Abstract
This study was performed to evaluate the efficacy of two levels of dietary oregano essential oil (OEO) on growth performances, biochemical, hematological parameters, and intestinal histomorphology in Japanese quail. A total number of 99 quail chicks were divided into three groups (33 quails per group): Control, OEO 150 mg kg-1, and OEO 300 mg kg-1 treated groups for 42 days. Feed conversion ratio (FCR), feed intake, weight gain, and edible organ weight were recorded. Biochemical and hematological parameters were determined. Histomorphological examination of hepatic and intestinal tissues was performed. FCR was significantly improved and feed intake was significantly decreased in OEO 150 mg kg-1 group compared to control. No detectable changes were observed in the lipid profile. Meanwhile, total protein, albumin, globulin, and H/L ratio were significantly increased in OEO 300 mg kg-1 at day 21. Uric acid and creatinine were significantly increased in the OEO 300 mg kg-1 group at day 42. A significant increase was observed in the whole thickness of the small intestine in the OEO 150 mg kg-1 group besides a significant increase in villi length, width, and crypt depth. Vacuolar and fatty degeneration of the hepatocytes along with Kupffer cell hypertrophy was observed in OEO 300 mg kg-1 group. It was concluded that OEO 150 mg kg-1 improved the quail’s performance, intestinal histomorphometry as well as hematological parameters with no negative impacts on biochemical parameters.
Key Words: Biochemical changes, Hematological parameters, Intestinal histomorphometry, Oregano essential oil, Quail
Introduction
Over the past three decades, interests in quail farming have arisen in many parts of the world for both meat and egg production and this could be due to their high production rate and resistance to diseases. Additionally, quail farming provides both reliable and practical solutions for animal protein shortage problem in many developing countries,1,2 whereas, it can be easily reared in these countries due to their small size that does not require a large space area. Moreover, Japanese quails have been used recently as experimental animals for the production of vaccines for diseases that are naturally resistant like New castle disease.2
In addition to medicinal plants, essential oils have recently attracted significant interest as feed additives for improving poultry performance.3 Many researchers are looking for using natural alternatives instead of antibiotics growth promoters to improve poultry production4,5 which are considered “Generally Recognized as Safe” (GRAS) by the United States Food and Drug Administration.6
Oregano essential oil (OEO) is a volatile oil obtained by steam distillation of oregano plant (Origanum vulgare subsp. hirtum) that is a popular spice in the Mediterranean countries.7 OEO contains more than 30 ingredients, most of which are phenolic compounds that exert various activities. The major components of OEO are carvacrol and thymol which are responsible for its various activities.8 OEO was proven to have antioxidant9,10 and anti-inflammatory11 activities. Moreover, it has been shown to have positive effects on poultry performance, digestion,12,13 immunity14 and digestibility.15
The OEO efficacy of quail growth performance and gut histomorphometry remains questionable. As well as being the small intestine, the primary site of enzymatic breakdown and absorption and the animal growth rate is closely related to the intestinal development in rapidly growing species like quails.1 Also, the blood biochemical parameters furnish beneficial information about the bird's health state and are often helpful in revealing health disorders already in the preclinical stage. Hematological parameters have no standard references for avian, however, they can act as a mirror for the intensity of metabolism which is reflected in changes of the blood parameters.16 Furthermore, improved immunity is very important to prevent infectious diseases that positively reflect poultry production17 as well as a balanced internal environment with a reduction of stress that reflected on heterophils/lymphocytes (H/L) ratio.18 Also, oxidative status and antioxidants reserve play a role in maintaining growth and performance of birds.19
The main goal of the present trial reported herein was to investigate the comparative efficacy of two doses of OEO on growth performance, biochemical and hematological parameters as well as intestinal histomorphometry and histological changes in liver tissue of Japanese quails.
Materials and Methods
Birds and experimental treatments. In the current experiment, ninety-nine, two-weeks-old quails (Coturnix coturnix Japonica) with an average weight of 43.70 ± 0.96 g were randomly divided equally into three groups with three replicates of 11 birds each. Experimental groups were categorized as follow: Control (birds received standard diet) and the other treatments were received a standard diet with 150 and 300 mg kg-1 OEO (Semieterik Oil Co., Mersin, Turkey) containing 80% carvacrol, according to Basmacıoğlu et al.20 The experimental protocols and techniques in this study were approved by the institutional research ethics committee at the Faculty of Veterinary Medicine, Suez Canal University (approval No: 2018064). The experimental diet was prepared to fulfill the nutritional requirements of growing quail as recommended by NRC21 and shown in Table 1.
Table 1.
Composition of quail's standard basal diet
| Ingredients | Concentration (g kg -1 diet) |
|---|---|
| Soya meal | 319.60 |
| Ground yellow corn | 557.50 |
| Corn gluten | 74.50 |
| Fish meal | 10.00 |
| Wheat bran | 10.00 |
| Limestone | 13.00 |
| Dicalcium phosphate | 7.00 |
| Lysine | 1.70 |
| DL–methionine | 0.07 |
| Sodium chloride | 3.00 |
| Mineral and vitamin premix* | 3.00 |
* The premix Supplied for each kilogram diet: 12,000 and 2000 IU vitamin A and D3 respectively; 1000 mg vitamin E, 100 mg vitamin K, 0.50 mg vitamin B2, 0.15 mg vitamin B6, 1000 mg pantothenic acid, 2.00 mg niacin, 60.00 mg choline chloride, 3.00 mg iron, 6.00 mg manganese, 0.40 mg copper, 5.00 mg zinc, 1.00 mg vitamin B1, 0.001 mg vitamin B12, 1.00 mg folic acid, 0.05 mg biotin, 0.30 mg iodine, 0.10 mg cobalt and 0.01 mg selenium.
Performance Parameters. Body weight and feed intake for each bird were recorded on 21 and 42 days of the experiment, while the cumulative feed intake and weight gain were determined at the end of the experimental period. Growth performance parameters such as body weight gain and feed conversion ratio (FCR) were determined on days 21 and 42 of the experimental period. The average FCR was calculated all over the experimental period.
Edible organs weights. At the end of the experiment (day 42), edible organs, liver, heart, gizzard, and spleen were removed and weighed immediately after slaughtering and dissection of quails. Their relative weights were obtained concerning the body weight.
Blood samples. Two blood samples were obtained from 15 quails per group by slaughtering, under the effect of isoflurane inhalation anesthesia, on days 21 and 42 of the experiment. The first ones were allowed to clot then centrifuged for 15 min at 3,000 rpm to obtain the sera that were kept in tubes at –20.00 ˚C for biochemical analysis. The second sample was obtained on EDTA tubes for leukocytes count.
Serum biochemical parameters. Individual serum samples were analyzed for high-density lipoproteins (HDL), low-density lipoproteins (LDL), triglycerides (TG), total cholesterol (TC), total protein (TP), albumin, uric acid, creatinine, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) using commercial colorimetric kits (Diamond Diagnostic, Egypt). The absorbance wavelengths were 505, 620, 505, 505, 546, 578, 505, 505, 340 and 340, respectively. Globulin level was obtained by subtracting albumin from total protein. All the procedures of analysis were carried out according to manufacturers’ protocol.
The serum reduced glutathione (GSH). GSH in serum were assayed using a kinetic enzymatic kit (BioVision, USA) according to manufacturer instructions.
Leukocyte counts and H/L ratio. Total and differential leukocyte counts were performed according to the standard avian guidelines of Ritchie et al.22 then Heterophils/ lymphocytes (H/L) ratio was calculated.
Histomorphometric analysis. Five birds from each group were decapitated on 42 day under the effect of isoflurane inhalation anesthesia. Specimens from duodenum at the midpoint of the pancreatic loop, jejunum, and ileum were obtained for gut histomorphological measurements. Additionally, liver samples were taken for evaluation of the hepatic histological changes. Each tissue sample from each treatment was briefly immersed directly in 10.00% neutral buffered formalin. Thereafter, fixed specimens were dehydrated in graded concentrations of ethyl alcohol, then embedded in molten paraffin wax and sectioned at 5.00 to 7.00 µm, routine histological processing was carried out according to the method of Suvarna et al.23 The prepared paraffin sections were stained with hematoxylin and eosin and finally examined by light microscopy.
The intestinal histomorphometric parameters included thickness of intestinal mucosa (MU), submucosal thickness (SM), tunica muscularis thickness (MS), tunica serosa thick-ness (SR), villus height (VH), crypt depth (CD), villi width (VW) at the crypt/villus junction and villus height. Crypt depth ratio (VC) was evaluated in the duodenum, jejunum, and ileum under blind conditions.24 For histomorphometric study, three sections from each intestinal part were used. Additionally, six microscopic areas from each section were used for histomorphometric investigation. Representative photo-micrographs were captured by light microscopy (BX41 research optical photomicroscope fitted with an Olympus DP25 digital camera; Olympus, Tokyo, Japan) using calibration software, CellSens image analysis program (version 1.5; Olympus) and image J Basics (version. 1.38; Millersville University, Millersville, USA).
Statistical analysis. The results were shown as mean ± SEM. Repeated measures of variance was used for analysis of growth performance, feed intake, biochemical parameters and reduced glutathione. Results were considered significant and highly significant when p-values were less than 0.05 and 0.01, respectively. All the obtained measurements were statistically analyzed by SPSS (version 12.0; IBM Corp., Armonk, USA).25
Results
Growth performance. Results of performance as 3 stages were described in Table 2, the first one was on days 1 to 21 of an experiment while the second one has lied between 21 to 42 days of the experiment and finally the third one could be considered as a cumulative stage which represented the average period (1 to 42 days). The first stage was characterized by a significant (p < 0.05) improvement in birds treated with OEO 150 mg kg-1 in both weight gain and FCR. On the other hand, the second stage indicated a decline in weight gain in both treatments compared to control which was not significant in OEO 150 mg kg-1 and significant in OEO 300 mg kg-1. Also, FCR did not show significant changes among the different treatments. There were no significant differences in cumulative weight gain and final body weight (1 to 42 days) among the treated and control groups. However, FCR showed a different pattern with a significant (p < 0.05) increase in OEO 150 mg kg-1 group compared to control and OEO 300 mg kg-1 group.
Table 2.
Effect of two different doses of oregano essential oil (OEO; 150 and 300 mg kg-1) on growth performance in quails on edible organs weight at the end of the experimental period
| Age of the birds | Parameters | Control | OEO 150 mg kg -1 | OEO 300 mg kg -1 |
|---|---|---|---|---|
| At 14-21 day | Feed intake (g) | 334.70 ± 12.00 | 269.70 ± 10.20 | 311.60 ± 13.00 |
| Weight gain (g) | 77.66 ± 3.36b | 89.61 ± 1.10a | 69.10 ± 3.70b | |
| Feed conversion ratio | 4.31 ± 0.22a | 3.01 ± 0.04b | 4.51 ± 0.24a | |
| At 21-42 day | Feed intake (g) | 624.80 ± 10.50 | 563.7 ± 11.2 | 531.70 ± 9.30 |
| Weight gain (g) | 110.00 ± 7.12a | 102.50 ± 7.37ab | 90.58 ± 3.49b | |
| Feed conversion ratio | 5.68 ± 0.48 | 5.50 ± 0.23 | 5.87 ± 0.45 | |
| Average period (14-42 day) | Feed intake (g) | 923.50 ± 8.00 | 804.50 ± 7.40 | 933.7 ± 10.00 |
| Weight gain (g) | 186.58 ± 5.62 | 190.19 ± 3.74 | 180.60 ± 7.76 | |
| Feed conversion ratio | 4.95 ± 0.15a | 4.23 ± 0.09b | 5.17 ± 0.26a | |
| Final BW (g) | 245.38 ± 6.16 | 233.5 ± 4.85 | 230.71 ± 8.86 | |
| At 42 day | Liver absolute weight (g) | 4.52 ± 0.47 | 5.37 ± 0.74 | 6.02 ± 0.53 |
| Liver relative weight (%) | 1.93 ± 0.24 | 2.26 ± 0.26 | 2.48 ± 0.18 | |
| Heart absolute weight (g) | 4.37 ± 0.43 | 4.55 ± 0.19 | 4.41 ± 0.20 | |
| Heart relative weight (%) | 0.84 ± 0.02 | 0.91 ± 0.01 | 0.85 ± 0.03 | |
| Gizzard absolute weight (g) | 1.96 ± 0.08 | 2.23 ± 0.12 | 2.02 ± 0.14 | |
| Gizzard relative weight (%) | 1.83 ± 0.12 | 1.92 ± 0.06 | 1.82 ± 0.03 |
ab Different letters in each raw represent a significant difference at p < 0.05.
Serum biochemical analysis. There were no significant differences in HDL, LDL, total cholesterol, and triglycerides (TG) levels among treated groups. Globulin and total protein (TP) showed significant (p < 0.05) increase during the first stage in OEO 300 mg kg-1 group compared to both control and 150 mg kg-1 groups while they did not alter statistically different during 21 to 42 days of the experiment. Albumin level revealed no significant alteration between the three groups. Serum ALT and AST exhibited significant (p < 0.05) increase in OEO 300 mg kg-1 at 42 day. Serum uric acid revealed a significant (p < 0.05) increment in OEO 300 mg kg-1 group compared to control during the last 3 weeks of the experiment. Moreover, serum creatinine was significantly (p < 0.05) increased in OEO 300 mg kg-1 group compared to control during whole the experimental period (Table 3).
Table 3.
Effect of two different doses of oregano essential oil (OEO; 150 and 300 mg kg-1) on serum lipid profile, protein levels, uric acid, creatinine, reduced glutathione (GSH), leukocytes counts and H/L ratio
| Parameters | Days | Control | OEO 150 mg kg -1 | OEO 300 mg kg -1 |
|---|---|---|---|---|
| High density lipoproteins (mg dL -1 ) | 14-21 | 95.00 ± 1.50 | 100.50 ± 1.60 | 105.00 ± 5.40 |
| 21-42 | 96.00 ± 0.63 | 98.50 ± 0.64 | 97.25 ± 0.85 | |
| L ow density lipoproteins (mg dL -1 ) | 14-21 | 43.71 ± 14.11 | 23.57 ± 12.66 | 26.94 ± 12.41 |
| 21-42 | 38.32 ± 6.50 | 28.88 ± 6.60 | 41.71 ± 25.78 | |
| T otal cholesterol (mg dL -1 ) | 14-21 | 163.10 ± 17.42 | 132.60 ± 19.64 | 149.10 ± 11.41 |
| 21-42 | 182.50 ± 11.52 | 157.40 ± 10.71 | 174.40 ± 21.29 | |
| T riglycerides (mg dL -1 ) | 14-21 | 137.90 ± 6.86 | 132.00 ± 15.27 | 109.80 ± 6.98 |
| 21-42 | 240.6 ± 45.20 | 150.10 ± 26.23 | 167.30 ± 26.65 | |
| Total protein (g dL -1 ) | 14-21 | 2.75 ± 0.25b | 3.50 ± 0.29b | 4.50 ± 0.65a |
| 21-42 | 3.80 ± 0.58 | 4.00 ± 0.71 | 4.75 ± 0.75 | |
| Albumin (mg dL -1 ) | 14-21 | 0.70 ± 0.03b | 0.97 ± 0.02a | 0.98 ± 0.03a |
| 21-42 | 1.34 ± 0.25 | 1.27 ± 0.08 | 1.53 ± 0.18 | |
| Globulin (mg dL -1 ) | 14-21 | 2.05 ± 0.28b | 2.53 ± 0.28b | 3.77 ± 0.60a |
| 21-42 | 2.46 ± 0.63 | 2.73 ± 0.78 | 3.23 ± 0.59 | |
| Aspartate Aminotransferase (U L-1) | 14-21 | 173.30 ± 11.42 | 159.90 ± 11.68 | 194.50 ± 3.23 |
| 21-42 | 188.30 ± 1.73b | 172.7 ± 6.85b | 201.70 ± 7.90a | |
| A lanine aminotransferase (U L -1 ) | 14-21 | 15.23 ± 0.99 | 12.90 ± 1.60 | 14.77 ± 0.57 |
| 21-42 | 17.43 ± 0.95b | 16.73 ± 1.39b | 25.00 ± 0.57a | |
| Uric acid (mg dL -1 ) | 14-21 | 1.75 ± 0.85 | 2.50 ± 0.51 | 2.52 ± 0.50 |
| 21-42 | 3.00 ± 0.58b | 4.50 ± 1.26 ab | 12.00 ± 3.89a | |
| Creatinine (mg dL -1 ) | 14-21 | 0.80 ± 0.27b | 2.50 ± 0.65 ab | 3.00 ± 0.91a |
| 21-42 | 2.00 ± 0.71b | 3.75 ± 1.11 ab | 6.50 ± 1.55a | |
| glutathione (mg mL -1 ) | 14-21 | 12.83 ± 0.72b | 13.78 ± 1.26b | 20.38 ± 0.81a |
| 21-42 | 13.50 ± 0.56b | 18.67 ± 0.61a | 7.63 ± 0.59c | |
| Total leukocyte count (×10 3 µL -1 ) | 14-21 | 14.00 ± 0.60 | 16.60 ± 1.80 | 16.60 ± 0.80 |
| 21-42 | 28.60 ± 2.80 | 27.30 ± 1.40 | 33.30 ± 1.70 | |
| Heterophils (%) | 14-21 | 33.30 ± 1.70 | 30.00 ± 0.60 | 28.60 ± 2.80 |
| 21-42 | 30.60 ± 1.20b | 34.30 ± 0.80ab | 38.00 ± 1.50a | |
| Lymphocytes (%) | 14-21 | 58.00 ± 1.50 | 56.30 ± 1.70 | 54.60 ± 1.40 |
| 21-42 | 56.30 ± 1.80b | 62.30 ± 0.80a | 61.00 ± 1.20a | |
| Eosinophils (%) | 14-21 | 5.80 ± 0.90 | 4.60 ± 0.30 | 4.80 ± 0.40 |
| 21-42 | 5.30 ± 0.30 | 5.00 ± 0.60 | 4.30 ± 0.30 | |
| Monocytes (%) | 14-21 | 5.00 ± 0.50 | 4.00 ± 0.60 | 4.60 ± 0.30 |
| 21-42 | 4.60 ± 0.60 | 4.00 ± 0.50 | 4.00 ± 0.60 | |
| Basophils (%) | 14-21 | 0.33 ± 0.30 | 0.66 ± 0.30 | 0.66 ± 0.30 |
| 21-42 | 0.33 ± 0.03 | 0.66 ± 0.33 | 1.00 ± 0.00 | |
| H/L ratio | 14-21 | 0.57 ± 0.13 | 0.53 ± 0.35 | 0.52 ± 0.20 |
| 21-42 | 0.54 ± 0.06b | 0.55 ± 0.10b | 0.62 ± 0.12a |
abc Different letters in each column represent a significant difference at p < 0.05.
Edible organs. There were no statistical differences in the liver, heart, gizzard, and spleen relative weights among treated and control groups on day 42 of the experiment (Table 2).
The serum reduced glutathione (GSH). Serum GSH exhibited a significant (p < 0.05) increase in OEO 300 mg kg-1 on days 1 to 21 of the experiment compared to control and OEO 150 mg kg-1 groups. However, it was significantly (p < 0.05) declined in OEO 300 mg kg-1 compared to control at 21-42 days. The level of GSH showed significant (p < 0.05) promotion in OEO 150 mg kg-1 compared to control and OEO 300 mg kg-1 on days 21 to 42.
Leukocyte count. The total leukocyte counts showed no significant difference between control and treated groups. However, heterophils % revealed a significant (p < 0.05) increase in OEO 300 mg kg-1 group compared to the control group during the last three weeks of the experiment (on day 42). Lymphocytes % revealed a significant (p < 0.05) increase in OEO 150 mg kg-1 and OEO 300 mg kg-1 groups compared to control. Meanwhile, the H/L ratio was significantly (p < 0.05) increased in the OEO 300 mg kg-1 group compared to the control and OEO 150 mg kg-1 groups (Table 3).
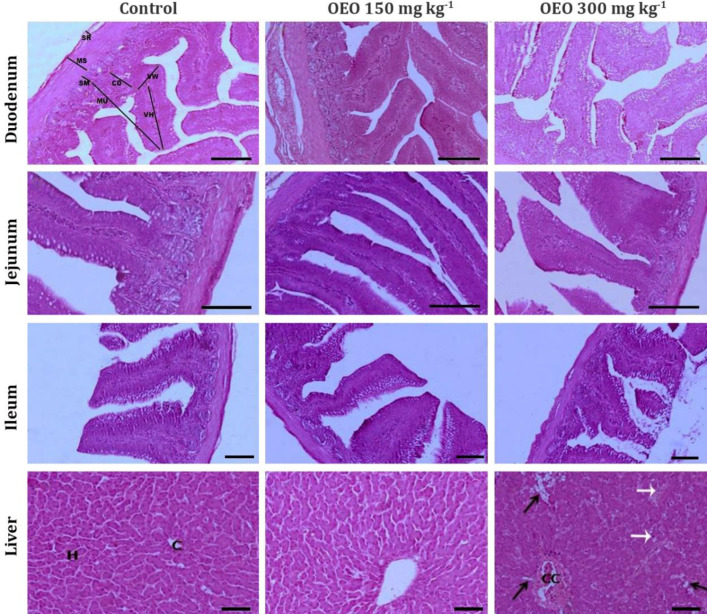
Histomorphological evaluations. The small intestine of quail has three structurally and functionally different regions which are clearly defined microscopically: The duodenum, the jejunum, and the ileum. The intestinal wall comprised three well-differentiated tunics: An inner mucosa, submucosa, middle masculosa, and an outer serosa. The intestinal tunica mucosa forms intestinal villi which project toward its lumen. Each villus is lined by absorptive columnar cells and numerous goblet cells scattered in-between. Crypts of Lieberkühn (intestinal glands) extend from the base of the villi into the underlying lamina propria. The myogenic zone was made up of few, loosely arranged and circularly oriented smooth myocytes surrounded by outer longitudinal arranged smooth muscle fibers. The intestinal outermost covering, tunica serosa, was formed of an outer mesothelial layer and a subjacent loose connective tissue layer (Fig. 1).
Fig. 1.
Light microscopic photomicrographs showed morphological characteristics of the parameters evaluated in particular regions of the quail alimentary tract. OEO: Oregano essential oil, VH: Villus height, VW: Villus width was near the crypt, CD: Crypt depth, MU: Thickness of intestinal mucosa, SM: Submucosa, MS: Tunica musculosa, and SR: Tunica serosa. H: Hepatocytes, C: Central vein, CC: Congested, and dilated central vein. Black arrows indicate fatty degeneration of hepatocytes, and white arrows show Kupffer cell hypertrophy, (H & E, Scale bar = 50 μm).
This study indicated that the addition of a low dose of oregano essential oil (OEO 150 mg kg-1) to quail diet resulted in a significant (p < 0.05) increase of duodenum, jejunum and ileum wall thickness (Fig. 2). However, the thickness of the intestinal wall showed a marked decrease of in-group subjected to OEO 300 mg kg-1 compared to control. According to Figure 1, the use of OEO 150 mg kg-1 promoted significant (p < 0.05) increase in villus height, width, and crypt depth when compared to control in all intestinal segments. The measurements of villi and crypts in histological sections were significantly (p < 0.05) short and shallow in OEO 300 mg kg-1 treated quails. Quails fed a diet supplemented with OEO 300 mg kg-1 showed side effects on the histological criteria of the intestinal integrity presented by slightly villus erosion associated with a reduction in the number of enterocytes lining the intestinal villi (Fig. 1).
Fig. 2.
Thickness (μm) of intestinal tunics including A) Mucosa, B) Submucosa, C) Muscularis, D) Serosa and E) Total wall thickness among different groups. abc Different letters on the same bar represent a significant difference (p < 0.05).
Concerning liver sections, control and OEO 150 mg kg-1 group showed normal hepatic architecture. Polygonal-shaped hepatic cells were arranged in cords surrounding the central veins (Fig. 1). Consistent changes were noticed in livers of OEO 300 mg kg-1 treated birds, moderate alterations in the hepatic parenchyma and, vacuolar and fatty degeneration of the hepatocytes along with Kupffer cell hypertrophy. Moderate lymphocytic infiltration was noticed especially around the dilated central veins (Fig. 1).
Discussion
The development and the use of growth-promoting additives from natural sources are needed due to the increased consumer awareness of food safety. Phytogenic feed additives have gained a high interest within the feed industry as possible natural alternatives to antibiotic growth promoters where they are believed to be safer and healthier with minimal hazards.26 Current study investigated the effect of two dose levels of OEO (150 mg kg-1 and 300 mg kg-1), as phytogenic feed additive, on growth promotion, some immune and biochemical parameters of growing Japanese quails.
According to the results of the current study, weight gain was significantly increased despite the lower feed intake at 150 mg kg-1 group compared to control and 300 mg kg-1 group. These results partially coincided with Bozkurt et al.27 who found that the supplementing of dietary OEO additives to broiler chickens’ diet did not affect feed intake, however, improved body weight gain and FCR. On the other hand, Basmacıoğlu et al.20 demonstrated different finding which revealed no effect of OEO treatment on feed intake and FCR. This positive growth-promoting effect could be attributed to the improved digestibility of nutrients due to the enhanced production of digestive secretions, stimulation of blood circulation, the antioxidant properties,28 and reduced levels of pathogenic bacteria by OEO that may enhance immune status.29 Moreover, OEO active ingredient, carvacrol, was proven to enhance villus height in the duodenum, jejunum, and ileum of birds, thus, improves the entire absorptive process of nutrients30 despite the reduced feed intake. However, OEO 300 mg kg-1 revealed a significant decrement in weight gain during the last three weeks of the experiment compared to control. These results are in agreement with Cross et al.31 and Abdel-Wareth14 who demonstrated the negative effects of high doses of OEO on poultry performance. They attributed such effect to carvacrol, a major active principle of the OEO, that has a dominant smell and taste and may affect performance by reducing appetite which is reflected by decreased cumulative feed intake in this group compared to control. Moreover higher dose of OEO ingredients may result on alteration of beneficial intestinal flora population (not tested) and the integrity of intestinal mucosa, as noted in the current study, that reflected negatively on absorption, weight gain and FCR.32 Thus, confirming the importance of specifying the dose level of OEO in the diet to obtain favorable response.33
Current results denoted no significant difference in serum HDL, LDL, TC, and TG levels between groups. These results were similar to those found by Sarica et al. and Traesel et al.34,35 The TP showed a significant increase during the first stage in OEO 300 mg kg-1 group compared to control and OEO150 mg kg-1 groups while they were not altered statistically during the last three weeks of the experiment. The increase in total protein could be attributed to the increase of serum total globulin as noted in the present study. These results coincided with Tollba et al. and Abdel-Ghany and Ismail.36,37 It is worth to mention that OEO 300 mg kg-1 have humeral immunity promoting effect at the first stage due to poor antioxidant power of OEO at lower dose38 that needs higher concentration. This was augmented by the significant increment in GSH level as an antioxidant reserve at 21 day in OEO 300 mg kg-1 group. The antioxidant power of the OEO higher dose could promote the humeral immune response indicated by the increased precursor of the immunoglobulin family.8,13,39 This effect was reversed at the 6th week of the experimental period that may be related to the stress induced by longer-term exposure to OEO 300 mg kg-1 on GIT and renal function as noted by the reduced antioxidant reserve of GSH in our results. Albumin level revealed no significant alteration between the three groups on day 42. These results were consistent with those of Traesel et al.35 Comparable serum albumin in both OEO groups (150 mg kg-1 and 300 mg kg-1) with control values excluded the occurrence of severe hepatic insufficiency in the present study as albumin synthesis occurs inside the liver tissue.40 However, OEO 300 mg kg-1 induced some focal hepatic deterioration that resulted in elevated serum AST and ALT that may not reach the degree of severe insufficiency. Serum uric acid and creatinine were significantly increased in the OEO 300 mg kg-1 group compared to control on day 42 and within the whole experimental period, respectively. These results were in agreement with those of Traesel et al.35 who reported the adverse effect of high doses of essential oils including OEO on the kidney. The elevated serum creatinine and uric acid were prominent indicators for renal injury or nephrotoxicity in this group that seemed to be time and dose-dependent. There were no significant differences in the liver, heart, gizzard, and spleen relative weights among treated groups and control. These results were in agreement with Sadi Cetingul et al. and Bulbul et al.41,42
Concerning the effect of diet, OEO on quail’s non-specific immune response, the TLC did not show any statistical differences among treated and control groups. Percentages of heterophils and lymphocytes were significantly increased in the OEO 300 mg kg-1 group. These results coincided with Tollba et al.36 The increased heterophils percentage and the increase in H/L ratio agreed with Yesilbag et al.43 and it was indicative of a stressful condition of the bird noted by decreased GSH level as an antioxidant reserve. Such stressful conditions could influence the hypothalamic-pituitary-adrenal axis to stimulate cortisol production.44 Cortisol decrease heterophils chemotaxis and enhance its mobilization to blood circulation and extend their life there.45 However, the lymphocytes seemed to be positively influenced by the two tested doses of OEO that may indicate a positive effect of the OEO on the nonspecific immune response.
The use of OEO at a level of 150 mg kg-1 significantly increased length and width of intestinal villi as well as whole intestinal wall thickness which was used as indicators of the digestive and absorptive capacity of the small intestine. The highest wall thickness, length, and depth of intestinal villi were obtained in OEO 150 mg kg-1. It was recorded that an increased intestinal villus height was parallel to the increased digestive and absorptive functions of the intestine due to increased absorptive surface area, expression of brush border enzymes, and nutrient transport systems.46 Our results coincided with that of Reisinger et al.47 who recorded a positive effect of herbal essential oil mixture including OEO on crypt depth and the ratio of height to crypt depth in the small intestine. High level of OEO 300 mg kg-1 resulted in a significantly shorter and shallower villi and crypts measurements with some impairments in the intestinal integrity represented by slight villus erosion associated with a reduction in the number of enterocytes lining the intestinal villi. The high dose of OEO could deteriorate the intestinal pH below an optimum level that could reverse the positive effects that were obtained in the OEO150 mg kg-1 group. The deteriorated histological picture and histomorphometric analysis of the intestine in OEO 300 mg kg-1 group denoted impairment in nutrient reabsorption that was reflected negatively on weight gain especially in the last 3 weeks of the experimental period. Overall, it seemed that OEO had a gut and hematological positive effects in a dose of 150 mg kg-1 while increasing the dose to 300 mg kg-1 was a stressful condition to the bird especially near the end of the experimental period. This means that the effect of OEO treatment was dose and time-dependent.
In conclusion, OEO feeding to quails as feed additives (150 mg kg-1) improved the intestinal histomorphometry and digestive capacity as well as biochemical and hematological parameters with no negative impact on quail health especially during the first stage of the experiment. An increase in the level of OEO to 300 mg kg-1 was considered a stress to the birds’ renal function and intestinal integrity, thus, depleting GSH as an anti-oxidant reserve. All these deteriorations produced an adverse effect on quail’s performance around the end of the experimental period. Hence, OEO could be used as a feed additive with special regard to its amount and exposure duration.
Acknowledgments
This work did not receive any financial support.
Conflict of interest
None of the authors has any financial interest or any possible conflict of interest related to the manuscript.
References
- 1.Shanaway MM. Quail production systems: a review, Food & Agriculture Organization of the United Nations. Rome, Italy. 1994:1–135. [Google Scholar]
- 2.Minvielle F, Kayang BB, Inoue-Murayama M, et al. Microsatellite mapping of QTL affecting growth, feed consumption, egg production, tonic immobility and body temperature of Japanese quail. BMC genomics. 2005;6:87. doi: 10.1186/1471-2164-6-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee KW, Everts H, Kappert HJ, et al. Cinnamaldehyde, but not thymol, counteracts the carboxymethyl cellulose-induced growth depression in female broiler chickens. Int J Poult Sci. 2004;3(9):608–612. [Google Scholar]
- 4.Adil S, Banday T, Bhat GA, et al. Response of broiler chicken to dietary supplementation of organic acids. J Cent Eur Agric. 2011;12(3):498–508. [Google Scholar]
- 5.Khan RU, Naz S, Nikousefat Z, et al. Thymus vulgaris: alternative to antibiotics in poultry feed. World Poultry Sci J. 2012;68(3):401–408. [Google Scholar]
- 6.Vazquez RS, Dunford NT. Bioactive components of Mexican oregano oil as affected by moisture and plant maturity. J Essent Oil Res. 2005;17(6):668–671. [Google Scholar]
- 7.De Falco E, Mancini E, Roscigno G, et al. Chemical composition and biological activity of essential oils of Origanum vulgare L subsp vulgare L under different growth conditions. Molecules. 2013;18(12):14948–14960. doi: 10.3390/molecules181214948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Botsoglou NA, Florou-Paneri P, Christaki E, et al. Effect of dietary oregano essential oil on performance of chickens and on iron-induced lipid oxidation of breast, thigh and abdominal fat tissues. Br Poult Sci . 2002;43(2):223–230. doi: 10.1080/00071660120121436. [DOI] [PubMed] [Google Scholar]
- 9.Bampidis VA, Christodoulou V, Florou-Paneri P, et al. Effect of dried oregano leaves versus neomycin in treating newborn calves with colibacillosis. J Vet Med A Physiol Pathol Clin Med. 2006;53(3):154–156. doi: 10.1111/j.1439-0442.2006.00806.x. [DOI] [PubMed] [Google Scholar]
- 10.Park JH, Kang SN, Shin D, et al. Antioxidant enzyme activity and meat quality of meat type ducks fed with dried oregano (Origanum vulgare L) powder. Asian-Australas J Anim Sci. 2015;28(1):79–85. doi: 10.5713/ajas.14.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Afarineshe Khaki MR, Pahlavan Y, Sepehri GH, et al. Antinociceptive effect of aqueous extract of Origanum vulgare l in male rats: Possible involvement of the GABAergic system. Iran J Pharm Res. 2013;12(2):407–413. [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang KY, Yan F, Keen CA, et al. Evaluation of micro-encapsulated essential oils and organic acids in diets for broiler chickens. Int J Poult Sci. 2005;4(9):612–619. [Google Scholar]
- 13.Hashemipour H, Kermanshahi H, Golian A, et al. Effect of thymol and carvacrol feed supplementation on performance, antioxidant enzyme activities, fatty acid composition, digestive enzyme activities, and immune response in broiler chickens. Poult Sci. 2013;92(8):2059–2069. doi: 10.3382/ps.2012-02685. [DOI] [PubMed] [Google Scholar]
- 14.Abdel-Wareth AAA. Effect of thyme, oregano and their major active components on performance and intestinal microbial populations of broilers. PhD Thesis. Germany: Institute of Animal Science. Rheinische Friedrich-Wilhelms-University. Bonn; 2011. [Google Scholar]
- 15.Hernández F, Madrid J, García V, et al. Influence of two plant extracts on broilers performance, digestibility, and digestive organ size. Poult Sci. 2004;83(2):169–174. doi: 10.1093/ps/83.2.169. [DOI] [PubMed] [Google Scholar]
- 16.Meluzzi A, Primiceri G, Giordani R et al. Determination of blood constituents reference values in broilers. Poult Sci. 1992;71:337–345. doi: 10.3382/ps.0710337. [DOI] [PubMed] [Google Scholar]
- 17.Piotrowska A, Burlikowska K, Szymeczko R. Changes in blood chemistry in broiler chickens during the fattening period. Folia Biol (Krakow) 2011;59(3-4):183–187. doi: 10.3409/fb59_3-4.183-187. [DOI] [PubMed] [Google Scholar]
- 18.Chen HL, Li DF, Chang BY, et al. Effects of Chinese herbal polysaccharides on the immunity and growth performance of young broilers. Poult Sci. 2003;82(3):364–370. doi: 10.1093/ps/82.3.364. [DOI] [PubMed] [Google Scholar]
- 19.Toghyani M, Toghyani M, Gheisari A, et al. Growth performance, serum biochemistry and blood hematology of broiler chicks fed different levels of black seed (Nigella sativa) and peppermint (Mentha piperita) Livest Sci. 2010;129(1-3):173–178. [Google Scholar]
- 20.Basmacıoğlu H, Tokuşoğlu O, Ergül M. The effect of oregano and rosemary essential oils or alpha-tocopheryl acetate on performance and lipid oxidation of meat enriched with n-3 PUFA’s in broilers. S Afr J Anim Sci. 2004;34(3):197–210. [Google Scholar]
- 21.Nutrient Requirement of Poultry (NRC) 9th Rev. Washington DC, USA: National Academy Press; 1994. p. 176. [Google Scholar]
- 22.Ritchie BW, Harrison JG, Harrison RL. Avian medicine. Florida, USA: Winger’s Publishing Inc; 1994. p. 1353. [Google Scholar]
- 23.Suvarna SK, Layton C, Bancroft JD. Bancroft’s theory and practice of histological techniques. 8th ed. Elsevier ; 2018. p. 672. [Google Scholar]
- 24.Iji PA, Saki AA, Tivey DR. Intestinal development and body growth of broiler chicks on diets supplemented with non-starch polysaccharides. Anim Feed Sci Technol. 2001;89:175–188. [Google Scholar]
- 25.Argyrous G. Statistics for research with a guide to SPSS. 2nd ed. London, UK: Sage Publications ; 2005. p. 416. [Google Scholar]
- 26.Sadek KM, Ahmed HA, Ayoub M, et al. Evaluation of Digestarom and thyme as phytogenic feed additives for broiler chickens. Eur Poult Sci. 2014;78:1–12. [Google Scholar]
- 27.Bozkurt M, Küçükyılmaz K, Çatlı AU, et al. Effect of dietary mannan oligosaccharide with or without oregano essential oil and hop extract supplementation on the performance and slaughter characteristics of male broilers. S Afr J Anim Sci. 2009;39(3):223–232. [Google Scholar]
- 28.Fonseca-García I, Escalera-Valente F, Martínez-González S, et al. Effect of oregano oil dietary supplementation on production parameters, height of intestinal villi and the antioxidant capacity in the breast of broiler. Austral J Vet Sci. 2017;49(2):83–89. [Google Scholar]
- 29.Roofchaee A, Irani M, Ebrahimzadeh MA, et al. Effect of dietary oregano (Origanum vulgare L) essential oil on growth performance, cecal microflora and serum antioxidant activity of broiler chickens. Afr J Biotechnol. 2011;10(32):6177–6183. [Google Scholar]
- 30.Franz C, Baser KHC, Windisch W. Essential oils and aromatic plants in animal feeding – a European perspective. A review. Flavour Fragr J. 2010;25(5):327–340. [Google Scholar]
- 31.Cross DE, Mc Devitt RM, Hillman K, et al. The effect of herbs and their associated essential oils on performance, dietary digestibility and gut microflora in chickens from 7 to 28 days of age. Br Poult Sci. 2007;48(4):496–506. doi: 10.1080/00071660701463221. [DOI] [PubMed] [Google Scholar]
- 32.Brenes A, Roura E. Essential oils in poultry nutrition: Main effects and modes of action. Anim Feed Sci Tech. 2010;158(1-2):1–14. [Google Scholar]
- 33.Mathlouthi N, Bouzaienne T, Oueslati I, et al. Use of rosemary, oregano, and a commercial blend of essential oils in broiler chickens: in vitro antimicrobial activities and effects on growth performance. J Anim Sci. 2012;90(3):813–823. doi: 10.2527/jas.2010-3646. [DOI] [PubMed] [Google Scholar]
- 34.Sarica S, Corduk M, Yarim GF, et al. Effects of novel feed additives in wheat-based diets on performance, carcass and intestinal tract characteristics of quail. S Afr J Anim Sci. 2009;39(2):144–157. [Google Scholar]
- 35.Traesel CK, Wolkmer P, Schmidt C, et al. Serum biochemical profile and performance of broiler chickens fed diets containing essential oils and pepper. Comp Clin Path. 2011;20:453–460. [Google Scholar]
- 36.Tollba AAH, Shabaan SAM, Abdel-Mageed MAA. Effects of using aromatic herbal extract and blended with organic acids on productive and physiological performance of poultry 2 - the growth during cold winter stress. Egypt Poult Sci J. 2010;30(1):229–248. [Google Scholar]
- 37.Abdel-Ghany WA, Ismail M. Tackling experimental colisepticaemia in broiler chickens using phytobiotic essential oils and antibiotic alone or in combination. Iran J Vet Res. 2014;15(2):110–115. [Google Scholar]
- 38.Bulbul A, Bulbul T, Biricik H, et al. Effects of various levels of rosemary and oregano volatile oil mixture on oxidative stress parameters in quails. Afr J Biotechnol. 2012;11(7):1800–1805. [Google Scholar]
- 39.Nazar FN, Magnoli AP, Dalcero AM, et al. Effect of feed contamination with aflatoxin B1 and administration of exogenous corticosterone on Japanese quail biochemical and immunological parameters. Poult Sci. 2012;91(1):47–54. doi: 10.3382/ps.2011-01658. [DOI] [PubMed] [Google Scholar]
- 40.Diaz González FH, da Silva SC. P Blood biochemical profile. In: Diaz González FH, da Silva SC., editors. Introduction to veterinary clinical biochemistry. 2nd ed. Porto Alegre, Brazil: Graphic of the Federal University of Rio Grande do Sul ; 2006. p. 358. [Google Scholar]
- 41.Sadi Cetingul I, Bayram I, Burhaneddin Akkaya A, et al. Utilisation of oregano (Origanum Onites) in laying quails (Coturnix coturnix japonica) (2): The effects of oregano on performance, carcass yield, liver and some blood parameters. Arch Zootech. 2007;10:57–65. [Google Scholar]
- 42.Bulbul T, Ozdemir V, Bulbul A. Use of sage (Salvia triloba L) and laurel (Laurus nobilis L) oils in quail diets. Eurasian J Vet Sci. 2015;31(2):95–101. [Google Scholar]
- 43.Yesilbag D, Gezen SS, Biricik H, et al. Effect of a rosemary and oregano volatile oil mixture on performance, lipid oxidation of meat and hematological parameters in Pharaoh quails. Br Poult Sci. 2012;53(1):89–97. doi: 10.1080/00071668.2012.654763. [DOI] [PubMed] [Google Scholar]
- 44.Salka Minka N, Adieza AA, Hassan FB, et al. Effects of melatonin and transportation on rectal temperature, heterophil/lymphocyte ratio and behaviour of Japanese male quails (Coturnix japonica) N Y Sci J. 2012;5(6):52–59. [Google Scholar]
- 45.Davis JM, Albert JD, Tracy KJ, et al. Increased neutrophil mobilization and decreased chemotaxis during cortisol and epinephrine infusions. J Trauma. 1991;31(6):725–731. [PubMed] [Google Scholar]
- 46.Amat C, Planas JM, Moretó M. Kinetics of hexose uptake by the small and large intestine of the chicken. Am J Physiol Regul Integr Comp Physiol. 1996;271:R1085–R1089. doi: 10.1152/ajpregu.1996.271.4.R1085. [DOI] [PubMed] [Google Scholar]
- 47.Reisinger N, Steiner T, Nitsch S, et al. Effects of a blend of essential oils on broiler performance and intestinal morphology during coccidial vaccine exposure. J Appl Poult Res. 2011;20:272–283. [Google Scholar]