Abstract
Understanding factors affecting ME availability for productive processes is an important step in optimal feed formulation. This study compared a modelling methodology with the comparative slaughter technique (CST) to estimate energy partitioning to heat production and energy retention (RE) and to investigate differences in heat dissipation. At hatch, 50 broilers were randomly allocated in one of 4 pens equipped with a precision feeding station. From day 14 to day 45, they were either fed with a low-ME (3,111 kcal/kg ME) or a high-ME (3,383 kcal/kg ME) diet. At day 19, birds were assigned to pair-feeding in groups of 6 with lead birds eating ad libitum (100%) and follow birds eating at either 50, 60, 70, 80, or 90% of the paired lead's cumulative feed intake. Heat production and RE were estimated by CST and with a nonlinear mixed model explaining daily ME intake (MEI) as a function of metabolic BW and average daily gain (ADG). The energy partitioning model predicted MEI = (145.10 + u) BW0.83 + 1.09 × BW−0.18 × ADG1.19 + ε. The model underestimated heat production by 13.4% and overestimated RE by 22.8% compared with the CST. The model was not able to distinguish between net energy for gain values of the diets (1,448 ± 18.5 kcal/kg vs. 1,493 ± 18.0 kcal/kg for the low-ME and high-ME diet, respectively), whereas the CST found a 148 kcal/kg difference between the low-ME and high-ME diets (1,101 ± 22.5 kcal/kg vs. 1,249 ± 22.0 kcal/kg, respectively). The estimates of the net energy for gain values of the 2 diets decreased with increasing feed restriction. The heat increment of feeding did not differ between birds fed with the low- or high-ME diet (26% of MEI). Additional measurements on heat dissipation, physical activity, and immune status indicated that the energetic content of the diet and feed restriction affect some parameters (shank temperature, feeding station visits) but not others (leukocyte counts, heterophil to lymphocyte ratio, and immune cell function).
Key words: maintenance, energetic modeling, net energy
Introduction
Efficient and sustainable poultry production requires accurate estimation of productive (retained) energetic values of feed ingredients and complete diets. Currently, metabolizable energy corrected for zero nitrogen retention (MEn) is the most commonly used energy value for ingredients and diets for the broiler industry. There is an ongoing debate between researchers on whether the industry would benefit from a net energy (NE) system over the ME system (Wu et al., 2018, Zuidhof, 2019) because of misunderstanding of definitions and disagreement on whether a NE system would enhance efficiency and profitability of diet formulation. As defined by Fraps and Carlyle (1939) and later by NRC (1981), the NE for gain (NEg) value for a feed, also called productive energy, is defined as the amount of energy stored by the chicken for a given amount of feed fed higher than that necessary for maintenance requirements. ME used for maintenance (MEm) is equivalent to total heat production (HP; Fraps and Carlyle, 1939, NRC, 1981, Latshaw and Moritz, 2009), therefore, NEg value for feed is equal to ME minus total HP from all sources divided by the weight of the feed consumed (Zuidhof, 2019). The purpose of the NEg value for feed is to characterize the quantity of the energy in the feed that is retained in the body and not released as heat. Net energy for gain is a property of the feed and expressed, for example, as kcal/kg.
Minimizing HP at the same level of ME intake will maximize the availability of energy for energy retention (RE; Zuidhof, 2019). Traditionally, it has been assumed that the energy requirement per unit of growth (g) is constant (Spratt et al., 1990, Rabello et al., 2006). Yet, depending on the composition and efficiency of energy retention (e.g., fat vs. lean tissue), energy partitioned to gain changes (Kielauowski, 1965). In addition, total HP can depend on the ingredients and composition of the diet. For example, the efficiency of the use of ME for gain was 45.4 kcal per 100 g gain greater in birds fed with diets containing sunflower oil than in those fed with isoproteic tallow–containing diets, which could be the result of a reduction in HP (Sanz et al., 2000). In addition, dietary NEg increased by 12.5% with supplementation of plant extracts carvacrol, cinnamaldehyde, and capsicum, which was hypothesized to result from a change in intestinal microbiome (Bravo et al., 2014). In addition, RE increased from 53.4 kcal/D in a control diet deficient in ME to 70.3 kcal/D in the same diet supplemented with phytase (Olukosi et al., 2008).
To evaluate the effects of animal, dietary, or environmental effects on HP, the partitioning of ME intake to HP and RE needs to be estimated. Total HP can be calculated indirectly by measuring RE through the comparative slaughter technique (CST; Fraps, 1946). Heat production can be also estimated through respiration calorimetry (Birkett and de Lange, 2001, Wu et al., 2018). Romero et al. (2009) proposed a mathematical method based on the work by Byerly et al. (1980) and Schulman et al. (1994), not assuming linearity and adjusting the energy requirement per unit of gain at different rates of gain. Mathematical modelling methods would be less invasive and less expensive as they do not require euthanizing animals (CST) or keeping them in respiratory units (respiration calorimetry). It would also be possible to relate estimated HP to ME intake per unit of metabolic BW and calculate a diet-specific heat increment of feeding (HIF), by comparing the slopes of the linear regression of individual HP on ME intake of different diets (Romero et al., 2011). Increased feed intake increases HIF (Liu et al., 2017). Heat increment of feeding is often expressed as a percentage of ME intake or in kcal and part of total HP (NRC, 1981). As level of feed intake can vary between individuals, quantifying ingredient- and nutrient-specific change in HIF can be an important measure to explain a portion of the HP that causes variation in ME availability for RE. Higher feed intake or higher ME intake in broilers fed with diets with an increased ME:CP ratio have sometimes led to increased total HP (Buyse et al., 1992), whereas others found reduced total HP (MacLeod, 1997). Heat increment of feeding has also been suggested to regulate voluntary feed intake in broilers (Swennen et al., 2004); however, this could not yet be confirmed (Swennen et al., 2006, Swennen et al., 2007). Yet, the literature has not studied diet-specific HIF at different levels of ME intake or diet composition. It was suggested that HIF would be higher for diets with a low ME:CP ratio at higher levels of intake. Overconsumption of CP over ME could result in deamination of excess amino acids releasing heat and an energy source for the bird (Musharaf and Latshaw, 1999, Gous and Morris, 2005).
Hence, the objective of this study was two-fold: 1) to evaluate the accuracy of a novel mathematical modelling methodology for energy partitioning to determine HP, RE, and NEg from ME intake compared with the CST and 2) to estimate diet-specific HIF by comparing the slope of the linear regression of HP on ME intake of 2 energetically different diets. It was hypothesized that the mathematical model would estimate similar values for HP and RE compared with the CST, including estimating a comparable NEg value of the diets. In addition, it was hypothesized that birds fed with the low-ME diet would have a higher HIF at increased levels of feed intake compared with the high ME treatment. Physiological adaptations affecting ME partitioning were investigated, which included evaluation of shank skin temperature and humoral immunological parameters. Body composition and feeding station visit frequency were evaluated to study the underlying potential causes of differences in total HP.
Material and methods
Experimental Design
The animal protocol for this study was approved by the Animal Care and Use Committee for Livestock of University of Alberta and followed principles established by the Guidelines and Policies of the Canadian Council on Animal Care (CCAC, 2009). The experiment was conducted as randomized block design of a 2 × 6 factorial arrangement of treatments with 50 broilers in 4 pens (blocks) fed with an isonitrogenous low-ME (3,111 kcal/kg ME) or a high-ME (3,383 kcal/kg ME) grower diet from day 14 and were provided ad libitum feeding or received 50, 60, 70, 80, 90% of ad libitum from day 19. The main experimental design was n = 25 per diet treatment with groups of birds fed at different levels. Pens were randomly assigned to the low-ME or high-ME grower diet, and birds within pens were randomly assigned restriction treatments. Individual bird was used as an experimental unit.
Animals and Housing
One-day-old Ross x Ross 308 feather-sexed male broilers purchased from Lilydale Hatchery (Edmonton, Alberta, n = 50), were randomly allocated in one of 4 wood shavings–covered floor pens, all equipped with a precision feeding (PF) station allowing individual feed distribution in a group housed setting, for detailed information refer to the study by Zuidhof et al., 2016, Zuidhof et al., 2017. At placement, birds were neck tagged for individual identification and trained to use the stations from 0 to 10 D of age. During this time, feeder space was limited. From day 0 to day 13, a starter diet was provided ad libitum. From day 14 to day 45, grower diets were fed at different levels using a PF station. At day 10, birds received a radio-frequency identification tag and were transitioned to individual feeding, which was fully implemented at day 14. To create a robust model, a wide range of energy intakes were implemented. At day 19, 2 birds per pen were assigned to ad libitum treatment and used as lead birds. Ten other birds per pen were coupled randomly to one of the 2 lead birds per pen and received 50, 60, 70, 80, or 90% of its lead's cumulative feed intake, creating graded levels of energy intake.
Experimental Diets
Diets were formulated on a least-cost basis and comparable with commercially available wheat–soybean meal–based diets in the Canadian Prairie provinces. The ingredient composition, calculated and analyzed ME, and nutrient content of the starter and grower diets are shown in Table 1. Celite was used as an index for digestibility determination to determine ileal energy digestibility.
Table 1.
Ingredient and nutritional composition of the starter (day 0–day 14) and grower (day 15–day 35) diets fed to broilers in the current experiment.
| Starter | Low-ME grower | High-ME grower | |
|---|---|---|---|
| Ingredient composition, g/kg | |||
| Corn, ground | 75.00 | 179.68 | 180.07 |
| Wheat, ground | 317.37 | 444.21 | 377.64 |
| Soybean meal (48% CP) | 175.00 | 289.49 | 310.12 |
| Faba beans, ground | 80.00 | - | - |
| Wheat cracks, ground | 80.00 | - | - |
| Wheat, whole (14.5% CP) | 75.00 | - | - |
| Meat and bone meal | 67.00 | - | - |
| Canola meal | 50.00 | - | - |
| Canola, whole | 40.00 | - | - |
| Animal fat | 22.00 | - | - |
| Canola oil | 22.51 | 68.30 | |
| Limestone | 5.00 | 10.12 | 9.92 |
| MHA1 | 2.70 | - | - |
| Salt, NaCl | 2.60 | 3.57 | 3.64 |
| Dicalcium Phosphate | - | 15.17 | 15.44 |
| L-Lysine HCL | 1.80 | 0.44 | - |
| Enzyme2 | 1.00 | - | - |
| Poultry trace mineral premix3 | 1.00 | - | - |
| Broiler vitamin premix3 | 1.00 | - | - |
| Broiler grower premix4 | - | 4.99 | 5.00 |
| Choline liquid 70% | 0.85 | - | - |
| Choline chloride premix5 | - | 4.99 | 5.00 |
| DL-Methionine | - | 1.29 | 1.36 |
| L-Threonine | 0.70 | 0.07 | - |
| Bacitracin MD | 0.50 | - | - |
| Monensin premix 20% | 0.50 | - | - |
| Coban | - | 0.50 | 0.51 |
| Vitamin E 5000 IU/kg | - | 3.00 | 3.00 |
| 25-OH Vitamin D3 | 0.40 | - | - |
| Copper sulfate | 0.40 | - | - |
| Ethoxyquin, 66% | 0.18 | - | - |
| Celite | - | 19.96 | 20.01 |
| Calculated composition, as fed basis | |||
| MEn, kcal/kg | 3,073 | 2,900 | 3,150 |
| CP, % | 23.16 | 22.00 | 22.00 |
| Lys, % | 1.25 | 1.12 | 1.12 |
| PCD6 Lys, % | 1.10 | 0.96 | 0.96 |
| PCD Met, % | 0.51 | 0.41 | 0.42 |
| PCD Met + Cys, % | 0.83 | 0.73 | 0.73 |
| Analyzed composition, as fed basis | |||
| Dry Matter | 87.8 | 87.3 | 86.1 |
| ME, kcal/kg | - | 3,111 | 3,383 |
| CP, % | 25.7 | 25.2 | 24.7 |
| Fat, % | 7.5 | 3.9 | 7.9 |
Methionine hydroxy analogue: 84% Ca salt of 2-hydroxy-4-(methylthio)butanoic acid, Novus International, Inc., St. Charles, MO.
Avizyme 1,302 feed enzyme for use in poultry diets containing at least 20% wheat (Danisco Animal Nutrition, Marlborough, Wiltshire, UK).
Combined poultry trace mineral premix and broiler vitamin premix contributed per kg of diet: vitamin A, 10,000 IU; vitamin D3, 4,000 IU; vitamin E, 50 IU; vitamin K3, 3.1 mg; riboflavin, 10 mg; thiamine, 2 mg; pyridoxine, 5 mg; vitamin B12, 0.02 mg; niacin, 65 mg; D-pantothenic acid, 15 mg; folic acid, 2.0 mg; biotin, 0.2 mg; iron, 80 mg; copper, 15 mg; manganese, 110 mg; zinc, 100 mg; iodine, 2 mg; selenium, 0.3 mg.
Contributed per kg of diet: vitamin A, 10,000 IU; vitamin D3, 4,000 IU; vitamin E, 50 IU; vitamin K3, 4 mg; riboflavin, 10 mg; thiamine, 4 mg; pyridoxine, 5 mg; vitamin B12, 0.02 mg; niacin, 65 mg; D-pantothenic acid, 15 mg; folic acid, 2.0 mg; biotin, 0.2 mg; iron, 80 mg; copper, 20 mg; manganese, 120 mg; zinc, 100 mg; iodine, 1.65 mg; selenium, 0.3 mg; choline, 2.64 mg.
Contributed per kilogram of diet 400 mg/kg choline.
Prececal digestible.
Data Collection
From day 0 to day 13, birds were weighed manually on a daily basis to ensure growth and verify the use of the PF system. Birds that were not gaining weight or were gaining weight slowly were trained individually. After individual feeding had been fully implemented at day 14, the PF system recorded individual BW and feed intake on a per visit basis. Feed intake and visit frequency was checked on a daily basis to ensure all birds were accessing the PF system. Shank temperature measurements were taken from all birds on day 22, 28, 35, and 42 with a handheld infrared camera. The highest temperature detected by the camera was recorded, focused on the posterior side of the shank area. The camera recorded the exact time the temperature measurement was taken, and this was aligned with feed intake data from the PF system. At day 45, 3 mL blood samples were collected in EDTA-coated vacutainer tubes from the brachial vein of each bird and shortly after, all birds were killed by cervical dislocation. All birds that died or were culled during the experiment were recorded (n = 1). The abdominal fat pad (including fat adhering to the proventriculus and gizzard), filled gastrointestinal tract (GIT), breast muscle (combined pectoralis major and pectoralis minor), heart, legs without skin (combined thigh and drum), and liver weight were recorded during dissection. Intestinal content was collected from the distal part of the ileum and stored at −20°C before analysis. After removal of all intestinal content, the empty GIT was weighed. The GIT consisted of the complete digestive tract including the pancreas, from 2 cm anterior to the crop up to but not including the bursa, with fat adhering to the proventriculus and gizzard removed. The sex of each bird was confirmed by visual inspection of the gonads at the time of dissection. All the dissected parts including empty carcass were collected in plastic bags and stored by −20°C before further sample processing.
Carcass and Digesta Composition Analysis
After pressure cooking and grinding of complete carcass, representative subsamples were taken and stored at −20°C before proximate analysis. Samples were dried at 60°C to determine carcass moisture. Dried samples were reground in a coffee grinder before energy content measurement and proximate analysis. Duplicate 1-g pellets of dried carcass sample were analyzed for energetic content in a bomb calorimeter (IKA Calorimeter System with C5000 control). Carcass samples were analyzed in duplicate for determination of total carcass dry matter, crude protein, lipid, and ash using standard chemical analysis procedures (Horwitz, 1980). Ileal digesta samples were pooled per restriction treatment within diet treatment. Dried ileal digesta samples and feed samples were analyzed following the same protocol. In addition, acid insoluble ash was analyzed in ileal digesta and feed samples. Samples were burned at 500°C overnight and then hydrolyzed with 4 M HCl at 110°C for 2 h. After centrifuging at 3,000 rpm for 8 min at 20°C, supernatant was discarded, and ash was burned overnight at 500°C. Acid insoluble ash was calculated as the weight of the ash divided by the dry matter weight of the initial sample times 100%. The ME value of the diets was calculated by the following equation (Scott and Boldaji, 1997):
where GE is the gross energy (kcal/kg) of the sample and AIA is the concentration of acid insoluble ash in the sample, all expressed on a dry matter basis.
Leukocytes
Peripheral blood leukocyte composition analysis was only performed on samples from the most extreme feed intake treatments – the 50% feed restricted and ad libitum fed birds. Directly after collection, blood smears were stained using the Hema 3 staining set (Fisher Scientific) as per the manufacturer's specifications. Slides were air-dried before observation by bright field microscopy. Photomicrographs were taken using a Leica DM1000 microscope, and images were acquired using QCapture software. Two hundred fifty cells were counted to estimate the heterophil/lymphocyte ratio.
Energy Partitioning Methods
Two methods were used to determine HP and RE in this study, the CST and a mathematical model explaining energy intake as a function of BW and gain. For the CST carcass gross energy, content at day 14 was estimated from individual live weight using the regression equations from the study carried out by Wolynetz and Sibbald (1985) based on 10-day-old broilers, where total carcass energy (kcal) = −181.2 kcal + 1,995.9 kcal/kg × BW (kg). For each individual, RE was calculated by subtracting the estimated carcass gross energy content at day 14 from the measured carcass gross energy content at day 45. Individual total HP was calculated as follows:
where FI is feed intake (g) over the experimental period (day 14–day 45) and MEdiet is the analyzed ME content of the diet (kcal/g). The mathematical model used to predict energy partitioning to HP and RE was based on previous work of Romero et al. (2009) and used by others (Pishnamazi et al., 2008, Hadinia et al., 2018). The following model was defined in the NLMIXED procedure in SAS (version 9.4.; SAS Institute Inc., Cary, NC, 2012): MEId = (a + u) × BWb + c × BWd × ADGe + ε, u ∼ N(0, Vu), MEId ∼ N(μ,V), where MEId = daily ME intake (kcal/D), BW = body weight (kg), ADG = average daily gain (g/D) calculated over a 4-D period, ε = residual error. The random term u was associated with each bird, variance parameters V and Vu were estimated in the regressions. The estimated equation was MEId = (145.10 + u) BW0.83 + 1.09 × BW−0.18 × ADG1.19 (P < 0.001 for all parameters; Table 2). The first part of the equation, (145.10 + u) × BW0.83, represented the partitioning of the daily ME intake towards maintenance, that is, HP. The second part of the equation, 1.09 × BW−0.18 × ADG1.19, reflected the partitioning of daily ME intake towards gain, that is, RE. Estimated HP and RE per 4-D period were summed to reflect total HP and total RE over the experimental period (day 14–day 45). For both the CST as the model method, NEg of the diets (kcal/kg) was calculated by dividing RE by the cumulative feed intake over the experimental period.
Table 2.
Regression coefficients of the nonlinear model1 estimating daily ME intake as a function of BW and average daily gain.
| Parameter | Estimate | SEM | t-value | P > t |
|---|---|---|---|---|
| a | 145.00 | 8.48 | 17.10 | <0.001 |
| b | 0.83 | 0.04 | 19.06 | <0.001 |
| c | 1.09 | 0.37 | 2.97 | 0.005 |
| d | −0.18 | 0.05 | -3.75 | <0.001 |
| e | 1.19 | 0.07 | 17.07 | <0.001 |
| V | 399.39 | 32.69 | 12.22 | <0.001 |
| Vu | 151.41 | 44.79 | 3.38 | 0.001 |
Equation: MEId = (a + u) BWb + 1.09 × BWd × ADGe MEId ∼ N(μ,V), u ∼ N(0, Vu), where MEId = daily ME intake (kcal/D), BW = body weight (kg), and ADG = average daily gain (g/D). Bayesian information criterion = 3,422.
Statistical Analysis
Animals that had to be culled before the end of the experiment because of a neurological abnormality (crooked neck, n = 1) and sexing errors (females, n = 2) were removed from the data set for all analyses. All ANOVA were conducted using the MIXED procedure of SAS with the Kenward–Roger method specified in the DDFM option (version 9.4.; SAS Institute Inc., Cary, NC, 2012). Tukey's range test was used to compare treatment means, and differences were considered significant at P ≤ 0.05. The model used for BW at day 45, cumulative feed intake, cumulative energy intake, cumulative gain, and cumulative feed conversion ratio included diet treatment, feed restriction treatment, and their interaction as fixed effects. The model used for dissection and carcass composition data included diet treatment, feed restriction treatment, and their interaction as fixed effects and BW as a covariate to account for BW differences. The model used to determine difference in HP and RE between the diets, the difference between the mathematical model and CST method in HP and RE included the diet treatment, and the difference between NEg included the diet treatment and feed restriction treatment and their interaction as fixed effects. The difference in HIF between the diets was tested by evaluating the slope of the linear regression of individual daily HP per metabolic BW on average daily ME intake per metabolic BW for the modeling method. The first iteration used a model including diet treatment as a fixed effect, ME intake as a covariate, and their interaction. Because the interaction was not significant for either methods, it was omitted from the model, and the results show the equation with diet treatment as a fixed effect, and ME intake per metabolic BW as a covariate. The model used to test the effect of diet treatments on shank temperature included age, diet treatment, and their interaction as fixed effects and a covariate for ME intake during the 6, 12, 24, or 48 h before the temperature measurement. The model used to test differences between the number of station visits per 24 h, the number of meals per 24 h, the meal to visit ratio, and meal size, included age, diet treatment, and feed restriction treatment as fixed effects and all their interactions. The model used to test differences in percentages of leukocytes included diet treatment as a fixed effect and ME intake from day 14 to day 45 as a covariate.
Results and discussion
Diet Analysis and Bird Performance
The analyzed ME content of the grower diets was higher than the originally calculated composition (Table 1; 3,111 vs. 2,900 kcal/kg for the low-ME diet and 3,383 vs. 3,150 kcal/kg for the high-ME diet). The diets were formulated on MEn basis. The differences between analyzed and formulated energy levels could have resulted from variation in feed ingredients ME or be part of the nitrogen correction. However, as it was intended to create a difference in ME and the actual ME difference between the diets was 272 kcal/kg, it was not expected that this would alter the inference. BW at day 45 did not differ between birds fed with the high-ME diet and the low-ME diet (Table 3). This is consistent with results from the study by Leeson et al. (1996), who found that BW at day 49 did not differ between ad libitum fed broilers fed with diets ranging in ME between 2,700 kcal/kg and 3,300 kcal/kg. As anticipated, restricting feed intake reduced BW and gain to day 45 (Table 3, P < 0.001). Cumulative feed intake from day 14 to day 45 was lower in birds fed with the high-ME diet than in birds fed with the low-ME diet (2,988 g vs. 3,099 g, P = 0.047). However, cumulative ME intake was higher in the high-ME treatment than that in the low-ME treatment (10,108 kcal vs. 9,641 kcal, P = 0.012). Earlier studies concluded that broilers were able to control their feed intake in ad libitum situations based on desire to normalize energy intake (Leeson et al., 1996); hence, with an increment of dietary ME, feed intake was reduced. Other studies concluded that broiler fed with a diet with a higher ME:CP ratio overconsumed ME to meet CP requirements (Swennen et al., 2004). As the diets were isonitrogenous, the high-ME diet had a higher ME:CP ratio than the low-ME diet (13.70 kcal/g vs. 12.35 kcal/g). Therefore, birds fed with the high-ME diet could have overconsumed ME to meet their CP requirement. In the present study, the ad libitum fed birds were paired with feed-restricted birds, thus feed intake differences between ad libitum fed birds fed with the high-ME or the low-ME diets were also imposed on the feed-restricted birds.
Table 3.
BW at day 45, cumulative feed intake (CFI), total ME intake (MEI), cumulative gain (Gain), and feed conversion ratio (FCR) of broilers fed with either a low-ME (3,111 kcal/kg) or a high-ME (3,383 kcal/kg) diet from day 14 to day 45.1
| Effect | Diet | Feed intake | BW (g) | SEM | CFI (g) | SEM | MEI (kcal) | SEM | Gain (g) | SEM | FCR | SEM |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diet | Low ME | 2,280 | 36.2 | 3,099a | 38.6 | 9,641b | 126 | 1,881 | 34.6 | 1.659 | 0.0168 | |
| High ME | 2,261 | 35.2 | 2,988b | 37.6 | 10,108a | 123 | 1,863 | 33.7 | 1.616 | 0.0163 | ||
| Feed intake | 50% | 1,639f | 65.2 | 2,071f | 69.6 | 6,721f | 228 | 1,227f | 62.3 | 1.694 | 0.0302 | |
| 60% | 1,843e | 65.2 | 2,444e | 69.6 | 7,924e | 228 | 1,468e | 62.3 | 1.666 | 0.0302 | ||
| 70% | 2,156d | 60.4 | 2,870d | 64.5 | 9,310d | 211 | 1,749d | 57.7 | 1.643 | 0.0279 | ||
| 80% | 2,414c | 65.2 | 3,247c | 69.6 | 10,531c | 228 | 2,013c | 62.3 | 1.619 | 0.0302 | ||
| 90% | 2,675b | 60.4 | 3,622b | 64.5 | 11,751b | 211 | 2,280b | 57.7 | 1.593 | 0.0279 | ||
| 100% | 2,896a | 54.0 | 4,008a | 57.7 | 13,012a | 189 | 2,494a | 51.6 | 1.609 | 0.0250 | ||
| Source of variation | ––––––––––––––––––––––––––––––––– P - value –––––––––––––––––––––––––––––––– | |||||||||||
| Diet | 0.70 | 0.047 | 0.012 | 0.70 | 0.07 | |||||||
| Feed intake | <0.001 | <0.001 | <0.001 | <0.001 | 0.16 | |||||||
| Diet × Feed intake | 0.92 | 0.97 | 0.75 | 0.95 | 0.70 | |||||||
a-fLSMeans within column and effect lacking a common superscript differ (P ≤ 0.05).
Birds were pair-fed through a precision feeding system with lead birds eating ad libitum (100%) and followers were allowed to eat either 50, 60, 70, 80, or 90% of the paired lead's cumulative feed intake.
BW-corrected breast muscle (P = 0.028) and liver weight (P = 0.002) were higher in birds fed with the low-ME diet than those in birds fed with the high-ME diet (Table 4). BW-corrected fat pad weight was higher in birds fed with the high-ME diet (P = 0.014). However, feed intake treatment did not affect any weights of the BW-corrected carcass components. Carcass crude fat percentage was higher in birds fed with the high-ME diet than that in birds fed with the low-ME diet (8.8 vs. 7.1%, P < 0.001; Table 5), and crude fat percentages increased gradually with increasing feed intake (P < 0.001). BW-corrected fat pad weight was the same for all feed intake treatments; therefore, the increase in crude fat retention could have occurred in other body tissues. Overall, bird performance was consistent with the literature investigating differences in dietary energy and feed restriction (Leeson et al., 1996, Swennen et al., 2004).
Table 4.
Individual BW-corrected breast, fat pad, liver, legs without skin, heart, gastro-intestinal tract (GIT), and empty GIT weight of broilers fed either a low-ME (3,111 kcal/kg) or high-ME (3,383 kcal/kg) diet from day 14 to day 45.1
| Effect | Diet | Feed intake | Breast (g) | SEM | Fat pad (g) | SEM | Liver (g) | SEM | Legs (g) | SEM | Heart (g) | SEM | GIT (g) | SEM | Empty GIT (g) | SEM |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diet | Low ME | 477a | 6.3 | 14.2b | 1.9 | 43.5a | 0.85 | 475 | 6.5 | 9.7 | 0.5 | 181 | 8.4 | 119 | 3.4 | |
| High ME | 457b | 6.3 | 21.0a | 1.9 | 39.6b | 0.84 | 478 | 6.5 | 10.3 | 0.5 | 167 | 8.4 | 120 | 3.4 | ||
| Feed intake | 50% | 415 | 22.7 | 21.3 | 6.9 | 46.2 | 3.04 | 467 | 23.3 | 9.2 | 1.8 | 230 | 30.3 | 146 | 12.2 | |
| 60% | 432 | 17.8 | 17.7 | 5.4 | 43.6 | 2.39 | 462 | 18.3 | 9.0 | 1.4 | 205 | 23.7 | 127 | 9.6 | ||
| 70% | 451 | 11.4 | 18.7 | 3.5 | 41.1 | 1.53 | 479 | 11.7 | 10.3 | 0.9 | 182 | 15.2 | 117 | 6.1 | ||
| 80% | 478 | 11.5 | 14.1 | 3.5 | 41.6 | 1.55 | 474 | 11.9 | 10.4 | 0.9 | 173 | 15.4 | 120 | 6.2 | ||
| 90% | 506 | 14.6 | 20.5 | 4.4 | 38.2 | 1.97 | 475 | 15.0 | 11.2 | 1.1 | 142 | 19.5 | 110 | 7.9 | ||
| 100% | 521 | 19.1 | 13.4 | 5.8 | 38.6 | 2.57 | 499 | 19.7 | 9.9 | 1.5 | 114 | 25.6 | 96 | 10.3 | ||
| Covariable | –––––––––––––––––––––––––––––––––––––– BW (g/kg) ––––––––––––––––––––––––––––––––––––––– | |||||||||||||||
| BW | 162 | 29.0 | 23.8 | 8.8 | 25.2 | 3.90 | 187 | 29.8 | 4.3 | 2.3 | 125 | 38.7 | 56 | 15.6 | ||
| Source of variation | ––––––––––––––––––––––––––––––––––––––– P - value –––––––––––––––––––––––––––––––––––––––– | |||||||||||||||
| Diet | 0.028 | 0.014 | 0.002 | 0.73 | 0.42 | 0.26 | 0.78 | |||||||||
| Feed intake | 0.16 | 0.60 | 0.51 | 0.69 | 0.72 | 0.36 | 0.16 | |||||||||
| Diet × Feed intake | 0.82 | 0.91 | 0.36 | 0.92 | 0.51 | 0.39 | 0.44 | |||||||||
| BW | <0.001 | 0.011 | <0.001 | <0.001 | 0.063 | 0.003 | 0.001 | |||||||||
a,bLSMeans within column and effect lacking a common superscript differ (P ≤ 0.05).
Birds were pair-fed through a precision feeding system with lead birds eating ad libitum (100%) and followers were allowed to eat either 50, 60, 70, 80, or 90% of the paired lead's cumulative feed intake.
Table 5.
Carcass crude protein (CP), crude fat (CF), ash, and moisture as percentage of BW at day 45 of broilers fed with either a low-ME (3,111 kcal/kg) or a high-ME (3,383 kcal/kg) diet from day 14 to day 45.1
| Effect | Diet | Feed intake | CP (%) | SEM | CF (%) | SEM | Ash (%) | SEM | Moisture (%) | SEM |
|---|---|---|---|---|---|---|---|---|---|---|
| Diet | Low ME | 20.5 | 0.13 | 7.1b | 0.26 | 3.1 | 0.05 | 70.2a | 0.34 | |
| High ME | 20.8 | 0.12 | 8.8a | 0.26 | 3.1 | 0.04 | 68.1b | 0.33 | ||
| Feed intake | 50% | 20.6 | 0.23 | 5.7c | 0.47 | 3.2 | 0.08 | 71.4a | 0.61 | |
| 60% | 20.6 | 0.23 | 6.9b,c | 0.47 | 3.2 | 0.07 | 69.9a,b | 0.61 | ||
| 70% | 20.7 | 0.21 | 7.8b | 0.44 | 3.1 | 0.08 | 69.5b | 0.57 | ||
| 80% | 20.7 | 0.23 | 7.6b | 0.47 | 3.2 | 0.08 | 69.4b | 0.61 | ||
| 90% | 20.6 | 0.21 | 9.6a | 0.44 | 3.0 | 0.07 | 67.6c | 0.57 | ||
| 100% | 20.6 | 0.19 | 10.1a | 0.39 | 2.9 | 0.06 | 67.1c | 0.51 | ||
| Source of variation | –––––––––––––––––––––––––– P - value ––––––––––––––––––––––––––– | |||||||||
| Diet | 0.072 | <0.001 | 0.529 | <0.001 | ||||||
| Feed intake | 0.995 | <0.001 | 0.077 | <0.001 | ||||||
| Diet × Feed intake | 0.50 | 0.76 | 0.47 | 0.83 | ||||||
a-cLSMeans within column and effect lacking a common superscript differ (P ≤ 0.05).
Birds were pair-fed through a precision feeding system with lead birds eating ad libitum (100%) and followers were allowed to eat either 50, 60, 70, 80, or 90% of the paired lead's cumulative feed intake.
Energy Partitioning and Net Energy
The nonlinear mixed model underestimated HP by 13.4% and overestimated RE by 22.8% compared with the CST (Table 6). Nonetheless, neither method detected differences in HP and RE between the low- and high- ME diet. Figure 1 and Figure 2 show the relationship between the model methodology and the CST in determining individual measurements for HP and RE. The model estimated the NEg of the feed 31.5% higher for the low-ME diet and 19.5% higher for the high-ME diet compared with the CST (Table 7). The NEg values estimated with the CST were on average 615 kcal/kg lower than the results from the studies by Fraps and Carlyle (1939) and Fraps (1946). Fraps (1946) found an average NEg value of 1,938 kcal/kg using the CST for an all mash grower diet. The NEg values calculated from reported feed intake and RE from a more recent publication from Wu et al. (2018) ranged from 1,258 to 1,407 kcal/kg in 3 different experiments, which is 83 to 232 kcal/kg higher than, but similar to, the current results. Wu et al. (2018) used Ross 308 broilers, the same strain as the current experiment. It needs to be considered that since 1946 (Fraps, 1946), broilers have been bred intensively for growth and efficiency and feed ingredients have changed over the years, which may have affected the biological energetic efficiency of broilers. For example, residual MEm (a measurement of biological energetic efficiency) was lower in a 1978 broiler strain than in a 1957 strain (Zuidhof et al., 2014). Thus, it would have been expected that the NEg content of the diet would have increased as broilers became more efficient. However, previous studies only used ad libitum fed birds to determine NEg content of the diet, whereas our present study used several levels of feed intake. Even though the NEg calculation corrected for individual feed intake, feed restriction reduced the NEg value of the feed (Table 7). The reason for this could be that a higher proportion of ME goes towards maintenance when gain is constrained. Following this reasoning, environmental factors limiting feed intake therefore decrease NEg of the feed.
Table 6.
Heat production (HP) and retained energy (RE) for broilers fed either a low-ME (3,111 kcal/kg) or a high-ME (3,383 kcal/kg) diet from day 14 to day 45 as calculated with the comparative slaughter technique (CST) or a mathematical nonlinear model (model1) and the difference between the model and the CST method (Δ).
| Diet | HP |
RE |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CST | SEM | Model | SEM | Δ | SEM | CST | SEM | Model | SEM | Δ | SEM | |
| ––––––––––––––––––––––––––––––––––––––––––– kcal ––––––––––––––––––––––––––––––––––––––––––– | ||||||||||||
| Low ME | 6,264 | 234 | 5,301 | 217 | −963b | 68 | 3,606 | 265 | 4,659 | 261 | 1,053a | 67 |
| High ME | 6,387 | 229 | 5,659 | 213 | −728a | 67 | 3,951 | 259 | 4,620 | 256 | 669b | 66 |
| Source of variation | –––––––––––––––––––––––––––––––––––––––––––––– P - value –––––––––––––––––––––––––––––––––––– | |||||||||||
| Diet | 0.71 | 0.24 | 0.018 | 0.36 | 0.91 | <0.001 | ||||||
a,bLSMeans within column lacking a common superscript differ (P < 0.05).
The estimated equation was MEId = (145.10 + u) BW0.83 + 1.09 × BW−0.18 × ADG1.19 and u ∼ N(0, Vu), MEId ∼ N(μ,V), where MEId = daily ME intake (kcal/D), BW = body weight (kg), and ADG = average daily gain (g/D). The error term u was associated with each bird, variance parameters V and Vu were estimated in the regressions. The first part of the equation, (145.10 + u) × BW0.83, represented HP, the second part of the equation (1.09 × BW−0.18 × ADG1.19) represented RE. Estimated HP and RE per period were summed to reflect total HP and total RE over the experimental period (day 14 to day 45).
Figure 1.
Retained energy (RE) estimated by a nonlinear equation explaining ME intake as a function of metabolic BW and gain (model) compared with the RE estimated by the comparative slaughter technique (CST) of broilers fed with either a low-ME (3,111 kcal/kg) or high-ME (3,383 kcal/kg) diet from day 14 to day 45, where the model overestimated RE. The solid gray line indicates where the model would have estimated the same value as the CST.
Figure 2.
Total heat production (HP) estimated by a nonlinear equation explaining ME intake as a function of metabolic BW and gain (model) versus that calculated through the comparative slaughter technique (CST) of broilers fed with either fed a low-ME (3,111 kcal/kg) or high-ME (3,383 kcal/kg) diet from day 14 to day 45, where the model underestimated HP. The solid gray line indicates where the model would have estimated the same value as the CST.
Table 7.
Net energy for gain (NEg) value of the feed for broilers fed with either a low-ME (3,111 kcal/kg) or a high-ME (3,383 kcal/kg) diet from day 14 to day 45 as calculated with the comparative slaughter technique (CST) or a mathematical nonlinear model (model1) and the difference between the model and the CST method (Δ).2
| Effect | Diet | Feed intake | NEg model | SEM | NEg CST | SEM | ΔNEg | SEM |
|---|---|---|---|---|---|---|---|---|
| ––––––––––––––––––––––––––––– kcal/kg ––––––––––––––––– | ||||||||
| Diet | Low ME | 1,448 | 18.5 | 1,101b | 22.5 | 346a | 20.1 | |
| High ME | 1,493 | 18.0 | 1,249a | 22.0 | 244b | 19.6 | ||
| Feed intake | 50% | 1,367d | 33.4 | 996c | 40.6 | 371a | 36.3 | |
| 60% | 1,423c,d | 33.4 | 1,099b,c | 40.6 | 324a,b | 36.3 | ||
| 70% | 1,454b,c,d | 30.9 | 1,163b | 37.6 | 291a,b,c | 33.6 | ||
| 80% | 1,500a,b,c | 33.4 | 1,169b | 40.6 | 331a,b | 36.3 | ||
| 90% | 1,543a | 30.9 | 1,307a | 37.6 | 237b,c | 33.6 | ||
| 100% | 1,534a,b | 27.6 | 1,317a | 33.6 | 216c | 30.1 | ||
| Source of variation | –––––––––––––––––––––––––––––– P - value ––––––––––––––– | |||||||
| Diet | 0.09 | <0.001 | 0.001 | |||||
| Feed intake | 0.002 | <0.001 | 0.020 | |||||
| Diet x Feed intake | 0.82 | 0.87 | 0.78 | |||||
a-dLSMeans within column and effect lacking a common superscript differ (P ≤ 0.05).
The estimated equation was MEId = (145.10 + u) BW0.83 + 1.09 × BW−0.18 × ADG1.19 and u ∼ N(0, Vu), MEId ∼ N(μ,V), where MEId = daily ME intake (kcal/D), BW = body weight (kg), and ADG = average daily gain (g/D). The error term u was associated with each bird, variance parameters V and Vu were estimated in the regressions. The first part of the equation, (145.10 + u) × BW0.83, represented HP, the second part of the equation (1.09 × BW−0.18 × ADG1.19) represented RE. Estimated HP and RE per period were summed to reflect total HP and total RE over the experimental period (day 14–day 45).
Birds were pair-fed through a precision feeding system with lead birds eating ad libitum (100%) and followers were allowed to eat either 50, 60, 70, 80, or 90% of the paired lead’s cumulative feed intake.
This is the first time that a nonlinear mixed model was evaluated against the CST for calculating NEg values for diets. Comparing the current results to the literature is challenging as many authors do not properly define or calculate the NEg value of feeds. In some literature, NEg has also been defined as NEg plus the energy requirement for maintenance of the body in healthy, fasting, nonreproductive, nonmoving, and thermal neutral state (NE for maintenance [NEm], basal metabolic rate, or fasting heat production), divided by the amount of feed consumed (Carré et al., 2014, Noblet et al., 2015, Wu et al., 2018). Or otherwise stated, NEg is the ME minus the heat increment, where heat increment is the heat produced in excess of NEm. However, NEm is affected by animal and environmental factors (Liu et al., 2017). In addition, it is very resource intensive to define NEm and practically not relevant to measure. More of interest is the effect of the diet on MEm, which varies with feeding level, environmental temperature, activity, immune status, and any other factor that could affect HP more than NEm. In the current experiment, the requirement for MEm was 145.10 kcal/BW0.83. Considering a BW range from 0.5 to 1.5 kg (82–203 kcal), the estimate for MEm is similar to estimations in the literature of 81 to 187 kcal (Noblet et al., 2015) but lower compared others of 117 with 266 kcal (Zuidhof et al., 2014). However, for higher BW (1.5–3.0 kg), the current estimates of MEm (258–361 kcal) were higherthan estimates of 214 to 304 kcal (Noblet et al., 2015) but still, lower than estimates of 330 to 447 kcal (Zuidhof et al., 2014). The current nonlinear mixed model may have partitioned ME not completely accurately to HP and RE. The model may have partitioned energy used for gain but lost as heat, towards the second part of the equation, 1.09 × BW−0.18 × ADG1.19, as this energy is required to establish gain. However, energy used for product formation is theoretically included in the portion of MEm (Zuidhof, 2019). Further studies are needed to improve the current model, potentially providing a solution to the previously described issue. Figure 3 shows the average ME requirement per g of gain as a function of BW and average daily gain. There was a decrease in the ME requirement per g of gain with increasing BW, especially at low levels of gain. This is in contrast with Romero et al. (2009), who found an increment in the ME requirement for gain at greater BW in adult broiler breeders. It is hypothesized that either 1) the efficiency of gain increased in juvenile birds with increased BW or 2) juvenile birds predominantly deposited lean tissue at very low gain and high BW in the current situation of severe feed restriction. The energy density of lean issue is lower than fat because protein has a lower energy content than fat (5.5 vs. 9.2 kcal/g) and because lean tissue contains about 75% water (Leeson and Summers, 2001). As Hadinia et al. (2018) calculated, the energy requirement per g of lean tissue is approximately 1.38 kcal. This is consistent with the current estimates in Figure 3 at high BW and low gain. In addition, birds with low gain had decreased carcass crude fat content and increased moisture content compared with ad libitum fed birds and similar CP content (Table 5); hence, the relative deposition of lean tissue would have been higher in the most feed restricted treatment. Consistent with Romero et al. (2009), ME requirement of gain increased with greater gains, likely because the relative deposition of fat increased, resulting in an increase in the ME requirement of gain.
Figure 3.
ME requirement per gram of average daily gain (ADG), as a function of body weight (BW) and ADG, as predicted by a nonlinear model explaining ME intake as a function of metabolic BW and gain of broilers from day 14 to day 45. The estimated equation was MEId = (145.10 + u) BW0.83 + 1.09 × BW−0.18 × ADG1.19 and u ∼ N(0, Vu), MEId ∼ N(μ,V), where MEId = daily ME intake (kcal/D), BW = body weight (kg), and ADG = average daily gain (g/D). The error term u was associated with each bird. The second part of the equation (1.09 × BW−0.18 × ADG1.19) represented retained energy (gain) per day.
Heat Increment of Feeding
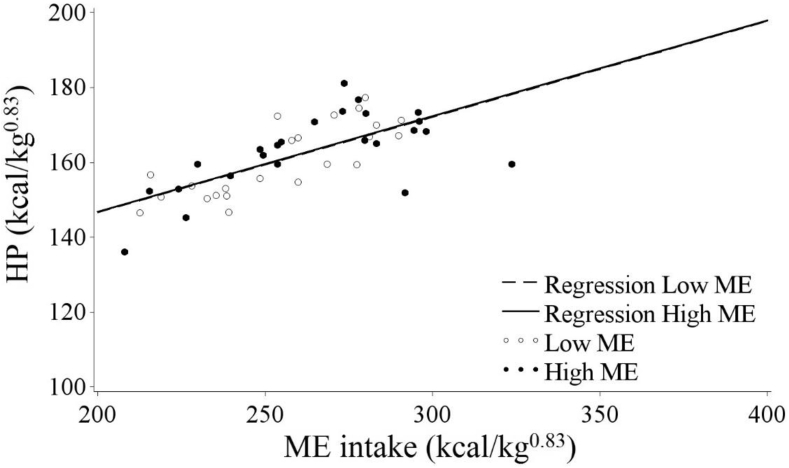
To investigate diet-specific HIF, the slopes of the linear regression of individual HP on ME intake of both diets were compared. Figure 4 shows the relationship between the ME intake and HP, and the regression lines for the low-ME and high-ME diets did not differ (intercept P = 0.23; slope P = 0.24). As HIF did not differ significantly between diets, the difference in slope was omitted in the final analysis. The HIF was estimated at 26% of the ME intake. This is consistent with results from Swennen et al. (2004), who found that the HIF did not differ between isoenergetic diets with low protein (12.6% CP, 10.6% fat) or low fat (24.4% CP; 0.4% fat) content. The HIF for those diets was estimated between 20 and 23% of the ME intake. Geraert et al. (1990) found HIF to be between 15.9 and 20.9% of the ME intake for diets differing in protein content, and they also concluded that HIF did not significantly differ between the diets. Koh and Macleod (1999) found a wider range of the HIF between 7.3 and 35.9% of ME intake depending on ambient temperature, but they did not report diet composition. Although the method of determining HIF in the previously mentioned studies (indirect calorimetry in respiratory cells) differed largely from the current methodology (using a mathematical approach), the outcomes of the current modeling methodology are in the same range.
Figure 4.
Linear regression of heat production (HP) and average daily ME intake (ME intake) per unit of metabolic BW (kg0.83) as estimated by the comparative slaughter technique of broilers fed with either a low-ME (3,111 kcal/kg) or high-ME (3,383 kcal/kg) diet from day 14 to day 45. Linear regression equations were: HP = 95.64 kcal + 0.26 × ME intake for the Low ME diet and HP = 95.44 kcal + 0.26 × ME intake for the High ME diet.
As mentioned earlier, it was suggested that HIF would be higher for diets with a low ME:CP ratio at higher levels of intake. Overconsumption of CP over ME could result in deamination of excess amino acids releasing heat and an energy source for the bird (Musharaf and Latshaw, 1999, Gous and Morris, 2005). However, the difference in dietary energy or protein content may have not been large enough to detect such an effect.
Heat Dissipation
Broilers can manage heat loss via thermoregulatory physiological responses. The temperature of the shank was used as an indicator of the control of heat transfer to their environment through conduction, radiation, and convection (Richards, 1971). There was a significant positive relationship between the temperature of the shank and the energy intake 6, 12, 24, or 48 before the measurement, which varied between 0.85°C/100 kcal and 2.74°C/100 kcal (Table 8). This indicated that increased energy intake resulted in an increment in shank temperature. Zhou and Yamamoto (1997) found that shank temperature increased with 0.26°C/100 kcal. The difference between results from Zhou and Yamamoto (1997) and the current result may originate from the difference in study design; Zhou and Yamamoto (1997) used short-term feed restriction on 49- to 70-day-old ad libitum fed broilers, whereas the present study used longer term feed restriction at a younger age. Birds fed with the low-ME diet had on average a 0.72°C lower shank temperature compared with birds fed with the high-ME diet (Table 8). It could be possible that birds regulated the heat loss through regulating blood flow through the skin on their shank (Richards, 1971), where birds fed with the low-ME diet were losing relatively less heat compared with birds fed with the high-ME diet. The differences in shank temperature may have also been related to bird fatness because of the insulative properties of fat in the body skin. Skin fat accounts for 60% of total body fat of ad libitum fed broiler chickens and 6.1 to 7.5% of the total BW (Ferrini et al., 2008). In addition, birds fed with diets with lower GE:CP ratios (14–18 kcal/g) had a reduced hypodermis thickness compared with birds fed with diets with a higher GE:CP ratio (16–20 kcal/g), which was linked to a decreased adipose tissue deposition in the skin (Kafri et al., 1986). In the current experiment, the low-ME birds had less fat as a percentage of their BW compared with high-ME birds (Table 5). Birds fed with the low-ME diet, with a lower ME:CP ratio, may have had to reduce heat loss through their shanks to compensate for the relative higher heat loss through the body skin compared with birds fed with the high-ME diet. However, it is unclear how quantitative feed restriction affects percentage of skin fat relative to total body fat or abdominal fat in broilers.
Table 8.
Temperature of the surface of the shank of broilers fed with either a low-ME diet (3,111 kcal/kg) or a high-ME diet (3,383 kcal/kg) from day 14 to day 45, measured at 22, 28, 45, or 42 day of age, and analyzed with a covariate for ME intake during the 6, 12, 24, or 48 h before the temperature measurement.
| Effect | Diet | Age | Covariate 6 h |
Covariate 12 h |
Covariate 24 h |
Covariate 48 h |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Temperature | SEM | Temperature | SEM | Temperature | SEM | Temperature | SEM | |||
| –––––––––––––––––––––––––––––––––––––– °C –––––––––––––––––––––––––––––––––––––– | ||||||||||
| Diet | Low ME | 35.53b | 0.25 | 35.66b | 0.24 | 35.65b | 0.23 | 35.67b | 0.22 | |
| High ME | 36.45a | 0.25 | 36.33a | 0.24 | 36.33a | 0.23 | 36.32a | 0.22 | ||
| Age | 22 D | 38.55a | 0.37 | 39.12a | 0.36 | 39.04a | 0.34 | 39.60a | 0.35 | |
| 28 D | 33.42c | 0.36 | 33.33c | 0.34 | 33.56c | 0.32 | 33.67c | 0.32 | ||
| 35 D | 36.33b | 0.36 | 36.02b | 0.34 | 35.78b | 0.33 | 35.57b | 0.33 | ||
| 42 D | 35.67b | 0.37 | 35.50b | 0.35 | 35.58b | 0.33 | 35.12b | 0.33 | ||
| Covariate | ––––––––––––––––––––––––––––––––––– °C/100 kcal –––––––––––––––––––––––––––––––––– | |||||||||
| ME intake | 2.74 | 0.50 | 2.34 | 0.30 | 1.56 | 0.17 | 0.85 | 0.09 | ||
| Source of variation | –––––––––––––––––––––––––––––––––––––– P - value ––––––––––––––––––––––––––––––––– | |||||||||
| Diet | 0.011 | 0.050 | 0.035 | 0.042 | ||||||
| Age | <0.001 | <0.001 | <0.001 | <0.001 | ||||||
| Diet x Age | 0.21 | 0.42 | 0.41 | 0.31 | ||||||
| ME intake | <0.001 | <0.001 | <0.001 | <0.001 | ||||||
a-cLSMeans within column and effect lacking a common superscript differ (P ≤ 0.05).
The shank temperature also varied with age, although the environmental temperature did so as well. The environmental temperature at day 22, day 28, day 35, and day 42 was 26.3°C, 21.8°C, 21.8°C, and 21.2°C, respectively. Heat loss rate is depending on the difference between the skin temperature and the environmental temperature (Yahav et al., 2005). It is therefore suggested that the increased shank temperature at day 22 was a result of the higher environmental temperature and unrelated to animal factors.
In the current experiment, HP may have also been affected by physical activity of the birds. Although no observational data for behavior were obtained, frequency of station visits is related to at least one type of physical activity. There was a significant effect of the feed intake treatment on the frequency of station visits (Table 9). Birds that were more severely restricted, visited the feeding station more often than ad libitum fed birds. This could indicate that the motivation to visit the feeding station was higher in the feed-restricted birds than in the ad libitum fed birds. However, this could also have resulted in an increase in HP of the feed-restricted birds compared with the ad libitum fed birds, as HP increases with increased physical activity (MacLeod et al., 1982, MacLeod et al., 1988). It was shown that the energy expenditure for locomotion activities in laying hens is about 20 to 25% of HP and that the total energy expenditure increased by about 53 to 65% when moving at a speed of 1–2 km/h (Kampen, 1976). In addition, the increased rate of HP during the light period compared with the dark period associated with standing was estimated to be about 18% of daily HP at 31.12 kcal/D per hen (Li et al., 1991). Therefore, it is hypothesized that feed-restricted birds could have had an increased energy expenditure for physical activity, which decreased the availability of energy for gain, in addition to the limitation of available nutrients as a direct result of the feed restriction. Research in broilers has also shown that resting energy expenditure is higher during the photoperiod than during the scotoperiod (Kim et al., 2014), where it was estimated that HP in the photoperiod was 15.80 kcal/D and HP in the scotoperiod was 7.59 kcal/D for each broiler. As birds on the 50% restricted treatment visited the PF system on average 3.9 times during the scotoperiod, whereas the ad libitum fed birds only visited the station 0.4 times during the scotoperiod (data not shown), feed-restricted birds could have had an increased time of activity during the scotophase and therefore a decrease in the time resting at a low rate of HP in comparison with the ad libitum fed birds.
Table 9.
Number of times birds accessed the feeding station (visits), number of daily meals, daily meal to visit (M:V) ratio, and meal size for broilers fed either a low-ME diet (3,111 kcal/kg) or a high-ME diet (3,383 kcal/kg) from day 14 to day 451.2
| Effect | Diet | Feed intake | Visits (#) | SEM | Meals (#) | SEM | M:V ratio (%) | SEM | Meal size (g) | SEM |
|---|---|---|---|---|---|---|---|---|---|---|
| Diet | Low ME | 61.7 | 1.12 | 14.3b | 0.20 | 43.8b | 0.96 | 8.4a | 0.08 | |
| High ME | 61.6 | 1.06 | 15.9a | 0.19 | 46.9a | 0.91 | 7.7b | 0.07 | ||
| Feed intake | 50% | 122.3a | 2.01 | 8.8e | 0.36 | 8.9f | 1.72 | 9.1a | 0.14 | |
| 60% | 86.1b | 1.98 | 10.5d | 0.36 | 20.0e | 1.70 | 9.1a | 0.14 | ||
| 70% | 57.4c | 1.84 | 13.4c | 0.33 | 27.9d | 1.58 | 8.0b | 0.13 | ||
| 80% | 49.1d | 2.01 | 17.6b | 0.36 | 49.0c | 1.72 | 7.3c,d | 0.14 | ||
| 90% | 33.2e | 1.84 | 18.5b | 0.33 | 66.6b | 1.58 | 7.7b,c | 0.13 | ||
| 100% | 21.8f | 1.64 | 21.7a | 0.30 | 99.5a | 1.40 | 7.0d | 0.11 | ||
| Diet × Feed intake | Low ME | 50% | 117.8b | 3.09 | 8.8g | 0.56 | 10.4g,h | 2.65 | 9.1a,b | 0.21 |
| 60% | 91.8c | 2.62 | 10.0f,g | 0.47 | 16.2g | 2.25 | 9.5a | 0.18 | ||
| 70% | 63.8e | 2.62 | 13.2e | 0.47 | 25.1e,f | 2.25 | 8.2c | 0.18 | ||
| 80% | 42.0g | 3.09 | 17.8c | 0.56 | 54.4c | 2.65 | 7.4d,e | 0.21 | ||
| 90% | 34.4g,h | 2.62 | 15.7d | 0.47 | 57.0c | 2.25 | 8.8b | 0.18 | ||
| 100% | 20.4i | 2.33 | 20.3b | 0.42 | 99.6a | 2.00 | 7.3e | 0.16 | ||
| High ME | 50% | 126.8a | 2.57 | 8.8g | 0.46 | 7.5h | 2.21 | 9.0a,b | 0.18 | |
| 60% | 80.4d | 2.97 | 11.0f | 0.54 | 23.8f | 2.55 | 8.8b | 0.20 | ||
| 70% | 50.9f | 2.57 | 13.6e | 0.46 | 30.7e | 2.21 | 7.8c,d | 0.18 | ||
| 80% | 56.3f | 2.57 | 17.5c | 0.46 | 43.6d | 2.21 | 7.3d,e | 0.18 | ||
| 90% | 32.0h | 2.57 | 21.3b | 0.46 | 76.1b | 2.21 | 6.5f | 0.18 | ||
| 100% | 23.1i | 2.30 | 23.0a | 0.42 | 99.4a | 1.97 | 6.7f | 0.16 | ||
| Source of variation | ––––––––––––––––––––––––––––– P - value –––––––––––––––––––––––––– | |||||||||
| Diet | 0.93 | <0.001 | 0.021 | <0.001 | ||||||
| Feed intake | <0.001 | <0.001 | <0.001 | <0.001 | ||||||
| Diet × Feed intake | <0.001 | <0.001 | <0.001 | <0.001 | ||||||
| Age3 | 0.07 | <0.001 | <0.001 | <0.001 | ||||||
| Age × Diet | 0.14 | 0.33 | 0.91 | 1.00 | ||||||
| Age × Feed intake | 0.98 | 0.93 | 0.96 | 1.00 | ||||||
| Age × Diet × Feed intake | 1.00 | 1.00 | 0.95 | 1.00 | ||||||
a-iLSMeans within column and effect lacking a common superscript differ (P ≤ 0.05).
Data till the end of day 44 were included as birds were euthanized at day 45.
Birds were pair-fed through a precision feeding system with lead birds eating ad libitum (100%) and followers were allowed to eat either 50, 60, 70, 80, or 90% of the paired lead’s cumulative feed intake.
Number of meals decreased with age from 17.5 at day 20 to 11.8 at day 44; M:V ratio decreased with age from 59.7% at day 20 to 39.2% at day 44; meal size increased with age from 4.3 g at day 20 to 11.5 g at day 44.
The effect of feed restriction on visit frequency depended on the diet treatment. Whereas ad libitum birds fed with the low-ME and high-ME diet did not differ, the 50% restricted birds fed with the high-ME diet visited the feeding station 9 more times per day compared with the 50% restricted birds fed with the low-ME diet. This may be related to the link between meal size and number of meals and the physical property of the 2 feeds. Meal size was 0.7 g smaller, and birds had 1.6 meals more per day in the high-ME treatment compared with the low-ME treatment (Table 9). From observation, the pellets of the high-ME diet had a lower quality than those the low-ME diet, which resulted in more fines. It is known that broilers fed with a mash need more time to eat than broilers fed with pellets (Nir et al., 1994). As the PF station had a set amount of time per meal, the meal size could therefore have been reduced, requiring birds fed with the high-ME diet to visit the PF system more often. Alternatively, the high-ME diet could also have been less palatable, or the intake of the High ME diet resulted more immediately in signaling of endocrinological satiety mechanisms and therefore a slower rate of intake.
There was no effect of diet or ME intake on the number of peripheral leukocytes or the heterophil to lymphocyte (H:L) ratio (Table 10). High H:L ratios are used as an indicator of chronic stress, and the number of leukocytes provides one indicator of the systemic immune status (More Bayona et al., 2017). Higher H:L ratios have been observed in restricted birds than in ad libitum fed birds in some studies (Maxwell et al., 1992, Hocking et al., 1993, Hocking et al., 1996, Savory et al., 1993) but not in other studies (van Niekerk et al., 1988, Katanbaf et al., 1989, Maxwell et al., 1990, Savory et al., 1996, Jong et al., 2002). The H:L ratios in the present study are higher than results from the study by Maxwell et al (1992). These authors found that at D 42, the H:L ratio was 0.53 for ad libitum and 0.76 for feed-restricted birds, owing to a significant change in the proportion of lymphocytes (57.0 and 47.4% for ad libitum and feed-restricted birds, respectively). The difference in the H:L ratio between the current results and results from the study by Maxwell et al (1992) could originate from differences in strain, rearing conditions, or health status, but this could also indicate that all birds in the present study were under chronic stress. In recent years, more attention has been paid to strategies that can take advantage of nutrition to modulate the immune system due to the prohibition of feed-added antibiotics in some regions of the world (Korver, 2012). It is still to be defined what proportion of the ME intake is partitioned to maintain and develop the innate and acquired immune system in healthy poultry. Klasing (2007) indicated that at maintenance, a young broiler uses about 0.5% of the body's lysine for leukocytes, antibodies, and accessory proteins and that the resting immune system uses about 1.2% of the lysine intake in a healthy growing broiler chick. In addition, a difference in the immune system between feed-restricted and ad libitum birds may also originate from differences in the responsiveness of leukocytes (More Bayona et al., 2017). In this regard, an assessment of immune function would provide a deeper understanding of immune changes due to feed restriction. Thus, it is recommended that further research studies the maintenance requirements and energetic costs of the immune system in poultry.
Table 10.
Number of total leukocytes and heterophil, lymphocyte, combined monocyte and macrophage percentages of total leukocyte number, and heterophil to lymphocyte (H/L) ratio in blood samples taken at day 45 of birds fed with low- (3,111 kcal/kg) or high-ME (3,383 kcal/kg) diets from day 14 to day 45.
| Diet | Total leukocytes (#) | SEM | Heterophils (%) | SEM | Lymphocytes (%) | SEM | Monocytes and Macrophages (%) | SEM | H/L ratio | SEM |
|---|---|---|---|---|---|---|---|---|---|---|
| Low ME | 32,197 | 2,100 | 43.5 | 3.3 | 40.9 | 4.3 | 15.6 | 2.8 | 1.2 | 0.2 |
| High ME | 28,049 | 1,817 | 39.2 | 3.1 | 44.3 | 4.0 | 17.0 | 2.6 | 1.0 | 0.2 |
| Source of variation | –––––––––––––––––––––––––––––––––––––––––––– P - value –––––––––––––––––––––––––––––––––––––––– | |||||||||
| Diet | 0.16 | 0.37 | 0.58 | 0.73 | 0.54 | |||||
| ME intake1 | 0.31 | 0.46 | 0.98 | 0.29 | 0.88 | |||||
P - values for the covariable ME intake was not significant, therefore the regression coefficient was not shown. Mean ME intake was 9,641 ± 126 kcal for the low-ME diet and 10,108 ± 123 kcal for the high-ME diet over the experimental period.
Conclusions
The nonlinear model provided a noninvasive real-time method to measure HP and RE in broilers. However, the model was not able to distinguish the NEg values of the 2 diets. Estimates of the NEg values increased when feed intake was reduced. The HIF could be determined with the modeling methodology and was in the range of values in the literature. Additional measurements on heat dissipation, physical activity, and immune status indicated that the energetic content of the diet and feed restriction affect some parameters (shank temperature, feeding station visits) but not others (leukocyte counts, H:L ratio, and immune cell function). Further research is needed to understand dietary factors affecting ME available for productive processes, including more comprehensive analysis on the energy expenditure on activity and immunity.
Acknowledgments
Financial support from Danisco Animal Nutrition (Marlborough, UK) is gratefully acknowledged. Special thanks Chris Ouellette and Mark Fedorak for their excellent technical support throughout the experiment. Thanks to the staff of the Poultry Research Centre (Edmonton, Alberta) for their technical support. Poultry Research Centre stakeholder contributions, which made this research possible, are gratefully acknowledged.
Conflict of Interest Statement: The authors declare that there is no conflict of interest
References
- Birkett S., de Lange K. Limitations of conventional models and a conceptual framework for a nutrient flow representation of energy utilization by animals. Br. J. Nutr. 2001;86:647–659. doi: 10.1079/bjn2001441. [DOI] [PubMed] [Google Scholar]
- Bravo D., Pirgozliev V., Rose S.P. A mixture of carvacrol, cinnamaldehyde, and capsicum oleoresin improves energy utilization and growth performance of broiler chickens fed maize-based diet. J. Anim. Sci. 2014;92:1531–1536. doi: 10.2527/jas.2013-6244. [DOI] [PubMed] [Google Scholar]
- Buyse J., Decuypere E., Berghman L., Kühn E.R., Vandesande F. Effect of dietary protein content on episodic growth hormone secretion and on heat production of male broiler chickens. Br. Poult. Sci. 1992;33:1101–1109. doi: 10.1080/00071669208417552. [DOI] [PubMed] [Google Scholar]
- Byerly T.C., Kessler J.W., Gous R.M., Thomas O.P. Feed requirements for egg production. Poult. Sci. 1980;59:2500–2507. [Google Scholar]
- Carré B., Lessire M., Juin H. Prediction of the net energy value of broiler diets. Animal. 2014;8:1395–1401. doi: 10.1017/S175173111400130X. [DOI] [PubMed] [Google Scholar]
- CCAC . Canadian Council on Animal Care; Ottawa, ON, Canada: 2009. CCAC Guidelines on: The Care and Use of Farm Animals in Research, Teaching and Testing. [Google Scholar]
- Ferrini G., Baucells M.D., Esteve-García E., Barroeta A.C. Dietary polyunsaturated fat reduces skin fat as well as abdominal fat in broiler chickens. Poult. Sci. 2008;87:528–535. doi: 10.3382/ps.2007-00234. [DOI] [PubMed] [Google Scholar]
- Fraps G. Composition and productive energy of poultry feeds and rations. Tex. Agric. Exp. Stn. Bull. 1946;No 678:5–37. [Google Scholar]
- Fraps G., Carlyle E. The utilization of the energy of feed by growing chickens. Tex. Agric. Exp. Stn. Bull. 1939;No 571:5–43. [Google Scholar]
- Geraert P.A., Macleod M.G., Larbier M., Leclercq B. Nitrogen metabolism in genetically fat and lean chickens. Poult. Sci. 1990;69:1911–1921. doi: 10.3382/ps.0691911. [DOI] [PubMed] [Google Scholar]
- Gous R.M., Morris T.R. Nutritional interventions in alleviating the effects of high temperatures in broiler production. Worlds Poult. Sci. J. 2005;61:463–475. [Google Scholar]
- Hadinia S.H., Carneiro P.R.O., Ouellette C.A., Zuidhof M.J. Energy partitioning by broiler breeder pullets in skip-a-day and precision feeding systems. Poult. Sci. 2018;97:4279–4289. doi: 10.3382/ps/pey283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocking P.M., Maxwell M.H., Mitchell M.A. Welfare assessment of broiler breeder and layer females subjected to food restriction and limited access to water during rearing. Br. Poult. Sci. 1993;34:443–458. [Google Scholar]
- Hocking P.M., Maxwell M.H., Mitchell M.A. Relationships between the degree of food restriction and welfare indices in broiler breeder females. Br. Poult. Sci. 1996;37:263–278. doi: 10.1080/00071669608417858. [DOI] [PubMed] [Google Scholar]
- Horwitz W. 13th ed. Horwitz Association of Official Analytical Chemists; Washington, DC: 1980. Official Methods of Analysis of the Association of Official Analytical Chemists. [Google Scholar]
- Jong I.C.D., Voorst S.V., Ehlhardt D.A., Blokhuis H.J. Effects of restricted feeding on physiological stress parameters in growing broiler breeders. Br. Poult. Sci. 2002;43:157–168. doi: 10.1080/00071660120121355. [DOI] [PubMed] [Google Scholar]
- Kafri I., Jortner B.S., Cherry J.A. Skin breaking strength in broilers: relationship with skin thickness. Poult. Sci. 1986;65:971–978. [Google Scholar]
- van Kampen M. Activity and energy expenditure in laying hens: 2. The energy cost of exercise. J. Agric. Sci. 1976;87:81–84. [Google Scholar]
- Katanbaf M.N., Dunnington E.A., Siegel P.B. Restricted feeding in early and late-feathering chickens. 1. Growth and physiological responses. Poult. Sci. 1989;68:344–351. doi: 10.3382/ps.0680344. [DOI] [PubMed] [Google Scholar]
- Kielauowski J. Estimates of the energy cost of protein deposition in growing animals. In: Blaxter K.L., editor. Proceedings of the Symposium on Energy Metabolism. Acad. Press; London, UK: 1965. pp. 13–20. [Google Scholar]
- Kim C., Lee S., Lee S.-J. Effects of light color on energy expenditure and behavior in broiler. Asian-australas. J. Anim. Sci. 2014;27:1044–1049. doi: 10.5713/ajas.2012.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klasing K.C. Nutrition and the immune system. Br. Poult. Sci. 2007;48:525–537. doi: 10.1080/00071660701671336. [DOI] [PubMed] [Google Scholar]
- Koh K., Macleod M.G. Effects of ambient temperature on heat increment of feeding and energy retention in growing broilers maintained at different food intakes. Br. Poult. Sci. 1999;40:511–516. doi: 10.1080/00071669987287. [DOI] [PubMed] [Google Scholar]
- Korver D.R. Implications of changing immune function through nutrition in poultry. Anim. Feed Sci. Technol. 2012;173:54–64. [Google Scholar]
- Latshaw J.D., Moritz J.S. The partitioning of metabolizable energy by broiler chickens. Poult. Sci. 2009;88:98–105. doi: 10.3382/ps.2008-00161. [DOI] [PubMed] [Google Scholar]
- Leeson S., Caston L., Summers J.D. Broiler response to diet energy. Poult. Sci. 1996;75:529–535. doi: 10.3382/ps.0750529. [DOI] [PubMed] [Google Scholar]
- Leeson S., Summers J.D. Nutrition of the Chicken. University Books; Guelph, ON: 2001. Energy; pp. 34–99. [Google Scholar]
- Li Y., Ito T., Yamamoto S. Diurnal variation in heat production related to some physical activities in laying hens. Br. Poult. Sci. 1991;32:821–827. doi: 10.1080/00071669108417407. [DOI] [PubMed] [Google Scholar]
- Liu W., Lin C.H., Wu Z.K., Liu G.H., Yan H.J., Yang H.M., Cai H.Y. Estimation of the net energy requirement for maintenance in broilers. Asian-australas. J. Anim. Sci. 2017;30:849–856. doi: 10.5713/ajas.16.0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod M.G. Effects of amino acid balance and energy: protein ratio on energy and nitrogen metabolism in male broiler chickens. Br. Poult. Sci. 1997;38:405–411. doi: 10.1080/00071669708418010. [DOI] [PubMed] [Google Scholar]
- MacLeod M.G., Jewitt T.R., Anderson J.E.M. Energy expenditure and physical activity in domestic fowl kept on standard and interrupted lighting patterns. Br. Poult. Sci. 1988;29:231–244. doi: 10.1080/00071668808417048. [DOI] [PubMed] [Google Scholar]
- MacLeod M.G., Jewitt T.R., White J., Verbrugge M., Mitchell M.A. Proceedings of the 9th Symposium on Energy Metabolism, European Association of Animal Production. European Assocation of Animal Production; Lillehammer, Norway: 1982. The contribution of locomotor activity to energy expenditure in the domestic fowl; pp. 297–300. [Google Scholar]
- Maxwell M.H., Hocking P.M., Robertson G.W. Differential leucocyte responses to various degrees of food restriction in broilers, turkeys and ducks. Br. Poult. Sci. 1992;33:177–187. doi: 10.1080/00071669208417455. [DOI] [PubMed] [Google Scholar]
- Maxwell M.H., Robertson G.W., Spence S., McCorquodale C.C. Comparison of haematological values in restricted-and ad libitum-fed domestic fowls: White blood cells and thrombocytes. Br. Poult. Sci. 1990;31:399–405. doi: 10.1080/00071669008417270. [DOI] [PubMed] [Google Scholar]
- More Bayona J.A., Karuppannan A.K., Barreda D.R. Contribution of leukocytes to the induction and resolution of the acute inflammatory response in chickens. Dev. Comp. Immunol. 2017;74:167–177. doi: 10.1016/j.dci.2017.04.018. [DOI] [PubMed] [Google Scholar]
- Musharaf N.A., Latshaw J.D. Heat increment as affected by protein and amino acid nutrition. Worlds Poult. Sci. J. 1999;55:233–240. [Google Scholar]
- van Niekerk T., Kantanbaf M.N., Dunnington E.A., Siegel P.B. Behavior of early and late feathering broiler breeder hens reared under different feeding regimes. Arch. Fuer Gefluegelkunde Ger. FR. 1988 [Google Scholar]
- Nir I., Twina Y., Grossman E., Nitsan Z. Quantitative effects of pelleting on performance, gastrointestinal tract and behaviour of meat-type chickens. Br. Poult. Sci. 1994;35:589–602. doi: 10.1080/00071669408417724. [DOI] [PubMed] [Google Scholar]
- Noblet J., Dubois S., Lasnier J., Warpechowski M., Dimon P., Carré B., van Milgen J., Labussière E. Fasting heat production and metabolic BW in group-housed broilers. Animal. 2015;9:1138–1144. doi: 10.1017/S1751731115000403. [DOI] [PubMed] [Google Scholar]
- NRC . 2nd rev. ed. National Academy Press; Washington DC: 1981. Nutritional Energetics of Domestic Animals and Glossary of Energy Terms. [Google Scholar]
- Olukosi O.A., Cowieson A.J., Adeola O. Energy utilization and growth performance of broilers receiving diets supplemented with enzymes containing carbohydrase or phytase activity individually or in combination. Br. J. Nutr. 2008;99:682–690. doi: 10.1017/S0007114507815807. [DOI] [PubMed] [Google Scholar]
- Pishnamazi A., Renema R.A., Zuidhof M.J., Robinson F.E. Effect of initial full feeding of broiler breeder pullets on carcass development and body weight variation. J. Appl. Poult. Res. 2008;17:505–514. [Google Scholar]
- Rabello C.B.V., Sakomura N.K., Longo F.A., Couto H.P., Pacheco C.R., Fernandes J.B.K. Modelling energy utilisation in broiler breeder hens. Br. Poult. Sci. 2006;47:622–631. doi: 10.1080/00071660600963628. [DOI] [PubMed] [Google Scholar]
- Richards S.A. The significance of changes in the temperature of the skin and body core of the chicken in the regulation of heat loss. J. Physiol. 1971;216:1–10. doi: 10.1113/jphysiol.1971.sp009505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero L.F., Zuidhof M.J., Renema R.A., Naeima A., Robinson F.E. Effects of maternal energy efficiency on broiler chicken growth, feed conversion, residual feed intake, and residual maintenance metabolizable energy requirements. Poult. Sci. 2011;90:2904–2912. doi: 10.3382/ps.2011-01665. [DOI] [PubMed] [Google Scholar]
- Romero L.F., Zuidhof M.J., Renema R.A., Robinson F.E., Naeima A. Nonlinear mixed models to study metabolizable energy utilization in broiler breeder hens. Poult. Sci. 2009;88:1310–1320. doi: 10.3382/ps.2008-00102. [DOI] [PubMed] [Google Scholar]
- Sanz M., Flores A., Lopez-Bote C.J. The metabolic use of energy from dietary fat in broilers is affected by fatty acid saturation. Br. Poult. Sci. 2000;41:61–68. doi: 10.1080/00071660086411. [DOI] [PubMed] [Google Scholar]
- Savory C.J., Carlisle A., Maxwell M.H., Mitchell M.A., Robertson G.W. Stress, arousal and opioid peptide-like immunoreactivity in restricted and ad libitum fed broiler breeder fowls. Comp. Biochem. Physiol. A. Physiol. 1993;106:587–594. [Google Scholar]
- Savory C.J., Hocking P.M., Mann J.S., Maxwell M.H. Is broiler breeder welfare improved by using qualitative rather than quantitative food restriction to limit growth rate? Anim. Welf. 1996;5:105–127. [Google Scholar]
- Schulman N., Tuiskula-Haavisto M., Siitonen L., Mäntysaari E.A. Genetic variation of residual feed consumption in a selected Finnish egg-layer population. Poult. Sci. 1994;73:1479–1484. doi: 10.3382/ps.0731479. [DOI] [PubMed] [Google Scholar]
- Scott T.A., Boldaji F. Comparison of inert markers [chromic oxide or insoluble ash (Celite)] for determining apparent metabolizable energy of wheat- or barley-based broiler diets with or without enzymes. Poult. Sci. 1997;76:594–598. doi: 10.1093/ps/76.4.594. [DOI] [PubMed] [Google Scholar]
- Spratt R.S., Bayley H.S., McBride B.W., Leeson S. Energy metabolism of broiler breeder hens: 1. The partition of dietary energy intake. Poult. Sci. 1990;69:1339–1347. doi: 10.3382/ps.0691339. [DOI] [PubMed] [Google Scholar]
- Swennen Q., Delezie E., Collin A., Decuypere E., Buyse J. Further investigations on the role of diet-induced thermogenesis in the regulation of feed intake in chickens: comparison of age-matched broiler versus layer cockerels. Poult. Sci. 2007;86:895–903. doi: 10.1093/ps/86.5.895. [DOI] [PubMed] [Google Scholar]
- Swennen Q., Janssens G. P. j, Collin A., Le Bihan-Duval E., Verbeke K., Decuypere E., Buyse J. Diet-induced thermogenesis and glucose oxidation in broiler chickens: influence of genotype and diet composition. Poult. Sci. 2006;85:731–742. doi: 10.1093/ps/85.4.731. [DOI] [PubMed] [Google Scholar]
- Swennen Q., Janssens G.P.J., Decuypere E., Buyse J. Effects of substitution between fat and protein on feed intake and its regulatory mechanisms in broiler chickens: energy and protein metabolism and diet-induced thermogenesis. Poult. Sci. 2004;83:1997–2004. doi: 10.1093/ps/83.12.1997. [DOI] [PubMed] [Google Scholar]
- Wolynetz M.S., Sibbald I.R. Prediction of initial carcass composition in comparative slaughter experiments. Poult. Sci. 1985;64:681–687. [Google Scholar]
- Wu S.B., Swick R.A., Noblet J., Rodgers N., Cadogan D., Choct M. Net energy prediction and energy efficiency of feed for broiler chickens. Poult. Sci. 2018;98:1222–1234. doi: 10.3382/ps/pey442. [DOI] [PubMed] [Google Scholar]
- Yahav S., Shinder D., Tanny J., Cohen S. Sensible heat loss: the broiler’s paradox. Worlds Poult. Sci. J. Camb. 2005;61:419–434. [Google Scholar]
- Zhou W.T., Yamamoto S. Effects of environmental temperature and heat production due to food intake on abdominal temperature, shank skin temperature and respiration rate of broilers. Br. Poult. Sci. 1997;38:107–114. doi: 10.1080/00071669708417949. [DOI] [PubMed] [Google Scholar]
- Zuidhof M.J. A review of dietary metabolizable and net energy: Uncoupling heat production and retained energy. J. Appl. Poult. Res. 2019;28:231–241. [Google Scholar]
- Zuidhof M.J., Fedorak M.V., Kirchen C.C., Lou E.H.M., Ouellette C.A., Wenger I.I. PrecisionZX, Inc., assignee. Pat. No. US10,506,793 B2; 2019. United States patent: System and method for feeding animals. [Google Scholar]
- Zuidhof M.J., Fedorak M.V., Ouellette C.A., Wenger I.I. Precision feeding: Innovative management of broiler breeder feed intake and flock uniformity. Poult. Sci. 2017;96:2254–2263. doi: 10.3382/ps/pex013. [DOI] [PubMed] [Google Scholar]
- Zuidhof M.J., Schneider B.L., Carney V.L., Korver D.R., Robinson F.E. Growth, efficiency, and yield of commercial broilers from 1957, 1978, and 2005. Poult. Sci. 2014;93:2970–2982. doi: 10.3382/ps.2014-04291. [DOI] [PMC free article] [PubMed] [Google Scholar]