Background/Objective
Systemic lupus erythematosus (SLE) is an inflammatory, chronic, and multisystemic disease, which may be associated with a wide range of neuropsychiatric manifestations, including cognitive impairment. Cognitive evaluations based on screening tests might identify early SLE-related cognitive alterations. The aim of this study was to evaluate and to compare the efficacy of three screening tests (Montreal Cognitive Assessment [MoCA], Mini Mental State Examination [MMSE], Cognitive Symptom Inventory [CSI]) against the gold standard (neuropsychological battery), in order to identify the most efficient screening test for cognitive impairment in patients with SLE.
Methods
This observational cross-sectional study recruited 44 patients, from August to December 2017, who were diagnosed with SLE according to the Systemic Lupus International Collaborating Clinics (SLICC) Criteria 2012, and had no medical or psychiatric comorbidities. The patients were evaluated using the MoCA, MMSE, CSI, and the gold standard. Spearman’s correlation and area under the curve analysis were performed; p < 0.05 was considered significant.
Results
The MoCA test showed the highest correspondence with the gold standard (AUC = 99.4%, p < 0.001), sensitivity (84%), and specificity (100%). This was followed by the MMSE (AUC = 92.6%, p < 0.001; sensitivity, 54.8%; specificity, 100%) and the CSI (AUC = 30.6%, p < 0.05; sensitivity, 54.8%; specificity, 30.76%).
Conclusion
The MoCA is a brief, easily applied screening test that is highly effective for detecting cognitive impairment in SLE patients. It could be useful in clinical follow-up as a tool for early detection of cognitive alterations.
Key Words: cognitive impairment, neuropsychiatric lupus, neuropsychological battery, screening tests
Systemic lupus erythematosus (SLE) is an inflammatory, chronic, and multisystemic disease, with up to 60% of patients showing neuropsychiatric manifestations.1–5 Neuropsychiatric involvement could affect the patients’ quality of life negatively, resulting in poor cognitive development, disability, and death.1,2,5
In the 19 neuropsychiatric syndromes described by the American College of Rheumatology (ACR) in 1999,6,7 one of the most frequent is cognitive impairment (present in up to 65% of SLE patients8), which can be associated with alterations in simple or complex attention, memory, visual–spatial processing, language, reasoning and problem solving, psychomotor speed, and executive functions.9–11 This could affect the self-concept of patients as well as their ability to communicate.5
Hence, there is a need to assess cognitive impairment appropriately. The neuropsychological battery (gold standard) is time-consuming to implement.7,10,12 Consequently, we considered it necessary to evaluate the use of other screening tools in comparison with the neuropsychological battery. The aim of our study was therefore to evaluate and compare the efficacy of three screening tests for cognitive impairment (the Mini Mental State Examination [MMSE],13,14 the Montreal Cognitive Assessment [MoCA],15,16 and the Cognitive Symptom Inventory [CSI]17) against the gold standard7,12 to identify the most efficient screening test, in patients with SLE.
MATERIALS AND METHODS
In this observational cross-sectional study, we recruited SLE patients who attended the outpatient rheumatology clinic of the Hospital Civil Juan I, Menchaca, Guadalajara, from August to December 2017. All patients were able to read and write Spanish fluently, with a minimum of 6 years of elementary school, and were legally capable of giving informed consent. The study was approved by the relevant institutional review board (002017-188).
We excluded patients who had cerebral vascular disease, hypertension, dyslipidemia, diabetes, thyroid pathology, a history of head trauma with loss of alertness or established neurological damage, drug abuse or dependence, or underlying psychiatric illness, as evaluated by a complete medical examination and a semi-structured psychiatric interview (MINI Plus 7.0). A complete medical examination was performed and subsequently the neuropsychological battery was applied, followed by the MoCA, MMSE (validated in Spanish language18,19), and the CSI (translated into Spanish by the LUMINA group17,20).
The MoCA test evaluates attention, concentration, executive functions, memory, language, visuospatial cognitive capacity, abstraction, calculation, and orientation. A score ≤25 is considered positive for cognitive impairment.
The MMSE measures cognitive impairment in people with dementia or delirium. It evaluates orientation, learning-evocation, attention, language, and visual-spatial construction. The result of this test is given as follows: no deterioration (30–26 points), doubtful or possible deterioration (25 points), mild to moderate dementia (24–10), moderate to severe dementia (9–6), and severe dementia (<6 points).
The CSI comprises 21 questions that aim to determine the ability of the person to perform certain cognitive tasks during activities of daily living. Each of the questions is scored on a scale of 0 to 4, and thus the maximum score is 84 points. A higher score indicates more severe cognitive impairment.
The neuropsychological battery we used comprised the following: the Matrix Wechsler Adult Intelligence Scale-Fourth Edition (WAIS-IV) test, Vocabulary WAIS-IV test, Analogies WAIS-IV test, Rey–Osterrieth complex figure test, Test de Aprendizaje Verbal España-Complutense (TAVEC), Semantic and phonemic fluency tests, D2 test, Stroop test, Trail Making test A and B, Paced Auditory Serial Addition test (PASAT), Digit-Symbol coding WAIS-IV test, letter–number sequencing WAIS-IV test, and Finger Tapping test. Using this battery, we evaluated visual, verbal, and abstract reasoning, visual and verbal memory, language, attention (selective, divided, and sustained), processing speed, and motor control.
Statistical Analyses
Results are given as mean ± standard deviation (SD) or percentages, as appropriate. The results of the screening instruments and of the neuropsychological battery were compared by χ2 tests. To calculate the sensitivity and specificity of each screening instrument, the area under the curve (AUC) was determined. Spearman’s correlation coefficient was employed to evaluate the relationship between all the applied instruments. For all statistical analyses, SPSS v.24 (IBM®) software was used and a p-value less than 0.05 was considered statistically significant.
RESULTS
The clinical and demographic characteristics of the 44 SLE patients are shown in Table 1.
TABLE 1.
Clinical and Demographic Characteristics in Lupus Patients
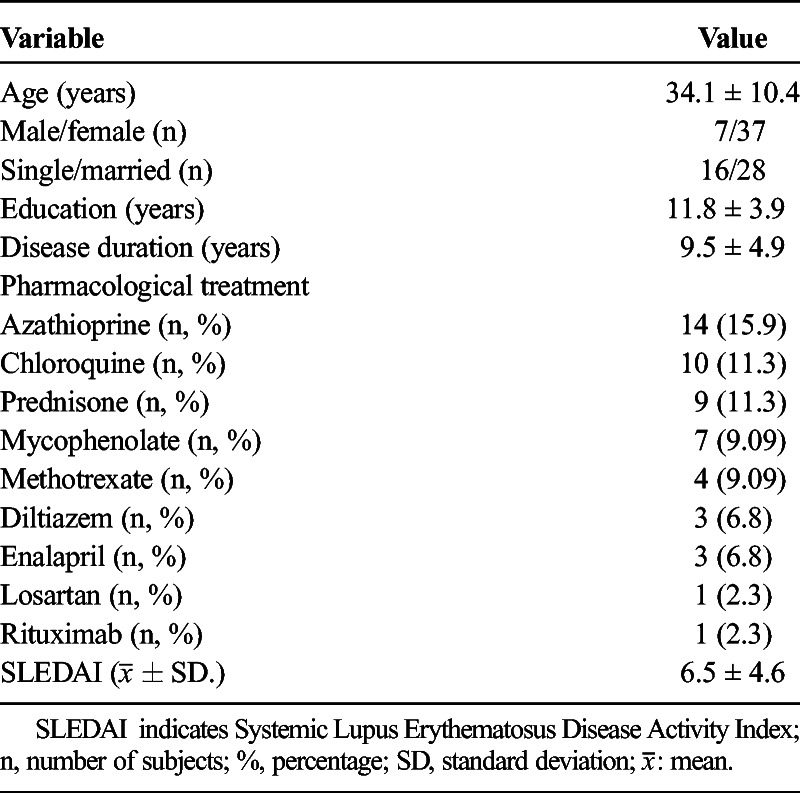
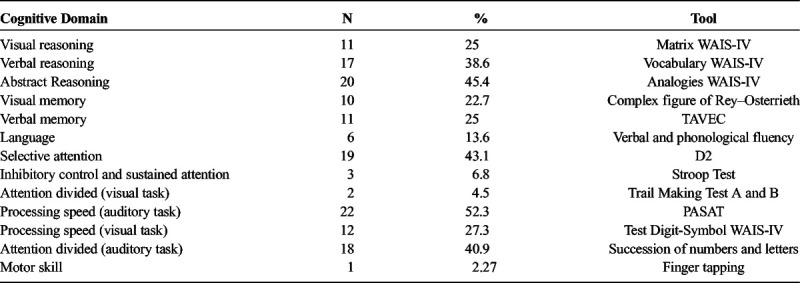
Using the gold standard, we identified cognitive impairment in at least one domain in 31 patients (70.4%). The most frequently affected cognitive domain was processing speed (evaluated with the PASAT), with 23 affected patients (52.3%), including 4 men (9.1%) and 19 women (43.18%). The least frequently affected domain was motor control (evaluated with the Finger Tapping test), with only 1 patient affected (2.27%) (Table 2).
TABLE 2.
Number of Affected Patients for Each Cognitive Domain, Obtained Through the Neuropsychological Evaluation
The CSI classified 26 patients (59.1%) with cognitive impairment, with a minimum score of 26, maximum score of 30, and an average score of 37.1 ± 1.2 (SD). This instrument showed a sensitivity of 54.8% and a specificity of 30.76% for identifying cognitive impairment in SLE patients.
On the other hand, from 17 MMSE detected patients (38.6% of SLE group) with cognitive impairment showed 24 ± 1 average score, being 22 minimum and 25 as maximum score. This instrument has presented a 54.8% sensitivity and a 100% specificity.
Moreover, from 26 MoCA cognitive impairment detected patients (59.1% of SLE group) a score of 21.5 ± 1.7 was observed, with 19 as minimum and 24 as maximum score. A 84% sensitivity and a 100% specificity were obtained with this instrument.
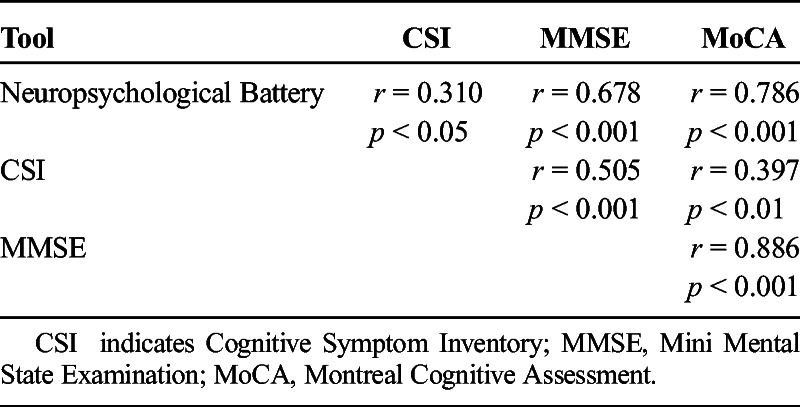
Of the three screening tools, the MoCA presented the strongest correlation with the gold standard (AUC = 99.4%, rs = 0.786, p < 0.001), followed by the MMSE, which showed a moderate correlation (AUC = 92.6%, rs = 0.505, p < 0.001). Of the three screening tests, the CSI showed the least significant correlation with the gold standard (AUC = 30.6%; rs = 0.310, p < 0.05, Table 3).
TABLE 3.
Correlations Between the Instruments Used to Assess Cognitive Impairment
No correlation was observed between age and the screening test scores, or the number of affected domains as evaluated by the gold standard. On the other hand, disease duration correlated positively with affected domains (rs = 0.319, p = 0.009), and negatively with the MMSE (rs = −0.336, p = 0.026) and MoCA (rs = −0.356, p = 0.018) scores. No correlation was found between the SLE disease activity index and any of the instruments employed, including the gold standard neuropsychological battery.
DISCUSSION
In our study, the MoCA test was the screening instrument that identifie the largest number of patients with cognitive impairment, followed by the MMSE and CSI, similar to the findings of previous reports.10,21–23 In addition, the three instruments correlated with the gold standard neuropsychological battery findings, in the same order (MoCA > MMSE > CSI), providing concurrent validity. Our study is robust, as the application of all three screening tools for assessing cognitive impairment as compared to the gold standard has not been reported previously.
Nantes et al. conducted a study in 98 patients with SLE, in which the MoCA and MMSE were compared against the Hopkins Verbal Learning Test-Revised.10 The MoCA test showed the highest sensitivity, while the MMSE presented highest specificity. In our study, the highest sensitivity and specificity were obtained with the MoCA test. Such differences might be due to our use of the gold standard recommended by the cognitive subcommittee of the ACR.7,10
Hanly et al. compared the results of the Hamilton scales for anxiety and depression, the CSI, and a neuropsychological battery. They found that the CSI items were more strongly related to the anxious and affective symptoms, than with cognitive deterioration.22 In a study carried out by D’Amico et al. in 86 Argentinean SLE patients, in which the CSI and a neuropsychological battery were applied, the authors reported poor validity of the CSI,24 in agreement with our results.
The MoCA test proved to be highly useful for the detection of cognitive impairment in patients with SLE. This test is a brief, simple, sensitive, and specific test, that does not require special training or additional economic cost. In the context of clinical follow-up, the MoCA can be used without excessively compromising consultation time, and may facilitate referral of patients with cognitive impairment to a formal neuropsychological evaluation; this would enable early diagnosis and timely treatment.
When we analyzed the cognitive domains from the neuropsychological battery, we found that processing speed (auditory task) was the most severely affected in SLE patients. In this context, two factors are important: the SLE per se and the concurrent therapy. Cognitive deterioration is an accepted part of the neuropsychiatric manifestations of SLE.7,8,17,25
One caveat of our study is that we did not include neuropsychiatric SLE7 patients for this evaluation, and did not perform functional magnetic brain resonance imaging or cerebrospinal fluid auto-antibody detection.3,26 Nevertheless, we found a positive correlation between disease duration and the affected domains as evaluated by implementing the gold standard.
It would be interesting to investigate the influence of pharmacological treatment and cognitive impairment in SLE in future. The main candidates is glucocorticoid therapy, which has an impact on cognitive impairment.8,27 Nonetheless, this was not the aim of our study. Unlike other previous studies, our study explored the relationship between formal neuropsychological assessments, performed with a battery similar to that recommended by the ACR, and three different screening tests for cognitive impairment in people with SLE. In the context of a clinical follow-up, knowing this may assist in helping patients to avoid forgetting to take their medication, by facilitating early diagnosis and treatment of cognitive impairment.
For future research, we suggest that the sample size should be increased and that the temporal stability (test–retest) of the evaluations should be determined. Additionally, pharmacological treatment, which can have a negative impact on cognition in general, should be considered.
CONCLUSION
The MoCA is a brief, simple, sensitive, and specific test, requiring no special training or additional costs to implement, that could be useful in a clinical context to facilitate the evaluation of patients with SLE for cognitive impairment, enabling early diagnosis and treatment. Due to the high rate of cognitive impairment in SLE patients, we therefore encourage rheumatologists to apply the MoCA test as a valuable and easily implemented tool for detecting cognitive impairment as part of an integrated approach in SLE.

Footnotes
None of the authors have any conflicts of interest to declare.
Conflict of interest and ethical and legal aspects: There is no conflict of interest in the application of the tests carried out on the study group that compromised the results on the part of the researchers or the study participants.
This investigation did not imply risks for the health of the patients since no intervention or invasive procedures were applied; only scales of measurement were applied to assess their cognitive function, for which informed consent was requested from the patients prior to commencement, as well as that of two witnesses other than the researcher, strictly following the guidelines of the Helsinki Declaration.
REFERENCES
- 1.Hanly JG, Su L, Farewell V, et al. Prospective study of neuropsychiatric events in systemic lupus erythematosus. J Rheumatol. 2009;36:1449–1159. [DOI] [PubMed] [Google Scholar]
- 2.Kozora E, Hanley JG, Lapteva L, et al. Cognitive dysfunction in systemic lupus erythematosus: past, present, and future. Arthritis Rheum. 2008;58:3286–3298. [DOI] [PubMed] [Google Scholar]
- 3.Liu S, Cheng Y, Lao Y, et al. Clinical factors associated with brain volume reduction in systemic lupus erythematosus patients without major neuropsychiatric manifestations. Front Psychiatry. 2018;9:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahdavi Adeli A, Haghighi A, Malakouti SK. Prevalence of cognitive disorders in patients with systemic lupus erythematosus: a cross-sectional study in Rasoul-e-Akram Hospital, Tehran, Iran. Arch Iran Med. 2016;19:257–261. [PubMed] [Google Scholar]
- 5.Moorthy LN, Petersen MGE, Hasset A, et al. Impact of lupus on school attendance and performance. Lupus. 2010;19:620–627. [DOI] [PubMed] [Google Scholar]
- 6.Cervera R, Khamashta MA, Font J, et al. Morbidity and mortality in systemic lupus erythematosus during a 5-year period. A multicenter prospective study of 1,000 patients. European Working Party on Systemic Lupus Erythematosus. Medicine (Baltimore). 1999;78:167–175. [DOI] [PubMed] [Google Scholar]
- 7.The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum. 1999;42:599–608. [DOI] [PubMed] [Google Scholar]
- 8.Butt BA, Farman S, Khan SE, et al. Cognitive dysfunction in patients with systemic lupus erythematosus. Pak J Med Sci. 2017;33:59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dorman G, Micelli M, Cosentino V, et al. [Cognitive dysfunction in systemic lupus erythematosus in relation to disease activity and damage]. Medicina (B Aires). 2017;77:257–260. [PubMed] [Google Scholar]
- 10.Nantes SG, Su J, Dhaliwal A, et al. Performance of screening tests for cognitive impairment in systemic lupus erythematosus. J Rheumatol. 2017;44:1583–1589. [DOI] [PubMed] [Google Scholar]
- 11.Leslie B, Crowe SF. Cognitive functioning in systemic lupus erythematosus: a meta-analysis. Lupus. 2018;p. 961203317751859. [DOI] [PubMed] [Google Scholar]
- 12.Hanly JG. Diagnosis and management of neuropsychiatric SLE. Nat Rev Rheumatol. 2014;10:338–347. [DOI] [PubMed] [Google Scholar]
- 13.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 14.Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. [DOI] [PubMed] [Google Scholar]
- 15.Bertsias GK, Boumpas DT. Pathogenesis, diagnosis and management of neuropsychiatric SLE manifestations. Nat Rev Rheumatol. 2010;6:358–367. [DOI] [PubMed] [Google Scholar]
- 16.Adhikari T, Piatti A, Luggen M. Cognitive dysfunction in SLE: development of a screening tool. Lupus. 2011;20:1142–1146. [DOI] [PubMed] [Google Scholar]
- 17.Ad Hoc Committee on Lupus Response Criteria: Cognition Sub-committee, Mikdashi JA, Esdaile JM, et al. Proposed response criteria for neurocognitive impairment in systemic lupus erythematosus clinical trials. Lupus 2007;16:418–425. [DOI] [PubMed] [Google Scholar]
- 18.Gil L, Ruiz de Sánchez C, Gil F, et al. Validation of the Montreal Cognitive Assessment (MoCA) in Spanish as a screening tool for mild cognitive impairment and mild dementia in patients over 65 years old in Bogotá, Colombia. Int J Geriatr Psychiatry. 2015;30:655–662. [DOI] [PubMed] [Google Scholar]
- 19.Rosselli D, Ardila A, Pradilla G, et al. [The Mini-Mental State Examination as a selected diagnostic test for dementia: a Colombian population study. GENECO]. Rev Neurol. 2000;30:428–432. [PubMed] [Google Scholar]
- 20.Alarcón GS, Cianfrini L, Bradley LA, et al. Systemic lupus erythematosus in three ethnic groups. X. Measuring cognitive impairment with the cognitive symptoms inventory. Arthritis Rheum. 2002;47:310–319. [DOI] [PubMed] [Google Scholar]
- 21.Benedict RH, Shucard JL, Zivadinov R, et al. Neuropsychological impairment in systemic lupus erythematosus: a comparison with multiple sclerosis. Neuropsychol Rev. 2008;18:149–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanly JG, Su L, Omisade A, et al. Screening for cognitive impairment in systemic lupus erythematosus. J Rheumatol. 2012;39:1371–1377. [DOI] [PubMed] [Google Scholar]
- 23.Tani C, Palagini L, Moraes-Fontes MF, et al. Neuropsychiatric questionnaires in systemic lupus erythematosus. Clin Exp Rheumatol. 2014;32:S-59–64. [PubMed] [Google Scholar]
- 24.D’Amico MA, Romero JD, Rodriguez G, et al. Estudio multicéntrico de deterioro cognitivo en lupus eritematoso sistémico: ECLES. Rev Arg Reumatol. 2015;26:28–32. [Google Scholar]
- 25.Bertsias GK, Ioannidis JP, Aringer M, et al. EULAR recommendations for the management of systemic lupus erythematosus with neuropsychiatric manifestations: report of a task force of the EULAR standing committee for clinical affairs. Ann Rheum Dis. 2010;69:2074–2082. [DOI] [PubMed] [Google Scholar]
- 26.Sato T, Fujii T, Yokoyama T, et al. Anti-U1 RNP antibodies in cerebrospinal fluid are associated with central neuropsychiatric manifestations in systemic lupus erythematosus and mixed connective tissue disease. Arthritis Rheum. 2010;62:3730–3740. [DOI] [PubMed] [Google Scholar]
- 27.Kenna HA, Poon AW, de los Angeles CP, et al. Psychiatric complications of treatment with corticosteroids: review with case report. Psychiatry Clin Neurosci. 2011;65:549–560. [DOI] [PubMed] [Google Scholar]


