Supplemental Digital Content is available in the text.
Key Words: ALK-positive NSCLC, non–small cell lung cancer, uncommon sites of metastases, genomics
Abstract
Introduction:
Anaplastic lymphoma kinase (ALK) gene rearrangements are observed in about 4% to 8% non–small cell lung cancer (NSCLC). ALK+ tumors have been associated with increased pleural and pericardial disease. Our primary objective was to determine the uncommon sites of metastasis of ALK+ NSCLC. Secondary objectives included study of coexisting mutations and factors impacting survival of ALK+ NSCLC.
Methods:
All patients with metastatic ALK+ NSCLC at the City of Hope Cancer Center in Duarte, California from 2010 to 2017 were selected for retrospective chart review. The demographic variables were collected. The molecular statuses of patients were evaluated through commercially available platforms for next-generation sequencing. Three-dimensional volumetric images were generated for the primary lesion and different sites of metastasis.
Results:
Sixty two patients with ALK+ NSCLC were identified from 2010 to 2017. The median age was 59 with 36 (58%) female individuals and only 20 (32%) smokers. Twenty four patients had uncommon sites of metastasis which were thyroid, soft tissue, chest and abdominal wall, spleen, peritoneum, omentum, kidney, and ovary. Common characteristics of the primary lesions were right upper lobe location (N=23 [37%]), oval shape (N=22 [35%]), irregular margins (N=26 [42%]), solid lesions (N=27 [44%]), presence of pleural contact or effusion (N=22 [35%]). Twenty four patients had next-generation sequencing testing which showed coexisting mutations such as TP53 (N=8), EGFR (N=5), KRAS (N=3). Patients with uncommon sites of metastasis had a decreased median survival compared with common sites (39 vs. 82 m, P=0.046).
Conclusion:
In NSCLC, ALK rearrangements may not be mutually exclusive mutations and can present with unique radiographic patterns. Patients with uncommon sites of metastasis may have worse outcomes.
Lung cancer is one of the most common types of solid malignancy with roughly around 1.6 million diagnoses worldwide each year. It is also the leading cause of cancer deaths worldwide.1 Lung cancer is subclassified into small cell lung cancer (SCLC) and non-SCLC (NSCLC). NSCLC is further subcategorized into adenocarcinoma, squamous cell carcinoma, and others such as large cell, sarcomatoid, and neuroendocrine carcinoma. Up to 69% of the patients with advanced lung cancer have actionable mutations. The majority of them are KRAS (25%), EGFR sensitizing (17%), anaplastic lymphoma kinase (ALK) (7%), MET (3%), HER-2 (2%), ROS1 (2%), BRAF (2%), RET (2%), NTRK1 (1%), PIK3CA (1%), and MEK1 (1%).2 In addition, 31% patients are found to have unknown oncogenic driver mutations for which we do not have any current targets.2
ALK gene was initially described in 1994 in anaplastic large cell lymphomas while cloning of t2,5 (p23; q35) translocation. It was also shown that ALK gene is expressed in small intestine, testes, and brain—but not in normal lymphoid cells and has similarities to insulin receptors family of kinase. Increased expression of truncated ALK theoretically lead to increased malignant transformation and non-Hodgkin’s lymphoma.3 The association of the ALK gene with NSCLC was reported in 2007 when a small inversion within chromosome 2p that juxtaposes the 5′ end of the echinoderm microtubule-associated protein-like 4 (EML4) gene with the 3′ end of the ALK gene that led to the production of oncogene EML4-ALK in NSCLC cells was discovered. This novel fusion oncogene leads to aberrant expression of ALK resulting in production of the EML4-ALK fusion protein that is constitutively active and results in increased downstream signaling pathways that leads to increased tumor growth, cell proliferation, and survival.4
ALK gene rearrangements are observed in about 4% to 8% of the patient’s with lung cancer.4 Because of its low incidence there is limited knowledge about its clinical, radiographic features and molecular profile which may be different from traditional lung cancer. In general, EML4-ALK-mutated patients present at a younger age, in women with never or light smoking (<10 pack-years) history.5–7 Up to 55% of the patients with all NSCLC present with stage IV disease with poor prognosis. Unique patterns and uncommon sites of metastasis of ALK+ tumors with their coexisting driver mutations such as EGFR, KRAS, and others have not been extensively reviewed in the literature. The diagnosis and treatment in NSCLC has becoming more individualized with the advancement of precision medicine.
In this study we aim to delineate the clinical and genomic features of ALK+ NSCLC. The primary objective of this study is to determine the uncommon patterns of metastasis of ALK+ NSCLC. Secondary objectives are study of other relevant mutations which coexist in patients with ALK gene rearrangements which may be of clinical significance, provide groundwork to establish further radiological advancement in ALK+ tumors and determine factors impacting survival of ALK+ tumors.
METHODS
Patient Selection
All patients who presented to City of Hope (COH) Cancer Center in Duarte, CA between 2010 and 2017 with ALK rearrangements were selected for retrospective chart review. Patients with nonmetastatic disease were excluded from the study. ALK testing was performed by fluorescence in situ hybridization (FISH) analysis, immunohistochemistry (IHC), or next-generation sequencing (NGS). The molecular status of each patient was evaluated through commercially available platforms for NGS at the request and discretion of the treating physician. The different platforms of NGS were (1) H&E stain, polymerase chain reaction, (2) OncImmune (OncImmune, De Soto, KS), (3) Ion Torrent Kaiser (Kaiser Permanente, Oakland, CA), (4) FoundationOne (Foundation Medicine, Cambridge, MA), (5) Guardant 360 (Guardant Health, Redwood City, CA), (6) Onco48 (COH, Duarte, CA), (7) Oncotype DX (Genomic Health, Redwood City, CA), (8) Snapshot NGS (COH), and (9) ResponseDx: Lung (Cancer Genetics, Los Angeles, CA). The demographic variables which were collected by the authors include age, sex, race, date of birth, vital status, histological diagnosis, number of prior lines of therapy, types of therapy and number of sites of metastasis (Table 1).
TABLE 1.
Baseline Characteristics of Patients With ALK+ NSCLC

Radiological Data
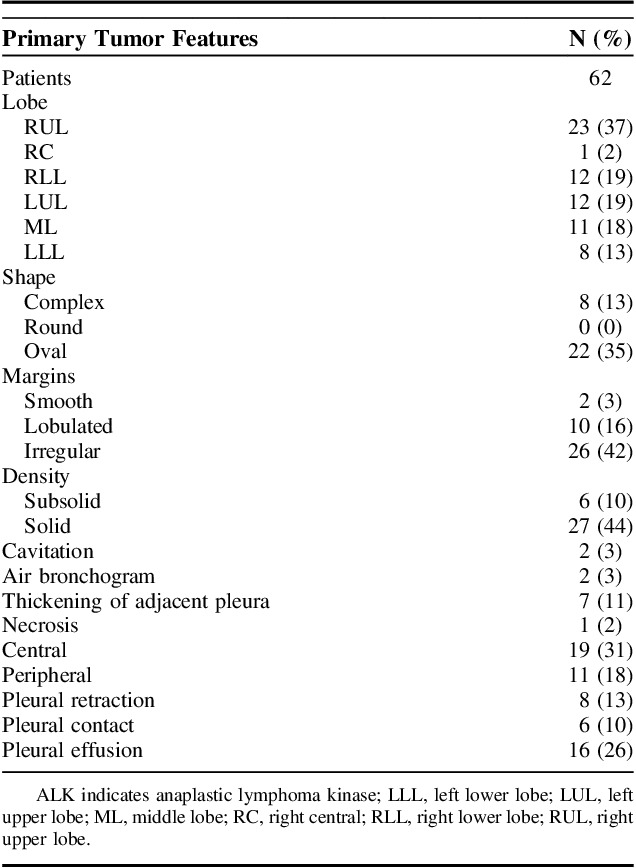
The radiologic images were reviewed by 2 experienced radiologists at COH for the patterns of the spread of primary lung cancer and different sites of metastasis. For each patient, multimodality assessments were performed using computed tomography (CT), PET-CT, and MRI. Different sites of the metastasis that appeared at any time during the disease course from the time of initial diagnosis till dates of last contact were noted (Fig. 1). The definition of uncommon metastases were metastatic sites excluding the brain, bone, liver, adrenal glands, thoracic cavity, and distant lymph nodes. Subsequently the characteristics of the primary malignancy including the location of the primary tumor, the shape of the tumor, density in the tumor borders, presence of cavitary lesions and air bronchograms, presence of fibrosis and emphysema, pleural retraction, pleural contact, and pleural effusions were also obtained (Table 2).
FIGURE 1.
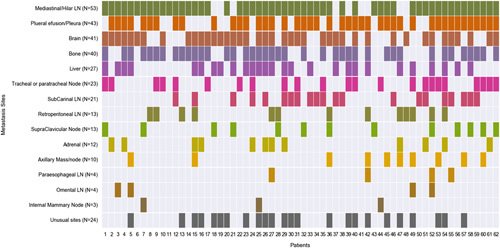
Different metastatic sites that appeared at any time during the disease course including common sites (brain, bone, liver, adrenal glands, and thoracic cavity) and uncommon sites.
TABLE 2.
Primary ALK+ Tumor Radiographic Characteristics of the Study Population
Data Analysis
The heat maps in the Figure 1 were created using Seaborn, a statistical Python visualization library.8 The original data were first organized in a comma-separated value file format and each mutation or metastatic site was cataloged from reports. The text-based genomic mutations or metastatic site were then coded to a number such as where the number 1 was coded to symbolize a substitution mutation. Pandas was used to populate the data to Seaborn and generate the heatmap.9 The Kaplan-Meier survival curve (Fig. 2) was created using Lifelines, a survival analysis Python library.10
FIGURE 2.
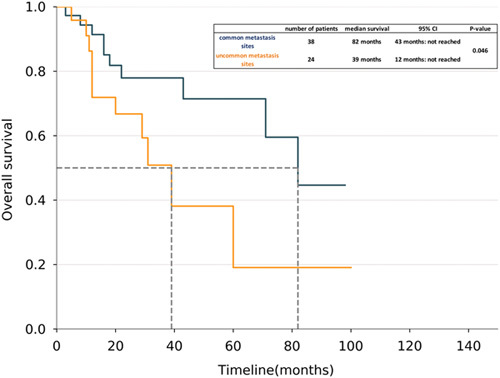
The Kaplan-Meier survival curves for patients with uncommon metastasis sites (N=24, 11 deaths) and for patients with common metastasis sites (N=38, 10 deaths). Patients with uncommon metastasis sites had a lower survival rate than patients with common metastasis sites. There was a significant difference between the two groups (log-rank test, HR 2.41; P=0.046).
CT imaging technique: images through the chest, abdomen, and pelvis that were performed on a GE 16 slice MDCT scanner were reconstructed at 2.5 and 5 mm thickness. All DICOM images then transferred into PACS which were analyzed by radiologist and then transferred into Vital images three-dimensional (3D) advanced imaging system for the 3D advanced imaging. 3D volumetric quantification was performed using voxel-based density method. 3D Imaging data set was transferred into Excel sheet for the analysis.
All patients in the study were consented under IRB study numbers 11237, 07047, and 16052.
RESULTS
At the COH Cancer Center we found the incidence of patients with metastatic ALK+ NSCLC was 7% during the study period of 2010 to 2017 which is similar to national average. The results of the baseline characteristics of the ALK+ NSCLC patients are shown in Table 1. Sixty two patients who met the inclusion criteria of metastatic disease were identified. The median age of the study group was 59 years and majority of the patients were female individuals (N=36 [58%]) and nonsmokers (N=42 [68%]) which is consistent with previously reported data.5,7,11,12 At COH most common ethnicities were Caucasian and Asian at 52% (N=32) and 37% (N=23), respectively. Majority of the ALK+ tumors were adenocarcinomas (N=59 [95%]), however 3 patients had adenosquamous carcinoma as well. Previous treatments included chemotherapy (N=57 [92%]), crizotinib (N=59 [95%]), alectinib (N=25, [40%]), brigatinib (N=7 [11%]), immunotherapy (N=11 [18%]), or clinical trials (N=15 [24%]). Majority (N=35 [56%]) of patients in our study had <4 lines of therapy (Table 1).
The sites of metastasis are shown in the Figure 1. Most patients had metastasis in mediastinal or hilar lymph nodes (n=53). In total, 43/62 patients also had either pleural metastasis or evidence of pleural effusion on imaging. Brain metastases were present in 41 patients (66%). Other common sites of metastasis such as bone, liver, regional lymph nodes, and adrenal gland were also noted. In total, 24 patients in the study group had uncommon sites of metastasis during their disease course. The physiologic landscape of the uncommon sites of metastasis is shown in Supplemental Figure 1 (Supplemental Digital Content 1, http://links.lww.com/AJCO/A235). The uncommon sites of metastasis in this study were leptomeningeal, epidural, thyroid, soft tissue, chest wall, spleen, peritoneum, omentum, kidney, ovarian, and abdominal wall. Most common uncommon site of metastasis was soft tissue (N=7) (Supplemental Fig. 1, Supplemental Digital Content 1, http://links.lww.com/AJCO/A235). Common soft tissue sites were metastasis in musculoskeletal sites including shoulder, hip muscles, and paraspinal metastasis. Patients with uncommon sites of metastasis (N=24) received similar number lines of treatment (3.5±1.9 vs. 3.2±1.9) and were followed for a shorter duration of time since diagnosis (25.8±21.1 vs. 34.4±27.8, Supplemental Table 1, Supplemental Digital Content 2, http://links.lww.com/AJCO/A236). This suggested that appearance of uncommon metastasis sites was not solely depended on duration of follow-up for a patient.
The radiographical features of the primary lung lesion are shown in Table 2. Majority of the patients had right upper lobe lesion (N=23 [37%]) with solid density (N=27 [44%]) and oval shaped tumor (N=22 [35%]). Margins were mostly irregular (N=26 [42%]) or lobulated (N=10 [16%]). We also noted that majority of the ALK+ tumors had pleural thickening, retraction, contact or effusion (N=37 [60%]). We have shown some examples of 3D volumetric images of uncommon sites of metastasis in the Supplemental Figure 2 (Supplemental Digital Content 3, http://links.lww.com/AJCO/A237). The details of the cases are listed in the panel.
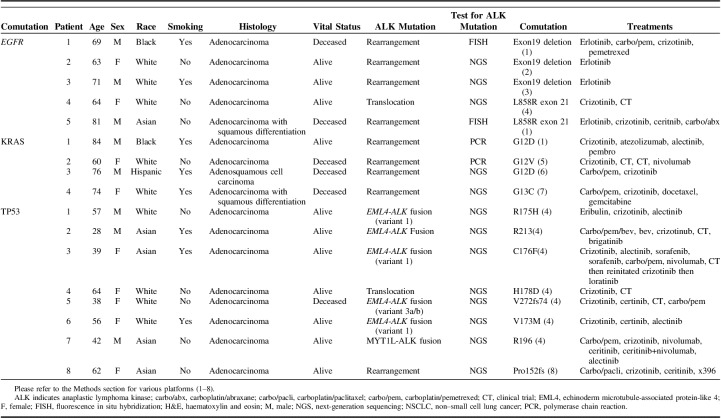
Mutations associated with ALK+ NSCLC are listed in Table 3, with 38 patients tested by IHC/FISH, and 24 patients tested by NGS. Mutations associated with uncommon sites of metastasis are shown schematically in the Supplemental Figure 3 (Supplemental Digital Content 4, http://links.lww.com/AJCO/A238). Notably 8 patients had the coexisting TP53 mutations, 5 had EGFR mutations, and 4 had KRAS mutations. The demographics, clinical characteristics, and treatment received by these patients are shown in Table 4. Four of 5 patients with EGFR mutations received erlotinib as initial treatment but interestingly 2 of 5 patients received treatment with crizotinib as well. We also found several other mutations of unknown significance that may impact tumor proliferation or development of resistance.
TABLE 3.
Molecular Profile of Patients With Next-generation Sequencing (N=24)
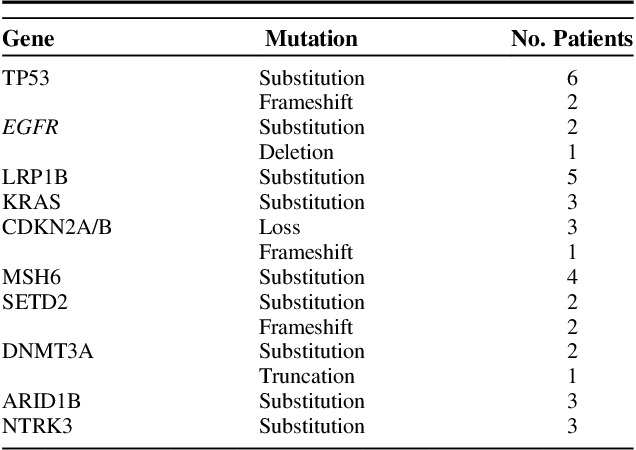
TABLE 4.
ALK+ NSCLC Patients With Overlapping EGFR, KRAS, and TP53 Mutations
In the univariate survival analysis, number of metastatic sites (hazard ratio [HR], 1.66; 95% confidence interval [CI], 1.3-2.11; P<0.001) and uncommon sites of metastasis (HR, 2.41; 95% CI, 1.00-5.77; P<0.04) had statistically significant impact on overall survival. In the multivariate analysis the number of metastatic sites (HR, 2.04; 95% CI, 1.40-2.96; P<0.001) (Supplemental Table 2, Supplemental Digital Content 5, http://links.lww.com/AJCO/A239) and coexistence of KRAS mutation (HR, 5.17; 95% CI, 1.27-21.1; P=0.02) impacted survival. In the Kaplan-Meier survival curve of ALK+ NSCLC, patients with uncommon sites of metastasis had decreased median survival of 39 months (95% CI, 12 mo to NR) compared with the median survival of 82 months (95% CI, 43 mo to NR) in patients with no uncommon sites of metastasis with a statistically significant P-value of 0.046 (Fig. 2).
DISCUSSION
In metastatic NSCLC molecular profiling is commonly used to identify oncogenic driver mutations and is associated with improved first-line progression-free survival (PFS) and overall survival.13 Even though ALK+ NSCLC comprises only up to 4% to 8% of all NSCLC there are 40,000 new cases diagnosed every year. ALK gene rearrangement is typically diagnosed with IHC, FISH, RT-polymerase chain reaction or with NGS. In a small cohort (N=47), we have previously demonstrated that there can be a discordance of up to 35% between ALK detection among NGS and FISH testing14 and therefore standardized ALK testing is needed. To date 4 ALK inhibitors: crizotinib, ceritinib, alectinib, and brigatinib, are FDA approved for frontline or second-line treatment of ALK+ NSLC.15–18 In the presented study 59 (95%) patients received crizotinib at some point during the treatment course. Seven patients were currently on brigatinib at the time of the study conclusion. Despite the availability of ALK inhibitors, 57 (92%) patients in the study received chemotherapy at some point. This suggests that third or fourth-line treatment is still not standardized.
In the current study we found different ALK alterations such as rearrangements (N=30), fusions (N=9, variant 1 [N=3], variant 2 [N=2], and variant 3 [N=1]), and translocation (N=3). Previous studies have shown that variant 1 is the most common subtype.19,20 Most prospective clinical studies assessing the response of ALK inhibitors have largely been carried out with no discriminations between variants. Few retrospective studies have shown different conclusions when comparing patient response based on the ALK variant. One study showed EML4-ALK variant 1 had longer PFS with pemetrexed but no PFS difference among variants when treated with platinum-based chemotherapy and ALK inhibitors.20 In contrast, Yoshida et al21 showed that patients with variant 1 showed a better response to crizotinib than patients with other EML4-ALK variants. In the current study different ALK alterations did not have any individual effect on survival. However, due to heterogeneity of ALK testing we had insufficient numbers of variants to make any conclusive evidence. The impact of these different alterations on development of tumor resistance and the patterns of metastasis should be investigated in future.
Metastases to the uncommon sites can be present in 7% patients with NSCLC irrespective of oncogenic driver mutations.22 In our study we have shown that patients with uncommon sites of metastasis tend to have worse outcomes with median survival of 39 months compared with 82 months with the patients with common sites of metastasis. Even though presence of uncommon sites of metastasis was significant in the univariate survival analysis it did not correlate with poor survival in the multivariate analysis (Supplemental Table 2, Supplemental Digital Content 5, http://links.lww.com/AJCO/A239). Our analysis showed that the number of metastatic sites and coexisting KRAS mutations may impact survival. Hence the reasons of poor survival in patients with uncommon metastasis are not entirely clear at this point. This could be possible due to coexisting mutations or aggressive nature of the disease. Another possible explanation is that the patients with uncommon sites have different mechanisms of metastases and there are tumor heterogeneity that exists within the primary and metastatic sites.23 Because of small number of patients and lack of the comparison group it is difficult to draw conclusive evidence and further prospective studies with increased number of patients are needed to better delineate the impact of individual variables on outcomes.
There is paucity of literature reporting incidence of uncommon sites of metastasis, specifically in ALK+ NSCLC. Unusual metastatic sites have been reported in only 9 of 213 NSCLC (all subtypes) patients including intestine (N=3), spleen (N=2), cervical lymph node (N=2), pancreas (N=1), and kidney (N=1).24 Another study showed that ALK+ NSCLC patients had increased median number of metastatic sites (mean=3.6 sites) compared with EGFR, KRAS, and triple-negative patients (mean around 2.5 sites).25 There was increased incidence of pleural metastasis in ALK patients compared with triple-negative cohort.25 In a study of 39 ROS+ NSCLC and 196 ALK+ NSCLC patients Gainor et al26 demonstrated that when compared with ROS1+ tumors, ALK+ tumors have significantly increased rates of extrathoracic metastasis at initial diagnosis. There are other case reports of ALK+ NSCLC patients with metastasis to uncommon visceral organs. West et al reported a 50-year-old female with stage IV ALK+ NSCLC who developed crizotinib resistant metastatic lesion in her adnexa apart from her initial metastatic sites of liver and bone.27 Another study showed a 62-year-old non-smoking Caucasian woman presented with epigastric pain, and was found to have NSCLC when a gastric ulcer was biopsied after EGD was performed.28 In 2017 another case of a 29-year-old woman with subcutaneous nodule adjacent to her scapula which was initially treated as folliculitis without any improvement in symptoms and then was later found to be biopsy-proven NSCLC was reported.29 In contrast to the sporadic case reports and nonselective cohorts, in our study we found that metastasis in uncommon sites can be present in up to one third of the patients with ALK+ lung cancer. It is also worth noting that majority of our patients had regional lymph node metastasis either in mediastinum or hilar lymph nodes (85%). Besides the common lymph node metastasis we also found that patient had metastasis in retroperitoneal (N=13), paraesophageal (N=4), omental lymph nodes (N=4), and internal mammary lymph nodes (N=3). There was increased incidence of brain metastases in up to 66% of ALK+ patients compared with the previously reported 30% to 50% brain metastasis in all NSCLC.30–32 ALK+ NSCLC also has increased tendency to metastasize in soft tissue or chest/abdominal wall (42%) compared with other uncommon sites (Supplemental Fig. 1, Supplemental Digital Content 1, http://links.lww.com/AJCO/A235).
In the current study that uncommon sites of metastasis were identified at any point of patient follow-up and patients with uncommon site of metastasis received additional lines of treatment compared with patients with only common sites of metastasis. At the same time these patients with uncommon sites of metastasis were followed for a shorter duration of time since diagnoses (Supplemental Table 2, Supplemental Digital Content 5, http://links.lww.com/AJCO/A239). This suggested that uncommon metastasis may appear as a result of treatment resistance or progression of disease and further correlative studies are needed to describe this. Whether similar patterns of metastasis are seen in larger cohorts of other subsets such as EGFR and KRAS mutant NSCLC remains to be seen.
In general EGFR mutations are associated with female individuals, nonsmokers with increased groundglass opacities, absence of emphysema and pleural retraction where KRAS mutations are associated with increased smoking, round-shaped primary lesion, and nodules in the known tumor lobes. In contrast ALK tumors were associated with young age, central location, increased pleural effusion, extranodal invasion, and lymphangitis which is consistent with the current study (Table 2).33,34 Newer 3D imaging techniques have led to possible correlations between the biological behavior of the tumor and CT scan characteristics of the primary lesion.35,36 For example, the proportion of solid tumors size compared with the whole tumor size on 3D CT evaluation significantly correlates with tumor invasiveness which in turn can be useful to differentiate malignant tumor from benign lesions.37 However, there is a paucity of data studying the role of 3D imaging the metastatic setting. Yamamoto et al developed an imaging biomarker which showed strong discriminatory power for ALK+ status in patients who were younger than 60 years of age and had central tumor location, absence of pleural tail, and large pleural effusion.38 Our study explores the multimodality data by using the 3D volumetric imaging which helps in diagnostic and prognostic accuracy. Standard CT imaging is widely used based on 2D tumor morphological assessment but due to variations in CT protocol and slice thickness there are question marks for the accuracy of the tumor metastatic pattern. The introduction of 3D technology allows pixel-based information on each slice with histogram charts which is critical to define the tumor patterns on each slice. Moreover, the reconstruction of the CT images explores the tumor heterogeneous characteristic of tumor morphology. This current study shows that there is prospective to utilize 3D volumetric images and mutation analysis to create histograms of patients which could identify uncommon sites of metastasis, assess treatment response, and help in better understanding of cellular and molecular properties of NSCLC which may be the future direction of precision medicine.
Historically it has been known that the coexisting mutations are only present in 1% to 3.2% NSCLC patients,13,39,40 however, there is recent emerging prospective data suggesting 12.3% rate of coexisting mutations in nonsquamous NSCLC.41 In a review, 100 cases with concomitant EGFR mutation and ALK rearrangement in NSCLC were reported.42 Blakely et al43 described that tumor genomic complexity increases with EGFR TKI and there are coexisting mutations in CTNNB1 and PIK3CA that promote tumor metastasis or limited treatment response. Another study showed 12 of 6637 EGFR-mutated patients had either additional KRAS or EML-ALK mutations. It was interesting to note that patients with KRAS mutations had papillary, solid, acinar imaging phenotype compared with solid, cribriform, and micropapillary patterns in ALK patients.44 In addition, the reported rate of coexisting mutations can be biased due to variations in number of mutations analyzed, different NGS techniques and sequencing errors.45,46 In the current study 8 patients had concomitant TP53 mutation, 5 EGFR, 4 KRAS, and several other patients with mutations of unknown significance. All the patients with uncommon sites of metastasis also had concomitant mutations (Supplemental Fig. 3, Supplemental Digital Content 4, http://links.lww.com/AJCO/A238). In spite of the presence of other mutations majority of the patients were treated with ALK TKI (Table 4). Several case reports and short series have shown conflicting responses with different TKI in patients with coexisting mutations47–50 and it will be interesting to see the future treatment guidelines in these patients.
Our study has some limitations due to its retrospective design and small cohort of patients. Currently due to paucity of the reported literature there is no standard definition of uncommon or unusual sites of metastasis based on presentation percentage or some reproducible cutoff. In the current study we chose to define the “uncommon sites” as any metastatic site which is beyond the common site of brain, bone, liver, adrenal glands, and thoracic cavity. Several of the patients were treated at an outside institution before they were referred to COH leading to lack of NGS testing and heterogeneous treatment course. We did not have a comparison group of EGFR or KRAS-positive NSCLC patients to evaluate the uncommon metastatic sites in those cohorts. In addition, patients had different NGS panel which check for different set of genomic mutations so incidence of the coexisting mutations could have low. Despite of these limitations this is one of the largest cohorts of a rare subtype of NSCLC highlighting uncommon patterns of metastasis and coexisting mutations in ALK+ tumors.
In conclusion, patients with metastatic ALK+ NSCLC tend to have more uncommon sites of metastasis when compared with previously reported NSCLC metastatic studies. Patients with uncommon sites of metastasis may have worse outcomes compared with patients with common sites of metastasis. Better understanding of associated mutations is needed to assess their role in prognosis and treatment. Further prospective studies comparing uncommon metastatic sites in other molecular variants of NSCLC are needed.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.amjclinicaloncology.com.
Footnotes
This study was supported by NIH/NCI P30CA033572 and NIH/NCI 1U54CA209978-01A1.
The authors declare no conflicts of interest.
REFERENCES
- 1.Forman D, Ferlay J, Stewart B, et al. The global and regional burden of cancer. World Cancer Report. 2014;2014:16–53. [Google Scholar]
- 2.Hirsch FR, Scagliotti GV, Mulshine JL, et al. Lung cancer: current therapies and new targeted treatments. Lancet. 2016;389:299–311. [DOI] [PubMed] [Google Scholar]
- 3.Morris SW, Kirstein MN, Valentine MB, et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science. 1994;263:1281–1284. [DOI] [PubMed] [Google Scholar]
- 4.Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4–ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–566. [DOI] [PubMed] [Google Scholar]
- 5.Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non–small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009;27:4247–4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. National Institutes of Health. Crizotinib US product insert. Available at: http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2a51b0de-47d6-455e-a94c-d2c737b04ff7. [Google Scholar]
- 7.Laktionov KK, Reutova EV, Sarantseva KV. ALK-positive non-small cell lung cancer and its treatment: a literature review. J Pharmateca. 2015;18. Available at: https://pharmateca.ru/en/archive/article/32148.
- 8.Waskom M, Botvinnik O, Hobson P, et al. Seaborn: statistical data visualization. 2014. Available at: https://seaborn.pydata.org/. Accessed May 15, 2017. [Google Scholar]
- 9.McKinney W. Pandas: a foundational Python library for data analysis and statistics. Python High Performance Sci Comput. 2011. Available at: https://www.dlr.de/sc/Portaldata/15/Resources/dokumente/pyhpc2011/submissions/pyhpc2011_submission_9.pdf. [Google Scholar]
- 10.Davidson-Pilon C. Survival analysis with lifelines. 2018. Available at: https://lifelines.readthedocs.io/en/latest/Survival%20analysis%20with%20lifelines.html.
- 11.Solomon B, Varella-Garcia M, Camidge DR. ALK gene rearrangements: a new therapeutic target in a molecularly defined subset of non-small cell lung cancer. J Thorac Oncol. 2009;4:1450–1454. [DOI] [PubMed] [Google Scholar]
- 12.Shaw AT, Solomon B. Targeting anaplastic lymphoma kinase in lung cancer. Clin Cancer Res. 2011;17:2081–2086. [DOI] [PubMed] [Google Scholar]
- 13.Barlesi F, Mazieres J, Merlio J-P, et al. Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT). Lancet. 2016;387:1415–1426. [DOI] [PubMed] [Google Scholar]
- 14.Ali SM, Hensing T, Schrock AB, et al. Comprehensive genomic profiling identifies a subset of crizotinib-responsive ALK-rearranged non-small cell lung cancer not detected by fluorescence in situ hybridization. Oncologist. 2016;21:762–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mok T, Kim D-W, Wu Y-L, et al. First-line crizotinib versus pemetrexed–cisplatin or pemetrexed–carboplatin in patients (pts) with advanced ALK-positive non-squamous non-small cell lung cancer (NSCLC): results of a phase III study (PROFILE 1014). J Clin Oncol. 2014;371:2167–2177. [Google Scholar]
- 16.Soria J-C, Tan DS, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet. 2017;389:917–929. [DOI] [PubMed] [Google Scholar]
- 17.Hida T, Nokihara H, Kondo M, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet. 2017;390:29–39. [DOI] [PubMed] [Google Scholar]
- 18.Kim D-W, Tiseo M, Ahn M-J, et al. Brigatinib in patients with crizotinib-refractory anaplastic lymphoma kinase–positive non–small-cell lung cancer: a randomized, multicenter phase II trial. J Clin Oncol. 2017;35:2490–2498. [DOI] [PubMed] [Google Scholar]
- 19.Sabir SR, Yeoh S, Jackson G, et al. EML4-ALK variants: biological and molecular properties, and the implications for patients. Cancers. 2017;9:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cha YJ, Kim HR, Shim HS. Clinical outcomes in ALK-rearranged lung adenocarcinomas according to ALK fusion variants. J Transl Med. 2016;14:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshida T, Oya Y, Tanaka K, et al. Differential crizotinib response duration among ALK fusion variants in ALK-positive non–small-cell lung cancer. J Clin Oncol. 2016;34:3383–3389. [DOI] [PubMed] [Google Scholar]
- 22.Niu F-Y, Zhou Q, Yang J-J, et al. Distribution and prognosis of uncommon metastases from non-small cell lung cancer. BMC Cancer. 2016;16:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salgia R, Kulkarni P. The genetic/non-genetic duality of drug ‘resistance’ in cancer. Trends Cancer. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu Q, Wang Y, Liu H, et al. Treatment outcome for patients with primary NSCLC and synchronous solitary metastasis. Clin Transl Oncol. 2013;15:802–809. [DOI] [PubMed] [Google Scholar]
- 25.Doebele RC, Lu X, Sumey C, et al. Oncogene status predicts patterns of metastatic spread in treatment‐naive nonsmall cell lung cancer. Cancer. 2012;118:4502–4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gainor JF, Tseng D, Yoda S, et al. Patterns of metastatic spread and mechanisms of resistance to crizotinib in ROS1-positive non–small-cell lung cancer. JCO Precis Oncol. 2017;1:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.West AH, Yamada SD, MacMahon H, et al. Unique metastases of ALK mutated lung cancer activated to the adnexa of the uterus. Case Rep Clin Pathol. 2014;1:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diem S, Früh M, Rodriguez R, et al. EML4-ALK-Positive pulmonary adenocarcinoma with an unusual metastatic pattern: a case report. Case Rep Oncol. 2013;6:316–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tokat F, Zeren H, Barut P, et al. EML4-ALK-positive lung adenocarcinoma presenting an unusual metastatic pattern in a 29-year-old woman who is alive and well in her third year follow up: a case report. Respir Med Case Rep. 2017;22:117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yousefi M, Bahrami T, Salmaninejad A, et al. Lung cancer-associated brain metastasis: molecular mechanisms and therapeutic options. Cellular Oncol. 2017;40:419–441. [DOI] [PubMed] [Google Scholar]
- 31.Gavrilovic IT, Posner JB. Brain metastases: epidemiology and pathophysiology. J Neurooncology. 2005;75:5–14. [DOI] [PubMed] [Google Scholar]
- 32.Sørensen J, Hansen H, Hansen M, et al. Brain metastases in adenocarcinoma of the lung: frequency, risk groups, and prognosis. J Clin Oncol. 1988;6:1474–1480. [DOI] [PubMed] [Google Scholar]
- 33.Park J, Kobayashi Y, Urayama KY, et al. Imaging characteristics of driver mutations in EGFR, KRAS, and ALK among treatment-naive patients with advanced lung adenocarcinoma. PloS One. 2016;11:e0161081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rizzo S, Petrella F, Buscarino V, et al. CT radiogenomic characterization of EGFR, K-RAS, and ALK mutations in non-small cell lung cancer. Eur Radiol. 2016;26:32–42. [DOI] [PubMed] [Google Scholar]
- 35.Tsutani Y, Miyata Y, Yamanaka T, et al. Solid tumors versus mixed tumors with a ground-glass opacity component in patients with clinical stage IA lung adenocarcinoma: prognostic comparison using high-resolution computed tomography findings. J Thorac Cardiovas Surg. 2013;146:17–23. [DOI] [PubMed] [Google Scholar]
- 36.Matsuguma H, Oki I, Nakahara R, et al. Comparison of three measurements on computed tomography for the prediction of less invasiveness in patients with clinical stage I non–small cell lung cancer. Ann Thorac Surg. 2013;95:1878–1884. [DOI] [PubMed] [Google Scholar]
- 37.Shikuma K, Menju T, Chen F, et al. Is volumetric 3-dimensional computed tomography useful to predict histological tumour invasiveness? Analysis of 211 lesions of cT1N0M0 lung adenocarcinoma. Interact Cardiovasc Thorac Surg. 2016;22:831–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamamoto S, Korn RL, Oklu R, et al. ALK molecular phenotype in non–small cell lung cancer: CT radiogenomic characterization. Radiology. 2014;272:568–576. [DOI] [PubMed] [Google Scholar]
- 39.Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311:1998–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li S, Li L, Zhu Y, et al. Coexistence of EGFR with KRAS, or BRAF, or PIK3CA somatic mutations in lung cancer: a comprehensive mutation profiling from 5125 Chinese cohorts. Br J Cancer. 2014;110:2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martorell PM, Huerta M, Quilis AC, et al. Coexistence of EGFR, KRAS, BRAF, and PIK3CA mutations and ALK rearrangement in a comprehensive cohort of 326 consecutive spanish nonsquamous NSCLC patients. Clin Lung Cancer. 2017;18:e395–e402. [DOI] [PubMed] [Google Scholar]
- 42.Russo GL, Imbimbo M, Corrao G, et al. Concomitant EML4-ALK rearrangement and EGFR mutation in non-small cell lung cancer patients: a literature review of 100 cases. Oncotarget. 2017;8:59889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blakely CM, Watkins TB, Wu W, et al. Evolution and clinical impact of co-occurring genetic alterations in advanced-stage EGFR-mutant lung cancers. Nat Genet. 2017;49:1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee T, Lee B, Choi Y-L, et al. Non-small cell lung cancer with concomitant EGFR, KRAS, and ALK mutation: clinicopathologic features of 12 cases. J Pathol Translational Med. 2016;50:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Won J, Keam B, Koh J, et al. Concomitant ALK translocation and EGFR mutation in lung cancer: a comparison of direct sequencing and sensitive assays and the impact on responsiveness to tyrosine kinase inhibitor. Ann Oncol. 2014;26:348–354. [DOI] [PubMed] [Google Scholar]
- 46.Lee JK, Kim TM, Koh Y, et al. Differential sensitivities to tyrosine kinase inhibitors in NSCLC harboring EGFR mutation and ALK translocation. Lung Cancer. 2012;77:460–463. [DOI] [PubMed] [Google Scholar]
- 47.Ulivi P, Chiadini E, Dazzi C, et al. Nonsquamous, non-small-cell lung cancer patients who carry a double mutation of EGFR, EML4-ALK or KRAS: frequency, clinical-pathological characteristics, and response to therapy. Clin Lung Cancer. 2016;17:384–390. [DOI] [PubMed] [Google Scholar]
- 48.Chen X, Zhang J, Hu Q, et al. A case of lung adenocarcinoma harboring exon 19 EGFR deletion and EML4-ALK fusion gene. Lung Cancer. 2013;81:308–310. [DOI] [PubMed] [Google Scholar]
- 49.Rossi G, Baldi L, Barbieri F, et al. Concomitant EGFR and KRAS mutations in ALK-rearranged lung cancer. Ann Oncol. 2015;26:1035–1036. [DOI] [PubMed] [Google Scholar]
- 50.Baldi L, Mengoli MC, Bisagni A, et al. Concomitant EGFR mutation and ALK rearrangement in lung adenocarcinoma is more frequent than expected: report of a case and review of the literature with demonstration of genes alteration into the same tumor cells. Lung Cancer. 2014;86:291–295. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.amjclinicaloncology.com.