Abstract
Polyvinyl chloride (PVC) has toxic effects through the induction of oxidative stress in the body and testicles. Vitamin E (Vit E) is a dietary compound that functions as an antioxidant scavenging toxic free radicals. The present study aimed to probe the protective effect of Vit E against PVC-induced reprotoxicity in male rats. In this experimental study, 24 male rats were randomly divided into four groups (n=6) including control, Vit E (150 mg kg-1 per day; orally), PVC (1000 mg kg-1 per day; orally) and PVC + Vit E. After 40 days, rats were euthanized and epididymal sperms characteristics, embryo development and malondialdehyde (MDA) and testosterone levels were examined. The PVC decreased sperm count, motility and viability as well as testosterone level and increased sperms with damaged chromatin in comparison with controls. Also, the percentages of fertilization, two-cell embryos and blastocysts as well as MDA levels were decreased in PVC-treated rats. However, Vit E improved PVC-induced alterations in aforesaid parameters. The results indicated that PVC can reduce fertility potential in male rats probably through androgen and sperm quality and quantity reductions, while Vit E can exert protective effects in PVC-related reproductive toxicities.
Key Words: Embryology, Polyvinyl chloride, Rat, Spermatology, Vitamin E
Introduction
Polyvinyl chloride (PVC) is used as a raw material in the manufacture of chemical and plastic industries including disposable containers and plastic tubes, cables and wires, floor coverings, film photography, automotive electronics and toys.1 Due to the increasing production of the above-mentioned products, PVC production will also increase, resulting in more PVC exposure.2 Since PVC is not chemically bonded to the polymer, it is removed at the time of production and use of the polymer. Therefore, it is transmitted to humans through air, water, food and even using medical devices.3 One of the most important uses of PVC is in the manufacture of medical devices and laboratory equipment.4 Blood storage bags, injection and hemodialysis devices and chip tubes contain large quantities of PVC. A lot of the materials used to make PVC industries are found in the blood and tissues of patients having a frequent transfusion.5 However, the main source of human exposure to PVC is food.6 Most of the previous studies have been focused on the effects of PVC on the liver because the first organ that is damaged by its exposure is the liver. In PVC exposed cases, the risk of liver angiosarcoma developing was 11 to 16 times and the risk of brain cancer developing was four times higher than normal people.7 It has been shown that PVC exerts hepatotoxic, teratogenic, mutagenic and carcinogenic effects and produces renal, pulmonary and reproductive dysfunctions. The liver and testes appear to be the target organs of PVC toxicity. The PVC and other plasticizers have been reported to cause testicular atrophy associated with a decreased zinc concentration in the testes. Also, PVC can decrease the serum level of testosterone.8 Other studies have also shown that PVC causes apoptosis in testis, induces oxidative stress and reduces the activity of the antioxidant enzyme.9,10 Since oxidative stress in the testicle is one of the main causes of apoptosis in the germ cells, it has a high concentration of antioxidants such ascorbic acid and vitamin E (Vit E).10 These antioxidants protect the germ cells against DNA oxidative damage and play an important role in spermatogenesis.11 These facts show that the mechanism of defense against oxidative stress plays a vital role in preserving spermatogenesis and preventing testicular atrophy.
The reactive oxygen species (ROS) can damage the DNA of sperm in a variety of ways, such as changing the structure of DNA strands, the abnormal corrosion of DNA and re-matching the chromosomes.12 Awareness of the damage to the DNA of sperm will be beneficial for the adoption of a suitable method to establish treatment and laboratory assists strategies. Sperms used in the laboratory issues are usually exposed to oxidative stress resulting in DNA damage in a large number of sperms.13 As a result; there is the possibility of selecting sperm with a high risk of DNA damage.14
Today, due to the increasing use of plastic products, an increase in the consumption of PVC and more exposure to this environmental pollutant became a matter of great concern. Several treatments have been proposed to reduce the oxidative stress levels induced by PVC in testicular tissue. Vitamin E as a potent antioxidant acting against free radicals has a protective effect against the oxidative damage of DNA and can inhibit the lipid peroxidation in cell membranes via restricting free radicals adverse effects.15
Therefore, in the present experimental study, rats were given PVC as an oxidative stress inducer and Vit E as an antioxidant defense system promoter. This study aimed to determine the protective effect of Vit E against PVC-induced spermatotoxicity, embryotoxicity and testosterone concentration changes.
Materials and Methods
Animals and treatment. In this study, 24 male adult Wistar rats weighing 180 ± 20 g were purchased from authorized laboratory animal breeding center (Laboratory Animal House, Urmia University, Urmia, Iran). They were housed in a specific pathogen-free environment under standard conditions of temperature (22.00 ± 2.00 ˚C), relative humidity (50.00 ± 10.00%) and light (12 hr light/dark), fed with a standard pellet diet and had free access to water. Animal work was conducted in compliance with Guidelines for the Humane Care and Use of Laboratory Animals using protocols approved by the Urmia University (No. 2.PAD.299, 2017). Following two weeks, the rats were divided into four groups (n=6) including control, Vit E (150 mg kg-1 per day) (Jaber Ebn Hayan, Tehran, Iran), PVC (1000 mg kg-1 per day) and PVC (1000 mg kg-1 per day) + Vit E (150 mg kg-1 per day). The oral administration method was used and the duration of this study was considered as 40 days. The chemicals were purchased from Sigma (St. Louis, USA) unless otherwise stated. The PVC (Merck, Darmstadt, Germany) and Vit E were dissolved in normal saline and olive oil (Mazo Light, Tehran, Iran), respectively.16
Sampling. At the end of the treatment period, the rats were euthanized with 75.00 mg kg-1 ketamine (Alfasan, Woerden, The Netherlands) and 10.00 mg kg-1 xylazine (Alfasan, Woerden, The Netherlands), both intra-peritoneally (IP). Through a caudal abdominal incision, the reproductive system was exposed. The epididymis was carefully separated from the testis and placed in a 1.00 mL of modified rat 1-cell embryo culture medium (mR1ECM; Sigma) with 4 mg mL-1 bovine serum albumin (BSA).
Sperm characteristics. Epididymal sperms were collected by chopping one caudal epididymis in 1 mL of mR1ECM medium and incubated for 60 min at 37.00 ˚C in an atmosphere of 5.00% CO2 incubator to allow sperms to swim out of the epididymal tubules. To assess the sperm motility, one drop of sperm suspension was placed on a microscope slide and a coverslip was placed over the droplet. At least 10 microscopic fields were observed at 400× magnification using a phase-contrast microscope and motile sperms percentages were calculated.17
The epididymal sperm counts were obtained by the standard hemocytometric method as described previously.18 Briefly, after dilution of epididymal sperm to 1:20 in distilled water, approximately 10.00 μL of the diluted specimen was transferred to each of the counting chambers of the hemocytometer. The cells were sedimented during this time and counted with a light microscope at 400× magnification. The sperm count was expressed as the number of sperm per milliliter.
Acridine orange (AO) staining was used to assess the cauda epididymal sperm DNA denaturation. For the analysis of sperm DNA integrity with fluorescence microscopy, thick smears were fixed in Carnoy’s fixative (methanol: acetic acid; 1: 3) for at least 2 hr. The slides were stained for 5 min and gently rinsed with deionized water. Two-hundred sperms were evaluated and sperm heads with denatured chromatin displayed an orange-red fluorescence compared to those with intact chromatin emitting green fluorescence.19
Evaluation of sperm abnormalities was performed as described previously.20 Sperm smears were prepared on clean and grease-free slides, allowed to air-dry overnight, stained with 1.00% eosin-Y/ 5.00% nigrosin and examined at 400x magnification for morphological abnormalities. Teratozoospermia index (TZI) was defined as the number of abnormalities present per abnormal spermatozoon. Each abnormal spermatozoon can have one to four abnormalities including head, neck/midpiece and tail defects or presence of cytoplasmic residues. The spermatozoa were recorded as normal or abnormal and distributed into specific groups (head, neck/midpiece and tail defects or cytoplasmic residues groups). The total number of abnormalities was then added together and divided by the number of abnormal spermatozoa.21
Oocyte collection. Female rats were super-ovulated with 25.00 IU of PMSG (Folligon, Boxmeer, Netherlands) hormone and 15.00 IU of hCG (Folligon, Boxmeer, The Netherlands) hormone, both IP. Twelve to sixteen hr after hCG injection, oocytes were harvested through the dissecting of the oviduct. The mR1ECM with 4.00 mg mL-1 BSA was placed in the 5.00% CO2 incubator at 37.00 ˚C overnight.One fertilization drop (500 μL), two wash drops (150 μL) and two overnight culture drops (150 μL) of the medium were put into 3×3 cm Petri dish and drops were covered by mineral oil. The oocytes were put in the fertilization drop and then capacitated sperms (1 × 106 per mL) were added to the fertilization drop. After 4 hr, the percentage of fertilized oocytes was examined under the inverted microscope and fertilized oocytes (zygotes) were transferred to fresh medium after washing. Meanwhile, the percentages of two-cell embryos and blastocysts were examined.22
Preparation of mR1ECM: Stock A. To prepare the stock, 0.239 g potassium chloride, 6.420 g sodium chloride, 1.352 g glucose, 0.075 g penicillin G, 0.050 g streptomycin and 1.900 mL sodium lactate were dissolved in 100 mL distilled water and the solution was sterilized by filtration through 0.20 μm filter and stored at 4.00 ˚C as stock A.
Preparation of mR1ECM: Stock B. The reagents including 0.102 g magnesium chloride and 0.294 g calcium chloride were dissolved in distilled water and adjusted to the volume of 100 mL. The solution was sterilized by filtration through a 0.20 μm filter and stored at 4.00 ˚C as stock B. All reagents (10.00 mL stock A, 10 mL stock B, 0.210 g sodium, 0.0055 g sodium pyruvate, 0.0146 g L-glutamine, 2.00 mL of essential amino acids and 1.00 mL non-essential amino acids) were dissolved in distilled water and adjusted to the volume of 100 mL. The osmotic pressure was adjusted to about 310 mOsm. The solution was sterilized by filtration through a 0.20 μm filter and stored at 4.00 ˚C.
Malondialdehyde (MDA) level determination. After homogenizing the testes, the levels of MDA were evaluated. To this end, 0.20 g of the testicular tissue was transferred to 0 °C 0.05M phosphate buffer with pH=7.40 (10.00% w/v) and ground by mortar and pestle. Then, the resulting solution was centrifuged at 1000 rpm. Afterward, 150 μg of the supernatant of the centrifuged specimen was removed and 300 μg of 10.00% trichloroacetic acid was added and centrifuged at 1000 rpm, 4 °C, for 10 min. Then, 300 μL of the supernatant was transferred to the test tube and incubated with 300 μL of 0.67% thiobarbituric acid at 100 °C for 25 min. After 5 min of cooling the solution, the pink color resulting from the reaction between MDA and thiobarbituric acid appeared and evaluated with a spectrophotometer at 535 nm wavelength. The concentration of MDA was calculated using the MDA absorption coefficient and expressed as nmol per g tissue.23
Testosterone level measurement. To measure the changes in serum levels of testosterone, blood samples were collected and centrifuged and sera were stored at –80.00 °C until testing. The concentrations of testosterone were measured by an ELISA method using a commercial kit (Ideal Tashkhis Atieh, Tehran, Iran).
Statistical analysis. Statistical analysis was performed using one-way ANOVA and Bonferroni test via the computer-based statistical programs (SPSS; version 24.0, IBM Corp., Armonk, USA). A p-value of less than 0.05 was considered statistically significant.
Results
Sperm count. The number of sperm in the PVC receiving group was significantly reduced compared to the control and Vit E groups (p < 0.05). However, the number of sperm was not significantly different in the PVC + Vit E group compared to the control and Vit E groups (p > 0.05; Table 1).
Table 1.
Effect of polyvinyl chloride (PVC) and vitamin E (Vit E) on epididymal sperm characteristics in different groups (Mean ± SEM).
| Groups | Sperm count (×10 6 mL -1 ) | Sperm motility (%) | Sperm viability (%) | Sperms with damaged DNA (%) |
|---|---|---|---|---|
| Control | 34.25 ± 2.71 a | 88.25 ± 3.01 a | 89.00 ± 2.41 a | 5.53 ± 0.40 a |
| Vit E | 36.50 ± 3.09 a | 86.75 ± 2.01 a | 86.50 ± 2.90 ab | 4.36 ± 0.67 a |
| PVC | 25.00 ± 1.29 b | 73.50 ± 3.22 b | 73.75 ± 2.92 b | 11.70 ± 1.76 b |
| PVC + Vit E | 27.50 ± 1.19 ab | 79.50 ± 1.32 ab | 79.50 ± 1.32 ab | 9.05 ± 0.80 ab |
ab Different letters indicate a significant difference between groups in each column (p < 0.05).
Sperm motility. The percentage of sperm motility in the PVC receiving group was significantly lower than the control and Vit E groups (p < 0.05). However, the administration of Vit E plus PVC resulted in an improvement in the percentage of motile sperms, which showed no significant difference compared to the control and Vit E groups (p > 0.05; Table 1).
Sperm viability. The percentage of sperm viability was significantly reduced due to PVC treatment (p < 0.05). However, the administration of Vit E in combination with PVC could improve the sperm viability, which was not significantly different compared to the control group (p > 0.05; Table 1 and Fig. 1A).
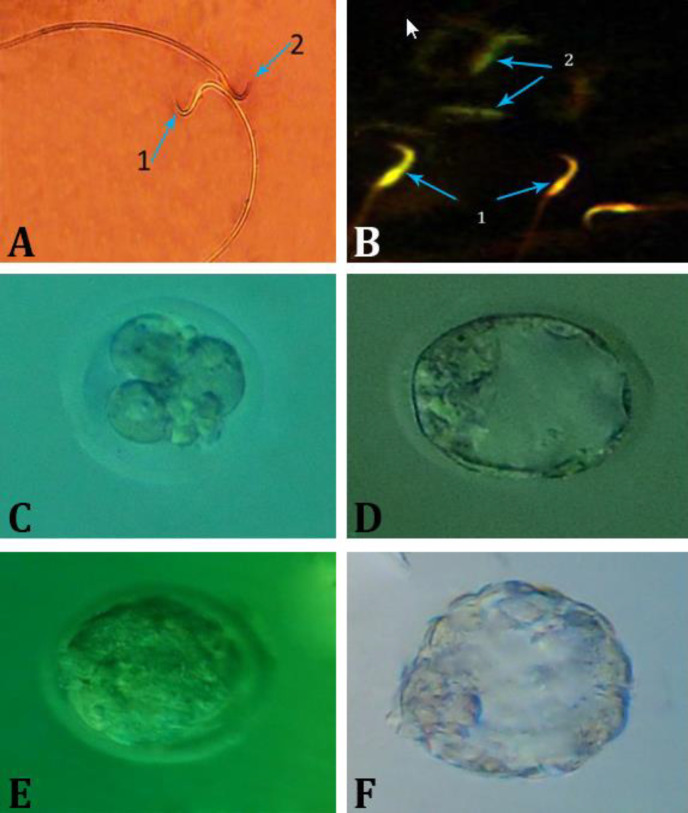
Fig. 1.
A) Photomicrograph of epididymal sperms stained with eosin-nigrosin in polyvinyl chloride (PVC) group. Live sperm (1) is not stained and dead sperm (2) appears pink (×1000); B) Sperms with the orange-colored head (1) having damaged chromatin and green-colored sperms with healthy chromatin (2) are seen in the PVC group (acridine orange staining technique, 1000×); C) Arrested 4-cell embryo; D) Blastocyst stage; E) Morula stage; F) Hatching embryo
Sperm DNA damage. To investigate the sperms with abnormal DNA via AO staining, it was found that in the PVC group, the number of sperms with abnormal DNA increased significantly compared to the control and Vit E groups (p < 0.05; Table 1 and Fig. 1B). In PVC + Vit E group, this increase was not significantly different compared to the control group (p > 0.05).
In vitro fertilization (IVF) outcome. Table 2 shows the IVF results in the experimental groups. According to the results, the zygote percentage in the PVC receiving group was significantly lower than that of the control and Vit E groups (p < 0.05). While, in the PVC + Vit E group, the zygote percentage was not significantly different from that of control and Vit E groups (p > 0.05). The mean of two-cell embryos in the PVC group showed a significant decrease compared to the control and Vit E groups (p < 0.05). Vitamin E significantly improved this alteration and there was no significant difference compared to the control and Vit E groups (p > 0.05). A significant reduction in the percentage of blastocysts was observed in the PVC group (p < 0.05). However, there was no significant difference in the percentage of blastocysts in the PVC + Vit E group compared to the control and Vit E groups (p > 0.05).
Table 2.
Effect of polyvinyl chloride (PVC) and vitamin E (Vit E) on in vitro fertilization outcomes in different groups (Mean ± SEM)
| Groups | Zygote (%) | Two-cell embryos (%) | Blastocysts (%) | Hatching embryos (%) | Arrested embryos (%) |
|---|---|---|---|---|---|
| Control | 85.57 ± 2.01 a | 85.63 ± 1.63 a | 72.86 ± 1.86 a | 57.77 ± 3.00 a | 11.36 ± 2.09 a |
| Vit E | 80.93 ± 2.14 a | 84.11 ± 2.54 a | 70.78 ± 2.45 a | 54.57 ± 1.60 ab | 16.56 ± 0.70 ab |
| PVC | 59.41 ± 1.23 b | 71.88 ± 0.93 b | 52.94 ± 2.94 b | 45.49 ± 1.38 b | 22.96 ± 2.96 b |
| PVC + Vit E | 71.04 ± 4.56 ab | 81.42 ± 1.42 a | 56.00 ± 4.00 b | 50.14 ± 1.57 ab | 18.34 ± 2.34 ab |
ab Different letters indicate a significant difference between groups in each column (p < 0.05).
The rate of completely hatched blastocysts was significantly lower in PVC-received rats compared to the controls (p < 0.05), while this ratio in PVC + Vit E group, was nearly restored to the control levels (p > 0.05).
The percentage of arrested embryos in the PVC group was significantly lower than the control and Vit E groups (p < 0.05). However, in PVC + Vit E group, this parameter did not show a significant difference compared to control and Vit E groups (p > 0.05; Table 2 and Figs. 1C-1F).
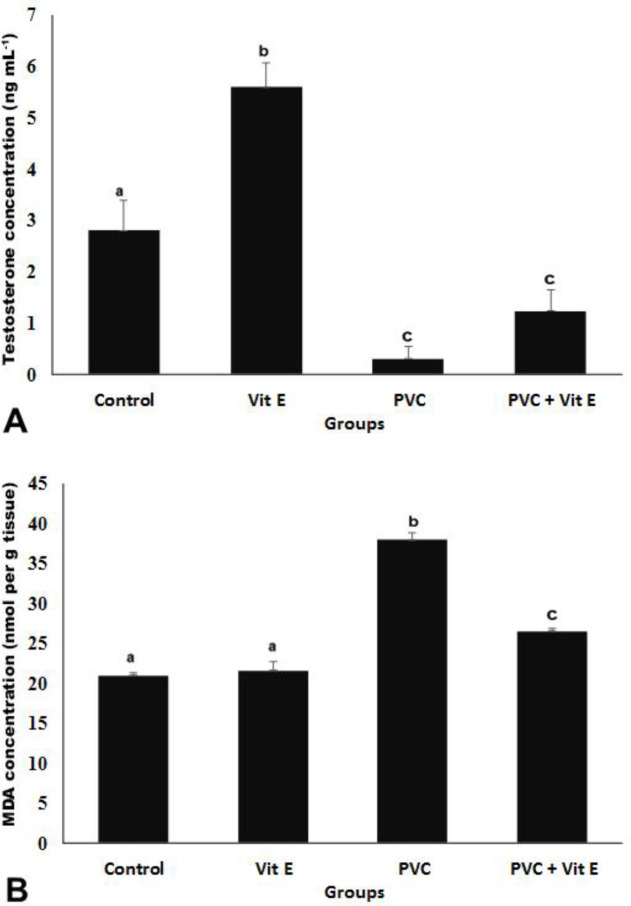
Testosterone level. Testosterone levels in PVC group significantly decreased compared to the control group, while the testosterone concentration was improved in PVC + Vit E group. However, there was a significant difference compared to the control group (p < 0.05; Fig. 2A).
Fig. 2.
Effect of vitamin E (Vit E) and polyvinyl chloride (PVC) on concentrations of A) testosterone and B) malondialdehyde (MDA) in the testis of different experimental groups (Mean ± SEM). abc Different letters indicate a significant difference between groups in each column (p < 0.05).
Malondialdehyde level. The MDA concentration significantly reduced in PVC group compared to controls, while it was significantly improved in PVC + Vit E group compared to the PVC-only group (p < 0.05; Fig. 2B).
Discussion
The PVC, an environmental contaminant, has been shown to interfere with normal reproductive processes leading to decreased sperm count and motility and semen quality.24 Conventionally, semen quality is typically measured by assessing sperm count, motility, morphology and vitality. Recently, sperm chromatin DNA integrity has also been considered as an important parameter for evaluating semen quality and might be correlated with other measures.25 Sperm DNA fragmentation and decondensation are being increasingly recognized as indicating parameters of early and late failures in reproductive technologies, respectively.26 In the present study, PVC caused a significant decrease in sperm count and percentage of live sperms. Studies have shown that PVC induces oxidative stress, reduces the activity of antioxidant enzymes and thus causes apoptosis in testicular tissue.27 One possible mechanism is that ROS generation might be correlated with PVC-induced calcium (Ca) entry, potentially through the Ca-mediated activation of the nicotinamide adenine dinucleotide phosphate complex.28 An experimental study using comet assay has shown that in vitro exposure to PVC induces DNA damage through the ROS generation in mouse Leydig cells.29 Oxidative stress may induce DNA damages in spermatozoa.30 The mechanism by which PVC-induced ROS generation causes damage to sperm DNA requires further investigations. Oxidative stress can cause DNA damage and apoptosis in the sperm resulting in reduced fertility.31 Previous studies have shown that the use of antioxidants in the culture medium improves the fertility ratio and implantation.32 Also, other reports have confirmed the role of antioxidants in reducing DNA damage and improving the proportion of blastocysts development in mice.33 The administration of Vit E (alpha-tocopherol), a potent antioxidant, showed a beneficial effect on spermatozoon by protecting the acrosome and improving mitochondrial activity. Vitamin E has been shown to react with free radicals via producing the alpha-tocopherol radical. This radical, once produced, can neutralize the existing free radicals.34 Alternatively, decreased sperm count can be associated with decreased testosterone secretion as the spermatogenesis process depends on the action of testosterone.35 It has been shown that the administration of PVC causes the destruction of Leydig cells and consequently decreases testosterone levels.36 In the present study, the concentration of testosterone was significantly reduced in the PVC group, indicating a toxic effect of PVC on Leydig cells. The initiation of spermatogenesis at puberty and the maintenance of this process in adults are testosterone-dependent. Testosterone is required for the completion of meiosis, differentiation of the spermatids and maturation and conversion of round spermatids to elongated ones.37 It has been confirmed that the conversion of round spermatids to elongated ones is suppressed when testosterone in the interstitial fluid is only 5% of the control group concentration.38 In addition, it has been demonstrated that sperm DNA damage leads to decreased fertilization rates and/or embryo cleavage.39 Because structurally abnormal spermatozoa are the major source of ROS production in semen, embryo development arrest in PVC-administered rats may be attributed to the adverse effects of ROS-producing damaged spermatozoa during in vitro insemination of oocytes.40 In the current study, concomitant administration of Vit E to PVC receiving rats noticeably improved the PVC-induced negative changes in the sperm parameters and embryo development. The protection offered by Vit E against PVC evoked reproductive toxicity is likely thanks to its ability to suppress oxidative stress through ROS over-production inhibition. Similar to our findings, it has been shown that Vit E with anti-inflammatory and antioxidant activities improves sperm parameters and embryo development.41
Collectively, in investigating the potential protective effects of Vit E in PVC-related reproductive toxicities, Vit E through its antioxidant functions provides a marked protective effect against PVC-induced sperm impairment and embryotoxicity. Further studies will be needed to disclose the applicability of Vit E in clinical trials.
Acknowledgments
Authors would like to sincerely thank the members of the Department of Biology, Faculty of Science and Department of Basic Sciences, Faculty of Veterinary Medicine, Urmia University, Urmia, Iran as well as Urmia University Research Council for the approval and support of this research.
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
- 1.LaDou J. Current occupational and environmental medicine. 3rd ed. California, USA: McGraw Hill Professional; 2003. p. 258. [Google Scholar]
- 2.Keys DA, Wallace DG, Kepler TB, et al. Quantitative evaluation of alternative mechanisms of blood and testes disposition of di (2-ethylhexyl) phthalate and mono (2-ethylhexyl) phthalate in rats. Toxicol Sci. 1999;49(2):172–185. doi: 10.1093/toxsci/49.2.172. [DOI] [PubMed] [Google Scholar]
- 3.Zare Z, Eimani H, Mohammadi MO, et al. Histo-pathological study of di-(2-ethylhexyl) phthalate (DEHP) on testes in mouse. J Mazandaran Univ Med Sci. 2009;19(71):52–59. [Google Scholar]
- 4.Latini G, Ferri M, Chiellini F. Material’s degradation in PVC medical devices, DEHP leaching and neonatal outcomes. Curr Med Chem. 2010;17(26):2979–2989. doi: 10.2174/092986710792064992. [DOI] [PubMed] [Google Scholar]
- 5.Ishihara M, Itoh M, Miyamoto K, et al. Spermatogenic disturbance induced by di-(2-ethylhexyl) phthalate is significantly prevented by treatment with antioxidant vitamins in the rat. Int J Androl. 2000;23(2):85–94. doi: 10.1046/j.1365-2605.2000.00212.x. [DOI] [PubMed] [Google Scholar]
- 6.Dirven HA, van den Broek PH, Arends AM, et al. Metabolites of the plasticizer di (2-ethylhexyl) phthalate in urine samples of workers in polyvinylchloride processing industries. Int Arch Occup Environ Health. 1993;64(8):549–554. doi: 10.1007/BF00517699. [DOI] [PubMed] [Google Scholar]
- 7.Wagoner JK. Toxicity of vinyl chloride and poly (vinyl chloride): A critical review. Environ Health Perspect. 1983;52:61–66. doi: 10.1289/ehp.835261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parmar D, Srivastava SP, Singh GB, et al. Effect of testosterone on the testicular atrophy caused by di (2-ethylhexyl) phthalate (DEHP) Toxicol Lett. 1987;36(3):297–308. doi: 10.1016/0378-4274(87)90199-8. [DOI] [PubMed] [Google Scholar]
- 9.Mathur PP, Dcruz SC. The effect of environmental contaminants on testicular function. Asian J Androl. 2011;13(4):585–591. doi: 10.1038/aja.2011.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kasahara E, Sato EF, Miyoshi M, et al. Role of oxidative stress in germ cell apoptosis induced by di (2-ethyl-hexyl) phthalate. Biochem J. 2002;365(3):849–856. doi: 10.1042/BJ20020254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fraga CG, Motchnik PA, Shigenaga MK, et al. Ascorbic acid protects against endogenous oxidative DNA damage in human sperm. Proc Natl Acad Sci USA. 1991;88(24):11003–11006. doi: 10.1073/pnas.88.24.11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kodama H, Yamaguchi R, Fukuda J, et al. Increased oxidative deoxyribonucleic acid damage in the spermatozoa of infertile male patients. Fertil Steril. 1997;68(3):519–524. doi: 10.1016/s0015-0282(97)00236-7. [DOI] [PubMed] [Google Scholar]
- 13.Aitken RJ. Reactive oxygen species as mediators of sperm capacitation and pathological damage. Mol Reprod Dev. 2017;84(10):1039–1052. doi: 10.1002/mrd.22871. [DOI] [PubMed] [Google Scholar]
- 14.Lobascio AM, De Felici M, Anibaldi M, et al. Involve-ment of seminal leukocytes, reactive oxygen species, and sperm mitochondrial membrane potential in the DNA damage of the human spermatozoa. Andrology. 2015;3(2):265–270. doi: 10.1111/andr.302. [DOI] [PubMed] [Google Scholar]
- 15.El-Demerdash FM. Antioxidant effect of vitamin E and selenium on lipid peroxidation, enzyme activities and biochemical parameters in rats exposed to aluminium. J Trace Elem Med Biol. 2004;18(1):113–121. doi: 10.1016/j.jtemb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Sadeghi A, Farokhi F, Najafi G, et al. Protective effect of vitamin E against polyvinyl chloride induced damages and oxidative stress in rat testicular tissue. J Kermanshah Univ Med Sci. 2017;21(3):96–102. [Google Scholar]
- 17.Selvakumar E, Prahalathan C, Sudharsan PT, et al. Chemoprotective effect of lipoic acid against cyclophosphamide-induced changes in the rat sperm. Toxicology. 2006;217(1):71–78. doi: 10.1016/j.tox.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 18.Zambrano E, Rodriguez-Gonzalez GL, Guzman C, et al. A maternal low protein diet during pregnancy and lactation in the rat impairs male reproductive development. J Physiol. 2005;563(1):275–284. doi: 10.1113/jphysiol.2004.078543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erenpreiss J, Bars J, Lipatnikova V, et al. Comparative study of cytochemical tests for sperm chromatin integrity. J Androl. 2001;22(1):45–53. [PubMed] [Google Scholar]
- 20.Wyrobek AJ, Gordon LA, Burkhart JG, et al. An evaluation of the mouse sperm morphology test and other sperm tests in nonhuman mammals A report of the US environmental protection agency gene-tox program. Mutat Res. 1983;115(1):1–72. doi: 10.1016/0165-1110(83)90014-3. [DOI] [PubMed] [Google Scholar]
- 21.Menkveld R, Wong WY, Lombard CJ, et al. Semen parameters, including WHO and strict criteria morphology, in a fertile and subfertile population: An effort towards standardization of in-vivo thresholds. Hum Reprod. 2001;16(6):1165–1171. doi: 10.1093/humrep/16.6.1165. [DOI] [PubMed] [Google Scholar]
- 22.Suckow MA, Weisbroth SH, Franklin CL. The laboratory rat. 2nd ed. New York, USA: Elsevier Academic Press; 2006. pp. 165–173. [Google Scholar]
- 23.Esterbauer H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990;186:407–421. doi: 10.1016/0076-6879(90)86134-h. [DOI] [PubMed] [Google Scholar]
- 24.Huang LP, Lee CC, Fan JP, et al. Urinary metabolites of di (2-ethylhexyl) phthalate relation to sperm motility, reactive oxygen species generation, and apoptosis in polyvinyl chloride workers. Int Arch Occup Environ Health. 2014;87(6):635–646. doi: 10.1007/s00420-013-0905-6. [DOI] [PubMed] [Google Scholar]
- 25.Esteves SC. Clinical relevance of routine semen analysis and controversies surrounding the 2010 World Health Organization criteria for semen examination. Int Braz J Urol. 2014;40(4):433–453. doi: 10.1590/S1677-5538.IBJU.2014.04.02. [DOI] [PubMed] [Google Scholar]
- 26.Cohen-Bacrie P, Belloc S, Menezo YJ, et al. Correlation between DNA damage and sperm parameters: A prospective study of 1,633 patients. Fertil Steril. 2009;91(5):1801–1805. doi: 10.1016/j.fertnstert.2008.01.086. [DOI] [PubMed] [Google Scholar]
- 27.Mathur PP, Huang L, Kashou A, et al. Environmental toxicants and testicular apoptosis. Open Reprod Sci J. 2011;3(1):114–124. [Google Scholar]
- 28.Palleschi S, Rossi B, Diana L, et al. Di (2-ethylhexyl) phthalate stimulates Ca2+ entry, chemotaxis and ROS production in human granulocytes. Toxicol Lett. 2009;187(1):52–57. doi: 10.1016/j.toxlet.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 29.Erkekoglu P, Rachidi W, Yuzugullu OG, et al. Evaluation of cytotoxicity and oxidative DNA damaging effects of di (2-ethylhexyl)-phthalate (DEHP) and mono (2-ethylhexyl)-phthalate (MEHP) on MA-10 Leydig cells and protection by selenium. Toxicol Appl Pharmacol. 2010;248(1):52–62. doi: 10.1016/j.taap.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 30.Aitken RJ, De Iuliis GN, Finnie JM, et al. Analysis of the relationships between oxidative stress, DNA damage and sperm vitality in a patient population: development of diagnostic criteria. Hum Reprod. 2010;25(10):2415–2426. doi: 10.1093/humrep/deq214. [DOI] [PubMed] [Google Scholar]
- 31.Hughes CM, Lewis SE, McKelvey-Martin VJ, et al. The effects of antioxidant supplementation during Percoll preparation on human sperm DNA integrity. Hum Reprod. 1998;13(5):1240–1247. doi: 10.1093/humrep/13.5.1240. [DOI] [PubMed] [Google Scholar]
- 32.Catt JW, Henman M. Toxic effects of oxygen on human embryo development. Hum Reprod. 2000;15(2):199–206. doi: 10.1093/humrep/15.suppl_2.199. [DOI] [PubMed] [Google Scholar]
- 33.Wang X, Falcone T, Attaran M, et al. Vitamin C and vitamin E supplementation reduce oxidative stress–induced embryo toxicity and improve the blastocyst development rate. Fertil Steril. 2002;78(6):1272–1277. doi: 10.1016/s0015-0282(02)04236-x. [DOI] [PubMed] [Google Scholar]
- 34.Ouchi A, Nagaoka SI, Abe K, et al. Kinetic study of the aroxyl radical-scavenging reaction of α-tocopherol in methanol solution: Notable effect of the alkali and alkaline earth metal salts on the reaction rates. J Phys Chem. 2009;113(40):13322–13331. doi: 10.1021/jp906425r. [DOI] [PubMed] [Google Scholar]
- 35.Sharpe RM, Donachie K, Cooper I. Re-evaluation of the intratesticular level of testosterone required for quantitative maintenance of spermatogenesis in the rat. J Endocrinol. 1988;117(1):19–26. doi: 10.1677/joe.0.1170019. [DOI] [PubMed] [Google Scholar]
- 36.Pan G, Hanaoka T, Yoshimura M, et al. Decreased serum free testosterone in workers exposed to high levels of di-n-butyl phthalate (DBP) and di-2-ethylhexyl phthalate (DEHP): a cross-sectional study in China. Environ Health Perspect. 2006;114(11):1643–1648. doi: 10.1289/ehp.9016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Odonnell L, McLachlan RI, Wreford NG, et al. Testosterone promotes the conversion of round spermatids between stages VII and VIII of the rat spermatogenic cycle. Endocrinology. 1994;135(6):2608–2614. doi: 10.1210/endo.135.6.7988449. [DOI] [PubMed] [Google Scholar]
- 38.Sun YT, Wreford NG, Robertson DM, et al. Quantitative cytological studies of spermatogenesis in intact and hypophysectomized rats: Identification of androgen-dependent stages. Endocrinology. 1990;127(3):1215–1223. doi: 10.1210/endo-127-3-1215. [DOI] [PubMed] [Google Scholar]
- 39.Jalali AS, Najafi G, Hosseinchi M, et al. Royal jelly alleviates sperm toxicity and improves in vitro fertilization outcome in stanozolol-treated mice. Iran J Reprod Med. 2015;13(1):15–22. [PMC free article] [PubMed] [Google Scholar]
- 40.Gil-Guzman E, Ollero M, Lopez MC, et al. Differential production of reactive oxygen species by subsets of human spermatozoa at different stages of maturation. Hum Reprod. 2001;16(9):1922–1930. doi: 10.1093/humrep/16.9.1922. [DOI] [PubMed] [Google Scholar]
- 41.Hassa H, Gurer F, Tanir HM, et al. Effect of cigarette smoke and alpha-tocopherol (vitamin E) on fertilization, cleavage, and embryo development rates in mice: An experimental in vitro fertilization mice model study. Eur J Obstet Gynecol Reprod Biol. 2007;135(2):177–182. doi: 10.1016/j.ejogrb.2007.05.020. [DOI] [PubMed] [Google Scholar]