Abstract
This study aimed to elucidate the mechanism by which adding Saccharomyces cerevisiae–derived yeast fermentate to the feed (XPC) or drinking water reduces stress in poultry. Day-old male Cobb 500 broiler chicks were assigned to 1 of 3 treatments: stressed control (CS), stressed + XPC (1.25 kg/metric ton feed, day 0–43; XPC), or stressed + AviCare (160 mL/100 L drinking water, day 0–43; AVI). All birds were spray-vaccinated for coccidiosis (day 0), raised on reused litter, spray-vaccinated for Newcastle/Bronchitis (day 18), and exposed to heat stress (32°C–34°C) and feed/water withdrawal for 12 h (day 18). Blood samples were collected to assess plasma corticosterone (CORT) and heterophil/lymphocyte (H/L) ratio (60 birds/treatment; day 40); plasma biochemistry and growth hormone (12 birds/treatment; day 38); and serum serotonin and plasma prolactin, thyroid hormones, antioxidant capacity, and selected cytokines (12 birds/treatment; day 39). Composite asymmetry scores were obtained from 60 birds/treatment on day 41. Organs were collected from 20 birds/treatment on day 43 to measure gene expression of CYP1A2 and melanocortin 2 receptor (MC2R) in the adrenal glands and IL10 and AvBD1 in the spleen. Serotonin was lower in CS than XPC (P = 0.049), whereas AVI was intermediate. Plasma interleukin (IL)-1β was higher in AVI than CS (P = 0.009) and XPC (P = 0.009). The CS treatment had higher CORT than AVI (P = 0.013) and XPC (P = 0.037) and higher H/L ratios than AVI (P = 0.026) and XPC (P = 0.034). Expression of CYP1A2, MC2R, and IL10 was lower (P < 0.05) in XPC and AVI compared with CS. Furthermore, IL10 expression was lower in XPC than AVI (P < 0.05). Adding yeast fermentate to the feed or drinking water reduced measures of stress and MC2R gene expression in birds exposed to acute and rearing stressors. However, differences in IL10 gene expression and circulating serotonin and IL-1β suggest that supplementing yeast fermentate in the feed is slightly more effective than supplementation via the drinking water in mitigating the physiological effects associated with the stress response in broilers.
Key words: stress, broilers, yeast fermentate, gene expression, cytokines
Introduction
Poultry are exposed to a variety of stressors associated with modern rearing practices, including vaccination, handling, heat stress, and feed withdrawal. Whether these stressors occur singly or simultaneously, they have the potential to stimulate the stress response and negatively affect bird growth and well-being. The stress response begins when the hypothalamic-pituitary-adrenal axis is stimulated to release adrenocorticotropic hormone (ACTH), which then binds to the melanocortin 2 receptor (MC2R) in the adrenal cortex and signals the production of the stress hormone corticosterone (CORT) (Mormède et al., 2007, Virden and Kidd, 2009). Corticosterone regulates the immune response and shifts metabolic processes toward catabolism to increase readily available energy (Beard and Mitchell, 1987, Mormède et al., 2007). However, chronic stimulation of the hypothalamic-pituitary-adrenal axis can slow growth rate and suppress the immune response, thereby increasing susceptibility to infection (McFarlane et al., 1989, Bessei, 2006, Burkholder et al., 2008). Reducing environmental stressors is therefore an important goal in broiler production.
Previous research has demonstrated a variety of beneficial outcomes to feeding Saccharomyces cerevisiae–derived yeast fermentation products to livestock, including improving reproductive performance in multiparous sows (Kim et al., 2010). When added to the formula, yeast fermentate improved weight gain and intestinal development and reduced symptoms of Salmonella enterica infection in dairy calves (Brewer et al., 2014). In poultry, dietary yeast fermentate has been shown to improve growth performance as well as increase antibody production after vaccination (Cortés-Coronado et al., 2016) and reduce the severity and incidence of intestinal lesions in ducks (Labib et al., 2014) and laying hens (Lensing et al., 2012). There is also evidence that adding yeast fermentate to either the feed or drinking water improves small intestine histomorphology in Pekin ducks exposed to cyclic heat stress (Nelson and Archer, 2019). In addition, yeast fermentate has been shown to reduce measures of short-term and long-term stress in heat-stressed turkeys (Bartz, 2016) and broiler chickens (Price et al., 2018), and in broilers exposed to acute or rearing stress (Nelson et al., 2018). It has been proposed that the metabolites in yeast fermentation products may help balance the immune and stress responses (Firman et al., 2013). Yeast fermentation metabolites may also improve antioxidant status by acting as reductants toward free radical (Jensen et al., 2008).
However, there is insufficient research on the effects of adding yeast fermentate to the feed or drinking water on other physiological parameters in broilers exposed to acute and chronic stress. Furthermore, the mode of action by which yeast fermentate reduces measures of stress is poorly understood. Therefore, the objective of this study was to elucidate the mechanism of action by which supplementing yeast fermentate in the feed or drinking water reduces measures of stress in broilers exposed to acute or chronic stressors.
Materials and methods
Animal Husbandry
All procedures were carried out in accordance with the guidelines established by Texas A&M Institutional Animal Care and Use Committee (AUP #2016-0004). There were 3 treatments: stressed and un-supplemented control (CS), stressed and supplemented with AviCare (Diamond V Mills, Cedar Rapids, IA; 160 mL/100 L drinking water, day 0–43; AVI), and stressed and supplemented with Original XPC (Diamond V Mills; 1.25 kg/metric ton feed, day 0–43; XPC). Birds were housed at the Texas A&M University Poultry Science Teaching, Research, and Extension Center. Pens were assigned to a given treatment using a randomized complete block design. On day 0, 25 male day-of-hatch Cobb 500 broilers were placed in each pen (n = 300 per treatment). Pens measured 0.91 × 1.83 m and were lined with 4 to 6 cm of re-used pine shavings. Building temperature was maintained at 31°C on day 0 to 7, reduced to 29°C on day 8 to 14, and then allowed to decrease 2.8°C each week until ambient temperature was reached. Birds were provided 24 h of light day 0 to 3 and 20 h of light followed by 4 h of darkness day 4 to 43.
One tube feeder and 1 waterer consisting of a 18.93 L bucket with 4 nipples on the bottom were hung in each pen, and their heights were adjusted as birds grew. Feed and water were provided ad libitum except during the 12 h acute stress challenge on day 18. Birds received standard broiler diets which were mixed at the Texas A&M Poultry Research Center Feed Mill. Birds were fed a crumbled starter diet day 0 to 18, a pelleted grower diet day 19 to 27, and a pelleted finisher diet day 28 to 42. Birds in the AVI treatment received fresh AviCare from a stock solution daily; all other treatments received fresh water without yeast fermentate as needed.
Stress Challenges
All birds were raised on re-used litter and were spray-vaccinated for coccidiosis (COCCIVAC-B52, Merck, Kenilworth, NJ) on day 0. On day 18, all treatments were spray-vaccinated for Newcastle/Bronchitis (COMBOVAC-30, Merck) and then exposed to a 12 h period of heat stress and feed/water withdrawal (20:00 on day 18–08:00 on day 19). Wire barriers were placed in each pen to crowd birds and produce enough collective body heat to increase litter temperatures to 32°C to 34°C. Building lights remained on to mimic daylight conditions.
Stress Measures
On day 38, 12 birds per treatment received an injection of ACTH in the thigh muscle; after 1 h, blood was collected via wing vein venipuncture and stored in a heparin vacutainer (367884, BD Medical, Franklin Lakes, NJ) for analysis of plasma CORT. Blood was collected again on day 40 from a separate group of 5 birds per pen (n = 60 per treatment) and analyzed for CORT. A drop of blood from each sample was also used to make a blood smear to determine heterophil/lymphocyte (H/L) ratio. Vacutainers were stored in an ice bath, whereas remaining blood samples were collected and then centrifuged (Centrifuge 5804, Eppendorf, Hamburg, Germany) at 4000 RPM for 15 min. The plasma layer was then poured off into a labeled 2 mL microcentrifuge tube and stored at −20°C until analysis. Samples were thawed at 4°C overnight before analysis. Plasma CORT concentration was obtained using a 96-well commercial ELISA kit (ADI-901-097, Enzo Life Sciences, Inc., Farmingdale, NY); absorbance was read at 450 nm using a microplate absorbance reader (Tecan Sunrise, Tecan Trading AG, Switzerland) and analyzed using the Magellan Tracker software program. Dry blood smear slides were stained with a neat stain hematology stain kit (Cat. #25034, Poly Sciences, Inc., Warrington, PA). Heterophil/lymphocyte ratio was determined by individually counting heterophils and lymphocytes up to a total of 100 cells per slide at 40x magnification using an oil immersion lens under microscopy (89,404-886, VWR International, Radnor, PA).
On day 41, physical asymmetry measurements were collected from 5 birds per pen (n = 60 per treatment). Middle toe length (MTL), metatarsal length (ML), and metatarsal width (MW) in millimeters were obtained on the left and right legs using Craftsman IP54 digital calipers (Sears Holdings, Hoffman Estates, IL). A composite asymmetry score (ASYM) for each bird was calculated using the following formula: (|L-R|MTL + |L-R|ML + |L-R|MW) ÷ 3.
Additional Blood Parameters
On day 38, blood was collected from 12 birds per treatment and separated into 2 heparin vacutainers. Half of the sample from each bird was sent to Texas A&M Veterinary Medical Diagnostic Laboratory for a plasma chemistry panel with electrolytes, which measured the following components: total protein, glucose, alkaline phosphatase, creatine kinase activity (CK), aspartate aminotransferase, uric acid, cholesterol, calcium, phosphorus, sodium, chloride, and sodium/potassium ratio. The other half of the sample was used to analyze growth hormone (GH; Advanced BioChemicals, Lawrenceville, GA). On day 39, blood samples from 12 birds per treatment were collected to determine serum serotonin. Building lights were turned off and noise kept to a minimum so as to mitigate increased serotonin secretion due to handling stress. Samples were stored in clot-activator serum separation vacutainers (367981, BD Medical), allowed to clot for 24 h, and then spun down at 4000 RPM for 15 min. Serum was then transferred to microcentrifuge tubes and stored at −20°C until analysis using a commercial ELISA kit (900-175, Enzo Life Sciences).
On day 39, blood samples were collected to determine the plasma level of various enzymes and hormones from 12 birds per treatment using individual ELISA kits. These included prolactin (PRL; Ch1686, Advanced BioChemicals, Lawrenceville, GA), total triiodothyronine (T3; 74C5DC, Genway Biotech, Inc., San Diego, CA), total thyroxine (T4; 511F19, Genway Biotech Inc.), CK (MAK116, Sigma-Aldrich, St. Louis, MO), superoxide dismutase activity (SOD; EIASODC, ThermoFisher Scientific, Waltham, MA), and ferric-reducing antioxidant power (FRAP; EIAFECL2, ThermoFisher Scientific). In addition, samples were assayed for the cytokines interleukin (IL)-1α (Ch1767, Advanced BioChemicals), IL-1β (Ch0539, Advanced BioChemicals), IL-2 (Ch0120, Advanced BioChemicals), IL-6 (Ch0228, Advanced BioChemicals), IL-10 (Ch1621, Advanced BioChemicals), IL-12 (Ch1651, Advanced BioChemicals), and tumor necrosis factor-α (TNF-α; Ch0215, Advanced BioChemicals).
Blood samples used to determine FRAP were stored in spray-coated heparin vacutainers (367884, BD Medical); those used to determine IL-1α, IL-1β, IL-2, IL-6, IL-10, IL-12, TNF-α, T3, T4, SOD, growth hormone, PRL, and CK were stored in spray-coated EDTA vacutainers (367861, BD Medical). All whole blood samples were centrifuged at 4000 RPM for 15 min. Plasma was then transferred to a microcentrifuge tube and stored at −20°C until analysis. Samples assigned to SOD assay had a slightly different procedure; after the initial centrifugation, the red blood cell pellet was isolated and re-suspended in 5x volume of ice-cold distilled water. The sample was centrifuged at 10,000 RPM for 10 min, and the resulting supernatant was transferred to a microcentrifuge tube and stored at −20°C until analysis. All samples were thawed overnight at 4°C before assay.
Organ Collection and Gene Expression Analysis
On day 43, 20 birds from each treatment were humanely euthanized, and the spleen and adrenal glands were collected and stored in 15 mL centrifuge tubes with 5 to 10 volumes RNAlater (AM7020, ThermoFisher Scientific). Samples were kept at −20°C until further processing, at which time samples were removed from the RNAlater, and individual spleens were sliced into smaller segments. Tissue samples were then transferred to microcentrifuge tubes and stored at −80°C. Next, batches of 20 samples were thawed at 4°C overnight. We isolated total RNA from sections of spleen tissue using the TRIzol Reagent method (ThermoFisher Scientific), and samples were quantified on a Nanodrop Spectrophotometer (ThermoFisher Scientific). The quality of the RNA isolates was checked using the Agilent Bioanalyzer 2100 (Agilent, Santa Clara, CA) with the RNA 6000 Nano Kit following the manufacturer's protocol.
We only retained samples with an RNA Integrity Number above 7, and poor quality samples were reextracted until we met this threshold. We performed reverse transcription reactions using the SuperScript VILO Master Mix (ThermoFisher Scientific). We used pooled cDNA samples as templates for primer testing. We designed the primers using the NCBI Primer-BLAST online tool. We set the amplicon size to between 200 and 300 bp, and only primers spanning exon-junctions were included and synthesized for assays (Integrated DNA Technologies, Coralville, IA). Primers were tested on a pooled cDNA sample using the PowerUP SYBR Green Master Mix (ThermoFisher Scientific) as recommended by the manufacturer. Primer pairs with 90 to 110% efficiency were retained, and dissociation curves (evidence of amplification) were inspected.
The RT-qPCR reactions were performed using the PowerUP SYBR Green Master Mix protocol. We assayed expression of CYP1A2 and MC2R in adrenal tissues and IL-10 and DEF1β expression in the spleen. The gene phosphoglycerate kinase 1 was used as the control locus. The expression levels of the 4 target genes and the control (housekeeping gene) were determined by amplification reactions performed on the ABI 7900 HT (Applied Biosystems, Foster City, CA) real-time PCR system. For each locus, we assayed expression in triplicate reaction tubes, and the results were used for plotting and statistical analysis using the ΔΔCT method.
Statistical Analysis
Stress and blood parameters were analyzed using one-way ANOVA in Minitab 17.1.0 with treatment as the main effect and pen as a random effect, and mean separation was performed using Fisher's LSD. Gene expression data were analyzed using one-way ANOVA followed by Tukey's HSD in R (SAS Institute). A significant difference was defined as P < 0.05.
For the qPCR data, we calculated the log2 fold change of expression for each target gene using the ΔΔCT method (Livak and Schmittgen, 2001) in Microsoft Excel. We also tested for statistical significance of gene expression among experimental groups using a one-way ANOVA, using the R statistical platform (R Core Team, 2019), with the treatments as the independent variable and the ΔΔCT values as the dependent variable. A significant difference was defined as P < 0.05.
Results
Stress Measures
Data for basal (serum serotonin and CORT) and chronic (H/L ratio and ASYM) stress measures are shown in Table 1. Treatment had an effect on plasma CORT after 42 D of growth (P = 0.029). Corticosterone was lower in both AVI (P = 0.013) and XPC (P = 0.037) than in CS birds. There was no difference among treatments in CORT production 1 h after ACTH injection (P = 0.957). XPC birds had higher serum serotonin than CS birds (P = 0.049). As for measures of chronic stress, H/L ratios were lower (P = 0.044) in both AVI (P = 0.026) and XPC (P = 0.034) than in CS birds. Composite asymmetry was not affected by treatment (P = 0.302).
Table 1.
Stress Measures and Blood Data After 42 D of Growth in Male Broiler Chickens.
| Measure | Units | CS | AVI1 | XPC2 | Pooled SEM |
|---|---|---|---|---|---|
| Adrenal exhaustion test | |||||
| CORT after ACTH injection | pg/mL | 25,896.94 | 24,677.8 | 25,151.42 | 2,899.48 |
| Acute stress | |||||
| CORT | pg/mL | 1,807.68a | 1,156.58b | 1,273.2b | 172.15 |
| Serotonin | ng/mL | 8,512.31a | 9,668.62a,b | 11,392.95b | 983.42 |
| Chronic stress | |||||
| H/L Ratio | 0.68a | 0.5b | 0.51b | 0.053 | |
| ASYM | 1.44 | 1.13 | 1.05 | 0.17 | |
| Plasma chemistry | |||||
| Phosphorus | mg/dL | 6.72a | 6.21b | 6.55a,b | 0.14 |
| Total Protein | g/dL | 3.47 | 3.42 | 3.35 | 0.14 |
| Calcium | mg/dL | 12.24 | 10.18 | 9.88 | 0.94 |
| Glucose | mg/dL | 268.67 | 262.92 | 270.17 | 5.65 |
| Uric Acid | mg/dL | 5.37 | 5.9 | 5.81 | 0.46 |
| Cholesterol | mg/dL | 136.42 | 139.25 | 138.55 | 5.01 |
| Potassium | mEq/L | 7.13 | 6.29 | 7.06 | 0.44 |
| Sodium | mEq/L | 150.58 | 152.83 | 151.17 | 0.95 |
| Chloride | mEq/L | 112.17 | 113.83 | 113.17 | 1.09 |
| Na/K Ratio | 21.92 | 24.68 | 22.82 | 1.21 | |
| Growth and metabolism | |||||
| T3 | ng/mL | 0.79 | 0.79 | 0.78 | 0.003 |
| T4 | μg/mL | 0.01 | 0.01 | 0.01 | 0 |
| GH | ng/mL | 0.63 | 0.71 | 1.02 | 0.2 |
| Prolactin | ng/mL | 52.98 | 67.74 | 59.09 | 6.55 |
| ALP | U/L | 2,641.92a,b | 3,526.08a | 2,243.83b | 386.9 |
| AST | U/L | 479 | 498.5 | 426.27 | 50.02 |
| Antioxidative capability | |||||
| CK | U/L | 31,657.83 | 30,196.33 | 32,346 | 2,918.74 |
| FRAP | μM Fe2+ | 76.38 | 74.1 | 77.42 | 4.3 |
| SOD | Inhibition rate (%) | 45.44 | 49.24 | 54.7 | 4.28 |
| Cytokine production | |||||
| IL-1α | pg/mL | 174.25 | 211.08 | 179.09 | 25.42 |
| IL-1β | pg/mL | 974.87a | 1,142.01b | 975.58a | 40.38 |
| IL-2 | pg/mL | 88.19 | 54.27 | 94.31 | 17.71 |
| IL-6 | pg/mL | 60.96 | 120.05 | 63.5 | 20.65 |
| IL-10 | pg/mL | 66.53 | 61.7 | 53.33 | 14.61 |
| IL-12 | pg/mL | 78.61 | 87.07 | 190.23 | 31.76 |
| TNF-α | pg/mL | 214.41 | 187.04 | 158.95 | 22.81 |
a,bValues with different superscripts within the same row indicate a significant difference at P < 0.05.
Abbreviations: ACTH, adrenocorticotropic hormone; ALP, alkaline phosphatase; AST, aspartate aminotransferase; ASYM, asymmetry score; CK, creatine kinase activity; CORT, corticosterone; CS, stressed control; FRAP, ferric-reducing antioxidant power; GH, growth hormone; H/L, heterophil/lymphocyte; IL, interleukin; Na/K, sodium/potassium ratio; SOD, superoxide dismutase activity; T3, total triiodothyronine; T4, total thyroxine; TNF-α, tumor necrosis factor-α.
Received XPC in the feed (1.25 kg/metric ton) day 0 to 42.
Received AviCare in the drinking water (160 mL/100L) day 0 to 42.
Plasma Chemistry and Electrolytes
Data for the plasma chemistry panel with electrolytes are shown in Table 1. Phosphorus was higher (P = 0.062) in CS birds than AVI (P = 0.021). Alkaline phosphatase was higher (P = 0.075) in XPC than AVI (P = 0.027). Treatment had no effect on plasma levels of total protein (P = 0.845), aspartate aminotransferase (P = 0.612), calcium (P = 0.478), glucose (P = 0.636), uric acid (P = 0.720), cholesterol (P = 0.918), potassium (P = 0.384), sodium (P = 0.251), chloride (P = 0.620), or sodium/potassium ratio (P = 0.287).
Metabolism and Growth
Data for indicators of metabolism and growth are shown in Table 1. Treatment had no effect on T3 (P = 0.635), T4 (P = 0.609), growth hormone (P = 0.407), or PRL (P = 0.347).
Antioxidative Capability
Data for markers of antioxidative capability are shown in Table 1. When measured in the plasma chemistry panel, CK was not affected by treatment (P = 0.873); this agreed with results from the independent ELISA (P = 0.699). There were no differences among treatments in FRAP (P = 0.872) or SOD (P = 0.322).
Cytokines
Data for cytokines are shown in Table 1. Treatment had an effect on plasma levels of IL-1β (P = 0.011). IL-1 β was higher in AVI than both CS (P = 0.009) and XPC (P = 0.009). However, there were no differences among treatments in plasma concentrations of IL-1α (P = 0.563), IL-2 (P = 0.299), IL-6 (P = 0.353), IL-10 (P = 0.817), IL-12 (P = 0.230), or TNF- α (P = 0.344).
Gene Expression
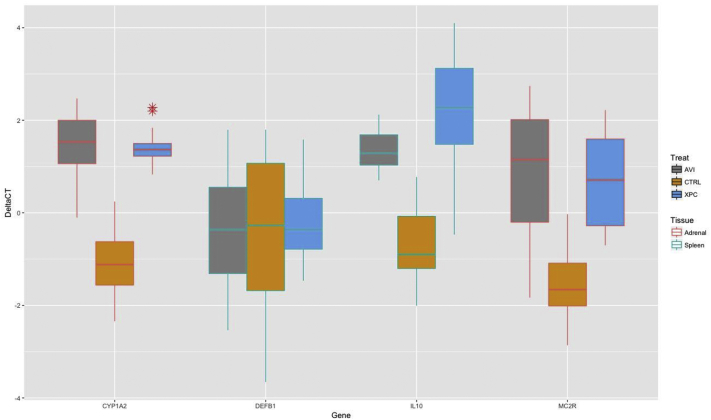
Data for ΔΔCT of gene expression of each treatment compared with the housekeeping gene is shown in Figure 1. Expression of CYP1A2 differed between treatments (P < 0.001) and was lower in XPC (P < 0.01) and AVI (P < 0.01) compared with CS. CYP1A2 expression did not differ between AVI and XPC (P > 0.05). MC2R expression also differed by treatment (P < 0.001) and was lower in XPC (P < 0.01) and AVI (P < 0.01) but did not differ between AVI and XPC (P > 0.05). Treatment differences were observed for IL10 (P < 0.001), wherein XPC (P < 0.01) and AVI (P < 0.01) were lower than CS, and XPC was lower (P < 0.05) than AVI. Treatments did not differ in expression of DEFB1 (P = 0.71).
Figure 1.
Expression values (ΔΔCT) of CYP1A2 and MC2R in the adrenal gland and IL-10 and DEFB1 in the spleen of broiler chickens after 43 D of growth. Higher (more positive) values represent lower abundance of gene expression.
Discussion
The objective of this study was to determine how adding yeast fermentate to the feed (XPC) or drinking water (AVI) would affect measures of stress, growth, immune function, and antioxidant capacity in broilers exposed to acute and chronic stressors over a 42 D rearing period. Moreover, this research aimed to clarify the mechanism by which yeast fermentate has been shown to reduce measures of stress in poultry exposed to acute and chronic stress. Primary findings suggest that both modes of administration reduce measures of acute and chronic stress by reducing gene expression of the ACTH receptor on adrenocortical cells, whereas supplementing yeast fermentate in the feed rather than in the drinking water may be more effective in modulating measures of immune activity.
To confirm that broilers supplemented with yeast fermentate retained their capacity to produce CORT in response to stress, a subset of birds from each treatment were injected with ACTH, and blood samples were collected 1 h postinjection. Indeed, results showed that there were no treatment differences in plasma CORT 1 h after ACTH injection, indicating that adrenal exhaustion was not of concern. After 38 D of growth, however, both XPC and AVI had lower plasma CORT compared with CS. Both XPC and AVI also had lower H/L ratios than the CS treatment. This suggests that dietary inclusion of yeast fermentate reduces measures of both short- and long-term stress. Similar results have also been reported in heat-stressed broilers (Price et al., 2018). Previous studies reported lower ASYM in broilers supplemented with yeast fermentate in the feed or drinking water and exposed to many of the same stressors used in this experiment (Nelson et al., 2018). However, in this study there were no treatment differences in ASYM. Plasma CORT and H/L ratio may be more reliable measures of stress than bilateral asymmetry.
Alkaline phosphatase is involved in bone mineralization, where its primary role is to catalyze the hydrolysis of monophosphate esters at high pH (Weiss et al., 1986, Golub and Boesze-Battaglia, 2007). Its activity may increase in states of calcium and vitamin D deficiency and bone degradation induced by parathyroid hormone and CORT (Hurwitz and Griminger, 1961). Plasma CORT was lower in both XPC and AVI than CS. However, alkaline phosphatase was higher in AVI than XPC, and phosphorus was higher in AVI than CS. Adding yeast fermentate to the drinking water may have affected bone deposition and phosphorus metabolism differently than including yeast fermentate in the feed.
The DEFB1 gene encodes avian β-defensin 1, an antimicrobial peptide which induces microbial cell lysis by disrupting cell membrane integrity (Pan and Yu, 2014). Probiotic administration (Akbari et al., 2008) and necrotic enteritis (Hong et al., 2012) have been shown to reduce DEFB1 expression in the spleen. On the other hand, proinflammatory cytokines such as IL-1β are known to up-regulate β-defensin expression (Hong et al., 2012). Birds supplemented with yeast fermentate in the feed had lower plasma IL-1β and IL10 gene expression than birds in the CS treatment. Alternatively, IL-10 has been shown to reduce inflammation by downregulating IL-1β (Plunkett et al., 2001). In this study, expression of IL-10 was lower in XPC and AVI compared with CS; furthermore, IL10 gene expression was lower in XPC than in AVI. Although plasma IL-10 did not differ between treatments, XPC had numerically lower IL-10 values than both CS and AVI. This differs from previous research showing that XPC increased IL-10 gene expression in 28-day-old broilers (Chou et al., 2017). Additionally, plasma concentrations of IL-1β were higher in AVI than XPC. Therefore, XPC may be more effective than AVI in regulating the immune response during exposure to normal rearing stressors. Serotonin serves as a link between the hippocampus and immune cells, thereby playing a role in regulation of both the stress response and the immune response. In this study, serum serotonin was higher in XPC than CS, whereas AVI was intermediate. Perhaps XPC consumption played a role in regulating the immune response by increasing serotonin levels.
The cytochrome P450 (CYP) enzymes are responsible for catalyzing oxidative metabolism of various drugs and steroids (Reed et al., 2007). Increased CYP1A2 gene expression has been associated with carcinogenic and mutagenic effects in the liver (Gallagher et al., 1996), as well as oxidative stress and inflammation (Hussain et al., 2014). Although measures of antioxidative capacity did not differ between treatments, adding yeast fermentate to the feed or drinking water appeared to reduce CYP1A2 expression, indicating positive effects on regulation of inflammatory processes and production of reactive oxygen species. CYP1A2 is also involved in metabolism of aflatoxin B1 to its epoxide form, a carcinogenic intermediary metabolite (Gallagher et al., 1996). Perhaps fermentation metabolite supplementation could alleviate the negative effects of mycotoxin consumption in poultry. Expression of MC2R in the adrenal glands was also reduced in both AVI and XPC compared to CS. The MC2R gene encodes the MC2R, which is expressed in the adrenal cortex and binds exclusively to ACTH, whereupon it stimulates glucocorticoid production (Gantz and Fong, 2003). Reduced plasma CORT in birds supplemented with yeast fermentate in either the feed or drinking water is therefore a direct result of reduced MC2R gene expression.
Adding yeast fermentate to either the feed or drinking water consistently reduces measures of short- and long-term stress in broiler chickens by reducing MC2R expression. However, the different routes of administration yielded variable results with regard to cytokine production and phosphorus utilization. Birds consuming XPC may have responded better to chronic stress than AVI as indicated by increased serotonin, reduced plasma IL-1β, and reduced IL10 expression. Antioxidative capacity was not significantly affected, but CYP1A2 expression was reduced with supplementation in either the feed or drinking water. In conclusion, while both routes of administration reduced susceptibility to basal and chronic stress, supplementing yeast fermentate in the feed may be more effective than supplementation via the drinking water in regulating inflammatory processes.
Acknowledgements
The authors would like to thank Mohamed Ibrahim for performing genomic analysis. They would also like to thank the undergraduate students who helped with bird care and data collection.
References
- Akbari M.R., Haghighi H.R., Chambers J.R., Brisbin J., Read L.R., Sharif S. Expression of antimicrobial peptides in cecal tonsils of chickens treated with probiotics and infected with Salmonella enterica serovar Typhimurium. Clin. Vaccin. Immunol. 2008;15:1689–1693. doi: 10.1128/CVI.00242-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz B.M. University of North Carolina; Raleigh, NC: 2016. Effects of Induced Stress on turkey Hens Supplemented with Yeast Derived Fermentation Products. Thesis. [Google Scholar]
- Beard C.W., Mitchell B.W. Influence of environmental temperatures on the serologic responses of broiler chickens to inactivated and viable Newcastle disease vaccines. Avian Dis. 1987;31:321–326. [PubMed] [Google Scholar]
- Bessei W. Welfare of broilers: a review. Worlds Poult. Sci. J. 2006;62:455–466. [Google Scholar]
- Brewer M.T., Anderson K.L., Yoon I., Scott M.F., Carlson S.A. Amelioration of salmonellosis in pre-weaned dairy calves fed Saccharomyces cerevisiae fermentation products in feed and milk replacer. Vet. Microbiol. 2014;172:248–255. doi: 10.1016/j.vetmic.2014.05.026. [DOI] [PubMed] [Google Scholar]
- Burkholder K.M., Thompson K.L., Einstein M.E., Applegate T.J., Patterson J.A. Influence of stressors on normal intestinal microbiota, intestinal morphology, and susceptibility to Salmonella Enteritidis colonization in broilers. Poult. Sci. 2008;87:1734–1741. doi: 10.3382/ps.2008-00107. [DOI] [PubMed] [Google Scholar]
- Chou W.K., Park J., Carey J.B., McIntyre D.R., Berghman L.R. Immunomodulatory effects of Saccharomyces cerevisiae fermentation product supplementation on immune gene expression and lymphocyte distribution in immune organs in broilers. Front. Vet. Sci. 2017;4:37. doi: 10.3389/fvets.2017.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortés-Coronado R.F., Gómez-Rosales S., Angeles M. de L., Casaubon-Huguenin M.T., Sørensen-Dalgaard T. Influence of a yeast fermented product on the serum levels of the manna-binding lectin and the antibodies against the Newcastle disease virus in Ross broilers. J. Appl. Poult. Res. 2016;26:38–49. [Google Scholar]
- Firman J.D., Moore D., Broomhead J., McIntyre D. Effects of dietary inclusion of a Saccharomyces cerevisiae fermentation product on performance and gut characteristics of male turkeys to market weight. Int. J. Poult. Sci. 2013;12:141–143. [Google Scholar]
- Gallagher E.P., Kunze K.L., Stapleton P.L., Eaton D.L. The kinetics of aflatoxin B1 oxidation by human cDNA-expressed and human liver microsomal cytochromes P450 1A2 and 3A4. Toxicol. Appl. Pharmacol. 1996;141:595–606. doi: 10.1006/taap.1996.0326. [DOI] [PubMed] [Google Scholar]
- Gantz I., Fong T.M. The melanocortin system. Am. J. Physiol. Endocrinol. Metab. 2003;284:E468–E474. doi: 10.1152/ajpendo.00434.2002. [DOI] [PubMed] [Google Scholar]
- Golub E.E., Boesze-Battaglia K. The role of alkaline phosphatase in mineralization. Curr. Opin. Orthop. 2007;18:444–448. [Google Scholar]
- Hong Y.H., Song W., Lee S.H., Lillehoj H.S. Differential gene expression profiles of β-defensins in the crop, intestine, and spleen using a necrotic enteritis model in 2 commercial broiler chicken lines. Poult. Sci. 2012;91:1081–1088. doi: 10.3382/ps.2011-01948. [DOI] [PubMed] [Google Scholar]
- Hurwitz S., Griminger P. The response of plasma alkaline phosphatase, parathyroids and blood and bone minerals to calcium intake in the fowl. J. Nutr. 1961;73:177–185. [Google Scholar]
- Hussain T., Al-Attas O.S., Al-Daghri N.M., Mohammed A.A., De Rosas E., Ibrahim S., Vinodson B., Ansari M.G., El-Din K.I.A. Induction of CYP1A1, CYP1A2, CYP1B1, increased oxidative stress and inflammation in the lung and liver tissues of rats exposed to incense smoke. Mol. Cell. Biochem. 2014;391:127–136. doi: 10.1007/s11010-014-1995-5. [DOI] [PubMed] [Google Scholar]
- Jensen G.S., Patterson K.M., Yoon I. Yeast culture has anti-inflammatory effects and specifically activates NK cells. Comp. Immunol. Microbiol. Infect. Dis. 2008;31:487–500. doi: 10.1016/j.cimid.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Kim S.W., Brandherm M., Newton B., Cook D.R., Yoon I., Fizner G. Effect of supplementing Saccharomyces cerevisiae fermentation product in sow diets on reproductive performance in a commercial environment. Can. J. Anim. Sci. 2010;90:229–232. [Google Scholar]
- Labib Z.M., Elsamadony H.A., El Gebaly L.S., Zoghbi A.F. Immunopathological studies on ducks experimentally infected with Duck Virus Enteritis and Salmonella Enteritidis with special references to the effect of XPC prebiotic. (Abstract) Zag. Vet. J. 2014;42:41–62. [Google Scholar]
- Lensing M., van der Klis J.D., Yoon I., Moore D.T. Efficacy of Saccharomyces cerevisiae fermentation product on intestinal health and productivity of coccidian-challenged laying hens. Poult. Sci. 2012;91:1590–1597. doi: 10.3382/ps.2011-01508. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔcT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- McFarlane J.M., Curtis S.E., Shanks R.D., Carmer S.G. Multiple concurrent stressors in chicks. 1. Effect on weight gain, feed intake, and behavior. Poult. Sci. 1989;68:501–509. doi: 10.3382/ps.0680501. [DOI] [PubMed] [Google Scholar]
- Mormède P., Andanson S., Aupérin B., Beerda B., Guémené D., Malmkvist J., Manteca X., Manteuffel G., Prunet P., van Reenen C.G., Richard S., Veissier E. Exploration of the hypothalamic-pituitary-adrenal function as a tool to evaluate animal welfare. Physiol. Behav. 2007;92:317–339. doi: 10.1016/j.physbeh.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Nelson J.R., Archer G.S. Effect of yeast fermentate supplementation on intestinal health and plasma biochemistry in heat-stressed Pekin ducks. Animals. 2019;9:790. doi: 10.3390/ani9100790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson J.R., McIntyre D.R., Pavlidis H.O., Archer G.S. Reducing stress susceptibility of broiler chickens by supplementing a yeast fermentation product in the feed or drinking water. Animals. 2018;8:173. doi: 10.3390/ani8100173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D., Yu Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes. 2014;5:108–119. doi: 10.4161/gmic.26945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plunkett J.A., Yu C.G., Easton H.A., Bethea J.R., Yezierski R.P. Effects of interleukin-10 (IL-10) on pain behavior and gene expression following excitotoxic spinal cord injury in the rat. Exp. Neurol. 2001;168:144–154. doi: 10.1006/exnr.2000.7604. [DOI] [PubMed] [Google Scholar]
- Price P.T., Byrd J.A., Alvarado C.Z., Pavlidis H.O., McIntyre D.R., Archer G.S. Utilizing original XPCTM in feed to reduce stress susceptibility of broilers. Poult. Sci. 2018;0:1–5. doi: 10.3382/ps/pex386. [DOI] [PubMed] [Google Scholar]
- Reed K.M., Mendoza K.M., Coulombe R.A., Jr. Structure and genetic mapping of the Cytochrome P450 gene (CYP1A5) in the Turkey (Meleagris gallopavo) Cytogenet. Genome Res. 2007;116:104–109. doi: 10.1159/000097426. [DOI] [PubMed] [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2019. R: A Language and Environment for Statistical Computing, version 3.0.2; p. 2013. [Google Scholar]
- Virden W.S., Kidd M.T. Physiological stress in broilers: Ramifications on nutrient digestibility and responses. J. Appl. Poult. Res. 2009;18:201. [Google Scholar]
- Weiss M.J., Henthorn P.S., Lafferty M.A., Slaughter C., Raducha M., Harris H. Isolation and characterization of a cDNA encoding a human liver/bone/kidney-type alkaline phosphatase. Proc. Natl. Acad. Sci. USA. 1986;83:7182–7186. doi: 10.1073/pnas.83.19.7182. [DOI] [PMC free article] [PubMed] [Google Scholar]