Abstract
During the first week after hatch, young chicks are vulnerable to pathogens as the immune system is not fully developed. The objectives of this study were to determine if supplementing the starter diet with a microencapsulated feed additive containing citric and sorbic acids, thymol, and vanillin affects in vitro functional activity of peripheral blood leukocytes (PBLs). Day-old chicks (n = 800) were assigned to either a control diet (0 g/metric ton [MT]) or a diet supplemented with 500 g/MT of the microencapsulated additive. At 4 D of age, peripheral blood was collected (100 birds per treatment), and heterophils and monocytes isolated (n = 4). Heterophils were assayed for the ability to undergo degranulation and production of an oxidative burst response while nitric oxide production was measured in monocytes. Select cytokine and chemokine mRNA expression levels were also determined. Statistical analysis was performed using Student t test comparing the supplemented diet to the control (P ≤ 0.05). Heterophils isolated from chicks fed the microencapsulated citric and sorbic acids, thymol, and vanillin had higher (P ≤ 0.05) levels of degranulation and oxidative burst responses than those isolated from chicks on the control diet. Heterophils from the supplemented chicks also had greater (P ≤ 0.05) expression of IL10, IL1β, and CXCL8 mRNA than those from control-fed chicks. Similarly, nitric oxide production was significantly (P ≤ 0.05) higher in monocytes isolated from birds fed the supplement. The cytokine and chemokine profile in monocytes from the supplement-fed chicks showed a significant (P ≤ 0.05) drop in IL10 mRNA expression while IL1β, IL4, and CXCL8 were unchanged. In conclusion, 4 D of supplementation with a microencapsulated blend made up of citric and sorbic acids, thymol, and vanillin enhanced the in vitro PBL functions of degranulation, oxidative burst, and nitric oxide production compared with the control diet. Collectively, the data suggest feeding broiler chicks a diet supplemented with a microencapsulated blend of citric and sorbic acids, thymol, and vanillin may prime key immune cells making them more functionally efficient and acts as an immune-modulator to boost the inefficient and undeveloped immune system of young chicks.
Key words: botanicals, feed additive, microencapsulated, organic acid, peripheral blood leukocyte
Introduction
The mandatory or voluntary removal of antibiotic growth promoters from poultry production in conjunction with increasing regulations has contributed to an enormous growth in the quest for suitable products to take their place in the poultry industry and throughout animal agriculture as a whole (Castanon, 2007). In addition to the governmental regulations, consumers and advocacy groups are further pressuring food animal industries to find alternative approaches to replace antibiotic use. In 2017, the animal feed additive market was valued near $20M USD and is projected to surpass $31M USD by 2025 (https://www.alliedmarketresearch.com/animal-feed-additives-market). According to that report, the poultry industry is the current and projected leading feed additive–consuming animal sector surpassing swine, ruminants, and aquatic species. Feed additives used by the poultry industry include, but are not limited to, organic acids, essential oils, minerals, plant metabolites, amino acids, medicinal herbs, nondigestible oligosaccharides, and food industry or other natural by-products; many of which have been recently reviewed (Zeng et al., 2015, Gessner et al., 2017, Micciche et al., 2018, Suresh et al., 2018, Yadav and Jha, 2019).
There are 2 divisions of an immune response: innate and acquired immunity. The innate immune system is recognized as a first line of defense, but in reality, the innate response is extremely important and responsible for much more than merely serving as a fist-line defense mechanism in the host. Cells of the innate immune system have key pattern recognition receptors responsible for recognizing pathogen and danger-associated molecular patterns that include lipoproteins, polysaccharides, nucleic acids, and glycolipids (Medzhitov and Janeway, 2000, Akira et al., 2001, Schnare et al., 2001, Athman and Philpott, 2004, Netea et al., 2004). As a result of this recognition, there is an immediate activation of defense-oriented intracellular signaling pathways which initiates diverse microbicidal killing mechanisms, cytokine and chemokine release, and costimulatory molecule production which are all required to activate the acquired response (Medzhitov and Janeway, 1997, Takeuchi and Akira, 2007). The complex interplay between innate and adaptive immune responses in the host can essentially coordinate the appropriate long-term protective response (Iwasaki and Medzhitov, 2015).
Young chicks are highly susceptible to poultry and foodborne pathogens. Once colonized with a foodborne pathogen, such a Salmonella or Campylobacter, the birds may be carriers for the duration of their life, therefore increasing the potential for carcass contamination (Hermans et al., 2011). In chickens, cells of the innate immune system include B1-type lymphocytes that produce natural antibodies, heterophils, and macrophages, and B2 and T lymphocytes are associated with acquired immunity (Klasing, 2007, Genovese et al., 2013). Heterophils and monocytes are found circulating in the blood and are the primary peripheral blood leukocytes (PBLs). Functionally, heterophils phagocytize foreign pathogens, promote chemotaxis and adhesion, undergo degranulation, generate a respiratory burst, and produce cytokines and chemokines (Genovese et al., 2013). Monocytes and macrophages are mononuclear phagocytes responsible for antigen processing and presentation to cells of the acquired response. Recognition of microbial components by pattern recognition receptor initiates signaling cascades which, in turn, initiate the expression of genes responsible for the production of reactive oxygen and nitrogen intermediates, cytokines, and costimulatory molecules (Werling and Jungi, 2003, Werling et al., 2004).
Over the years, our laboratories have used an array of diverse feed additives to help protect chickens of all ages against key foodborne pathogens including Salmonella (Lowry et al., 2005, Grilli et al., 2011, Kogut et al., 2012, Kogut et al., 2013) and Campylobacter (Grilli et al., 2013). Administration of prebiotics or antimicrobial peptides to young chicks enhances heterophil functional efficiency that corresponds to increased resistance to colonization and organ invasion by Salmonella (Lowry et al., 2005, Kogut et al., 2012, Kogut et al., 2013). The poultry industry has used organic acids in the diet and water supply to enhance growth and performance for many years (Khan and Iqbal, 2016) which significantly lowered Salmonella (Grilli et al., 2011) and Campylobacter colonization in market-age broilers (Grilli et al., 2013).
A complex interplay exits between early dietary interventions and the development of immune competence; however, 2 recent reviews highlight that much of the research is directed toward the impact of dietary interventions on lymphoid tissue (Taha-Abdelaziz et al., 2018) or gastrointestinal integrity (Adedokun and Olojede, 2018). The vast majority of studies evaluating feed additives in poultry focus on performance and growth parameters with little regard for determining the mode of action and impact on immune function outside the gut. The objectives of the present study were to determine if supplementing a broiler chicken's diet with a microencapsulated feed additive containing organic acids, thymol, and vanillin impacted the functional activity of PBL, including heterophils and monocytes, isolated from young chicks.
Materials and methods
Experimental Chickens
Day-of-hatch by-product breeder chicks (n = 800 total chicks) were obtained from a commercial hatchery (Timpson, TX) and placed in floor pens containing wood shavings and provided supplemental heat and ad libitum access to water. Chicks assigned to the control group were allowed ad libitum access to a balanced, unmedicated corn and soybean meal–based starter diet. The feed contained 23% crude protein and 3,200 kcal of ME/kg, and all other nutrient levels met or exceeded the established requirements (National Research Council, 1994). Chicks assigned to the treated group were given free access to the same chick starter diet supplemented with 500 g/metric ton (MT) of a microencapsulated blend of citric (25%) and sorbic (16.7%) acids, thymol (1.7%), and vanillin (1.0%) (AviPlusP; Vetagro S.p.A., Reggio Emilia, Italy). The birds did not receive any medications during the course of the study. The experiments were conducted in accordance with the recommended code of practice for the care and handling of poultry and followed the ethical principles according to the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011). The US Department of Agriculture, Agricultural Research Service Institutional Animal Care and Use Committee approved all experimental procedures (protocol #2017007).
PBL Isolation
Heterophils and monocytes were isolated from the peripheral blood of 100 chickens per group 4 D after hatch as previously described (Swaggerty et al., 2003a). Cell isolations were conducted on 4 separate occasions with chicks from different flocks to ensure reproducibility (100 chicks × 4 replicate experiments = 400 chicks per treatment group). Briefly, blood was collected in vacutainer tubes containing disodium ethylenediaminetetraacetic acid (EDTA) (BD vacutainer; Becton, Dickinson and Company, Franklin Lakes, NJ) and mixed thoroughly. The blood and EDTA were then pooled and mixed with 1% methylcellulose (1:1 v/v) and centrifuged at 25 × g for 15 min. The supernatant was diluted with Ca2+- and Mg2+-free Hanks balanced salt solution, carefully layered onto a discontinuous Histopaque gradient (specific gravity 1.077/1.119), and centrifuged at 250 × g for 60 min. The heterophils were collected from the 1.119 gradient layer and washed with RPMI-1640. Heterophil preparations were consistently 95% pure and 95% viable. Monocytes, the peripheral blood mononuclear cells (PBMCs), were collected from the layer at the 1.077/1.119 interface and washed with RPMI-1640. Heterophils were counted and diluted in RPM I-1640 to the appropriate concentration for each assay. The PBMC were counted and resuspended in RPMI-1640 containing gentamicin (50 μg/mL) and then diluted to appropriate concentrations for each assay. All reagents and chemicals were purchased from Sigma Chemical Company, St. Louis, MO, unless otherwise noted.
Bacteria
A poultry isolate of Salmonella enterica Enteritidis (S. Enteritidis; #97-11771) was obtained from the National Veterinary Services Laboratory (Ames, IA). The S. Enteritidis was cultured in tryptic soy broth (Difco Laboratories, Becton Dickinson Co., Sparks, MD) overnight at 41°C. Stock S. Enteritidis (1 × 109 cfu/mL) was prepared in clear RPMI-1640 as previously described (Swaggerty et al., 2003a). Opsonized S. Enteritidis (OpSE) was prepared with normal chicken serum as previously described (Kogut et al., 2001). The heat-killed S. Enteritidis (HKSE) was prepared by incubating a stock culture of 1 × 108 cfu/mL in PBS at 75°C for 15 min as previously described (He et al., 2007).
Oxidative Burst Assay
The oxidative burst assay quantifies reactive oxygen species produced by heterophils by measuring the oxidation of 2′,7′-dichlorofluorescin-diacetate to fluorescent DCF as previously described (He et al., 2005). Briefly, heterophils (1 × 107 cells/mL) were stimulated with phorbol myristate acetate (PMA, 1 μg/mL) in 2-mL microcentrifuge tubes containing 10 μg/mL of 2′,7′-dichlorofluorescin-diacetate for 1 h at 39°C in 5% CO2 and 95% humidity. After incubation, aliquots (150 μL; n = 4 wells) were dispensed into a black 96-well plate, and the relative fluorescent units measured (485/530 nm) using a fluorescence microplate reader (Genios Plus Plate Reader, TECAN U.S. Inc., Raleigh, NC).
Degranulation Assay
Heterophil degranulation was measured by quantifying β-glucuronidase activity in culture medium after stimulation as previously described (Swaggerty et al., 2003b). Briefly, heterophils (8 × 106 mL) were stimulated with OpSE (300 μL of the stock culture) at 39°C for 1 h on a rocker platform in 5% CO2 and 95% humidity. After incubation, the cells were pelleted by centrifugation at 10,000 × g for 2 min at 4°C and supernatants collected for the assay. An aliquot of supernatant (25 μL; n = 8 wells) was incubated with freshly prepared substrate (50 μL; 10 mmol 4-methylumbelliferyl-β-d-glucuronide and 0.1% Triton X-100 in 0.1 mol sodium acetate buffer) in a black 96-well plate for 4 h at 39°C. The reaction was stopped by adding 200 μL of stop solution (0.05 mol glycine and 5 mmol EDTA; pH 10.4) to each well. Liberated 4-methylumbelliferone was measured fluorometrically (355/460 nm) using a fluorescence microplate reader (Genios Plus Plate Reader).
Monocyte Culture and Stimulation for the Nitric Oxide Assay
The PBMC (2 mL aliquots; 1 × 107 cells/mL) were transferred to a 12-well round-bottom plate and incubated at room temperature for 2 h. After incubation, nonadherent cells were removed by washing 3 times with RPMI-1640. The adherent monocytes were cultured overnight in complete DMEM medium (Dulbecco's Modified Eagles Medium containing 10% chicken serum, antibiotics [100 U penicillin/mL and 100 μg streptomycin/mL], and 1.5 mmol L-glutamine). Before stimulation, the cells were washed once with fresh media. Monocytes were then stimulated with HKSE by adding 50 μL of HKSE stock prepared in PBS to a final 1-mL cell culture medium and incubated for 72 h at 39°C in a 5% CO2 and 95% humidity incubator as previously described (He et al., 2007).
Nitrite Assay
Nitrite, a stable metabolite of nitric oxide (NO), produced by activated monocytes was measured by the Griess assay (Green et al., 1982). Briefly, culture supernatant (100 μL) from each well was transferred to 4 wells of a new 96-well flat-bottom plate and combined with 50 μL of 1% sulfanilamide and 50 μL of 0.1% naphthylenediamine (both prepared in 2.5% phosphoric acid solution). After 10 min incubation at room temperature, the nitrite concentration was determined by measuring optical density (OD550) of each well using a microplate reader (Genios Plus Plate Reader). Sodium nitrite was used as a standard to determine nitrite concentrations in the cell-free medium.
RNA Isolation for Quantitative Real-Time RT-PCR
Heterophils and monocytes (8 × 106 mL) were stimulated (separately) with HKSE (300 μL of the stock culture) at 39°C for 1 h on a rocker platform in 5% CO2 and 95% humidity. After incubation, the cells were pelleted by centrifugation at 10,000 × g for 2 min at 4°C, and the supernatant discarded. The cells were resuspended in lysis buffer (Qiagen RNeasy mini RNA extraction kit; Qiagen Inc., Valencia, CA) and frozen at −20°C. The samples were then homogenized (QIAshredder columns; Qiagen Inc., Valencia, CA), total RNA extracted according to the manufacturer's instructions, eluted in 50 μL water, and stored at −80°C until further use.
Quantitative Real-Time RT-PCR
Cytokine and chemokine mRNA expressions in heterophils and monocytes isolated from control-fed and supplement-fed chicks were determined using quantitative real-time RT-PCR as previously described (Kaiser et al., 2000). All primer and probe sequences used in the study are provided elsewhere (Kaiser et al., 2000, Kogut et al., 2003, Rothwell et al., 2004, Swaggerty et al., 2004). The quantitative real-time RT-PCR was performed using the Taqman RNA-to-CT 1-Step Kit (Applied Biosystems, Foster City, CA). Amplification and detection of products were performed using the StepOnePlus Real-Time PCR System (Applied Biosystems). All samples were standardized using 28S RNA. Results for each group were calculated as 40-cycle threshold (CT) for nonstimulated and stimulated samples and the data shown as fold-change from nonstimulated. Fold change is calculated as 2ˆ(stimulated corrected mean – nonstimulated corrected mean).
Statistical Analyses
Anticoagulated blood from 100 chickens per group was pooled, and heterophils and monocytes were isolated (n = 4). Each blood collection and cell isolation was replicated on 4 separate occasions; therefore, cells were pooled from a total of 400 chickens for each group (control-fed and supplement-fed chicks). Statistical analyses (Student t test) were performed using Microsoft Excel Version 16 (P < 0.05).
Results
Heterophil-Mediated Oxidative Burst
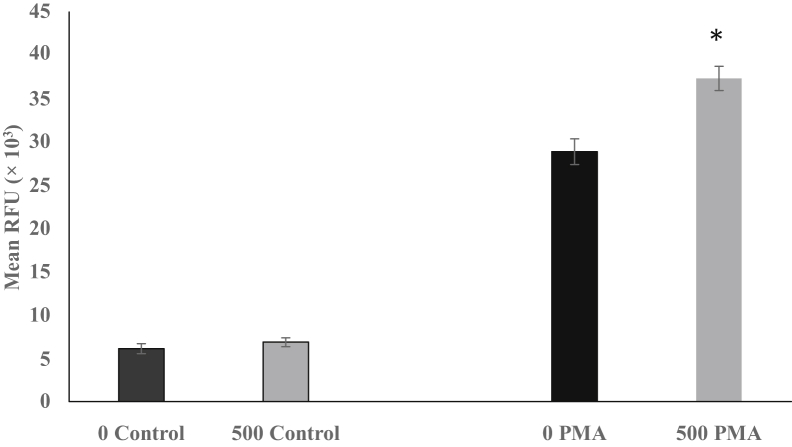
Oxidative burst was measured in heterophils isolated from chicks fed a control diet and those fed a diet supplemented with a microencapsulated blend of citric and sorbic acids, thymol, and vanillin (Figure 1). After stimulation with PMA, heterophils from the supplement-fed chicks had significantly (P < 0.05) higher relative fluorescent unit than heterophils from chicks on the control diet (37.33 and 28.86 × 103, respectively). There were no differences in the nonstimulated cells from control (6.1 × 103) or supplement (6.8 × 103)-fed chicks.
Figure 1.
Oxidative burst response in heterophils isolated from 4-day-old chicks fed a control diet or a diet supplemented with 500 g/metric ton (MT) of a microencapsulated blend of organic acids and botanicals. Heterophils were assayed for the ability to undergo an oxidative burst response and the mean relative fluorescent units ([RFU] × 103) determined. Nonstimulated heterophils from control (0 control) and supplement-fed (500 control) chicks were compared. After stimulation with phorbol myristate acetate (PMA), heterophils isolated from control-fed chicks (0 PMA) were compared with those isolated from supplement-fed chicks (500 PMA). All comparisons were made between either controls (0 and 500) or PMA-stimulated (0 and 500) heterophils using Student t test, and significance is noted with ∗ (P ≤ 0.05). Data are given as mean ± SEM of 4 separate replications.
Heterophil-Mediated Degranulation
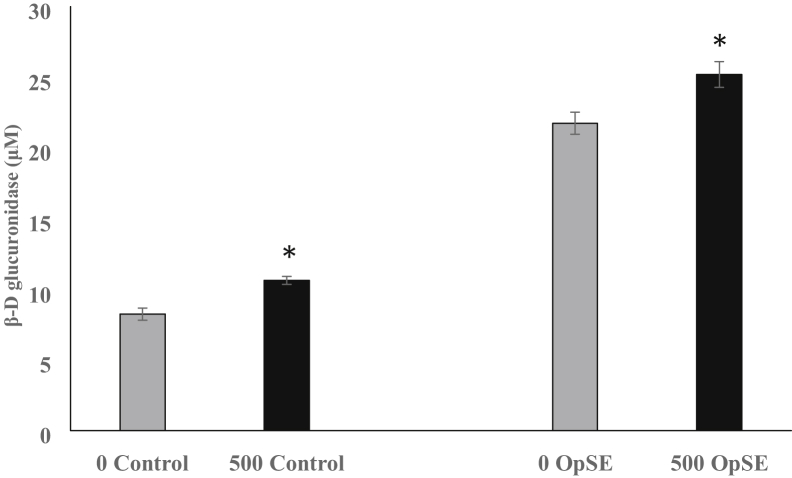
Degranulation was measured in heterophils isolated from chicks fed a control diet and those fed a diet supplemented with a microencapsulated blend of citric and sorbic acids, thymol, and vanillin (Figure 2). After stimulation with OpSE, heterophils from the supplement-fed chicks released significantly (P < 0.05) more b-D-glucuronidase (25.2 ± 0.9 μmol) than the 21.7 ± 0.43 μmol released by heterophils isolated from chicks on the control diet. In addition, the basal levels detected in nonstimulated heterophils from the supplement-fed chicks was higher (P < 0.05) than those in nonstimulated heterophils from control fed chicks (10.6 ± 0.2 and 8.2 ± 0.4, respectively)
Figure 2.
Degranulation of heterophils isolated from 4-day-old chicks fed a control diet or a diet supplemented with 500 g/metric ton (MT) of a microencapsulated blend of organic acids and botanicals. Heterophils were assayed for the ability to degranulate and the amount (μmol) of released b-D glucuronidase measured. Nonstimulated heterophils from control (0 control) and supplement-fed (500 control) chicks were compared. After stimulation with opsonized S. Enteritidis (OpSE), heterophils isolated from control-fed chicks (0 OpSE) were compared with those isolated from supplement-fed chicks (500 OpSE). All comparisons were made between either controls (0 and 500) or OpSE-stimulated (0 and 500) heterophils using Student t test, and significance is noted with ∗ (P ≤ 0.05). Data are given as mean ± SEM of 4 separate replications.
Monocyte Production of Nitric Oxide
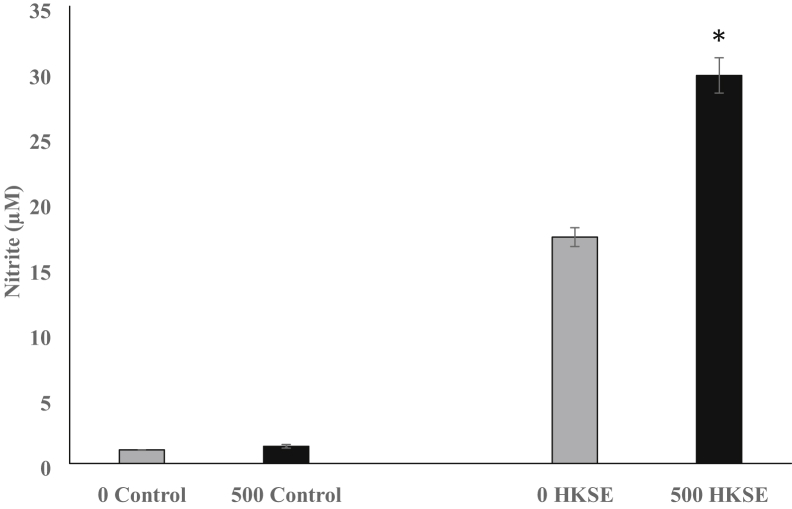
NO production was measured in monocytes isolated from chicks fed a control diet and those fed a diet supplemented with a microencapsulated blend of citric and sorbic acids, thymol, and vanillin (Figure 3). After stimulation with HKSE, monocytes from the supplement-fed chicks produced more (P < 0.05) nitrite (29.7 ± 1.3 μmol) than monocytes from control-fed chicks (17.3 ± 0.7 μmol). The basal levels of nitrite detected in nonstimulated monocytes from control- or supplement-fed chicks were comparable (1.0–1.31 μmol).
Figure 3.
Nitric oxide production by monocytes isolated from 4-day-old chicks fed a control diet or a diet supplemented with 500 g/metric ton (MT) of a microencapsulated blend of organic acids and botanicals. Monocytes were assayed for the ability to generate a nitric oxide response, and the amount (μmol) of nitrite released was measured. Nonstimulated monocytes from control (0 control) and supplement-fed (500 control) chicks were compared. After stimulation with heat-killed S. Enteritidis (HKSE), monocytes isolated from control-fed chicks (0 HKSE) were compared with those isolated from supplement-fed chicks (500 HKSE). All comparisons were made between either controls (0 and 500) or HKSE-stimulated (0 and 500) heterophils using Student t test, and significance is noted with ∗ (P ≤ 0.05). Data are given as mean ± SEM of 4 separate replications.
Cytokine and Chemokine mRNA Expression
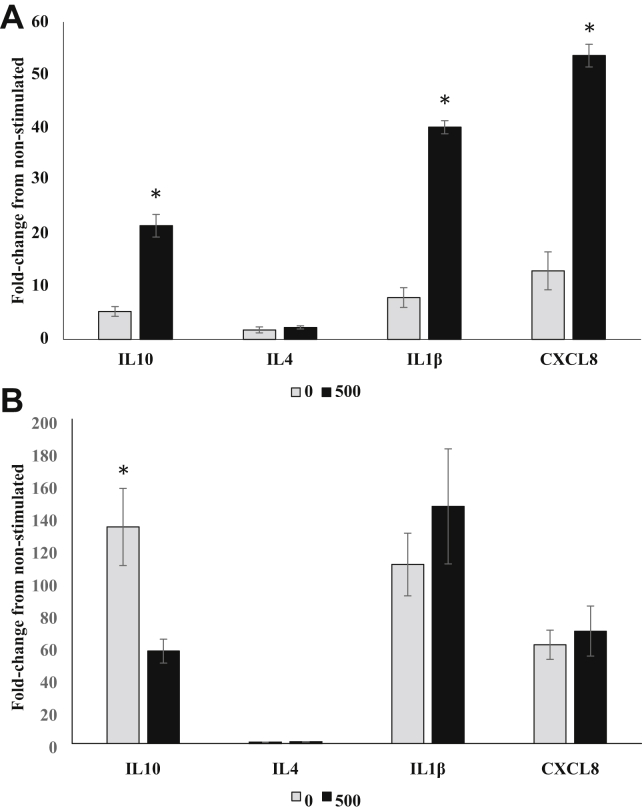
The mRNA expression levels of select cytokines (IL4, IL10, and IL1b) and a chemokine (CXCL8) were measured in heterophils and monocytes isolated from chicks fed a control diet and those fed a diet supplemented with a microencapsulated blend of citric and sorbic acids, thymol, and vanillin (Figure 4). The data for both cell populations are shown as fold-change from stimulated over nonstimulated for each group. The heterophil data are shown in Figure 4A, and the monocyte data are shown in Figure 4B. Heterophils from supplement-fed chicks after stimulation with HKSE had a 53-fold increase in CXCL8 mRNA expression compared with a 5.3-fold increase observed in stimulated heterophils from chicks on the control diet (P < 0.05). Similarly, mRNA expression of IL1b was significantly greater (P < 0.05) in heterophils from supplemented chicks than that in controls (40.1- and 21.5-fold, respectively). The mRNA expression of IL10 was higher (P < 0.05) in heterophils from supplement-fed chicks than that in those from control-fed chicks (21.5- and 5.3-fold, respectively). After stimulation with HKSE, there were no differences in IL4 mRNA expression between the 2 groups of heterophils (P > 0.05).
Figure 4.
Cytokine and chemokine mRNA expression of heterophils (A) and monocytes (B) after stimulation with heat-killed S. Enteritidis. Cytokine and chemokine mRNA expression in heterophils and monocytes isolated from control-fed and supplement-fed chicks were determined using quantitative real-time RT-PCR. Results for each group were calculated as 40-cycle threshold (CT) for nonstimulated and stimulated samples, and the data shown as fold-change from nonstimulated. Fold change is calculated as 2ˆ(stimulated corrected mean – nonstimulated corrected mean). All analyses were made using Student t test, and significance is noted with ∗ (P ≤ 0.05). Data are given as mean ± SEM of 4 separate replications.
Monocytes isolated from supplement-fed chicks had a 56.9-fold increase in IL10 mRNA expression after stimulation with HKSE; however, this was significantly (P < 0.05) less than the 133.5-fold increase observed in HKSE-stimulated monocytes from control-fed chicks. No differences were observed in IL4, CXCL8, or IL1b mRNA expression between monocytes isolated from control- or supplement-fed chicks (P > 0.05).
Discussion
Young chickens have immature and inefficient immune systems at hatch, and as soon as the birds are placed in a pen or house, they will be rapidly exposed to numerous poultry and foodborne pathogens. One way to enhance the immature immune system of young chicks is to start the birds on a diet supplemented with an immunomodulatory feed additive at placement. In this study, immediate dietary supplementation with a microencapsulated blend of organic acids, vanillin, and thymol increased the functional capabilities of heterophils and monocytes isolated from 4-day-old chicks compared to cells isolated from chicks on a nontreated control diet. There are numerous primary and review articles in the literature supporting the use of natural products for use as an alternative to antibiotics and a means to improve performance, animal welfare, and robustness in poultry (Zeng et al., 2015, Abd El-Hack et al., 2016, Jazi et al., 2018, Yadav and Jha, 2019). The EU Commission and European Food Safety Authority have recognized the microencapsulated blend of organic acids and natural botanicals used in the present study (AviPlus P; identification number 4d3) for its ability to improve growth rate and feed efficiency in healthy poultry. Furthermore, the findings presented herein indicate that AviPlus P may also provide beneficial immunological support by boosting the innate immune response of young chicks.
In vitro heterophil function can be a good indicator for whether a group of birds will be either resistant or susceptible to various poultry and foodborne pathogens (Genovese et al., 2013). In the present study, heterophils isolated from chicks whose diet was supplemented with 500 g/MT of a microencapsulated blend of organic acids, vanillin, and thymol demonstrated an improved ability to degranulate and produce an oxidative burst response after stimulation with OpSE and PMA, respectively. Although it is not the same organic acid evaluated in our study, dietary supplementation with ascorbic acid has been shown to increase in vitro heterophil function, specifically the ability to kill bacteria (Andreasen and Frank, 1999). Additional studies have also shown feeding prebiotics or antimicrobial peptides to young chicks can also enhance heterophil functional efficiency (Lowry et al., 2005, Kogut et al., 2012). Our data also indicate the supplement's affect went beyond the gut and had a direct impact on PBL function including heterophil-mediated degranulation and oxidative burst.
Enhanced in vitro heterophil function has been observed in different lines of chickens and is generally associated with increased resistance against Salmonella (Ferro et al., 2004, He et al., 2005, Swaggerty et al., 2005a, Redmond et al., 2011), Campylobacter (Li et al., 2008), Enterococcus (Swaggerty et al., 2005b), and coccidiosis (Swaggerty et al., 2011). Unlike the present study, none of the aforementioned challenge trials used a feed additive; however, dietary supplementation with organic acids and botanicals significantly lowers Salmonella (Grilli et al., 2011) and Campylobacter (Grilli et al., 2013) colonization in market-age broilers. Although not considered in the studies by Grilli et al., it is possible that an increase in heterophil function contributed to the observed decline in Salmonella and Campylobacter colonization, but additional studies are required to confirm this hypothesis. In vitro studies also show thymol, one of the components of AviPlus P, reduces Salmonella and Campylobacter numbers in cecal contents (Johny et al., 2010). The experimental approach used in the present study was not designed to determine which compound in the blend directly impacted heterophil function. There are studies that evaluate specific organic acids and natural compounds, but most of those trials tend to monitor cell numbers in the form of the heterophil:lymphocyte ratio (Jazi et al., 2018, Nelson et al., 2018, Salah et al., 2018) instead of actual cell function as we measured in this study. Collectively, the findings of this study suggest that dietary supplementation from a very young age could provide immunological benefits to the bird in the form of early immunomodulation of PBL, including heterophils.
Heterophils isolated from our supplement-fed chicks had changes in regulatory (IL10) and proinflammatory cytokine (IL1ß) and chemokine (CXCL8) mRNA expression after stimulation with HKSE while monocytes had lower expression of IL10, a key regulatory cytokine. The changes after stimulation indicate increased activity similar to a “priming” effect. Treatment of splenic macrophages with thymol followed by lipopolysaccharide (LPS) stimulation results in a significant increase in IL1ß expression compared with controls (Chauhan et al., 2014), which is in agreement with our findings suggesting cellular activation and active response to a foreign challenge. Other feed additive studies support the idea of a “primed” immune system. For example, supplementing the diet of young chicks with cationic peptides increases heterophil and monocyte mRNA expression of key proinflammatory cytokines and chemokines after stimulation (Kogut et al., 2012), which translates to increased resistance against Salmonella colonization in a challenge model (Kogut et al., 2013). Outside of the studies conducted by our laboratory, the authors are unaware of reports addressing PBL activity in broilers given a feed additive containing organic acids and botanicals, so most comparisons discussed herein are related to data generated in specific tissues and not PBL. Similar to what we observed at the cellular (heterophil) level, a study by Liu et al. shows addition of carvacrol, an essential oil compound, to the diet increases IL1ß and IL8 (CXCL8) mRNA expression in gut samples after in vivo exposure to LPS (Liu et al., 2019). Carvacrol was not included in the dietary blend used in the present study, but the data are consistent that cytokine and chemokine mRNA expression was impacted by the inclusion of essential oils to the diet and indicates tissues and peripheral blood cells can be primed to be more responsive after in vivo challenge with LPS or in vitro stimulation with Salmonella as we observed. These similarities are likely due to the fact that thymol and carvacrol share the same structure with the exception for the position of the OH group. No changes were observed in IL4, and it is likely that IL10, a key regulatory cytokine (Rothwell et al., 2004), influenced these complex signaling interactions. Moving forward, future experiments will consider effects at the gut level including cytokine and chemokine expression.
As observed with heterophils, monocytes isolated from supplement-fed chicks also had increased functional activity compared with those isolated from control-fed chicks (Figure 3). After stimulation with HKSE, higher levels of NO were observed. These data are in accordance with a report showing that in vitro administration of Nigella sativa seed extract increases NO production by monocytes (Elmowalid et al., 2013), indicating monocytes are sensitive to the immunostimulatory effects of diverse natural products. The monocytes in the present study were exposed to HKSE, therefore indicative of M1 activation because the response is directed toward eliminating a pathogenic threat, whereas a response more toward healing would be characterized as M2 activation (Italiani and Boraschi, 2014, Ley, 2017). We observed increased mRNA expression of IL1ß and IL10 in heterophils, as well as NO production in monocytes isolated from the peripheral blood, and similar results have been reported in a chicken-derived macrophage cell line (HD-11) after treatment with a natural feed additive (Han et al., 2017). Athough outside the scope of the present study, there are reports that show NO production can impact monocyte and macrophage maturation and therefore may have an influence over subsequent macrophage populations residing in various tissues (Mills et al., 2000). Future studies could evaluate macrophage population in tissues from control and supplement-fed chickens to determine if all sub-sets are immunologically more responsive or if the enhanced function is limited to monocytes circulating in the peripheral blood as observed herein. As the innate immune response is critical for regulating acquired immunity (Iwasaki and Medzhitov, 2015), activation of monocytes and the subsequent impact on host signaling pathways may prove beneficial by producing a quick response against potential pathogens. Collectively, our data indicate that dietary supplementation of a microencapsulated blend containing organic acids and natural botanicals beginning at hatch provided immunological benefits to the young chicks by increasing the functional activity of PBL, including heterophils and monocytes.
Acknowledgements
The authors thank M. Reiley Street, Jr. (U.S. Department of Agriculture, Agricultural Research Service [USDA/ARS], College Station, TX) for outstanding technical support and assistance with daily animal care. This research was supported, in part, by Vetagro S.p.A, Italy (Agreement number 58-3091-8-005) and the USDA/ARS (3091-32000-035-00D). USDA is an equal opportunity provider and employer. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA.
Conflict of Interest Statement: The authors did not provide a conflict of interest statement.
References
- Abd El-Hack M.E., Alagawany M., Farag M.R., Tiwari R., Karthik K., Dhama K., Zorriehzahra M.J., Adel M. Beneficial impacts of thymol essential oil on health and production of animals, fish and poultry: a review. J. Essent. Oil Res. 2016;28:365–382. [Google Scholar]
- Adedokun S.A., Olojede O.C. Optimizing gastrointestinal integrity in poultry: the role of nutrients and feed additives. Front. Vet. Sci. 2018;5:348. doi: 10.3389/fvets.2018.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S., Takada H., Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- Andreasen C.B., Frank D.E. The effects of ascorbic acid on in vitro heterophil function. Avian Dis. 1999;43:656–663. [PubMed] [Google Scholar]
- Athman R., Philpott D. Innate immunity via Toll-like receptors and Nod proteins. Curr. Opin. Microbiol. 2004;7:25–32. doi: 10.1016/j.mib.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Castanon J.I.R. History of the use of antibiotic as growth promoters in European poultry feeds. Poult. Sci. 2007;86:2466–2471. doi: 10.3382/ps.2007-00249. [DOI] [PubMed] [Google Scholar]
- Chauhan A.K., Jakhar R., Paul S., Kang S.C. Potentiation of macrophage activity by thymol through augmenting phagocytosis. Int. Immunopharmacol. 2014;18:340–346. doi: 10.1016/j.intimp.2013.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmowalid G., Amar A.M., Ahmad A.A. Nigella sativa seed extract: 1. Enhancement of sheep macrophage immune functions in vitro. Res. Vet. Sci. 2013;95:437–443. doi: 10.1016/j.rvsc.2013.02.015. [DOI] [PubMed] [Google Scholar]
- Ferro P.J., Swaggerty C.L., Kaiser P., Pevzner I.Y., Kogut M.H. Heterophils isolated from chickens resistant to extraintestinal Salmonella enteritidis infection express higher levels of pro-inflammatory cytokine mRNA following infection than heterophils from susceptible chickens. Epidemiol. Infect. 2004;132:1029–1037. doi: 10.1017/s0950268804002687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese K.J., He H., Swaggerty C.L., Kogut M.H. The avian heterophil. Dev. Comp. Immunol. 2013;41:334–340. doi: 10.1016/j.dci.2013.03.021. [DOI] [PubMed] [Google Scholar]
- Gessner D.K., Ringseis R., Eder K. Potential of plant polyphenols to combat oxidative stress and inflammatory processes in farm animals. J. Anim. Physiol. Anim. Nutr. (Berl) 2017;101:605–628. doi: 10.1111/jpn.12579. [DOI] [PubMed] [Google Scholar]
- Green L.C., Wagner D.A., Glogowski J., Skipper P.L., Wishnok J.S., Tannenbaum S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Grilli E., Tugnoli B., Formigoni A., Massi P., Fantinati P., Tosi G., Piva A. Microencapsulated sorbic acid and nature-identical compounds reduced Salmonella Hadar and Salmonella Enteritidis colonization in experimentally infected chickens. Poult. Sci. 2011;90:1676–1682. doi: 10.3382/ps.2011-01441. [DOI] [PubMed] [Google Scholar]
- Grilli E., Vitari F., Domeneghini C., Palmonari A., Tosi G., Fantinati P., Massi P., Piva A. Development of a feed additive to reduce caecal Campylobacter jejuni in broilers at slaughter age: from in vitro to in vivo, a proof of concept. J. Appl. Microbiol. 2013;114:308–317. doi: 10.1111/jam.12053. [DOI] [PubMed] [Google Scholar]
- Han D., Lee H.T., Lee J.B., Kim Y., Lee S.J., Yoon J.W. A Bioprocessed polysaccharide from Lentinus edodes Mycelia cultures with Turmeric protects chicks from a Lethal challenge of Salmonella gallinarum. J. Food Prot. 2017;80:245–250. doi: 10.4315/0362-028X.JFP-16-306. [DOI] [PubMed] [Google Scholar]
- He H., Genovese K.J., Nisbet D.J., Kogut M.H. Synergy of CpG oligodeoxynucleotide and double-stranded RNA (poly I:C) on nitric oxide induction in chicken peripheral blood monocytes. Mol. Immunol. 2007;44:3234–3242. doi: 10.1016/j.molimm.2007.01.034. [DOI] [PubMed] [Google Scholar]
- He H., Lowry V.K., Swaggerty C.L., Ferro P.J., Kogut M.H. In vitro activation of chicken leukocytes and in vivo protection against Salmonella enteritidis organ invasion and peritoneal S. enteritidis infection-induced mortality in neonatal chickens by immunostimulatory CPG oligodeoxynucleotide. FEMS Immunol. Med. Microbiol. 2005;43:81–89. doi: 10.1016/j.femsim.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Hermans D., Van Deun K., Martel A., Van Immerseel F., Messens W., Heyndrickx M., Haesebrouck F., Pasmans F. Colonization factors of Campylobacter jejuni in the chicken gut. Vet. Res. 2011;42:82. doi: 10.1186/1297-9716-42-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Italiani P., Boraschi D. From monocytes to M1/M2 macrophages: Phenotypical vs. Functional Differentiation. Front. Immunol. 2014;5:514. doi: 10.3389/fimmu.2014.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki A., Medzhitov R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 2015;16:343–353. doi: 10.1038/ni.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazi V., Foroozandeh A.D., Toghyani M., Dastar B., Rezaie Koochaksaraie R., Toghyani M. Effects of Pediococcus acidilactici, mannan-oligosaccharide, butyric acid and their combination on growth performance and intestinal health in young broiler chickens challenged with Salmonella Typhimurium. Poult. Sci. 2018;97:2034–2043. doi: 10.3382/ps/pey035. [DOI] [PubMed] [Google Scholar]
- Johny A.K., Darre M.J., Donoghue A.M., Donoghue D.J., Venkitanarayanan K. Antibacterial effect of trans-cinnamaldehyde, eugenol, carvacol, and thymol on Salmonella Enteritidis and Campylobacter jejuni in chicken cecal contents in vitro. J. Appl. Poult. Res. 2010;19:237–244. [Google Scholar]
- Kaiser P., Rothwell L., Galyov E.E., Barrow P.A., Burnside J., Wigley P. Differential cytokine expression in avian cells in response to invasion by Salmonella typhimurium, Salmonella enteritidis, and Salmonella gallinarum. Microbiol. 2000;146:3217–3226. doi: 10.1099/00221287-146-12-3217. [DOI] [PubMed] [Google Scholar]
- Khan S.H., Iqbal J. Recent advances in the role of organic acids in poultry nutrition. J. Appl. Anim. Res. 2016;44:359–369. [Google Scholar]
- Klasing K.C. Nutrition and the immune system. Br. Poult. Sci. 2007;48:525–537. doi: 10.1080/00071660701671336. [DOI] [PubMed] [Google Scholar]
- Kogut M.H., Genovese K.J., He H., Swaggerty C.L., Jiang Y. Modulation of chicken intestinal immune gene expression by small cationic peptides as feed additives during the first Week post-hatch. Clin. Vacc. Immunol. 2013;20:1440–1448. doi: 10.1128/CVI.00322-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogut M.H., Genovese K.J., He H., Swaggerty C.L., Jiang Y.W. BT cationic peptides: small peptides that modulate innate immune responses of chicken heterophils and monocytes. Vet. Immunol. Immunopathol. 2012;145:151–158. doi: 10.1016/j.vetimm.2011.10.023. [DOI] [PubMed] [Google Scholar]
- Kogut M.H., Genovese K.J., Lowry V.K. Differential activation of signal transduction pathways mediating phagocytosis, oxidative burst, and degranulation by chicken heterophils in response to stimulation with opsonized Salmonella enteritidis. Inflammation. 2001;25:7–15. doi: 10.1023/a:1007067426499. [DOI] [PubMed] [Google Scholar]
- Kogut M.H., Rothwell L., Kaiser P. Differential regulation of cytokine gene expression by avian heterophils during receptor-mediated phagocytosis of opsonized and non-opsonized Salmonella enteritidis. J. Interferon Cytokine Res. 2003;23:319–327. doi: 10.1089/107999003766628160. [DOI] [PubMed] [Google Scholar]
- Ley K. M1 means kill; M2 means heal. J. Immunol. 2017;199:2191–2193. doi: 10.4049/jimmunol.1701135. [DOI] [PubMed] [Google Scholar]
- Li X., Swaggerty C.L., Kogut M.H., Chiang H., Wang Y., Genovese K.J., He H., Stern N.J., Pevzner I.Y., Zhou H. The paternal effect of Campylobacter jejuni colonization in ceca in broilers. Poult. Sci. 2008;87:1742–1747. doi: 10.3382/ps.2008-00136. [DOI] [PubMed] [Google Scholar]
- Liu S.D., Song M.H., Yun W., Lee J.H., Kim H.B., Cho J.H. Effect of carvacrol essential oils on immune response and inflammation-related genes expression in broilers challenged by lipopolysaccharide. Poult. Sci. 2019;98:2026–2033. doi: 10.3382/ps/pey575. [DOI] [PubMed] [Google Scholar]
- Lowry V.K., Farnell M.B., Ferro P.J., Swaggerty C.L., Bahl A., Kogut M.H. Purified beta-glucan as an abiotic feed adative up-regulates the innate immune response in immature chickens against Salmonella enterica serovar Enteritidis. Int. J. Food Microbiol. 2005;98:309–318. doi: 10.1016/j.ijfoodmicro.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Medzhitov R., Janeway C.A., Jr. Innate immunity: the virtues of a nonclonal system of recognition. Cell. 1997;91:295–298. doi: 10.1016/s0092-8674(00)80412-2. [DOI] [PubMed] [Google Scholar]
- Medzhitov R., Janeway C.A., Jr. Innate immune recognition: mechanisms and pathways. Immunologic. Rev. 2000;173:89–97. doi: 10.1034/j.1600-065x.2000.917309.x. [DOI] [PubMed] [Google Scholar]
- Micciche A.C., Foley S.L., Pavlidis H.O., McIntyre D.R., Ricke S.C. A review of prebiotics against Salmonella in poultry: current and future potential for Microbiome research Applications. Front. Vet. Sci. 2018;5:191. doi: 10.3389/fvets.2018.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills C.D., Kincaid K., Alt J.M., Heilman M.J., Hill A.M. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 2000;164:6166–6173. doi: 10.4049/jimmunol.164.12.6166. [DOI] [PubMed] [Google Scholar]
- National Research Council . National Academy Press; Washington DC: 1994. Nutrient Requirements of Poultry; pp. 19–34. [Google Scholar]
- National Research Council . 8th ed. National Academies Press; Washington DC: 2011. Guide for the Care and Use of Laboratory Animals. [Google Scholar]
- Nelson J.R., McIntyre D.R., Pavlidis H.O., Archer G.S. Reducing stress susceptibility of broiler chickens by supplementing a Yeast Fermentation product in the feed or Drinking water. Animals (Basel) 2018;8:173. doi: 10.3390/ani8100173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea M.G., van der Graaf C., Van der Meer J.W., Kullberg B.J. Toll-like receptors and the host defense against microbial pathogens: bringing specificity to the innate-immune system. J. Leukoc. Biol. 2004;75:749–755. doi: 10.1189/jlb.1103543. [DOI] [PubMed] [Google Scholar]
- Redmond S.B., Chuammitri P., Andreasen C.B., Palic D., Lamont S.J. Genetic control of chicken heterophil function in advanced intercross lines: associations with novel and with known Salmonella resistance loci and a likely mechanism for cell death in extracellular trap production. Immunogenet. 2011;63:449–458. doi: 10.1007/s00251-011-0523-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell L., Young J.R., Zoorob R., Whittaker C.A., Hesketh P., Archer A., Smith A.L., Kaiser P. Cloning and characterization of chicken IL-10 and its role in the immune response to Eimeria maxima. J. Immunol. 2004;173:2675–2682. doi: 10.4049/jimmunol.173.4.2675. [DOI] [PubMed] [Google Scholar]
- Salah A.S., El-Tarabany M.S., Ali M.A. Impact of dietary supplementation with a synbiotic, organic acids or their combination on growth performance, carcass traits, economic efficiency, jejunum histomorphometry and some blood indicies of broiler chickens. Anim. Prod. Sci. 2018;59:1318–1326. [Google Scholar]
- Schnare M., Barton G.M., Holt A.C., Takeda K., Akira S., Medzhitov R. Toll-like receptors control activation of adaptive immune responses. Nat. Immunol. 2001;2:947–950. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- Suresh G., Das R.K., Kaur Brar S., Rouissi T., Avalos Ramirez A., Chorfi Y., Godbout S. Alternatives to antibiotics in poultry feed: molecular perspectives. Crit. Rev. Microbiol. 2018;44:318–335. doi: 10.1080/1040841X.2017.1373062. [DOI] [PubMed] [Google Scholar]
- Swaggerty C.L., Ferro P.J., Pevzner I.Y., Kogut M.H. Heterophils are associated with resistance to systemic Salmonella enteritidis infection in genetically distinct lines of chickens. FEMS Immunol. Med. Microbiol. 2005;43:149–154. doi: 10.1016/j.femsim.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Swaggerty C.L., Lowry V.K., Ferro P.J., Pevzner I.Y., Kogut M.H. Disparity in susceptibility to vancomycin-resistant Enterococcus organ invasion in commercial broiler chickens that differ in innate immune responsiveness. Food Agric. Immunol. 2005;16:1–15. [Google Scholar]
- Swaggerty C.L., Genovese K.J., He H., Duke S.E., Pevzner I.Y., Kogut M.H. Broiler breeders with an efficient innate immune response are more resistant to Eimeria tenella. Poult. Sci. 2011;90:1014–1019. doi: 10.3382/ps.2010-01246. [DOI] [PubMed] [Google Scholar]
- Swaggerty C.L., Kogut M.H., Ferro P.J., Rothwell L., Pevzner I.Y., Kaiser P. Differential cytokine mRNA expression in heterophils isolated from Salmonella - resistant and -susceptible chickens. Immunol. 2004;113:139–148. doi: 10.1111/j.1365-2567.2004.01939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaggerty C.L., Pevzner I.Y., Ferro P.J., Crippen T.L., Kogut M.H. Association between in vitro heterophil function and the feathering gene in commercial broiler chickens. Avian Pathol. 2003;32:483–488. doi: 10.1080/0307945031000154071. [DOI] [PubMed] [Google Scholar]
- Swaggerty C.L., Pevzner I.Y., Lowry V.K., Farnell M.B., Kogut M.H. Functional comparison of heterophils isolated from commercial broiler chickens. Avian Pathol. 2003;32:95–102. doi: 10.1080/0307945021000070769. [DOI] [PubMed] [Google Scholar]
- Taha-Abdelaziz K., Hodgins D.C., Lammers A., Alkie T.N., Sharif S. Effects of early feeding and dietary interventions on development of lymphoid organs and immune competence in neonatal chickens: a review. Vet. Immunol. Immunopathol. 2018;201:1–11. doi: 10.1016/j.vetimm.2018.05.001. [DOI] [PubMed] [Google Scholar]
- Takeuchi O., Akira S. Signaling pathways activated by microorganisms. Curr. Opin. Cell Biol. 2007;19:185–191. doi: 10.1016/j.ceb.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Werling D., Hope J.C., Howard C.J., Jungi T.W. Differential production of cytokines, reactive oxygen and nitrogen by bovine macrophages and dendritic cells stimulated with Toll-like receptor agonists. Immunol. 2004;111:41–52. doi: 10.1111/j.1365-2567.2003.01781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werling D., Jungi T.W. TOLL-like receptors linking innate and adaptive immune response. Vet. Immuno.L Immunopathol. 2003;91:1–12. doi: 10.1016/s0165-2427(02)00228-3. [DOI] [PubMed] [Google Scholar]
- Yadav S., Jha R. Strategies to modulate the intestinal microbiota and their effects on nutrient utilization, performance, and health of poultry. J. Anim. Sci. Biotechnol. 2019;10:2. doi: 10.1186/s40104-018-0310-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Z., Zhang S., Wang H., Piao X. Essential oil and aromatic plants as feed additives in non-ruminant nutrition: a review. J. Anim. Sci. Biotechnol. 2015;6:7. doi: 10.1186/s40104-015-0004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]