modulatory activities (Hooper et al., 2012, Diaz Carrasco et al., 2019, Maki et al., 2019). For example, the functions of Bacteroides thetaiotamicron seem to be associated with modulation of gene expression; in addition to inducing the expression of the bactericidal gut protein Angiogenin (Ang) 4 in mice, this microorganism has been shown to upregulate different genes related to mucosal barrier integrity (Hooper et al., 2001; Zocco et al., 2007). Probiotics enhance the expression of antimicrobial peptides in the gut, such as defensins, and organic acids modulate the gut microbial community through the inhibition of pathogenic bacteria (Akbari et al., 2008, Malik et al., 2016). Therefore, these nutraceutical compounds are tools being explored to improve intestinal health in poultry without the need for antibiotics (Sugiharto, 2016, Maki et al., 2019).
The Ribonuclease (RNase) A Superfamily is a vertebrate-specific gene family that encodes cationic peptides secreted by immune cells and epithelial tissues, with cytotoxic, anthelmintic, antibacterial, antiviral and antifungal activity (Schwartz et al., 2018). Mouse Ang4 is a member of this superfamily, localized in Paneth cell granules, which is secreted into the intestinal lumen together with other secretory granule contents, such as lysozyme, in response to bacterial signals (Hooper et al., 2003). In the absence of Paneth cells, goblet cells have been identified as the cellular source of Ang4 in mouse colon during Trichuris muris infection (Forman et al., 2012). Paneth cells have been detected in chicken small intestinal crypts through lysozyme c expression (Wang et al., 2016). However, other lysozyme-positive cells have also been observed along the villus in the duodenum, such as goblet cells and rod-shaped cells, and in cecum and colon (Bar-Shira and Friedman, 2018).
The gene encoding angiogenin is present in nearly all vertebrates (Sheng and Xu, 2016). Moreover, whereas humans, nonhuman primates, gray short-tailed opossums, and rabbits harbour a single gene, rats, pigs, and painted turtles have 2, cattle 3, zebrafish 5, and mice 6 (Zhang and Rosenberg, 2002, Sheng and Xu, 2016, Schwartz et al., 2018). It has been shown that mouse Ang 1 is primarily expressed in the liver and exhibits antimicrobial activity against Candida albicans and Streptococcus pneumoniae. By contrast, murine Ang4 expression is almost restricted to the intestine and is enhanced by commensal bacteria such as B. thetaiotamicron; this protein also presents bactericidal activity against Enterococcus faecalis and Listeria monocytogenes (Hooper et al., 2003). In addition, mouse Ang4 is angiogenic, like mouse Ang1, as determined by a thoracic aorta assay (Crabtree et al., 2007).
In chickens, expression of a RNase A/angiogenin related-protein was found in normal and avian myeloblastosis virus (AMV)-transformed myelomonocytic cells, and overexpression of the chicken RNase superfamily related (RSFR) gene was reported in normal bone marrow cells and bone marrow–derived AMV-transformed monoblasts (Klenova et al., 1992, Nakano and Graf, 1992). These were later renamed chicken leukocyte RNase A-1 (RNase A/angiogenin related-protein) and A-2 (RSFR), mapped to chromosome 6 and documented to share 81% amino acid sequence identity. Besides, both RNases were detected in the cytoplasm of cells in bone marrow and in ∼80% of peripheral blood granulocytes and are structurally related to the mammalian angiogenin lineage, although only leukocyte RNase A-2 has angiogenic activity ex vivo. This RNase also demonstrated antibacterial activity against Escherichia coli and Staphylococcus aureus, being implicated in the angiogenic and bactericidal activities of chicken peripheral blood granulocytes (Nitto et al., 2006). However, the biological activity of this type of proteins within the gastrointestinal tract (GIT) remains unexplored in chickens.
The objectives of this study were to identify and characterize the expression of a new chicken gene, Angiogenin-4 (chAng4), possibly involved in the innate immune response in the gut as has been observed in mammals, and to determine the effect of probiotic and organic acid supplementation on chAng4 expression pattern across the GIT. The investigation of the antimicrobial potential of chAng4 might provide insight into a nonpharmacological alternative to antibiotic growth promoters in poultry production.
Materials and methods
All animal procedures were approved by the University of Manitoba Animal Care Protocol Management and Review Committee, and chickens were handled in accordance with the guidelines described by the Canadian Council on Animal Care (CCAC, 1993).
Probiotic and Organic Acid Blend
A probiotic and organic acid blend (Acid-Pak 4-way, Alltech Inc., Nicholasville, KY) was added to drinking water at a dosage of 1 g/L. The species used included Lactobacillus acidophilus, Lactobacillus casei, Enterococcus faecium, and Saccharomyces cerevisiae at a concentration of 108 CFU/g for each strain. The organic acids were sorbic (1%) and citric acid (0.2%). All birds were provided with access to a standard corn-wheat soybean meal diet and water ad libitum. Chlorinated drinking water was used in this trial and the basal diet was previously reported by Rodríguez-Lecompte et al. (2012).
Animal Trial
A total of 300 1-day-old Ross-308 chicks, obtained from a commercial hatchery (Carleton Hatchery, Grunthal, MB, Canada), were randomly assigned to 3 treatments: no treatment (control, T1) or the blend of probiotics and organic acids for either 7 (T2) or 14 D (T3). There were 5 replications (pens) per treatment and 20 birds per pen. Chicks were vaccinated at the hatchery against infectious bronchitis (Massachusetts-type H120) and Marek's disease (HVT strain FC-126), and on day 12 post-hatch against infectious bursal disease (intermediate strain D-78).
On day 1, five birds were randomly selected from the 3 treatment groups (n = 300), sacrificed by cervical dislocation, and tissue sections of crop, proventriculus, duodenum, jejunum, ileum, cecal tonsils, and bursa of Fabricius were collected for chAng4 gene expression analysis. Then, on day 11 and 22, five broilers were randomly selected from each treatment (1 bird/pen) and tissue samples were collected as described previously. The first and second sampling dates were chosen to observe the response of a developing immune system, and the third sampling date was selected to observe the response of a developed immune system (Bar-Shira et al., 2003).
RNA Isolation, Reverse Transcriptase PCR, and Cloning
The tissue samples collected from crop, proventriculus, duodenum, jejunum, ileum, cecal tonsils, and bursa of Fabricius were placed in liquid nitrogen for 1 min and then frozen at −80°C until RNA extraction. RNA extraction and cDNA synthesis were performed as described by Rodríguez-Lecompte et al. (2012). The obtained cDNA containing the full coding region of chAng4 was amplified by PCR using primer pairs previously reported by Hooper et al. (2003) and Jing et al. (2009), corresponding to mouse Ang4 and chicken β-actin sequences, respectively(Table 1). The resulting products were stained with ethidium bromide, loaded to a 1.5% agarose gel and separated using agarose gel electrophoresis. PCR-amplified cDNA was cloned into the pGEM-T Easy vector (Promega, Fisher Scientific, WI) as described by Jing et al. (2009). Ten randomly selected clones were sent to McMaster University (Hamilton, ON, Canada) for sequencing. Sequences of the amplified products and the respective reference mRNA were aligned for comparative analysis with the NCBI Local Alignment Search Tool (BLAST).
Table 1.
Pairs of primers used for RT-PCR and qRT-PCR.
| Genes | Primer sequence | Accession no. | Annealing temp. (°C) |
|---|---|---|---|
| chAng4 | F: TGTTGGAAGAGATGACAATGAGCCC | EU818941.1 | 60.0 |
| R: GGGCCTGCTGTCTACGGACTGATA | |||
| mAng-41 | F: GGGAATTCCATATGCAGAATGAAAGGTAC- GAAAAATTCCTAC |
AY219870.1 | 60.0 |
| R: CCTTGGATCCCTACGGACTGATAAAAGAC- TCATCG | |||
| β-actin2 | F: CAACACAGTGCTGTCTGGTGGTA | X00182 | 61.0 |
| R: ATCGTACTCCTGCTTGCTGATCC |
Pair of primers for this gene previously reported by Hooper et al. (2003)1 and Jing et al. (2009)2.
Quantitative Reverse Transcriptase PCR
Total RNA isolation and cDNA synthesis were performed from sections of all tissue samples as previously mentioned. SYBR Green-based qRT-PCR was conducted for chAng4 and β-actin as housekeeping gene, using a thermocycler (CFX Connect Real-Time PCR, Bio-Rad, Mississauga, ON, Canada) on a 96-well plate using a total reaction volume of 25 μL as described by Pfaffl and Hageleit (2001). chAng4 primers were designed according to the obtained amplicon sequence (Table 1), and thermal cycling conditions were previously reported by Rodríguez-Lecompte et al. (2012). All samples were amplified in triplicate and the mean was used for further analysis.
Statistical Analysis
This trial is a completely randomized design with the chicken as experimental unit. Quantification of gene expression and calculation of the amplification efficiency of target and reference genes were done as described by Rodríguez-Lecompte et al. (2012). Relative expression was calculated using Pfaffi equation, based on the expression of the housekeeping gene β-actin (Pfaffiand Hageleit, 2001). Data were analyzed with the MIXED procedure of SAS (SAS Inst. Inc., Cary, NC). Scheffe's test was used for multiple comparisons between means and the significance level was defined at P-value <0.05.
Results
Chicken Ang4 Identification
Considering the previous finding and characterization of Ang4 in the mouse, primers reported by Hooper et al. (2003) for this species were used and the same expression pattern was considered to identify and characterize Ang4 in chicken gastrointestinal tissue.
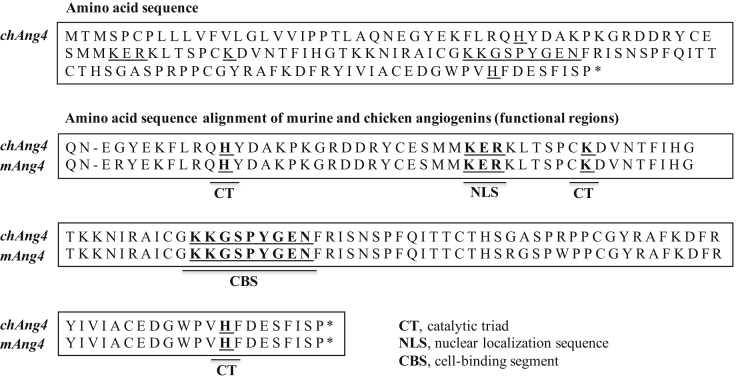
In this research, chAng4 gene was amplified and cloned successfully. Additional sequence analysis was performed indicating a 435-bp length (coding sequence) with 99% nucleotide and 97% amino acid sequence identity to mouse Ang4 (GenBank accession numbers: NM_177544.4 and AAH42938.2, respectively), being expressed in the GIT of chickens (Figure 1). The cloned gene was further characterized and our results reveal the presence of essential catalytic areas associated with their predicted bactericidal activity (Figure 2).
Figure 1.
Full-length nucleotide sequence (5′–3′) of chicken Angiogenin-4. Start and stop codons are shown in bold. Numbers indicate the coding-sequence length (bp). Total length of 435 bp.
Figure 2.
Predicted amino acid sequences and amino acid alignment of murine and chicken angiogenins 4 (functional regions). ∗ = 3′ end of the corresponding sequence. GenBank accession number for mouse Ang4 = AAH42938.2; GenBank accession number for chAng4 = NP_001157124.1.
Tissue Distribution
Because Ang4 mRNA represented a previously uncharacterized transcript, we initially established the molecular base of commensal host-bacterial interaction in the chicken gut. The first step was to characterize this gene on intestinal epithelial and lymphocytic cells (bursa of Fabricius) under nonpathological conditions and secondly in the presence or absence of commensal bacteria (probiotics) and organic acids, at different time periods. Time-course changes in mRNA levels of chAng4 during the all production cycle in broilers were investigated.
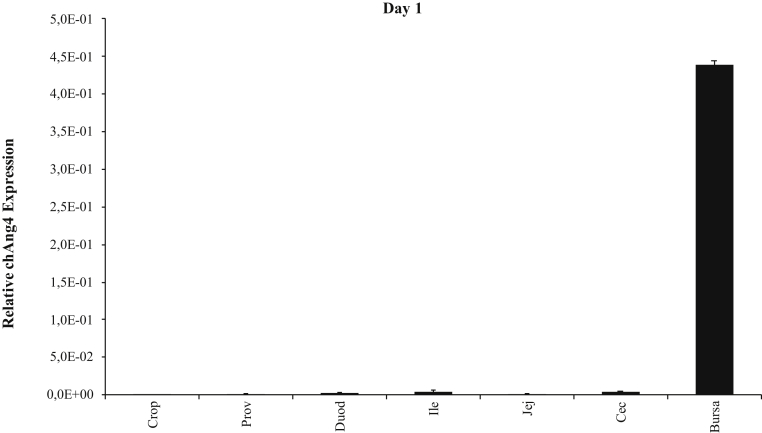
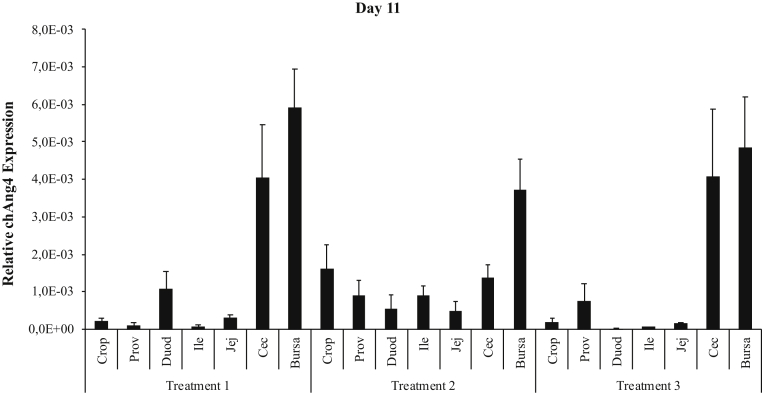
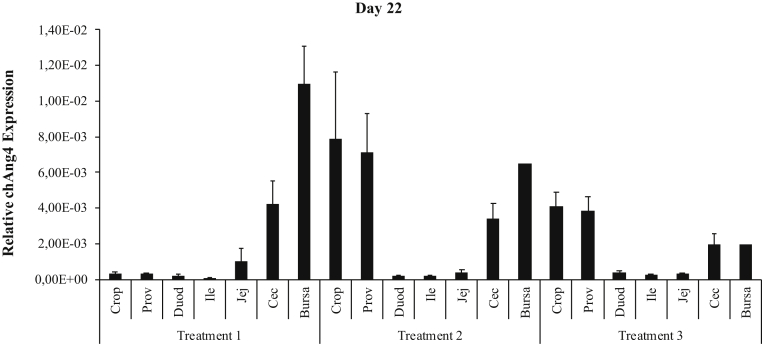
The qRT-PCR analysis showed that the highest expression of chAng4 was in the bursa of Fabricius for birds on day 1 post-hatch (Figure 3), and it was also the tissue with highest expression on day 11 in all treatment (Figure 4) groups. chAng4 expression in bursa of Fabricius was higher in nontreated birds (T1) than in those treated with probiotics and organic acids on day 11 and 22 (Figures 4 and 5), and on day 22 in the case of the cecal tonsils (Figure 5). However, the gene expression level in cecal tonsils was similar on day 11 for T1 and T3 (Figure 4). chAng4 mRNA expression in crop, proventriculus, ileum, and jejunum was higher in birds treated for 7 D (T2) than in those treated for 14 D (T3) and the nontreated (T1) ones on day 11 (Figure 4). The highest expression of chAng4 in duodenum was in nontreated birds on day 11, decreasing in those broilers treated for a longer period of time (T2 and T3) (Figure 4). chAng4 expression in crop and proventriculus was higher in birds treated for 7 D (T2) than in those treated for 14 D (T3) and the nontreated ones on day 11 and 22 (Figures 4 and 5). The highest chAng4 expression level in crop and proventriculus was observed in birds treated for 7 D on day 22 (Figure 5).
Figure 3.
Relative expression of chAng4 in the gastrointestinal tract of day-old broiler chickens. chAng4 gene expression was normalized to that of the housekeeping gene. Bars represent means ± SEM (n = 5). Abbreviations: Prov, proventriculus; Duod, duodenum; Ile, ileum; Jej, jejunum; Cec, cecum.
Figure 4.
Relative expression of chAng4 in the gastrointestinal tract of broiler chickens supplemented with a combination of probiotics and organic acids, on day 11. T1 = birds that did not receive the blend of probiotics and organic acids; T2 = birds that received the blend of probiotics and organic acids for 7 D; and T3 = birds that received the blend of probiotics and organic acids for 14 D. chAng4 gene expression was normalized to that of the housekeeping gene. Bars represent means ± SEM (n = 15). Abbreviations: Prov, proventriculus; Duod, duodenum; Ile, ileum; Jej, jejunum; Cec, cecum.
Figure 5.
Relative expression of chAng4 in the gastrointestinal tract of broiler chickens supplemented with a combination of probiotics and organic acids, on day 22. T1 = birds that did not receive the blend of probiotics and organic acids; T2 = birds that received the blend of probiotics and organic acids for 7 D; and T3 = birds that received the blend of probiotics and organic acids for 14 D. chAng4 gene expression was normalized to that of the housekeeping gene. Bars represent means ± SEM (n = 15). Abbreviations: Prov, proventriculus; Duod, duodenum; Ile, ileum; Jej, jejunum; Cec, cecum.
Discussion
Innate intestinal defenses are important for protection against ingested and commensal microbes. Intestinal epithelial cells are the major producers of substances with antimicrobial activity in the intestine; the most abundant and diverse of these are the defensins. The RNase A superfamily also includes important antimicrobial proteins involved in host-defense mechanisms in vertebrates; however, they have not been reported in avian species (Schwartz et al., 2018). Here we show the successful amplification, cloning, and molecular characterization of a new chicken RNase A Ang4 gene found for the first time, to the best of our knowledge, in a nonmammalian species, from intestinal epithelial and lymphoid cells under nonpathological and commensal associated conditions.
According to Klenova et al. (1992), significantly lower RSFR mRNA expression was observed in spleen, thymus, bursa of Fabricius, and liver cells, in comparison to bone marrow (Klenova et al., 1992, Nakano and Graf, 1992). Although chAng4 expression in blood marrow and peripheral blood cells was not evaluated in the present study, lymphoid tissue, especially present in the bursa and cecal tonsils, and secretory epithelium of the GIT seem to be important for chAng4 expression and production in chickens.
In comparison to mice, chAng4 expression does not appear to be exclusively crypt-dependent as it was observed in all sampled intestinal luminal tissues on day 1, and mainly in the bursa of Fabricius. B cell progenitors derived from extrabursal tissue that are committed to the B cell lineage and have undergone Ig gene rearrangement colonize the bursal mesenchyme from about day 8 to 14 of incubation (Le Douarin et al., 1975, Houssaint et al., 1976, Ratcliffe et al., 1986, Reynaud et al., 1992). Only B cell precursors expressing functional BCR complexes migrate into bursal follicles, where they rapidly proliferate during the final week of embryonic development (Lydyard et al., 1976, Reynolds, 1987, Thompson and Neiman, 1987, McCormack et al., 1989). At hatch, more than 90% of bursal cells are IgM+ B cells and the growth rate of this organ slows thereafter, with a high proportion of cells dying by apoptosis (Reynaud et al., 1987, Lassila, 1989, Motyka and Reynolds, 1991). Only IgM-expressing bursal B cells migrate to the secondary lymphoid organs, including the gut-associated lymphoid tissue (GALT), in the absence of intrabursal antigen from day 18 of embryogenesis until sexual maturity (Davani et al., 2014, Ko et al., 2018). This may explain the increased chAng4 expression level in bursa on day 1, rather than in the GIT, when compared to day 11 and 22. The few lymphocytes that populate the intestinal tract at this time can also contribute to low expression levels of chAng4 in the small intestine, which could explain the expression level observed in duodenum and jejunum on day 1 (Jeurissen et al., 1999, Friedman et al., 2003).
It has been reported that the level of B cells at hatch and shortly afterward is greatest in the cecal tonsils. However, some functional immaturity of GALT lymphocytes at hatch has also been suggested (Bar-Shira et al., 2003). The first stage of B cell functional maturity occurs during the first week after hatching and it is related to intestinal bacterial colonization, potentially explaining the increased chAng4 expression in duodenum of nontreated birds, and ileum and jejunum after 7 D of supplementation on day 11 in our results (Honjo et al., 1993, Bar-Shira et al., 2003). In addition, maturation and complete development of GALT require gut microbial colonization, which can explain the predominant expression of chAng4 in the bursa, connected to the hindgut lumen by the bursal duct, and higher expression levels in cecal tonsils in comparison to day 1 (Hegde et al., 1982, Honjo et al., 1993, Arakawa et al., 2002).
The lymphoid component appears to have more relevance than the secretory component of epithelial cells; nevertheless, possible Paneth and goblet cell production of chAng4 cannot be excluded. In chickens, goblet cells containing predominantly acid mucin appear before hatch and their numbers increase with development in duodenum, jejunum, and ileum. This pattern alters with acid and neutral mucin-containing goblet cells at hatch, whose proportions remain constant during the first-week post-hatch (Uni et al., 2003). The presence of neutral mucins in ileal and jejunal goblet cells of day-old chicks has been associated with increased intestinal maturity, and changes in acidic mucin composition on day 4 post-hatch in response to intestinal bacterial colonization have been suggested as a protective mechanism during early development (Forder et al., 2007). Furthermore, lysozyme positive cells, in addition to Paneth cells in chicken small intestinal crypts, have been observed early post-hatch along the villus in the duodenum, cecum, and colon (Wang et al., 2016, Bar-Shira and Friedman, 2018). These observations may explain the chAng4 expression found in all tissues on day 1, together with the GALT development as previously discussed.
Bacterial products such as lipopolysaccharide promote the release of Ang4 in mice (Hooper et al., 2003). In chickens, intestinal retroperistalsis has been suggested as an important mechanism for exogenous antigen sampling in the bursa and cecal tonsils (Sorvari et al., 1977, Oláh and Glick, 1978). The constant B-cell encounter with antigens may be related to the continuous chAng4 expression localized mainly in these tissues on day 11 and 22. Similarly, the esophageal tonsil, at the junction of the esophagus and proventriculus, and the lymphoid tissue in the crop and proventriculus are constantly exposed to undigested antigens that stimulate the immune system, thereby possibly explaining the increased expression of chAng4 in crop and proventriculus in birds treated for 7 D (Oláh et al., 2003, Vaughn et al., 2008, Casteleyn et al., 2010). Moreover, the proportion of lymphoid tissue in the crop seems to be inducible, as observed in specific-pathogen-free white leghorn chickens infected with Salmonella enteritidis (Vaughn et al., 2008). Spheniscins, ß-defensins with broad activity spectrum that have been found in preserved stomach contents of king penguins (Aptenodytes patagonicus) are another example of evolutionarily inducible host immune mechanisms, possibly secreted in response to the presence of and exposition time to exogenous microorganisms (Thouzeau et al., 2003).
Chicken's GIT is a diverse microbiological environment associated with an active relationship between microbial groups and their host. The presence and persistence of those diverse bacterial groups are age, environment, food, and pH dependent (Xi et al., 2019). pH conditions along the GIT have an effect on specific physiological functions such as digestion and absorption, control and prevention of pathogen attachment, and intestinal health status (Oakley et al., 2014); therefore, it is possible to infer that some bacterial species are more prevalent than others in each section of the tract. Regarding the GIT pH conditions, it has been demonstrated that acidic pH enhances the activity of many antimicrobial peptides (Malik et al., 2016). In our research, this is consistent with higher chAng4 expression levels in crop and proventriculus of treated birds on day 22, with lower pH values, compared with those in the small intestine (Farner, 1942, Mabelebele et al., 2014, Nkukwana et al., 2015). Besides, the present study showed that the administration of a probiotic and organic acid blend during the first 7-day post-hatching increased chAng4 expression mainly in crop and proventriculus.
The microbiota of the chicken GIT influences gut health, productivity, and disease. The most abundant bacterial species vary in the different chicken GIT sections (crop, gizzard, duodenum, ileum, cecum, and feces), and their dominance and abundance depend on the feed, batch age, sex, and other microbiota influencing variables (Stanley et al., 2014). It has been observed that germ-free mice colonization with B. thetaiotamicron and unfractionated microbiota, harvested from conventionally raised mice, increases Ang4 expression throughout the small bowel. B. thetaiotamicron is an important component of the mouse distal intestinal microbiota, therefore indicating that the expression of Ang4 is enhanced by at least one normal member of the microbiota (Hooper et al., 2001, Hooper et al., 2003). Firmicutes, bacteroidetes, proteobacteria, and actinobacteria have been found to be the most common phyla in chicken cecum, which is consistent with their possible role in the regulation of Ang4 production in intestinal epithelial cells (Gallo and Hooper, 2012, Oakley et al., 2014). In this study, chAng4 expression was observed in all tissues of nontreated birds and the levels in crop, proventriculus, ileum, and jejunum in T2 birds were higher than in T1 and T3 birds on day 11, which suggests that the expression of this protein in the GIT is modulated by microbial exposure. Similar to murine Ang4, ß-defensins are produced in response to bacterial products, and a reduction in microbial exposure results in lower intestinal expression of these antimicrobial peptides at early growing period of birds (Diaz-Carrasco et al., 2019).
During the development of the gut microbial ecosystem, antigen-specific B cells learn to regulate their responses to resident microorganisms, and the gut microflora is also able to induce tolerance to certain microbial epitopes and to potentiate the immune response against others (Zocco et al., 2007). This may help to explain the lower expression levels of chAng4 in the small intestine on day 22, in comparison to day 11, implying that chAng4 could participate in intestinal innate immunity. Our results can also be explained by the probiotic (L. acidophilus, L. casei, S. faecium, and S. cerevisiae) and organic acid (sorbic and citric acid) supplementation. In the intestine, Lactobacilli produce a wide variety of short-chain fatty acids such as butyric, propionic, and acetic acids, which are bacteriostatic either directly or by reducing the pH of the intestinal environment, enhance epithelial integrity, and act on the mucosal immune system (Sugiharto, 2016). Short-chain fatty acids have an effect on tolerogenic dendritic cells, regulating the production of the anti-inflammatory cytokine IL-10 by T-regulatory cells, and enhancing group 3 innate lymphoid cell-derived IL-22 production, that stimulate Paneth cells to secrete Ang4 and some acute-phase proteins (Walker et al., 2013). In addition, Lactobacilli produce lactic acid that can lower the GIT pH further, and bacteriocins with microbicidal or microbiostatic properties (Sugiharto, 2016, Dittoe et al., 2018). Organic acids are commonly produced by beneficial bacteria, that along with organic acid supplements can decrease intestinal pH, depress intestinal bacterial populations, and consequently reduce the trigger factor for immune stimulation (van der Wielen et al., 2000, Dittoe et al., 2018).
In conclusion, the newly characterized chAng4 gene was 97% and 99% identical to mouse Ang4 in the amino acid and nucleotide sequences, respectively, suggesting it as a mediator of innate defense in the GIT, and was predominantly expressed in the GALT, particularly in the bursa of Fabricius and cecal tonsils. Intestinal bacterial colonization seems to modulate the expression of this gene, and the administration of the probiotic and organic acid blend for 7 D maintained higher chAng4 expression levels over time, compared to the 14-D course of supplementation. In chickens, studies concerning the biological function of this protein and the induction of its expression by specific members of the microbiota are lacking and are necessary to determine the potential of chAng4 as an antimicrobial peptide alternative to antibiotics in the poultry industry.
Acknowledgments
The authors would like to thank Beatrice Despres for her technical support and advice, Alltech Inc., the Canadian Poultry Research Council (CPRC) for the financial support for this research and Natural Sciences and Engineering Research Council of Canada (NSERC) discovery grant.
References
- Akbari M.R., Haghighi H.R., Chambers J.R., Brisbin J., Read L.R., Sharif S. Expression of antimicrobial peptides in cecal tonsils of chickens treated with probiotics and infected with Salmonella enterica serovar Typhimurium. Clin. Vaccine Immunol. 2008;15:1689–1693. doi: 10.1128/CVI.00242-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa H., Kuma K., Yasuda M., Ekino S., Shimizu A., Yamagishi H. Effect of environmental antigens on the Ig diversification and the selection of productive V-J joints in the bursa. J. Immunol. 2002;169:818–828. doi: 10.4049/jimmunol.169.2.818. [DOI] [PubMed] [Google Scholar]
- Bar-Shira E., Friedman A. Innate immune functions of avian intestinal epithelial cells: response to bacterial stimuli and localization of responding cells in the developing avian digestive tract. PLoS One. 2018;13:e0200393. doi: 10.1371/journal.pone.0200393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Shira E., Sklan D., Friedman A. Establishment of immune competence in the avian GALT during the immediate post-hatch period. Dev. Comp. Immunol. 2003;27:147–157. doi: 10.1016/s0145-305x(02)00076-9. [DOI] [PubMed] [Google Scholar]
- Casteleyn C., Doom M., Lambrechts E., van den Broeck W., Simoens P., Cornillie P. Locations of gut-associated lymphoid tissue in the 3-month-old chicken: a review. Avian Pathol. 2010;39:143–150. doi: 10.1080/03079451003786105. [DOI] [PubMed] [Google Scholar]
- Crabtree B., Holloway D.E., Baker M.D., Acharya K.R., Subramanian V. Biological and structural features of murine angiogenin-4, an angiogenic protein. Biochemistry. 2007;46:2431–2443. doi: 10.1021/bi062158n. [DOI] [PubMed] [Google Scholar]
- Davani D., Pancer Z., Ratcliffe M.J. Ligation of surface Ig by gut-derived antigen positively selects chicken bursal and peripheral B cells. J. Immunol. 2014;192:3218–3227. doi: 10.4049/jimmunol.1302395. [DOI] [PubMed] [Google Scholar]
- Diaz Carrasco J.M., Casanova N.A., Fernández Miyakawa M.E. Microbiota, gut health and chicken productivity: what is the connection? Microorganisms. 2019;7:374. doi: 10.3390/microorganisms7100374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittoe D.K., Ricke S.C., Kiess A.S. Organic acids and potential for modifying the avian gastrointestinal tract and reducing pathogens and disease. Front. Vet. Sci. 2018;5:216. doi: 10.3389/fvets.2018.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farner D.S. The hydrogen ion concentration in avian digestive tracts. Poult. Sci. 1942;21:445–450. [Google Scholar]
- Forder R.E.A., Howarth G.S., Tivey D.R., Hughes R.J. Bacterial modulation of small intestinal goblet cells and mucin composition during early posthatch development of poultry. Poult. Sci. 2007;86:2396–2403. doi: 10.3382/ps.2007-00222. [DOI] [PubMed] [Google Scholar]
- Forman R.A., deSchoolmeester M.L., Hurst R.J., Wright S.H., Pemberton A.D., Else K.J. The goblet cell is the cellular source of the anti-microbial angiogenin 4 in the large intestine post Trichuris muris infection. PLoS One. 2012;7:e42248. doi: 10.1371/journal.pone.0042248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman A., Bar-Shira E., Sklan D. Ontogeny of gut associated immune competence in the chick. Worlds Poul Sci. J. 2003;59:209–219. [Google Scholar]
- Gallo R.L., Hooper L.V. Epithelial antimicrobial defence of the skin and intestine. Nat. Rev. Immunol. 2012;12:503–516. doi: 10.1038/nri3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde S.N., Rolls B.A., Turvey A., Coates M.E. Influence of gut microflora on the lymphoid tissue of the chicken (Gallus domesticus) and Japanese quail (Coturnix coturnix japonica) Comp. Biochem. Physiol. A. Physiol. 1982;72:205–209. [Google Scholar]
- Honjo K., Hagiwara T., Itoh K., Takahashi E., Hirota Y. Immunohistochemical analysis of tissue distribution of B and T cells in germfree and conventional chickens. J. Vet. Med. Sci. 1993;55:1031–1034. doi: 10.1292/jvms.55.1031. [DOI] [PubMed] [Google Scholar]
- Hooper L.V., Littman D.R., Macpherson A.J. Interactions between the microbiota and the immune system. Science. 2012;336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper L.V., Stappenbeck T.S., Hong C.V., Gordon J.I. Angiogenins: a new class of microbicidal proteins involved in innate immunity. Nat. Immunol. 2003;4:269–273. doi: 10.1038/ni888. [DOI] [PubMed] [Google Scholar]
- Hooper L.V., Wong M.H., Thelin A., Hansson L., Falk P.G., Gordon J.I. Molecular analysis of commensal host-microbial relationships in the intestine. Science. 2001;291:881–884. doi: 10.1126/science.291.5505.881. [DOI] [PubMed] [Google Scholar]
- Houssaint E., Belo M., Le Douarin N.M. Investigations on cell lineage and tissue interactions in the developing bursa of Fabricius through interspecific chimeras. Dev. Biol. 1976;53:250–264. doi: 10.1016/0012-1606(76)90227-x. [DOI] [PubMed] [Google Scholar]
- Jeurissen S.H., Wagenaar F., Janse E.M. Further characterization of M cells in gut-associated lymphoid tissues of the chicken. Poult. Sci. 1999;78:965–972. doi: 10.1093/ps/78.7.965. [DOI] [PubMed] [Google Scholar]
- Jing M., Tactacan G.B., Rodriguez-Lecompte J.C., Kroeker A., House J.D. Molecular cloning and tissue distribution of reduced folate carrier and effect of dietary folate supplementation on the expression of reduced folate carrier in laying hens. Poult. Sci. 2009;88:1939–1947. doi: 10.3382/ps.2009-00032. [DOI] [PubMed] [Google Scholar]
- Klenova E.M., Botezato I., Laudet V., Goodwin G.H., Wallace J.C., Lobanenkov V.V. Isolation of a cDNA clone encoding the RNase-superfamily-related gene highly expressed in chicken bone marrow cells. Biochem. Biophys. Res. Commun. 1992;185:231–239. doi: 10.1016/s0006-291x(05)80980-5. [DOI] [PubMed] [Google Scholar]
- Ko K.H., Lee I.K., Kim G., Gu M.J., Kim H.Y., Park B.C., Park T.S., Han S.H., Yun C.H. Changes in bursal B cells in chicken during embryonic development and early life after hatching. Sci. Rep. 2018;8:16905. doi: 10.1038/s41598-018-34897-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassila O. Emigration of B cells from chicken bursa of Fabricius. Eur. J. Immunol. 1989;19:955–958. doi: 10.1002/eji.1830190527. [DOI] [PubMed] [Google Scholar]
- Le Douarin N.M., Houssaint E., Jotereau F.V., Belo M. Origin of hemopoietic stem cells in embryonic bursa of Fabricius and bone marrow studied through interspecific chimeras. Proc. Natl. Acad. Sci. U S A. 1975;72:2701–2705. doi: 10.1073/pnas.72.7.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydyard P.M., Grossi C.E., Cooper M.D. Ontogeny of B cells in the chicken. I. Sequential development of clonal diversity in the bursa. J. Exp. Med. 1976;144:79–97. doi: 10.1084/jem.144.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabelebele M., Alabi O.J., Ng`ambi J.W., Norris D., Ginindza M.M. Comparison of gastrointestinal tracts and pH values of digestive organs of Ross 308 broiler and indigenous Venda chickens fed the same diet. Asian J. Anim. Vet. Adv. 2014;9:71–76. [Google Scholar]
- Maki J.J., Klima C.L., Sylte M.J., Looft T. The microbial pecking order: utilization of intestinal microbiota for poultry health. Microorganisms. 2019;7:376. doi: 10.3390/microorganisms7100376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik E., Dennison S.R., Harris F., Phoenix D.A. pH dependent antimicrobial peptides and proteins, their mechanisms of action and potential as therapeutic agents. Pharmaceuticals. 2016;9:67. doi: 10.3390/ph9040067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack W.T., Tjoelker L.W., Barth C.F., Carlson L.M., Petryniak B., Humphries E.H., Thompson C.B. Selection for B cells with productive IgL gene rearrangements occurs in the bursa of Fabricius during chicken embryonic development. Genes Dev. 1989;3:838–847. doi: 10.1101/gad.3.6.838. [DOI] [PubMed] [Google Scholar]
- Motyka B., Reynolds J.D. Apoptosis is associated with the extensive B cell death in the sheep ileal Peyer's patch and the chicken bursa of Fabricius: a possible role in B cell selection. Eur. J. Immunol. 1991;21:1951–1958. doi: 10.1002/eji.1830210825. [DOI] [PubMed] [Google Scholar]
- Nakano T., Graf T. Identification of genes differentially expressed in two types of v-myb-transformed avian myelomonocytic cells. Oncogene. 1992;7:527–534. [PubMed] [Google Scholar]
- Nitto T., Dyer K.D., Czapiga M., Rosenberg H.F. Evolution and function of leukocyte RNase A ribonucleases of the avian species, Gallus gallus. J. Biol. Chem. 2006;281:25622–25634. doi: 10.1074/jbc.M604313200. [DOI] [PubMed] [Google Scholar]
- Nkukwana T.T., Muchenje V., Masika P.J., Mushonga B. Intestinal morphology, digestive organ size and digesta pH of broiler chickens fed diets supplemented with or without Moringa oleifera leaf meal. S. Afr. J. Anim. Sci. 2015;45:362–370. [Google Scholar]
- Oakley B.B., Lillehoj H.S., Kogut M.H., Kim W.K., Maurer J.J., Pedroso A., Lee M.D., Collett S.R., Johnson T.J., Cox N.A. The chicken gastrointestinal microbiome. FEMS Microbiol. Lett. 2014;360:100–112. doi: 10.1111/1574-6968.12608. [DOI] [PubMed] [Google Scholar]
- Oláh I., Glick B. The number and size of the Follicular epithelium (FE) and follicles in the bursa of Fabricius. Poult. Sci. 1978;57:1445–1450. doi: 10.3382/ps.0571445. [DOI] [PubMed] [Google Scholar]
- Oláh I., Nagy N., Magyar A., Palya V. Esophageal tonsil: a novel gut-associated lymphoid organ. Poult. Sci. 2003;82:767–770. doi: 10.1093/ps/82.5.767. [DOI] [PubMed] [Google Scholar]
- Pfaffl M., Hageleit M. Validities of mRNA quantification using recombinant RNA and recombinant DNA external calibration curves in real-time RT-PCR. Biotechnol. Lett. 2001;23:275–282. [Google Scholar]
- Ratcliffe M.J., Lassila O., Pink J.R., Vainio O. Avian B cell precursors: surface immunoglobulin expression is an early, possibly bursa-independent event. Eur. J. Immunol. 1986;16:129–133. doi: 10.1002/eji.1830160204. [DOI] [PubMed] [Google Scholar]
- Reynaud C.A., Anquez V., Grimal H., Weill J.C. A hyperconversion mechanism generates the chicken light chain preimmune repertoire. Cell. 1987;48:379–388. doi: 10.1016/0092-8674(87)90189-9. [DOI] [PubMed] [Google Scholar]
- Reynaud C.A., Imhof B.A., Anquez V., Weill J.C. Emergence of committed B lymphoid progenitors in the developing chicken embryo. EMBO J. 1992;11:4349–4358. doi: 10.1002/j.1460-2075.1992.tb05534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds J.D. Mitotic rate maturation in the Peyer's patches of fetal sheep and in the bursa of Fabricius of the chick embryo. Eur. J. Immunol. 1987;17:503–507. doi: 10.1002/eji.1830170411. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Lecompte J.C., Yitbarek A., Brady J., Sharif S., Cavanagh M.D., Crow G., Guenter W., House J.D., Camelo-Jaimes G. The effect of microbial-nutrient interaction on the immune system of young chicks after early probiotic and organic acid administration. J. Anim. Sci. 2012;90:2246–2254. doi: 10.2527/jas.2011-4184. [DOI] [PubMed] [Google Scholar]
- Schwartz L., Cohen A., Thomas J., Spencer J.D. The immunomodulatory and antimicrobial properties of the vertebrate Ribonuclease A Superfamily. Vaccines (Basel) 2018;6:76. doi: 10.3390/vaccines6040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng J., Xu Z. Three decades of research on angiogenin: a review and perspective. Acta Biochim. Biophys. Sin. (Shangai) 2016;48:399–410. doi: 10.1093/abbs/gmv131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorvari R., Naukkarinen A., Sorvari T.E. Anal sucking-like movements in the chicken and chick embryo followed by the transportation of environmental material to the bursa of Fabricius, caeca and caecal Tonsils. Poult. Sci. 1977;56:1426–1429. doi: 10.3382/ps.0561426. [DOI] [PubMed] [Google Scholar]
- Stanley D., Hughes R.J., Moore R.J. Microbiota of the chicken gastrointestinal tract: influence on health, productivity and disease. Appl. Microbiol. Biotechnol. 2014;98:4301–4310. doi: 10.1007/s00253-014-5646-2. [DOI] [PubMed] [Google Scholar]
- Sugiharto S. Role of nutraceuticals in gut health and growth performance of poultry. J. Saudi Soc. Agric. Sci. 2016;15:99–111. [Google Scholar]
- Thompson C.B., Neiman P.E. Somatic diversification of the chicken immunoglobulin light chain gene is limited to the rearranged variable gene segment. Cell. 1987;48:369–378. doi: 10.1016/0092-8674(87)90188-7. [DOI] [PubMed] [Google Scholar]
- Thouzeau C., Le Maho Y., Froget G., Sabatier L., Le Bohec C., Hoffmann J.A., Bulet P. Spheniscins, avian ß-defensins in preserved stomach contents of the king penguin, Aptenodytes patagonicus. J. Biol. Chem. 2003;278:51053–51058. doi: 10.1074/jbc.M306839200. [DOI] [PubMed] [Google Scholar]
- Uni Z., Smirnov A., Sklan D. Pre- and posthatch development of goblet cells in the broiler small intestine: effect of delayed access to feed. Poult. Sci. 2003;82:320–327. doi: 10.1093/ps/82.2.320. [DOI] [PubMed] [Google Scholar]
- van der Wielen P.W., Biesterveld S., Notermans S., Hofstra H., Urlings B.A., van Knapen F. Role of volatile fatty acids in development of the cecal microflora in broiler chickens during growth. Appl. Environ. Microbiol. 2000;66:2536–2540. doi: 10.1128/aem.66.6.2536-2540.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn L.E., Holt P.S., Gast R.K. Cellular assessment of crop lymphoid tissue from specific-pathogen-free white leghorn chickens after Salmonella enteritidis challenge. Avian Dis. 2008;52:657–664. doi: 10.1637/8369-052308-Reg.1. [DOI] [PubMed] [Google Scholar]
- Walker C.R., Hautefort I., Dalton J.E., Overweg K., Egan C.E., Bongaerts R.J., Newton D.J., Cruickshank S.M., Andrew E.M., Carding S.R. Intestinal intraepithelial lymphocyte-enterocyte crosstalk regulates production of bactericidal Angiogenin 4 by Paneth Cells upon microbial challenge. PLoS One. 2013;8:e84553. doi: 10.1371/journal.pone.0084553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Li J., Li J., Jr., Li R.X., Lv C.F., Li S., Mi Y.L., Zhang C.Q. Identification of the Paneth cells in chicken small intestine. Poult. Sci. 2016;95:1631–1635. doi: 10.3382/ps/pew079. [DOI] [PubMed] [Google Scholar]
- Xi Y., Yan J., Li M., Ying S., Shi Z. Gut microbiota dysbiosis increase the risk of visceral gout in goslings through translocation of gut-derived lipopolysaccharide. Poult. Sci. 2019;98:5361–5373. doi: 10.3382/ps/pez357. [DOI] [PubMed] [Google Scholar]
- Zhang J., Rosenberg H.F. Diversifying selection of the tumor-growth promoter angiogenin in primate evolution. Mol. Biol. Evol. 2002;19:438–445. doi: 10.1093/oxfordjournals.molbev.a004099. [DOI] [PubMed] [Google Scholar]
- Zocco M.A., Ainora M.E., Gasbarrini G., Gasbarrini A. Bacteroides thetaiotaomicron in the gut: molecular aspects of their interaction. Dig. Liver Dis. 2007;39:707–712. doi: 10.1016/j.dld.2007.04.003. [DOI] [PubMed] [Google Scholar]