Abstract
The present study determined the effects of in ovo feeding (IOF) of N-acetyl-L-glutamate (NAG) on early intestinal development and growth performance of broilers. A total of 702 fertile broiler eggs were randomly divided into 3 treatments: 1) non-punctured control group, 2) saline-injected control group, and 3) NAG solution–injected group (1.5 mg/egg). At 17.5 D of incubation, 300 μL of each solution was injected into each egg of injected groups. Results indicated that the hatchability and healthy chicken rate were not affected by NAG injection (P > 0.05). Chicks from NAG solution–injected group had significantly decreased average daily feed intake and feed conversion ratio during 1–14 D than those in the non-punctured control group (P < 0.05). Compared with the non-punctured control group, IOF of NAG significantly increased the density of goblet cells in jejunum at hatch, duodenum at 7 D, and ileum at 14 D; decreased crypt depth in jejunum at hatch; and increased villus height in duodenum and jejunum and villus height:crypt depth ratio in duodenum at 7 D (P < 0.05). The intestinal mRNA expression of Na+-dependent neutral amino acid transporter, peptide transporter, and excitatory amino acid transporter 3 did not differ between groups at 7 or 14 D. However, the mRNA expression level of rBAT in jejunum significantly increased in the NAG solution–injected group than in the non-punctured control group at 7 D (P < 0.05). In conclusion, IOF of NAG (1.5 mg/egg) accelerated the early intestinal development by enhancing intestinal immune and absorption function, thereby positively affecting the feed efficiency for the first 2 wk post-hatch.
Key words: N-acetyl-L-glutamate, intestinal development, growth performance, intestinal morphology, nutrient transporter
Introduction
The gastrointestinal tract is an essential system of organs for animal growth because it performs vital functions in nutrient digestion and absorption (Huycke and Tabin, 2018). Although the development of the intestine, a precise and ordered process, occurs through incubation, the functional abilities do not begin to develop until amniotic fluid is orally consumed by the chick embryo during the late incubation period. Avian embryo development is determined by the nutrients within the egg (Ohta et al., 1999). However, in the late period of incubation, dramatic physiological change and huge energy expenditure occur in the chick embryo, which decreases the content of nutrients, such as amino acid (AA) (De Oliveira et al., 2008). Moreover, nutrient deficit at the late phase of incubation may limit the development of embryo and post-hatch growth performance of chickens (Ohta et al., 1999). After hatching, modern broilers may fast within 36 to 72 h (Zhang et al., 2018a). Delayed feeding increases malnutrition (Kadam et al., 2013) and hinders the maturation of the intestine of chicks (Gao et al., 2017a), which negatively affects the development of the small intestine, such as decrease in the villus area, crypt size, crypt proliferation, number of crypt per villus, and rate of enterocyte migration (Geyra et al., 2001a). Accumulating studies show that in ovo supplementation with nutrients at the late phase of incubation could provide nutrients for the intestine and improve the nutritional status between late incubation and first feeding (Gao et al., 2017b).
Relative to fat and water, the amount of AAs is only enough to satisfy the needs of hatch and development of the embryo (Al-Murrani, 1978, Ohta et al., 1999). In addition, the extra nutrients injected into the amnion at the late phase of incubation may be delivered to the intestine for improving its development (Willemsen et al., 2010). In ovo feeding (IOF) of AAs at the late phase of incubation has been shown to stimulate intestine development and improve growth performance of broiler chicks (Gao et al., 2017b, Nazem et al., 2017). In ovo feeding of arginine (Arg) increases the villus height (VH) and decreases the crypt depth (CD) in duodenum of broiler embryos and post-hatch hatchlings (Gao et al., 2017c), and IOF of methionine increases VH, width, area, enterocyte height, and goblet cell density of embryos (Nazem et al., 2017). In addition, in ovo supplementation with threonine improves growth performance of chickens by accelerating the morphological and functional development of the intestinal mucosa (Filho et al., 2019). Thus, IOF of AAs may be an effective way to improve intestinal development of broiler embryos and hatchlings (Al-Murrani, 1982).
N-Acetyl-L-glutamate (NAG) is a catalyst of carbamoyl phosphate synthase-Ι (CPS-Ι), and the carbamoyl phosphate synthesized by CPS-Ι plays an important role in Arg synthesis. An early study showed that the addition of 0.1 mmol/L NAG to incubation medium increased pyrroline-5-carboxylate (P5C) synthase activity in enterocytes of suckling pigs; thus, NAG is an activator of P5C synthase in enterocytes (Wu et al., 2004). N-Acetyl-L-glutamate concentration in enterocytes decreased progressively with increased growth of the suckling pigs, as was used to synthesize citrulline and Arg in the intestine (Wu et al., 2004), which showed that low NAG levels are responsible for the limited intestinal synthesis of citrulline and Arg (Wu et al., 2007). N-Acetyl-L-glutamate was believed to play an important role in regulating intestinal synthesis of citrulline and Arg by modulating P5C synthase and CPS-Ι activities (Wu et al., 2004). Thus, it is reasonable to assume that in ovo administration of NAG may improve early intestinal development and growth performance of broilers after hatching. N-Carbamylglutamate (NCG), a synthetic metabolically stable analogue of NAG, had been observed to stimulate Arg synthesis with NAG (Zhang et al., 2018b). Several studies have investigated the effects of NCG on intestine and growth performance of animals. For example, dietary supplementation with NCG increases the expression of intestinal AA transporters in weaned Huanjiang mini-pig piglets (Yang et al., 2013), enhances piglet growth (Wu et al., 2004), and improves the intestinal function of Hu sucking lambs with intrauterine growth restriction (Zhang et al., 2018b). However, little information is available on the practical application of NAG in broiler production. Therefore, the present study investigated the effects of IOF of NAG at 17.5 D of incubation (DOI) on hatchability, growth performance, and early morphological and functional development of the intestine of broilers in the first 2 wk post-hatch.
Materials and methods
Incubation
Fertile eggs from Ross 308 breeder hens aged 35 wk were obtained from a commercial source. The eggs had an average weight of 59.28 ± 0.07 g and were stored in an egg storage facility under commercial conditions. The eggs were warmed to room temperature (25°C) before being set (Zhang et al., 2018a) and incubated in a micro-computer automatic incubator (Model: BLF-J3520, Chengdu Beili Agricultural Technology Co., Ltd., Chengdu, China) at Nankou Experimental Base of Chinese Academy of Agricultural Sciences (Beijing, China). After setting the eggs, the incubator temperature was raised from 25°C to 37.8°C at a rate of 3°C per hour. The eggs were incubated under standard conditions (37.8°C and 60% relative humidity) according to constant temperature incubation program and turned through 90° per 1.5 h until 17.5 DOI. At 10 and 17 DOI, unfertilized and dysplastic eggs were eliminated after candling, and all eggs were transferred to the hatching basket with the injection site remained open after injection at 17.5 DOI.
Treatment Solutions and Injection Procedure
A total of 702 alive embryo eggs were weighed and randomly allocated to 3 treatment groups, consisting of 6 replicates of 39 eggs each at 17.5 DOI. The 3 treatment groups included the non-punctured control group (NC group); 8.5 g/L saline-injected control group (SC group), 8.5 g of NaCl was dissolved in 1 L sterile distilled water (the concentration of physiological saline of the poultry); and 5.0 g/L NAG solution–injected group (NAG group), 5.0 g of NAG (855642; Sigma-Aldrich Inc., St. Louis, MO) was dissolved in 1 L of 8.5 g/L NaCl diluent solution. The dose of NAG was selected on the basis of previous experiment result (J. Wang, unpublished data) in our laboratory, and NAG has been reported to be safe (Wu et al., 2004). The solutions were freshly prepared and warmed in the incubator for 30 min before in ovo injection. At 17.5 DOI, except eggs in the NC group, eggs taken from setter trays were punctured at their blunt end after cleaning with 75% ethanol, and then 0.3 mL of injectant was injected into the amnion of each egg in SC and NAG groups through a pinhole with a 25 mm needle, which contains saline solution or NAG solution, respectively.
Treatments and Management
On the day of hatch, hatchlings were weighed and gender of hatchling was identified by feather sexing. Twelve healthy males from each replicate whose body weight was close to the average body weight (BW) of the replicate (±2 g) were selected. Then, a total of two hundred sixteen healthy male birds were distributed with 3 treatment groups and 6 replicates with 12 chicks per treatment group and transferred to the chicken house. Chicks were allowed free access to feed and water in a temperature-controlled room. All birds were fed with the same diet and managed according to the routine procedure. A corn-soybean meal diet was formulated based on the National Research Council (1994), with 23% crude protein, 3,000 kcal/kg ME, 1.25% digestible lysine, 0.92% digestible methionine + cystine, and 0.82% digestible Thr. Broilers are raised in accordance with Ross 308 broiler management guide (Aviagen 2015). Average daily food intake (ADFI), average daily weight gain (ADG), and feed conversion ratio (FCR) were recorded during the experimental phase. The feeding trial lasted for 14 D.
Data and Sample Collection
At 19.5 DOI, 6 live embryonated eggs chosen from each treatment were terminated. Egg weight (EW), embryo bodyweight (EBW, included yolk), and yolk sac weight (YSW) of each selected egg were determined, and their weight relative to set egg weight (SEW) (EW/SEW × 100, YSW/SEW × 100) was calculated as percentages. At day of hatch, the number of hatched birds and healthy chicks were recorded. Hatched chicks as a percentage of total fertile eggs (hatchability), healthy chicks as a percentage of total hatched chicks (rate of healthy chicks), and the ratio of BW to SEW were computed.
At the day of hatch, 7 D of age, and 14 D of age (d), 6 chicks from each treatment were weighted and euthanized by cervical dislocation. The duodenum, jejunum, and ileum were separated. The length of each bowel segments was measured with a soft ruler, the length of the intestine was recorded, and intestinal relative length was calculated relative to BW, intestinal relative length = intestinal length (cm)/body weight (Kg) (Shirkey et al., 2006). Approximately 1 cm and 1 g samples of the middle part of each intestinal segment were collected for histology and mRNA expression assay, respectively.
Histology Analyses
Samples of the intestine were fixed in 4% (vol/vol) buffered formaldehyde, dehydrated in ethanol and xylol alcohol, cleaned, and embedded in paraffin. Four-micrometer sections were stained with hematoxylin–eosin. Stained samples were observed under an Olympus BX43 microscope with a magnification of 400×. The VH, CD, and density of goblet cells were measured with medical image analysis software Image-Pro Plus 7.0. The VH was measured from the crypt mouth to the villus tip. The CD was measured from the base to the border between the villi and the crypt. The area of goblet cells in different intestines was determined from the length and width of the goblet cell “cup” in cross-sections of the villi, and the number of goblet cells per 100 villous mucosal columnar cells was measured.
Quantification of Nutrient Transporter mRNA With Real-Time PCR
Total RNA was extracted from duodenum and jejunum tissues with the TransZol Up Plus RNA Kit (ER501, Biohuafu Co., Ltd.) and transcribed to cDNA with TransScript II All-in-One First-Strand cDNA Synthesis SuperMix for qPCR (AH341, Biohuafu Co., Ltd.). Real-time PCR was carried out by using a LightCycler 96Roche Real-time PCR Instrument with TransStart Top Green qPCR SuperMix (AQ131, TransGen Biotech Co., Ltd.). The program used was as follows: 95°C for 10 min; 0 cycles of 94°C for 10 s, 60°C for 10 s, and 72°C for 10 s. The primers used for the real-time PCR were synthesized by Tianyi Huiyuan (Tianyi Huiyuan Biotechnology Co., Ltd., Beijing, China). Gene expression was measured by relative expression with GAPDH serving as an endogenous control. The average value of 3 replicates of each sample were used for PCR analysis. The relative mRNA expression levels were calculated with the 2−ΔΔCt method (Livak and Schmittgen, 2001). Primer sequences are shown in Table 1.
Table 1.
Primer sequence of target and reference genes.
| Gene | Function | Forward primer (5′-3′) | Reverse primer (3′-5′) | GenBank number | Length (bp) |
|---|---|---|---|---|---|
| B0AT | Na+-dependent neutral amino acid transporter | TGCGTAGGGTTTTGTGTTGG | AACTCCAGACTCCCACACTG | XM_419056 | 184 |
| Pept1 | Dipeptide and tripeptide transporter | ACACGTTTGTTGCTCTGTGC | GACTGCCTGCCCAATTGTAT | NM_204365 | 122 |
| EAAT3 | Neutral amino acid transporter | ACCCTTTTGCCTTGGAAACT | TTGAGATGTTTGCGTGAAG | XM_424930 | 122 |
| rBAT | Cationic and zwitterionic amino acid transporter | CTACCAGGTCTACCCTCGTTC | TTCCCATAGACACTCACCCA | XM_426125 | 414 |
| GAPDH | Housekeeping gene | ATCCGGACCCTCCATTGTC | AGCCATGCCAATCTCGTCTT | NM_205518 | 120 |
Abbreviations: B0AT, solute carrier family 6, member 19 (SLC6A19); GAPDH, glyceraldehyde-3-phosphate dehydrogenase; Pept1, peptide transporter-1 (SLC15A1); EAAT3, excitatory amino acid transporter 3 (SLC1A1); rBAT, solute carrier family 3, member1 (SLC3A1).
Statistical Analyses
Data were analyzed by one-way ANOVA using SAS, version 9.4 (SAS Institute, 2013), and differences were considered statistically significant at P ≤ 0.05. Duncan's multiple range test was used to detect significant differences between individual means when the treatment effect (in ovo injection) was significant. The results were expressed as mean and SEM.
Results
Embryonic Characteristics at 19.5 DOI and Hatching Characteristics
The data of the incubation performance and the various embryonic characteristics are shown in Table 2. The SEW did not differ within the 3 treatments. In ovo feeding of NAG had no effects on EW, EBW, YSW, YSW/EBW, hatchability, and rate of healthy chicks at hatch (P > 0.05).
Table 2.
Effects of in ovo feeding of N-acetyl-L-glutamate (NAG) on embryonic characteristics at 19.5 D of incubation and hatchability of broilers.1
| Variables | Treatment groups |
SEM5 | P-value | ||
|---|---|---|---|---|---|
| NC2 | SC3 | NAG4 | |||
| SEW6(g) | 59.46 | 59.22 | 59.15 | 0.07 | 0.132 |
| 17.5 DOI EW7(g) | 53.87 | 55.96 | 53.67 | 0.53 | 0.149 |
| 19.5 DOI EW8 (g) | 53.21 | 55.57 | 53.03 | 0.59 | 0.150 |
| EBW9 (g) | 43.68 | 45.93 | 43.91 | 0.55 | 0.194 |
| YSW10 (g) | 9.09 | 10.73 | 9.29 | 0.31 | 0.056 |
| YSW/EBW (%) | 20.85 | 23.38 | 21.14 | 0.57 | 0.148 |
| Hatchability (%) | 84.62 | 84.62 | 80.34 | 1.65 | 0.502 |
| Rate of healthy chicks (%) | 97.64 | 94.34 | 92.62 | 1.19 | 0.225 |
| BW11/SEW (%) | 71.20 | 69.83 | 70.17 | 0.28 | 0.162 |
N = 6, one embryo from per replicate (39 eggs).
NC: non-punctured control group.
SC: saline-injected control group (eggs injected with 300 μL of saline water at 17.5 D of incubation).
NAG: NAG solution–injected group (eggs injected with 300 μL of saline water containing NAG 1.5 mg/egg at 17.5 D of incubation).
SEM: standard error of the mean.
SEW: set egg weight.
17.5 DOI WE: egg weight at 17.5 DOI.
19.5 DOI EW: egg weight at 19.5 DOI.
EBW: embryo body weight at 19.5 DOI.
YSW: yolk sac weight at 19.5 DOI.
BW: average body weight of hatchlings.
Growth Performance
Table 3 shows the initial weight (IW), final weight (FW), ADG, ADFI, and FCR of broilers during 1–14 D post-hatch. Between the treatment groups, no significant treatment effects were observed on the IW, FW, and ADG of broilers in the phase of 1–14 D. The ADFI in the NC group was significantly higher (P < 0.05) than that of the NAG group. Compared with the NC group, in ovo supplementation with NAG significantly improved the FCR of broilers (P < 0.05).
Table 3.
Effects of in ovo feeding of N-acetyl-L-glutamate (NAG) on growth performance of 14-day-old broilers.1
| Variables | Treatment groups |
SEM5 | P-value | ||
|---|---|---|---|---|---|
| NC2 | SC3 | NAG4 | |||
| IW (g)6 | 42.15 | 40.87 | 41.47 | 0.34 | 0.299 |
| FW (g)7 | 408.01 | 412.77 | 390.57 | 8.09 | 0.564 |
| ADG (g) | 24.98 | 24.35 | 23.03 | 0.52 | 0.352 |
| ADFI (g) | 31.28a | 29.44a,b | 27.18b | 0.70 | 0.048 |
| FCR (g/g)8 | 1.253a | 1.210a,b | 1.181b | 0.011 | 0.013 |
a–bMeans within a row without common superscript differ significantly (P < 0.05).
N = 6, 12 birds in one replicate.
NC: non-punctured control group.
SC: saline-injected control group (eggs injected with 300 μL of saline water at 17.5 D of incubation).
NAG: NAG solution–injected group (eggs injected with 300 μL of saline water containing NAG 1.5 mg/egg at 17.5 D of incubation).
SEM: standard error of the mean.
IW: the initial weight of hatchlings at hatch.
FW: the weight of 14-day-age broilers.
FCR: feed conversion ratio.
Intestinal Length
The intestinal length and relative length of the intestine of 14-day-age broilers are shown in Table 4. No statistical differences were observed on the intestinal length and relative length of the intestine between the treatment groups. The length of the intestine increased from the duodenum to the ileum and then the jejunum in our experiment.
Table 4.
Effects of in ovo feeding of N-acetyl-L-glutamate (NAG) on intestinal length and relative length of the intestine of broilers at 14 D post-hatch.1
| Item | Variables | Treatment groups |
SEM5 | P-value | ||
|---|---|---|---|---|---|---|
| NC2 | SC3 | NAG4 | ||||
| Intestinal length (cm) | Duodenum | 18.60 | 18.70 | 17.50 | 0.36 | 0.382 |
| Jejunum | 41.25 | 38.00 | 37.25 | 0.85 | 0.163 | |
| Ileum | 39.00 | 35.00 | 34.50 | 0.92 | 0.154 | |
| Relative length of the intestine6 (cm/kg) | Duodenum | 45.12 | 46.04 | 44.19 | 1.28 | 0.856 |
| Jejunum | 99.93 | 93.38 | 93.45 | 1.87 | 0.314 | |
| Ileum | 91.73 | 85.93 | 86.65 | 2.12 | 0.584 | |
N = 6, one chick from each replicate (11 chicks).
NC: non-punctured control group.
SC: saline-injected control group (eggs injected with 300 μL of saline water at 17.5 D of incubation).
NAG: NAG solution–injected group (eggs injected with 300 μL of saline water containing NAG 1.5 mg/egg at 17.5 D of incubation).
SEM: standard error of the mean.
Relative length of the intestine = intestinal length (cm)/the body weight (kg).
Intestinal Morphology
The VH, CD, and VH/CD in the small intestine of broilers at hatch, 7 D, and 14 D are presented in Table 5, Table 6, Table 7. At hatch, a significantly smaller CD was observed in the jejunum of birds supplemented with NAG compared with the NC group (P < 0.05). No significant difference was observed in other items (Table 4).
Table 5.
Effects of in ovo feeding of N-acetyl-L-glutamate (NAG) on the intestinal morphology of broilers at hatch.1
| Item | Variables | Treatment groups |
SEM5 | P-value | ||
|---|---|---|---|---|---|---|
| NC2 | SC3 | NAG4 | ||||
| Duodenum | VH6 (μm) | 568.72 | 555.40 | 552.55 | 19.09 | 0.941 |
| CD7 (μm) | 95.17 | 97.38 | 101.12 | 2.63 | 0.673 | |
| VH/CD8 | 6.00 | 5.73 | 5.49 | 0.19 | 0.580 | |
| Jejunum | VH6 (μm) | 409.58 | 423.25 | 388.20 | 21.56 | 0.828 |
| CD7 (μm) | 88.17a | 76.27b | 74.96b | 2.34 | 0.025 | |
| VH/CD8 | 4.61 | 5.54 | 5.18 | 0.24 | 0.270 | |
| Ileum | VH6 (μm) | 250.48 | 246.68 | 234.30 | 4.31 | 0.364 |
| CD7 (μm) | 58.35 | 58.68 | 58.40 | 1.88 | 0.998 | |
| VH/CD8 | 4.38 | 4.23 | 4.01 | 0.15 | 0.650 | |
a–bMeans within a row without common superscript differ significantly (P < 0.05) among the groups.
N = 6, one chickling from each replicate (38 fertile eggs).
NC: non-punctured control group.
SC: saline-injected control group (eggs injected with 300 μL of saline water at 17.5 D of incubation).
NAG: NAG solution–injected group (eggs injected with 300 μL of saline water containing NAG 1.5 mg/egg at 17.5 D of incubation).
SEM: standard error of the mean.
VH: villus height.
CD: crypt depth.
VH/CD = villus height (μm)/crypt depth (μm).
Table 6.
Effects of in ovo feeding of N-acetyl-L-glutamate (NAG) on intestinal morphology of broilers at 7 D post-hatch.1
| Item | Variables | Treatment groups |
SEM5 | P-value | ||
|---|---|---|---|---|---|---|
| NC2 | SC3 | NAG4 | ||||
| Duodenum | VH6 (μm) | 873.14b | 985.54b | 1,031.58a | 26.54 | 0.029 |
| CD7 (μm) | 169.70 | 184.20 | 165.88 | 4.82 | 0.283 | |
| VH/CD8 | 5.18b | 5.37b | 6.25a | 0.17 | 0.008 | |
| Jejunum | VH6 (μm) | 569.67b | 563.02b | 631.98a | 12.40 | 0.021 |
| CD7 (μm) | 147.75 | 165.32 | 145.73 | 3.97 | 0.060 | |
| VH/CD8 | 3.90a,b | 3.43b | 4.35a | 0.15 | 0.023 | |
| Ileum | VH6 (μm) | 578.66a | 489.62b | 595.92a | 17.19 | 0.012 |
| CD7 (μm) | 142.50 | 136.38 | 153.34 | 3.11 | 0.067 | |
| VH/CD8 | 4.07 | 3.90 | 3.73 | 0.09 | 0.309 | |
a–bMeans within a row without common superscript differ significantly (P < 0.05) among the groups.
N = 6, one chick was selected from each replicate.
NC: non-punctured control group.
SC: saline-injected control group (eggs injected with 300 μL of saline water at 17.5 D of incubation).
NAG: NAG solution–injected group (eggs injected with 300 μL of saline water containing NAG 1.5 mg/egg at 17.5 D of incubation).
SEM: standard error of the mean.
VH: villus height.
CD: crypt depth.
VH/CD = villus height (μm)/crypt depth (μm).
Table 7.
Effects of in ovo feeding of N-acetyl-L-glutamate (NAG) on intestinal morphology of broilers at 14 D post-hatch.1
| Item | Variables | Treatment groups |
SEM5 | P-value | ||
|---|---|---|---|---|---|---|
| NC2 | SC3 | NAG4 | ||||
| Duodenum | VH6 (μm) | 1,411.08 | 1,330.58 | 1,392.30 | 37.68 | 0.706 |
| CD7 (μm) | 242.25 | 238.56 | 257.80 | 3.74 | 0.052 | |
| VH/CD8 | 5.83 | 5.61 | 5.41 | 0.19 | 0.698 | |
| Jejunum | VH6 (μm) | 900.15 | 1,016.82 | 909.27 | 28.08 | 0.172 |
| CD7 (μm) | 207.87 | 193.48 | 195.32 | 4.70 | 0.421 | |
| VH/CD8 | 4.37 | 5.28 | 4.68 | 0.18 | 0.106 | |
| Ileum | VH6 (μm) | 617.30 | 686.90 | 706.18 | 22.66 | 0.254 |
| CD7 (μm) | 193.75 | 167.37 | 167.30 | 7.57 | 0.273 | |
| VH/CD8 | 3.34 | 4.11 | 4.25 | 0.19 | 0.095 | |
a–bMeans within a row without common superscript differ significantly (P < 0.05) among the groups.
N = 6, one chick was selected from each replicate.
NC: non-punctured control group.
SC: saline-injected control group (eggs injected with 300 μL of saline water at 17.5 D of incubation).
NAG: NAG solution–injected group (eggs injected with 300 μL of saline water containing NAG 1.5 mg/egg at 17.5 D of incubation).
SEM: standard error of the mean.
VH: villus height.
CD: crypt depth.
VH/CD = villus height (μm)/crypt depth (μm).
At 7 D of age, greater VH in the duodenum and jejunum was observed in chicks hatched from the NAG injection group compared with the NC or SC group (P < 0.05). In ovo feeding of NAG significantly increased VH/CD in the duodenum of broilers at 7 D compared with other groups (P < 0.05). In the NAG group, VH/CD in the jejunum of chickens was higher than that in the SC group (P < 0.05), which was similar to the NC group (P > 0.05). No effect was observed on other variables between the treatment groups (Table 6). N-acetyl-L-glutamate supplementation in ovo had no effect on intestinal morphology of 14-day-age broilers (Table 7).
Goblet Cell Density
The effects of IOF of NAG on goblet cell density in the intestine are listed in Table 8. At hatch, the density of goblet cells in the jejunum significantly increased (P < 0.05) in broiler chickens from NAG group when compared with the NC group although no significant difference was observed on the density of goblet cells between the NAG group and the SC group. At 7 D, IOF of NAG increased goblet cell density in the duodenum compared with the NC group (P < 0.05). At 14 D, the density of goblet cells in the ileum was higher significantly in the NAG group than in the NC group (P < 0.05).
Table 8.
Effects of in ovo feeding of N-acetyl-L-glutamate (NAG) on goblet cell density (%) in the intestine of broilers at hatch, 7 D, and 14 D.1
| Variables | Treatment groups |
SEM5 | P-value | ||
|---|---|---|---|---|---|
| NC2 | SC3 | NAG4 | |||
| At hatch | |||||
| Duodenum | 20.6 | 22.1 | 21.4 | 0.009 | 0.801 |
| Jejunum | 12.0b | 12.1a,b | 15.6a | 0.007 | 0.027 |
| Ileum | 13.3 | 13.3 | 12.3 | 0.005 | 0.623 |
| At 7 D | |||||
| Duodenum | 21.6b | 26a,b | 29.2a | 0.012 | 0.028 |
| Jejunum | 20.8a | 15.6b | 21.8a | 0.009 | 0.001 |
| Ileum | 16.2 | 14 | 14.6 | 0.005 | 0.173 |
| At 14 D | |||||
| Duodenum | 32 | 27.8 | 29.6 | 0.012 | 0.373 |
| Jejunum | 20.9 | 23.9 | 24.1 | 0.008 | 0.154 |
| Ileum | 14.6b | 18a,b | 22.4a | 0.011 | 0.005 |
a–bMeans within a row without common superscript differ significantly (P < 0.05) among the groups.
N = 6, one chick was selected from each replicate.
NC: non-punctured control group.
SC: saline-injected control group (eggs injected with 300 μL of saline water at 17.5 D of incubation).
NAG: NAG solution–injected group (eggs injected with 300 μL of saline water containing NAG 1.5 mg/egg at 17.5 D of incubation).
SEM: standard error of the mean.
mRNA Expression of Nutrient Transporters in the Jejunum and Duodenum
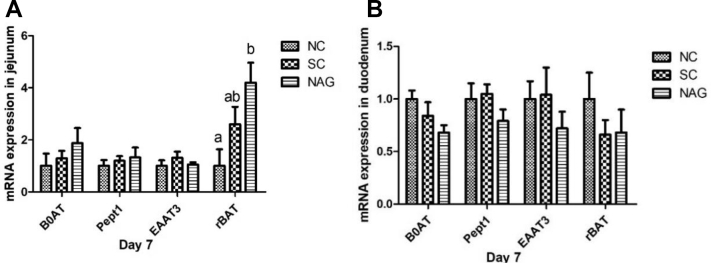
The mRNA expression levels of nutrient transporters in the jejunum and duodenum at 7 and 14 D are shown in Figure 1 and Table 9. The results showed that compared with other groups, IOF of NAG significantly increased (P < 0.05) the mRNA expression level of rBAT in the jejunum only at 7 D but not at 14 D. No significant differences were observed in the relative mRNA expression level of Na+-dependent neutral amino acid transporter (B0AT), di- and tri-peptide transporters 1 (Pept1), and excitatory amino acid transporter 3 (EAAT3) in the jejunum and duodenum (P > 0.05).
Figure 1.
Effects of in ovo feeding of N-acetyl-L-glutamate (NAG) on relative mRNA expression of nutrient transporters in the jejunum (A) and duodenum (B) of broilers at 7 D post-hatch. The mRNA expression levels were determined by quantitative real-time PCR and calculated relative to the GAPDH gene. Data are presented with means ± SEM (standard error) of 6 replicates. NC = non-punctured control group; SC = saline-injected control group (eggs injected with 300 μL of saline water at 17.5 D of incubation); NAG = NAG solution–injected group (eggs injected with 300 μL of saline water containing NAG 1.5 mg/egg at 17.5 D of incubation); B0AT = solute carrier family 6, member 19 (SLC6A19); Pept1 = peptide transporter-1 (SLC15A1); EAAT3 = excitatory amino acid transporter 3 (SLC1A1); rBAT = solute carrier family 3, member1 (SLC3A1).Values within the same gene without common superscript (a-b) differed significantly (P < 0.05).
Table 9.
Effects of in ovo feeding of N-acetyl-L-glutamate (NAG) on the mRNA expression of nutrient transporters of broilers at 14 D post-hatch.1
| Item | Variables | Treatment groups |
SEM5 | P-value | ||
|---|---|---|---|---|---|---|
| NC2 | SC3 | NAG4 | ||||
| Jejunum | B0AT | 1.00 | 2.39 | 3.66 | 0.55 | 0.191 |
| Pept1 | 1.00 | 2.69 | 2.09 | 0.34 | 0.577 | |
| EAAT3 | 1.00 | 3.50 | 2.09 | 0.97 | 0.082 | |
| rBAT | 1.00 | 2.06 | 1.42 | 0.37 | 0.482 | |
| Duodenum | B0AT | 1.00 | 0.93 | 0.60 | 0.14 | 0.554 |
| Pept1 | 1.00 | 0.82 | 0.97 | 0.13 | 0.254 | |
| EAAT3 | 1.00 | 0.80 | 0.65 | 0.08 | 0.835 | |
| rBAT | 1.00 | 0.27 | 0.47 | 0.16 | 0.154 | |
Abbreviations: B0AT, solute carrier family 6, member 19 (SLC6A19); Pept1, peptide transporter-1 (SLC15A1); EAAT3, excitatory amino acid transporter 3 (SLC1A1); rBAT, solute carrier family 3, member1 (SLC3A1).
N = 6, one chick was selected from each replicate.
NC: non-punctured control group.
SC: saline-injected control group (eggs injected with 300 μL of saline water at 17.5 D of incubation).
NAG: NAG solution–injected group (eggs injected with 300 μL of saline water containing NAG 1.5 mg/egg at 17.5 D of incubation).
SEM: standard error of the mean.
Discussion
Embryonic Growth and Growth Performance
The nutrition in eggs is the only nutrient source for the development of chicken embryos. Nutrition deficiency eventually occurs at the last phase of incubation (Filho et al., 2019), which may restrict embryonic growth and reduce the hatchability of chicken eggs (Shafey et al., 2012). During the development of avian embryos, the yolk is the first primary nutrition source and the sole energy supply (Romanoff, 1960). Thus, embryo development is dependent on the utilization of yolk nutrition (Ohta et al., 1999). In our study, the similar yolk sac weight among groups suggested that IOF of solution containing NAG or saline did not affect the absorption or utilization of yolk during the late phase of incubation. The results of embryonic characteristics examined at 19.5 DOI indicated that all embryos, including those injected with solution, grew normally. Similar results were observed in a previous study with L-ascorbic acid injection (Zhang et al., 2018a). Greater synthesis and lower degradation of proteins were observed in embryos injected with nutrition (Ohta and Kidd, 2001). Thus, nutritional supplementation by in ovo administration is beneficial to increase the BW of fowls during hatching (Al-Murrani, 1982, Tangara et al., 2010, Gaafar et al., 2013). Chicks with greater BW during hatching are believed to have an innate advantage in growth. This theory has been supported by the fact that IOF of carbohydrates and β-hydroxy-β-methyl-butyrate increased the BW of chicks during hatching, and the weight of chickens in the treatment group was always higher than that in the control group (Uni et al., 2005). Supplementation with Arg in ovo affected post-hatch growth of broilers in the pattern described previously, and the superior BW lasted until the fourth week (Nayak et al., 2016).
In our study, although no differences of BW at hatch were noticed, IOF of NAG improved feed efficiency at 1–14 D by decreasing feed intake compared with the NC group. Accumulating studies show that in ovo supplementation with nutrients not only increases BW of birds but also improves feed efficiency of chicks. The chicks that consumed the amniotic fluid with the feeding solution during incubation may have advantage in terms of nutrient utilization post-hatch (Uni and Ferket, 2004). The results in the present study are consistent with an early study on Arg, which showed that IOF of Arg significantly improved the feed efficiency of chickens at days 1–7 without affecting the hatching weight of broilers (Gao et al., 2017b). Feed efficiency improvement was also observed in intrauterine growth restriction suckling lamb fed diet containing NCG, a functional analogue of NAG (Zhang et al., 2018b). In addition, IOF of Arg (Saki et al., 2013) and other critical AAs (Bhanja and Mandal, 2005) had been reported to ameliorate the feed efficiency. These observations demonstrated that IOF of functional AAs has the potential to beneficially affect the post-hatch growth performance.
Intestinal Morphology, Goblet Cell Density, and Nutrient Transporters
The small intestine plays an important role in nutrition absorption, digestion, and assimilation because the greatest digestion and absorption occur in this organ (Nazem et al., 2017). Early intestinal development is vital to maximize the growth potential of chicks (Cheled-Shoval et al., 2011). Therefore, any improvement of early intestinal morphology and functional capacity is beneficial for broiler growth performance (Cheled-Shoval et al., 2011). For example, IOF of Arg increased the weight of jejunum and lipase activity in the jejunum of 7-day-age broilers, and the ADG of chickens from Arg in ovo group was significantly increased during days 1–21 (Gao et al., 2017b). In ovo supplementation with Thr positively affects VH, CD, and VH/CD in the jejunum of broilers at hatch and improves the growth performance of chickens during days 1–7, 7–14, and 1–21 (Filho et al., 2019). In the present study, although IOF of NAG did not affect the length of the intestine, it positively affected intestinal morphology, the density of goblet cells, and mRNA expression level of the nutrient transporters.
The first response in the small intestine to NAG supplementation was the increased density of goblet cells in the jejunum at hatch. The long-term effect of in ovo NAG supplementation on goblet cell density in the small intestine lasted for 14 D. Increased goblet cell density was beneficial for intestinal immunity and nutrient absorption function because goblet cells can secrete chicken-lactose-lectin and produce many neutral and acidic mucin proteins. Chicken-lactose-lectin, which is present in the embryo muscle and adult intestine (Beyer and Barondes, 1982), regulates lymphocyte proliferation (Lipsick et al., 1980). In addition, neutral and acidic mucin proteins secreted by goblet cells are an important component of the intestinal mucus layer (Smirnov et al., 2006). As a part of the innate host response system, the mucus layer protects the epithelial cells from harmful constituents in the enterocoel, plays a role in nutrient absorption, and prevents gastrointestinal pathologies (Forstner et al., 1995). Functional development of chicken goblet cells occurs in the late period of incubation and immediate post-hatch period (Smirnov et al., 2006), and any events that occur at an early age have a significant impact on intestine function later (Van Zijderveld et al., 1992). Because the amniotic fluid is swallowed by the embryo in the vital period of intestinal development, intraamniotic nutrient supply increases goblet cell density (Cheled-Shoval et al., 2011, Nazem et al., 2017). In agreement with our results, increased intestinal density of goblet cells had been observed in pigs fed with NCG-supplemented diet (Wu et al., 2010). In addition, the results showed that the goblet cell density in the duodenum increased most rapidly from hatch to 14 D in our study.
The second response of the intestine to IOF of NAG was the improvement of intestinal morphology characterized by the lower CD in the jejunum at hatch and higher VH in the duodenum and jejunum at 7 D post-hatch. Normal morphology of the intestine is vital for nutrient digestion and absorption (Yao et al., 2012). Therefore, increased VH is indicative of better growth in broilers. The increase in VH indicates an increase in the number of epithelial cells, which is beneficial to the growth of broilers (Geyra et al., 2001a, Geyra et al., 2001b). Furthermore, increased VH, a sign of accelerated protein synthesis, indicates the accelerated proliferation rate of intestinal epithelial cells, which is beneficial for intestinal development. Unquestionably, the increase in VH increases the area of the intestinal tract for digesting nutrition, and a study showed that structures with higher villus and lower crypt are more suitable for digestion and absorption (Pluske et al., 1996). The VH increases at 17 DOI during incubation. During the last period of incubation, energy consumption is large, and the nutrient content drops significantly (De Oliveira et al., 2008), which may limit the development of the intestinal tract of embryos. Moreover, during this period, in ovo supplementation with nutrients contributes to intestinal development. The increased VH induced by in ovo NAG supplementation may be associated with Arg metabolism. Dietary supplementation of NCG, a metabolic analogue of NAG, can increase VH in pigs (Wu et al., 2010), which could be explained by the theory that NCG promotes the synthesis of Arg via stimulating the conversion of glutamine and proline into citrulline in the small intestine (Wu et al., 2008). In addition, improved VH was also reported by the in ovo administration of Arg in broilers (Gao et al., 2017b, Gao et al., 2017c). Therefore, in ovo NAG supplementation may stimulate intestinal development through Arg. However, more investigations are needed to support this theory. In the present study, in ovo NAG administration did not induce changes of intestinal length of broilers. The intestinal length of broilers increased from the duodenum to the ileum and then jejunum in our study, which is consistent with a previous study (Saki et al., 2011).
Nutrient transporters in the intestinal mucosa play a major role in nutrient absorption (Smirnov et al., 2006). In the breeding of broiler chickens, the cost of daily food is the highest, especially protein (Miska and Fetterer, 2016). Protein is broken down to small peptides and AAs in the small intestine. Then, dipeptide and tripeptide are transported by enterocytes via Pept1 or are catabolized to AA by aminopeptidase N. Free AA is transported into enterocytes via several AA transporters located in the brush border membrane with varying specificities for different AAs (Miska et al., 2014). B0AT transports neutral AA into the enterocyte, and EAAT3 is an important anionic AA transporter with affinity for glutamate (Hundal and Taylor, 2009), which is the main fuel source of intestinal epithelial cells (Wu, 1998). rBAT, the heavy chain of dibasic and neutral AA transporters (Li et al., 2008), composes heterodimer with b0,+AT to transfer cationic and zwitterionic AAs into the enterocyte (Broer, 2008). To investigate the effects of IOF of NAG on the ability of broilers to absorb protein in the small intestine, we determined the mRNA expression level of transporters.
During the incubation, chicken embryos consume nutrients from a lipid-rich yolk; thus, the expression levels of intestinal brush border membrane-bound nutrient transporters are low (Li et al., 2008). In the late phase of incubation, the expression of intestinal nutrient transporters enables chick embryos to extract nutrients from amniotic fluid (Uni and Ferket, 2004). After hatch, broilers absorb nutrients from a carbohydrate- and protein-rich diet. To accommodate the shift in food types, chickens adjust their metabolic machinery to access nutrition better. The mRNA expression of Pept1, EAAT3, and rBAT increases from 18 DOI to 14 D of age (Gilbert et al., 2007), which indicates that these transporters are important for chickens to adjust to diet. Our results showed that in ovo supplementation with NAG significantly increased the mRNA expression level of rBAT in the jejunum at 7 D post-hatch, which indicates that in ovo NAG supplementation helped broilers to be prepared to adjust to the change of nutrient supply. In our study, the mRNA expression level of transporters associated with nutrient absorption in the NAG group was upregulated, which may contribute to the accelerating maturation of intestinal mucosa (Li et al., 2008). In addition, the increased expression of nutrient transporter protein seems to be accompanied by changes in intestinal morphology, which is more conducive to the absorption of nutrients. Elevated expression of nutrient transporters, better nutrition-absorbing intestinal structures, and increased goblet cell density were helpful for broilers to improve nutrient absorption efficiency, adjust to feed earlier, increase the area to absorb enough nutrients, and boost immunity to protect individuals from pathogens, enabling broilers to absorb nutrients from feed more efficiently. The aforementioned effects of IOF of NAG contributed to the improvement of feed efficiency during day 1 to 14.
Nutritional modulation-induced improvement of the growth performance is often accompanied by the improvement of intestinal function in broilers (Boroojeni et al., 2019, Kazemi et al., 2019). However, whether nutrition regulation first improves intestinal function and then affects the growth performance of broilers remains to be elucidated. In the present study, the effects of IOF of NAG on broilers were first reflected in the improvement of intestinal morphology and function and then in the growth performance. The influence model of NAG on broilers was similar to that of plant extracts observed in an early study, in which dietary plant extracts did not affect the growth performance in the first 2 wk but increased the BW at 28 D and the feed efficiency at 15 to 42 D by increasing the abundance of firmicutes in intestinal microbiota from 14 D, as evidenced by the decomposition of polysaccharides and the production of butyrate (Zhu et al., 2019). These results demonstrated that the morphological or functional changes induced by dietary exposure usually occur before the alteration of growth, which would need a period of time to manifest. However, NAG administration in ovo improved the feed efficiency by increasing nutrient transporter expression and ameliorating the intestinal morphology. In addition, another reason for the improvement of feed efficiency may be related to the embryonic metabolism or chick quality. Chick quality and post-hatch performance may be affected by the embryo eggshell temperature (Joseph et al., 2006, Hulet et al., 2007, Zhai et al., 2011, Pulikanti et al., 2012, Ipek et al., 2014). It is possible that NAG can modulate the embryo metabolism and heat production, which may positively affect growth. The effects of IOF of NAG on embryo shell temperature change deserve further investigation.
In conclusion, the amniotic injection of NAG at 17.5 DOI (1.5 mg/egg) increased the density of goblet cells, promoted intestinal development, and improved intestinal immune and absorption function, therefore improving the feed efficiency in the first 2 wk post-hatch. The optimal time, site, and dosage of IOF of NAG warrant further study.
Acknowledgments
This work was financially supported by the National Key R&D program of China (2018YFD0500403-4), Beijing Innovation Consortium of Agriculture Research System Poultry-related Science and Technology Team, and the Agricultural Science and Technology Innovation Program, China (ASTIP).
Conflict of Interest Statement: The authors did not provide a conflict of interest statement.
Contributor Information
Haijun Zhang, Email: zhanghaijun@caas.cn.
Zhigang Song, Email: naposong@qq.com.
References
- Al-Murrani W.K. Maternal effects on embryonic and post-embryonic growth in poultry. Br. Poult. Sci. 1978;19:277–281. doi: 10.1080/00071667808416476. [DOI] [PubMed] [Google Scholar]
- Al-Murrani W.K. Effect of injecting amino acids into the egg on embryonic and subsequent growth in the domestic fowl. Br. Poult. Sci. 1982;23:171–174. doi: 10.1080/00071688208447943. [DOI] [PubMed] [Google Scholar]
- Aviagen . 2015. Ross 308 Broiler Management Pocket Guide. Aviagen Ltd., Newbridge, UK. Accessed May 2019.http://en.aviagen.com/assets/Tech_Center/BB_Resources_Tools/Pocket_Guides/Ross-Broiler-Pocket-Guide-2015-EN.pdf [Google Scholar]
- Beyer E.C., Barondes S.H. Secretion of endogenous lectin by chicken intestinal goblet cells. J. Cell Biol. 1982;92:28–33. doi: 10.1083/jcb.92.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhanja S.K., Mandal A.B. Effect of in ovo Injection of critical amino acids on pre- and post-hatch growth, immunocompetence and development of digestive organs in broiler chickens. Asian-australas. J. Anim. Sci. 2005;18:524–531. [Google Scholar]
- Boroojeni F.G., Manner K., Rieger J., Calvo E.P., Zentek J. Evaluation of a microbial muramidase supplementation on growth performance, apparent ileal digestibility, and intestinal histology of broiler chickens. Poult. Sci. 2019;98:2080–2086. doi: 10.3382/ps/pey556. [DOI] [PubMed] [Google Scholar]
- Broer S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol. Rev. 2008;88:249–286. doi: 10.1152/physrev.00018.2006. [DOI] [PubMed] [Google Scholar]
- Cheled-Shoval S.L., Amit-Romach E., Barbakov M., Uni Z. The effect of in ovo administration of mannan oligosaccharide on small intestine development during the pre- and posthatch periods in chickens. Poult. Sci. 2011;90:2301–2310. doi: 10.3382/ps.2011-01488. [DOI] [PubMed] [Google Scholar]
- De Oliveira J.E., Uni Z., Ferket P.R. Important metabolic pathways in poultry embryos prior to hatch. Worlds Poult. Sci. J. 2008;64:488–499. [Google Scholar]
- Forstner J.F., Oliver M.G., Sylvester F.A. Raven Press; New York, NY: 1995. Production, structure and biologic relevance of gastrointestinal mucins. Pages 71-88 in Infections of the Gastrointestinal Tract. R. L. Guerrant, ed. [Google Scholar]
- Gaafar K.M., Selim S.A., Elballal S.S. Effect of in-ovo administration with two levels of amino acids mixture on the performance of Muscovy ducks. Emir. J. Food Agric. 2013;25:58–65. [Google Scholar]
- Gao T., Zhao M., Zhang L., Li J., Yu L., Lv P., Gao F., Zhou G. Effects of in ovo feeding of l-arginine on the development of lymphoid organs and small intestinal immune barrier function in posthatch broilers. Anim. Feed Sci. Tech. 2017;225:8–19. [Google Scholar]
- Gao T., Zhao M., Zhang L., Li J., Yu L., Lv P., Gao F., Zhou G. Effect of in ovo feeding of l-arginine on the hatchability, growth performance, gastrointestinal hormones, and jejunal digestive and absorptive capacity of posthatch broilers. J. Anim. Sci. 2017;95:3079–3092. doi: 10.2527/jas.2016.0465. [DOI] [PubMed] [Google Scholar]
- Gao T., Zhao M.M., Li Y.J., Zhang L., Li J.L., Yu L.L., Gao F., Zhou G.H. Effects of in ovo feeding of L-arginine on the development of digestive organs, intestinal function and post-hatch performance of broiler embryos and hatchlings. J. Anim. Physiol. Anim. Nutr. 2017;102:e166–e175. doi: 10.1111/jpn.12724. [DOI] [PubMed] [Google Scholar]
- Geyra A., Uni Z., Sklan D. The effect of fasting at different ages on growth and tissue dynamics in the small intestine of the young chick. Br. J. Nutr. 2001;86:53–61. doi: 10.1079/bjn2001368. [DOI] [PubMed] [Google Scholar]
- Geyra A., Uni Z., Sklan D. Enterocyte dynamics and mucosal development in the posthatch chick. Poult. Sci. 2001;80:776–782. doi: 10.1093/ps/80.6.776. [DOI] [PubMed] [Google Scholar]
- Gilbert E.R., Li H., Emmerson D.A., Webb K.E., Wong E.A. Developmental regulation of nutrient transporter and enzyme mRNA abundance in the small intestine of broilers. Poult. Sci. 2007;86:1739–1753. doi: 10.1093/ps/86.8.1739. [DOI] [PubMed] [Google Scholar]
- Hulet R., Gladys G., Hill D., Meijerhof R., El-Shiekh T. Influence of egg shell embryonic incubation temperature and broiler breeder flock age on posthatch growth performance and carcass characteristics. Poult. Sci. 2007;86:408–412. doi: 10.1093/ps/86.2.408. [DOI] [PubMed] [Google Scholar]
- Hundal H.S., Taylor P.M. Amino acid transceptors: gate keepers of nutrient exchange and regulators of nutrient signaling. AM. J. Physiol-endoc M. 2009;296:e603–e613. doi: 10.1152/ajpendo.91002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huycke T.R., Tabin C.J. Chick midgut morphogenesis. Int. J. Dev. Biol. 2018;62:109–119. doi: 10.1387/ijdb.170325ct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ipek A., Sahan U., Baycan S.C., Sozcu A. The effects of different eggshell temperatures on embryonic development, hatchability, chick quality, and first-week broiler performance. Poult. Sci. 2014;93:464–472. doi: 10.3382/ps.2013-03336. [DOI] [PubMed] [Google Scholar]
- Joseph N.S., Lourens A., Moran E.T., Jr. The effects of suboptimal eggshell temperature during incubation on broiler chick quality, live performance, and further processing yield. Poult. Sci. 2006;85:932–938. doi: 10.1093/ps/85.5.932. [DOI] [PubMed] [Google Scholar]
- Kadam M.M., Barekatain M.R., Bhanja S K., Iji P.A. Prospects of in ovo feeding and nutrient supplementation for poultry: the science and commercial applications—a review. J. SCI. FOOD AGR. 2013;93:3654–3661. doi: 10.1002/jsfa.6301. [DOI] [PubMed] [Google Scholar]
- Kazemi S.A., Ahmadi H., Torshizi M.A.K. Evaluating two multistrain probiotics on growth performance, intestinal morphology, lipid oxidation and ileal microflora in chickens. J. Anim. Physiol. Anim. Nutr. 2019;00:1–9. doi: 10.1111/jpn.13124. [DOI] [PubMed] [Google Scholar]
- Li H., Gilbert E.R., Zhang Y., Crasta O., Emmerson D.A., Webb K.E., Wong E.A. Expression profiling of the solute carrier gene family in chicken intestine from the late embryonic to early post-hatch stages. Anim. Genet. 2008;39:407–424. doi: 10.1111/j.1365-2052.2008.01744.x. [DOI] [PubMed] [Google Scholar]
- Lipsick J.S., Beyer E.C., Barondes S.H., Kaplan N.O. Lectins from chicken tissues are mitogenic for Thy-1 negative murine spleen cells. Biochem. Biophys. Res. Commun. 1980;97:56–61. doi: 10.1016/s0006-291x(80)80133-1. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Miska K.B., Fetterer R.H., Wong E.A. The mRNA expression of amino acid transporters, aminopeptidase N, and the di-and tri-peptide transporter PepT1 in the embryo of the domesticated chicken (Gallus gallus) shows developmental regulation. Poult. Sci. 2014;93:2262–2270. doi: 10.3382/ps.2014-03983. [DOI] [PubMed] [Google Scholar]
- Miska K.B., Fetterer R.H. The mRNA expression of amino acid and sugar transporters, aminopeptidase, as well as the di- and tri-peptide transporter PepT1 in the intestines of Eimeria infected broiler chickens. Poult. Sci. 2016;96:465–473. doi: 10.3382/ps/pew303. [DOI] [PubMed] [Google Scholar]
- Moreira Filho A.L.B., Ferket P.R., Malheiros R.D., Oliveira C.J.B., Aristimunha P.C., Wilsmann D.E., Givisiez P.E.N. Enrichment of the amnion with threonine in chicken embryos affects the small intestine development, ileal gene expression and performance of broilers between 1 and 21 days of age. Poult. Sci. 2019;98:1363–1370. doi: 10.3382/ps/pey461. [DOI] [PubMed] [Google Scholar]
- Nayak N., Rajini R.A., Ezhilvalavan S., Sahu A.R., Kirubaharan J.J. Influence of in-ovo arginine feeding on post-hatch growth performance and Economics of broilers. Indian J. Anim. Res. 2016;6:585–591. [Google Scholar]
- Nazem M.N., Sajjadian S.M., Kheirandish R., Mohammadrezaei H. Histomorphometric analysis of the small intestine of broiler chick embryos injected in ovo with methionine. Anim. Prod. Sci. 2017;59:133–139. [Google Scholar]
- National Research Council . 9th rev. Natl. Acad. Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Ohta Y., Kidd M.T. Optimum site for in ovo amino acid injection in broiler breeder eggs. Poult. Sci. 2001;80:1425. doi: 10.1093/ps/80.10.1425. [DOI] [PubMed] [Google Scholar]
- Ohta Y., Tsushima N., Koide K., Kidd M.T., Ishibashi T. Effect of amino acid injection in broiler breeder eggs on embryonic growth and hatchability of chicks. Poult. Sci. 1999;78:1493–1498. doi: 10.1093/ps/78.11.1493. [DOI] [PubMed] [Google Scholar]
- Pulikanti R., Peebles E.D., Zhai W., Bennett L.W., Gerard P.D. Physiological relationships of the early posthatch performance of broilers to their embryo and eggshell characteristics. Poult. Sci. 2012;91:1552–1557. doi: 10.3382/ps.2011-02065. [DOI] [PubMed] [Google Scholar]
- Pluske J.R., Thompson M.J., Atwood C.S., Bird P.H., Williams I.H., Hartmann P.E. Maintenance of villus height and crypt depth, and enhancement of disaccharide digestion and monosaccharide absorption, in piglets fed on cows' whole milk after weaning. Br. J. Nutr. 1996;76:409–422. doi: 10.1079/bjn19960046. [DOI] [PubMed] [Google Scholar]
- Romanoff A.L. The avian embryo. Structural and functional development. Avian Dis. 1960;4:541–542. [Google Scholar]
- Saki A., Haghighat M., Khajali F. Supplemental arginine administered in ovo or in the feed reduces the susceptibility of broilers to pulmonary hypertension syndrome. Br. Poult. Sci. 2013;54:575–580. doi: 10.1080/00071668.2013.811716. [DOI] [PubMed] [Google Scholar]
- Saki A.A., Matin H.R.H., Zamani P., Tabatabai M.M., Vatanchian M. Various ratios of pectin to cellulose affect intestinal morphology, DNA quantitation, and performance of broiler chickens. Livest. Sci. 2011;139:237–244. [Google Scholar]
- Shafey T.M., Alodan M.A., Alruqaie I.M., Abouheif M.A. In ovo feeding of carbohydrates and incubated at a high incubation temperature on hatchability and glycogen status of chicks. South Afr. J. Anim. Sci. 2012;42:210–220. [Google Scholar]
- Shirkey T.W., Siggers R.H., Goldade B.G., Marshall J.K., Drew M.D., Laarveld B.L., Van Kessel A.G. Effects of commensal bacteria on intestinal morphology and expression of proinflammatory cytokines in the gnotobiotic pig. Exp. Biol. Med. 2006;231:1333–1345. doi: 10.1177/153537020623100807. [DOI] [PubMed] [Google Scholar]
- Smirnov A., Tako E., Ferket P.R., Uni Z. Mucin gene expression and mucin content in the chicken intestinal goblet cells are affected by in ovo feeding of carbohydrates. Poult. Sci. 2006;85:669. doi: 10.1093/ps/85.4.669. [DOI] [PubMed] [Google Scholar]
- Tangara M., Chen W., Xu J., Huang F., Peng J. Effects of in ovo feeding of carbohydrates and arginine on hatchability, body weight, energy metabolism and perinatal growth in duck embryos and neonates. Br. Poult. Sci. 2010;51:602–608. doi: 10.1080/00071668.2010.520303. [DOI] [PubMed] [Google Scholar]
- Uni Z., Ferket P.R., Tako E., Kedar O. In ovo feeding improves energy status of late-term chicken embryos. Poult. Sci. 2005;84:764–770. doi: 10.1093/ps/84.5.764. [DOI] [PubMed] [Google Scholar]
- Uni Z., Ferket R.P. Methods for early nutrition and their potential. Worlds Poult. Sci. J. 2004;60:101–111. [Google Scholar]
- Willemsen H., Debonne M., Swennen Q., Everaert N., Careghi C., Han H., Bruggeman V., Tona K., Decuypere E. Delay in feed access and spread of hatch: importance of early nutrition. Worlds Poult. Sci. J. 2010;66:177–188. [Google Scholar]
- Wu G., Knabe D.A., Sung Woo K. Arginine nutrition in neonatal pigs. J. Nutr. 2004;134:2783S–2790S. doi: 10.1093/jn/134.10.2783S. [DOI] [PubMed] [Google Scholar]
- Wu G. Intestinal mucosal amino acid catabolism. J. Nutr. 1998;128:1249–1252. doi: 10.1093/jn/128.8.1249. [DOI] [PubMed] [Google Scholar]
- Wu G., Bazer F.W., Davis T.A., Jaeger L.A., Johnson G.A., Kim S.W., Knabe D.A., Meininger C.J., Spencer T.E., Yin Y.L. Important roles for the arginine family of amino acids in swine nutrition and production. Livest. Sci. 2007;112:8–22. [Google Scholar]
- Wu X., Zheng R., Gao Y., Yin Y., Zhou X., Wang L., Geng M., Hou Y., Wu G. Dietary supplementation with l -arginine or N-carbamylglutamate enhances intestinal growth and heat shock protein-70 expression in weanling pigs fed a corn- and soybean meal-based diet. Amino Acids. 2010;39:831–839. doi: 10.1007/s00726-010-0538-y. [DOI] [PubMed] [Google Scholar]
- Yang H.S., Fu D.Z., Kong X.F., Wang W.C., Yang X.J., Nyachoti C.M., Yin Y.L. Dietary supplementation with N-carbamylglutamate increases the expression of intestinal amino acid transporters in weaned Huanjiang mini-pig piglets. J. Anim. Sci. 2013;91:2740–2748. doi: 10.2527/jas.2012-5795. [DOI] [PubMed] [Google Scholar]
- Yao K., Xi P., Wang J., Lei J., Hou Y., Wu G. Alpha-ketoglutarate inhibits glutamine degradation and enhances protein synthesis in intestinal porcine epithelial cells. Amino Acids. 2012;42:2491–2500. doi: 10.1007/s00726-011-1060-6. [DOI] [PubMed] [Google Scholar]
- Zhai W., Gerard P.D., Pulikanti R., Peebles E.D. Effects of in ovo injection of carbohydrates on embryonic metabolism, hatchability, and subsequent somatic characteristics of broiler hatchlings. Poult. Sci. 2011;90:2134–2143. doi: 10.3382/ps.2011-01418. [DOI] [PubMed] [Google Scholar]
- Zhang H., Kec E., Durojaye O.A., Fatemi S.A., Peebles E.D. Effects of in ovo administration of L-ascorbic acid on broiler hatchability and its influence on the effects of pre-placement holding time on broiler quality characteristics. Poult. Sci. 2018;97:1941–1947. doi: 10.3382/ps/pey040. [DOI] [PubMed] [Google Scholar]
- Zhang H., Zhao F., Peng A., Dong L., Wang M., Yu L., Loor J.J., Wang H. Effects of dietary l-arginine and N-carbamylglutamate supplementation on intestinal Integrity, immune function, and Oxidative status in intrauterine-growth-Retarded suckling lambs. J. Agric. Food Chem. 2018;66:4145–4154. doi: 10.1021/acs.jafc.8b00726. [DOI] [PubMed] [Google Scholar]
- Zhu N., Wang J., Yu L., Zhang Q., Chen K., Liu B. Modulation of growth performance and intestinal microbiota in chickens fed plant extracts or Virginiamycin. Front. Microbiol. 2019;10:1333. doi: 10.3389/fmicb.2019.01333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Zijderveld F.G., Bemmel Z.V., Anakotta J. Comparison of four different enzyme-linked immunosorbent assays for serological diagnosis of Salmonella enteritidis infections in experimentally infected chickens. J. Clin. Microbiol. 1992;30:2560–2566. doi: 10.1128/jcm.30.10.2560-2566.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]