Abstract
Two experiments were conducted to evaluate the effects of functional oils containing cashew nutshell and castor oil on turkey performance and intestinal morphology. In experiment 1, 585 hatchlings were randomly placed in 15 replicate floor pens, (13 poults/pen) with recycled litter and provided feed and water ad libitum. Birds were randomly assigned to 3 dietary treatments from 1 to 12 wk: nonmedicated control, 0.15% functional oils, and 66-ppm monensin. From wk 13 to 20, each initial treatment group was further divided into 3 treatments—control (no additive), 0.15% of functional oils, or 20 ppm of virginiamycin to produce 9 different treatments, 5 replicate pens per treatment. Data on feed weights were collected weekly, and body weight bi-weekly. At termination (20 wk), birds were euthanized, and their meat was processed to determine mass of carcass sections and meat quality, while intestinal samples were collected for histology. In experiment 1, toms fed monensin or functional oils were 10.5 and 4.5% heavier (P < 0.05), respectively, than the controls at 12 wk. Birds fed monensin had a 4% improvement (P < 0.05) in feed conversion as compared to the other treatments. Neither virginiamycin nor the functional oils affected bird performance when fed from 13 to 20 wk. The jejunum villi surface area at 3 wk was most enhanced (P < 0.05) for the poults fed monensin. Supplementation with functional oils significantly reduced leg yield and thiobarbituric-acid reactive substances of white meat after 7 D of storage (P < 0.05). There were no effects on performance or carcass characteristics in experiment 2. While additional confirmatory studies are needed, functional oils in the diet of turkey toms may be a viable alternative to antibiotic growth promotants.
Key words: functional oil, growth promoter, growth performance, antioxidant, turkey
Introduction
Under current production systems, turkeys have traditionally been protected from pathogenic microorganisms by vaccination and antibiotics. For decades, subtherapeutic doses of antibiotics have commonly been used within the poultry industry to improve production performance and reduce pathogenic microbial challenges. However, negative consumer perception and increased regulatory guidance prohibiting the use of growth promoters or antibiotics within the animal production industry have led to the search for natural alternatives. In addition, it is of paramount importance to find alternative production practices and antibiotic alternatives to control pathogenic microorganisms within animal food production systems because of the problem of antibiotic resistance.
A commercial mixture of functional oils containing cashew nut shell liquid and castor oil (Essential; Oligo Basics Agroindustrial Ltda., Cascavel, Brazil) has previously been shown to successfully protect broiler chickens against coccidiosis challenge (Murakami et al., 2014) and improve broiler performance in supplemented chickens (Bess et al., 2012). These extracted oils are defined as “functional oils” because of their action beyond their nutritional value and status as neither spices nor essences (Bess et al., 2012). Castor oil is a triglyceride in which 90% of the fatty acid chains are ricinoleic acid and has anti-inflammatory activity (Vieira et al., 2001) and activity against some fungi and gram-positive bacteria (Novak et al., 1961). The active components of extracted cashew nutshell liquid are alkylphenols, which have been demonstrated to have antioxidant (Trevisan et al., 2005), molluscicidal (Kubo et al., 1986), antitumor (Itokawa et al., 1989), and antimicrobial (Kubo et al., 2003) properties. Moreover, some of the active components of extracted cashew nutshell liquid have been shown to inhibit several enzymes, such as α-glucosidase, invertase, aldose reductase (Toyomizu et al., 1993), tyrosinase (Kubo et al., 1994), and xanthine oxidase (Masuoka and Kubo, 2004), and may improve meat quality or freshness.
Current studies by Torrent et al. (2019) demonstrated that, at both moderate and high ambient temperatures, broilers supplemented with Essential (1.5 kg/ton) had significantly greater body weight gain at 42 D of age (P < 0.01) than the controls. Their study also demonstrated a reduction in breast yield in broilers subjected to heat stress and fed a conventional diet, whereas breast yields were not reduced in heat-stressed broilers fed a diet supplemented with Essential. At both moderate and high ambient temperatures, breast yields of birds were significantly increased (P < 0.01) by Essential supplementation in comparison to the nonsupplemented controls (Torrent et al., 2019). However, no studies to date have examined the effects of functional oils as an alternative feed additive for commercial turkey toms or the impact of antioxidant and anti-inflammatory functional oils on poultry carcass and meat characteristics. Therefore, in this study, we aimed to examine the effect of functional oils, Essential feed supplementation, on commercial turkey toms' growth performance, meat characteristics, and intestinal morphology.
Materials and methods
Experiment 1
Birds and Housing
Five hundred and eighty-five one-day-old male turkeys (hybrid converter) were randomly assigned to 45 pens (9.29 m2/pen), 15 replicate pens per treatment with 13 poults per pen. For the first 12 wk, each pen was randomly assigned to one of 3 dietary treatments: 1) control (conventional soybean meal + corn diet with no additive included), 2) inclusion of 0.15% of a commercial mixture of functional oils (Essential; Oligo Basics Agroindustrial Ltda., Cascavel, Brazil) in conventional soybean meal + corn diet, or 3) 66 ppm of monensin (Coban; Elanco Animal Health, Greenfield, IN) in a conventional soybean meal + corn diet. Both the functional oils and the growth promotants (either monensin or virginiamycin) were blended with the vitamin mineral premix before incorporating them into the final mix. The conventional soybean meal + corn diet was used as the experimental negative control diet, while the monensin (0 to 12 wk) and virginiamycin (13 to 20 wk) were used as a positive control and antibiotic growth promotant for experimental comparison.
Each one of the initial treatments was further divided into 3 treatments for week 13 to week 20 (Table 1)—control (no additive added), 0.15% of functional oils, or 20 ppm of virginiamycin (antibiotic Stafac; Phibro Animal Health, Westport, CT)—to produce 9 different treatments for the overall study, 5 replicate pens per treatment. Diets were formulated according to the age of the birds (Table 2): prestarter (0–2 wk, mash), starter (3–4 wk, crumble), developer 1 (5–8 wk, pellet), developer 2 (9–12 wk, pellet), grower (13–16 wk, pellet), and finisher (17–20 wk, pellet).
Table 1.
Experiment 1 treatment experimental design (1 to 20 wk).
| 1 to 12 wk | Number of pens | 13 to 20 wk | Number of pens |
|---|---|---|---|
| Control | 15 | Control | 5 |
| Control | 0 | Virginiamycin3 | 5 |
| Control | 0 | Functional oils2 | 5 |
| Monensin1 | 15 | Control | 5 |
| Monensin1 | 0 | Virginiamycin3 | 5 |
| Monensin1 | 0 | Functional oils2 | 5 |
| Functional oils2 | 15 | Control | 5 |
| Functional oils2 | 0 | Virginiamycin3 | 5 |
| Functional oils2 | 0 | Functional oils2 | 5 |
Antibiotic, 66 ppm Coban, Elanco Animal Health, Greenfield, IN.
0.15% Essential, Oligo Basics Agroindustrial Ltda., Cascavel Brazil.
Antibiotic, 20 ppm Stafac, Phibro Animal Health, Westport, CT.
Table 2.
Ingredients and calculated nutrient values of the experimental diets.
| Dietary component | Feed phase (weeks of age) |
|||||
|---|---|---|---|---|---|---|
| 0–2 | 3–4 | 5–8 | 9–12 | 13–16 | 17–20 | |
| Ingredients | % of Diet | |||||
| Corn | 40.33 | 47.07 | 53.35 | 57.67 | 64.16 | 66.35 |
| Soybean meal | 47.81 | 41.08 | 31.90 | 27.23 | 20.35 | 17.06 |
| Poultry meal | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Poultry fat | 1.23 | 1.15 | 5.05 | 6.09 | 6.82 | 8.16 |
| Dicalcium phosphate (18.5) | 3.05 | 2.84 | 2.11 | 1.88 | 1.67 | 1.59 |
| Limestone | 1.11 | 1.16 | 1.10 | 1.00 | 0.96 | 0.90 |
| Methionine | 0.37 | 0.41 | 0.25 | 0.13 | 0.16 | 0.15 |
| Sodium chloride | 0.34 | 0.34 | 0.34 | 0.34 | 0.34 | 0.34 |
| Mineral premix1 | 0.20 | 0.20 | 0.20 | 0.20 | 0.15 | 0.15 |
| Choline chloride 60% | 0.20 | 0.22 | 0.17 | 0.14 | 0.08 | 0.05 |
| Lysine | 0.17 | 0.29 | 0.31 | 0.15 | 0.15 | 0.08 |
| Vitamin premix2 | 0.15 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Sodium selenite premix3 0.06% | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| L-threonine | 0.00 | 0.11 | 0.07 | 0.00 | 0.00 | 0.00 |
| Nutrients | ||||||
| Crude protein, % | 29.6 | 27.0 | 23.0 | 20.9 | 18.0 | 16.5 |
| Crude fat, % | 3.75 | 3.79 | 7.73 | 8.83 | 9.67 | 11.02 |
| Calcium, % | 1.45 | 1.40 | 1.20 | 1.10 | 1.00 | 0.95 |
| Total phosphorus, % | 1.08 | 1.01 | 0.84 | 0.77 | 0.71 | 0.70 |
| Available P, % | 0.80 | 0.75 | 0.70 | 0.60 | 0.55 | 0.48 |
| Sodium, % | 0.18 | 0.18 | 0.18 | 0.18 | 0.18 | 0.18 |
| Potassium, % | 1.50 | 1.18 | 1.10 | 1.02 | 0.83 | 0.67 |
| Chloride, % | 0.29 | 0.32 | 0.32 | 0.29 | 0.30 | 0.27 |
| Arginine, % | 2.07 | 1.78 | 1.66 | 1.56 | 1.30 | 1.08 |
| Lysine, % | 1.85 | 1.70 | 1.60 | 1.40 | 1.20 | 0.90 |
| Methionine, % | 0.80 | 0.75 | 0.67 | 0.59 | 0.53 | 0.43 |
| Methionine + cysteine, % | 1.25 | 1.15 | 1.05 | 0.95 | 0.85 | 0.70 |
| Threonine, % | 1.15 | 1.05 | 0.95 | 0.90 | 0.74 | 0.62 |
| Tryptophan, % | 0.35 | 0.30 | 0.27 | 0.26 | 0.21 | 0.17 |
| Metabolizable energy poultry, kcal/kg | 2,850 | 2,900 | 3,200 | 3,300 | 3,400 | 3,500 |
| Na + K-Cl, MEq/kg | 354 | 289 | 267 | 257 | 207 | 174 |
| Choline, mg/kg | 2,720 | 2,700 | 2,570 | 2,570 | 2,025 | 1,285 |
Each kilogram of mineral premix (0.1% inclusion) supplied the following per kg of complete feed: 60 mg Zn as ZnSO4.H2O; 60 mg Mn as MnSO4.H2O; 40 mg Fe as FeSO4.H2O; 5 mg Cu as CuSO4; 1.25 mg I as Ca(IO3)2; 1 mg Co as CoSO4.
Each kilogram of vitamin premix (0.1% inclusion) supplied the following per kg of complete feed: vitamin A, 13,200 IU; cholecalciferol, 4,000 IU; alpha-tocopherol, 66 IU; niacin, 110 mg; pantothenic acid, 22 mg; riboflavin, 13.2 mg; pyridoxine, 8 mg; menadione, 4 mg; folic acid, 2.2 mg; thiamin, 4 mg; biotin, 0.253 mg; vitamin B12, 0.04 mg; ethoxyquin, 100 mg.
NaSeO3 premix provided 0.3 mg Se/kg of complete feed.
Turkeys were fed ad libitum until 20 wk of age. Individual body weights and pen feed consumption data were determined at 2, 4, 6, 8, 12, 14, 16, 18, and 20 wk of age. Mortality was recorded and used to adjust pen feed conversion data. The lighting program provided 23 h of light per day during the first 2 wk and natural light from then on. Litter from a previous flock with a new layer of soft pine shavings was used in all pens. Subsequently, clean shavings were added to each pen as needed to maintain an acceptable litter quality.
Morphometric Intestinal Characteristics
Morphometric intestinal characteristics were analyzed at 4 D of age using 4 birds per treatment, and at 11 and 21 D of age, using 6 birds per treatment. Approximately 5-cm-long samples were removed from the jejunum, opened longitudinally, and fixed immediately in a buffered 10% formalin solution for 48 h. Samples were then washed in 70% ethanol to remove the fixing solution, dehydrated in increasing alcohol concentrations, clarified in xylol, and embedded in paraffin. Semi-seriated 5-μm transverse thick histological sections were stained with hematoxilin-eosin (Behmer et al., 1976), and microscope slides were assembled with Canada balsam. Light-microscope (LEICA-DMR; Leica Camera AG, Solms, Germany) was used to visualize stained sections on slides, and images were captured by Image Tools to measure the villus height, upper villi width, bottom villi width, crypt depth, and muscularis mucosae thickness. The villi surface was calculated using 10 readings per replicate per variable, according to the formula: villi surface = [(upper villi width + bottom villi width)/2] × villus height.
Carcass and Meat Characteristic
At 11 and 21 D, 6 birds per treatment were weighed and euthanized, and the pectoralis major and pectoralis minor breast muscle sections were then dissected and weighed. At 20 wk of age, 15 toms from the treatment that had been fed functional oils during the whole experiment, and 6 toms from the treatment that had been fed monensin during the first 12 wk and virginiamycin from wk 13 to wk 20 were also weighed and euthanized and then dissected to weigh their legs and breasts (pectoralis major and minor).
The water-holding capacity of the meat was estimated by measuring the drip loss of the raw meat after storage at 2°C. A sample of the pectoralis major was weighed 24 h postmortem, placed immediately in a plastic bag, and stored at 2°C for 4 D. The sample was subsequently wiped with absorbent paper and weighed. The difference in weight corresponded to the drip loss and was expressed as a percentage (Berri et al., 2008). Deboned breast muscle (pectoralis major) was used to measure color with a portable colorimeter (Minolta Corp., Ramsey, NJ). Samples were numbered, placed into sterile polyethylene bags, packed on ice, and transported to the laboratory (Allen et al., 1998). Color values were recorded in triplicate for L∗ (lightness), a∗(red), and b∗(yellow).
Lipid peroxidation was assessed by measuring the concentration of thiobarbituric-acid reactive substances (TBARS) expressed as milligrams of malonaldehyde per kilogram of sample (Spanier and Traylor, 1991). Fifteen samples from the treatment that had been fed functional oils during the whole experiment and 6 from the treatment that had been fed monensin during the first 12 wk and virginiamycin from wk 12 to wk 20 were used to determine lipid peroxidation (Corzo et al., 2009) in red (gastrocnemius) and white meat (pectoralis major).
Statistical Analyses
Experiment 1
Data from the first 12 wk and carcass and meat quality data were analyzed with an ANOVA using JMP 8.0 (SAS Institute Inc., 2009) at a significance level P < 0.05. Data from 13 to 20 wk were analyzed using a factorial design with type of additive during 1 to 12 wk (control, monensin, and functional oils) and type of additive from 13 to 20 wk (control, virginiamycin, and functional oils) as factors. Differences between treatment means were evaluated using Tukey's test.
Experiment 2
To examine the effects of feeding 0.15% functional oils during the last 4 to 6 wk of growth, ninety-six turkeys, previously fed a control diet, were randomly assigned to one of 3 treatments (4 pens/treatment): control (no additives) from 16 to 22 wk, 0.15% functional oils (Essential) supplemented from 18 to 22 wk, and 0.15% functional oils (Essential) supplemented from 16 to 22 wk. Feed and water were provided ad libitum for consumption. Body weights, feed intake, and feed conversion ratio (FCR) adjusted for mortality were determined at 2-wk intervals. Four birds per pen were used to determine carcass and meat characteristics at 22 wk. Water-holding capacity (determined 4 and 7 D postmortem), breast meat color (determined 1, 4, and 7 D postmortem), and lipid peroxidation (determined 7 D postmortem) were assessed using the same methods as in experiment 1.
Performance and meat quality data were analyzed with an ANOVA using JMP 8.0 (SAS Institute Inc., 2009) at a significance level P < 0.05. When a parameter was analyzed multiple times, a factorial design was used with number of wk of functional oil supplementation and day of measurement as factors. Differences between treatment means were evaluated using Tukey's test. Mortality data were arcsine transformed before analysis to achieve normality.
Results
Experiment 1
The treatment effects on growth performance at 12 wk are summarized in Table 3. At 2 wk of age, poults supplemented with functional oils were heavier than the nonmedicated and medicated controls, respectively (381 g vs. 353 g and 373 g, P < 0.05). However, the birds supplemented with monensin became the heaviest by 4 wk (1056 g, 1251 g, and 1143 g for control, monensin, and functional oils, respectively; all treatments differed P < 0.05). At 12 wk, toms fed monensin were significantly heavier (P < 0.01) than those fed functional oils or the control diet. However, FCR (g:g) at 12 wk was significantly improved (P < 0.01) only in toms supplemented with monensin. There were no significant differences in percent mortality between treatment groups during the experiment, which were 6.7, 4.6, and 5.6% mortality in the control, monensin, and functional oils treatment groups, respectively.
Table 3.
Effects of feeding monensin1 or functional oils2 on body weight, feed intake, feed conversion, and mortality (%) at 12 wk of age (experiment 1).
| Treatment | Body weight (kg) | Feed intake (kg) | Feed conversion ratio (g:g) | Mortality (%) |
|---|---|---|---|---|
| Control | 8.59c | 16.93 | 2.005a | 6.67 |
| Monensin1 | 9.49a | 17.50 | 1.868b | 4.64 |
| Functional oils2 | 8.96b | 17.32 | 1.964a | 5.64 |
| SEM | 0.31 | 1.12 | 0.099 | 2.65 |
a,bValues in the same column without a superscript in common differ statistically (P < 0.05).
Antibiotic, 66 ppm Coban, Elanco Animal Health, Greenfield, IN.
0.15% Essential, Oligo Basics Agroindustrial Ltda., Cascavel, Brazil.
Table 4 summarizes the growth performance of toms as affected by the carry-over effects of treatments subjected 1 to 12 wk, and the treatments subjected 13 to 20 wk. At 20 wk, toms fed monensin were still significantly heavier than the controls (P < 0.05) but were not significantly different than toms fed the functional oils. The feed intakes and the FCR from 1 to 20 wk were not affected by either virginiamycin or functional oils. However, the FCR from 13 to 20 wk for monensin was significantly larger than that of the controls and functional oils. Neither virginiamycin nor the functional oils affected any performance variable from 13 to 20 wk.
Table 4.
Effects of functional oils1 and virginiamycin2 on BW, feed intake, and feed conversion of turkey toms at 20 wk (experiment 1).
| 1–12 wk treatments | Control | 13–20 wk treatments |
P value |
||||
|---|---|---|---|---|---|---|---|
| Functional oils1 | Virginiamycin2 | Average | SEM | 1–12 wk | 13–20 wk | ||
| BW (kg) | |||||||
| Control | 19.55 | 19.74 | 20.04 | 19.78b | |||
| Monensin3 | 19.98 | 20.45 | 20.60 | 20.34a | |||
| Functional oils1 | 20.23 | 20.42 | 19.84 | 20.18a,b | |||
| Average | 19.93 | 20.20 | 20.16 | 20.10 | 0.56 | 0.03 | 0.38 |
| Feed Intake (kg) | |||||||
| Control | 27.73 | 29.44 | 29.20 | 28.79 | |||
| Monensin3 | 27.73 | 28.49 | 28.11 | 28.11 | |||
| Functional oils1 | 28.54 | 29.61 | 26.47 | 28.20 | |||
| Average | 28.00 | 29.18 | 27.93 | 28.37 | 1.83 | 0.55 | 0.12 |
| Feed conversion ratio at 13–20 wk (g:g) | |||||||
| Control | 3.350 | 3.392 | 3.446 | 3.396b | |||
| Monensin3 | 3.664 | 3.668 | 4.414 | 3.915a | |||
| Functional oils1 | 3.252 | 2.968 | 2.784 | 3.001b | |||
| Average | 3.422 | 3.343 | 3.548 | 3.438 | 0.613 | >0.01 | 0.66 |
| Feed conversion ratio at 1–20 wk (g:g) | |||||||
| Control | 2.617 | 2.616 | 2.644 | 2.626 | |||
| Monensin3 | 2.586 | 2.621 | 2.747 | 2.651 | |||
| Functional oils1 | 2.718 | 2.483 | 2.651 | 2.617 | |||
| Average | 2.640 | 2.574 | 2.681 | 2.632 | 0.262 | 0.93 | 0.53 |
| Mortality 13–20 wk (%) | |||||||
| Control | 23.08 | 15.38 | 18.46 | 18.97 | |||
| Monensin3 | 23.08 | 18.46 | 23.08 | 21.54 | |||
| Functional oils1 | 23.08 | 12.09 | 23.84 | 19.67 | |||
| Average | 23.08 | 15.31 | 21.79 | 20.06 | 1.81 | 0.61 | 0.30 |
| Mortality 1–20 wk (%) | |||||||
| Control | 27.69 | 23.08 | 26.15 | 25.64 | |||
| Monensin3 | 27.69 | 23.08 | 27.69 | 26.15 | |||
| Functional oils1 | 27.69 | 18.24 | 28.59 | 24.84 | |||
| Average | 27.69 | 21.46 | 27.48 | 25.54 | 5.12 | 0.95 | 0.38 |
a,bValues in the same column without a superscript in common differ statistically (P < 0.05).
0.15% Essential, Oligo Basics Agroindustrial Ltda., Cascavel, Brazil.
Antibiotic, 20 ppm Stafac, Phibro Animal Health, Westport, CT.
Antibiotic, 66 ppm Coban, Elanco Animal Health, Greenfield, IN.
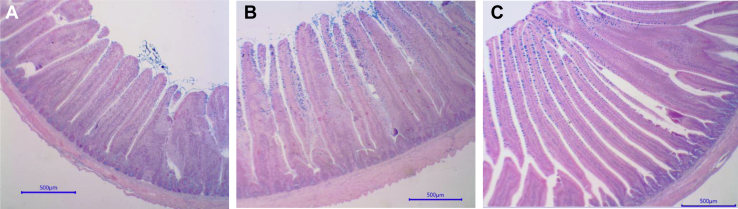
Relative to the control diet, both monensin and functional oils significantly increased the thickness of the jejuna muscularis mucosae at 4 D by 28% (P < 0.05) and significantly decreased (P < 0.05) the crypt depth by approximately 21% at 11 D of age (Table 5). The surface area of jejunal villi of birds fed monensin was significantly smaller than that of the controls (P < 0.05), whereas that of birds fed functional oils were not different from either of the other treatments at 11 D (Table 5). In contrast, at 21 D, the jejunal villi surface area of monensin-fed birds was significantly greater (P < 0.05) than that of the controls. At 21 D, dietary inclusion of functional oils also significantly increased (P < 0.05) the villi height in comparison to the other treatments, and monensin and functional oils both increased (P < 0.05) the crypt depth by approximately 14% relative to the control (Figure 1). At 21 D, monensin significantly increased (P < 0.05) both the villi and crypt widths relative to those of the functional oils–fed group. Interestingly, the villi surface area and the crypt depths at 21 D followed the same pattern as the live weights, being the greatest for the poults fed monensin.
Table 5.
Effects of supplementation with monensin1 and functional oils2 on histological variables of jejunum mucosa in turkey toms at 4, 11, and 21 D of age (experiment 1).
| Treatment | Jejunum villi (μm) |
Crypt depth (μm) | Muscularis thickness (μm) | Surface area (μm2) | ||
|---|---|---|---|---|---|---|
| Height | Upper width | Bottom width | ||||
| 4 D | ||||||
| Control | 761 | 113 | 162 | 96 | 68b | 355 |
| Monensin1 | 860 | 116 | 173 | 92 | 88a | 343 |
| Functional oils2 | 760 | 104 | 167 | 84 | 88a | 316 |
| SEM | 77 | 6 | 14 | 5 | 8 | 25 |
| 11 D | ||||||
| Control | 933 | 135 | 228 | 134a | 114 | 433a |
| Monensin1 | 786 | 129 | 187 | 106b | 111 | 260b |
| Functional oils2 | 974 | 135 | 223 | 104b | 122 | 372a,b |
| SEM | 64 | 10 | 20 | 6 | 6 | 31 |
| 21 D | ||||||
| Control | 1,046b | 156a,b | 234a,b | 107b | 107 | 429b |
| Monensin1 | 1,213b | 192a | 293a | 124a | 135 | 617a |
| Functional oils2 | 1,637a | 136b | 218b | 122a | 126 | 549a,b |
| SEM | 68 | 12 | 18 | 3 | 9 | 47 |
a,bValues in the same column without a superscript in common differ statistically (P < 0.05).
Antibiotic, 66 ppm Coban, Elanco Animal Health, Greenfield, IN.
0.15% Essential, Oligo Basics Agroindustrial Ltda., Cascavel, Brazil.
Figure 1.
Effects of supplementation with Monensin (Antibiotic, 66 ppm Coban; Elanco Animal Health, Greenfield, IN) and functional oils (0.15% Essential, Oligo Basics Agroindustrial Ltda., Cascavel, Brazil) on histological morphology of jejunum mucosa in turkey toms experiment 1. Each panel is representative of jejunal morphological characteristics of birds from each treatment group in experiment 1: A = control, B = monensin, and C = functional oils. For 12 wk, 585 male turkeys were randomly assigned to one of 3 dietary treatments: (1) control, (2) 0.15% of functional oils in basal diet, or (3) 66 ppm of monensin in a basal diet. At week 13 to week 20, each of 3 treatments were subdivided into 3 additional treatment groups (control-no additive added, 0.15% of functional oils, or 20 ppm of virginiamycin). Jejunal morphometric characteristics using standard histological processes and staining methods were used at 21 D of age with 6 birds per treatment. Villi surface area was calculated using 10 readings per replicate per variable, using the following equation: villi surface = [(UVW + BVW)/2] × VH. Abbreviations: BVW, bottom villi width; UVW, upper villi width; VH, villus height.
Whole breast yield was 14% greater (P < 0.05) at 11 D and 10% (P < 0.05) greater at 21 D in monensin-fed birds compared with the controls (Table 6). However, supplementation with functional oils marginally increased whole breast yield by only 8 and 9% at 11 D and 21 D, respectively, only being significant at 21 D (P < 0.05). The improvement in breast yield was primarily due to the increase in the pectoralis major because supplementation with monensin or functional oils increased (P < 0.05) the proportion of this muscle relative to control both at 11 and 21 D. The pectoralis minor was significantly increased by functional oils dietary supplementation in comparison to the controls only at 21 D (P < 0.05). Supplementation with functional oils significantly decreased (P < 0.05) the percentage yield of the leg mass, with a marginal (P = 0.11) percentage increase in breast yield (Table 7).
Table 6.
Effects of the supplementation with monensin1 and functional oils2 on breast meat yield (% live weight) of turkey toms at 11 and 21 D (experiment 1).
| Treatments | 11 D of age |
21 D of age |
||||
|---|---|---|---|---|---|---|
| Whole breast | Pectoralis major | Pectoralis minor | Whole breast | Pectoralis major | Pectoralis minor | |
| % of Live body weight | ||||||
| Control | 11.17b | 8.52b | 2.65 | 14.65b | 11.47b | 3.17b |
| Monensin | 12.77a | 10.11a | 2.67 | 16.14a | 12.81a | 3.33a,b |
| Functional oils | 12.12a,b | 9.62a | 2.50 | 15.97a | 12.58a | 3.40a |
| SEM | 0.27 | 0.25 | 0.09 | 0.24 | 0.21 | 0.06 |
a,bValues in the same column without a superscript in common differ statistically (P < 0.05).
Antibiotic, 66 ppm Coban, Elanco Animal Health, Greenfield, IN.
0.15% Essential, Oligo Basics Agroindustrial Ltda., Cascavel, Brazil.
Table 7.
Effects of the supplementation of functional oils1 and virginamycin2 on the percentage of breast and leg meat yield, and color measurements (L∗, a∗, b∗), drip loss, and TBARS of the breast meat at 20 wk from turkey toms (experiment 1).
| Meat yield and quality measurement | Functional oils1 | Virginiamycin2 | P value | SEM |
|---|---|---|---|---|
| Carcass (%) | ||||
| Breast | 25.14 | 24.26 | 0.11 | 0.38 |
| Leg | 20.77 | 21.71 | 0.03 | 0.30 |
| Breast meat color | ||||
| L∗ | 48.07 | 47.15 | 0.07 | 0.34 |
| a∗ | 3.27 | 3.21 | 0.83 | 0.17 |
| b∗ | 1.55 | 1.4 | 0.41 | 0.13 |
| % Drip loss after 7 D of storage | 2.40 | 3.39 | 0.12 | 0.42 |
| TBARS (mg malonaldehyde/kg) | ||||
| Red meat | 3.48 | 3.59 | 0.60 | 0.11 |
| White meat | 2.66 | 3.11 | 0.02 | 0.13 |
Abbreviation: TBARS, thiobarbituric-acid reactive substances.
0.15% Essential, Oligo Basics Agroindustrial Ltda., Cascavel, Brazil.
Stafac, Phibro Animal Health, Westport, CT.
Supplementation with functional oils significantly improved the antioxidant status of the meat as it significantly decreased (P < 0.05) the TBARS of the white meat with marginal (P = 0.07) increase in the lightness (L∗) of the meat and no differences in (P = 0.12) drip loss after 7 D of storage when compared to the monensin/virginiamycin treatment (Table 7). No other differences were observed in any other meat quality characteristic.
Experiment 2
Although supplementation with functional oils during the last 6 vs. 4 or 0 wk of growth marginally improved the average daily gain and FCR, no significant differences (P < 0.05) were seen in any of the performance parameters supplemented for either 0, 4, or 6 wk (Table 8).
Table 8.
Effect of feeding functional oils1 for 0, 4, or 6 wk at the end of the finishing period on turkey tom BW, feed intake, and feed conversion at a slaughter age of 22 wk (experiment 2).
| Performance characteristics | 0 wk | 4 wk | 6 wk | P value |
|---|---|---|---|---|
| Final BW (kg) | 22.04 | 21.61 | 21.32 | 0.80 |
| Feed intake (kg/D) | 1.11 | 1.11 | 1.04 | 0.65 |
| ADG (g/D) | 194 | 205 | 208 | 0.90 |
| Feed:gain (g/g) | 8.23 | 8.47 | 7.98 | 0.51 |
Abbreviation: ADG, average daily gain.
0.15% Essential, Oligo Basics Agroindustrial Ltda., Cascavel, Brazil.
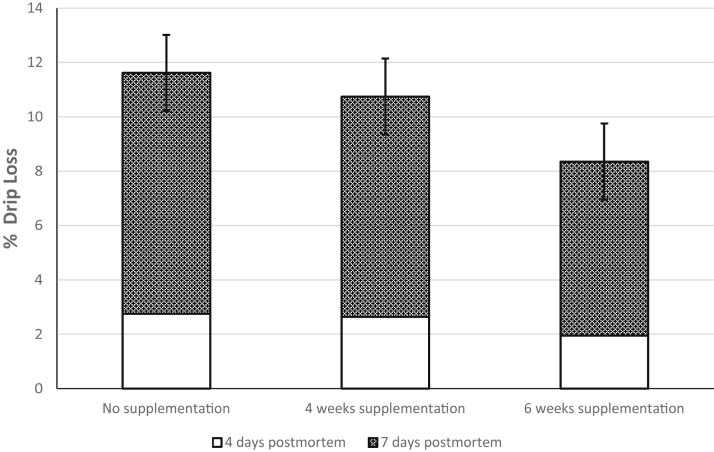
Supplementation with functional oils did not significantly affect the color characteristics of the meat. However, day of color measurement,1, 4, or 7 D postmortem, affected L∗, a∗, and b∗ values (Table 9). All color parameters were greatest at day 4 postmortem compared with day 1 and 7 postmortem. While not significant, dietary supplementation with functional oils resulted in a marginal decrease in drip loss (P < 0.08; Figure 2). However, no differences were seen among TBARS values across treatment groups.
Table 9.
Effects of feeding functional oils1 for 0, 4, or 6 wk at the end of the finishing period on turkey tom meat color characteristics, 1, 4, and 7 D postmortem (experiment 2).
| Color measurement | Supplementation period (wks) | Time postmortem (D) |
||
|---|---|---|---|---|
| 1 | 4 | 7 | ||
| L∗ | ||||
| 0 | 49.5 | 51.9 | 50.2 | |
| 4 | 49.3 | 51.6 | 49.6 | |
| 6 | 49.9 | 52.2 | 50.1 | |
| Average 0–6 wks | 49.6b | 51.9a | 50.0b | |
| a∗ | ||||
| 0 | 2.59 | 3.99 | 3.73 | |
| 4 | 2.69 | 4.07 | 3.86 | |
| 6 | 2.62 | 3.84 | 3.96 | |
| Average 0–6 wks | 2.64b | 3.97a | 3.85a | |
| b∗ | ||||
| 0 | 2.37 | 4.14 | 3.94 | |
| 4 | 2.19 | 3.91 | 3.81 | |
| 6 | 2.26 | 3.78 | 3.54 | |
| Average 0–6 wks | 2.28b | 3.95a | 3.76b | |
a,bValues in the same column without a superscript in common differ statistically (P < 0.05).
0.15% Essential, Oligo Basics Agroindustrial Ltda., Cascavel, Brazil.
Figure 2.
Effects of supplementation with functional oils (0.15% Essential, Oligo Basics Agroindustrial Ltda., Cascavel, Brazil) during the last 4 or 6 wk before slaughter on % drip loss in 22-wk-old turkey toms (P < 0.08) (experiment 2).
Discussion
Although both functional oils and monensin improved weight gain during the first 12 wk of experiment 1, only monensin improved feed:gain ratios, with the greatest improvement with monensin at wk 4. Mortality did not differ among treatment groups during the first 12 wk (5.65% across treatments) with the use of recycled poultry litter, which may have presented birds with subclinical coccidiosis challenge during this experimental timeframe. No clinical signs of coccidiosis were seen in the birds of this study. However, the growth performance and enhancement of the intestinal absorptive surface area in response to dietary supplementation with functional oils and monensin parallel coccidia studies conducted in birds fed diets supplemented with monensin (Cabel et al., 1991, Chapman and Saleh, 1999, Bozkurt et al., 2016).
Monensin is a carboxylic ionophore that has been used as a growth promotant and has been shown to be very effective in controlling coccidiosis in turkeys (Cabel et al., 1991), so improvements in live weight and FCR could have been associated with its anticoccidial effects. Studies have demonstrated that monensin supplementation in Eimeria-infected turkeys to be beneficial at 3 wk of age (Chapman and Saleh, 1999); therefore, it is logical to expect a response in live weight with monensin supplementation at 4 wk. Functional oils have also been shown to protect chickens against coccidiosis challenge (Murakami et al., 2014), although no research with functional oils has been carried out on their efficacy against Eimeria species infecting turkeys. The smaller improvement in body weight gain at 12 wk with functional oils relative to monensin suggests that while functional oils may improve growth performance, they are not as efficacious. However, neither experiment 1 (13 to 20 wk) nor experiment 2 (16 to 22 wk) showed any treatment effects on performance parameters of older toms.
The higher villi surface and lower crypt values in the ileum at 21 D for the treatments supplemented with monensin and functional oils indicate an improved absorptive surface area when compared to the control (Table 5 and Figure 1). Although there is no research published on the effects of either monensin or functional oils on the intestinal morphology of turkeys, it has been shown that when broiler chickens were experimentally challenged with coccidiosis, both monensin and oregano oils improved the surface area and decreased the crypt depth (Bozkurt et al., 2016). In the event of subclinical coccidiosis challenge due to the utilization of recycled poultry liter in this study, the increased jejunal absorptive area could be explained by the action of monensin and functional oils.
In both experiments, the water-holding capacity of meat improved with functional oil supplementation. Essential oils contain antioxidants, and earlier studies by Young et al. (2003) demonstrated that supplementation with antioxidants, such as α-tocopherol, increases meat lightness color and reduces the TBARS of poultry meat, as breast meat lightness color values and breast meat pH are negatively correlated (Barbut, 1993, Allen et al., 1997). The water-holding capacity of poultry meat decreases as pH decreases, and low water-holding capacity increases cooking losses and drip loss (Froning et al., 1978, Barbut, 1993, Northcutt et al., 1994). Meat pH was not measured in this study; however, if the higher lightness values yielded by the birds fed functional oils were correlated with lower meat pH, this low pH did not translate into an increased drip loss. Drip loss decreased in both experiments 1 and 2 (29 and 39%, 7 D postmortem, respectively). The improved antioxidant status of the meat (TBARS) from turkeys fed functional oils may be due to the antioxidant properties of the cashew nut shell liquid (Amorati et al., 2001, Trevisan et al., 2005) and the anti-inflammatory properties of ricinoleic acid, the primary fatty acid found in castor oil (Vieira et al., 2001).
Although dietary inclusion of functional oils has been shown to improve the carcass characteristics of beef (Purevjav et al., 2013), such differences have not been found in chicken carcass characteristics (Bess et al., (2012). However, in this study, turkey carcass characteristics were observed to be altered by dietary functional oils in experiment 1, while absent in experiment 2. This effect might have been due to the antioxidant and anti-inflammatory properties of functional oils, which might have reduced the heat stress experienced by the birds during the last part of this experiment in the summer months. Heat stress is known to reduce breast and increase leg meat yields (Veldkamp et al., 2000). The lack of these effects in experiment 2 could be due to cooler environmental temperatures during the experiment and thus the lack of heat stress on the birds or the shorter duration of feeding functional oils. Moreover, these experimental differences between experiment 1 and 2 may have been due to differences in the duration of the experimental diets of functional oils or monensin (experiment 1, 12 wk; experiment 2, 7 wk).
In conclusion, supplementation with functional oils improved the body weight of turkey poults at 12 wk relative to the controls, although not as much as with monesin. Supplementation of functional oils also improved carcass yield of whole breast sections and pectoralis major breast sections relative to the controls, thus suggesting that supplementing turkey feed with functional oils could serve as a partial alternative to supplementing with antibiotic growth promoters.
Acknowledgments
This research was supported by a sponsored grant from Oligo Basics USA, LLC (Cary, NC), the North Carolina Agricultural Foundation, Inc. (Raleigh, NC), and USDA-NIFA Project No. NC06343 Nutrient and by-product utiliation and health of turkeys and broilers. The authors thank Prestage Farms Inc. (Clinton, NC) for the donation of poults used in this study. Finally, the authors gratefully acknowledge the students and staff of the Prestage Department of Poultry Science, North Carolina State University, staff of North Carolina State University Feed Mill, and Dr. Marilyn Mayer for their contributions to this study.
Conflict of Interest Statement: J. Torrent is an employee of Oligo Basics USA, LLC.
References
- Allen C.D., Russell S.M., Fletcher D.L. The relationship of broiler breast meat color and pH to shelf life and odor development. Poult. Sci. 1997;76:1042–1046. doi: 10.1093/ps/76.7.1042. [DOI] [PubMed] [Google Scholar]
- Amorati R.G.F., Pedulli L., Valgimigli, Attanasi O.A., Filippone P., Fiorucci C., Saladino R. Absolute rate constants for the reaction of peroxyl radicals with cardanol derivatives. J. Chem. Soc. Perkin Trans. 2001;2:2142–2146. [Google Scholar]
- Barbut S. Colour measurements for evaluating the pale soft exudative (PSE) occurrence in Turkey meat. Food Res. Intl. 1993;26:39–43. [Google Scholar]
- Behmer A.O., Tolosa E.M.C., Freitas-Neto A.G. 1.ed. EUSPE; São Paulo: 1976. Manual de técnicas para histologia normal e patológicas; p. 239. [Google Scholar]
- Berri C., Besnard J., Relandeau C. Increasing dietary lysine increases final pH and decreases drip loss of broiler breast meat. Poult. Sci. 2008;87:480–484. doi: 10.3382/ps.2007-00226. [DOI] [PubMed] [Google Scholar]
- Bess E., Favero A., Vieira S.L., Torrent J. The effect of functional oils on broiler diets of varying energy levels. J. Appl. Poult. Res. 2012;21:567–578. [Google Scholar]
- Bozkurt M., Ege G., Aysul N., Aksit H., Tüzün E., Küçükylmz K., Borum A.E., Uygun M., Aksit D., Simsek E., Seyrek K., Kocer B., Bintas E., Orojpur A. Effect of anticoccidal monensin with oregano essential oil on broilers experimentally challenged with mixed Eimeria spp. Poult. Sci. 2016;95:1858–1868. doi: 10.3382/ps/pew077. [DOI] [PubMed] [Google Scholar]
- Cabel M.C., Norton R.A., Yazwinsky T.A., Waldroup P.W. Efficacy of different anticoccidials against experimental coccidiosis in large white turkeys. Poult. Sci. 1991;70:289–292. doi: 10.3382/ps.0700289. [DOI] [PubMed] [Google Scholar]
- Chapman H.D., Saleh E. Effects of different concentrations of monensin and monensin withdrawal upon the control of coccidiosis in the Turkey. Poult. Sci. 1999;78:50–56. doi: 10.1093/ps/78.1.50. [DOI] [PubMed] [Google Scholar]
- Corzo A., Schilling M.W., Loar R.E., II, Jackson V., Kin S., Radhakrishnan V. The effects of feeding distillers dried grains with soluble on broiler meat quality. Poult. Sci. 2009;88:432–439. doi: 10.3382/ps.2008-00406. [DOI] [PubMed] [Google Scholar]
- Froning G.W., Babji A.S., Mather F.B. The effect of preslaughter temperature, stress, struggle and anesthetization on color and textural characteristic of Turkey muscle. Poult. Sci. 1978;57:630–633. [Google Scholar]
- Itokawa H., Totsuka N., Nakahara K., Maezuru M., Takeya K., Kondo M. A quantitative structure-activity relationship for antitumor activity of long-chain phenols from Ginko biloba L. Chem. Pharm. Bull. 1989;37:1619–1621. doi: 10.1248/cpb.37.1619. [DOI] [PubMed] [Google Scholar]
- Kubo I., Kints-Hori I., Yokokawa Y. Tyrosinase inhibitors from anacardium Occidental fruits. J. Nat. Prod. 1994;57:545–551. doi: 10.1021/np50106a021. [DOI] [PubMed] [Google Scholar]
- Kubo I., Nihei K., Tsujimoto K. Antibacterial action of anacardic acids against methicillin resistant Staphylococcus aureus (MRSA) J. Agric. Food Chem. 2003;51:7624–7628. doi: 10.1021/jf034674f. [DOI] [PubMed] [Google Scholar]
- Kubo I., Komatsu S., Ochi M. Molluscacides from the cashew anacardium occidentale and their large-scale isolation. J. Agric. Food Chem. 1986;34:970–973. [Google Scholar]
- Masuoka N., Kubo I. Characterization of xanthine oxidase inhibition by anacardic acids. Biochem. Biophys. Acta. 2004;1688:245–249. doi: 10.1016/j.bbadis.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Murakami A.E., Eyng C., Torrent J. Effects of functional oils on coccidiosis and apparent metabolizable energy in broiler chickens. Asian Australas. J. Anim. Sci. 2014;27:981–989. doi: 10.5713/ajas.2013.13449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northcutt J.K., Foegeding E.A., Edens F.W. Water-holding properties of thermally preconditioned chicken breast and leg meat. Poult. Sci. 1994;73:308–316. doi: 10.3382/ps.0730308. [DOI] [PubMed] [Google Scholar]
- Novak A.F., Clark G.C., Dupuy H.P. Antimicrobial activity of some ricinoleic and oleic acid derivatives. J. Am. Oil Chemists’ Soc. 1961;38:321–324. [Google Scholar]
- Purevjav T., Hoffman M.P., Ishdorj A., Conover A.J., Jedlicka M.E., Prusa K., Torrent J., Pusillo G.M. Effects of functional oils and monensin on cattle finishing programs. Prof. Anim. Sci. 2013;29:426–434. [Google Scholar]
- SAS Institute Inc . 2nd ed. SAS Institute Inc.; Cary, NC: 2009. JMP 8 User Guide. [Google Scholar]
- Spanier A.M., Traylor R.D. A rapid, direct chemical assay for the quantitative determination of thiobarbituric acid reactive substances in raw, cooked, and cooked/stored muscle foods. J. Muscle Foods. 1991;2:165–176. [Google Scholar]
- Torrent J., Arce Menocal J., López Coello C., Ávila González E. Effects of functional oils on performance and carcass characteristics of broilers under two different temperature environments. Poult. Sci. Pii. 2019;98:5855–5861. doi: 10.3382/ps/pez235. [DOI] [PubMed] [Google Scholar]
- Toyomizu M., Sugiyama S., Jin R.L., Nakatsu T. α-Glucosidase and aldose reductase inhibitors: constituents of cashew, Anacardium occidentale, nutshell liquids. Phytother. Res. 1993;7:252–254. [Google Scholar]
- Trevisan M.T., Pfundstein B., Haubner R., Wurtele G., Spiegelhalder B., Bartsch H., Owen R.W. Characterization of alkyl phenols in cashew (Anacardium occidentale) products and assay of their antioxidant capacity. Food Chem. Toxicol. 2005;44:188–197. doi: 10.1016/j.fct.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Veldkamp T., Kwakkel R.P., Ferket P.R., Simons P.C.M., Noordhuizen J.P.T.M., Pijpers A. Effects of ambient temperature, arginine-to-lysine ratio, and electrolyte balance on performance, carcass, and blood parameters in commercial male turkeys. Poult. Sci. 2000;79:1608–1616. doi: 10.1093/ps/79.11.1608. [DOI] [PubMed] [Google Scholar]
- Vieira C., Fetzer S., Sauer S.K., Evangelista S., Averbeck B., Kress M., Reeh P.W., Cirillo R., Lippi A., Maggi C.A., Manzini S. Pro- and anti-inflammatory actions of ricinoleic acid: similarities and differences with capsaicin. Naunyn-schmiedeberg’s Arch. Pharmacol. 2001;364:87–95. doi: 10.1007/s002100100427. [DOI] [PubMed] [Google Scholar]
- Young J.F., Stagsted J., Jensen S.K., Karlsson A.H., Henckel P. Ascorbic acid, α-tocopherol, and oregano supplements reduce stress-induced deterioration of chicken meat quality. Poult. Sci. 2003;82:1343–1351. doi: 10.1093/ps/82.8.1343. [DOI] [PubMed] [Google Scholar]