Abstract
White striping (WS) and woody breast (WB) have been previously associated with older and heavier birds. However, there is limited information supporting the association between these 2 muscle conditions and growth parameters. The objectives of this study were 1) to investigate the relationship between WS and WB using different growth production factors and 2) to propose a predictive model that uses growth production factors to investigate the incidence and severity of WS and WB. A combined database of 4,332 broilers pooled from 7 research experiments conducted from 2016 to 2017 at Texas A&M University was used in this study. Parameters such as sex, age (4 wk, 6 wk, and 8 wk), strain (standard A vs. high-breast-yield [B and C]), live weight categories (500 g increments), and breast weight categories (250 g increments) were included in the model. Results showed that WS was 12% more likely to be present in non-WB fillets. The association between WS and WB suggests a moderate relationship between the ranks of both outcome variables (ρ = 0.57, P < 0.0001). Variables such as age, live weight, and sex were not as important as breast weight and strain in the severity prediction of WS and WB. Butterfly fillets above 750 g and with high-breast-yielding strains were more likely associated with higher severity of WS and WB scores. No post hoc variable selection was performed. Both models show good discrimination. The WS model produced an uncorrected area under the curve (AUC) of 0.739, with a bootstrap corrected estimate of 0.736. The WB model produced an uncorrected AUC of 0.753 and a bootstrap corrected estimate of 0.752. Therefore, the growth production factors analyzed in this study indicated that there is a moderate relationship between WS and WB myopathies and were jointly predictive of the severity of WS and WB. Potentially other factors not included in this study may play a major role in the relationship of these 2 myopathies. More research should be done to investigate this possibility.
Key words: association, prediction model, woody breast, white striping, broilers
Introduction
Chicken meat is a popular protein around the world. A recent consumer survey showed that versatility and properties related to healthy eating (i.e., low fat) were the main reasons consumers preferred chicken to other protein sources (Neth and Parker, 2018). Since 2012, there has been an increased demand for fresh and deli chicken products and the trends indicate further growth in this area over the next few years (National Chicken Council, 2018, Neth and Parker, 2018).
To fulfill the growing demand and make the production more efficient, selection criteria of broiler chickens have been changing through genetic selection, improved management practices, and nutrition (Zuidhof et al., 2014, Bodle et al., 2018). However, several studies have shown that genetic selection for high breast development in broilers has led to muscle fiber hypertrophy, which is causing structural, functional, and metabolic changes (Dransfield and Sosnicki, 1999, Velleman and Nestor, 2003, Kuttappan et al., 2012b, Kuttappan et al., 2013, Petracci and Cavani, 2012).
White striping (WS) and woody breast (WB) are recent meat quality issues found in fast-growing heavy broilers (Sihvo et al., 2014). The exact etiology of these conditions remains unknown. However, some associated quality issues include lower water-holding capacity, higher cook loss percentage, lower marinade pick-up percentage, and differences in mechanical and sensory texture (higher values of crunchiness, chewiness, and fibrousness) compared to normal fillets (Mudalal et al., 2014, Soglia et al., 2017, Aguirre et al., 2018). Recently, the Food Safety and Inspection Services (2018) sent disposition instructions for WS and WB poultry conditions. This notice explains that the severe inflammatory tissues associated with WS and WB must be trimmed during inspection. These new dispositions plus the consumer's unwillingness to eat WS/WB meat is causing an economic impact for the poultry industry (Kuttappan et al., 2012a). In this regard, it is imperative to explore new alternatives to identify the cause of the incidence and severity of these conditions.
Predictive models are widely used in clinical studies as an important decision-making tool across various disease procedures in medicine (Michaelson et al., 2011, Aziz et al., 2016, McGirt et al., 2017). For example, McGirt et al. (2017) created predictive models for the efficient selection of patients, based on patient-specific factors, who need spinal surgery. Predictive models, trained on the appropriate data, can be a key decision-making tool. The following study pooled data sets from several nutritional studies performed at Texas A&M University to investigate the relationship between WS and WB using different growth production factors and to propose a predictive model that uses growth production factors to investigate the incidence and severity of WB and WS.
Materials and methods
Database
All the projects included in the database used in the present study were approved by the Texas A&M's Institutional Animal Care and Use Committee. A combined database of 4,332 broilers from 7 different research experiments conducted during 2016 to 2017 at Texas A&M University was used in this study. Broilers from each study were conventionally processed under similar conditions in a pilot scale processing facility at Texas A&M University. Upon completion of the debone process, breast fillets were weighed, palpated, and scored for WS and WB. Breast fillets were scored for WS using normal (0) without any white lines, mild (1), moderate (1.5), and severe (≥2.5) as described by Kuttappan et al. (2012a). The total number of observations for each category was as follows: 0 (782), 0.5 (186), 1 (1,725), 1.5 (973), 2 (561), 2.5 (93), 3 (4). Breast fillets presenting WB were categorized according to Tijare et al. (2016) as normal (0) without any hardness, mild (1) hardness present mainly on the cranial region, moderate (2) hardness throughout the fillet with some flexibility in the mid-caudal region, and severe (3) hardness in the cranial/caudal region with no flexibility. The total number of observations in each category was as follows: 0 (973), 0.5 (125), 1 (1,591), 1.5 (2), 2 (1,193), 3 (440). In addition, breast fillets were scored in 0.5 increments, when necessary, and because of small cell counts, some of the cells were combined. One person was in charge of the WS and WB categorization for all the nutritional studies.
The distribution of the birds is presented in Tables 1 and 2. Factors analyzed for the predictive model included sex, age, strain, live weight categories, and breast weight categories. The full data set consisted of 4,332 observations. A complete case analysis was performed and observations with missing outcome or covariate values were excluded from each analysis. Ultimately, 4,320 observations were used for the WS analysis and 4,321 observations for the WB analysis. Thus, only minimal information was lost because of the exclusion of observations with missing data.
Table 1.
Frequency analysis and the probability of occurrence of white striping (WS) degrees of severity with respect to the growth production variables included in the prediction model.
| Variable | WS frequency1 |
Total | ||||
|---|---|---|---|---|---|---|
| 0 | 1 | 1.5 | 2 | 2.5 | ||
| Sex (%) | ||||||
| Female | 237 (50) | 186 (40) | 30 (6) | 14 (3) | 3 (1) | 470 |
| Male | 545 (14) | 1,725 (45) | 943 (24) | 547 (14) | 90 (2) | 3,853 |
| Age (%) | ||||||
| 4 wk | 342 (61) | 218 (39) | 0 | 0 | 0 | 560 |
| 6 wk | 410 (12) | 1,637 (46) | 929 (26) | 472 (13) | 93 (3) | 3,541 |
| 8 wk | 30 (14) | 56 (26) | 44 (20) | 89 (41) | 0 | 219 |
| Strain (%) | ||||||
| Standard breast yield A | 559 (29) | 860 (44) | 311 (16) | 163 (8) | 44 (3) | 1,937 |
| High breast yield B | 31 (16) | 104 (55) | 33 (17) | 18 (9) | 4 (2) | 190 |
| High breast yield C | 192 (9) | 947 (43) | 629 (29) | 380 (17) | 49 (2) | 2,197 |
| Live weight (%) | ||||||
| <2,000 g | 274 (70) | 116 (30) | 2 (1) | 0 | 0 | 392 |
| >2,000– < 2,500 g | 99 (37) | 153 (57) | 15 (6) | 2 (1) | 0 | 269 |
| >2,500–<3,000 g | 122 (23) | 272 (50) | 94 (17) | 46 (9) | 5 (1) | 539 |
| >3,000–<3,500 g | 200 (13) | 732 (47) | 386 (25) | 205 (13) | 43 (3) | 1,566 |
| >3,500–<4,000 g | 80 (6) | 589 (43) | 424 (31) | 226 (17) | 44 (3) | 1,363 |
| >4,000 g | 6 (3) | 49 (25) | 52 (27) | 82 (42) | 5 (3) | 194 |
| Breast weight (%) | ||||||
| <500 g | 287 (67) | 132 (31) | 6 (1) | 2 (0) | 0 | 427 |
| >500– < 750 g | 394 (21) | 1,036 (55) | 322 (17) | 99 (5) | 17 (1) | 1,868 |
| >750– < 1,000 g | 96 (5) | 722 (39) | 602 (32) | 386 (21) | 68 (4) | 1,874 |
| >1,000 g | 3 (2) | 19 (13) | 33 (23) | 74 (52) | 12 (9) | 141 |
n (% is based on the total number within each row).
Table 2.
Frequency analysis and the probability of occurrence of woody breast (WB) degrees of severity with respect to the growth production variables included in the prediction model.
| Variable | WB frequency1 |
Total | |||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ||
| Sex (%) | |||||
| Female | 258 (55) | 158 (33) | 44 (9) | 10 (2) | 470 |
| Male | 715 (19) | 1,558 (41) | 1,151 (30) | 430 (11) | 3,854 |
| Age (%) | |||||
| 4 wk | 338 (60) | 197 (35) | 22 (4) | 3 (1) | 560 |
| 6 wk | 619 (17) | 1,464 (41) | 1,090 (31) | 372 (11) | 3,545 |
| 8 wk | 16 (7) | 55 (25) | 83 (38) | 65 (30) | 219 |
| Strain (%) | |||||
| Standard breast yield A | 734 (38) | 771 (40) | 327 (17) | 103 (5) | 1,937 |
| High breast yield B | 43 (23) | 77 (41) | 59 (31) | 11 (6) | 190 |
| High breast yield C | 196 (9) | 868 (40) | 809 (37) | 324 (15) | 2,197 |
| Live weight (%) | |||||
| <2,000 g | 265 (68) | 119 (30) | 7 (2) | 1 (0) | 392 |
| >2,000– <2,500 g | 124 (46) | 117 (43) | 26 (10) | 3 (1) | 270 |
| >2,500–<3,000 g | 149 (28) | 191 (35) | 146 (27) | 53 (10) | 539 |
| >3,000–<3,500 g | 282 (18) | 681 (43) | 449 (29) | 154 (10) | 1,566 |
| >3,500–<4,000 g | 148 (11) | 559 (41) | 488 (36) | 168 (12) | 1,363 |
| >4,000 g | 5 (3) | 49 (25) | 79 (41) | 61 (31) | 194 |
| Breast weight (%) | |||||
| <500 g | 302 (71) | 118 (28) | 8 (2) | 0 | 428 |
| >500– < 750 g | 555 (30) | 861 (46) | 371 (20) | 91 (5) | 1,878 |
| >750– < 1,000 g | 115 (6) | 727 (39) | 755 (40) | 277 (15) | 1,874 |
| >1,000 g | 0 | 9 (6) | 60 (43) | 72 (51) | 141 |
n (% is based on the total number within each row).
Statistical Analysis
Frequency Analysis and Correlation Coefficient Values
Frequency analysis was used to investigate how overall, and each variable was distributed within each WS and WB severity score. The monotonic association between both myopathies scores was tested using Spearman's rank correlation coefficient. Correlation coefficients between 0.10 to 0.39 were considered as weak, values between 0.40 to 0.69 were moderate, and values between 0.70 to 0.89 were strong (Schober et al., 2018).
Predictive Models
Because outcomes were not equally spaced ordinal ranks (i.e., WB = 2 is not necessarily 2 times as bad as WB = 1, WB = 3 is not necessarily 3 times as bad as WB = 1, etc.), proportional odds ordinal logistic regression models would provide an appropriate and parsimonious modeling method to investigate the relationship between predictors and outcomes. P-values were derived using Wald statistics. No variable selection was performed. Variable importance was measured by how each variable contributed to the overall chi-square. The effect of predictors on the probability of broilers having high myopathy severity scores was reported as an odds ratio (OR), 95% CI, and P-values. Variables with odds ratio >1 increase the odds of having a high myopathy severity, odds ratio = 1 does not affect the odds of the outcome, and variables with odds ratio <1 decrease the odds (are protective). Importantly, these measures give the increase in the odds ratio for a 1-unit increase in the predictor variable. The probabilities of having a WS and WB > 1 for 2 hypothetical broilers were calculated to provide an example use of the models.
Model Validation
Model calibration was investigated using calibration plots and a discrimination measure of model performance was computed by 500-sample bootstrap resampling method to estimate the likely performance of the model on a new sample of broilers. Discrimination was expressed as a generalized area under the curve (AUC), which measures how well the model can identify the outcome levels apart. A value of 0.5 indicates no predictive discrimination and a value of 1.0 indicates perfect discrimination (Harrell et al., 1996).
The analysis was performed in R using the rms package (Harrell, 2018). P < 0.05 was considered statistically significant.
Results and discussion
Frequencies and Correlation Coefficients Values
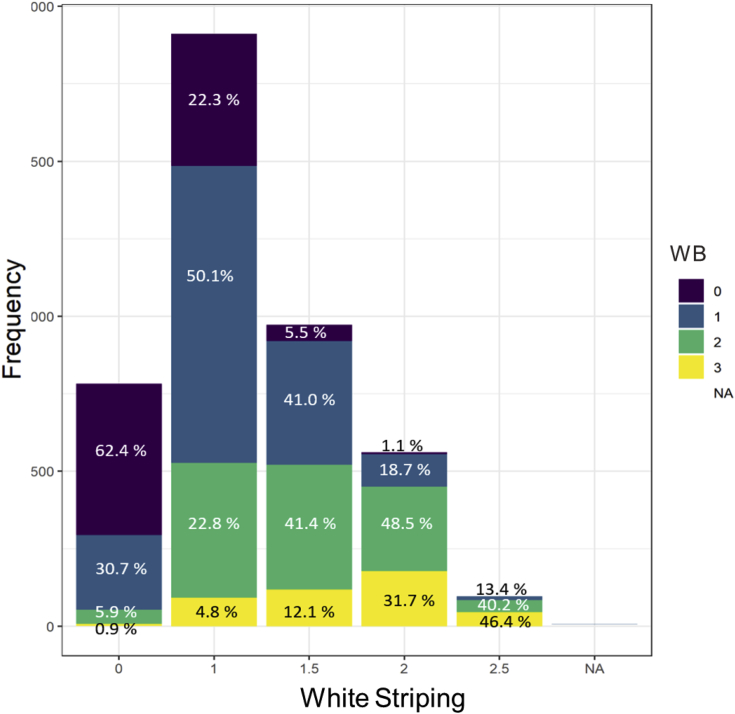
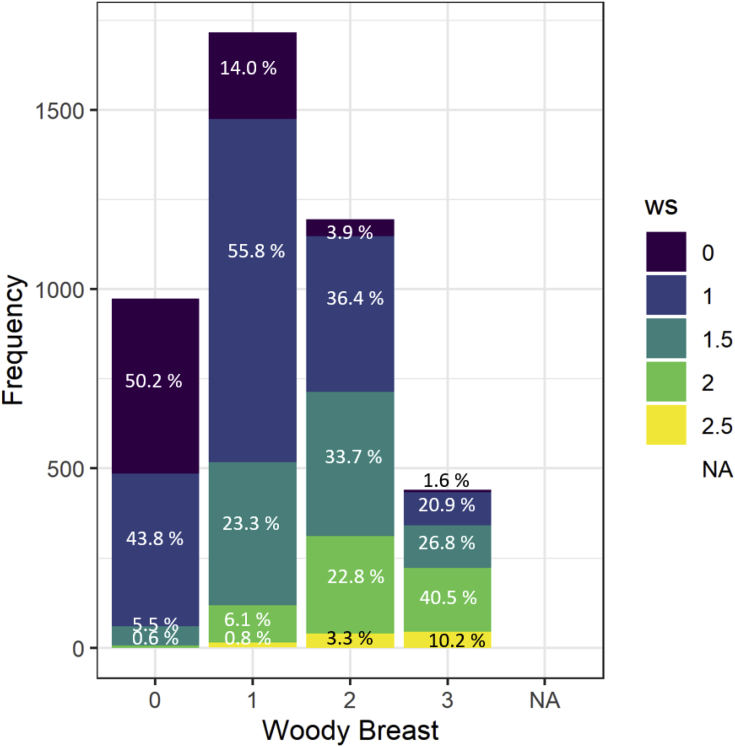
Frequency analysis of the occurrence of each WB scores within each WS scores category is shown in Figure 1. Results suggested that 62% of normal WS fillets (WS = 0) presented no WB. Among that breast fillets containing severe WS scores, 0% were normal, 13.4% mild, 40.2% moderate, and 46.4% had severe WB scores. In addition, frequency analysis of the occurrence of each WS scores within each WB scores category is presented in Figure 2. Data suggested that 50.2% of normal WB fillets (WB = 0) exhibited no WS scores. As WB severity scores increased, there was a decreasing trend of fillets that did not present WS (WS = 0), whereas there is a slightly increasing trend of severe scores of WS with the increased severity of WB scores. Similarly, it was estimated that 98.4% of severe WB fillets (WB = 3) presented some degree of WS, where 28.4% were normal to mild, 67.5% moderate, and 10.2% severe. Data demonstrated that WS was approximately more likely to be present in non-WB fillets, than WB present in non-WS breast fillets. These results are in agreement with Bowker et al. (2019). In this regard, Griffin et al. (2017) investigated the progressive changes and abnormal clinical presentation during Pectoralis major post-hatch growth and reported that WS was the first myopathy to be present in breast fillets at day 16.
Figure 1.
Frequency of breast fillets with different white striping scores and percentage of woody breast scores within each white striping score category.
Figure 2.
Frequency of breast fillets with different woody breast scores and percentage of white striping scores within each woody breast score category.
The association between WS and WB outcomes suggests a positive moderate relationship (ρ = 0.57, P = < 0.0001) between the ranks of both outcome variables (Table 3). In a recent study, Bowker et al. (2019) investigated the association between WS/WB and found similar results (rs = 0.55, P < 0.0001) in 2,600 breast fillets collected from a commercial processing plan. The results obtained also demonstrate that regardless of the growing method (commercial or in a controlled research environment), the association between both myopathies is similar.
Table 3.
Spearman's correlation coefficient between white striping (WS) and woody breast (WB) muscle conditions.
| Trait | ρ | P-value |
|---|---|---|
| WSWB | 0.57 | <0.0001 |
Predictive Models
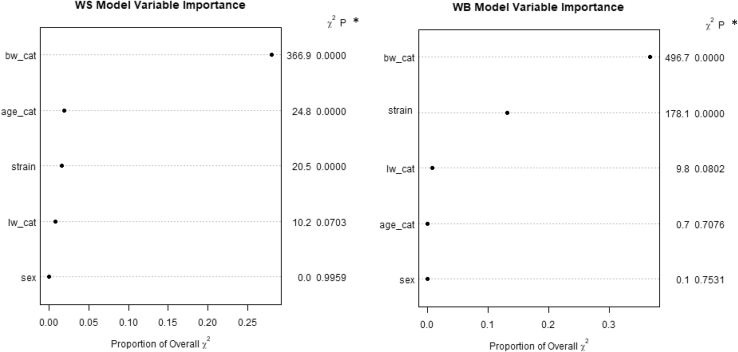
To better understand the relationship between predictors (variables) and outcomes (WS and WB), a proportional odds ordinal logistic regression model was fit to each outcome to determine the predictive ability of the study predictor variables. Statistical models for WS and WB are presented in Figure 3 (Table S1). Variables sex (P = 0.995) and live wt category (P = 0.07) in the WS model, and sex (P = 0.753), age (P = 0.707), and live wt category (P = 0.08) in the WB model, were not significant. As the primary objective of the study was to assess whether and to what degree each outcome could be predicted by the predictor variables, there is no need to simplify the model by performing variable selection. The full model consists of only 5 variables and is not greatly simplified by removing any of them. In addition, automatic variable selection via P-values, although widely used in procedures such as stepwise regression, can be dangerous and lead to incorrect inferences if not done correctly (Whittingham et al., 2006, Heinze et al., 2017). In addition, Figure 3 illustrates the importance of each predictor included in the WS and WB model. Each plot shows how important each variable was measured by how much it contributes to the overall Wald chi-square statistic. The x-axis is the amount of the chi-square statistic the variable accounts for. The higher the amount, the higher the importance of the predictor in the model. By this measure, breast wt was the most important predictor in the severity of WS, and breast wt together with strain in WB, whereas the least important for both outcomes was sex.
Figure 3.
Variable importance plots on the incidence of white striping (WS) and woody breast (WB) measured by Wald chi-square. ∗P = P–value, X2 = chi-square.
Whether factors increase or decrease the odds of moving into a higher level of the outcomes and by how much are shown in Table 4. Results obtained from this study showed that breast fillets above 750 g were 4.28 times (95% CI, 3.69–4.96) in WB and 3.59 (95% CI, 3.09–4.16) times in WS more likely to have higher myopathy severity scores compared to lighter fillets (P = < 0.0001), whereas fillets above 1,000 g were associated with the highest odds of increased WB (OR, 23.92; 95% CI, 15.91–35.97) and WS (OR, 13.90; 95% CI, 9.31–20.76) severity (P = < 0.0001). On the other hand, fillets weighing less than 500 g were 0.25 (95% CI, 0.16–0.37) times in WB and 0.37 (0.25–0.56) times in WS less likely to present high severity myopathy scores (P = < 0.0001). The proportional odds ordinal logistic regression model obtained in the present study demonstrated that breast weight was the most important predictor in the severity of WS and WB. These support previous research where moderate and severe degrees of WS were found in heavier fillets (Kuttappan et al., 2013). In addition, Griffin et al. (2017) documented the macroscopic changes occurring with age/growth in the breast muscle and found that breast muscle yield was the primary predictor for the severity of WB. It has been hypothesized that accelerated muscle growth reduced the interstitial space between the epimysium (connective tissue sheath) and the P. major muscle leading to muscle damage (Sihvo et al., 2014, Soglia et al., 2015).
Table 4.
White striping and woody breast odds ratio (OR) for variables1 included in the model.
| Dependent variable | White striping |
Woody breast |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Sex = m | 1.0 | 0.77 – 1.29 | 0.99 | 1.04 | 0.80 – 1.35 | 0.75 |
| Age = 6 wk | 3.08 | 1.88 – 5.03 | <0.0001 | 1.07 | 0.65 – 1.76 | 0.77 |
| Age = 8 wk | 2.21 | 1.23 – 3.94 | 0.007 | 0.95 | 0.53 – 1.70 | 0.88 |
| Strain B | 1.34 | 1.17 – 1.53 | <0.0001 | 2.47 | 2.15 – 2.83 | <0.0001 |
| Strain C | 1.44 | 1.04 – 1.99 | 0.02 | 2.49 | 1.81 – 3.42 | <0.0001 |
| Live wt = < 2,000 g | 0.85 | 0.53 – 1.35 | 0.5 | 1.33 | 0.83 – 2.13 | 0.22 |
| Live wt = > 2,500 – < 3,000 g | 0.98 | 0.63 – 1.53 | 0.93 | 1.64 | 1.05 – 2.56 | 0.02 |
| Live wt = > 3,000 – <3,500 g | 1.25 | 0.79 – 1.96 | 0.32 | 1.38 | 0.87 – 2.16 | 0.16 |
| Live wt = > 3,500 – <4,000 g | 1.34 | 0.84 – 2.13 | 0.2 | 1.29 | 0.81 – 2.05 | 0.26 |
| Live wt = > 4,000 g | 1.68 | 0.96 – 2.92 | 0.06 | 1.34 | 0.76 – 2.32 | 0.3 |
| Breast wt = < 500 g | 0.37 | 0.25 – 0.56 | <0.0001 | 0.25 | 0.16 – 0.37 | <0.0001 |
| Breast wt = > 750 – < 1,000 g | 3.59 | 3.09 – 4.16 | <0.0001 | 4.28 | 3.69 – 4.96 | <0.0001 |
| Breast wt = > 1,000 g | 13.90 | 9.31 – 20.76 | <0.0001 | 23.92 | 15.91 – 35.97 | <0.0001 |
Variables not presented in the table were used as a reference.
Furthermore, in the WS model, 6 wk (OR, 3.08; 95% CI, 1.88–5.03; P = <0.0001) and 8 wk (OR, 2.21; 95% CI, 1.23–3.94; P = 0.007) of age were associated with greater probability of higher severity of WS scores compared to 4-wk-old broilers. As for strain, results showed than strain B (OR, 1.34; 95% CI, 1.17–1.53; P = <0.0001) and C (OR, 1.44; 95% CI, 1.04–1.99; P = 0.02) which are bred to be high-breast-yielding broilers were associated with higher odds of increasing WS severity scores compared to strain A. Live wt categories and sex were not associated with the increase in WS severity scores (P > 0.05).
As for the WB model, the second most important attribute was strain, strain B (OR, 2.47; 95% CI, 2.15–2.83) and C (OR, 2.49; 95% CI, 1.81–3.42) were associated with higher odds of presenting increased severity of WB scores (P = < 0.0001) than strain A. In live wt categories, only broilers weighing >2,500–<3,000 g (OR, 1.64; 95% CI, 1.05– 2.56) were associated with the highest odds of having higher WB severity scores (P = 0.02). Although the rest of the categories showed OR higher than 1, the confidence intervals contain the relative risk of 1.00; therefore, the association between WB and age categories was not considered significant which is confirmed by the P > 0.05. Age categories and sex also showed no association with the increase in severity scores of WB (P > 0.05).
In regards to the live wt predictor, data showed a strong positive correlation between breast wt and live wt (ρ = 0.78; P = < 0.0001), and live wt and age (ρ = 0.75; P = < 0.0001). Care should be taken when individually interpreting the odds ratios provided by the models. For instance, when breast weight is removed from the model, the effect of live weight goes from having lower probability of increasing the severity of the outcome (protective against risk) to increasing risk. Thus, it is only jointly that these estimated odds ratios can be used to predict the outcomes. In addition, variable importance is conditional on the variables in the model. This means that variables might not look important because another variable is included but will become so if that variable is excluded. For instance, if breast weight is excluded then age and live weight become relatively more important. Therefore, considering the high correlation between these variables, the results from the present study showed that overall live wt and age can influence the severity of WS and WB myopathy scores. Similar results can be observed in the frequency analysis, where normal fillets gradually decrease with the increase in age and live wt. Moreover, in a previous study, Kuttappan et al. (2013) and Petracci et al. (2013) reported that heavier birds and thicker breast fillets are most likely to show WS than lighter birds. These results are also consistent with those of Griffin et al. (2017) who reported that the first documented case of WB was at day 23, and more severe characteristics were observed from day 30 onward including thick white striations.
Strain is the second and third more important variable in the severity of WB and WS, respectively. High-breast-yield strains seemed to have more impact on the severity of WB score compared to WS score. Analogous to our results, Bailey et al. (2015) reported that genetic selection has a role in the expression of both myopathies; however, it is not the main driving force. Moreover, Kuttappan et al., (2013) and Trocino et al. (2015) reported that differences between strains could be influenced by growth pattern. In addition, results in this study showed that, regardless of the strain, there is some degree of WS and/or WB present in breast fillets.
Sex was not significant variable in the prediction of WS and WB. Moreover, it also had significantly lower odds of increasing both myopathies severity. Although there is a large disparity in the sample size of females and males, results from this study are similar to previous research where gender had no significant effect on WS (Kuttappan et al. 2013) and WB (Chen et al., 2019) occurrence.
Hypothetical Examples
The hypothetical case scenarios represent examples of how the combination of different variables predict better or worse outcomes. Based on the proposed model, 2 hypothetical examples were generated and their predicted probabilities of having a score of WS and WB > 1 were computed. As detailed in Table 5, hypothetical broiler A is a 4-wk-old male with a standard breast yield B, 2,200 g live weight, 750 g breast weight. The baseline score for WS and WB was 1, respectively. The average scores of WS and WB are the scores multiplied by the probability of a broiler with the provided characteristics falling into that category added together. As a result, the average WS score was 1.01 and for WB was 1.61. The probability of being in a category above the baseline score in WB was 32.9% and 21.6% for WS. These results stand in contrast to those of broiler B (Table 5), who had an average WS score of 1.73 and 2.37 WB, and the probability of having scores above the baseline was higher (WB 89.6%, WS 84.2%).
Table 5.
Hypothetical case scenarios comparing 2 different broilers (1 and 2) to predict the probability of incidence of white striping (WS) and woody breast (WB) > score 1, derived from the predictive model.
| Variables | Broiler 1 | Broiler 2 |
|---|---|---|
| Sex | Male | Male |
| Age | 4 wk. | 8 wk. |
| Strain | Standard breast yield | High breast yield |
| Live weight | 2,200 | 4,000 |
| Breast weight | 750 | 1,000 |
| Baseline WS/WB | Score 1 | Score 1 |
| Average WS level | 1.01 | 1.73 |
| Average WB level | 1.61 | 2.365 |
| Probability of WS > 1 | 21.6% | 84.2% |
| Probability of WB > 1 | 32.9% | 89.6% |
Model Performance and Validation
The WS model produced an uncorrected AUC of 0.739, and a bootstrap corrected estimate of 0.736. The WB model produced an uncorrected AUC of 0.753 and a bootstrap corrected estimate of 0.752. The small difference between uncorrected and corrected estimates suggests that the models are not overfitting the data (Harrell et al., 1996). This is a promising sign for the model's ability to perform well on similar but new samples of data.
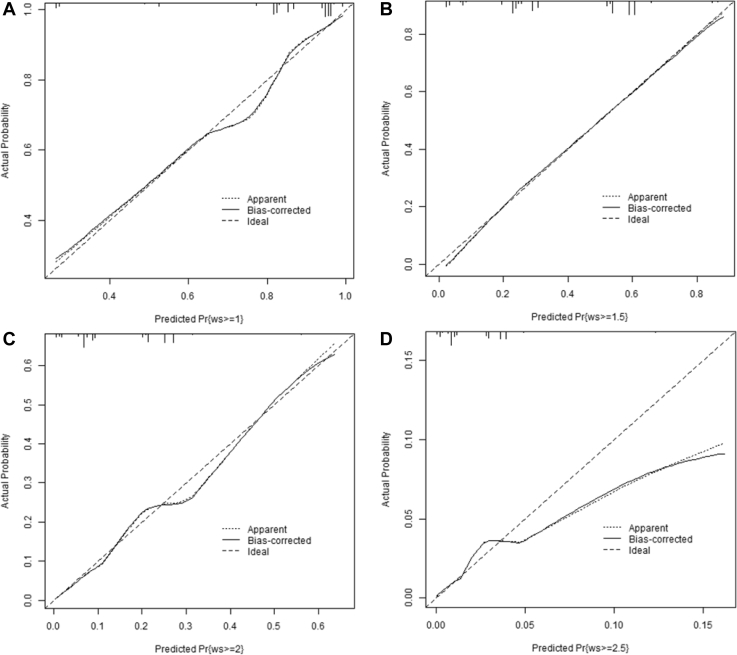
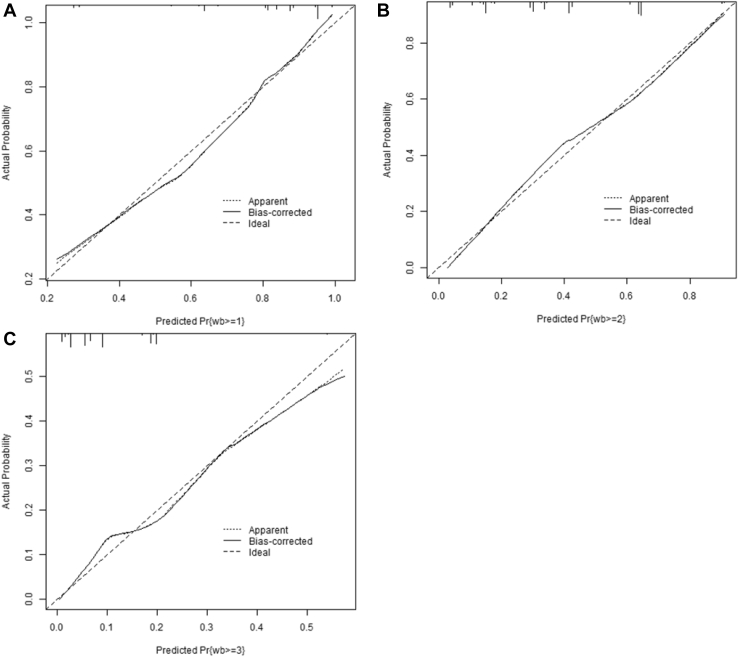
The calibration plots (Figures 4 and 5) suggest good calibration for all levels of each outcome except WS ≥ 2.5 (Figure 4D). This plot suggests slight overfitting (the model is providing probabilities that are higher than what would be expected from the observed data). This is due to the relatively small number of observations in the highest levels of the outcome. There are only 97 observations with WS = 2.5.
Figure 4.
Model validation plots using bootstrap calibration for the predictive probability of white striping (A) ≥1, (B) ≥1.5, (C) ≥2, and (D) ≥2.5.
Figure 5.
Model validation plots using bootstrap calibration for the predictive probability of woody breast (A) ≥1, (B) ≥2, and (C) ≥3.
Overall, the accuracy of the model's prediction probabilities is good (Harrell et al., 1996). Therefore, the predicted probabilities can likely be trusted in all cases except when trying to predict whether an observation will fall into the highest categories.
Conclusions
It was demonstrated that there is a positive moderate association between WB and WS. Growth production factors analyzed in this study significantly influence the severity of WS and WB myopathy scores. The application of the proposed model is that companies having data sets could plug in values for variables in this model and get predicted probability in the way that the hypothetical examples provided do. Most importantly, novel predictive models constructed with data sets hold a potential to reduce the severity of WB and WS by identifying important modifiable factors antemortem. The potential limitations of the present study can be associated with the combined data across experiments. Any inconsistencies in the protocol or deviations could make data sets not suitable for pooling. Finally, potentially other factors not included in this study can play a role in the predictive model of WB and WS severity and further research should be conducted.
Acknowledgments
Conflict of Interest Statement: The authors did not provide a conflict of interest statement.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.psj.2020.03.026.
Supplementary data
References
- Aguirre M.E., Owens C.M., Miller R.K., Alvarado C.Z. Descriptive sensory and instrumental texture profile analysis of woody breast in marinated chicken. Poult. Sci. 2018;97:1456–1461. doi: 10.3382/ps/pex428. [DOI] [PubMed] [Google Scholar]
- Aziz A., Shariat S.F., Roghmann F., Brookman-May S., Stief C.G., Rink M. Prediction of cancer-specific survival after radical cystectomy in pT4a urothelial carcinoma of the bladder: development of a tool for clinical decision-making. BJU Int. 2016;117:272–279. doi: 10.1111/bju.12984. [DOI] [PubMed] [Google Scholar]
- Bailey R.A., Watson K.A., Bilgili S.F., Avendano S. The genetic bases of pectoralis major myopathies in modern broiler chicken lines. Poult. Sci. 2015;94:2870–2879. doi: 10.3382/ps/pev304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodle B.C., Alvarado C., Shirley R.C., Mercier Y., Lee J.T. Evaluation of different dietary alterations in their ability to mitigate the incidence and severity of woody breast and white striping in commercial male broilers. Poult. Sci. 2018;97:3298–3310. doi: 10.3382/ps/pey166. [DOI] [PubMed] [Google Scholar]
- Bowker B., Zhuang H., Yoon S.C., Tasoniero G., Lawrence K. Relationships between attributes of woody breast and white striping myopathies in commercially processed broiler breast meat. J. Appl. Poult. Res. 2019;0:1–7. [Google Scholar]
- Chen L.R., Suyemoto M.M., Sarsour A.H., Cordova H.A., Oviedo-Rondón E.O., Wineland M., Barnes H.J., Borst L.B. Temporal characterization of wooden breast myopathy (“woody breast”) severity and correlation with growth rate and lymphocytic phlebitis in three commercial broiler strains and a random-bred broiler strain. Avian Pathol. 2019;48:319–328. doi: 10.1080/03079457.2019.1598541. [DOI] [PubMed] [Google Scholar]
- Dransfield E., Sosnicki A.A. Relationship between muscle growth and poultry meat quality. Poult. Sci. 1999;78:743–746. doi: 10.1093/ps/78.5.743. [DOI] [PubMed] [Google Scholar]
- Food Safety and Inspection Services Disposition Instructions for “Woody Breast” and “White Striping” Poultry Conditions. 2018. https://content.govdelivery.com/accounts/USFSIS/bulletins/203655d
- Griffin J.R., Moraes L., Wick M., Lilburn M.S. Onset of white striping and progression into wooden breast as defined by myopathic changes underlying Pectoralis major growth. Estimation of growth parameters as predictors for stage of myopathy progression. Avian Pathol. 2017;47:2–13. doi: 10.1080/03079457.2017.1356908. [DOI] [PubMed] [Google Scholar]
- Harrell F.E., Jr. Regression modeling Strategies. 2018. http://cran.nexr.com/web/packages/rms/index.html
- Harrell F.E., Kerry L., Mark D.B. Multivariable Prognostic models: issues in developing models, Evaluating Assumptions and Adequacy and measuring and reducing Errors. Stat. Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Heinze G., Wallisch C., Dunkler D. Variable selection – a review and recommendations for the practicing statistician. Biomet. J. 2017;60:431–449. doi: 10.1002/bimj.201700067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuttappan V.A., Lee Y., Erf G.F., Meullenet J.F., Owens C.M. Consumer acceptance of visual appearance of broiler breast meat with varying degrees of white striping. Poult. Sci. 2012;91:1240–1247. doi: 10.3382/ps.2011-01947. [DOI] [PubMed] [Google Scholar]
- Kuttappan V.A., Brewer V.B., Apple J.K., Waldroup P.W., Owens C.M. Influence of growth rate on the occurrence of white striping in broiler breast fillets. Poult. Sci. 2012;91:2677–2685. doi: 10.3382/ps.2012-02259. [DOI] [PubMed] [Google Scholar]
- Kuttappan V.A., Brewer V.P., Mauromoustakos A., McKee S.R., Emmert J.L., Meullenet J.F., Owens C.M. Estimation of factors associated with the occurrence of white striping in broilers breast fillets. Poult. Sci. 2013;92:811–819. doi: 10.3382/ps.2012-02506. [DOI] [PubMed] [Google Scholar]
- Michaelson J.S., Chen L.L., Bush D., Fong A., Smith B., Younger J. Improved web-based calculators for predicting breast cancinoma outcomes. Breast Cancer Res. Treat. 2011;128:827–835. doi: 10.1007/s10549-011-1366-9. [DOI] [PubMed] [Google Scholar]
- McGirt M.J., Bydon M., Archer K.R. An analysis from the Quality Outcomes Database, Part 1: disability, quality of life, and pain outcomes following lumbar spine surgery: predicting likely individual patient outcomes for shared decision-making. J. Neurosurg. Spine. 2017;27:357–369. doi: 10.3171/2016.11.SPINE16526. [DOI] [PubMed] [Google Scholar]
- Mudalal S., Lorenzi M., Soglia F., Cavani C., Petracci M. Implications of white striping and wooden breast abnormalities on quality traits of raw and marinated chicken meat. Animal. 2014;9:728–734. doi: 10.1017/S175173111400295X. [DOI] [PubMed] [Google Scholar]
- National Chicken Council Per Capita Consumption of Poultry and Livestock, 1965 to Estimated 2019, in Pounds. 2018. https://www.nationalchickencouncil.org/about-the-industry/statistics/per-capita-consumption-of-poultry-and-livestock-1965-to-estimated-2012-in-pounds/
- Neth J., Parker J. 2018 Chicken Consumption Survey: What Consumers Want. WATTAgNet. 2018. https://www.wattagnet.com/media/videos/play/85
- Petracci M., Cavani C. Muscle growth and poultry meat quality issues. Nutrients. 2012;4:1–12. doi: 10.3390/nu4010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petracci M., Mudalal S., Bonfiglio A., Cavani C. Occurrence of white striping under commercial conditions and its impact on breast meat quality in broiler chickens. Poult. Sci. 2013;92:1670–1675. doi: 10.3382/ps.2012-03001. [DOI] [PubMed] [Google Scholar]
- Schober P., Boer C., Schwarte L.A. Correlation coefficients: appropriate Use and Interpretation. Anesth. Analg. 2018;126:1763–1768. doi: 10.1213/ANE.0000000000002864. [DOI] [PubMed] [Google Scholar]
- Sihvo H.K., Immonen K., Puolanne E. Myodegeneration with fibrosis and regeneration in the pectoralis major muscle of broilers. Vet. Pathol. 2014;51:619–623. doi: 10.1177/0300985813497488. [DOI] [PubMed] [Google Scholar]
- Soglia F., Gao J., Mazzoni M., Puolanne E., Cavani C., Petracci M., Ertbjerg P. Superficial and deep changes of histology, texture and particle size distribution in broiler wooden breast muscle during refrigerated storage. Poult. Sci. 2017;96:3465–3472. doi: 10.3382/ps/pex115. [DOI] [PubMed] [Google Scholar]
- Soglia F., Mudalal S., Babini E., Di Nunzio M., Mazzoni M., Sirri F., Cavani C., Petracci M. Histology, composition, and quality traits of chicken Pectoralis major muscle affected by wooden breast abnormality. Poult. Sci. 2015;95:651–659. doi: 10.3382/ps/pev353. [DOI] [PubMed] [Google Scholar]
- Tijare V.V., Yang F.L., Kuttappan V.A., Alvarado C.Z., Coon C.N., Owens C.M. Meat quality of broiler breast fillets with white striping and woody breast muscle myopathies. Poult. Sci. 2016;95:2167–2173. doi: 10.3382/ps/pew129. [DOI] [PubMed] [Google Scholar]
- Trocino A., Piccirillo A., Birolo M., Radaelli G., Bertotto D., Filiou E., Petracci M., Xiccato G. Effect of genotype, gender and feed restriction on growth, meat quality and the occurrence of white striping and woody breast in broiler chickens. Poult. Sci. 2015;94:2996–3004. doi: 10.3382/ps/pev296. [DOI] [PubMed] [Google Scholar]
- Velleman S.G., Nestor K.E. Effect of selection for growth rate on myosin heavy chain temporal and spatial localization during Turkey breast muscle development. Poult. Sci. 2003;82:1373–1377. doi: 10.1093/ps/82.9.1373. [DOI] [PubMed] [Google Scholar]
- Whittingham M.J., Stephens A.P., Bradbury R.B., Freckleton R.P. Why do we still use stepwise modelling in ecology and behaviour? J. Anim. Eco. 2006;75:1182–1189. doi: 10.1111/j.1365-2656.2006.01141.x. [DOI] [PubMed] [Google Scholar]
- Zuidhof M.J., Schneider B.L., Carney V.L., Korver D.R., Robinson F.E. Growth, efficiency, and yield of commercial broilers from 1957, 1978, and 20051. Poult. Sci. 2014;93:2970–2982. doi: 10.3382/ps.2014-04291. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.