Abstract
Previous findings have shown that saffron (Crocus sativus L.) extract and its active constituents produce antinociceptive effects in the rat models of orofacial pain. In the present study, the central H2 histaminergic and alpha-2 adrenergic receptors involvement in crocetin-induced antinociception in orofacial formalin pain in rats was evaluated. The guide cannula was implanted into the fourth ventricle in ketamine-xylazine anesthetized rats. Subcutaneous injection of a diluted formalin solution (1.50%; 50.00 µL) into a vibrissae pad was used as a model of orofacial pain. Face rubbing behavior durations were recorded at 3 min blocks for 45 min. Formalin produced a biphasic pain response (first phase: 0-3 min and second phase: 15-33 min). Intra-fourth ventricle injections of crocetin (5.00 and 10.00 μg μL-1) suppressed, whereas yohimbine (10.00 μg μL-1) and naloxone (10.00 μg μL-1) increased the intensity of both phases of pain. Crocetin-induced antinociception was not prevented by central pretreatment with naloxone. However, the antinociceptive effect of crocetin (5.00 μg μL-1) was inhibited by prior administration of famotidine (10.00 μg μL-1) and yohimbine (10.00 μg μL-1). Our study showed that injection of crocetin into the cerebral fourth ventricle attenuated formalin-induced orofacial pain in rats. Central H2 histaminergic and alpha-2 adrenergic receptors, but not opioid receptors, might be involved in crocetin-induced antinociception.
Key Words: Crocetin, Famotidine, Fourth ventricle, Orofacial pain, Yohimbine
Introduction
Saffron is a derived plant product from the dried stigma of Crocus sativus flower being used in folk medicine for various purposes. Crocin, crocetin, safranal, and picrocrocin are the main components of saffron.1 In addition to their potent antioxidant properties, crocetin and its by-product crocin (digentibiose adduct of crocetin) have neuroprotective, anxiolytic and memory improvement activities.2-7 Furthermore, crocin also affects pain modulating mechanisms. For example, systemic and central administrations of crocin produced analgesic effects in various experimental models of pain in rodents.8-13 However; the analgesic property of crocetin has not been investigated yet.
Acute and chronic pains originating from the orofacial region are important in public health emerging in clinical conditions such as dental pain, migraine, and trigeminal neuralgia.14 Understanding the neural pathways mediating orofacial pain could improve treatment for the clinical conditions. Clavelou et al. have introduced the orofacial formalin test to study the pain mechanisms in the orofacial region.15 Thereafter, scholars frequently used this model with success to study the orofacial pain mechanisms.16-19
Increasing pieces of evidence highlighted the role of central histaminergic and adrenergic systems involved in the intrinsic modulation of pain. For example, intra-cerebroventricular injection of histamine produced antinociception in the formalin test in mice and rats.20,21 Also, prior microinjection of famotidine (a histamine H2 receptor antagonist) blocked dimaprit-induced anti-nociception.16 On the other hand, Pertovaara has suggested the involvement of the alpha-2 adrenergic system in local peripheral, spinal and supra-spinal modulating mechanisms of pain.22
In the present study, we aimed to investigate the central effect of crocetin, separately and in combination with naloxone (an opioid receptor antagonist), famotidine (an H2 histamine receptor antagonist) and yohimbine (an alpha-2 adrenergic receptor antagonist) in the formalin-induced orofacial pain in rats. Besides, we used the intra-fourth ventricle injection procedure because important structures in the descending regulation of noxious stimuli such as rostral ventromedial medulla and locus coeruleus (the main source of norepinephrine in the brain) are located near the fourth ventricle.23, 24
Materials and Methods
Animals. In the present study, 60 healthy adult male Wistar rats, weighing between 260 and 300 g, were divided into 10 groups with six rats in each group. Rats were randomly housed in polyethylene cages at 22.00 ± 2.00 ˚C on a 12 hr. light/dark cycle (lights on between 7:00 AM to 7:00 PM) with free access to food and water. Experiments were performed between 12:00 pm to 04:00 pm. Laboratory Animal Care and Use Center of the Faculty of Veterinary Medicine, Urmia University, Urmia, Iran approved the experimental protocol (AECVU-157-2018).
Chemicals. The following chemicals were used in this study: crocetin, yohimbine hydrochloride, naloxone hydrochloride, and famotidine hydrochloride (Sigma-Aldrich, St. Louis, USA). The chemicals were dissolved and diluted in artificial cerebrospinal fluid (aCSF) 30 min before intra-fourth ventricle administration. The aCSF solution (pH: 7.40) contains the following (in mM): 120 NaCl, 2.50 KCl, 26 NaHCO3, 1.20 NaH2PO4, 2 CaCl2, 1.00 MgCl2, 5.00 glucose, 10.00 sucrose, 0.40 ascorbic acid, 3.00 myo-inositol and 2.00 sodium pyruvate.25 The chemicals used for preparing aCSF and formalin were purchased from Merck (Darmstadt, Germany).
Stereotaxic surgery. To deliver the chemical agents into the fourth ventricle of the brain, a permanent guide cannula was implanted in the fourth ventricle of the brain. The animals were anesthetized with 80.00 mg kg-1 ketamine (Alfasan, Woerden, The Netherlands) and 8.00 mg kg-1 xylazine (Alfasan) and fixed in a stereotaxic frame (Stoelting Co., Wood Dale, USA). A 23-gauge, 14-mm guide cannula made of stainless steel was implanted in the fourth ventricle of the brain. The stereotaxic coordinates, according to Paxinos and Watson,26 were – 12.50 mm posterior to the bregma, 0 mm lateral to the midline, and 7.80 mm below the top of the skull. The guide cannula was fixed to the skull using two screws and dental acrylic (Acropars, Iran). At the end of the surgery, one 14-mm stainless steel stylet was inserted into the guide cannula to protect it from obstruction. All animals were allowed to recover from surgery for 10 days.
Intra-fourth ventricle injection. Intra-fourth ventricle microinjections of sterile aCSF, crocetin (2.50, 5.00 and 10.00 μg μL-1), famotidine (10.00 μg μL-1), yohimbine (10.00 μg μL-1) and naloxone (10.00 μg μL-1) were performed using a 30-gauge, 15-mm injection needle attached to a 5.00 µL Hamilton syringe. The volume of drug solution to be microinjected into the fourth ventricle was 1.00 µL and the microinjection was slowly made for 30 sec. After completion of each injection, the injection needle was left in place for a further 30 sec to facilitate diffusion of the drug solution. Crocetin was microinjected 4 min and naloxone, yohimbine, and famotidine were microinjected 8 min before orofacial pain induction.8 Doses for drug administration were obtained from previous studies8,21,27 and our preliminary experiments.
Orofacial formalin test. For induction of orofacial pain, each rat was placed in a plexiglass observation chamber (30.00 × 30.00 × 30.00 cm3) with a mirror mounted at 45° beneath the floor to allow an unobstructed view of the orofacial region. After a 30-min adaptation period, 50.00 µL of 1.50% diluted formalin solution was subcutaneously injected into the left side of the vibrissae pad just lateral to the nose using a 30-gauge injection needle. Face rubbing duration with ipsilateral forepaw was recorded in consecutive 3-min blocks for 45 min and considered as an index of nociception. Formalin injection induced a stereotyped response characterized by two well distinct phases.15,16 All the observers were blinded to the protocol of the study.
Locomotor activity. Locomotor activity was assessed in an electronic activity box (Borj Sanat, Tehran, Iran). The apparatus has consisted of a plexiglass chamber (40.00 × 40.00 × 40.00 cm3). To monitor the activity, animals were placed directly in one corner of the activity box. When an animal moved in the box, it caused beam breaks. The monitor of the apparatus showed the number of beam breaks in a 5-min session. To reduce the used animal number, 10 days after the pain test, the same rats were assessed for the locomotor activity.
Verification of cannula. At the end of each experiment, 1.00 μL of methylene blue solution (Merck) was injected into the fourth ventricle in anesthetized rats. Then, the rats were euthanized by intracardiac perfusion with 150 mg kg-1 ketamine and 20.00 mg kg-1 xylazine mixture. The brains were removed and placed in 10.00% formalin solution. After 48 hr, the brains were sectioned coronally (50-100 μm) and viewed under a loupe to observe the distribution of methylene blue in the fourth ventricle according to the atlas of Paxinos and Watson.26
Statistical analysis. Statistical comparisons were performed using GraphPad Prism Software (version 5.0; GraphPad, San Diego, USA). Data obtained from the subcutaneous injection of formalin into the vibrissae pad were analyzed by repeated measure ANOVA followed by Tukey’s post-hoc test. One-way ANOVA followed by Tukey’s post-hoc test was applied to compare the differences among experimental groups. In figures, all values were expressed as mean ± SEM. A value of p < 0.05 was considered statistically significant.
Results
Normal saline injection into the vibrissae pad did not produce any considerable nociceptive behavior, whereas subcutaneous injection of formalin into the vibrissae pad produced more face rubbing at the first and 6th-11th 3-min blocks compared to 2nd-5th and 12th-15th 3-min blocks (F(14,89) = 17.35; p < 0.05). Thus, formalin produced a typical biphasic (first phase: 0-3 min and second phase: 15-33 min) pattern of nociceptive behavior (data not shown).
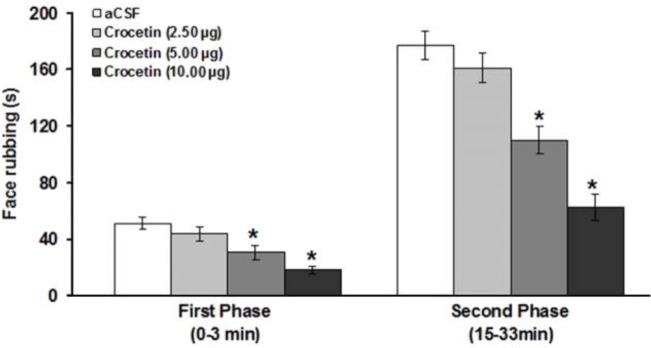
The effects of intra-fourth ventricle administration of crocetin on the formalin-induced orofacial pain were shown in Figure 1. While crocetin at a dose of 2.50 μg μL-1 produced no significant effects on pain response, at doses of 5.00 and 10.00 μg μL-1, it significantly attenuated the first (F(3,23) = 10.75; p < 0.05; Fig. 1) and second (F(3,23) = 26.70; p < 0.05; Fig. 1) phases of formalin-induced orofacial pain.
Fig. 1.
Effects of intra-fourth ventricle injection of crocetin on the formalin-induced orofacial pain. * indicates a significant difference (p < 0.05) in comparison with artificial cerebrospinal fluid (aCSF) treated group
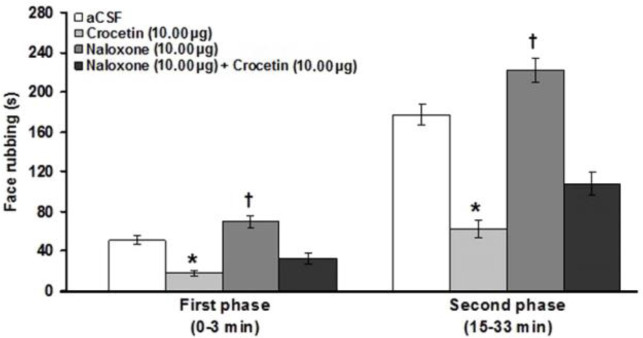
Intra-fourth ventricle microinjection of the naloxone significantly increased the first and (F(3,23) = 25.04; p < 0.05; Fig. 2 ) second (F(3,23) = 45.36; p < 0.05; Fig. 2 ) phases of pain. In addition, pretreatment with naloxone did not prevent the antinociceptive effects of crocetin (10.00 μg μL-1) in the first and the second phases of pain.
Fig. 2.
Effects of intra-fourth microinjection of naloxone and naloxone before crocetin (10.00 μg μL-1) on the formalin-induced orofacial pain. * indicates a significant decrease at p < 0.05 in comparison with artificial cerebrospinal fluid (aCSF) treated group. † indicates a significant increase (p < 0.05) compared to the aCSF treated group
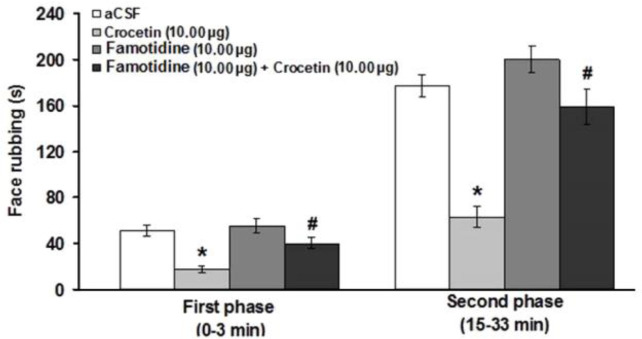
Intra-fourth ventricle administration of famotidine did not change the nociceptive response in the first and second phases of pain. However, famotidine prevented the antinociceptive effect induced by crocetin (10.00 μg μL-1) at first (F(3,23) = 13.17; p < 0.05) and second (F(3,23) = 27.05; p < 0.05) phases of pain (Fig. 3).
Fig. 3.
Effects of intra-fourth ventricle injection of famotidine and famotidine before crocetin on the formalin-induced orofacial pain. * indicates a significant decrease at p < 0.05 in comparison with artificial cerebrospinal fluid (aCSF) treated group. # indicates a significant difference (p < 0.05) compared to crocetin (10.00 μg μL-1) treated group
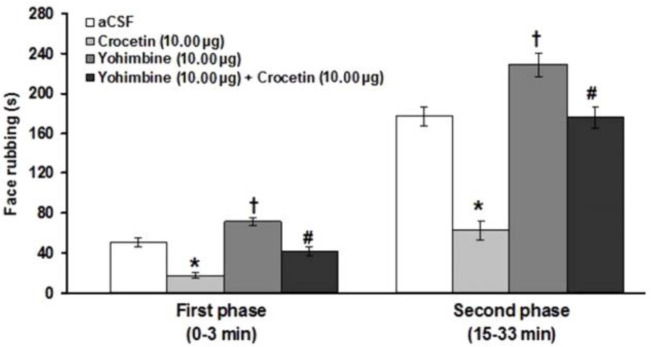
The results showed that yohimbine significantly increased the first (F(3,23) = 28.51; p < 0.05) and second (F(3,23) = 35.85; p < 0.05) phases of pain induced by formalin (Fig. 4). The suppressive effects of crocetin on the first (F(3,23) = 28.51; p < 0.05) and second (F(3,23) = 35.851; p < 0.05) phases of formalin-induced pain were significantly prevented by intra-fourth ventricle administration of yohimbine (Fig. 4).
Fig. 4.
Effects of intra-fourth ventricle injection of yohimbine and yohimbine before crocetin on the formalin-induced orofacial pain. * indicates a significant decrease at p < 0.05 in comparison with artificial cerebrospinal fluid (aCSF) treated group. † indicates a significant increase (p < 0.05) compared to the aCSF treated group. # indicates a significant difference (p < 0.05) compared to crocetin (10.00 μg μL-1) treated group
The number of beam breaks was 54.20 ± 6.40 after the intra-fourth ventricle administration of aCSF. All the above-mentioned treatments did not affect locomotor activity (data not shown).
Discussion
The present study showed that subcutaneous injection of formalin (1.50%) into the vibrissae pad of rats produced biphasic nociceptive behavior typically characterized by face rubbing. Face rubbing with the ipsilateral forepaw due to formalin injection into the upper lip has been mentioned as a specific nociceptive response.28 Formalin is known to produce biphasic pain behaviors related to different mechanisms: The first transient phase is associated with the direct effect of formalin on sensory C fibers and the second prolonged phase is caused by inflammatory processes and release of algesic mediators.29 However, our results were inconsistent with other observations indicating that formalin administration produces a typical biphasic pattern of face rubbing behavior.11,16,18,28
Our findings showed that the central injection of crocetin produced antinociception in formalin-induced orofacial pain. Although some scholars have shown an antinociceptive effect for crocin in animal models, the analgesic property of crocetin in inflammatory pain has not been investigated yet. There are shreds of evidence that systemic administration of crocin decreases nociceptive response in hind paw and orofacial formalin tests of pain and a rat model of carrageenan-induced hyperalgesia.11,12,30 Also, Karami et al. have shown that chronic neuropathic pain induced by spinal cord injury is suppressed by systemic administration of crocin.9 However, it was shown that crocin could not act as a bioactive molecule by itself in vivo through systemic administration and it hydrolyzed to crocetin before being incorporated into the blood circulation.31 Although there is not any report showing the central effect of crocetin on the nociceptive behavior, it has been shown that cerebral ventricle injection of crocin attenuates capsaicin-induced orofacial pain and acute trigeminal pain in rats.8,13 Most recently, Wang et al. have reported that spared nerve injury-induced neuropathic pain is attenuated via intrathecal administration of crocetin.32 However, the central mechanisms of crocetin induced-antinociception have not been clarified yet.
In this study, prior microinjection of naloxone did not change the antinociceptive effect of crocetin. The endogenous opioid system is one of the most studied innate pain-relieving systems. However, previous studies have shown that the anti-nociceptive effect of systemic and central administrations of crocin does not attribute to the opioid receptors. For example, it was shown that antinociception induced by central administration of crocin in acute corneal and capsaicin-induced orofacial pain models was not prevented by an opioid receptor antagonist, naloxone.8,13 Besides, systemic crocin induced antinociception in the hind paw and orofacial formalin test in rats was not sensitive to naloxone.11,12
In our study, the central administration of yohimbine increased the intensity of pain response. Moreover, yohimbine prevented crocetin-induced antinociception. This indicates that the central alpha-2-adrenoceptor is involved in mediating the effect of crocetin on the orofacial pain. Former studies have shown that alpha-2-adrenergic system activation produces local peripheral, spinal, and supra-spinal antinociception antagonized by an alpha-2-adrenergic blocker, yohimbine.22,33 Investigations have shown that the antinociceptive effect of intra-peritoneal administration of xylazine (an alpha-2 receptor agonist) is antagonized by yohimbine but not by naloxone (an opioid receptor antagonist) indicating that alpha-2-adrenoceptor, but not opioid receptor, mechanism is involved in this analgesic effect.33 Additionally, intrathecal injection of another alpha-2 agonist, clonidine, reduced licking activity in both phases of paw formalin pain in mice.34 Taherianfard and Khazaee have reported that micro-injection of xylazine and yohimbine into the lateral ventricle of the brain decreases and increases pain sensitivity in tail-flick test, respectively.27
On the other hand, our study showed that famotidine did not change the intensity of pain. However, prior microinjection of famotidine inhibited crocetin-induced antinociception. It has been well documented that activation of the central histaminergic system could be pain-alleviating in several animal models of pain study. Furthermore, this effect of histamine could be antagonized by histamine H2 blockers. For example, central administration of famotidine inhibited the suppressive effect of histamine in the formalin pain.21 At the level of the hippocampus, prior microinjection of ranitidine (another H2 blocker) prevented histamine-induced antinociception in orofacial formalin pain.35 However, this study showed that crocetin-induced analgesia is mediated by the H2 histaminergic system to some extent.
It is well known that analgesic mechanisms of many medicinal plants and their active substances are dependent on opioid and non-opioid pathways. In this context, many scholars have suggested that in the non-opioid pathways, the adrenergic, serotonergic, and cholinergic systems are involved in the analgesic effects of natural active substances. 19,36-38
In conclusion, the results of this study showed that the central administration of crocetin produced antinociception in the orofacial formalin test. Our findings showed that supra-spinally alpha-2-adrenoceptor and histamine H2 receptor activations by crocetin probably lead to this analgesia.
Acknowledgments
This study was financially supported by the Faculty of Veterinary Medicine, Urmia University, Urmia, Iran.
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- 1.Moshiri M, Vahabzadeh M, Hosseinzadeh H. Clinical applications of saffron (Crocus sativus) and its constituents: A review. Drug Res (Stuttg) 2015;65(6):287–295. doi: 10.1055/s-0034-1375681. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad AS, Ansari MA, Ahmad M, et al. Neuroprotection by crocetin in a hemi-parkinsonian rat model. Pharmacol Biochem Behav. 2005;81(4):805–813. doi: 10.1016/j.pbb.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Amin B, Nakhsaz A, Hosseinzadeh H. Evaluation of the antidepressant-like effects of acute and sub-acute administration of crocin and crocetin in mice. Avicenna J Phytomed. 2015;5(5):458–468. [PMC free article] [PubMed] [Google Scholar]
- 4.Tamaddonfard E, Farshid AA, Ahmadian E, et al. Crocin enhanced functional recovery after sciatic nerve crush injury in rats. Iran J Basic Med Sci. 2013;16(1):83–90. [PMC free article] [PubMed] [Google Scholar]
- 5.Tamaddonfard E, Farshid AA, Asri-Rezaee S, et al. Crocin improved learning and memory impairments in streptozotocin-induced diabetic rats. Iran J Basic Med Sci. 2013;16(1):91–100. [PMC free article] [PubMed] [Google Scholar]
- 6.Nam KN, Park YM, Jung HJ, et al. Anti-inflammatory effects of crocin and crocetin in rat brain microglial cells. Eur J Pharmacol. 2010;648(1-3):110–116. doi: 10.1016/j.ejphar.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Hosseinzadeh H, Noraei NB. Anxiolytic and hypnotic effect of Crocus sativus aqueous extract and its constituents, crocin and safranal, in mice. Phytother Res. 2009;23(6):768–774. doi: 10.1002/ptr.2597. [DOI] [PubMed] [Google Scholar]
- 8.Tamaddonfard E, Tamaddonfard S, Pourbaba S. Effects of intra-fourth ventricle injection of crocin on capsaicin-induced orofacial pain in rats. Avicenna J Phytomed. 2015;5(5):450–457. [PMC free article] [PubMed] [Google Scholar]
- 9.Karami M, Bathaie SZ, Tiraihi T, et al. Crocin improved locomotor function and mechanical behavior in the rat model of contused spinal cord injury through decreasing calcitonin gene related peptide (CGRP) Phytomedicine. 2013;21(1):62–67. doi: 10.1016/j.phymed.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 10.Amin B, Hosseinzadeh H. Evaluation of aqueous and ethanolic extracts of saffron, Crocus sativus L, and its constituents, safranal and crocin in allodynia and hyperalgesia induced by chronic constriction injury model of neuropathic pain in rats. Fitoterapia. 2012;83(5):888–895. doi: 10.1016/j.fitote.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 11.Erfanparast A, Tamaddonfard E, Taati M, et al. Effects of crocin and safranal, saffron constituents, on the formalin-induced orofacial pain in rats. Avicenna J Phytomed. 2015;5(5):392–402. [PMC free article] [PubMed] [Google Scholar]
- 12.Tamaddonfard E, Hamzeh-Gooshchi N. Effect of crocin on the morphine-induced antinociception in the formalin test in rats. Phytother Res. 2010;24(3):410–413. doi: 10.1002/ptr.2965. [DOI] [PubMed] [Google Scholar]
- 13.Tamaddonfard E, Hamzeh-Gooshchi N. Effect of intraperitoneal and intracerebroventricular injection of crocin on acute corneal pain in rats. Phytother Res. 2010;24(10):1463–1467. doi: 10.1002/ptr.3169. [DOI] [PubMed] [Google Scholar]
- 14.Sessle BJ. Peripheral and central mechanisms of orofacial inflammatory pain. Int Rev Neurobiol. 2011;97:179–206. doi: 10.1016/B978-0-12-385198-7.00007-2. [DOI] [PubMed] [Google Scholar]
- 15.Clavelou P, Pajot J, Dallel R, et al. Application of the formalin test to the study of orofacial pain in the rat. Neurosci Lett. 1989;103(3):349–353. doi: 10.1016/0304-3940(89)90125-0. [DOI] [PubMed] [Google Scholar]
- 16.Erfanparast A, Tamaddonfard E, Taati M, et al. Role of the thalamic submedius nucleus histamine H1 and H2 and opioid receptors in modulation of formalin-induced orofacial pain in rats. Naunyn Schmiedebergs Arch Pharmacol. 2015;388(10):1089–1096. doi: 10.1007/s00210-015-1143-0. [DOI] [PubMed] [Google Scholar]
- 17.Miranda HF, Sierralta F, Lux S, et al. Involvement of nitridergic and opioidergic pathways in the antinociception of gabapentin in the orofacial formalin test in mice. Pharmacol Rep. 2015;67(2):399–403. doi: 10.1016/j.pharep.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 18.Donatti AF, Araujo RM, Soriano RN, et al. Role of hydrogen sulfide in the formalin-induced orofacial pain in rats. Eur J Pharmacol. 2014;738:49–56. doi: 10.1016/j.ejphar.2014.05.023. [DOI] [PubMed] [Google Scholar]
- 19.de Carvalho EF, de Oliveira SK, Nardi VK, et al. Ilex paraguariensis promotes orofacial pain relief after formalin injection: Involvement of noradrenergic pathway. Pharmacognnosy Res. 2016;8(Suppl 1):S31–S37. doi: 10.4103/0974-8490.178643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tamaddonfard E, Rahimi S. Central effect of histamine and peripheral effect of histidine on the formalin-induced pain response in mice. Clin Exp Pharmacol Physiol. 2004;31(8):518–522. doi: 10.1111/j.1440-1681.2004.04033.x. [DOI] [PubMed] [Google Scholar]
- 21.Mojtahedin A, Tamaddonfard E, Zanboori A. Antinociception induced by central administration of histamine in the formalin test in rats. Indian J Physiol Pharmacol. 2008;52(3):249–254. [PubMed] [Google Scholar]
- 22.Pertovaara A. The noradrenergic pain regulation system: a potential target for pain therapy. Eur J Pharmacol. 2013;716(1-3):2–7. doi: 10.1016/j.ejphar.2013.01.067. [DOI] [PubMed] [Google Scholar]
- 23.Berridge CW, Waterhouse BD. The locus coeruleus–noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Rev. 2003;42(1):33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- 24.Bourne S, Machado AG, Nagel SJ. Basic anatomy and physiology of pain pathways. Neurosurg Clin N Am, 2014;25(4):629–638. doi: 10.1016/j.nec.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Richardson J, Cruz MT, Majumdar U, et al. Melanocortin signaling in the brainstem influences vagal outflow to the stomach. J Neurosci. 2013;33(33):13286–13299. doi: 10.1523/JNEUROSCI.0780-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3rd ed. San Diego, USA: Academic Press; 1997. Figures 67 and 68. [DOI] [PubMed] [Google Scholar]
- 27.Taherianfard M, Khazaee Z. Effect of xylazine and yohimbine on the phasic pain during the estrous cycle in the rat. Iran J Vet Res. 2006;7(4):33–39. [Google Scholar]
- 28.Raboisson P, Dallel R. The orofacial formalin test. Neurosci Biobehav Rev. 2004;28(2):219–226. doi: 10.1016/j.neubiorev.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Anderson LC, Vakoula A, Veinote R. Inflammatory hypersensitivity in a rat model of trigeminal neuropathic pain. Arch Oral Biol. 2003;48(2):161–169. doi: 10.1016/s0003-9969(02)00203-0. [DOI] [PubMed] [Google Scholar]
- 30.Tamaddonfard E, Farshid AA, Eghdami K, et al. Comparison of the effects of crocin, safranal and diclofenac on local inflammation and inflammatory pain responses induced by carrageenan in rats. Pharmacol Rep. 2013;65(5):1272–1280. doi: 10.1016/s1734-1140(13)71485-3. [DOI] [PubMed] [Google Scholar]
- 31.Asai A, Nakano T, Takahashi M, et al. Orally administered crocetin and crocins are absorbed into blood plasma as crocetin and its glucuronide conjugates in mice. J Agric Food Chem . 2005;53(18):7302–7306. doi: 10.1021/jf0509355. [DOI] [PubMed] [Google Scholar]
- 32.Wang X, Zhang G, Qiao Y, et al. Crocetin attenuates spared nerve injury-induced neuropathic pain in mice. J Pharmacol Sci, 2017;135: 141–147. doi: 10.1016/j.jphs.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 33.Romero TRL, de Castro Perez A, de Francischi JN, et al. Probable involvement of alpha(2C)-adrenoceptor subtype and endogenous opioid peptides in the peripheral antinociceptive effect induced by xylazine. Eur J Pharmacol. 2009;608(1-3):23–27. doi: 10.1016/j.ejphar.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 34.Kanui TI, Tjolsen A, Lund A, et al. Antinociceptive effects of intrathecal administration of alpha-adreno-ceptor antagonists and clonidine in the formalin test in the mouse. Neuropharmacology. 1993;32(4):367–371. doi: 10.1016/0028-3908(93)90158-y. [DOI] [PubMed] [Google Scholar]
- 35.Tamaddonfard E, Erfanparast A, Farshid AA, et al. Interaction between histamine and morphine at the level of the hippocampus in the formalin-induced orofacial pain in rats. Pharmacol Rep. 2011;63(2):423–432. doi: 10.1016/s1734-1140(11)70508-4. [DOI] [PubMed] [Google Scholar]
- 36.Parvizpur A, Ahmadiani A, Kamalinejad M. Spinal serotonergic system is partially involved in antinociception induced by Trigonella foenum-graecum (TFG) leaf extract. J Ethnopharmacol. 2004;95(1):13–17. doi: 10.1016/j.jep.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 37.Sawada LA, Monteiro VS, Rabelo GR, et al. Libidibia ferrea mature seeds promote antinociceptive effect by peripheral and central pathway: Possible involvement of opioid and cholinergic receptors. Biomed Res Int. 2014:508725. doi: 10.1155/2014/508725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao X, Xu Y, Zhao Q, et al. Curcumin exerts antinociceptive effects in a mouse model of neuro-pathic pain: Descending monoamine system and opioid receptors are differentially involved. Neuro-pharmacology. 2012;62(2):843–854. doi: 10.1016/j.neuropharm.2011.08.050. [DOI] [PubMed] [Google Scholar]