Abstract
The objective of this study was to investigate the effects of higher vitamins supplementation level on the performance, immunity, and intestinal microbiota of old laying hens. Twelve birds were randomly chosen from 312 healthy, 65-wk-old Hy-Line Brown layers for sampling after a 7-wk acclimation period. The remaining 300 hens were randomly allocated to 1 of 4 dietary treatments for a 13-wk feeding trial: basal diet (CON), basal diet with 2-fold supplementation level of lipid-soluble vitamins (LV), 2-fold supplementation level of water-soluble vitamins (WV), or 2-fold supplementation level of both lipid-soluble and water-soluble vitamins (BV), respectively. Compared with 72-wk-old laying hens, the 85-wk-old laying hens showed declined egg quality, which implied by inferior eggshell strength and yolk color (P < 0.05). However, after 13 wks feeding trial, the birds in WV group had higher yellowness of yolk color, and LV group had increased laying rate (P < 0.05) compared with CON. Meanwhile, WV and/or BV groups showed improved GSH/GSSG levels in liver and increased secretory immunoglobulin A concentrations in jejunum compared with CON (P < 0.05). In addition, higher dietary vitamin supplementation levels significantly altered the composition of intestinal microbiota, as evidenced by increased abundance of ileal Lactobacillus, whereas reduced richness of ileal Romboutsia, Turicibacter, and cecal Faecalibacterium (P < 0.05) in WV group and increased cecal Megasphaera and Phascolarctobacterium (P < 0.05) in LV group compared with CON group. In conclusion, higher vitamin supplementation levels in the diet could improve laying performance and egg quality of aged hens, which was closely correlated with the increased abundance of beneficial microbiota in the intestine.
Key words: laying hen, vitamin, egg quality, immunity, microbiota
Introduction
Generally, laying hens over 70-wk-old age have lower laying rate and poorer egg quality, which result in a great economic loss (Molnar et al., 2017, Rattanawut et al., 2018). Thus, it is imperative to decipher the intrinsic mechanism of performance deterioration of laying hens and establish an appropriate nutrition strategy for promoting the development of the laying hen industry. Importantly, attenuated antioxidant capacity and weak immune system are ascribed for the poor production performance of old laying hens (Claudio et al., 2000, Holmes et al., 2003, Wan et al., 2017). In an intensive breeding system, a variety of stresses inter alia higher breeding density, poor ventilation, and thermal stress add to more oxidative stress in laying hens (Park et al., 2009). Additionally, these stresses further reduce feed efficiency, causing immunosuppression and gut microbiota dysfunction (Zhu et al., 2019). Owing to the declined antioxidant and immune capacity (Katz et al., 2004), the aged hens lack the ability to adapt to external challenges, thereby falling short of their potential production capacity.
The prevailing dietary nutrient standards used in the industry may not fulfill the nutritional requirements of the aged-laying hens subjected to a variety of stresses. Therefore, lots of efforts are being employed to improve the productive performance of aged laying hens using nutritional interventions, including but not restricted to the supplementation of the diet with the exogenous enzymes, prebiotics and probiotics, minerals (calcium and zinc, etc.), and vitamins such as vitamin D (VD) and biotin (Abdelqader et al., 2013, Kim et al., 2013, Nascimento et al., 2014, Ghasemian and Jahanian, 2016, Tsai et al., 2016). Owing to their beneficial effects on reproduction, antioxidant activity and other physiological mechanisms (Combs Jr. and McClung, 2016), vitamins have justifiably attracted a lot of interest in the laying hen industry.
Dietary vitamins exert critical effects on eliminating free radicals and improving antioxidant levels and immune functions. As antioxidants in laying hens diet, vitamin C (VC) and vitamin E (VE) help protecting membrane phospholipids, cytosolic substances, and other components from oxidative damage (Combs Jr. and McClung, 2016). However, the advancing aging of laying hens is invariably accompanied by the reduced ability of VC synthesis and VD metabolism in the kidneys (Abe et al., 1982, Gan et al., 2018) and the attenuated absorption of vitamin B complex in the intestine (Pannerec et al., 2018). Some previous studies demonstrated that supplementation with a high concentration of VD/K in the diet of old laying hens enhanced the laying rate and the concentration of VD/K in the eggs (Park et al., 2005, Nascimento et al., 2014). Similarly, a higher inclusion rate of VC in 80-wk-old laying hens also improved antioxidant ability and immunity (Gan et al., 2018). Interestingly, some studies reported no effects on the production performance and egg quality of old laying hens when VD (Persia et al., 2013) or biotin (Daryabari et al., 2014) supplementation level was increased in the diet. The inconsistent results of supplementation with additional vitamins in the diet of old laying hens has attracted more attention on the application of vitamins in old laying hens.
The chicken gut is inhabited by a variety of microbiota that contribute to the gut homeostasis and protect the host from pathogens (Stanley et al., 2014, Zhu et al., 2019). The gut microbiota is also pivotal in regulating intestinal morphology and physiology, improving food digestion and energy recovery, contributing essential nutrients such as vitamins, and modulating the immune system of the host (Leblanc et al., 2013, Purchiaroni et al., 2013, Pan and Yu, 2014). The structure and abundance of intestinal microflora changes as the hen gets older (Cui et al., 2017). Diet is considered as a major factor influencing the composition and metabolism of the intestinal microbiota. Both macronutrients (carbohydrates, fats, and proteins) and micronutrients (vitamins, minerals, and trace elements) have pronounced effects on the gut microbiota (Scott et al., 2013, Biesalski, 2016). It is suggested that vitamin biosynthesis can be modulated by some commensal microbiota (Shapiro et al., 2014). Conversely, dietary vitamins also have a profound influence on the gut microbiota (Ribeiro et al., 2019). Furthermore, vitamin A (VA) and VD can modulate the gut microbiota by preserving the intestinal barrier integrity and intestinal immune status, thereby protecting the host from certain diseases (Riccio and Rossano, 2018).
However, very limited information is available regarding the effects of vitamins on the gut microbiota and almost no report exploring the influence of additional dietary vitamins supplementation on the intestinal microbiota in aged laying hens. Thus, we hypothesized that the dietary supplementation with vitamins over and above the recommended doses in feeding formula used in industry could enhance the laying performance, immunity, and beneficial microbes community in aged laying hens. A feeding experiment was conducted to explore the effects of dietary supplementation with high doses of vitamins on laying performance of old laying hens and the changes in immunity, antioxidant condition, and intestinal microbiota of old laying hens.
Materials and methods
Birds, Diets, and Management
All experimental procedures were approved by the Animal Care and Use Committee of China Agricultural University (No. AW05060202-1). A total of 312 healthy, 65-wk-old, Hy-Line Brown hens with similar laying performance were randomly allocated to 104 cages with 3 birds per cage and fed a commercial layer diet for a 7-wk transitional period. After 7 wks, 12 birds from 4 cages were randomly selected to collect samples of the magnum, shell gland, and kidney to determine the basal physiological indicators of layers at 72-wk-old. The remaining 300 birds were randomly allocated to 4 dietary treatments, with 5 replicates in each treatment. One replicate consisted of 5 cages with 15 layers. The control group (CON) was fed the basal diet with vitamins supplementation level according to the recommendation of the chicken feeding standard (Ministry of Agriculture of the PRC, 2004) with some modifications in production, which is presented at Supplementary Table 1. According to National Research Council (1987), the maximum tolerable dose of VA for laying hens is 40,000 IU/kg. Other vitamins are generally tolerated at intakes as great as 10- to 1,000-fold, the required level. However, supplementation with about 30,000 IU/kg VA did not influence the production performance of breeders (Yuan et al., 2014). Furthermore, taking the production cost into account, 1-fold more vitamins were added in the following groups: double levels of lipid-soluble vitamin content (LV), double levels of water-soluble vitamin content (WV), and double levels of lipid-soluble and water-soluble vitamins (BV).
The supplemented vitamins in different groups were first mixed with ground corn separately, following by mixing with premix and then subsequently mixed thoroughly with the other dietary ingredients. All the diets were prepared every 4 wks and then stored in a temperature-controlled room (<25°C) to prevent the inactivation of the vitamins. The composition and nutrient level of the basal diet and the exact vitamin concentrations of each treatment are shown in Table 1 and Supplementary Table 1. During the 13-wk experimental period, all the birds had free access to fresh tap water and assigned feed. The birds were housed in an environmentally controlled house with the temperature maintained at 24°C under 16:8-h light-dark cycle.
Table 1.
Composition and nutrient contents of the basal diet.
| Composition | Content, % | Nutrient content | |
|---|---|---|---|
| Corn | 60.44 | AME, MJ/kg | 10.93 |
| Wheat bran | 4.100 | Crude protein, % | 16.26 |
| Soybean meal | 21.60 | Lysine, % | 0.73 |
| Cotton meal | 2.000 | Methionine, % | 0.34 |
| Soybean oil | 0.500 | Methionine + Cystine, % | 0.65 |
| Calcium carbonate | 9.500 | Calcium, % | 3.63 |
| Calcium phosphate | 1.000 | Available phosphorus, % | 0.29 |
| Sodium chloride | 0.300 | Total phosphorus, % | 0.51 |
| Mineral premix2 | 0.150 | ||
| Vitamin premix1 | 0.200 | ||
| Phytase | 0.015 | ||
| Choline chloride (50%) | 0.100 | ||
| DL-Met | 0.080 | ||
| Flavomycin (4%) | 0.015 | ||
| Total | 100.00 |
Vitamin premix was produced following 4 different ration (Supplementary Table 1), and the basal diet (control group) was formulated based on the national standard with some modification, which is presented at supplemental.
Provided per kilogram of diet: Cu, 8 mg; Zn, 75 mg; Fe, 80 mg; Mn, 100 mg; Se, 0.15 mg; I, 0.35 mg.
At the end of the experiment, following 12 h fasting, 2 birds from each replicate (10 birds from each treatment) were randomly picked and sacrificed by intravenously injecting with 50 mg pentobarbital anesthesia per kilogram weight, using a 1 mL syringe. The body cavity was immediately opened to collect samples from the magnum, shell gland, liver, and kidney into RNAase-free tubes for gene analysis. The mucosa was scraped from 10 cm of the jejunum (proximal to Meckel's diverticulum), whereas the ileal and cecal digesta samples were collected by gently squeezing the intestinal contents from proximal to the distal end. All the samples were immediately snap-frozen in liquid nitrogen and stored into −80°C freezer until further analysis.
Egg Quality and Production Performance
At the end of 72 wks, 80 eggs were randomly collected for 2 consecutive day (40 eggs per D) for quality analysis. Similarly, on the last 2 D of the 13th wk, all the eggs from each treatment were selected for quality analysis. Eggshell strength, egg weight, egg yolk color, Haugh unit, and albumen height were tested using a digital egg tester (DET-6000, Nabel Co., Ltd., Kyoto, Japan). After getting rid of the residual egg white, the eggshell weight was obtained using a balance. The egg shell portion was calculated by eggshell weight dividing by the egg weight.
The production performance of the laying hens was measured from 81 to 85 wks of age. Daily laying rate and egg weight were determined per replicate unit. Feed offered, orts, and spillages were collected and weighed every 2 wks to determine the ADFI. The feed conversion ratio (FCR) was calculated by dividing ADFI by average egg weight.
The Secretory Immunoglobulin A Concentrations in Jejunal Mucosa
The Secretory Immunoglobulin A (sIgA) concentration in the jejunum was detected following the method described by Du et al. (2016). Briefly, the mucosa samples were thawed at room temperature and homogenized in 4 volumes of ice-cold PBS. The homogenate was centrifuged at 13,800 × g for 20 min at 4°C, and the supernatant was analyzed for sIgA and protein content by ELISA kit (Bethyl Laboratories Inc., Montgomery, TX) and BCA protein assay kit (Pierce Biotechnology, Rockford, IL) according to the manufacturer's protocol, respectively. The final sIgA concentrations were expressed as milligrams per gram protein.
Pyrosequencing of Ileal and Cecal Microbiota
DNA samples were extracted from ileal and cecal digesta using QIAamp DNA Stool Mini Kits (Qiagen Inc., Hilden, Germany), according to the manufacturers' instructions. The concentration and purity of the DNA samples were checked with gel electrophoresis. The microbial 16S rRNA sequences were amplified with universal primers 515 F (5′-GTG CCA GCM GCC GCG GTA A-3′) and 806 R (5′-GGA CTA CHV GGG TWT CTA AT-3′) targeting the V3-V4 region according to the PCR methods described by Wang et al. (2016). The PCR products were determined by 2% gel electrophoresis and purified with QIAquick Gel Extraction Kit (Qiagen Inc.). A library was constructed using Ion Plus Fragment Library Kit 48 rxns (Thermo Scientific Inc., Waltham, MA) and quantified by Qubit 2.0 Fluorometer (Thermo Fisher Scientific) to pool at equal concentrations. Pyrosequencing for 16S rRNA was carried out on the Illumina HiSeq2500 PE250 platform (Illumina, San Diego, USA). All of the procedures were conducted by Novogene Bioinformatics Technology Co. Ltd. (Beijing, China). The clean reads were obtained after the quality filtering by Cutadapt (V1.9.1, http://cutadapt.readthedocs.io/en/stable/) and Chimera removal by UCHIME algorithm (UCHIME Algorithm, http://www.drive5.com/usearch/manual/uchime_algo.html). Then, the clean reads were clustered into operational taxonomic units (OTU) using Uparse in QIIME software (Uparse v7.0.1001, http://drive5.com/uparse/) with a similarity threshold of 97%. The OTU were obtained from Mothur and were sorted from most to least abundant. The 16S rRNA gene sequences were aligned using the multiple sequence alignment method MUSCLE (Version 3.8.31, http://www.drive5.com/muscle/). And the OTU were further subjected to the taxonomy-based analysis by the Ramer-Douglas-Peucker algorithm using the Greengenes database (http://greengenes.lbl.gov). Alpha diversity (Shannon) and beta diversity (weighted UniFrac, principal coordinate analysis were analyzed using QIIME [Version 1.9.1]).
Quantification of mRNA Expression of the Genes
The gene expressions of avidin-related protein (AVR), ovalbumin, avidin, and tumor necrosis factor alpha (TNF-α) in the magnum, carbonic anhydrase (CA), plasma membrane Ca2+-ATPase, ovocleidin-116 (OC116), ovocalyxin-32 (OCX32), TNF-α, and vitamin D receptor in the shell gland and 1-α-hydroxylase, L-gulonolactone oxidase (GLO), and sodium-dependent vitamin C transporter (SVCT) 1 in the kidney were determined using the quantitative real-time polymerase chain reaction analysis as described before (Gan et al., 2018). In short, total RNA from the magnum, shell gland, and kidney were isolated by using TRIzol reagent (TAKARA Bio., Beijing, China), and cDNA synthesis was performed by using a PrimeScript RT reagent kit with gDNA eraser (TaKaRa Bio) according to the manufacturer's instructions. The primer sequences for the target and reference genes are shown in Supplementary Table 2. All the measurements were carried out in triplicate, and the average values were calculated. Relative expression levels of different genes were normalized to the expression of housekeeping gene GAPDH using the 2–ΔΔCt method (Livak and Schmittgen, 2001).
Antioxidant Capacity of the Liver
Malondialdehyde (MDA) and catalase (CAT) contents in the liver were detected by biochemical methods following the instructions provided with the reagent kits (MDA, A003; CAT, A007-1-1) purchased from Nanjing Jiancheng Bioengineering Institute of China. The glutathione (GSH) and glutathione disulfide (GSSG) contents in the liver were detected by the GSH/GSSG reagent kits (Beyotime, S0053, Shanghai, China) according to the manufacturer's guidelines, whereas the GSH/GSSG ratio was calculated by dividing GSH by GSSG.
Correlation Analysis
The Pearson's correlation analysis was performed to evaluate the potential link between alterations in intestinal microbiota composition and production performance and other health parameters with production performance of layers after feeding 13 wks of high levels of vitamins. The mean value from each replicate of the variable was used to conduct the correlation analysis in the present study. Namely, there were 20 samples for each variable. After running the correlation analysis between paired variables, Pearson's correlation coefficient was obtained, which ranged from -0.5 to 0.5 in the present study. Furthermore, the positive values denote positive linear correlation, whereas negative values denote negative linear correlation between 2 variables.
Statistical Analysis
Results are present as mean values and pooled standard errors. All the data were analyzed using SPSS statistical software (SPSS for Windows, version 22.0; SPSS Inc., Chicago, IL). For the Pearson's correlation analysis, the t test was used to establish if the correlation coefficient is statistically significant. One-way analysis of variance was conducted followed by Duncan's multiple comparisons for other parameters. Statistical differences were considered significant at P ≤ 0.05, and 0.05 < P ≤ 0.10 was viewed as a trend.
Results
Egg Quality and Production Performance
As present in Table 2, there is no difference on egg weight, Haugh unit, and albumen height between 72 and 85 wks old laying hens. However, the 72-wk-old laying hens had greater (P < 0.05) eggshell strength and the yolk color but decreased eggshell portion (P < 0.05) compared with that of 85-wk-old laying hens. After 13 wks feeding with high levels of vitamins, the 85-wk-old laying hens in different dietary treatments showed no differences on egg weight, Haugh unit, and albumen height, whereas dietary supplementation with WV increased the yolk color (P = 0.002) and supplementation with BV decreased (P < 0.001) the eggshell portion compared with CON group (Table 3). The laying rate was increased (P < 0.05, Table 4) in the LV group after the feeding trial compared with the CON group. However, there were no significant differences between FCR or ADFI among all the groups (Table 4).
Table 2.
The egg quality from layers in different ages.
| Items | N | Egg weight, g | Eggshell strength, kg/cm3 | Haugh unit | Albumen height, mm | Yolk color | Eggshell portion, % |
|---|---|---|---|---|---|---|---|
| 72 wks | 77 | 63.82 | 3.809a | 77.86 | 6.394 | 6.701a | 9.663b |
| 85 wks | 107 | 63.60 | 2.941b | 78.46 | 6.607 | 6.404b | 10.08a |
| SEM | 0.379 | 0.061 | 0.655 | 0.090 | 0.051 | 0.059 | |
| P-value | 0.773 | <0.001 | 0.657 | 0.242 | 0.004 | <0.001 |
a–bDifferent letters in the same column indicate significant differences (P < 0.05), and the same letter means no significant difference (P > 0.05).
Table 3.
The egg quality from layers in different treatments (85 wk old).
| Treatment | N | Egg weight, g | Eggshell strength, kg/cm3 | Haugh unit | Albumen height, mm | Yolk color | Eggshell portion, % |
|---|---|---|---|---|---|---|---|
| CON1 | 107 | 63.60 | 2.920 | 78.46 | 6.607 | 6.404b | 10.07a |
| LV | 107 | 63.27 | 2.977 | 78.32 | 6.558 | 6.427b | 10.05a |
| WV | 107 | 64.19 | 2.994 | 79.19 | 6.508 | 6.717a | 9.730a |
| BV | 114 | 64.01 | 2.913 | 79.90 | 6.707 | 6.559a,b | 9.340b |
| SEM | 0.274 | 0.035 | 0.480 | 0.068 | 0.033 | 0.060 | |
| P-value | 0.604 | 0.802 | 0.622 | 0.751 | 0.002 | <0.001 |
a–cDifferent letters in the same column indicate significant differences (P < 0.05), and the same letter means no significant difference (P > 0.05).
CON, control group with normal vitamins concentration; LV, reduplicated supplementation level of lipid-soluble vitamins; WV, reduplicated supplementation level of water-soluble vitamins; BV, reduplicated supplementation level of both lipid-soluble and water-soluble vitamins.
Table 4.
The production performance of laying hens in different treatments.
| Treatment | N | Laying rate, % | FCR, g:g | ADFI, g/D | Broken egg rate,% |
|---|---|---|---|---|---|
| CON1 | 5 | 73.83b | 2.05 | 105.8 | 0.937 |
| LV | 5 | 82.93a | 1.95 | 108.8 | 1.202 |
| WV | 5 | 81.34a,b | 1.93 | 108.0 | 0.849 |
| BV | 5 | 82.14a,b | 1.99 | 108.9 | 0.795 |
| SEM | 1.543 | 0.024 | 1.029 | 0.175 | |
| P-value | 0.045 | 0.135 | 0.725 | 0.871 |
a–cDifferent letters in the same column indicate significant differences (P < 0.05), and the same letter means no significant difference (P > 0.05).
Abbreviation: FCR, feed conversion ratio.
CON, control group with normal vitamins concentration; LV, reduplicated supplementation level of lipid-soluble vitamins; WV, reduplicated supplementation level of water-soluble vitamins; BV, reduplicated supplementation level of both lipid-soluble and water-soluble vitamins.
Gene Expressions in the Shell Gland, Magnum, and Kidney at 85-Week-Old Laying Hens
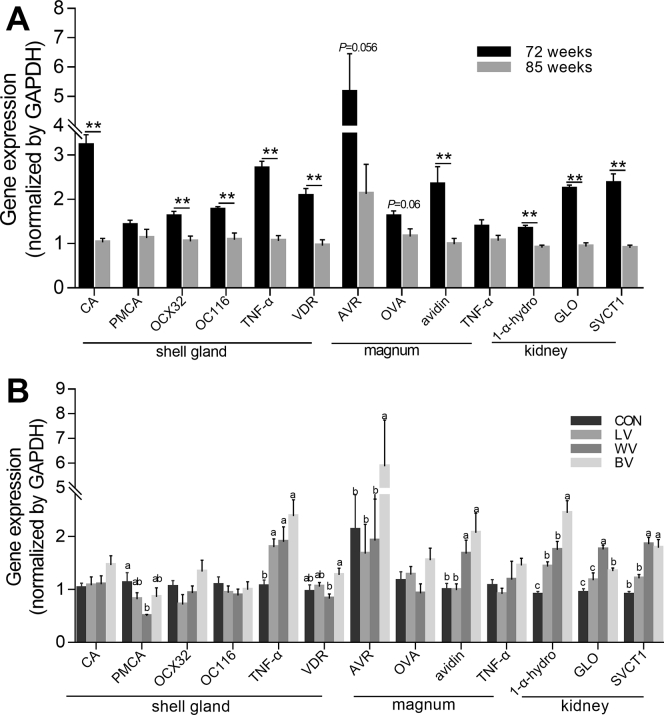
Carbonic anhydrase, OCX32, and OC116 plays vital roles in the eggshell formation at the shell gland (Nys et al., 2004). In the present study, compared with the 72-wk-old laying hens, the gene expressions of CA, OCX32, OC116, TNF-α, and vitamin D receptor in the shell gland of the 85-wk-old laying hens significantly decreased (P < 0.05; Figure 1). However, the TNF-α expression significantly increased (P < 0.05) when LV, WV individually, or in combination were supplemented for 13 wks in the diet of the old laying hens. In addition, the WV group had less PCMA gene expression than the CON group (P < 0.05). No significant effects were observed on other genes' expression levels in the shell gland between the vitamin-supplemented groups and the control.
Figure 1.
Gene expression in shell gland, magnum and kidney of different ages/treatments of the laying hens. (A) Gene expression of laying hens at 72 wks old (N = 12) and 85 wks old (N = 10). (B) Gene expression in different treatments of 85 wks old hens (N = 10). Within each panel, means without a common letter differ at P < 0.05. Abbreviations: CON, control group with normal vitamins concentration; LV, reduplicated supplementation level of lipid-soluble vitamins; WV, reduplicated supplementation level of water-soluble vitamins; BV, reduplicated supplementation level of both lipid-soluble and water-soluble vitamins; CA, carbonic anhydrase; PMCA, plasma membrane Ca2+-ATPase; OCX32, ovocalyxin-32; OC116, ovocleidin-116; TNF-α, tumor necrosis factor alpha; VDR, vitamin D receptor; AVR, avidin-related protein; OVA, ovalbumin; 1-α-hydro, 1-α-hydroxylase; GLO, L-gulonolactone oxidase; SVCT1, sodium-dependent vitamin C transporter 1.
In the magnum, the AVR and ovalbumin expressions tended to decrease (P = 0.056 and 0.060, respectively), whereas the expression of avidin significantly declined (P < 0.05) in the 85-wk-old laying hens compared with 72-wk-old hens. The BV group had greater (P < 0.05) expression of the AVR in the magnum than that of the CON group, meanwhile the expression of the avidin in BV and WV group were higher (P < 0.05) than that in the LV and the CON group.
The gene expression of the 1-α-hydroxylase, GLO, and SVCT1 in kidneys decreased (P < 0.05) during the aging process. However, the increased dietary vitamins supplementation to the birds for 13 wks reversed this trend and significantly increased the gene expression of 1-α-hydroxylase in the kidneys as compared with CON group. The GLO and SVCT1 expressions were significantly increased (P < 0.05) in the WV and BV groups compared with the CON group.
The Antioxidant Ability in Liver and the Immunity in Jejunum
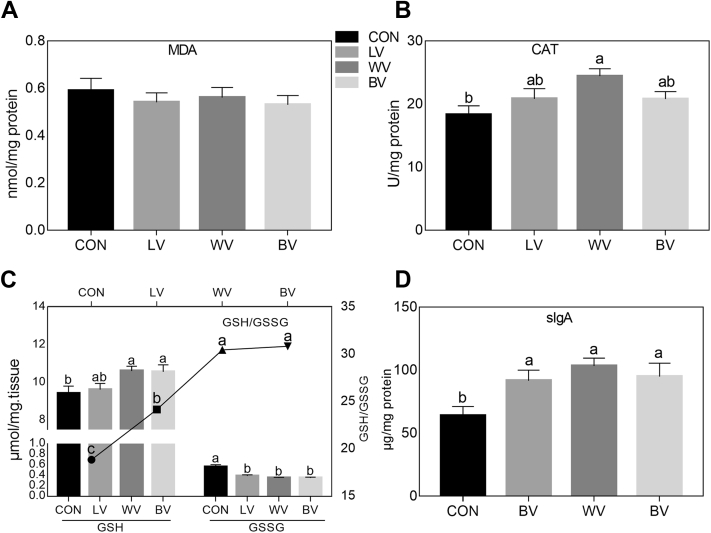
As shown in Figure 2B, compared with the CON group, CAT content in the liver of the 85-wks-old laying hens was significantly increased (P < 0.05) in WV group. In the present study, the GSH concentration was significantly higher in treatment groups WV and BV, whereas all 3 treatments had decreased GSSG contents. Therefore, the 3 increased vitamins supplementation treatments have higher GSH/GSSG ratio compared with the CON (P < 0.05; Figure 2C). The concentration of MDA was not influenced by dietary treatment. As indicated in Figure 2D, compared with the CON group, the levels of sIgA were significantly increased (P < 0.05) in all treatment groups after feeding 13 wks of high levels of vitamins.
Figure 2.
The contents of malondialdehyde (MDA) (A), antioxidant enzyme catalase (CAT) (B), and GSH/GSSG (C) in liver, and the secretory immunoglobulin A (sIgA) contents in the jejunum mucosa (D). Within each panel, means without a common letter differ at P < 0.05. N = 10. Abbreviations: CON, control group with normal vitamins concentration; LV, reduplicated supplementation level of lipid-soluble vitamins; WV, reduplicated supplementation level of water-soluble vitamins; BV, reduplicated supplementation level of both lipid-soluble and water-soluble vitamins.
Bacterial Community Richness and Biodiversity and β-Diversity
The microbiota of ileal and cecal digesta in the 4 treatments of 85-wk-old laying hens was analyzed by sequencing the bacterial 16S rRNA V3+V4 region. High-throughput pyrosequencing of the samples (n = 10 for each treatment) generated a total of 3,290,008 raw reads and 3,239,218 raw reads in ileal and cecal content, respectively. After removing the low-quality sequences, 3,099,034 and 3,015,087 clean reads were acquired from the 40 ileum and cecum digesta samples through Illumina miSequencing analysis, respectively. Based on 97% sequence similarity, a total of 8,450 and 33,936 OTU were identified and clustered.
This sequencing depth was sufficient for the coverage (>99%) of all OTU present in ileal and cecal samples and almost reflected the total microbial species richness, as demonstrated by the Rarefaction and rank abundance (Supplementary Figure 1). Notably, dietary vitamin supplementation did not affect alpha diversity (OS, Shannon, Simpson, ACE, and Chao1 indexes) of ileal and cecal microbiota (data not shown). There were 2,259, 2,091, 1,994, and 2,061 OTU obtained from the CON, LV, WV, and BV groups in ileum and 8,674, 8,231, 8,656, and 8,357 OTU from CON, LV, WV, and BV groups in cecum respectively, of which 287 and 1,041 were common across the 4 experimental groups in ileum and cecum, respectively (Supplementary Figure 2). Beta diversity analysis was depicted via principal co-ordinates analysis, where the distance means microbial diversity in different groups (Supplementary Figure 3A and B).
Characterization of Ileal and Cecal Microbiota in Laying Hens Fed Different Dietary Vitamins Concentrations
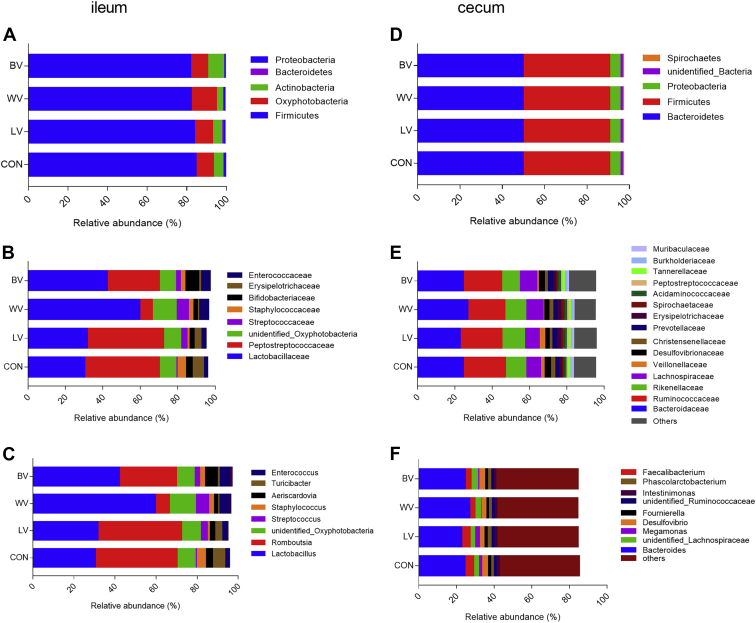
The relative abundance of ileal and cecal microbiota that occurred as more than 1% was determined at the phylum, family, and genus levels (Figure 3). The ileal microbiota was dominated by the phylum Firmicutes (regardless of dietary treatment) with the relative abundance of 84.4, 83.7, 82.0, and 81.7% in CON, LV, WV, and BV group, respectively. The dominant phyla in cecum were Bacteroidetes and Firmicutes accounting for 49.7, 49.7, 51.2, and 50.3% and 40.8%, 42.0%, 38.7%, and 40.6% in CON, LV, WV, and BV group, respectively (Figure 3D and Supplementary Table 4).
Figure 3.
Effects of dietary vitamins on ileal and cecal microbiota composition of laying hens. Microbial community bar plot at the (A, D) phylum, (B, E) family level, and (C, F) genus level. N = 10. Abbreviations: CON, control group with normal vitamins concentration; LV, reduplicated supplementation level of lipid-soluble vitamins; WV, reduplicated supplementation level of water-soluble vitamins; BV, reduplicated supplementation level of both lipid-soluble and water-soluble vitamins.
The dominant families within the ileal phylum Firmicutes consisted of Lactobacillaceae and Peptostreptococcaceae (Figure 3B and Supplementary Table 3). The main families belonging to cecum phyla Bacteroidetes and Firmicutes were the Bacteroidaceae, Ruminococcaceae, Lachnospiraceae, and Rikenellaceae (Figure 3E; Supplementary Table 4).
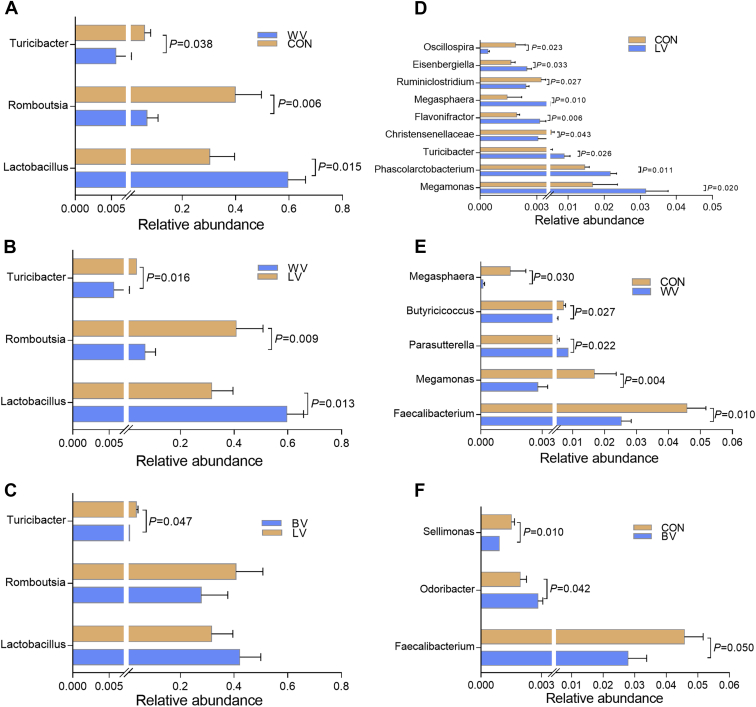
The differences among treatments in the microbial communities at the genus levels are shown in Figure 4 (ileum A–C, cecum D–F). Dietary vitamin concentrations exert a substantial effect on the microbiota community structure and abundance in ileum and cecum. Lactobacillus, the most abundant genera in the WV group was higher than that in the other 2 groups (CON group: P = 0.015; LV group: P = 0.013) in the ileum. In contrast, the Romboutsia contents in ileum in WV group occurred at a notably decreased level compared with CON and LV groups(P = 0.006, 0.009, respectively). However, the relative abundance of ileal Lactobacillus and Romboutsia had no significant difference between the BV and LV groups (P > 0.05) (Figure 4 and Supplementary Table 3).
Figure 4.
Comparison of the ileal and cecal microbial community in genus level of laying hens fed diet with different levels of vitamins (N = 10). A, B, C in ileum; D, E, F in cecum. Abbreviations: CON, control group; CON, control group with normal vitamins concentration; LV, reduplicated supplementation level of lipid-soluble vitamins; WV, reduplicated supplementation level of water-soluble vitamins; BV, reduplicated supplementation level of both lipid-soluble and water-soluble vitamins.
Dietary supplementation with extra vitamins did not affect the relative abundance of most abundant genus Bacteroides in the cecum (P > 0.05, Supplementary Table 4). However, compared with the control group, the relative abundance of genus Megasphaera and Phascolarctobacterium in LV group were significantly increased (P = 0.01 and 0.01, respectively), whereas the abundance of the Ruminiclostridium and Oscillospira were reduced (P < 0.05). Meanwhile, the genus Faecalibacterium in both WV and BV group exhibited lower abundance than that in the CON group (P = 0.01, 0.05, respectively, Figures 4E and 4F).
Correlation Between Alterations in Intestinal Microbiota Composition and Production Performance
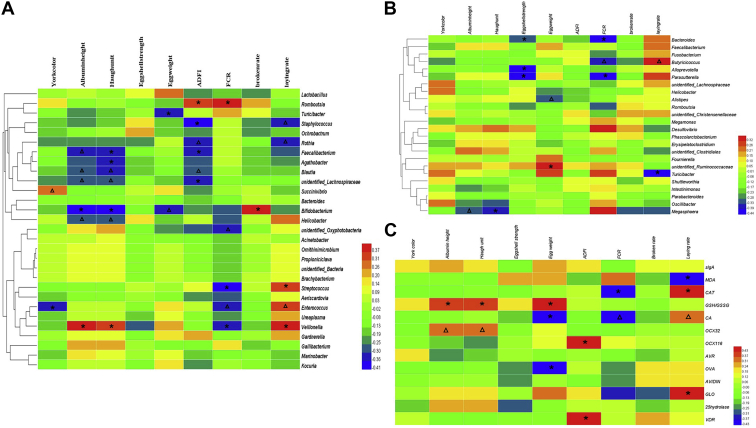
The intestinal microbiota is closely associated with production performance. In this experiment, ileal Streptococcus was positively related with laying rate, whereas cecal Turicibacter showed the opposite trend. At the same time, ileal Romboutsia and cecal Desulfovibrio showed positive correlation with FCR, whereas ileal Streptococcus, cecal Bacteroides, and Parasutterella were negatively correlated with FCR (Figures 5A and 5B, P < 0.05). The intestinal microbiota also shows relations with egg quality. Ileal Turicibacter was negatively related with egg weight, and ileal Faecalibacterium, Agathobacter, and Bifidobacterium, and cecal Megasphaera were negatively correlated with Haugh unit. Ileal Veillonella was positively correlated with Haugh unit and albumin height. Meanwhile, cecal Bacteroides, Alloprevotella, and Parasutterella were negatively related with eggshell strength (Figures 5A and 5B, P < 0.05).
Figure 5.
Heatmap of the Pearson rank correlations between the production performance and egg quality with intestinal microbiota, or with other health parameters in old laying hens (N = 20). The correlation between ileal microbiota and production performance (A), the correlation between cecal microbiota and production performance (B), and the relationship between other variables with production performance (C) of the old laying hens. ∗means P ≤ 0.05; Δmeans 0.05 < P ≤ 0.10. Abbreviation: FCR, feed conversion ratio.
As shown in Figure 5C, MDA concentration was negatively related with laying rate, whereas hepatic CAT concentration and GLO expression all have positive correlation with laying rate. Meanwhile, CAT showed negative relation with FCR. GSH/GSSG ratio was positively correlated with egg quality parameters, referring to the positive relation with egg weight, Haugh unit, and albumin height (P < 0.05). Ovocalyxin-32 expression also exhibited positive relation with Haugh unit and albumin height.
Discussion
The old laying hens usually have decreased immune capacity, intestinal dysfunction, poorer egg quality, and production performance, which causes substantial economic losses (Claudio et al., 2000, Liu et al., 2016, Zhu et al., 2019). The poor eggshell strength could cause higher egg broken rate and great economic loss during transportation of eggs. In the present work, dietary supplementation with WV reversed the declining trend in yolk color, which suggested that more water-soluble vitamins could promote more color pigment deposition in the yolk (Squires and Naber, 1993). At the same time, both WV and BV treatment reversed the decrease in avidin expression in magnum caused by the increased age. Avidin is well-known for its exceptionally strong and noncovalent binding with biotin. As a key antimicrobial protein in egg white, avidin plays vital roles in the maintenance of embryo viability, avian hatchling morphology, and immune phenotype (Diederich et al., 2015, Krkavcova et al., 2018). The increase of avidin and AVR in those 2 dietary groups could possibly because of the increased biotin contents. These findings were consistent with Daryabari et al (2014), who reported that supplementation with higher biotin increased the expression of AVR in the magnum, which could ward off the invasion of bacteria, thereby increasing the egg quality.
As the hen gets older, the ability to synthesize and absorb vitamins decreased as reflected by lower expression of 1-α-hydroxylase, GLO, and SVCT1 in the kidney of the 85-wk-old laying hens in the resent work. This was in agreement with our previous research (Gan et al., 2018). Additionally, adding more vitamins than used in the industry significantly increased the expression of 1-α-hydroxylase, GLO, and SVCT1, which implied the enhanced synthesis and metabolism ability in the old laying hens (Hooper et al., 2000). Interestingly, GLO expression showed positive correlation with laying rate, which means VC levels may be positively related to laying rate in old laying hens. The increased vitamin contents also contributed to the improved antioxidant ability and immune capacity, corresponding to the increased CAT content, much higher GSH/GSSG ratio, and increased jejunal sIgA levels. The antioxidant status in old laying hens is highly associated with production performance and egg quality. Importantly, the increased GSH together with VA, VE, and VC could also enhance the function of lymphocytes, thus supporting the immune system. Meanwhile, both VA and VE have positive effects on the reproductive system of the laying hens (Jiang et al., 2013, Yuan et al., 2014). Therefore, the higher concentration of VA and VE, together with higher antioxidant capacity could have contributed to a higher laying rate in LV group.
It is commonly accepted that diet is a major factor driving the composition and metabolism of the gut microbiota (Scott et al., 2013). Meanwhile, a favorable gut microbiota modulation through dietary manipulation can also improve the production performance of the aged laying hens. The gut microbiota is involved in the regulation of multiple host metabolic pathways, shaping the immune system, keeping the intestinal mucosa intact, and secreting certain enzymes and other metabolites that beneficially affect the host (Round and Mazmanian, 2009, Jeremy et al., 2012, Angelakis, 2017). In the present study, including double doses of vitamins than that used in industry did not alter the diversity of ileal and cecal microbiota as evidenced by the similar α-diversity index among different treatments of the 85-wk-old laying hens. However, the community structure and abundance of the microbiota in ileum and cecum were changed. The WV group significantly increased the abundance of Lactobacillus, whereas significantly decreased abundance of Turicibacter and Romboutsia than in CON group. Lactobacillus are heterogeneous, gram-positive rods or coccobacilli including a higher number of Generally Recognized As Safe species. Moreover, many of these strains are routinely used in food microbiology and human nutrition as food fermenter and probiotics (Spoelstra et al., 2012, Dec et al., 2014). Some previous studies indicated that feeding high levels of heavy metal led to notably higher numbers of Turicibacter in mice, creating dysbiosis with detrimental effects on the health of mice (Breton et al., 2013). In line with these findings, Turicibacter showed a negative correlation with egg weight and laying rate in this experiment.
Interestingly, the abundance of Romboutsia in the WV group was significantly lower than that in CON and LV groups. A previous research reported that Romboutsia ilealis had limited capacity to synthesize amino acids and vitamins. So, it adapts to a nutrient-rich environment abundantly supplied with carbohydrates, amino acids, and vitamins (Gerritsen et al., 2017). A surge in this genus hinted at the sufficient availability of nutrients in the ileum, including vitamins and other nutrients not being absorbed in the duodenum or jejunum. At the same time, it could be argued that more vitamins moved further down the intestine into the ceca and thus resulted in drastic changes in cecal microbial composition in the LV group. The abundance of some microbiota, such as Megamonas, Megasphaera, and Phascolarctobacterium in the LV group, were significantly higher than that in CON group. It has been reported that including VA in the diet of male infants makes a higher Megamonas abundance in the fecal microbiota (Huda et al., 2019). The abundance of Megamonas exceeded 3% in the cecum of the 85-wk-old laying hens. Moreover Megamonas could degrade non-starch polysaccharides to cellobiose and remove hydrogen, which could possibly benefit the other members of the microbial community in addition to the host by improving the recovery of energy from food (Sergeant et al., 2014). Both Phascolarctobacterium and Megasphaera belongs to the phylum Firmicutes. Phascolarctobacterium is a substantial acetate/propionate-producer. It is suggested to be positively correlated with good moods in humans. Additionally, it is correlated positively with body weight gain, fat mass, and plasma leptin levels in rat receiving a high-fat diet (Lecomte et al., 2015, Wu et al., 2017). Some species in Megasphaera genus (Besten et al., 2013) has been used as probiotic in ruminants. Meanwhile, this genus can also generate short-chain fatty acids (SCFA) by fermenting the indigestible fiber entering the cecum (Kim et al., 2014). Hence, an increase in Phascolarctobacterium and Megasphaera in cecum means more production of SCFA in the LV group. High amounts of SCFA lowers the pH of the cecum, which could inhibit the growth of pathogens and promote the abundance of bifidobacteria and lactobacilli (Jeremy K. Nicholson et al., 2012; Besten et al., 2013).
In conclusion, the inclusion of higher levels of dietary vitamins for 85-wk-old laying hens improved the production performance and egg quality, which is highly correlated with intestinal microbial. Greater dietary vitamins content contributed to enhanced antioxidant ability, immunity, as well as improved composition of gut microbiota, referring to increased beneficial bacteria abundance in particular Lactobacillus in the ileum along with Megamonas and Phascolarctobacterium in the cecum. Higher dietary vitamins supplementation was a feasible nutritional strategy to improve the health and production performance of aged laying hens.
Acknowledgments
The authors are grateful to the staff of the Department of Animal Science and Technology of the China Agricultural University for their valuable assistance in sample collecting. The research was financially supported by the State Key Development Program (2016YFD0501202).
Conflict of Interest Statement: The authors did not provide a conflict of interest statement.
Footnotes
Supplementary data associated with this article can be found in the online version at http://doi.org/10.1016/j.psj.2020.04.007.
Supplementary data
References
- Abdelqader A., Irshaid R., Al-Fataftah A.R. Effects of dietary probiotic inclusion on performance, eggshell quality, cecal microflora composition, and tibia traits of laying hens in the late phase of production. Trop. Anim. Health Prod. 2013;45:1017–1024. doi: 10.1007/s11250-012-0326-7. [DOI] [PubMed] [Google Scholar]
- Abe E., Horikawa H., Masumura T., Sugahara M., Kubota M., Suda T. Disorders of Cholecaiciferol metabolism in old egg-laying hens. J. Nutr. 1982;112:436–446. doi: 10.1093/jn/112.3.436. [DOI] [PubMed] [Google Scholar]
- Angelakis E. Weight gain by gut microbiota manipulation in productive animals. Microb. Pathog. 2017;106:162–170. doi: 10.1016/j.micpath.2016.11.002. [DOI] [PubMed] [Google Scholar]
- Biesalski H.K. Nutrition meets the microbiome: micronutrients and the microbiota. Ann. N. Y. Acad. Sci. 2016;1372:53–64. doi: 10.1111/nyas.13145. [DOI] [PubMed] [Google Scholar]
- Breton J., Massart S., Vandamme P., Brandt E.D., Pot B., Foligné B. Ecotoxicology inside the gut: impact of heavy metals on the mouse microbiome. BMC Pharmacol. Toxico. 2013;14:1–11. doi: 10.1186/2050-6511-14-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claudio F., Massimiliano B., Silvana V., Fabiola O., Maria D.L., Enzo O., Giovanna D.B. Inflamm-aging: an evolutionary perspective on immunosenescence. Ann. NY Acad. Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- Combs G.F., Jr, Mcclung J.P. Elsevier; Amsterdam, the Netherlands: 2016. The vitamins: fundamental aspects in nutrition and health. [Google Scholar]
- Cui Y., Wang Q., Liu S., Sun R., Zhou Y., Li Y. Age-related Variations in intestinal microflora of free-Range and caged hens. Front. Microbiol. 2017;8:1310. doi: 10.3389/fmicb.2017.01310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daryabari H., Akhlaghi A., Zamiri M.J., Mianji G.R., Pirsaraei Z.A., Deldar H., Eghbalian A.N. Reproductive performance and oviductal expression of avidin and avidin-related protein-2 in young and old broiler breeder hens orally exposed to supplementary biotin. Poult. Sci. 2014;93:2289–2295. doi: 10.3382/ps.2013-03862. [DOI] [PubMed] [Google Scholar]
- Dec M., Puchalski A., Urban-Chmiel R., Wernicki A. Screening of Lactobacillus strains of domestic goose origin against bacterial poultry pathogens for use as probiotics. Poult. Sci. 2014;93:2464–2472. doi: 10.3382/ps.2014-04025. [DOI] [PubMed] [Google Scholar]
- Diederich P., Hoffmann M., Hubbuch J. High-throughput process development of purification alternatives for the protein avidin. Biotechnol. Prog. 2015;31:957–973. doi: 10.1002/btpr.2104. [DOI] [PubMed] [Google Scholar]
- Du E., Wang W., Gan L., Li Z., Guo S., Guo Y. Effects of thymol and carvacrol supplementation on intestinal integrity and immune responses of broiler chickens challenged with Clostridium perfringens. J. Anim. Sci. Biotechnol. 2016;7:19. doi: 10.1186/s40104-016-0079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan L., Fan H., Nie W., Guo Y. Ascorbic acid synthesis and transportation capacity in old laying hens and the effects of dietary supplementation with ascorbic acid. J. Anim. Sci. Biotechnol. 2018;9:71. doi: 10.1186/s40104-018-0284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerritsen J., Hornung B., Renckens B., Van Hijum S., Martins Dos Santos V.a.P., Rijkers G.T., Schaap P.J., De Vos W.M., Smidt H. Genomic and functional analysis of Romboutsia ilealis CRIB(T) reveals adaptation to the small intestine. PeerJ. 2017;5:e3698. doi: 10.7717/peerj.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemian M., Jahanian R. Dietary mannan-oligosaccharides supplementation could affect performance, immunocompetence, serum lipid metabolites, intestinal bacterial populations, and ileal nutrient digestibility in aged laying hens. J. Anim. Sci. Biotechnol. 2016;213:81–89. [Google Scholar]
- Besten G.D., Eunen K.V., Groen A.K., Venema K., Reijngoud D., Bakker B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013;54:2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D.J., Thomson S.L., Wu J., Ottinger M.A. Reproductive aging in female birds. Exp. Gerontol. 2003;38:751–756. doi: 10.1016/s0531-5565(03)00103-7. [DOI] [PubMed] [Google Scholar]
- Hooper C.L., Maurice D.V., Lightsey S.F., Toler J.E. Factors affecting ascorbic acid biosynthesis in chickens. I. Adaptation of an assay and the effect of age, sex, and food deprivation. J. Anim. Physiol. Anim. Nutr. (Berl.). 2000;84:48–56. [Google Scholar]
- Huda M.N., Ahmad S.M., Kalanetra K.M., Taft D.H., Alam M.J., Khanam A., Raqib R., Underwood M.A., Mills D.A., Stephensen C.B. Neonatal vitamin A supplementation and vitamin A status are associated with gut microbiome composition in Bangladeshi infants in Early infancy and at 2 Years of age. J. Nutr. 2019;149:1075–1088. doi: 10.1093/jn/nxz034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeremy K., Nicholson E.H., James K., Burcelin R., Gibson G., Jia Wei, Pettersson S. Host-gut microbiota metabolic Interactions. Science. 2012;336:1262–1267. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- Jiang W., Zhang L., Shan A. The effect of vitamin E on laying performance and egg quality in laying hens fed corn dried distillers grains with solubles. Poult. Sci. 2013;92:2956–2964. doi: 10.3382/ps.2013-03228. [DOI] [PubMed] [Google Scholar]
- Katz J.M., Plowden J., Renshaw-Hoelscher M., Lu X., Tumpey T.M., Sambhara S. Immunity to influenza: the challenges of protecting an aging population. Immunol. Res. 2004;29:113–124. doi: 10.1385/IR:29:1-3:113. [DOI] [PubMed] [Google Scholar]
- Kim C.H., Paik I.K., Kil D.Y. Effects of increasing supplementation of magnesium in diets on productive performance and eggshell quality of aged laying hens. Biol. Trace Elem. Res. 2013;151:38–42. doi: 10.1007/s12011-012-9537-z. [DOI] [PubMed] [Google Scholar]
- Kim C.H., Park J., Kim M. Gut microbiota-derived short-chain Fatty acids, T cells, and inflammation. Immune Netw. 2014;14:277–288. doi: 10.4110/in.2014.14.6.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krkavcova E., Kreisinger J., Hyankova L., Hyrsl P., Javurkova V. The hidden function of egg white antimicrobials: egg weight-dependent effects of avidin on avian embryo survival and hatchling phenotype. Biol. Open. 2018;7:1–9. doi: 10.1242/bio.031518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc J.G., Milani C., De Giori G.S., Sesma F., Van Sinderen D., Ventura M. Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Curr. Opin. Biotechnol. 2013;24:160–168. doi: 10.1016/j.copbio.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Lecomte V., Kaakoush N.O., Maloney C.A., Raipuria M., Huinao K.D., Mitchell H.M., Morris M.J. Changes in gut microbiota in rats fed a high fat diet correlate with obesity-associated metabolic parameters. PLoS One. 2015;10:e0126931. doi: 10.1371/journal.pone.0126931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Cottrell J.J., Furness J.B., Rivera L.R., Kelly F.W., Wijesiriwardana U., Pustovit R.V., Fothergill L.J., Bravo D.M., Celi P., Leury B.J., Gabler N.K., Dunshea F.R. Selenium and vitamin E together improve intestinal epithelial barrier function and alleviate oxidative stress in heat-stressed pigs. Exp. Physiol. 2016;101:801–810. doi: 10.1113/EP085746. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Ministry of Agriculture of the PRC . 2004. Feeding standard of chicken, NY/T 33-2004. (in Chinese) [Google Scholar]
- Molnar A., Maertens L., Ampe B., Buyse J., Zoons J., Delezie E. Supplementation of fine and coarse limestone in different ratios in a split feeding system: effects on performance, egg quality, and bone strength in old laying hens. Poult. Sci. 2017;96:1659–1671. doi: 10.3382/ps/pew424. [DOI] [PubMed] [Google Scholar]
- Nascimento G.R.D., Murakami A.E., Guerra A., Ospinas-Rojas I.C., Ferreira M.F.Z., Fanhani J.C. Effect of different vitamin D sources and calcium levels in the diet of layers in the second laying cycle. Revista Brasileira de Ciência Avícola. 2014;16:37–42. [Google Scholar]
- National Research Council . National Academy Press; Washington, DC: 1987. Vitamin Tolerance of Animals. [Google Scholar]
- Nys Y., Gautron J., Garcia-Ruiz J.M., Hincke M.T. Avian eggshell mineralization: biochemical and functional characterization of matrix proteins. Comptes Rendus Palevol. 2004;3:549–562. [Google Scholar]
- Pan D., Yu Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes. 2014;5:108–119. doi: 10.4161/gmic.26945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannerec A., Migliavacca E., De Castro A., Michaud J., Karaz S., Goulet L., Rezzi S., Ng T.P., Bosco N., Larbi A., Feige J.N. Vitamin B12 deficiency and impaired expression of amnionless during aging. J. Cachexia Sarcopenia Muscle. 2018;9:41–52. doi: 10.1002/jcsm.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C.H., Namkung H., Ahn D.U., Paik I.K. Enrichment of vitamins D3, K and Iron in eggs of laying hens. Asian-aust. J. Anim. Sci. 2005;18:226–229. [Google Scholar]
- Park K.W., Rhee A.R., Um J.S., Paik I.K. Effect of dietary available phosphorus and organic acids on the performance and egg quality of laying hens. J. Appl. Poult. Res. 2009;18:598–604. [Google Scholar]
- Persia M.E., Higgins M., Wang T., Trample D., Bobeck E.A. Effects of long-term supplementation of laying hens with high concentrations of cholecalciferol on performance and egg quality. Poult. Sci. 2013;92:2930–2937. doi: 10.3382/ps.2013-03243. [DOI] [PubMed] [Google Scholar]
- Purchiaroni F., Tortora A., Gabrielli M., Bertucci F., Gigante G., Ianiro G., Ojetti V., Scarpellini E., Gasbarrini A. The role of intestinal microbiota and the immune system. Eur. Rev. Med. Pharmacol. Sci. 2013;17:323–333. [PubMed] [Google Scholar]
- Rattanawut J., Pimpa O., Yamauchi K.E. Effects of dietary bamboo vinegar supplementation on performance, eggshell quality, ileal microflora composition, and intestinal villus morphology of laying hens in the late phase of production. Anim. Sci. J. 2018;89:1572–1580. doi: 10.1111/asj.13080. [DOI] [PubMed] [Google Scholar]
- Ribeiro R., Nicoli J.R., Santos G., Lima-Santos J. Impact of vitamin deficiency on microbiota composition and immunomodulation: relevance to autistic spectrum disorders. Nutr. Neurosci. 2019:1–13. doi: 10.1080/1028415X.2019.1660485. [DOI] [PubMed] [Google Scholar]
- Riccio P., Rossano R. Diet, gut microbiota, and vitamins D + A in multiple Sclerosis. Neurotherapeutics. 2018;15:75–91. doi: 10.1007/s13311-017-0581-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Round J.L., Mazmanian S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott K.P., Gratz S.W., Sheridan P.O., Flint H.J., Duncan S.H. The influence of diet on the gut microbiota. Pharmacol. Res. 2013;69:52–60. doi: 10.1016/j.phrs.2012.10.020. [DOI] [PubMed] [Google Scholar]
- Sergeant M.J., Constantinidou C., Cogan T.A., Bedford M.R., Penn C.W., Pallen M.J. Extensive microbial and functional diversity within the chicken cecal microbiome. PLoS One. 2014;9:e91941. doi: 10.1371/journal.pone.0091941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro H., Thaiss C.A., Levy M., Elinav E. The cross talk between microbiota and the immune system: metabolites take center stage. Curr. Opin. Immunol. 2014;30:54–62. doi: 10.1016/j.coi.2014.07.003. [DOI] [PubMed] [Google Scholar]
- Spoelstra M.N., Mari A., Mendel M., Senga E., Van Rheenen P., Van Dijk T.H., Reijngoud D.J., Zegers R.G., Heikens G.T., Bandsma R.H. Kwashiorkor and marasmus are both associated with impaired glucose clearance related to pancreatic beta-cell dysfunction. Metabolism. 2012;61:1224–1230. doi: 10.1016/j.metabol.2012.01.019. [DOI] [PubMed] [Google Scholar]
- Squires M.W., Naber E.C. Vitamin Profiles of eggs as indicators of nutritional status in the laying hen: Riboflavin study. Poult. Sci. 1993;72:483–494. doi: 10.3382/ps.0720483. [DOI] [PubMed] [Google Scholar]
- Stanley D., Hughes R.J., Moore R.J. Microbiota of the chicken gastrointestinal tract: influence on health, productivity and disease. Appl. Microbiol. Biotechnol. 2014;98:4301–4310. doi: 10.1007/s00253-014-5646-2. [DOI] [PubMed] [Google Scholar]
- Tsai Y.H., Mao S.Y., Li M.Z., Huang J.T., Lien T.F. Effects of nanosize zinc oxide on zinc retention, eggshell quality, immune response and serum parameters of aged laying hens. Anim. Feed Sci. Technology. 2016;213:99–107. [Google Scholar]
- Wan Q.L., Shi X., Liu J., Ding A.J., Pu Y.Z., Li Z., Wu G.S., Luo H.R. Metabolomic signature associated with reproduction-regulated aging in Caenorhabditis elegans. Aging. 2017;9:447–463. doi: 10.18632/aging.101170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Li Z., Han Q., Guo Y., Zhang B., D'inca R. Dietary live yeast and mannan-oligosaccharide supplementation attenuate intestinal inflammation and barrier dysfunction induced by Escherichia coli in broilers. Br. J. Nutr. 2016;116:1878–1888. doi: 10.1017/S0007114516004116. [DOI] [PubMed] [Google Scholar]
- Wu F., Guo X., Zhang J., Zhang M., Ou Z., Peng Y. Phascolarctobacterium faecium abundant colonization in human gastrointestinal tract. Exp. Ther. Med. 2017;14:3122–3126. doi: 10.3892/etm.2017.4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J., Roshdy A.R., Guo Y., Wang Y., Guo S. Effect of dietary vitamin A on reproductive performance and immune response of broiler breeders. PLoS One. 2014;9:e105677. doi: 10.1371/journal.pone.0105677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L., Liao R., Wu N., Zhu G., Yang C. Heat stress mediates changes in fecal microbiome and functional pathways of laying hens. Appl. Microbiol. Biotechnol. 2019;103:461–472. doi: 10.1007/s00253-018-9465-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.