Abstract
Stress and leg weakness are detrimental to broiler production, health, and welfare. Traditional methods to evaluate stress may be stressful to the bird because they are invasive and require handling and restraint. Two studies examined the effects of light intensity and flooring on the following in broilers: 1) traditional methods for assessing stress using heterophil-to-lymphocyte ratios and serum corticosterone (CORT) concentrations, 2) noninvasive measures of stress from infrared thermography (IRT) eye and beak surface temperatures, and 3) latency-to-lie (LTL) test times of birds tested individually and in groups of 5. Day-of-hatch male broiler chicks were placed into 6 pens (N = 120 chicks/pen). At 1 wk, pens were allocated to 3 light intensity treatments (2, 5, or 10 lux). At 4 wk, half of the birds from each pen were moved to a pen with wire flooring and the same light intensity. At 1, 4, 5, and 8 wk, blood samples were collected and IRT images of the heads of 5 clinically healthy broilers from each pen were captured. In study 2, IRT images of the heads of birds that became lame in the wire flooring pens were taken. There were no treatment effects on the LTL times of birds tested in groups or individually (P > 0.05). On day 56 in study 1, birds on wire flooring had elevated heterophil-to-lymphocyte ratios and CORT concentrations (P ≤ 0.002) and depressed IRT eye and beak temperatures (P < 0.0001). In both studies, there were negative correlations between CORT concentrations and IRT beak surface temperatures (P < 0.05). Lame birds had lower IRT eye and beak surface temperatures than sound birds (P ≤ 0.004), and the IRT beak surface temperatures of lame birds were lower than their eye surface temperatures (P = 0.004) in study 2. These studies indicate that the IRT surface temperatures of the eye, and more distinctly of the beak, can be used as sensitive noninvasive indicators of stress.
Key words: infrared thermography, wire flooring, light intensity, stress, latency to lie
Introduction
Stress is a broad term contextually used to describe stimuli challenging homeostasis, emergency responses, and states of physiological imbalance (Siegel, 1995, Blas, 2015). Stress has been defined as “the biological response elicited when an individual perceives a threat to its homeostasis” (Moberg, 2000). An appropriate response to a stressor is essential for survival and malfunction of the stress response leads to decreased welfare (Blas, 2015). Selye (1950) described the stress response as the “general adaptation syndrome” where major organs within an individual respond to stress by supplying energy to allow adaptation to their environment. Chronic stress occurs when the body cannot maintain high energy status or return to homeostasis and negatively impacts welfare and production (Siegel, 1995, Virden and Kidd, 2009).
Several methods have been used to measure performance, behavioral, and physiological responses to stressful conditions in broilers (Siegel, 1995, Moberg, 2000). Established measures of broiler performance include BW gain, feed conversion ratio, and growth rate. Physiological and behavioral methods of measuring broiler stress may not be established definitively, exhibit repeatability, or the cause and effect relationship is not fully clarified (Manning et al., 2007). For example, behavioral evaluations by visual examination can be biased and the large variation inherent to measures of physiological stress can be misleading (Post et al., 2003, Stewart et al., 2005). Thus, there is a need for research to identify more robust measures of broiler welfare.
Environmental stressors activate the hypothalamo–pituitary–adrenal axis organ system, eliciting a cascade of biochemical responses resulting in the release of corticosterone (CORT; Siegel, 1995, Blas, 2015, Carsia, 2015). As CORT concentrations increase, the ratios of circulating blood heterophils to lymphocytes (H:L ratio) also increase (Siegel, 1980, Puvadolpirod, 1997, Shini et al., 2009, Virden and Kidd, 2009). Concentrations of CORT determined in blood plasma and/or sera are widely used in animal stress and welfare studies (Mormède et al., 2007, Scanes, 2016). However, the additional stress of restraint and pain during blood sampling may affect stress measures and result in misleading interpretations of welfare status (Mormède et al., 2007, Chloupek et al., 2011). Infrared thermography (IRT) is a noninvasive, noncontact method of determining the surface temperature of an object (Eddy et al., 2001). Biological applications of IRT in research measuring physiological phenomena in domestic chickens include noninvasive measures of metabolic heat production (Nääs et al., 2010, Yahav and Giloh, 2012), footpad dermatitis (Wilcox et al., 2009, Jacob et al., 2016), and handling stress (Edgar et al., 2013, Moe et al., 2017).
Light intensity is an environmental factor that affects broiler behavior, production, and welfare (Newberry et al., 1988, Olanrewaju et al., 2006, Deep et al., 2010, Deep et al., 2013, Rault et al., 2017). Traditionally, lower light intensities have been shown to improve production performance, feed conversion (Lien et al., 2008), and BW gain (Gross and Siegel, 1981, Lien et al., 2008). In addition, low light intensities can have behavioral benefits such as decreased aggression and cannibalism in layers (Leeson and Walsh, 2004). However, there is no evidence that light intensity affects stress as measured by the H:L ratio (Lien et al., 2007) or circulating concentrations of CORT (Olanrewaju et al., 2008, Olanrewaju et al., 2010, Olanrewaju et al., 2013, Olanrewaju et al., 2014, Rault et al., 2017). Thus, further research is necessary to determine the effects of light intensity on the homeostasis of broilers.
Light intensity may also influence the level of activity, time spent walking, and leg health of broilers (Newberry et al., 1988, Alvino et al., 2009, Deep et al., 2012). The latency-to-lie (LTL) test is a behavioral method to assess leg health in broilers by recording the amount of time a bird takes to sit after being placed in a standing position in tepid water. The LTL test provides a quantitative time measure that has an advantage over the qualitative subjectivity of gait scoring (Weeks et al., 2002). Previous research has found positive relationships between gait score and LTL time (Weeks et al., 2002, Berg and Sanotra, 2003, Aydin et al., 2015), whereas other studies have not (Webster et al., 2008). The LTL test is an indicator of leg strength; however, the environment in which the test is conducted may influence results. For instance, LTL times may be affected by the presence or absence of sensory stimuli or other birds during the test. The LTL test previously has been conducted using either groups (Weeks et al., 2002) or individual birds (Berg and Sanotra, 2003, Webster et al., 2008, Rault et al., 2017), and the authors are unaware of any previous studies LTL testing birds both individually and in groups.
The stress indices in the present study were collected from the same birds in which data have been previously reported (Weimer et al., 2019). Briefly, broiler chickens were raised on wire flooring to experimentally induce bacterial chondronecrosis with osteomyelitis (BCO) and to compare stress and behavioral measures to broilers raised on litter flooring. Raising broilers on wire flooring is reported to be a possible stressor in itself, with negative physiological and behavioral consequences of lameness (Wideman et al., 2012, Wideman and Prisby, 2013, Wideman, 2016) on welfare status. Environmental stressors elicit a natural stress response, as opposed to artificial endocrine interference methods used in laboratory studies (Puvadolpirod and Thaxton, 2000, El-Lethey et al., 2003, Weimer et al., 2018). In addition, stress-mediated immunosuppression has been reported to contribute to the pathogenesis of BCO of the proximal femoral and tibial heads (Wideman and Prisby, 2013).
Two studies (study 1 and study 2) examined the effects of 2 lux (lx), 5 lx, and 10 lx light intensity and litter vs. wire flooring on the following in broilers: 1) traditional methods for assessing level of stress (H:L ratios and serum CORT concentrations), 2) putative, noninvasive measures of stress from IRT eye and beak surface temperatures, and 3) the LTL test times when birds were tested individually (LTLI) and in groups of 5 penmates (LTLG). In addition, measures of stress were correlated with BW reported previously (Weimer et al. 2019), and it was expected that BW would have a negative relationship with stress levels.
Materials and methods
Animals and Facility
Male Cobb 500 broiler chicks (N = 720 chicks per study) were obtained from a commercial hatchery (Cobb-Vantress, Fayetteville, AR) and were randomly placed into 6 1.5-m-wide × 3-m-long pens (120 chicks per pen) in separate environmental chambers (1 pen per chamber) on clean wood shaving litter at the Poultry Environmental Research Laboratory at the University of Arkansas Poultry Research Farm. All procedures were approved by the Institutional Animal Care and Use Committee of University of Arkansas (IACUC #1604). Birds had ad libitum access to feed and water provided by tube feeders and nipple drinkers, respectively. Diets were formulated to meet minimum industry standards. A crumbled commercial starter diet was provided until day 28 when birds were switched to a pelleted commercial finisher diet. The photoperiod was 23L:1D for days 0 to 4; 20L:4D for days 5 to 14, and 18L:6D from day 15 through the end of each trial. Room temperatures were set at 33°C for day 0 to 3, 30°C for day 4 to 7, 26°C for day 8 to 14, and 24°C for day 15 through the end of trial. Ambient temperatures were measured using a thermometer (TM99A Thermistor Temperature Instrument; Cooper-Atkins, Middlefield, CT) and an infrared thermometer (IRT657; General Tools & Instruments LLC, Secaucus, NY). Study 1 was conducted from September to November 2015, and study 2 was conducted from April to June 2016.
Experimental Design
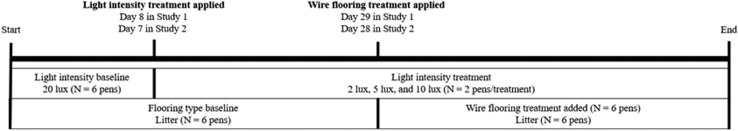
Studies followed a 3 × 2 split plot design with pen as the experimental unit and are summarized in Figure 1. Light intensity was the main plot factor with 3 light intensities (2, 5, or 10 lx) from incandescent light bulbs. The light intensity treatment was applied to the 6 pens with litter flooring (2 pens/light intensity treatment/study) on day 8 in study 1 and day 7 in study 2. The light intensity before treatments was 20 lx in each pen. The subplot factor was flooring type (litter or wire) and this was imposed on day 29 (study 1) and day 28 (study 2). At these times, half of the pen population (N = 50 birds) from each pen (6 pens) was moved to a separate pen with wire flooring (6 pens). Birds were provided twice as much space after the imposition of the wire flooring treatment, with a premove density of 32 kg/m2 to a postmove density of 16 kg/m2 per pen. The wire flooring pens had the same dimensions, bird density, and light intensity as the source litter pens. To account for the stress of handling and introduction to a novel pen, each litter flooring pen was randomly reassigned to a new litter flooring pen with the same light intensity. Thus, there were 12 total pens each containing 50 birds with 2 pen replicates for each light intensity and flooring treatment combination in each study. The rationale behind use of the wire flooring model was the following. First, a previous study demonstrated significant negative relationships between IRT leg surface temperatures with leg bone necrosis severity and lameness attributed to BCO (Weimer et al., 2019). Second, raising broilers on wire flooring is viewed as a stressor per se (Wideman and Pevzner, 2012, Wideman and Prisby, 2013), and the present study examined the effects of wire flooring on indicators of stress. Traditional (H:L ratios and CORT concentrations) and noninvasive stress measures (IRT eye and beak surface temperatures) were measured the day before and the day after the light intensity and wire flooring treatment imposition to determine if the light intensity or the wire flooring, per se, elicited acute stress responses.
Figure 1.
Timeline of light intensity and flooring treatment application in 2 studies.
Data Collection
Thermal images of the head were taken with an IRT camera (Fluke Ti400; Everette, WA) within 60 s of bird capture from the home pen of 5 randomly selected birds from each pen on day 7 (6 pens), 9 (6 pens), 28 (6 pens), 30 (12 pens), 38 (12 pens), and 56 (12 pens) in study 1 and day 6 (6 pens), 8 (6 pens), 27 (6 pens), 29 (12 pens), 40 (12 pens), and 55 (12 pens) in study 2. Samples were collected from different individual birds for each time point (N = 270 total birds sampled per study). The details of the IRT image-capture methodology have been reported (Weimer et al., 2019). Within each IRT image, shapes were made to isolate the pixels representing the surface temperatures of the eye and beak regions (Figure 2).
Figure 2.
Pixels of the right (A) eye and (B) beak were isolated on the image of each bird's head that was taken with an infrared thermography camera using SmartView (v 2.8) software.
Immediately after thermal image capture, blood was drawn from each bird. For day 7 and 9 (study 1) and day 6 and 8 (Study 2), blood samples were collected after rapid decapitation with scissors. For all subsequent blood sampling, blood was collected by venipuncture from the brachial vein within 90 s of capture. Each blood sample was immediately injected into K3EDTA-coated blood tubes and serum-separation tubes. Blood H:L ratios and serum CORT concentrations were determined by methods previously described (Weimer et al., 2018). Serum CORT ELISA interassay and intra-assay CV were <6%. Core body temperature was measured using a thermometer (TM99A Thermistor Temperature Instrument, Cooper Atkins, Middlefield, CT) inserted through the cloaca to a depth of 5 cm.
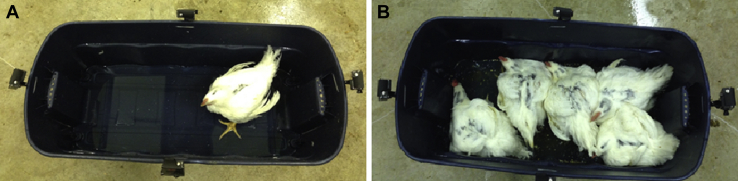
Birds from which blood samples were collected on day 56 (study 1) and day 55 (study 2) were tagged with spray paint for individual identification during the LTL tests. Latency-to-lie tests were conducted in the hallway of the facility on day 57 and 58 (study 1) and day 56 and 57 (study 2). The LTL test was a modified version from previous works (Weeks et al., 2002, Berg and Sanotra, 2003). Each bird was subjected to the LTLI and 5LTLG. The LTL test order was randomized to control for possible bias from previous LTL test experience. First, the 5 birds were removed from their pen and placed into a plastic storage tub containing litter in the hallway. Then, the birds were placed in a second 1.02-m-long × 0.51-m-wide plastic storage tub with 4 video cameras (GoPro Hero; San Mateo, CA) mounted to each side. A bucket of tepid (32°C) water was poured into the tub containing the bird(s) to a depth of 30 mm (Figure 3). A stopwatch was started to represent the test start time when each bird was standing after the water was introduced. If a bird was not standing after water was introduced, the bird was gently coaxed to stand and the test started. Birds that did not stand were considered lame and were removed from the study. The test ended, and the stopwatch was stopped when a bird sat for 3 s or longer and their LTL time was recorded. If the bird did not sit after 900 s, the test ended, and a LTL time of 900 s was recorded. Video recordings of each LTL test were uploaded to a computer and observed to verify LTL times.
Figure 3.
The latency-to-lie (LTL) test (A) individually (LTLI) and (B) in groups of 5 penmates (LTLG).
In study 2, IRT images of the heads of birds that became lame in the wire flooring pens (N = 46 birds) were taken to compare their IRT eye and beak surface temperatures with those of clinically healthy (sound) birds sampled from wire flooring pens on day 29 through day 57 (N = 90 birds). The BW of the birds sampled in the present study have been previously reported (Weimer et al., 2019) and were correlated with stress indices.
Statistical Analysis
Data were statistically analyzed using JMP software (version 13, SAS Institute Inc., Cary, NC), with the pen of birds as the experimental unit. The studies followed a 3 × 2 split plot design with light intensity as the main plot factor (2, 5, or 10 lx) and flooring type (litter or wire) as the subplot factor.
Data from study 1 and study 2 were analyzed separately because when study was included as a fixed effect in the model, study accounted for the majority of the variation in all models. All continuous data were analyzed for distribution normality. The H:L ratio and CORT concentration data had a log10 normal distribution and the model analyzed the log transformed data for these measures. Log-transformed means were back transformed to original values for reported results.
Separate mixed models were used to determine the main effects of light intensity, flooring type, age, and health status. The mixed model to analyze the effect of light intensity on H:L ratios, CORT concentrations, IRT eye and beak surface temperatures, and core body temperatures included the fixed effect of light intensity and the random effect of pen for day 7, 9, and 28 (study 1) and day 6, 8, and 27 (study 2). The split-plot mixed model to analyze the effects of light intensity, flooring treatment, and their interaction on H:L ratio, CORT concentration, IRT eye and beak surface temperatures, and core body temperature for day 30, 38, and 56 (study 1) and day 29, 40, and 55 (study 2) included the fixed effects of light intensity, flooring type, their interaction, and the random effect of pen. The same split-plot model was used to analyze the LTLI and LTLG test times. A one-way ANOVA was used to determine if the order in which the LTL test was conducted affected the subsequent LTL time (e.g., if the LTLI test affected LTLG times or vice versa). The mixed model to determine the effects of health status (sound vs. lame) on the IRT eye and beak surface temperatures of the birds from wire flooring pens (N = 6 pens) included the fixed effects of health status and head region. The linear relationships between BW and the untransformed data from the present studies were compared using Pearson's pairwise correlations. Significant means were separated post-hoc with least squared means (one main effect) and with Tukey's honest significant difference (2 main effects) where appropriate. Data were considered significant at P ≤ 0.05.
Results
Stress and Age
Owing to equipment malfunction, the H:L ratios on day 7 and 9 are missing in study 1. Prelight treatment (baseline, day 7) IRT eye temperatures of birds in 10 lx–designated pens were about 0.30°C higher than lower light intensities (P = 0.05; Table 1). On day 9, birds in 2-lx pens had at least 1.79°C lower IRT beak surface temperatures (P = 0.02) than higher light intensities. On day 9 and 28, the CORT levels of birds at 2 lx were at least 20% higher than at 5 lx (P ≤ 0.05). In study 2, prelight treatment (baseline, day 6), H:L ratios were higher for birds in 10 lx–designated pens than lower light intensities (P = 0.002). On day 27, birds at 10 lx had higher H:L ratios and CORT concentrations than birds at lower light intensities (P ≤ 0.05; Table 1).
Table 1.
Effects of light intensity on heterophil-to-lymphocyte (H:L) ratios, serum corticosterone (CORT) concentrations (ng/mL), and IRT eye and beak surface temperatures (°C), and core body temperature (°C; study 2 only) for broilers in pens with 2, 5, or 10 lx light intensity at 1 and 4 wk in 2 studies (N = 2 pens per study).
| Age (D) | Light intensity (lx)1 |
SEM | P-value | |||
|---|---|---|---|---|---|---|
| 2 | 5 | 10 | ||||
| Study 1 | ||||||
| 7 | H:L2 | - | - | - | - | - |
| CORT | 11.16 | 8.50 | 7.71 | 1.39 | NS | |
| Eye temp | 34.79b | 34.74b | 35.09a | 0.13 | 0.05 | |
| Beak temp | 32.00 | 32.40 | 33.31 | 0.77 | NS | |
| 9 | H:L | - | - | - | - | - |
| CORT | 10.71a | 7.28b | 8.63a,b | 1.28 | 0.05 | |
| Eye temp | 34.11 | 34.49 | 34.66 | 0.15 | 0.06 | |
| Beak temp | 29.67b | 31.52a | 31.46a | 0.49 | 0.02 | |
| 28 | H:L | 0.29 | 0.18 | 0.22 | 0.16 | NS |
| CORT | 15.83a | 8.25b | 10.41b | 1.27 | 0.0006 | |
| Eye temp | 32.88 | 32.70 | 33.17 | 0.20 | NS | |
| Beak | 32.17 | 33.18 | 33.39 | 0.56 | NS | |
| Study 2 | ||||||
| 6 | H:L | 0.06b | 0.04b | 0.22a | 0.03 | 0.002 |
| CORT | 13.56 | 11.45 | 14.43 | 1.24 | NS | |
| Eye temp | 34.84 | 35.10 | 34.90 | 0.17 | NS | |
| Beak temp | 34.29 | 33.24 | 33.41 | 0.56 | NS | |
| Core temp | 41.01 | 41.05 | 41.17 | 0.06 | NS | |
| 8 | H:L | 0.19 | 0.15 | 0.21 | 0.14 | NS |
| CORT | 13.09 | 12.20 | 11.43 | 1.19 | NS | |
| Eye temp | 34.05 | 33.96 | 34.24 | 0.25 | NS | |
| Beak temp | 32.17 | 32.32 | 32.86 | 0.61 | NS | |
| Core temp | 41.01 | 41.02 | 41.13 | 0.05 | NS | |
| 27 | H:L | 0.20a,b | 0.16b | 0.22a | 0.03 | 0.05 |
| CORT | 7.70b | 8.63b | 9.81a | 1.10 | 0.0007 | |
| Eye temp | 33.14 | 32.82 | 32.40 | 0.26 | NS | |
| Beak temp | 32.62 | 32.24 | 31.92 | 0.60 | NS | |
| Core temp | 41.43 | 41.48 | 41.38 | 0.05 | NS | |
a,bMeans not sharing the same superscript letter across each row differ at P ≤ 0.05.
Abbreviation: lx, lux.
Light intensities in all pens were 20 lux from day 0 to 7 in study 1 and day 0 through 6 in study 2 and changed to 2, 5, or 10 lx from day 8 in study 1 and day 7 in study 2 until the end of the trial.
Day 7 and 9 H:L ratios in study 1 were not run due to equipment malfunction.
In both studies, the H:L ratios for birds on wire flooring were higher (P ≤ 0.03) than for birds on litter on day 38 in study 1 and day 40 in study 2 (Table 2). In study 1 (day 38), birds in 10 lx wire flooring pens had the lowest IRT beak temperatures compared with birds at other light flooring treatments (P = 0.004), whereas in study 2 (day 40), this effect switched to the eye IRT surface temperatures (P = 0.05; Table 2).
Table 2.
Effects of light intensity and flooring type on heterophil-to-lymphocyte (H:L) ratios, serum corticosterone (CORT) concentrations (ng/mL), and IRT eye and beak surface temperatures (°C) of broilers in pens with light intensities of 2, 5, or 10 lx (L) on litter or wire flooring (F) in 2 studies.
| Flooring (F) 1 |
Litter |
Wire |
P-value2 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Light lx (L) | 2 | 5 | 10 | 2 | 5 | 10 | SEM | L | F | L∗F | |
| Age (D) | |||||||||||
| Study 1 | |||||||||||
| 30 | H:L | 0.26 | 0.37 | 0.34 | 0.24 | 0.24 | 0.35 | 0.20 | NS | NS | NS |
| CORT | 4.98 | 12.65 | 10.62 | 10.84 | 9.61 | 13.07 | 1.79 | NS | NS | NS | |
| Eye temp | 33.12 | 33.31 | 33.30 | 33.59 | 33.04 | 32.93 | 0.20 | NS | NS | 0.08 | |
| Beak temp | 33.50 | 33.44 | 33.86 | 34.05 | 33.71 | 32.44 | 0.50 | NS | NS | NS | |
| 38 | H:L | 0.16b | 0.18b | 0.13b | 0.28a | 0.32a | 0.40a | 0.12 | NS | 0.0001 | NS |
| CORT | 5.65 | 6.82 | 5.20 | 5.35 | 5.55 | 5.53 | 1.18 | NS | NS | NS | |
| Eye temp | 32.91 | 33.02 | 33.44 | 33.38 | 33.01 | 32.60 | 0.28 | NS | NS | 0.07 | |
| Beak temp | 33.68a | 33.31a | 33.97a | 33.09a | 33.97a | 29.84b | 0.62 | 0.04 | 0.003 | 0.004 | |
| Study 2 | |||||||||||
| 29 | H:L | 0.22 | 0.18 | 0.24 | 0.20 | 0.20 | 0.19 | 0.08 | NS | NS | NS |
| CORT | 6.79 | 6.91 | 7.31 | 6.38 | 6.62 | 6.72 | 1.18 | NS | NS | NS | |
| Eye temp | 33.83 | 33.50 | 33.91 | 34.28 | 33.75 | 33.88 | 0.23 | NS | NS | NS | |
| Beak temp | 33.60 | 32.94 | 32.93 | 34.06 | 32.53 | 34.36 | 0.59 | NS | NS | NS | |
| Core temp | 41.19 | 41.50 | 41.25 | 41.50 | 41.43 | 41.42 | 0.10 | NS | NS | NS | |
| 40 | H:L | 0.24b | 0.18b | 0.19b | 0.42a | 0.29a | 0.27a | 0.17 | NS | 0.03 | NS |
| CORT | 6.56 | 6.67 | 6.18 | 7.22 | 7.94 | 6.99 | 1.20 | NS | 0.06 | NS | |
| Eye temp | 34.16a | 34.14a | 33.92a | 34.04a | 33.87a | 32.84b | 0.22 | 0.004 | 0.01 | 0.05 | |
| Beak temp | 34.53 | 34.28 | 34.12 | 34.27 | 34.77 | 32.89 | 0.53 | NS | NS | NS | |
| Core temp | 41.47 | 41.49 | 41.54 | 41.59 | 41.39 | 41.32 | 0.09 | NS | NS | NS | |
a,bMeans not sharing the same superscript letter across each row differ at P ≤ 0.05.
Abbreviation: lx, lux.
Flooring treatments were litter in all pens until day 29 in study 1and day 28 in study 2 when half of the population from each litter pen (N = 6 pens) were moved to a wire flooring pen (N = 6 pens; 50 birds per pen) with the same light intensity as the corresponding source litter pen.
ANOVA P-values represent the overall effects, the main effects of light intensity (L) and flooring type (F) and the interaction of light intensity and flooring type (L∗F).
There were more prominent treatment effects on the stress response of the birds in study 1 on day 56 than in study 2 on day 55 (Table 3). In study 1, birds in wire flooring pens had higher H:L ratios and CORT concentrations (P < 0.01) and lower IRT eye surface temperatures (P < 0.0001) than litter pens. Birds in 10-lx pens had higher IRT eye and beak surface temperatures than birds at 2 and 5 lx (P ≤ 0.02). In study 2, birds in 10-lx pens had lower CORT concentrations than birds at 2 and 5 lx (P = 0.02). The core body temperature of birds on wire flooring at 10 lx was higher than at 2 lx (P = 0.03; Table 3).
Table 3.
Effects of light intensity and flooring type on heterophil-to-lymphocyte (H:L) ratios, serum corticosterone (CORT) concentrations (ng/mL), IRT eye and beak surface temperatures (°C), core body temperatures (°C), and the latency-to-lie (LTL) time (s) when tested individually (LTLI) and in groups of 5 pen mates (LTLG) of broilers in pens with 2, 5, or 10 lx light intensity (L) on litter or wire flooring (F) on day 56 to 58 in study 1 and day 55 to 57 in study 2.
| Flooring (F) |
Litter |
Wire |
P-value2 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Light lx (L) | 2 | 5 | 10 | 2 | 5 | 10 | SEM | L | F | L∗F |
| Study 1 | ||||||||||
| H:L | 0.32b | 0.30b | 0.36b | 0.59a | 0.78a | 0.60a | 0.26 | NS | 0.002 | NS |
| CORT | 5.91b | 5.57b | 5.63b | 6.89a | 7.19a | 6.56a | 1.16 | NS | 0.0004 | NS |
| Eye temp | 33.03a | 33.28a | 33.73a | 31.70c | 32.21b,c | 32.50b,c | 0.26 | 0.02 | 0.0001 | NS |
| Beak temp | 35.23a | 35.00a | 35.35a | 32.69b | 30.05c | 33.29a,b | 0.54 | 0.002 | 0.0001 | 0.03 |
| LTL1 | ||||||||||
| LTLG | 157.0 | 94.7 | 47.1 | 60.1 | 169.2 | 59.2 | 58.72 | NS | NS | NS |
| LTLI | 129.8 | 53.6 | 82.8 | 101.0 | 231.8 | 134.7 | 53.94 | NS | NS | NS |
| Study 2 | ||||||||||
| H:L | 0.24b | 0.32b | 0.40b | 0.48a | 0.45a | 0.40a | 0.20 | NS | 0.04 | NS |
| CORT | 7.17a | 7.23a | 6.41b | 7.64a | 7.57a | 7.11b | 1.09 | 0.02 | 0.02 | NS |
| Eye temp | 35.31 | 35.63 | 35.81 | 35.62 | 35.44 | 35.62 | 0.20 | NS | NS | NS |
| Beak temp | 36.29 | 37.18 | 36.96 | 36.96 | 36.27 | 36.95 | 0.33 | NS | NS | NS |
| Core temp | 41.83a,b | 41.96a,b | 42.03a,b | 41.58b | 41.83a,b | 42.07a | 0.12 | 0.03 | NS | NS |
| LTL | ||||||||||
| LTLG | 71.2 | 44.1 | 99.5 | 148.7 | 44.8 | 87.6 | 44.8 | NS | NS | NS |
| LTLI | 133.1 | 116.5 | 126.9 | 192.0 | 96.4 | 85.9 | 44.1 | NS | NS | NS |
a,bMeans not sharing the same superscript letter across each row differ at P ≤ 0.05.
Abbreviation: lx, lux.
The LTL test was conducted in the hallway of the facility on day 57 and 58 in study 1 and day 56 and 57 in study 2. Five birds were subjected to the LTL test individually (LTLI) and in groups of 5 (LTLG), and data are reported as means for the pen of birds.
ANOVA P-values represent the overall effects, the main effects of light intensity (L) and flooring type (F) and the interaction of light intensity and flooring type (L∗F).
The effects of age on stress indices (independent of light intensity and flooring treatments) exhibited broadly similar profiles within and across studies. H:L ratios tended to increase with age in both studies (P < 0.05; Table 4). Corticosterone concentrations were the highest on day 28 in study 1 (P < 0.0001), whereas CORT was the highest on day 6 in Study 2 (P < 0.0001; Table 4). In study 1, IRT eye surface temperatures decreased (P < 0.0001) from day 7 (34.90°C) to day 28 (32.92°C), then maintained a similar temperature to day 56. IRT beak surface temperatures exhibited a different response pattern from IRT eye surface temperatures with age in study 1, where beak temperatures decreased from day 7 to day 9, then increased with age to the highest temperatures on day 56 (33.63°C; P < 0.0001). In study 2, IRT eye and beak surface temperatures decreased (P < 0.0001) from day 6 to day 27, increased after the imposition of the flooring treatment on day 29 (P < 0.0001), and were the highest on day 55 (P < 0.0001). Core body temperatures increased with age in study 2 (P < 0.0001; Table 4).
Table 4.
Effects of age on the heterophil-to-lymphocyte (H:L) ratios, serum corticosterone (CORT) concentrations (ng/mL), IRT eye and beak surface temperatures (°C), and core body temperatures (°C) of broilers in pens with light intensities of 2, 5, or 10 lx and after day 29 were raised on litter or wire flooring (N = 6 pens for day 6 through 28; N = 12 pens for day 28 through 56).
| Age (D) | H:L1 | CORT | Eye temp | Beak temp | Core temp |
|---|---|---|---|---|---|
| Study 1 | |||||
| 7 | - | 9.12 ± 1.15b | 34.90 ± 0.16a | 32.60 ± 0.41b | - |
| 9 | - | 8.76 ± 0.13b | 34.39 ± 0.14b | 30.88 ± 0.38c | - |
| 28 | 0.22 ± 0.15b | 11.08 ± 1.13a | 32.92 ± 0.14c,d | 32.91 ± 0.38a,b | - |
| 30 | 0.29 ± 0.10b | 10.92 ± 0.10a | 33.22 ± 0.10c | 33.50 ± 0.27a,b | - |
| 38 | 0.25 ± 0.05b | 5.66 ± 0.10c | 33.06 ± 0.10c | 32.84 ± 0.27b | - |
| 56 | 0.58 ± 0.11a | 6.25 ± 0.10c | 32.74 ± 0.10d | 33.63 ± 0.27a | - |
| P value | 0.0001 | 0.0001 | 0.0001 | 0.0001 | - |
| Study 2 | |||||
| 6 | 0.09 ± 0.14c | 13.08 ± 1.10a | 34.94 ± 0.14b | 33.65 ± 0.33b,c | 41.07 ± 0.05c |
| 8 | 0.16 ± 0.13b | 12.22 ± 1.10a | 34.08 ± 0.14c | 32.45 ± 0.33d | 41.05 ± 0.05c |
| 27 | 0.19 ± 0.13b | 9.02 ± 1.10b | 32.79 ± 0.14d | 32.26 ± 0.33d | 41.44 ± 0.05b |
| 29 | 0.21 ± 0.10b | 6.78 ± 1.08c | 33.86 ± 0.10c | 33.40 ± 0.23c | 41.38 ± 0.04b |
| 40 | 0.25 ± 0.10b | 6.91 ± 1.08c | 33.83 ± 0.10c | 34.14 ± 0.23b | 41.47 ± 0.04b |
| 55 | 0.37 ± 0.10a | 7.17 ± 1.08c | 35.58 ± 0.10a | 36.65 ± 0.23a | 41.88 ± 0.04a |
| P value | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
a–dMeans not sharing the same superscript letter within each column differ at P ≤ 0.05.
Data are shown as mean ± SEM.
Abbreviation: lx, lux.
In study 1 day 7 and 9, H:L ratios were not determined because of equipment malfunction.
Table 5 shows the correlations between BW, stress indices, and LTL times. There were inconsistent correlations between the 2 studies and there were many more significant correlations in study 1 than in study 2. There was only one significant correlation in study 2, which was mirrored in study 1. This was a negative correlation between CORT concentrations and IRT beak surface temperatures (P < 0.05; Table 5). The remaining correlation results are for days 56 through 58 in study 1. BW was negatively correlated with H:L ratios, CORT concentrations, LTLG, and LTLI times (P < 0.01) and was positively correlated with IRT eye and beak surface temperatures (P < 0.05; Table 5). Heterophil-to-lymphocyte ratios were positively correlated with CORT concentrations (r = 0.41; P = 0.001), and both H:L ratios and CORT concentrations were negatively correlated with IRT beak surface temperatures and positively correlated with LTLI times (P < 0.05). The 2 LTL times were moderately positively correlated (P = 0.0006; Table 5).
Table 5.
Correlations (r) of BW (g), heterophil-to-lymphocyte (H:L) ratios, serum corticosterone (CORT) concentrations (ng/mL), eye surface temperatures (Eye temp, °C), and beak surface temperatures (Beak temp, °C), core body temperature (Core temp, °C), and the latency-to-lie (LTL, s) test times of broilers tested in groups (LTLG) and individually (LTLI) on day 56 to 58 in study 1 and day 55 to 57 in study 2.
| Correlations (r)1 |
||||||||
|---|---|---|---|---|---|---|---|---|
| BW | H:L | CORT | Eye temp | Beak temp | Core temp | LTLG | LTLI | |
| Study 1 | ||||||||
| BW | 1.00 | −0.37∗∗ | −0.50∗∗ | 0.36∗∗ | 0.64∗∗ | - | −0.26∗ | −0.30∗ |
| H:L | 1.00 | 0.41∗∗ | −0.20 | −0.43∗∗ | - | 0.03 | 0.32∗ | |
| CORT | 1.00 | −0.36∗∗ | −0.62∗∗ | - | 0.27∗ | 0.42∗∗ | ||
| Eye temp | 1.00 | 0.51∗∗ | - | 0.02 | 0.14 | |||
| Beak temp | 1.00 | - | −0.23 | −0.27∗ | ||||
| LTLG | - | 1.00 | 0.43∗∗ | |||||
| LTLI | - | 1.00 | ||||||
| Study 2 | ||||||||
| BW | 1.00 | −0.13 | 0.02 | 0.17 | 0.11 | 0.25 | −0.18 | 0.20 |
| H:L | 1.00 | 0.03 | −0.16 | 0.02 | −0.09 | 0.14 | 0.00 | |
| CORT | 1.00 | −0.01 | −0.34∗ | −0.06 | 0.03 | −0.07 | ||
| Eye temp | 1.00 | 0.25 | 0.18 | 0.01 | −0.02 | |||
| Beak temp | 1.00 | 0.14 | −0.26 | −0.15 | ||||
| Core temp | 1.00 | −0.18 | −0.13 | |||||
| LTLG | 1.00 | 0.10 | ||||||
| LTLI | 1.00 | |||||||
Pairwise correlations with asterisks indicate significance at ∗P ≤ 0.05; ∗∗P ≤ 0.01.
Lameness Effect on IRT Surface Temperatures
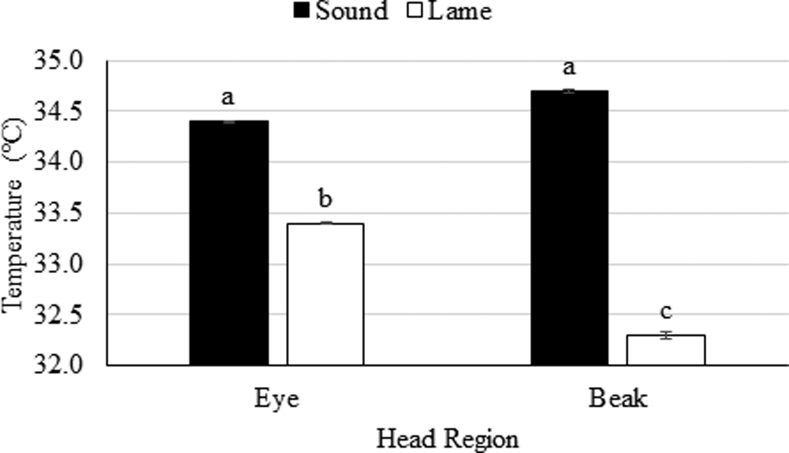
Figure 4 shows the IRT surface temperatures of the eye and beak of sound (N = 90 birds) and lame (N = 43 birds) birds from the wire flooring pens (N = 6 pens) in study 2. Lame birds had 1.0°C lower IRT eye and 2.4°C lower IRT beak surface temperatures than sound birds (P ≤ 0.004). There were no differences between the IRT eye and IRT beak surface temperatures of sound birds, but the beak surface temperatures of lame birds were 2.1°C lower than their IRT eye surface temperatures (P = 0.004; Figure 4).
Figure 4.
Surface temperatures (°C) of the eye and beak of sound or lame broilers between day 29 and 57 in study 2. Broilers were on wire flooring in pens with 2, 5, or 10 lux light intensity. Data are presented as a mean (±SEM) for each wire flooring pen of sound (N = 6 pens) and lame birds (N = 6 pens). a,b,cRegions with different letters differ in temperature at P ≤ 0.05.
Discussion
Environmental management is the key factor that affects the overall welfare of commercial broiler flocks (Dawkins et al., 2004). However, robust indices of stress remain enigmatic. This, in part, is owing to the inherent, stochastic variation of physiological stress measures (Dehnhard et al., 2003) as well as the dynamic differences and interactions between bird genetics, nutrition, environment, and management (Bessei, 2006). The present studies evaluated the effects of 2 experimentally controlled factors, light intensity (2, 5, and 10 lx) and flooring type (litter vs. wire), on the stress and leg health responses of broilers measured at different ages.
Stress indices were measured 24 h before and 24 h after applying the light intensity treatments (from 20 lx to 2, 5, or 10 lx) and these time points represented physiological baseline and acute stress response to the light treatment, respectively. The effects of light intensity were transient on the stress responses measured in both studies. Moreover, there were baseline light intensity differences in both studies. This may reflect the low number of replicates or that the magnitude of the differences in light intensity were not extreme enough to elicit response differences.
Stress indices were measured 24 h before and 24 h after the imposition of the wire flooring treatment (moving half of the population from litter flooring pens to wire flooring pens with the same light intensity) and these time points represented physiological baseline and acute stress response to the flooring treatment, respectively. Raising broilers on wire flooring is reported to induce stress-mediated immunosuppression and contribute to the pathogenesis of BCO of the proximal femoral and tibial head growth plates in broilers (Wideman and Prisby, 2013, Wideman, 2016). However, there were no light intensity or flooring type effects on stress indices in either study when they were measured 24 h after the imposition of the wire flooring treatment.
Increases in circulating CORT concentrations indicate the activation of the acute stress response, whereas increases in H:L ratios indicate chronic stress response of the immune system in chickens (Gross and Siegel, 1983; Shini et al., 2009; Weimer et al., 2018). The wire flooring increased H:L ratios and CORT concentrations of older birds in both of the present studies, indicating that they were indeed experiencing physiological levels of stress. However, the increase in the H:L ratios of birds on wire flooring was likely influenced by the immune response to BCO infection. Corticosterone concentrations generally decrease with age, and this has been found in birds raised in laboratory (Carsia, 2015) and commercial settings (Thaxton et al., 2005). The age-related changes of H:L ratios and CORT concentrations in broilers are both novel and confounding because the CORT concentrations and age-related response patterns differed between the 2 studies. Corticosterone concentrations decreased with age in study 2 but not in study 1. These differences may have been because of chick quality (e.g., breeder flock age, hatch window, and hatchery sanitation) or unidentified stressors, especially in study 1.
The LTL test has previously been conducted on birds in either in groups (Weeks et al., 2002) or individually (Berg and Sanotra, 2003, Webster et al., 2008, Rault et al., 2017), yet no previous work has tested the same birds individually and in groups. It was hypothesized that the LTL test would provide a quantitative determinant of leg health that was sensitive enough to scale the stress and pain experienced by broilers on wire flooring and/or with BCO. The first presumption of the LTL test was that birds would sit sooner if they were experiencing more leg pain than those that stood longer, and it was expected that birds on wire flooring would have shorter LTL times. Surprisingly, this was not the case as there were no differences in LTLI or LTLG between the birds raised on litter vs. wire flooring. The second presumption of the LTL test was that the LTL times would be shorter for birds tested individually than in groups because they would experience stress-induced self-analgesia or attentional shifts (Gentle, 2001) to the presence conspecifics. Previous work has characterized attentional shifts by evaluating the effects of a transient lameness (sodium urate injection) on the behavior of laying hen and found that the hens placed into individual cages displayed more pain-related behaviors than hens placed into pens with conspecifics (Gentle and Corr, 1995). In the present studies, the LTLG times for many of the birds were lower than LTLI times but not for all, and the large variation in LTL times lead to no statistical differences. It was further expected that LTL times would be moderately correlated between individuals and in groups. This was the case in study 1 but not study 2. The negative correlations between IRT beak temperature with H:L ratios and CORT concentrations indicate the LTLI test may have also been an indicator of stress in study 1.
Biological IRT research is centered on detecting responses in peripheral blood flow. Thermoregulatory control of the body is mediated by the hypothalamus (Esmay, 1978), and under acute stress, peripheral blood is sequestered to increase core temperature. This vasoconstrictive response of surface blood vessels results in a reduction in skin temperature (Siegel, 1995). Therefore, a colder skin surface temperature indicates a more severe stress response. Previous studies have demonstrated that handling stress increases heart rate and core body temperature (Cabanac and Aizawa, 2000) and decreases IRT surface temperature of the skin (Cabanac and Aizawa, 2000) and the eyes (Edgar et al., 2013) of chickens. Infrared thermography images were taken within 60 s of bird capture from the home pen in the present studies. However, the peripheral vasoconstriction due to stress from handling and/or being taken out of their home pen may have affected IRT body surface temperatures. In addition, IRT eye and beak surface temperatures exhibited different response patterns with age and were higher at market age in study 2 compared with study 1. Another study found the IRT surface temperatures of the head, eye, and comb of broiler breeders differed in their temperature change response pattern over a 20-min restraint stress (Edgar et al., 2013). Study 1 was conducted in the fall, and study 2 was conducted in the summer. Thermal images of the birds were taken in the same location in the hallway of the facility for both studies, and it is possible that hallway ambient air temperatures may have been higher in study 2 during IRT image capture than in study 1. Ambient air temperature of the IRT image capture area was not recorded in the present studies and should be in future studies.
There were no differences between the IRT eye and beak surface temperatures of sound birds, but the IRT beak surface temperatures of lame birds were lower than their eye surface temperatures in study 2. Similarly, lame birds also had reduced hock, shank, and foot IRT surface temperatures compared with sound birds (Weimer et al., 2019). No previous studies have evaluated the beak region in IRT research with chickens. The beak region isolated in the thermal image included the culmen, and this region of the upper beak receives pain signals as well as sympathetic and parasympathetic signals (Lunam, 2005, Kuenzel, 2007) from the brain. The IRT beak surface temperatures may have been sensitive to the pain experienced by the birds on the wire flooring or stress signals from handling for image capture. Corticosterone should be measured in lame birds to determine if IRT beak surface temperatures are indeed indicative of stress attributable to lameness in future studies.
The present data support the utility of IRT eye and beak surface temperatures as an indicator of stress in broilers. Lame broilers had reduced IRT surface temperatures of the eye and the beak (study 2), and these birds also had reduced hock, shank, and foot IRT surface temperatures (Weimer et al., 2019). There were several correlations between stress indices and LTL times in study 1. These were negative correlations between body weight with H:L ratios, CORT concentrations, individual and group LTL times, and positive correlations with IRT eye and beak surface temperatures. In other words, heavier birds were had lower physiological stress levels and sat sooner in the LTL test than lighter birds in study 1. Lameness incidence in study 1 was 38% higher than in study 2 (Weimer et al., 2019). Similarly, there were greater effects of wire flooring on the stress indices in study 1 than in study 2. These data suggest a more pronounced stress response of the birds in study 1, which likely contributed to their higher lameness incidence.
Conclusions
There were transient effects of both light intensity and flooring type on indices of stress. Some indices of stress, but not all, were correlated. Infrared thermography beak surface temperatures were moderately correlated with traditional stress measures (H:L ratios and CORT concentrations) in study 1 but not in study 2. In study 2, lame birds had lower IRT eye and beak surface temperatures compared with sound birds and lame bird IRT beak surface temperatures were lower than their eye temperatures. In both studies, there were negative correlations between CORT concentrations and IRT beak surface temperatures, indicating that beak surface temperature is an indicator of the stress response. The results from these studies provide evidence that lameness is stressful and that the IRT surface temperatures of the eye, and more distinctly of the beak, can be used as sensitive noninvasive indicators of stress in broilers.
Acknowledgments
The authors like to express gratitude to USDA/ARS and Dr. Sonia Tsai for Cell-Dyn use and technical assistance and to Dr. Douglas Aldridge and Dr. Dawn Koltes for sampling assistance.
Conflict of Interest Statement: The authors did not provide a conflict of interest statement.
References
- Alvino G.M., Archer G.S., Mench J.A. Behavioural time budgets of broiler chickens reared in varying light intensities. Appl. Anim. Behav. Sci. 2009;118:54–61. [Google Scholar]
- Aydin A., Bahr C., Berckmans D. Automatic classification of measures of lying to assess the lameness of broilers. Anim. Welf. 2015;24:335–343. [Google Scholar]
- Bessei W. Welfare of broilers: a review. Worlds Poult. Sci. J. 2006;62:455–466. [Google Scholar]
- Berg C., Sanotra G.S. Can a modified latency-to-lie test be used to validate gait-scoring results in commercial flocks? Anim. Welf. 2003;12:655–659. [Google Scholar]
- Blas J. Stress in birds. In: Scanes C.G., editor. Sturkie’s Avian Physiology. 6th ed. Academic Press; New York: 2015. pp. 769–803. [Google Scholar]
- Cabanac M., Aizawa S. Fever and tachycardia in a bird (Gallus domesticus) after simple handling. Physiol. Behav. 2000;69:541–545. doi: 10.1016/s0031-9384(00)00227-4. [DOI] [PubMed] [Google Scholar]
- Carsia R. Adrenals. In: Scanes C.G., editor. Sturkie’s Avian Physiology. 6th ed. Academic Press; New York: 2015. pp. 577–615. [Google Scholar]
- Chloupek P., Bedanova I., Chloupek J., Vecerek V. Changes in selected biochemical indices resulting from various pre-sampling handling techniques in broilers. Acta Vet. Scand. 2011;53:31. doi: 10.1186/1751-0147-53-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawkins M.S., Donnelly C.A., Jones T.A. Chicken welfare is influenced more by housing conditions than by stocking density. Nature. 2004;427:342–344. doi: 10.1038/nature02226. [DOI] [PubMed] [Google Scholar]
- Deep A., Schwean-Lardner K., Crowe T.G., Fancher B.I., Classen H.L. Effect of light intensity on broiler production, processing characteristics, and welfare. Poult. Sci. 2010;89:2326–2333. doi: 10.3382/ps.2010-00964. [DOI] [PubMed] [Google Scholar]
- Deep A., Schwean-Lardner K., Crowe T.G., Fancher B.I., Classen H.L. Effect of light intensity on broiler behaviour and diurnal rhythms. Appl. Anim. Behav. Sci. 2012;136:50–56. [Google Scholar]
- Deep A., Raginski C., Schwean-Lardner K., Fancher B.I., Classen H.L. Minimum light intensity threshold to prevent negative effects on broiler production and welfare. Br. Poult. Sci. 2013;54:686–694. doi: 10.1080/00071668.2013.847526. [DOI] [PubMed] [Google Scholar]
- Dehnhard M., Schreer A., Krone O., Jewgenow K., Krause M., Grossmann R. Measurement of plasma corticosterone and fecal glucocorticoid metabolites in the chicken (gallus domesticus), the great cormorant (phalacrocorax carbo), and the goshawk (accipiter gentilis) Gen. Comp. Endocrinol. 2003;131:345–352. doi: 10.1016/s0016-6480(03)00033-9. [DOI] [PubMed] [Google Scholar]
- Eddy A., Van Hoogmoed L., Snyder J. The role of thermography in the management of equine lameness. Vet. J. 2001;162:172–181. doi: 10.1053/tvjl.2001.0618. [DOI] [PubMed] [Google Scholar]
- Edgar J.L., Nicol C.J., Pugh C.A., Paul E.S. Surface temperature changes in response to handling in domestic chickens. Physiol. Behav. 2013;119:195–200. doi: 10.1016/j.physbeh.2013.06.020. [DOI] [PubMed] [Google Scholar]
- El-Lethey H., Huber-Eicher B., Jungi T.W. Exploration of stress-induced immunosuppression in chickens reveals both stress-resistant and stress-susceptible antigen responses. Vet. Immunol. Immunopathol. 2003;95:91–101. doi: 10.1016/s0165-2427(02)00308-2. [DOI] [PubMed] [Google Scholar]
- Esmay M.L. Principles of Animal Environment. AVI Publishing Co. Inc.; Westport, CT: 1978. Chapter 10; pp. 167–196. [Google Scholar]
- Gentle M.J., Corr S.A. Endogenous analgesia in the chicken. Neurosci. Lett. 1995;201:211–214. doi: 10.1016/0304-3940(95)12181-1. [DOI] [PubMed] [Google Scholar]
- Gentle M.J. Attention shifts alter pain perception in the chicken. Anim. Welf. 2001;10:S187–S194. [Google Scholar]
- Gross W.B., Siegel H.S. Long-term exposure of chickens to three levels of social stress. Avian Dis. 1981;25:312–325. [PubMed] [Google Scholar]
- Gross W.B., Siegel H.S. Evaluation of the heterophil/lymphocyte ratio as a measure of stress in chickens. Avian Dis. 1983;27:972–979. [PubMed] [Google Scholar]
- Jacob F.G., Baracho M.D.S., Nääs I.D.A., Souza R., Salgado D.D.A. The use of infrared thermography in the identification of pododermatitis in broilers. J. Braz. Assoc. Agric. Eng. 2016;36:253–259. [Google Scholar]
- Kuenzel W.J. Neurobiological basis of sensory perception: welfare implications of beak trimming. Poult. Sci. 2007;86:1273–1282. doi: 10.1093/ps/86.6.1273. [DOI] [PubMed] [Google Scholar]
- Leeson S., Walsh T. Feathering in commercial poultry. II. Factors influencing feather growth and feather loss. Worlds Poult. Sci. J. 2004;60:52–63. [Google Scholar]
- Lien R.J., Hess J.B., McKee S.R., Bilgili S.F., Townsend J.C. Effect of light intensity and photoperiod on live performance, heterophil-to-lymphocyte ratio, and processing yields of broilers. Poult. Sci. 2007;86:1287–1293. doi: 10.1093/ps/86.7.1287. [DOI] [PubMed] [Google Scholar]
- Lien R.J., Hess J.B., Bilgili S.F. Effect of light intensity on live performance and processing characteristics of broilers. Poult. Sci. 2008;87:853–857. doi: 10.3382/ps.2007-00277. [DOI] [PubMed] [Google Scholar]
- Lunam C.A. The anatomy and innervation of the chicken beak: effects of trimming and re-trimming. In: Glatz P.C., editor. Poultry Welfare Issues: Beak Trimming. Nottingham Unv. Press; Nottingham,UK: 2005. pp. 51–68. [Google Scholar]
- Manning L., Chadd S.A., Baines R.N. Key health and welfare indicators for broiler production. Worlds Poult. Sci. J. 2007;63:46–62. [Google Scholar]
- Moberg G.P. Biological response to stress: implications for animal welfare. In: Moberg G.P., Mench J.A., editors. The Biology of Animal Stress: Basic Principles and Implications for Animal Welfare. CAB International; Wallingford, UK: 2000. pp. 1–21. [Google Scholar]
- Moe R.O., Bohlin J., Flø A., Vasdal G., Stubsjøen S.M. Hot chicks, cold feet. Physiol. Behav. 2017;179:42–48. doi: 10.1016/j.physbeh.2017.05.025. [DOI] [PubMed] [Google Scholar]
- Mormède P., Andanson S., Aupèrin B., Beerda B., Guèmenè D., Malmkvist J., Manteca X., Manteuffel G., Prunet P., van Reenen C.G., Richard S., Veissier I. Exploration of the hypothalamic-pituitary-adrenal function as a tool to evaluate animal welfare. Physiol. Behav. 2007;92:317–339. doi: 10.1016/j.physbeh.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Nääs I.A., Romanini C.E.B., Neves D.P., Nascimento G.R., Vercellino R.A. Broiler surface temperature distribution of 42 day old chickens. Sci. Agric. 2010;67:497–502. [Google Scholar]
- Newberry R.C., Hunt J.R., Gardiner E.E. Influence of light intensity on behavior and performance of broiler chickens. Poult. Sci. 1988;67:1020–1025. doi: 10.3382/ps.0671020. [DOI] [PubMed] [Google Scholar]
- Olanrewaju H.A., Thaxton J.P., Dozier W.A., III, Purswell J., Roush W.B., Branton S.L. A review of lighting programs for broiler production. Int. J. Poult. Sci. 2006;5:301–308. [Google Scholar]
- Olanrewaju H.A., Thaxton J.P., Dozier W.A., III, Purswell J., Collier S.D., Branton S.L. Interactive effects of ammonia and light intensity on hematochemical variables in broiler chickens. Poult. Sci. 2008;89:2668–2677. doi: 10.3382/ps.2007-00486. [DOI] [PubMed] [Google Scholar]
- Olanrewaju H.A., Purswell J.L., Collier S.D., Branton S.L. Effect of ambient temperature and light intensity on physiological reactions of heavy broiler chickens. Poult. Sci. 2010;89:2668–2677. doi: 10.3382/ps.2010-00806. [DOI] [PubMed] [Google Scholar]
- Olanrewaju H.A., Purswell J.L., Collier S.D., Branton S.L. Interactive effects of photoperiod on blood physiological and biochemical reactions of broilers grown to heavy weights. Poult. Sci. 2013;92:1029–1039. doi: 10.3382/ps.2012-02792. [DOI] [PubMed] [Google Scholar]
- Olanrewaju H.A., Purswell J.L., Collier S.D., Branton S.L. Effects of genetic strain and light intensity on blood physiological variables of broilers grown to heavy weights. Poult. Sci. 2014;93:970–978. doi: 10.3382/ps.2013-03613. [DOI] [PubMed] [Google Scholar]
- Post J., Rebel M.J., ter Huurne A.A.H.M. Automated blood cell count: a sensitive and reliable method to study corticosterone-related stress in broilers. Poult. Sci. 2003;82:591–595. doi: 10.1093/ps/82.4.591. [DOI] [PubMed] [Google Scholar]
- Puvadolpirod S. Mississippi State University; Starkville, Mississippi: 1997. Physiological Stress Responses in Broiler Chicks. PhD Dissertation. [Google Scholar]
- Puvadolpirod S., Thaxton J.P. Models of physiological stress in chickens. 1. Response parameters. Poult. Sci. 2000;79:363–369. doi: 10.1093/ps/79.3.363. [DOI] [PubMed] [Google Scholar]
- Rault J.L., Clark K., Groves P.J., Cronin G.M. Light intensity of 5 or 20 lux on broiler behavior, welfare and productivity. Poult. Sci. 2017;96:779–787. doi: 10.3382/ps/pew423. [DOI] [PubMed] [Google Scholar]
- Scanes C.G. Biology of stress in poultry with emphasis on glucocorticoids and the heterophil to lymphocyte ratio. Poult. Sci. 2016;95:2208–2215. doi: 10.3382/ps/pew137. [DOI] [PubMed] [Google Scholar]
- Selye H. Stress and the general adaptation syndrome. Br. Med. J. 1950;1:1383–1392. doi: 10.1136/bmj.1.4667.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shini S., Shini A., Huff G.R. Effects of chronic and repeated corticosterone administration in rearing chickens on physiology, the onset of lay and egg production of hens. Physiol. Behav. 2009;98:73–77. doi: 10.1016/j.physbeh.2009.04.012. [DOI] [PubMed] [Google Scholar]
- Siegel H.S. Physiological stress in birds. BioScience. 1980;30:529–534. [Google Scholar]
- Siegel H.S. Stress, strains and resistance. Br. Poult. Sci. 1995;36:003–022. doi: 10.1080/00071669508417748. [DOI] [PubMed] [Google Scholar]
- Stewart M., Webster J.R., Schaefer A.L., Cook N.J., Scott S.L. Infrared thermography as a non-invasive tool to study animal welfare. Anim. Welf. 2005;14:319–325. [Google Scholar]
- Thaxton J.P., Stayer P., Ewing M., Rice J. Corticosterone in commercial broilers. J. Appl. Poult. Res. 2005;14:745–749. [Google Scholar]
- Virden W.S., Kidd M.T. Physiological stress in broilers: ramifications on nutrient digestibility and responses. J. Appl. Poult. Res. 2009;18:338–347. [Google Scholar]
- Webster A.B., Fairchild B.D., Cummings T.S., Stayer P.A. Validation of a three-point gait-scoring system for field assessment of walking ability of commercial broilers. J. Appl. Poult. Res. 2008;17:529–539. [Google Scholar]
- Weeks C.A., Knowles T.G., Gordon R.G., Kerr A.E., Peyton S.T., Tilbrook N.T. New method for objectively assessing lameness in broiler chickens. Vet. Rec. 2002;151:762–764. [PubMed] [Google Scholar]
- Weimer S.L., Wideman R.F., Scanes C.G., Mauromoustakos A., Christensen K.D., Vizzier-Thaxton Y. An evaluation of methods for measuring stress in broiler chickens. Poult. Sci. 2018;97:3381–3389. doi: 10.3382/ps/pey204. [DOI] [PubMed] [Google Scholar]
- Weimer S.L., Wideman R.F., Scanes C.G., Mauromoustakos A., Christensen K.D., Vizzier-Thaxton Y. The utility of infrared thermography for evaluating lameness attributable to bacterial chondronecrosis with osteomyelitis. Poult. Sci. 2019;98:1575–1588. doi: 10.3382/ps/pey538. [DOI] [PubMed] [Google Scholar]
- Wideman R.F., Hamal K.R., Stark J.M., Blankenship J., Lester H., Mitchell K.N., Lorenzoni G., Pevzner I. A wire-flooring model for inducing lameness in broilers: evaluation of probiotics as a prophylactic treatment. Poult. Sci. 2012;91:870–883. doi: 10.3382/ps.2011-01907. [DOI] [PubMed] [Google Scholar]
- Wideman R.F., Pevzner I. Dexamethasone triggers lameness associated with necrosis of the proximal tibial head and proximal femoral head in broilers. Poult. Sci. 2012;91:2464–2474. doi: 10.3382/ps.2012-02386. [DOI] [PubMed] [Google Scholar]
- Wideman R.F., Prisby R.D. Bone circulatory disturbances in the development of spontaneous bacterial chondronecrosis with osteomyelitis: a translational model for the pathogenesis of femoral head necrosis. Front. Endocrinol. 2013;3:183. doi: 10.3389/fendo.2012.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wideman R.F. Bacterial chondronecrosis with osteomyelitis and lameness in broilers: a review. Poult. Sci. 2016;95:3235–3344. doi: 10.3382/ps/pev320. [DOI] [PubMed] [Google Scholar]
- Wilcox C.S., Patterson J., Cheng H.W. Use of thermography to screen for subclinical bumblefoot in poultry. Poult. Sci. 2009;88:1176–1180. doi: 10.3382/ps.2008-00446. [DOI] [PubMed] [Google Scholar]
- Yahav S., Giloh M. Infrared thermography - applications in poultry biological research. In: Prakash R.V., editor. Infrared Thermography. IntechOpen Limited; London, UK: 2012. pp. 93–116. [Google Scholar]