Abstract
The effects of different rearing systems (RS) including cage rearing systems (CRS), litter rearing systems (LRS), and perforated plastic slate rearing systems (PSRS) on the productive performance, carcass traits, blood hematological and biochemical parameters, and humoral immunity in broiler chickens exposed to heat stress were investigated. A total of 270 1-day-old Avian 48 chicks were randomly assigned to 3 groups equally, each was divided into 9 replicates (each of 10 birds) housed in studied RS. Results revealed that CRS had higher (P < 0.001) body weight and weight gain at all experimental periods (except in the sixth wk for weight gain) followed by LRS. Birds housed in PSRS consumed lower (P < 0.001) feed than those in CRS (during the fourth to sixth and overall periods) and LRS (during all experimental periods except the second one). Best values of feed conversion ratio and European broiler index were shown in CR birds. All carcass traits were not altered by different RS except the percentages of dressing, liver, breast, and left filet, which were elevated (P < 0.05) in caged birds. Eosinophil, lymphocyte, basophil, and monocyte counts and phagocytic index and activity were reduced (P < 0.05 or P < 0.01) in LRS. Humoral immune response against the Newcastle disease virus and avian influenza were not differed. Birds in LRS showed higher (P < 0.05) serum cholesterol, uric acid, and lactate dehydrogenase as well as liver and muscle cholesterol contents. Lipid peroxidation was reduced (P < 0.05) in the LRS and PSRS groups, whereas superoxide dismutase was elevated (P < 0.05) in CRS and LRS. Thus, CRS and LRS were preferred for better growth performance and carcass traits of heat-stressed broilers, whereas CRS and PSRS were better in reducing tissue cholesterol under the conditions of our study.
Key words: broiler, rearing system, growth, oxidative status, humoral immunity
Introduction
Rearing systems of broiler chickens are crucial to influence their welfare, health, and production efficiency especially under heat stress conditions. Heat stress has strong and immediate impacts on the welfare and performance of birds (Alagawany et al., 2017, El-Kholy et al., 2017, El-Kholy et al., 2018, Farghly et al., 2018a, Farghly et al., 2018b, Farghly et al., 2018c, Abd El-Hack et al., 2019a), and these adverse effects are strongly influenced by interfering with different European broiler g and rearing systems. Floor constructions greatly contribute to heat gain or loss in summer and its effect is usually ignored. However, the use of an appropriate rearing system is often effective in controlling bedding temperature and obtaining an adequate thermal environment in the birds' living area (Farghly et al., 2018d). Three rearing systems including litter rearing system (LRS), cage rearing system (CRS), and perforated plastic slate rearing system (PSRS) are used in conventional production of broilers. Each of these rearing systems is characterized by several pros and cons. Moreover, costs of feeding instruments are cheap in LRS; however, this system requires more floor space, and the birds raised in it are more susceptible to respiratory and enteric diseases such as chronic respiratory disease, enteritis, and coccidiosis because of their contact with manure and the incidence of dust and wet litter. Cage rearing system addresses all problems of LRS because there is no need to use the litter in addition to increasing uniformity and production per unit area, improving feed efficiency, reducing labor cost/m2, increasing annual production owing to convenience of cleaning and disinfection operations, and the ease of transporting birds to slaughterhouses (Willis et al., 2002). Nevertheless, CRS has many obstacles and disadvantages also including high initial investment costs, deterioration of birds welfare, increase mortalities related to wing and leg disorders, incidence of perosis, leg and wing fractures caused by bone softening, and difficulty of management and controlling environmental factors at large-scale flocks (Sogunle et al., 2008, Moravej et al., 2012, Lacin et al., 2013, Shields and Greger, 2013). The plastic-slatted floors are cost-effective, very durable, and easy to install and clean and do not deteriorate or need rapid replacement (Farghly et al., 2018d, Çavuşoğlu et al., 2018).
Results of studies conducted to assess the effects of LRS and CRS on broiler performance were not always consistent. It has been reported that LRS improved growth performance of male broilers than CRS (Fortomaris et al., 2007, Santos et al., 2012). However, Al-Bahouh et al. (2012) and Wang et al. (2015) observed better performance and economic efficiency of birds reared in CRS. Moreover, Bahreiny et al. (2013) noticed no alterations in growth performance parameters of male and female broilers affected by LRS or CRS. Limited researches have been conducted to evaluate the impact of PSRS on broiler performance, meat quality, and other traits particularly under heat stress. Therefore, the present study was designed to investigate the impact of these 3 rearing systems (CRS, LRS, and PSRS) on growth performance, carcass traits, blood hematological and biochemical parameters, tissue cholesterol content, oxidative stress biomarkers, and humoral immunity in broiler chickens reared under heat stress.
Materials and methods
The experimental procedures were performed in accordance with the guidelines of the Animal Care and Ethics Committee at Animal Husbandry and Animal Wealth Development Department, Faculty of Veterinary Medicine, Damanhour University, Egypt.
Experimental Birds, Design, and Management
Three hundred twenty-four 1-day-old broiler chicks (Avian 48) were obtained from a local hatchery in Egypt and randomly assigned equally to 3 groups, each was divided into 9 replicates (each of 12 birds) to evaluate the impacts of different rearing systems (CRS, LRS, and PSRS) as a managerial tool to eliminate the deleterious effects of the heat stress on growth performance, carcass traits, blood hematological and biochemical parameters, tissue cholesterol content, oxidative stress biomarkers, and humoral immunity of broilers. Chicks of LRS (control birds) were housed in 1 m2 pen/replicate and brooded on wood shaving litter at a depth of 5 cm. In CRS, birds were caged in stainless steel batteries with dimensions of 100 × 90 × 45 cm (length × width × height)/pen/replicate. Birds of PSRS were brooded and raised in pens (1 m2 space/replicate) with plastic slate floor (50 × 50 cm) that consisted of holes (15 × 10 mm) and bridges (steel bars covered with plastic; width 3.5 mm). During the entire fattening period, the excreta were stored at a depth of approximately 20 cm under the slatted flooring. Pens of LRS and PSRS were separated with net walls of 1 m height. Under all RS, each pen was equipped with a separate feeder and conventional drinker, and water and feed were offered ad libitum. Starter (1 to 21 D) and grower (22 to 42 D) rations were formulated (Table 1) to meet the requirements of NRC (1994). The experiment was conducted under summer conditions (June to August). Chicks were brooded at 33°C, at the birds' level, during first 3 D of age, and then temperature reduced gradually to meet the natural ambient temperature and relative humidity (24.2°C to 37.4°C and 49.5 to 77.3% at midnight and midday, respectively). All chicks were subjected to the same vaccination program and exposed to 24 h constant light during all the experimental period.
Table 1.
Ingredients and composition of basal diet.
| Ingredients | Starter | Grower |
|---|---|---|
| Yellow corn | 59.50 | 62.5 |
| Soybean meal (44%) | 26.00 | 23.94 |
| Maize gluten meal (62%) | 9.00 | 7.00 |
| Vegetable oil | 1.50 | 2.50 |
| Limestone | 1.12 | 1.23 |
| Dicalcium Phosphate | 1.75 | 1.70 |
| Premix1 | 0.30 | 0.30 |
| NaCl (salt) | 0.30 | 0.30 |
| L-lysine | 0.36 | 0.36 |
| DL-Methionine | 0.17 | 0.17 |
| Calculated compositions2 | ||
| ME (kcal kg−1) | 3,055.00 | 3,120 |
| Crude protein | 22.10 | 20.20 |
| Calcium | 0.93 | 0.95 |
| Nonphytate phosphorus | 0.46 | 0.45 |
| Methionine | 0.3 | 0.3 |
| Lysine | 1.28 | 1.2 |
| TSAA | 0.98 | 0.90 |
Abbreviation: TSAA, total sulfur amino acids.
Provides each kg of diet: vitamin A, 12,000 IU; vitamin D3, 5,000 IU; vitamin E, 130.0 mg; vitamin K3, 3.605 mg; vitamin B1, 3.0 mg; vitamin B2, 8.0 mg; vitamin B6, 4.95 mg; vitamin B12, 0.17 mg; niacin, 60.0 mg; folic acid, 2.083 mg; D-biotin, 200.0 mg; calcium D-pantothenate, 18.333 mg; copper, 80 mg; iodine, 2.0 mg; selenium, 150.0 mg; iron, 80.0 mg; manganese, 100.0 mg; zinc, 80.0 mg; cobalt, 500.0 mg.
Calculated according to (NRC, 1994).
Growth Performance Traits
Individual live body weight/replicate was recorded at the beginning of each experimental week in early morning before receiving any feed. Weekly weight gain was calculated, and feed intake (FI) was also recorded weekly to estimate feed conversion ratio (FCR) (as g feed: g gain) on replication basis. Birds were monitored for mortality 3 times a day. European broiler index (EBI) was calculated for the overall period using the following equation: .
Carcass Characteristics
Before slaughtering, birds were deprived of feed for 12 h and weighed. One bird/replicate was slaughtered, scalded, wet plucked, and eviscerated. The liver, gizzard, heart, and spleen were separated and individually weighed, and the dressing percentage was determined as hot carcass weight/preslaughter weight × 100. Abdominal fat was collected from carcass and weighed. The blood, lungs, limbs, viscera, head, and neck were termed as the offal and discarded. The carcass was cut into separate parts including the shoulder (average of 2 shoulders’ weight), thigh (average of 2 thighs’ weight), breast (muscles with the sternum), and left filet (the deskinned left breast muscle), and each was weighed and proportioned to the preslaughter weight. Liver, breast, and thigh samples were immediately imbedded in liquid nitrogen and stored at −80°C till the analysis of the cholesterol content.
Blood Hematology, Biochemistry, and Tissue Cholesterol Content
At 42 D of age, 2 blood samples were collected from wing veins from 1 bird/replicate/group in separate labeled test tubes. For hematological observations, the first tube contained 3.2% sodium citrate solution to prevent blood from clotting. Hemoglobin and hematocrit values and erythrocyte, leucocyte, and differential leucocytic counts were determined. Phagocytic activity and index were determined as described by Kawahara et al. (1991). Blood collected in the second tube was allowed to clot and was then centrifuged at 4,500 × g for 15 min. The sera were collected and preserved at −80°C until the time of analysis. Aspartate aminotransferase, alanine aminotransferase, lactate dehydrogenase (LDH), urea, uric acid, creatinine, and total cholesterol were determined spectrophotometrically (Spectronic 1,201, Milton Roy, Ivyland, PA, USA) using commercial kits of Bio Diagnostic Co., Egypt, in accordance with the manufacturer's instructions. Serum oxidative stress biomarkers including malondialdehyde (MDA) content and superoxide dismutase (SOD) and glutathione peroxidase (GPx) activities were estimated using ELISA Kit of QuantiChrom (BioAssay Systems, USA and Cayman Chemical Company, USA). Cholesterol content in liver, breast, and thigh muscles samples was determined in accordance with the modified method of the one described by Dinh et al. (2008).
Estimation of Humoral Immune Response
Nine chicks from each group were randomly chosen, marked, and vaccinated with Newcastle disease virus (NDV) and avian influenza (H9N1 and H5N2) at 28 D of age. Blood samples were collected 14 D after immunization, kept till clotting, and then, centrifuged at 4,500 × g for 10 min, and the sera samples are stored at −80°C until determination of antibody titers. Serum antibody titers were determined by means of the hemagglutination inhibition test using ELISA test kit (FLOCKTYPE recNDV; Labor Diagnostik, Leipzig, Germany) as described by OIE (2009).
Statistical Analysis
Differences among experimental groups were analyzed by one-way ANOVA with the general linear models using SPSS software, version 18.0. The cage was the experimental unit for growth performance traits, while individuals' data were the experimental units for the rest of the parameters. The applied model was as follows:
where Yij = an observation, μ = the overall mean, Ti = effect of different rearing systems (CRS, LRS, and PSRS), and eij = random error. Shapiro–Wilk and Levene tests were used to test the normal distribution of data as well as homogeneity of variance. Tukey's multiple rang test was used to detect differences between groups at a significance level of P < 0.05.
Results
Growth Performance Traits
Interval growth performance traits were greatly affected by different rearing systems (Table 2). Live body weight during all experimental periods was significantly (P < 0.001) increased in CRS birds compared with LRS birds, whereas birds reared under PSRS recorded the lowest BW. The CRS birds also had higher (P < 0.01) BWG during all experimental periods (except sixth wk of age) and the overall one compared with other treatment groups. Birds of LRS consumed higher (P < 0.001) feed than CRS birds during the first, third, sixth, and overall periods, whereas birds housed in PSRS recorded the lowest FI during all periods. Better interval and total FCR and EBI values were observed in caged birds followed by PSRS. Dressing percentage and relative weights of the liver, breast and lift fillet were significantly (P < 0.05) elevated in CRS birds compared with birds of the other rearing system (Table 3). Relative weights of the gizzard, heart, spleen, abdominal fat, thigh, and shoulder were not influenced by studied rearing systems.
Table 2.
Effect of different rearing systems on growth, feed intake, and feed conversion ratio in broiler chickens.
| Traits | Rearing systems1 |
SEM2 | P-value | ||
|---|---|---|---|---|---|
| CRS | LRS | PSRS | |||
| First wk | |||||
| Chick body weight (g, day 0) | 40.30 | 40.35 | 40.35 | 0.201 | 0.993 |
| BW (g) | 164.1a | 165.1a | 157.3b | 0.899 | <0.001 |
| WG (g/bird/wk) | 123.8a | 124.8a | 116.9b | 0.913 | <0.001 |
| FI (g/bird/wk) | 182.5c | 187.8a | 185.8b | 0.395 | <0.001 |
| FCR (g feed/g gain) | 1.48b | 1.51b | 1.60a | 0.012 | <0.001 |
| Second wk | |||||
| BW (g) | 375.4a | 354.3b | 345.0c | 2.008 | <0.001 |
| WG (g/bird/wk) | 211.3a | 189.2b | 187.7b | 2.028 | <0.001 |
| FI (g/bird/wk) | 308.8 | 307.0 | 305.0 | 1.110 | 0.393 |
| FCR (g feed/g gain) | 1.46b | 1.63a | 1.63a | 0.016 | <0.001 |
| Third wk | |||||
| BW (g) | 662.6a | 625.6b | 617.8c | 2.895 | <0.001 |
| WG (g/bird/wk) | 287.3a | 271.3b | 272.8b | 2.035 | 0.001 |
| FI (g/bird/wk) | 417.5b | 427.8a | 420.0b | 1.149 | <0.001 |
| FCR (g feed/g gain) | 1.46b | 1.58a | 1.54a | 0.013 | <0.001 |
| Forth wk | |||||
| BW (g) | 1,211.8a | 1,117.7b | 1,068.3c | 8.712 | <0.001 |
| WG (g/bird/wk) | 549.2a | 492.1b | 450.5c | 6.823 | <0.001 |
| FI (g/bird/wk) | 855.8a | 868.8a | 763.8b | 9.890 | <0.001 |
| FCR (g feed/g gain) | 1.56b | 1.77a | 1.70a | 0.022 | <0.001 |
| Fifth wk | |||||
| BW (g) | 1,916.3a | 1,767.0b | 1,687.5c | 14.81 | <0.001 |
| WG (g/bird/wk) | 704.5a | 649.4b | 619.3b | 10.19 | 0.002 |
| FI (g/bird/wk) | 1,187.5a | 1,174.3a | 1,002.5b | 14.18 | <0.001 |
| FCR (g feed/g gain) | 1.70b | 1.83a | 1.64b | 0.029 | 0.022 |
| Sixth wk | |||||
| BW (g) | 2,366.4a | 2,199.0b | 2,103.2c | 16.88 | <0.001 |
| WG (g/bird/wk) | 450.2 | 432.0 | 415.7 | 12.72 | 0.550 |
| FI (g/bird/wk) | 848.8b | 885.0a | 777.5c | 6.507 | <0.001 |
| FCR (g feed/g gain) | 1.93 | 2.21 | 2.01 | 0.071 | 0.242 |
| Overall (day 0 to 42) | |||||
| WG (g/bird/wk) | 2,326.1a | 2,158.7b | 2,062.8c | 16.88 | <0.001 |
| FI (g/bird/wk) | 3,800.8b | 3,850.5a | 3,454.5c | 24.20 | <0.001 |
| FCR (g feed/g gain) | 1.64c | 1.79a | 1.68b | 0.011 | <0.001 |
| EBI | 339.1a | 288.6b | 293.7b | 3.890 | <0.001 |
Means carrying different superscripts within the same raw are significantly different (P < 0.05).
Abbreviations: CRS, cage rearing system; LRS, litter rearing system; PSRS, plastic slate rearing system.
Abbreviations: BW, body weixght; EBI, European Broiler Index; FCR, feed conversion ratio; FI, feed intake; SEM, standard error of means; WG, weight gain.
Table 3.
Effect of different rearing systems on organ weights (g/100 g body weight) in broiler chickens.
| Traits | Rearing systems1 |
SEM2 | P-value | ||
|---|---|---|---|---|---|
| CRS | LRS | PSRS | |||
| Dressing weight | 72.71a | 69.55b | 70.30b | 0.466 | 0.004 |
| Liver weight | 4.42a | 3.99b | 4.04a,b | 0.084 | 0.045 |
| Gizzard weight | 3.06 | 2.72 | 2.90 | 0.080 | 0.238 |
| Heart weight | 0.90 | 0.85 | 0.92 | 0.017 | 0.241 |
| Spleen weight | 0.22 | 0.20 | 0.21 | 0.006 | 0.371 |
| Abdominal fat weight | 1.44 | 1.77 | 1.82 | 0.092 | 0.187 |
| Breast weight | 28.08a | 25.85b | 25.80b | 0.443 | 0.041 |
| Thigh weight | 17.47 | 16.29 | 16.16 | 0.359 | 0.277 |
| Shoulder weight | 4.77 | 4.06 | 4.18 | 0.183 | 0.249 |
| Lift filet weight | 11.84a | 10.47b | 10.32b | 0.272 | 0.027 |
Means carrying different superscripts within the same raw are significantly different (P < 0.05).
Abbreviations: CRS, cage rearing system; LRS, litter rearing system; PSRS, plastic slate rearing system.
Abbreviation: SEM, standard error of means.
Hematological Observations
As presented in Table 4, rearing systems did not affect erythrocyte, total leucocyte, and heterophil (H) counts and values of hemoglobin and hematocrit. However, counts of eosinophils, lymphocytes (L), basophils, and monocytes and phagocytic index and activity were increased (P < 0.05) in the birds of CRS and PSRS compared with those of the birds in LRS. The H/L ratio was decreased in birds reared in CRS and PSRS compared to LRS birds.
Table 4.
Effect of different rearing systems on blood corpuscle, hematocrit, and hemoglobin concentration of broiler chickens.
| Traits | Rearing systems1 |
SEM2 | P-value | ||
|---|---|---|---|---|---|
| CRS | LRS | PSRS | |||
| Leukocyte count ( × 103/mm3) | 23.75 | 23.86 | 23.84 | 0.057 | 0.720 |
| Erythrocyte count ( × 106/mm3) | 3.19 | 3.15 | 3.21 | 0.018 | 0.413 |
| Hematocrit % | 29.26 | 29.20 | 29.02 | 0.076 | 0.432 |
| Hemoglobin (g/dl) | 14.03 | 14.00 | 14.10 | 0.046 | 0.671 |
| Eosinophils % | 8.54a | 8.16b | 8.44a | 0.060 | 0.014 |
| Lymphocytes % | 36.08a | 34.34b | 36.18a | 0.267 | 0.001 |
| Heterophils% | 23.28 | 23.42 | 23.40 | 0.075 | 0.744 |
| H/L Ratio | 0.645b | 0.682a | 0.647b | 0.006 | 0.002 |
| Basophils% | 1.10a | 1.04b | 1.08a | 0.009 | 0.003 |
| Monocytes% | 5.42a | 5.16b | 5.42a | 0.049 | 0.026 |
| Phagocytic index | 1.72a | 1.44b | 1.66a | 0.040 | 0.002 |
| Phagocytic activity | 16.30a | 15.10b | 16.40a | 0.206 | 0.005 |
Means carrying different superscripts within the same raw are significantly different (P < 0.05).
Abbreviations: CRS, cage rearing system; LRS, litter rearing system; PSRS, plastic slate rearing system.
Abbreviation: SEM, standard error of means.
Blood Biochemical Parameters
Hepatic and renal function biomarkers as affected by rearing systems are presented in Table 5. Serum levels of Aspartate aminotransferase, alanine aminotransferase and urea were not differed among experimental groups. However, the serum content of LDH and uric acid was elevated (P < 0.05) in the LRS group, whereas the creatinine level was increased (P < 0.01) in the PSRS group. Humoral immune response was not affected by different rearing systems; total antibody titers against NDV, H9N1, and H5N2 were not altered in birds reared in the CRS, LRS, or PSRS.
Table 5.
Effect of different rearing systems on hepatic and renal function biomarkers and humoral immunity of broiler chickens.
| Traits | Rearing systems1 |
SEM2 | P-value | ||
|---|---|---|---|---|---|
| CRS | LRS | PSRS | |||
| AST, U/L | 97.20 | 97.00 | 95.60 | 1.978 | 0.946 |
| ALT, U/L | 20.00 | 19.80 | 19.20 | 0.433 | 0.763 |
| LDH, U/L | 332.2b | 341.4a | 332.2b | 1.843 | 0.049 |
| Urea, mmol/L | 5.30 | 5.53 | 5.41 | 0.046 | 0.101 |
| Uric acid, μmol/L | 408.6b | 416.6a | 404.8b | 1.907 | 0.021 |
| Creatinine, mmol/L | 0.430b | 0.456b | 0.484a | 0.007 | 0.003 |
| NDV titer | 2.79 | 2.68 | 2.61 | 0.042 | 0.205 |
| AIV (H9N1) titer | 2.89 | 2.86 | 2.89 | 0.043 | 0.948 |
| AIV (H5N2) titer | 2.93 | 2.75 | 2.86 | 0.048 | 0.313 |
Means carrying different superscripts within the same raw are significantly different (P < 0.05).
Abbreviations: CRS, cage rearing system; LRS, litter rearing system; PSRS, plastic slate rearing system.
Abbreviations: AIV, avian Influenza virus; ALT, alanine aminotransferase; AST, aspartate aminotransferase; LDH, lactate dehydrogenase; NDV, Newcastle disease virus; SEM, standard error of means.
Tissue Cholesterol Content
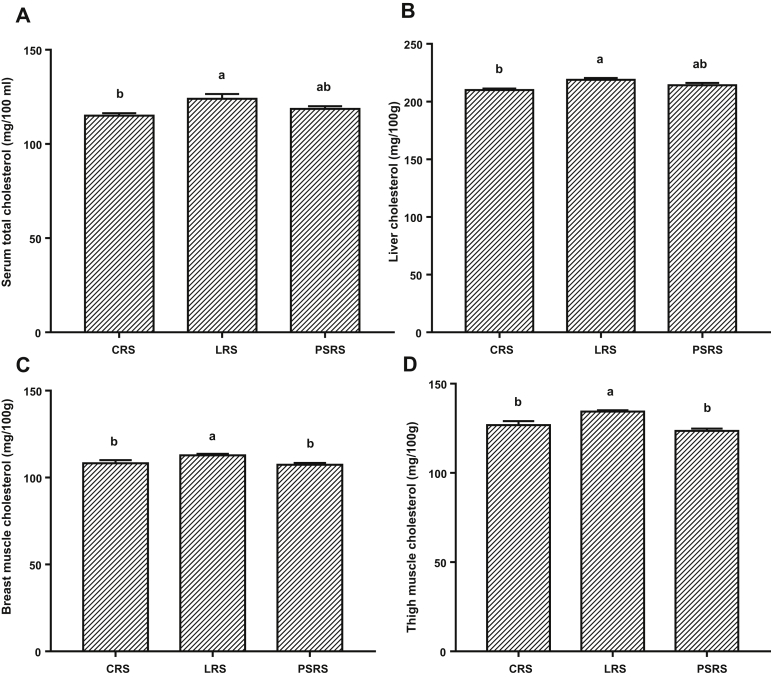
Data depicted in Figure 1 illustrate the impact of rearing systems on the serum, liver, breast, and thigh content of cholesterol. Cholesterol level in serum and liver tissue was reduced significantly (P < 0.05) in the CRS birds and numerically in the PSRS birds. Moreover, CRS and PSRS reduced (P < 0.05) cholesterol content of breast and thigh muscles.
Figure 1.
Effect of different rearing systems on cholesterol content of the (A) serum, (B) liver, (C) breast muscle, and (D) thigh muscle of broiler chickens. Data are presented as mean values with their standard errors. Values with different superscript letters are statistically different (P < 0.05). Abbreviations: CRS, cage rearing system; LRS, litter rearing system; PSRS, plastic slate rearing system.
Oxidative Stress Biomarkers
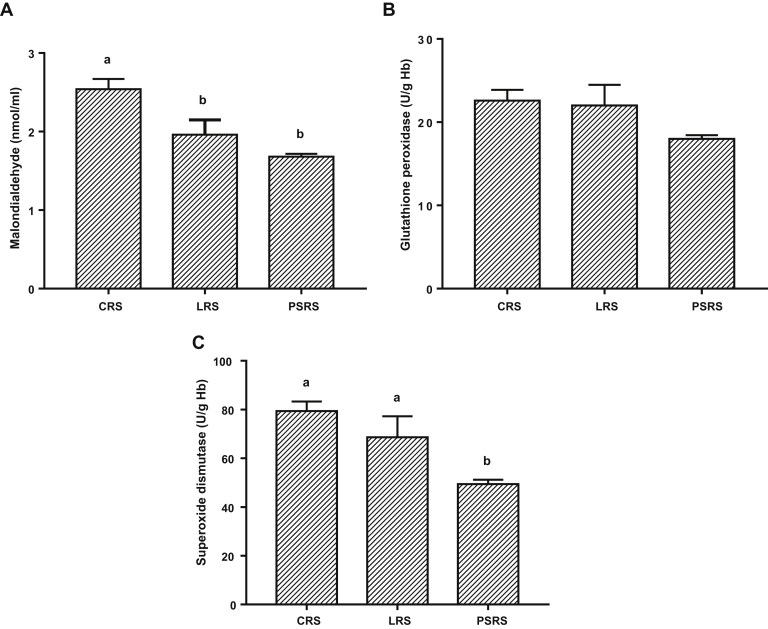
Serum levels of oxidative stress biomarkers are illustrated in Figure 2. Serum content of MDA, as a lipid peroxidation biomarker, was increased (P < 0.05) in CRS birds compared with that in birds of the other rearing systems. However, SOD activity was elevated (P < 0.05) in the CRS and LRS groups. Glutathione peroxidase activity was not significantly influenced.
Figure 2.
Effect of different rearing systems on serum oxidative stress biomarkers of broiler chickens. Data are presented as mean values with their standard errors. Values with different superscript letters are statistically different (P < 0.05). Abbreviations: CRS, cage rearing system; LRS, litter rearing system; PSRS, plastic slate rearing system.
Discussion
In the present results, CRS birds recorded the highest BW and BWG followed by LRS and PSRS birds. The LRS birds consumed higher feed than CRS birds, whereas PSRS birds recorded the lowest FI. The best FCR and EBI values were observed in caged birds followed by PSRS ones. These variations in growth performance among different rearing systems under heat stress may be becasue of the different ability of broilers to exchange their body heat with air, bedding floor materials, and the ground underneath. The possible reason for the increased growth of birds reared in CRS was their lack of direct contact with feces, which maintains better environmental hygiene and thus reduces the incidence of diseases. The aforementioned reasons may also explain the improvement of FCR and EBI in CRS and PSRS birds. Our results also revealed significant reduction in FI of PSRS birds compared with LRS ones. Increased amount of feed consumed by LRS birds may be because of the pebbles and grit swallowed by birds, which improve and accelerate the digestion process and subsequently speeds up the digesta rate of passage through the gastrointestinal tract. Similar to the present study, Al-Bahouh et al. (2012) and Şimşek et al. (2014) showed that caged broilers showed higher growth performance. Contrarily, Santos et al. (2012) and Lacin et al. (2013) found that broiler chicks reared in LRS had better body weight and lower FCR than those of caged birds. However, Sogunle et al. (2008) and Wang et al. (2015) reported insignificant differences in growth performance of broilers reared in CRS and LRS. Limited studies have been conducted to evaluate the effects of net and plastic slate rearing systems on broiler growth performance. Çavuşoğlu et al. (2018) and Chuppava et al. (2018) demonstrated that broiler chicks reared on slatted floor observed higher BW than those reared on litter. However, Almeida et al. (2018) observed no differences in weights of broiler chickens from the same gender reared on litter or plastic floors. These discrepancies in research results may be because of the differences in experimental conditions such as ambient temperature, season, and broiler strain and sex. In a comprehensive comparison of LRS and PSRS over 4 flocks for 8 mo, Li et al. (2017) reported that BW were numerically improved in PSRS birds in spring and winter, whereas it was higher in birds of LRS in fall and summer. Şimşek et al. (2014) also noticed higher BW of litter reared broilers in autumn, whereas no difference between litter and caged birds was recorded in summer and winter at 28 D of age. Moreover, Al-Bahouh et al. (2012) observed significant elevation in BW of caged Cobb 500 birds, whereas Indian River broilers reared on litter had higher BW than those in cages; however, BW of Ross birds were not affected by these rearing systems. Male broilers also recorded higher BW than female ones either when reared on wood shavings or plastic floors (Almeida et al., 2018).
The results obtained regarding carcass traits were partially in line with those obtained by Şimşek et al. (2014), who observed significant increase in breast weight of caged birds than floored birds, while carcass, thigh, wings, liver and spleen were not affected. The same results were reported by Bahreiny et al. (2013) who reported that breast weight was greater in male chickens reared in CRS. Contrarily, Sogunle et al. (2008) and Santos et al. (2012) documented that breast (%) were increased in floor birds than in caged birds. However, Wang et al. (2015) showed no significant alterations in carcass yield and breast relative weight, whereas thigh weight (%) was higher in birds reared in CRS than those reared in LRS and net rearing system. Other researches did not find any significant effects of different rearing systems on all examined carcass traits (Al-Bahouh et al., 2012, Almeida et al., 2018).
Blood biochemical and hematological parameters, leukocytes differentiation, and phagocytic activity are important tools for assessing long-term stress in poultry and their immune response to stressors (Abd El-Hack et al., 2019b, Saeed et al., 2019). Altan et al. (2000) reported that high temperature accentuates H/L ratio in broilers. Heat stress in poultry exerts several physiological manifestations including alternations in circulating leukocytes counts, and particularly, pronounced heterophilia and lymphocytopenia, which were considered as trustworthy stress indicators (Bin-Jumah et al., 2020). Our results revealed significant increase in counts of L, eosinophils, basophils, and monocytes and phagocytic index and activity and decrease in the H/L ratio in CRS and PSRS birds compared with those of LRS. These results suggest that rearing birds in cages and plastic slate improved their peripheral immunity. The present results agreed with those obtained by Farghly et al. (2018d) who stated that plastic and wooden slates reduced values of the H/L ratio and increased L count in turkey. Matur et al. (2015) noticed significant reduction in heterophils percentage and H/L ratio and increase in antibody production in layers reared in furnished cages compared with those reared in conventional cages. All blood biochemical parameters and antibody titers against NDV, H9N1, and H5N2 were not affected except LDH and uric acid that were elevated in the LRS group. These results are in accordance with the results of Sogunle et al. (2008) who noticed no alterations in blood biochemistry between caged and litter reared birds. The authors attributed this lack of significance to the good health conditions of birds during the period of the study.
Lower cholesterol content in serum, the liver and breast, and thigh muscles was recorded in caged birds followed by PSRS birds compared with LRS ones. To our knowledge, limited investigations studied the effect of flooring types on cholesterol and fat content of broiler muscles. Nevertheless, despite the lack of literature, it is obviously that different flooring systems greatly affect meat quality and fat and cholesterol contents. Where, Evans et al. (1976) reported that cage-reared broiler chickens showed low fat content of meat than those of floor-reared chicks. Moreover, Manohar et al. (2005) demonstrated that the litter-reared birds had higher level of cholesterol in thigh and breast meat than the cage-reared broilers irrespective of the stocking densities and age. This low-cholesterol content meets the market's need as this feature recently favored by health-conscious consumers.
Antioxidant status of birds was influenced by the type of floor; low lipid peroxidation was observed in LRS and PSRS birds, whereas serum SOD activity was elevated in CRS and LRS birds; however, GPx was not affected. Our results are in agreement with the findings of Zhao et al. (2009) who noticed significant reduction in MDA content in birds on plastic slats than those of wire netting birds; however, the activity of GPx in serum was not significantly differed. The same observations were reported by Şimşek et al. (2014) who recorded significant elevation in MDA content and catalase activity in the serum of CRS birds compared with that of LRS chicks in the summer season. The increase in MDA content in cage-reared birds might indicate the high susceptibility of these birds to heat stress and less ability to treat such circumstances that subsequently raise corticosterone synthesis (Şimşek et al., 2014). Zhao et al. (2009) speculated a positive correlation between the elevation of MDA content and the incidence of breast blisters that greatly impair the birds' welfare and reduce carcass grade.
Under the conditions of the present study, birds reared in CRS had better growth performance, carcass yield, and breast weight and breast meat yield followed by birds of LRS, while those of PSRS recorded the lowest values. Cage rearing system and PSRS were more effective in alleviating the adverse effect of heat stress than LRS as the H/L ratio was decreased and phagocytic index and activity were improved in birds of these rearing systems. Antioxidant defense system was also affected by studied flooring systems. Birds reared in CRS and PSRS recorded the lower content of cholesterol in breast and thigh muscles. Therefore, PSRS is suggested if needed to produce more healthy meat, but CRS is recommended for higher production efficiency.
Acknowledgments
This research was funded by the Deanship of Scientific Research at Princess Nourah Bint Abdulrahman University through the Fast Track Research Funding Program.
Conflicts of Interest Statement: All authors declare that they do not have any conflicts of interests that could inappropriately influence this manuscript.
Contributor Information
Mahmoud M. Abo Ghanima, Email: aboghoneima.mmyvet2@vetmed.dmu.edu.eg.
Mohamed E. Abd El-Hack, Email: dr.mohamed.e.abdalhaq@gmail.com.
References
- Abd El-Hack M.E., Abdelnour S.A., Taha A.E., Khafaga A.F., Arif M., Ayasan T., Swelum A.A., Aleya L., Abdel Daim M.M., Alkahtani S., Abukhalil M.H. Herbs as thermoregulatory agents: an overview. Sci. Total Environ. 2019;134399 doi: 10.1016/j.scitotenv.2019.134399. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., Alagawany M., Noreldin A.E. Sustainability of Agricultural Environment in Egypt: Part II. Springer; Cham: 2019. Managerial and nutritional trends to mitigate heat stress risks in poultry farms; pp. 325–338. [Google Scholar]
- Al-Bahouh M.E., Al-Nasser A.Y., Abdullah F.K., Ragheb G., Mashaly M.M. Production performance of different broiler breeds under different housing systems. Inter. J. Poult. Sci. 2012;11:190–195. [Google Scholar]
- Alagawany M., Farag M., Abd El-Hack M.E., Patra A. Heat stress: effects on productive and reproductive performance of quail. World's Poult. Sci. J. 2017;73:747–756. [Google Scholar]
- Almeida E.A.d., Sant’Anna A., Crowe T., Macari M., Furlan R. Poultry rearing on perforated plastic floors and the effect on air quality, growth performance, and carcass injuries–Experiment 2: heat stress situation. Poult. Sci. 2018;97:1954–1960. doi: 10.3382/ps/pey048. [DOI] [PubMed] [Google Scholar]
- Altan Ö., Altan A., Çabuk M., Bayraktar H. Effects of heat stress on some blood parameters in broilers. Turkish J. Vet. Anim. Sci. 2000;24:145–148. [Google Scholar]
- Bahreiny E., Dadvar P., Morovat M., Bujarpoor M. Effect of different level of energy to protein ratio and breeding system on performance and carcass characteristics of male and female broilers. Inter. J. Agric. 2013;3:597. [Google Scholar]
- Bin-Jumah M., Abd El-Hack M.E., Abdelnour S.A., Hendy Y.A., Ghanem H.A., Noreldin A.E., Khafaga A.F., Alsafy S.A., Shaheen H., Samak D., Momenah M.A., Allam A.A., Aleya L., Abdel-Daim M.M. The potentiality of using chromium as an attenuators agent of thermal stress in animals: a review. Sci. Total Environ. 2020;707:135996. doi: 10.1016/j.scitotenv.2019.135996. [DOI] [PubMed] [Google Scholar]
- Çavuşoğlu E., Petek M., Abdourhamane I.M., Akkoc A., Topal E. Effects of different floor housing systems on the welfare of fast-growing broilers with an extended fattening period. Arch. Anim. Breed. 2018;61:9–16. [Google Scholar]
- Chuppava B., Visscher C., Kamphues J. Effect of different flooring Designs on the performance and Foot Pad health in broilers and Turkeys. Animals. 2018;8:70. doi: 10.3390/ani8050070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh T., Blanton J., Jr., Brooks J., Miller M., Thompson L. A simplified method for cholesterol determination in meat and meat products. J. Food Compo. Anal. 2008;21:306–314. [Google Scholar]
- El-Kholy M.S., El-Hindawy M.M., Alagawany M., Abd El-Hack M.E., El-Sayed S.A.A. Use of acetylsalicylic acid as an allostatic modulator in the diets of growing Japanese quails exposed to heat stress. J. Therm. Biol. 2018;74:6–13. doi: 10.1016/j.jtherbio.2018.02.011. [DOI] [PubMed] [Google Scholar]
- El-Kholy M.S., Alagawany M., Abd El-Hack M.E., El-Sayed S.A.A. Dietary supplementation of chromium can alleviate negative impacts of heat stress on performance, carcass yield, and some blood hematology and chemistry indices of growing Japanese quail. Biol. Trace Elem. Res. 2017;179:148–157. doi: 10.1007/s12011-017-0936-z. [DOI] [PubMed] [Google Scholar]
- Evans D.G., Goodwin T.L., Andrews L.D. Chemical composition, carcass yield and tenderness of broilers as influenced by rearing methods and genetic strains. Poult. Sci. 1976;55:748–755. [Google Scholar]
- Farghly M.F.A., Alagawany M., Abd El-Hack M.E. Feeding time can alleviate negative effects of heat stress on performance, meat quality and health status of Turkey. Br. Poult. Sci. 2018;59:205–210. doi: 10.1080/00071668.2017.1413233. [DOI] [PubMed] [Google Scholar]
- Farghly M.F.A., Abd El-Hack M.E., Alagawany M., Saadeldin I.M., Swelum A.A. Ameliorating the deleterious effects of heat stress on growing Muscovy ducklings by feed withdraw and cold water. Poult. Sci. 2018;98:251–259. doi: 10.3382/ps/pey396. [DOI] [PubMed] [Google Scholar]
- Farghly M.F.A., Abd El-Hack M.E., Alagawany M., Saadeldin I.M., Swelum A.A. Wet feed and cold water as heat stress modulators in growing Muscovy ducklings. Poult. Sci. 2018;97:1588–1594. doi: 10.3382/ps/pey006. [DOI] [PubMed] [Google Scholar]
- Farghly M.F., Mahrose K.M., Cooper R.G., Ullah Z., Rehman Z., Ding C. Sustainable floor type for managing Turkey production in a hot climate. Poult. Sci. 2018;97:3884–3890. doi: 10.3382/ps/pey280. [DOI] [PubMed] [Google Scholar]
- Fortomaris P., Arsenos G., Tserveni-Gousi A., Yannakopoulos A. Performance and behaviour of broiler chickens as affected by the housing system. Archiv Fur Geflugelkunde. 2007;71:97. [Google Scholar]
- Kawahara E., Ueda T., Nomura S. In vitro phagocytic activity of white-spotted char blood cells after injection with Aeromonas salmonicida extracellular products. Fish Pathol. 1991;26:213–214. [Google Scholar]
- Lacin E., Coban O., Aksu M.I., Sabuncuoglu N., Das H. The effects of different breeding methods on fattening performance and parameters related to slaughter, carcass and some meat quality in broiler chickens. Kafkas Vet. Fak. Derg. 2013;19:283–289. [Google Scholar]
- Li H., Wen X., Alphin R., Zhu Z., Zhou Z. Effects of two different broiler flooring systems on production performances, welfare, and environment under commercial production conditions. Poult. Sci. 2017;96:1108–1119. doi: 10.3382/ps/pew440. [DOI] [PubMed] [Google Scholar]
- Manohar G.R., Mani K., Viswanathan K. Carcass yields and meat cholesterol as influenced by stocking density and system of rearing in commercial broilers. Indian J. Poult. Sci. 2005;40:255–258. [Google Scholar]
- Matur E., Eraslan E., Akyazi I., Ergul Ekiz E., Eseceli H., Keten M., Metiner K., Aktaran Bala D. The effect of furnished cages on the immune response of laying hens under social stress. Poult. Sci. 2015;94:2853–2862. doi: 10.3382/ps/pev297. [DOI] [PubMed] [Google Scholar]
- Moravej H., Alahyari-Shahrasb M., Baghani M., Shivazad M. Withdrawal or reduction of the dietary vitamin premix on bone parameters of broiler chickens in two rearing systems. South Afr. J. Anim. Sci. 2012;42:169–177. [Google Scholar]
- NRC . 9th ed. National Academy Press; Washington, DC: 1994. Nutrition Requirements of Poultry. [Google Scholar]
- OIE A. 5th ed. OIE; Tokyo, Japan: 2009. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. [Google Scholar]
- Saeed M., Abbas G., Alagawany M., Kamboh A.A., Abd El-Hack M.E., Khafaga A.F., Chao S. Heat stress management in poultry farms: a comprehensive overview. J. Therm. Biol. 2019;84:414–425. doi: 10.1016/j.jtherbio.2019.07.025. [DOI] [PubMed] [Google Scholar]
- Santos F.d.O., Santos Junior A., Oviedo-Rondon E., Ferket P. Influence of housing system on growth performance and Intestinal health of Salmonella-challenged broiler chickens. Embrapa Suínos e Aves-Artigo em periódico indexado (ALICE) 2012 [Google Scholar]
- Shields S., Greger M. Animal welfare and food safety aspects of confining broiler chickens to cages. Animals. 2013;3:386–400. doi: 10.3390/ani3020386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Şimşek Ü.G., Erişir M., Ciftci M., Seven P.T. Effects of cage and floor housing systems on fattening performance, oxidative stress and carcass defects in broiler chicken. Kafkas Vet. Fak. Derg. 2014;20:727–733. [Google Scholar]
- Sogunle O., Egbeyale L., Bajomo T., Bamigboje O., Fanimo A. Comparison of the performance, carcass characteristics and haematological parameters of broiler chicks reared in cage and floor. Pak. J. Biol. Sci. 2008;11:480–483. doi: 10.3923/pjbs.2008.480.483. [DOI] [PubMed] [Google Scholar]
- Wang Y., Ru Y., Liu G., Chang W., Zhang S., Yan H., Zheng A., Lou R., Liu Z., Cai H. Effects of different rearing systems on growth performance, nutrients digestibility, digestive organ weight, carcass traits, and energy utilization in male broiler chickens. Livest. Sci. 2015;176:135–140. [Google Scholar]
- Willis W.L., Murray C., Talbott C. Campylobacter isolation trends of cage versus floor broiler chickens: a one-year study. Poult. Sci. 2002;81:629–631. doi: 10.1093/ps/81.5.629. [DOI] [PubMed] [Google Scholar]
- Zhao F.R., Geng A.L., Li B.M., Shi Z.X., Zhao Y.J. Effects of environmental factors on breast blister incidence, growth performance, and some biochemical indexes in broilers. J. Appl. Poult. Res. 2009;18:699–706. [Google Scholar]