Abstract
Genistein is abundant in the corn-soybean meal feed. Little information is available about the effect of dietary genistein on the intestinal transcriptome of chicks, especially when suffering from intestinal injury. In this study, 180 one-day-old male ROSS 308 broiler chickens were randomly allocated to 3 groups, with 4 replicates (cages) of 15 birds each. The treatments were as follows: chicks received a basal diet (CON), a basal diet and underwent lipopolysaccharide-challenge (LPS), or a basal diet supplemented with 40 mg/kg genistein and underwent LPS-challenge (GEN). LPS injection induced intestinal injury and inflammatory reactions in the chicks. Transcriptomic analysis identified 7,131 differently expressed genes (3,281 upregulated and 3,851 downregulated) in the GEN group compared with the LPS group (P adjusted value < 0.05, |fold change| > 1.5), which revealed that dietary genistein exposure altered the gene expression profile and signaling pathways in the ileum of LPS-treated chicks. Furthermore, dietary genistein improved intestinal morphology, mucosal immune function, tight junction, antioxidant activity, apoptotic process, and growth performance, which were adversely damaged by LPS injection. Therefore, adding genistein into the diet of chicks can alter RNA expression profile and ameliorate intestinal injury in LPS-challenged chicks, thereby improving the growth performance of chicks with intestinal injury.
Key words: genistein, lipopolysaccharide, broiler, transcriptome, intestine
Introduction
Intestinal diseases are one of the main factors that plague human health and animal production. However, “prohibition of the antibiotic uses” aggravates the problem of intestinal diseases in poultry. As we all know, external stimulation or internal environmental disturbance can promote the proliferation of pathogenic microorganisms, thus destroying the intestinal barrier function (Mauro et al., 2013). Lipopolysaccharide (LPS) derives from the cell wall of gram-negative bacteria, such as Escherichia coli and Proteus. As an endotoxin, LPS can upregulate the expressions of TLR4-MyD88-IRAK, then activating NF-κB signaling pathway and MAP family enzyme through tumor necrosis factor receptor–associated factor-6, which further induces inflammatory reaction and oxidative stress (Fukata and Abreu, 2006). Therefore, it has been widely applied to establish a model of intestinal injury in chickens (Hou et al., 2010, Wang et al., 2017). It is necessary to develop new feed additives with therapeutic potential for disrupted intestinal homeostasis of LPS-challenged in broilers. In this study, LPS intraperitoneal injection was used to establish the intestinal injury model of chicks.
Genistein, a kind of natural phytoestrogens from soybeans, can be absorbed directly by intestinal epithelial cells through passive diffusion (Soukup et al., 2016). It is suggested that genistein inhibits the activity of tyrosine kinase and mitogen activated protein kinase (MAPK)/NF-κB signalling pathway, which play important roles in anti-inflammatory effects (Markovits et al., 1989, Byun et al., 2014). Similarly, a previous study indicated that genistein can inhibit NF-κB activation and IL-17 secretion in CD4+ T cells, which relieve the inflammation reaction (Kehlen et al., 1999). Tight junctions between intestinal epithelial cells constitute a barrier with selective permeability, which not only facilitates to transfer ions and solutes but also prevents intestinal microorganisms, antigens, and toxins from translocating into tissues. It is reported that genistein (300 M) can inhibit the invasion of pathogenic microorganisms (Listeria monocytogenes, Salmonella typhimurium, and E. coli) into Caco-2 cells through inhibiting tyrosine protein kinase, which is conducive to maintaining the integrity of tight junction (Wells et al., 1999). Further studies have suggested that genistein can inhibit tyrosine phosphorylation of intestinal tight junction protein and alleviate intestinal barrier dysfunction induced by oxidative stress and inflammatory factors (Noda et al., 2012).
Genistein, as a potential substitute for antibiotics, has practical application value in animal production (Iqbal et al., 2014). Rasouli and Jahanian (2015) suggested that adding genistein to the diet can improve the growth performance, feed intake, and body weight of ROSS broilers and reduce the feed-meat ratio (Rasouli and Jahanian, 2015). However, little information is known about the effect of dietary genistein on the intestinal transcriptome of chicks, especially when suffering from intestinal injury. Owing to the rapid advancement of sequencing technologies, omics are increasingly being applied to “unusual” species to generate information that allows better understanding of biological characteristics in fields ranging from metabolism to immune research. Therefore, the present research was designed to evaluate the effects of dietary genistein on mRNA expression landscapes in the ileum of LPS-treated chicks.
Materials and methods
Animals and Experimental Design
All procedures for animal handling were conducted under protocols approved by the Animal Welfare Committee of Nanjing Agricultural University (Nanjing, China, permit number NJAU/20191683-C1). In addition, all experiments were performed in accordance with approved relevant guidelines and regulations. A total of 180 one-day-old male ROSS 308 broiler chickens were randomly allocated to 3 groups, with 4 replicates of 15 birds each. The present experiment lasted for 21 D (from 1 to 21 D of age). The treatments were as follows: birds received a basal diet (CON, genistein monomer concentration = 0.72 ± 0.24 mg/kg), birds received a basal diet and underwent LPS-challenge (LPS), or birds received a basal diet supplemented with 40 mg/kg genistein and underwent LPS-challenge (GEN, genistein monomer concentration = 41.2 ± 3.3 mg/kg). GEN is a synthetic product from Kai Meng. Co. (Xi An, China) Chemical Plant with 99.9% purity. LPS is from E. coli (L2880; Sigma Aldrich Inc., St. Louis, MO). The basal diet was formulated based on the nutrient requirements given by the National Research Council (NRC, 1994; Table 1). The adding level of genistein in the current experiment is according to our previous researches (Lv et al., 2018a, Lv et al., 2018b, Lv et al., 2018c). All broilers were housed in wired cages in a temperature- and light-controlled room on a 23 h/D-lighting programme and routinely immunized. All broilers had ad libitum access to diet and water. The chickens were housed in wire cages under a standard, gradually decreasing temperature regimen that ranged from 35°C to 26°C. LPS was dissolved in 0.9% sterile saline solution. At 7:00 am of 17, 19, and 21 D, the LPS and GEN groups received an intraperitoneal injection of lipopolysaccharide solution at a dose of 1 mg/kg, whereas the CON group received sterile saline injection. The dosage and injection of LPS were referred to available findings (Tan et al., 2014, Chen et al., 2018).
Table 1.
Diet composition and nutrient levels.
| Ingredient | Feed formula (0–3 wk, %) |
|---|---|
| Corn | 53.28 |
| Soybean meal | 38.57 |
| Limestone | 1.05 |
| Soybean oil | 3.70 |
| Dicalcium phosphate | 1.98 |
| NaCl | 0.35 |
| 1Trace mineral premix | 0.30 |
| Choline chloride (50%) | 0.30 |
| DL-methionine | 0.22 |
| 2Vitamin premix | 0.02 |
| Santoquin | 0.03 |
| Lysine·HCl (8%) | 0.12 |
| Tatal | 100.00 |
| Avian metabolic energy MC/kg | 2.95 |
| Crude protein | 21.6 |
| Calcium | 1.05 |
| Tatal phosphorus | 0.70 |
| Available phosphorus | 0.45 |
| Methionine | 0.50 |
| Lysine | 1.15 |
| Met + Cys | 0.86 |
| Threonine | 0.80 |
The following was supplied per kg complete diet: Cu, 8 mg; Zn, 75 mg; Fe, 80 mg; Mn, 100 mg; Se, 0.15 mg; I, 0.35 mg.
The following was supplied per kg complete diet: vitamin A, 12,500 IU; vitamin D3, 2500 IU; vitamin E, 30 IU; vitamin K3, 2.65 mg; thiamine, 2 mg; riboflavin, 6 mg; vitamin B12, 0.025 mg; biotin, 0.0325 mg; folic acid, 1.25 mg; pantothenic acid, 12 mg; niacin, 50 mg.
Growth Performance and Sample Collection
On day 21, body weight and feed consumption were measured for each cage to calculate the feed conversion ratio (kg of feed consumed/kg of live BW), after 10-h feed deprivation. Three hours after injection of LPS at 21 D of age, 2 broilers in each replicate (8 broilers per treatment) with body weights close to the average were selected. Then, the chickens were sacrified by intravenous injection of pentobarbital sodium (30 mg/kg body weight) and jugular exsanguination. Sections of approximately 2 cm in length were cut off from the middle of each ileum. The ileac sections were promptly fixed in 4% paraformaldehyde for histological analyses. The midregion of the ileum (∼1 cm) was sampled, flushed gently with saline to remove digesta and immediately frozen in liquid nitrogen, and stored at −80°C for RNA extraction. Mucosa from the second half of the ileum (from Meckel's diverticulum to the midpoint of ileum) was scrapped, immediately frozen in liquid nitrogen, and stored at −20°C for subsequent determination of secretory immunoglobulin (sIgA) and total antioxidative capability (TAOC).
Intestinal Morphology Analysis
After fixation in 4% paraformaldehyde for 24 h, the ilea were soaked through a graded series of ethanol and xylene, embedded in paraffin, and sectioned at 5 μm with a Lecia RM2235 microtome (Leica Biosystems Inc., Buffalo Grove, IL). The sections were deparaffinized with xylene and rehydrated through graded dilutions of ethanol and stained with hematoxylin and eosin. The images of ilea were acquired using an Olympus simon-01 microscope (Olympus Optical Co., Ltd., Beijing, China). The values of villus height and crypt depth were measured 5 times from different villus and crypts per section from each broiler using the Image-Pro Plus software 6.0 (Media Cybernetics, Inc., Washington, DC). The average value of the 5 villus and crypts represent the value of each ileum sample.
TUNEL Assay and Assessments of TAOC, sIgA, and Antibody Titer of Newcastle Disease
Intestinal apoptosis was determined using a terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick-end labeling (TUNEL) assay with a TUNEL BrightGreen Apoptosis Detection Kit (Vazyme Biotech, Nanjing, China). TAOC and protein content of the ileal mucosae was detected using commercial reagent kits (Beyotime Biotechnology, Shanghai, China). The contents of sIgA were detected using ELISA commercial kit (Mlbio Co., Ltd., Shanghai, China). Te serum antibody titer against Newcastle disease (ND) viruses was determined using a commercial ELISA kit (IDEXX laboratories Inc., Westbrook, ME). All experimental procedures were performed according to the manufacturer's instructions. The result was normalized to protein concentration in each sample.
RNA Extraction and Qualification
TRIzol reagent (Invitrogen, Carlsbad, CA) was used to isolate the total RNA of each sample. The purity of RNA was checked using a NanoPhotometer R spectrophotometer (IMPLEN; Westlake Village, CA). The concentration of RNA was tested using Qubit R RNA Assay Kit in Qubit 2.0 Flurometer (Life Technologies, Carlsbad, CA). In addition, RNA integrity was assessed with RNA Nano 6000 Assay Kit in the Bioanalyzer 2100 system (Agilent Technologies, Santa Clara, CA). The samples with RNA integrity number scores higher than 8 were used in this study.
Process of High-Throughput RNA Sequencing
RNA Sequencing (RNA-Seq) of chick ileum was performed in Novogene Bioinformatics Institute (Beijing, China) on an Illumina HiSeq 4,000 platform, and 150 bp pairedend reads were generated after clustering of the index-coded samples. Standardized processes, including library preparation, sequencing, and transcriptome assembly are shown in Supplementary Data 1.
Expression Analysis by RNA-Seq and Quantitative PCR
The protein-coding gene expression levels in each sample were estimated according to fragments per kilo-base of exon per million fragments mapped and assessed with the analysis software Cufflinks v2.1.1 (Ghosh and Chan, 2016). P adjusted values were calculated using the Benjamini-corrected modified Fisher's exact test, Transcripts with a P adjusted value < 0.05, |fold change| > 1.5, were considered differentially expressed. The results of RNA sequencing were validated through qPCR. qPCR was performed using the LightCycler 480 real-time PCR system and SYBR Green PCR Master Mix (TaKaRa Biotechnology, Dalian, China). Total RNA was isolated from the ileac tissues using Trizol reagent (Invitrogen Life Technologies, Carlsbad, CA). cDNA was generated using the PrimeScript RT reagent kit with cDNA eraser (Takara, Dalian, China). The specific quantitative primers for 7 transcripts are listed in Supplementary Data 2. Quantitative PCR assays were carried out using the SYBR Premix Ex TaqTM kit (Takara, Dalian, China). The conditions were 95°C for 2 min followed by 40 cycles (95°C for 20 s, 60°C for 30 s, and 68°C for 30 s). Each experiment was performed in triplicate. Target gene expression was quantified using the 2-△△CT method and normalized to the expression of glyceraldehyde-3-phosphate dehydrogenase.
Gene Ontology, Kyoto Encyclopedia of Genes and Genomes, and Protein-Protein Interaction (PPI) Analysis
Differently expressed gene (DEG) lists were submitted to the databases of gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) for enrichment analysis of the significant overrepresentation of GO terms and KEGG-pathway categories (Ashburner et al., 2000, Minoru et al., 2008). In all tests, P adjusted values were calculated using the Benjamini-corrected modified Fisher's exact test, and values being <0.05 was taken as a threshold of significance. Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) is a widely used biologic database and Web resource of known and predicted protein-protein interactions. A network model was generated using the Cytoscape Web application using information gained from 4 levels of functional analysis: fold changes of genes/proteins, protein-protein interactions, KEGG pathway enrichment, and biologic process enrichment. A default confidence cutoff of 400 was used: Interactions with confidence scores above that threshold are shown as solid lines between genes/proteins, and the remaining interactions are shown as dashed lines.
Statistical Analysis
The results are expressed as means ± standard deviation or the means ± standard error of mean (for gene expression), and differences were considered significant when P < 0.05, as calculated by ANOVA with SPSS 11.0 for Windows (SPSS Inc., Chicago, IL).
Results
Growth Performance
The effects of GEN treatment on the growth performance of LPS-treated broilers are shown in Table 2. The results demonstrated that LPS injection significantly decreased the body weight gain and feed intake of chicks aged 1 to 21 D (P < 0.05). However, dietary genistein supplementation significantly increased the body weight gain and feed intake in comparision with the LPS group (P < 0.05). There were no significant effects of genistein and LPS treatment on the feed-gain ratio of chicks.
Table 2.
Effects of dietary genistein on the growth performance of chicks at 21 D of age.
| Treatment | Body weight gain (g) | Feed intake (g) | Feed-gain ratio |
|---|---|---|---|
| CON | 700 ± 19a | 959 ± 13a | 1.38 ± 0.11 |
| LPS | 548 ± 16c | 826 ± 13c | 1.51 ± 0.13 |
| LPS + GEN | 605 ± 38b | 863 ± 11b | 1.43 ± 0.30 |
| P value | 0.002 | <0.001 | 0.348 |
The data were expressed as the mean ± SD (n = 4 replicate cages).
a-cSuperscripts represent significant difference (P < 0.05).
Abbreviations: CON, nonchallenge control; GEN, lipopolysaccharide-challenged group fed diet supplemented with 40 mg/kg genistein; LPS, lipopolysaccharide-challenged group; SD, standard deviation.
Intestinal Morphological Analyses and TUNEL Assay
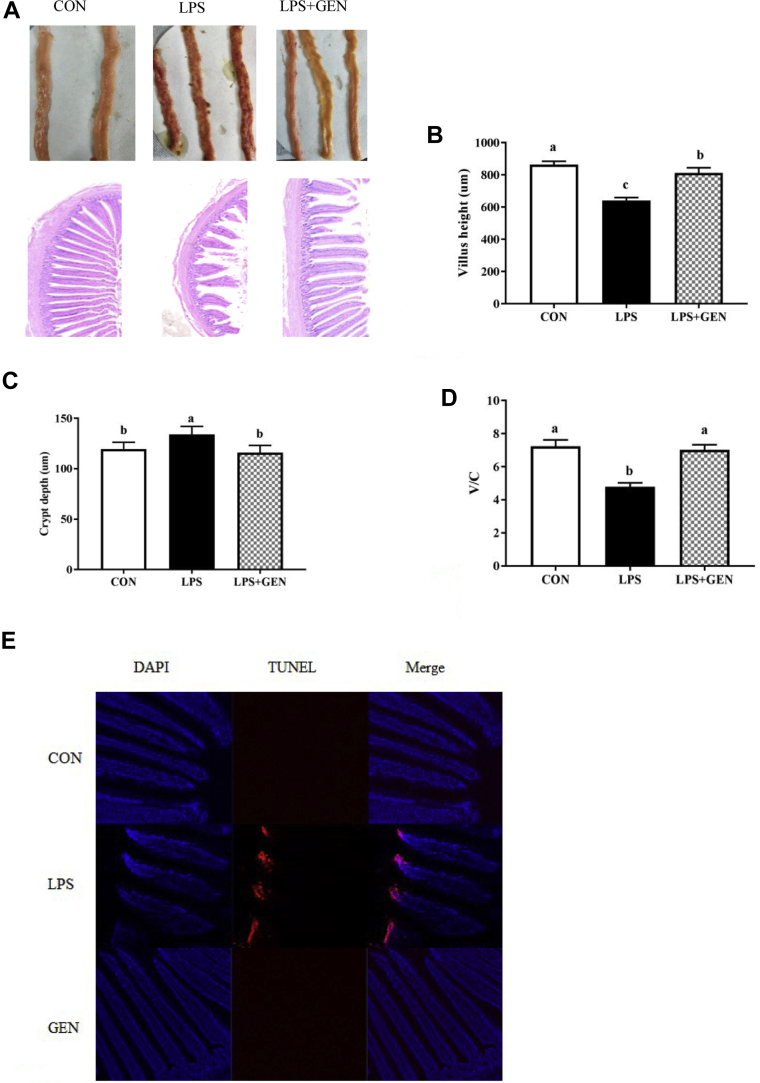
According to Figure 1, the intestinal barrier was significantly damaged by LPS injection. There were obvious bleeding points in the ileum of the LPS injected group. The slice of pathology showed that the ileac mucosa of chicks in the LPS group grew thinner, along with intestinal villus swelling and failing off (Figure 1A). Furthermore, LPS injection decreased the villus height and villus height-crypt depth ratio, along with the increased crypt depth, compared to the CON group (Figure 1, A–C). Dietary genistein significantly relieved the symptoms of intestinal bleeding and impaired villus morphology, which were induced by LPS challenge. As shown in Figure 1E, the apoptotic cells were primarily distributed to the apical region of ileac villus. LPS-challenged broilers exhibited a greater percentage in the ileac mucosae than the other groups. In contrast, the GEN group had a lower apoptotic index in the ileac mucosae than the LPS group.
Figure 1.
The effects of genistein on the villus morphology and apoptosis status of the ileum. (A) Representative photographs of the ileac appearance and cross section in chicken. (B) Comparison of ileum villus height between the 3 groups. (C) Comparison of ileum crypt depth between the 3 groups. (D) Comparison of ileum villus height/crypt depth between the 3 groups. Original magnification is 40×. (n = 8). (E) TUNEL assay of the ileac sections by immunofluorescence. Scale bar = 200 μm. a-cLetters represent significant differences (P < 0.05). Abbreviations: CON, nonchallenged broilers fed a basal diet; GEN, lipopolysaccharide-challenged group fed diet supplemented with 40 mg/kg genistein; LPS, LPS-challenged broilers fed a basal diet.
Levels of Serum sIgA, Antibody Titer of ND, and TAOC in the Ileac Mucosa
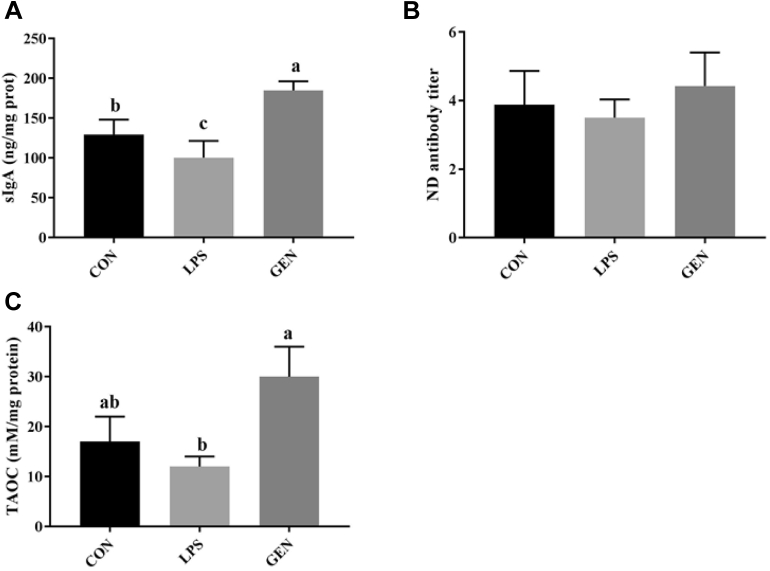
As shown in Figure 2A, LPS injection decreased the levels of sIgA in the ileac mucosa. However, the levels of sIgA in the ileum mucosa of GEN groups were significantly higher than those in the CON and LPS groups (P < 0.001). Similarly, dietary genistein supplementation reversed the decreased levels of TAOC induced by LPS injection (P < 0.05; Figure 2C). However, there is no significant difference in the ND level among the 3 groups, which might be due to missing the best immune response period after vaccination (Figure 2B).
Figure 2.
The effects of genistein on the immune indexes and antioxidative capability of LPS-treated chicks. (A) The effects of genistein on the serum level of sIgA. (B) The effects of genistein on the serum level of ND antibody titer. (C) The effects of genistein on the level of TAOC in the ileum. a-cLetters represent significant differences (P < 0.05). Abbreviations: CON, nonchallenge control; GEN, lipopolysaccharide-challenged group fed diet supplemented with 40 mg/kg genistein; LPS, lipopolysaccharidechallenged group; ND, antibody titer of Newcastle disease; sIgA, secretory immunoglobin A (ng/mg prot); TAOC, total antioxidative capability (mM/mg protein).
Quality Control of RNA-Seq Data and Identification of Transcripts Expressed in the Chick Ileums
In this study, we established 9 cDNA libraries from the ileums of chicks in the CON, LPS, and GEN groups, with 3 replicates in each group. An RNA-Seq quality control summary, including base content along reads, error rate distribution along reads, classification of raw data, and percent of genome regions, is shown in Supplementary Data 3A. We concluded that the quality of RNA-Seq data was reliable and qualified. RNA-Seq generated 55061878 to 72871048 raw reads for each library, with an average of 59821721, 67250158, and 63405653 paired-end reads for the CON, LPS, and GEN groups, respectively. Low-quality reads were filtered out, and the average numbers of clean reads were 57554141, 64675796, and 62046788 for the CON, LPS, and GEN groups, respectively. The clean reads were used for all further analyses. After assembly, a total of 20,733 mRNAs were obtained from the 3 groups. The average mapping rates were 86.17, 85.17, and 90.31% for the CON, LPS, and GEN groups, respectively (Table 3). The mapped reads of different regions of the genome are displayed in Supplementary Data 3B. The top 10 most abundantly expressed genes among the 3 groups, ranked by absolute abundance, were APOA1, COX1, COX3, ATP6, COII, GUCA2A, PLA2G2E, FABP2, UBB, and FTH1 (Supplementary Data 4).
Table 3.
Characteristics of the reads from 12 embryo liver libraries.
| Sample ID | raw_reads | clean_reads | error_rate (%) | Q20(%) | GC_pct (%) | Mapping ratio (%)1 |
|---|---|---|---|---|---|---|
| CON1 | 62224764 | 59541880 | 0.03 | 97.26 | 50.47 | 86.74 |
| CON2 | 55061878 | 53710768 | 0.03 | 97.72 | 51.6 | 86.39 |
| CON3 | 62178520 | 59409776 | 0.03 | 97.34 | 50.91 | 85.37 |
| LPS1 | 64366450 | 61450866 | 0.03 | 97.12 | 50.33 | 83.57 |
| LPS2 | 72871048 | 70213034 | 0.03 | 97.6 | 51.07 | 86.38 |
| LPS3 | 64512976 | 62363488 | 0.03 | 97.49 | 51.54 | 85.55 |
| GEN1 | 60596774 | 59548220 | 0.02 | 98.02 | 51.83 | 90.94 |
| GEN2 | 67568632 | 65994052 | 0.02 | 97.91 | 51.18 | 89.54 |
| GEN3 | 62051554 | 60598094 | 0.02 | 97.98 | 51.62 | 90.46 |
Units: reads, n.
Abbreviations: CON, nonchallenge control; GC, guanine-cytosine; GEN, lipopolysaccharide-challenged group fed diet supplemented with 40 mg/kg genistein; ID, identification; LPS, lipopolysaccharide-challenged group; Q20, Phred quality score.
Mapping ratio, mapped reads/all reads.
Identification of Differentially Expressed Genes and Venn Analysis
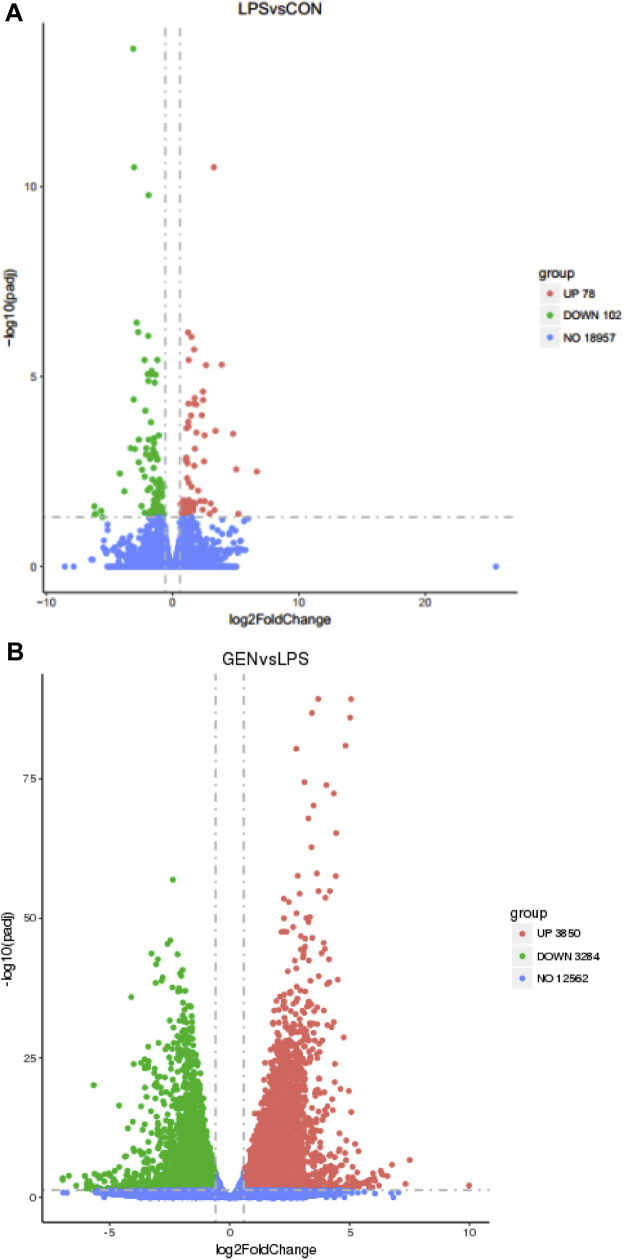
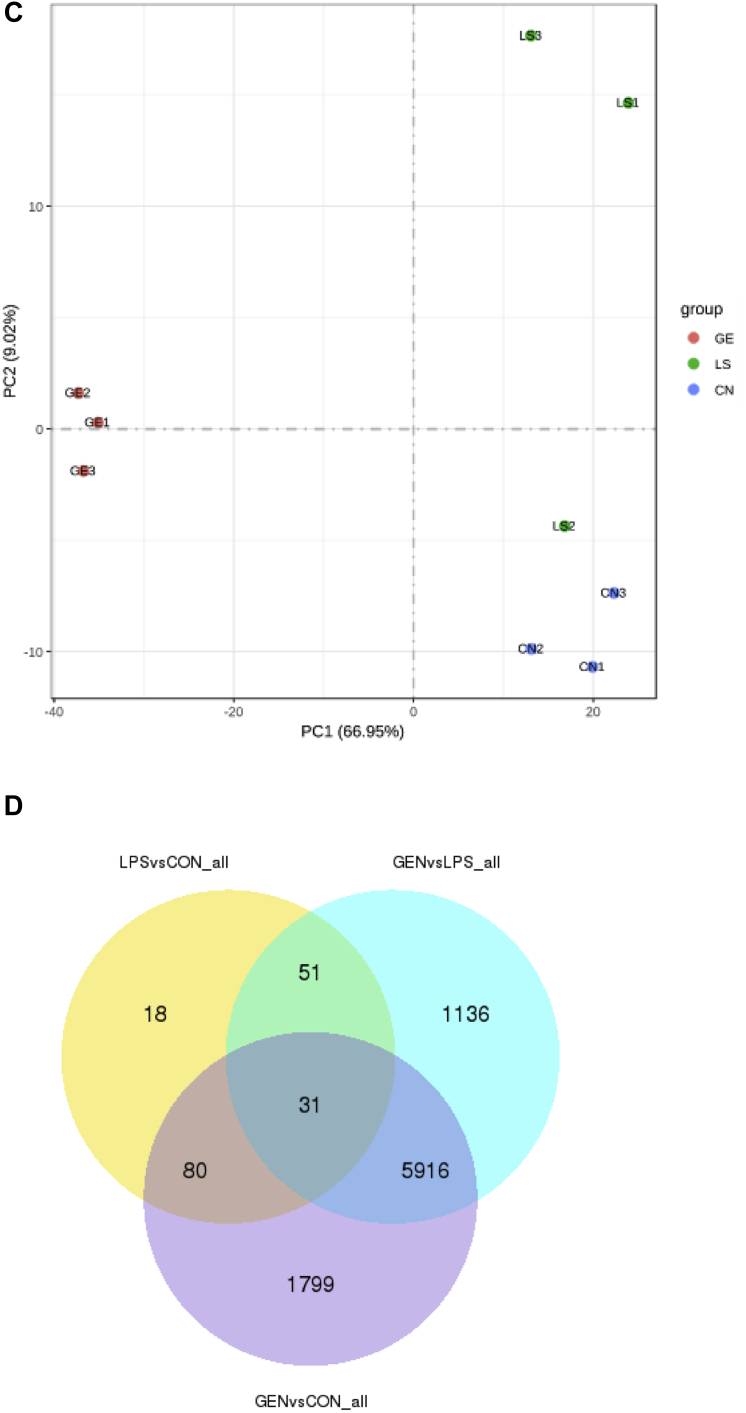
We identified 178 differentially expressed genes (100 upregulated and 78 downregulated) in the LPS group compared with the CON group with a |fold change| ≥ 1.5 (FDR ≤ 0.05; Figure 3A, 3B). Meanwhile, we identified 7,131 differentially expressed genes (3,281 upregulated and 3,851 downregulated) in the GEN group compared with the LPS group. The expression abundance and fold changes of DEGs are shown in Supplementary Data 4. We performed principal component analysis analysis of gene expression values (fragments per kilo-base of exon per million fragments mapped) in all samples. As shown in Figure 3C, the samples between groups were scattered, while samples within groups were clustered. Thirty-one genes were commonly differently expressed in the 3 groups. Dietary genistein treatment reversed the expressions of 51 genes, which differently expressed between LPS and CON groups (Figure 3D).
Figure 3.
(A) Scatter plot of DEGs (LPS vs. CON). Red points represent upregulated genes with |fold change| ≥1.5 and FDR ≤0.05. (B) Scatter plot of DEGs (GEN vs. LPS). Red points represent upregulated genes with |fold change| ≥1.5 and FDR ≤0.05. Green points represent downregulated genes with P value <0.05, fold change <0.67. Blue points represent genes with no significant difference. (C) Principal component analysis (PCA) using gene expressions (FPKM) of all samples. CN = CON, LS = LPS, GE = GEN. (D) Venn diagram of differentially expressed genes among the CON, LPS, and GEN groups. Abbreviations: CON, nonchallenge control; DEG, differently expressed gene; GEN, lipopolysaccharide-challenged group fed diet supplemented with 40 mg/kg genistein; LPS, lipopolysaccharide-challenged group.
Real-Time PCR Validation of Differential Gene Expression
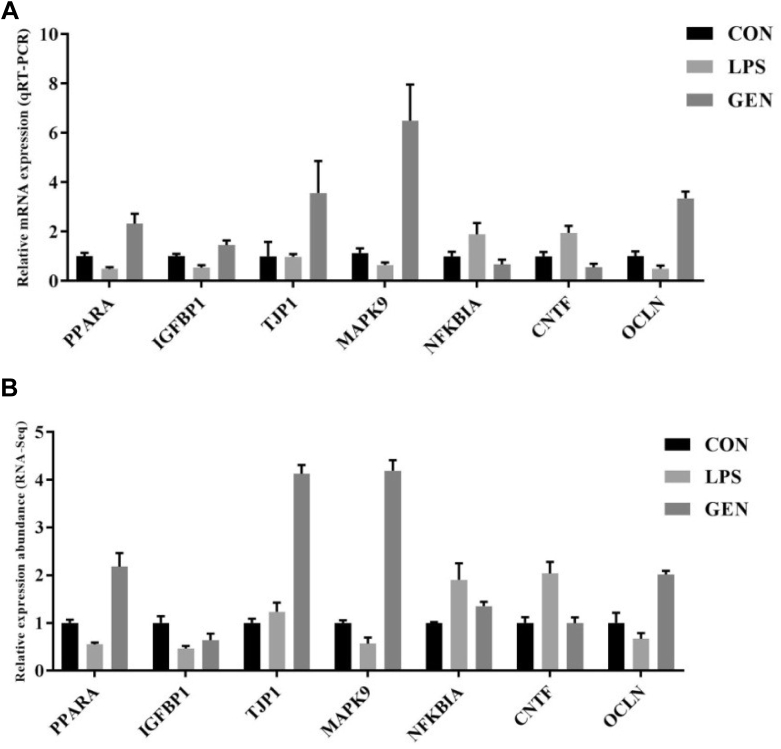
To confirm the accuracy of the RNA-Seq data, we randomly selected 7 genes. The expression levels of the selected genes were quantified using qRT-PCR, and the results were consistent with the findings obtained by RNA-Seq (Figure 4). For example, LPS injection decreased the mRNA expressions of IGFBP1 and PPARA in the ileum compared with those in the CON group, while genistein treatment increased IGFBP1 and PPARA expressions. The results suggested that RNA-Seq reliably identified differentially expressed mRNAs in the ileac transcriptome of chicks.
Figure 4.
(A) The relative gene expression abundance from RNA-seq data as determined by using Cufdiff software (n = 3). (B) The relative mRNA expressions of random selected genes in the chick ileum as determined by using qRT-PCR. Data are presented as mean value ± SEM (n = 8). Abbreviations: CNTF, ciliary neurotrophic factor; CON, nonchallenge control; GEN, lipopolysaccharide-challenged group fed diet supplemented with 40 mg/kg genistein; IGFBP1, insulin like growth factor binding protein 1; LPS, lipopolysaccharide-challenged group; MAPK9, mitogen-activated protein kinase 9; NFKBIA, NF-kappa-B inhibitor alpha; OCLN, Occludin; PPARA, peroxisome proliferator activated receptor α; SEM, standard error of mean; TJP1, tight binding protein 1.
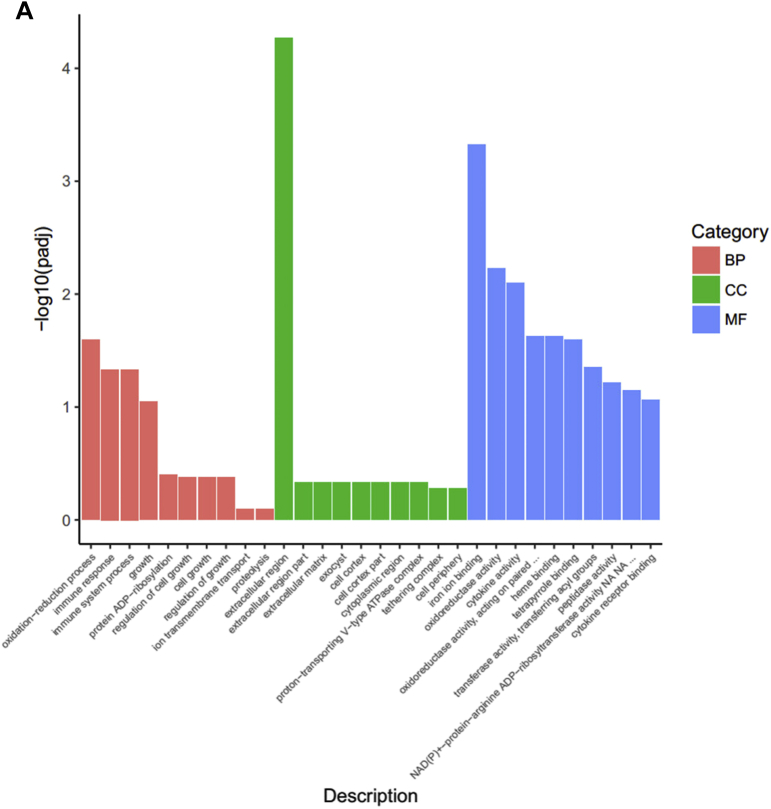
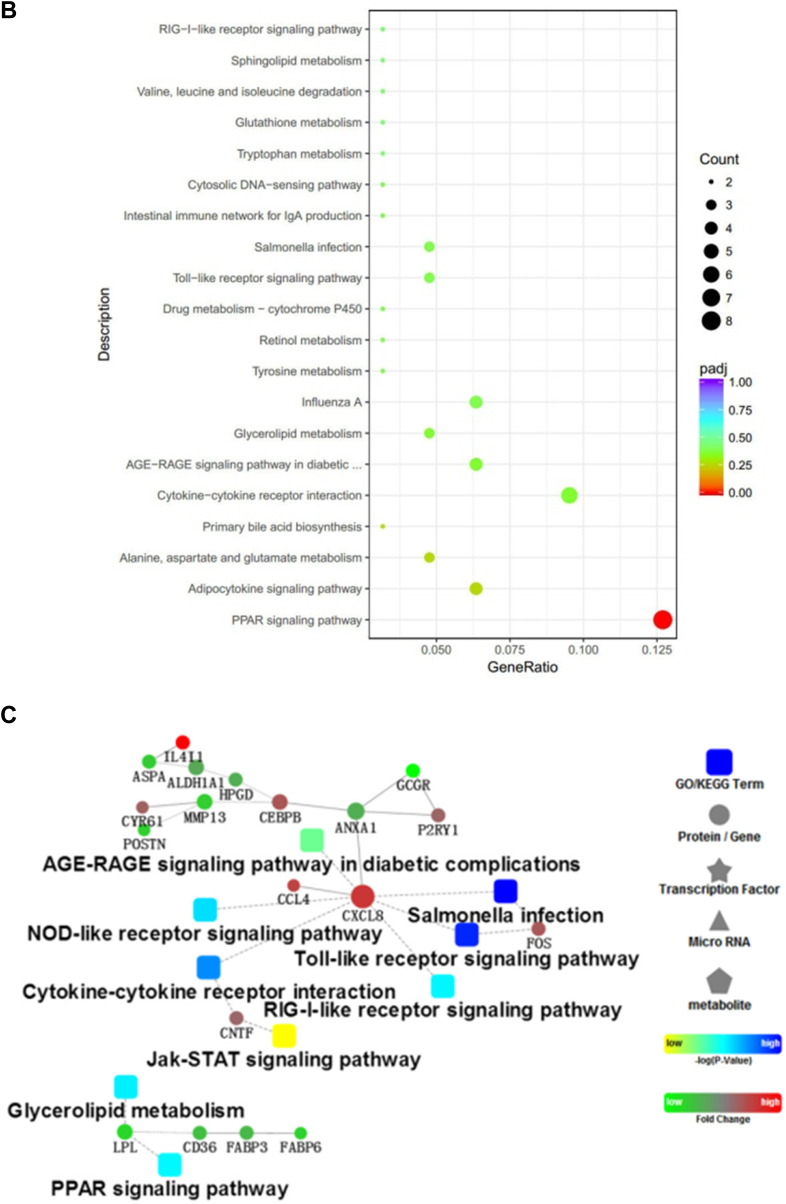
Gene Ontology Terms and KEGG and PPI Analysis Using DEGs Between the LPS and CON Groups
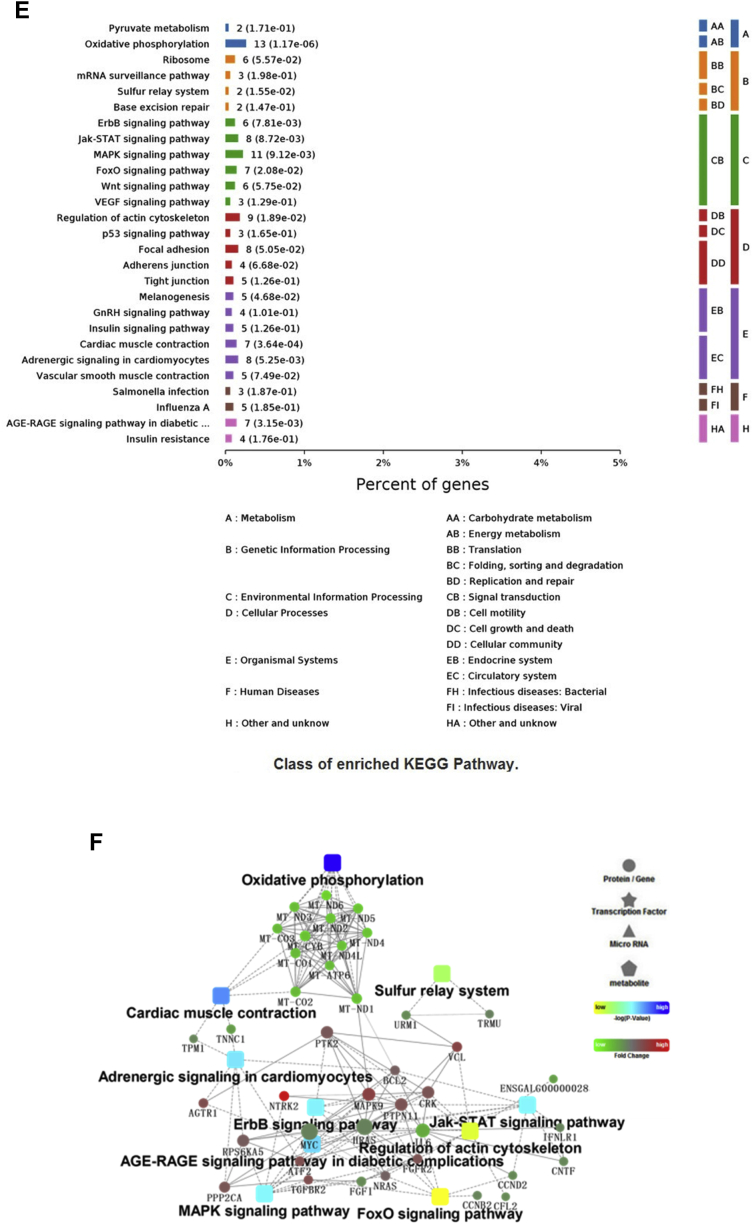
To better understand the network that regulates cell junctions and immune functions of LPS and genistein treatments, we conducted GO Biological Process, Cellular Component, and Molecular Function categories and KEGG pathway analysis using the genes that were differentially expressed between the LPS and CON groups, respectively. As shown in Figure 5A, GO analysis of Biological Process showed that the DEGs were significantly enriched into the terms, including oxidation-reduction process, immune response, growth prcocess, ion transmembrane transport, and proteolysis. Meanwhile, LPS injection influenced iron ion binding, oxidoreductase activity, and heme binding. From the enriched genes, we found that LPS injection made adverse effects on oxidation-reduction process (CYP2C23b|-1.66; CYP4B7|-3.08;DHCR24|-1.55;AGMO|-1.70;AOX1|-2.41;PTGR1|-1.04;CYP2C23a|-1.32;ALDH1A1|-0.98;CYP2J22|-1.56), immune response (CCL4|1.11;IL8|3.01;IL-18|1.63 IL15|-0.82), growth (IGFBP1|-1.10), oxidoreductase activity, and acted on paired donors, with incorporation or reduction of molecular oxygen (ENSGALG00000036831|-3.12; CYP2C23b|-1.66; CYP4B7|-3.08; CYP2C23a|-1.32; CYP2J22|-1.16). The top enriched terms are shown in Supplementary Data 5. As shown in Figure 5B, KEGG pathways analysis suggested that LPS injection inhibited peroxisome proliferator activated-receptors (PPAR) signaling pathway (PLIN1|-2.85; FABP6|-1.93; FABP3|-1.35; CD36|-1.49; LPL|-2.09; APOA1|-1.50; PPARA|-0.87), retinol metabolism (AOX1|-2.42; ALDH1A1|-1.52), and intestinal immune network for IgA production. Furthermore, LPS injection activated advanced glycation end products-receptor of advanced glycation end products (AGE-RAGE) signaling pathway in diabetic complications (NOX1|0.21; ENSGALG00000031430|1.74; IL8|3.01; PIM1|1.35), primary bile acid biosynthesis (AKR1D1|1.49; CH25H|1.45), influenza A pathway (IL8|3.01; IL-18|1.62; IL-18R1|0.89; NF-ΚBIA|0.92; SOCS3|2.93), Toll-like receptor signaling pathway (FOS|1.64; IL8|3.01; NF-ΚBIA|0.92), Salmonella infection (FOS|1.64; IL8|3.01; IL-18|1.62), cytokine-cytokine receptor interaction (IL13RA2|3.89; CNTF0.24|; IL8|3.01; IL-18|1.62), cell adhesion molecules (ENSGALG00000028341|2.40; ENSGALG00000031430|1.74; ENSGALG00000015032|1.23), and nucleotide binding oligomerization domain (NOD)-like receptor signaling pathway (IL8|3.01; IL-18|1.62; NF-ΚBIA|0.92). Accordingly, Cytoscape bioinformatics analysis of potential protein interactions for all DEGs between the LPS and GEN groups was performed (Figure 5C). The DEGs between the LPS and CON groups were significantly enriched into the AGE-RAGE signaling pathway in diabetic complications, NOD-like signaling pathway, Salmonella infection, Toll-like signaling pathway, cytokine-cytokine receptor interaction, retinoic acid-inducible gene I-like signaling pathway, Janus kinase-signal transducer and activator of transcription (JAT-STAT) signaling pathway, glycerolipid metabolism, and PPAR signaling pathway, which were all related to the core gene-CXCL8, CNTF, and LPL.
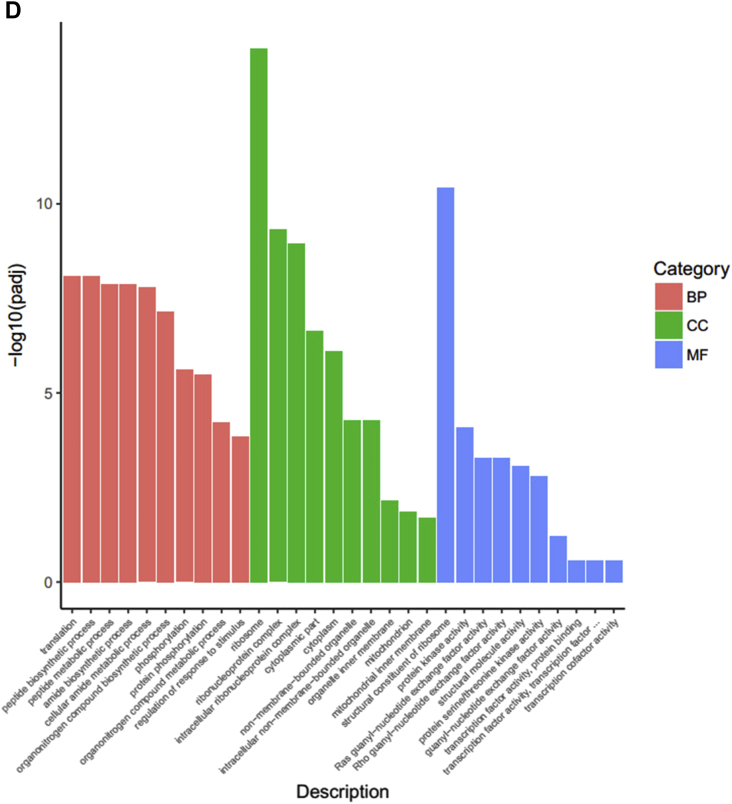
Figure 5.
(A, D) The top 10 enriched items in each main category (biologic process, cell component, and molecular function) of the GO database at all levels using DEGs of the LPS vs. CON groups and the GEN vs. LPS groups, respectively. (B, E) The top enriched KEGG items using DEGs of the LPS vs. CON groups and the GEN vs. LPS groups, respectively. (C, F) Protein-protein interaction analysis using DEGs of the LPS vs.CON groups and the GEN vs. LPS groups, respectively. Circular nodes represent genes/proteins; rectangles represent KEGG pathways or GO Biologic Process terms. The pathways are colored with a gradient from yellow to blue, in which yellow indicates a smaller P value, and blue indicates a larger P value. GO biologic processes are colored red. In the fold-change analysis, genes/proteins are colored red for upregulation or green for downregulation. Abbreviations: CON, nonchallenge control; DEG, differently expressed gene; GEN, lipopolysaccharide-challenged group fed diet supplemented with 40 mg/kg genistein; LPS, lipopolysaccharide-challenged group; GO, gene ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes.
Gene Ontology Terms, KEGG, and PPI Analysis Using DEGs Between GEN and LPS Groups
Among the Biological Process terms enriched for DEGs between the GEN and LPS groups, we found that GEN treatment enhanced translation, peptide biosynthetic process, cellular amide metabolic process, phosphorylation, and regulation of response to stimulus (Figure 5D). Total enriched terms are shown in Supplementary Data 6. Because of too much enriched terms and DEGs, we focus the significantly enriched terms at level 6. As shown in Table 4, genistein treatment did positive regulations in leukocyte activation, cellular response to growth factor stimulus, homotypic cell-cell adhesion, morphogenesis of an epithelium, programmed cell death, T-cell differentiation, MAPK cascade, lipid metabolic process, epithelial cell development, cell junction assembly, and adherens junction organization. Meanwhile, dietary genistein supplementation inhibited Wnt signaling pathway and I-κB kinase/NF-κB signaling. In addition, genistein treatment significantly upregulated the mRNA expressions of HNF4G, NR5A2, NR1D2, PPARA, HNF4beta, NR1H3, HNF4A, PPARG, NR3C1, RORA, ESRRG, RXRA, THRA, ESR1, and AR, which were enriched into cellular response to lipid (GO:0071396) and steroid hormone–mediated signaling pathway (GO:0043401). As shown in Figure 5E, KEGG analysis indicated that genistein treatment activated GnRH signaling pathway, insulin signaling pathway, AGE-RAGE signaling pathway in diabetic complications, tight junction, adherens junction, focal adhesion, Samonella infection, MAPK signaling pathway, Wnt signaling pathway, and forkhead box O signaling pathway. Also, the DEGs (MAPK9|7.21, IL6|0.1, MyD88|0.17) were enriched into Toll-like signaling pathway and NOD-like signaling pathway. Furthermore, the upregulated expressed genes, including H6PD, HEPH, CYP2U1, MICAL3, KDM5A, GLYR1, EGLN3, CYP7B1, OGFOD3, QSOX2, MICAL2. ALDH6A1, GPD2, DAO, VAT1, Nox4, DPYD, PXDN, ERO1B, KDM1B, MTHFR, TXNRD1, PDPR, RRM2B, KDM1A, GPD1L, MSRB1, ACOX2, CYP3A5, FA2H, NOS1, LOX, RETSAT, GYS2, and IL6, were enriched into oxidation-reduction process. In contract, genistein treatment downregulated the mRNA expressions of IL-12a, IL6, IL10RA, and IL11RA compared to LPS groups, which related to inflammatory reaction. Accordingly, PPI analysis of all DEGs between the GEN and LPS groups was performed (Figure 5F). The DEGs between the GEN and LPS groups were significantly enriched into oxidative phosphorylation, cardiac muscle contraction, sulfur relay system, adrenergic signaling cardiomyocytes, ErbB signaling pathway, JAK-STAT signaling pathway, AGE-RAGE signaling pathway in diabetic complications, regulation of actin cytoskeleton, MAPK signaling pathway, and forkhead box O signaling pathway, which were all related to the core gene-BCL2, PTK2, MAPK9, PTPN11, NRAS, VCL, IL6, and FGFR2.
Table 4.
GO cluster analysis (GEN vs. LPS).
| Levels | GO_Name | GO_ID | P value_adjusted | Gene_Count | Pop_Hit |
|---|---|---|---|---|---|
| 4, 5, 6 | Regulation of leukocyte activation | GO:0002694 | 6.62E-06 | 90 | 281 |
| 6 | Cellular response to growth factor stimulus | GO:0071363 | 6.96E-06 | 135 | 468 |
| 5, 6 | Homotypic cell-cell adhesion | GO:0034109 | 3.10E-05 | 98 | 324 |
| 5, 6 | Morphogenesis of an epithelium | GO:0002009 | 9.68E-05 | 115 | 405 |
| 5, 6 | Leukocyte cell-cell adhesion | GO:0007159 | 1.64E-04 | 87 | 291 |
| 5, 6 | Regulation of programmed cell death | GO:0043067 | 2.07E-04 | 256 | 1052 |
| 6 | Leukocyte differentiation | GO:0002521 | 3.55E-04 | 98 | 344 |
| 6 | T-cell differentiation | GO:0030217 | 7.83E-04 | 53 | 163 |
| 5, 6 | MAPK cascade | GO:0000165 | 8.21E-04 | 151 | 587 |
| 4, 5, 6 | Positive regulation of lipid metabolic process | GO:0045834 | 9.04E-04 | 31 | 80 |
| 5, 6 | I-κB kinase/NF-κB signaling | GO:0007249 | 3.22E-03 | 62 | 210 |
| 6 | Alpha-beta T-cell activation | GO:0046631 | 4.20E-03 | 31 | 87 |
| 5, 6 | Epithelial cell development | GO:0002064 | 5.28E-03 | 57 | 193 |
| 4, 5, 6 | Positive regulation of cell junction assembly | GO:1901890 | 7.16E-03 | 13 | 26 |
| 5, 6 | Regulation of Wnt signaling pathway | GO:0030111 | 7.43E-03 | 65 | 230 |
| 6 | Response to interleukin-1 | GO:0070555 | 1.08E-02 | 22 | 58 |
| 5, 6 | Positive regulation of adherens junction organization | GO:1903393 | 1.11E-02 | 12 | 24 |
Abbreviations: GEN, lipopolysaccharide-challenged group fed diet supplemented with 40 mg/kg genistein; LPS, lipopolysaccharide-challenged group; GO, gene ontology.
Discussion
In this experiment, LPS injection caused obvious pathological changes in the ileum of broilers, including hyperemia, bleeding spots, and intestinal wall growing thin. The destructed villus morphology and inflammatory reaction were induced by LPS injection, which decreased the growth performance of chicks. These phenotypes indicated that intestinal injury model had been successfully established, which is consistent with the previous researches (Wang et al., 2019b, Zhuang et al., 2019). In line with our expectations, genistein treatment alleviated LPS-induced intestinal damage and improved growth performance of chicks. The specific manifestations are improvement of intestinal morphology, mucosal immune function, tight junction, antioxidant activity, and apoptotic process.
Intestinal villus is the main site for nutrient absorption. Intestinal morphological integrity has great impacts on the animal health and growth performance. In the present experiment, LPS infection reduced the ileum villus height and damaged the villus structure, which definitely decreased the absorption capacity of intestines. Meanwhile, LPS infection reduced the crypt depth, suggesting that LPS hindered the normal development of intestinal crypt. Similarly, it is reported that LPS stress could inhibit the proliferation of intestinal epithelial cells, along with the enhanced apoptosis process, which resulted in morphological damage of intestinal villus (Liu et al., 2012). Interestingly, adding genistein into the diet could protect against LPS-induced morphology injury in the ileum of piglets, which was consistent with the beneficial effects of daidzein on the intestinal barrier (Zhu et al., 2015). What we stress here is that dietary genistein also improved the growth performance of chicks, which was decreased by LPS injection. The transcriptomic analysis of ileums revealed that LPS injection inhibited the growth process (Wang et al., 2019a). Furthermore, it promoted proteolysis process, along with the upregulated expressions of ADAMTS4 and MST1. Our previous study suggested that adding genistein into the diet of LBB hens can activate the GHIGFs-PI3K/Akt pathway in offspring chicks and increased the body weight gain (Lv et al., 2019). Similarly, dietary genistein treatment in the present study increased the expressions of growth-related genes (CRIM1/ESM1, IGFBP1, IGF1R, and IGF2R) compared with LPS treatment. As we all know, PPAR signaling pathway involves in the regulation of lipid metabolism and inflammatory gene expression (Aoyama, 1999, Zhang and Young, 2002). In the present study, genistein treatment alleviated the inhibitory effect of LPS on PPAR pathway, which is consistent with our previous research (Lv et al., 2019). Therefore, dietary genistein supplementation for broilers can alleviate the adverse effects of LPS on the intestinal morphology and growth performance.
Cytokines are the primary markers of injury or infection (Ertel et al., 1991). The increased expressions of proinflammatory cytokines and receptors suggested that LPS injection induced inflammatory response in the intestine of broilers. sIgA is the main immune barrier to prevent intestinal pathogens from colonizing into the intestinal mucosa, which further maintain the homeostasis of symbiotic bacteria (Fang et al., 2010). Polosukhin et al. (2011) observed a decrease in sIgA in the bronchoalveolar lavage fluid of patients with chronic obstructive pulmonary disease. It is believed that abnormal bronchial epithelial structure led to local sIgA deficiency (Polosukhin et al., 2011). In the present study, GO analysis indicated that LPS injection influenced intestinal immune network for IgA production in the intestine. Furthermore, the quantitative results of ELISA suggested that LPS challenge decreased the levels of sIgA in the ileum mucosa, which might be due to the damaged villus morphology and immune levels. However, dietary genistein increased the sIgA levels compared with the LPS group, which could enhance the barrier function of intestinal mucosa. Pattern recognition receptor can recognize various invasive pathogens, resulting in inflammation through specific signaling pathways (Petr and Monack, 2013). Toll-like receptors and Nod-like receptors are 2 major families of pattern recognition receptor, which can recognize LPS in the cell wall of gram-negative bacteria. In the present study, transcriptomic analysis indicated that LPS injection induced immune response in the chick ileum. It activated influenza A pathway (IL8|3.01; IL-18|1.62; IL-18R1|0.89; NF-ΚBIA|0.92; SOCS3|2.93), Toll-like receptor signaling pathway (FOS|1.64; IL8|3.01; NF-ΚBIA|0.92), Salmonella infection (FOS|1.64; IL8|3.01; IL-18|1.62), cytokine-cytokine receptor interaction (IL13RA2|3.89; CNTF|0.24; IL8|3.01; IL-18|1.62), and NOD-like receptor signaling pathway (IL8|3.01; IL-18|1.62; NF-ΚBIA|0.92). Furthermore, the expression of NF-ΚBIA increased significantly after LPS treatment, which activated the NF-κB signaling pathway. The result was consistent with the previous study (GomezCabrera et al., 2003, Wang et al., 2015). Meanwhile, LPS injection upregulated the expressions of IL8, IL-18, and IL13RA2 in the ileum. The main biological activity of IL-8 is to activate neutrophils. After contacting with IL-8, neutrophils undergo morphological changes and migrate to the reaction site, resulting in local inflammation in the body. IL-18 is the proinflammatory cytokine, which can induce the production of IFN-gamma from T cells. IL-18 can combine to IL12, then inhibit the production of IgE and IgG1 (Salagianni et al., 2007). These evidences further revealed that LPS challenge induced the inflammatory reactions in the ileum of chicks. In the present study, dietary genistein supplementation significantly decreased the expression of IL6 and inflammatory factor receptors (IL10RA, IL11RA, IL31RA, and IL17RA). Transcriptomic analysis further revealed that dietary genistein did positive regulations on leukocyte activation and T-cell differentiation. It inhibits Toll-like signaling pathway and NOD-like signaling pathway in the ileum. As we all know, Toll-like and NOD-like signaling pathways can activate NF-κB and MAPK signaling pathways, which promote the expressions of proinflammatory genes (Fukata et al., 2009). Similarly, genistein is reported to decrease the degradation of IκB–α, then inhibit tyrosine kinase and IL-17 expression (Kehlen et al., 1999). Therefore, the inhibited I-κB kinase/NF-κB signaling after genistein treatment is the main factor that alleviates inflammatory response. In the present study, dietary genistein significantly downregulated the mRNA expressions of INSR, EGFR, ABL1, ABL2, PTK2, PTK2B, and TEK in the ileum compared to the LPS group, which were enriched into the inhibited biological process of protein tyrosine kinase activity. Furthermore, genistein can induce tyrosine kinase phosphorylation, acting as a critical upstream signaling event in LPS-mediated c-Jun N-terminal kinase and NF-κB activation (Kang et al., 2001). The transcriptomic analysis indicated that dietary genistein supplementation upregulated the mRNA expressions of PTPRJ, PTPN3, PTPRU, PTPRK, PTPRB, PTPRN2, PTPN1, PTPRE, PTPN11, PTPN12, PTPRF, PTPN4, PTPRG, PTPRS, PTPN14, and PTPRC, which enhanced protein tyrosine phosphatase activity. Activation of tyrosine kinases induces downstream activation of MAPK, and inhibiting tyrosine kinase activity blocks MAPK activation (Purcell et al., 2003). Therefore, genistein, an inhibitor of tyrosine kinase, is effective in preventing LPS-induced NF-κB–dependent cytokine and MAPK cascade signaling.
Integrity of intestinal epithelial cells is mainly regulated by microtubules and actin. The junctions between epithelial cells include tight junction, gap junction, adherence junction, and desmosome junction. As we all know, LPS can destroy the junctions between intestinal cells (He et al., 2019). It is reported that genistein can inhibit phosphorylation of intestinal tight junction protein, which ensures the integrity of intestinal barrier (Suzuki and Hara, 2011). In addition, genistein can increase the expression of E-cadherin and maintain the stability of cytoskeleton (Rao et al., 2002, Ying and Simmen, 2009). In the present study, LPS injection significantly influenced cell adhesion molecules in the ileum, while dietary genistein treatment upregualted the mRNA expressions of TJP1and OCLN. Transcriptomic analysis indicated that genistein treatment significantly enhanced the biological processes, including positive regulation of homotypic cell-cell adhesion, morphogenesis of an epithelium, cell junction assembly, and adherens junction organization. KEGG analysis further revealed that genistein treatment enhanced tight junction genes (NRAS, PPP2Ca, YES1), adherens junction (YES1, TGFBR2, PTPN1, VCL), and focal adhesion (BCL2, PTK2, CRK, VCL, MAPK9). This is consistent with the reports that genistein can inhibit the invasion of pathogenic bacteria to Caco-2 cells, reducing the invasion and internalization of pathogenic bacteria (Wells et al., 1999). Therefore, dietary genistein supplementation protected against LPS-induced intestinal cell junction damage in broilers.
LPS can promote the production of reactive oxygen species through NF-κB signaling pathway in inflammatory cells, thereby impairing the antioxidant function (Haddad and Land, 2002). Transcriptomic analysis suggested that LPS injection significantly reduced oxidoreductase activity, doing adverse effects on the incorporation or reduction of molecular oxygen. Adding genistein into the diet significantly increased the total antioxidant capability in the ileum of broilers. This is consistent with the report that genistein (5 mg/kg) can improve antioxidant capacity and immune function of broilers (Kamboh et al., 2016). Accordingly, transcriptomic analysis showed that dietary genistein significantly enhanced oxidation-reduction process. Meanwhile, LPS treatment can induce apoptosis in the tissue through reactive oxygen species (Du et al., 2012). Transcriptomic analysis revealed that dietary genistein improved cell death process and significantly increased related-gene expression in the ileum. Similarly, TUNEL analysis in the present study suggested that dietary genistein supplementation significantly alleviated LPS-induced apoptosis in the ileum. Therefore, dietary genistein might alleviate LPS-induced intestinal injury through regulating apoptosis process.
In conclusion, dietary genistein supplementation altered the gene expression profile and signaling pathway in the ileum of LPS-challenged chicks. Furthermore, dietary genistein improved intestinal morphology, mucosal immune function, tight junction, antioxidant activity, and apoptotic process, which were adversely damaged by LPS injection. Therefore, adding genistein into the diet of chicks can promote the growth performance of chicks under intestinal injury.
Acknowledgments
This work was supported by Jiangsu Agricultural Science and Technology Innovation Fund (JASTIF), China, (CX(18)2002), and China Postdoctoral Science Foundation (2019M651867). The authors thank Jiangsu Hesheng Food Group Limited Company for providing the chicks. The authors also express that they are deeply indebted to Song Jin, Jiao Wang (Animal Disease Control Center of Changzhou) for their help for detection of the immune indexes.
Conflicts of Interest Statement: The authors declare that there are no conflicts of interest.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.psj.2020.03.020.
Contributor Information
Debing Yu, Email: yudebing@njau.edu.cn.
Fangxiong Shi, Email: fxshi@njau.edu.cn.
Supplementary data
References
- Aoyama T. PPAR and disorder of lipid metabolism (obesity. hyper lipidemia. fatty liver) Cell. 1999;31:9–12. [Google Scholar]
- Ashburner M., Ball C.A., Blake J.A., Botstein D., Butler H., Cherry J.M., Davis A.P., Dolinski K., Dwight S.S., Eppig J.T. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byun E.B., Sung N.Y., Yang M.S., Lee B.S., Song D.S., Park J.N., Kim J.H., Jang B.S., Choi D.S., Park S.H. Anti-inflammatory effect of gamma-irradiated genistein through inhibition of NF-κB and MAPK signaling pathway in lipopolysaccharide-induced macrophages. Food Chem. Toxicol. 2014;74:255–264. doi: 10.1016/j.fct.2014.08.019. [DOI] [PubMed] [Google Scholar]
- Chen Y., Zhang H., Cheng Y., Li Y., Wen C., Zhou Y. Dietary l-threonine supplementation attenuates lipopolysaccharide-induced inflammatory responses and intestinal barrier damage of broiler chickens at an early age. Br. J. Nutr. 2018;119:1254–1262. doi: 10.1017/S0007114518000740. [DOI] [PubMed] [Google Scholar]
- Du S.C., Ge Q.M., Lin N., Dong Y., Su Q. ROS-mediated lipopolysaccharide- induced apoptosis in INS-1 cells by modulation of Bcl-2 and Bax. Cell Mol Biol (Noisy-le-grand) 2012;58:1654–1659. [PubMed] [Google Scholar]
- Ertel W., Morrison M.H., Wang P., Ba Z.F., Ayala A., Chaudry I.H. The complex pattern of cytokines in sepsis. Association between prostaglandins, cachectin, and interleukins. Ann. Surg. 1991;214:141–148. doi: 10.1097/00000658-199108000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata M., M. T. Abreu. 2006. Toll-like receptors and inflammatory bowel disease. Pages 107-123 in Toll-like Receptors in Inflammation. L. A. O’Neill, E. Brint, eds. Birkhäuser Basel, Basel, Switzerland.
- Fukata M., Vamadevan A.S., Abreu M.T. Toll-like receptors (TLRs) and Nod-like receptors (NLRs) in inflammatory disorders. Semin. Immunol. 2009;21:242–253. doi: 10.1016/j.smim.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Ghosh S., Chan C.K. Analysis of RNA-Seq data using TopHat and Cufflinks. Methods Mol Biol. 2016;1374:339–348. doi: 10.1007/978-1-4939-3167-5_18. [DOI] [PubMed] [Google Scholar]
- GomezCabrera M.C., Steinhafel N., Vina J., Ji L.L. Activation of NFkB in rat skeletal muscle: effects of exercise, hydroperoxide and lipopolysaccharide. Med. Sci. Sports Exerc. 2003;35:534–544. [Google Scholar]
- Haddad J.J., Land S.C. Redox/ROS regulation of lipopolysaccharide-induced mitogen-activated protein kinase (MAPK) activation and MAPK-mediated TNF-alpha biosynthesis. Br. J. Pharmacol. 2002;135:520–536. doi: 10.1038/sj.bjp.0704467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C., Deng J., Hu X., Zhou S., Wu J., Xiao D., Darko K.O., Huang Y., Tao T., Peng M. Vitamin A inhibits the action of LPS on the intestinal epithelial barrier function and tight junction proteins. Food Funct. 2019;10:1235–1242. doi: 10.1039/c8fo01123k. [DOI] [PubMed] [Google Scholar]
- Hou Y., Wang L., Ding B., Liu Y., Zhu H., Liu J., Li Y., Wu X., Yin Y., Wu G. Dietary alpha-ketoglutarate supplementation ameliorates intestinal injury in lipopolysaccharide- challenged piglets. Amino Acids. 2010;39:555–564. doi: 10.1007/s00726-010-0473-y. [DOI] [PubMed] [Google Scholar]
- Iqbal M.F., Luo Y.H., Hashim M.M., Zhu W.Y. Evaluation of genistein mediated growth, metabolic and anti-inflammatory responses in broilers. Pak. J. Zool. 2014;46:317–327. [Google Scholar]
- Kamboh A.A., Hang S.Q., Khan M.A., Zhu W.Y. In vivo immunomodulatory effects of plant flavonoids in lipopolysaccharide-challenged broilers. Animal. 2016;10:1619–1625. doi: 10.1017/S1751731116000562. [DOI] [PubMed] [Google Scholar]
- Kang J.L., Lee H.W., Lee H.S., Pack I.S., Chong Y., Castranova V., Koh Y. Genistein prevents nuclear factor-kappa B activation and acute lung injury induced by lipopolysaccharide. Am. J. Respir. Crit. Care Med. 2001;164:2206–2212. doi: 10.1164/ajrccm.164.12.2104017. [DOI] [PubMed] [Google Scholar]
- Kehlen A., Thiele K., Riemann D., Rainov N., Langner J. Interleukin-17 stimulates the expression of IκBα mRNA and the secretion of IL-6 and IL-8 in glioblastoma cell lines. J. Neuroimmunol. 1999;101:1–6. doi: 10.1016/s0165-5728(99)00111-3. [DOI] [PubMed] [Google Scholar]
- Liu Y., Chen F., Odle J., Lin X., Jacobi S.K., Zhu H., Wu Z., Hou Y. Fish oil enhances intestinal integrity and inhibits TLR4 and NOD2 signaling pathways in weaned pigs after LPS challenge. J. Nutr. 2012;142:2017–2024. doi: 10.3945/jn.112.164947. [DOI] [PubMed] [Google Scholar]
- Lv Z., Fan H., Zhang B., Ning C., Xing K., Guo Y. Dietary genistein supplementation in laying broiler breeder hens alters the development and metabolism of offspring embryos as revealed by hepatic transcriptome analysis. FASEB J. 2018;32:4214–4228. doi: 10.1096/fj.201701457R. [DOI] [PubMed] [Google Scholar]
- Lv Z., Fan H., Zhang B., Xing K., Guo Y. Dietary genistein supplementation for breeders and their offspring improves the growth performance and immune function of broilers. Sci. Rep. 2018;8:5161–5173. doi: 10.1038/s41598-018-23530-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv Z., Xing K., Li G., Liu D., Guo Y. Dietary genistein alleviates lipid metabolism disorder and inflammatory response in laying hens with fatty liver syndrome. Front. Physiol. 2018;9:1493–1514. doi: 10.3389/fphys.2018.01493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv Z., Fan H., Song B., Li G., Liu D., Guo Y. Supplementing genistein for breeder hens alters the fatty acid metabolism and growth performance of offsprings by epigenetic modification. Oxidative Med. Cell Longevity. 2019;2019:9214209. doi: 10.1155/2019/9214209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovits J., Linassier C., Fossé P., Couprie J., Pierre J., Jacquemin-Sablon A., Saucier J.M., Pecq J.B., Le, Larsen A.K. Inhibitory effects of the tyrosine kinase inhibitor genistein on mammalian DNA topoisomerase II. Cancer Res. 1989;49:5111–5117. [PubMed] [Google Scholar]
- Mauro A.D., Neu J., Riezzo G., Raimondi F., Martinelli D., Francavilla R., Indrio F. Gastrointestinal function development and microbiota. Ital. J. Pediatr. 2013;39:15–26. doi: 10.1186/1824-7288-39-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoru K., Michihiro A., Susumu G., Masahiro H., Mika H., Masumi I., Toshiaki K., Shuichi K., Shujiro O., Toshiaki T. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008;36:480–484. doi: 10.1093/nar/gkm882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda S., Tanabe S., Suzuki T. Differential effects of flavonoids on barrier integrity in human intestinal Caco-2 cells. J. Agric. Food Chem. 2012;60:4628–4633. doi: 10.1021/jf300382h. [DOI] [PubMed] [Google Scholar]
- NRC . National Academy Press; Washington, DC: 1994. Nutrition Requirements of Poultry. 9th ed. [Google Scholar]
- Petr B., Monack D.M. Newly described pattern recognition receptors team up against intracellular pathogens. Nat. Rev. Immunol. 2013;13:551–565. doi: 10.1038/nri3479. [DOI] [PubMed] [Google Scholar]
- Polosukhin V.V., Cates J.M., Lawson W.E., Rinat Z., Milstone A.P., Massion P.P., Sebahat O., Ware L.B., Jae Woo L., Bowler R.P. Bronchial secretory immunoglobulin a deficiency correlates with airway inflammation and progression of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2011;184:317–329. doi: 10.1164/rccm.201010-1629OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell A.L., Sharma S.K., Bagnall M.W., Sutton M.A., Carew T.J. Activation of a tyrosine kinase-MAPK cascade enhances the induction of long-term synaptic facilitation and long-term memory in Aplysia. Neuron. 2003;37:473–484. doi: 10.1016/s0896-6273(03)00030-8. [DOI] [PubMed] [Google Scholar]
- Rao R.K., Shyamali B., Rao V.U., Karnaky K.J., Jr., Akshay G. Tyrosine phosphorylation and dissociation of occludin-ZO-1 and E-cadherin-beta-catenin complexes from the cytoskeleton by oxidative stress. Biochem. J. 2002;368:471–485. doi: 10.1042/BJ20011804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasouli E., Jahanian R. Improved performance and immunological responses as the result of dietary genistein supplementation of broiler chicks. Anim. Int. J. Anim. Biosci. 2015;9:1473–1480. doi: 10.1017/S1751731115000853. [DOI] [PubMed] [Google Scholar]
- Salagianni M., Loon W.K., Thomas M.J., Noble A., Kemeny D.M. An essential role for IL-18 in CD8 T cell-mediated suppression of IgE responses. J. Immunol. 2007;178:4771–4778. doi: 10.4049/jimmunol.178.8.4771. [DOI] [PubMed] [Google Scholar]
- Soukup S.T., Helppi J., Müller D.R., Zierau O., Watzl B., Vollmer G., Diel P., Bub A., Kulling S.E. Phase II metabolism of the soy isoflavones genistein and daidzein in humans, rats and mice: a cross-species and sex comparison. Arch. Toxicol. 2016;90:1335–1347. doi: 10.1007/s00204-016-1663-5. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Hara H. Role of flavonoids in intestinal tight junction regulation. J. Nutr. Biochem. 2011;22:401–408. doi: 10.1016/j.jnutbio.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Tan J., Liu S., Guo Y., Applegate T.J., Eicher S.D. Dietary L-arginine supplementation attenuates lipopolysaccharide-induced inflammatory response in broiler chickens. Br. J. Nutr. 2014;111:1394–1404. doi: 10.1017/S0007114513003863. [DOI] [PubMed] [Google Scholar]
- Wang J.W., Reiser M.A., Li K., Wang B., Lockey R.F. Lipopolysaccharide- responsive beige-like anchor is required for both activation and deactivation of NFkB. J. Allergy Clin. Immunol. 2015;135:152–163. [Google Scholar]
- Wang H., Liu Y., Shi H., Wang X., Zhu H., Pi D., Leng W., Li S. Aspartate attenuates intestinal injury and inhibits TLR4 and NODs/NF-kappaB and p38 signaling in weaned pigs after LPS challenge. Eur. J. Nutr. 2017;56:1433–1443. doi: 10.1007/s00394-016-1189-x. [DOI] [PubMed] [Google Scholar]
- Wang K., Chen G., Cao G., Xu Y., Wang Y., Yang C. Effects of Clostridium butyricum and Enterococcus faecalis on growth performance, intestinal structure, and inflammation in lipopolysaccharide-challenged weaned piglets. J. Anim. Sci. 2019;97:4140–4151. doi: 10.1093/jas/skz235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Wang W., Wang L., Yu C., Zhang G., Zhu H., Wang C., Zhao S., Hu C.-A.A., Liu Y. Lentinan modulates intestinal microbiota and enhances barrier integrity in a piglet model challenged with lipopolysaccharide. Food Funct. 2019;10:479–489. doi: 10.1039/c8fo02438c. [DOI] [PubMed] [Google Scholar]
- Wells C.L., Jechorek R.P., Kinneberg K.M., Debol S.M., Erlandsen S.L. The isoflavone genistein inhibits internalization of enteric bacteria by cultured Caco-2 and HT-29 enterocytes. J. Nutr. 1999;129:634–640. doi: 10.1093/jn/129.3.634. [DOI] [PubMed] [Google Scholar]
- Fang X., I L., Liu Y., Wen S., Tang L. Changes of intestinal sIgA and mucin in mice with intestinal dysbacteria. Chin. J. Microecol. 2010;1211:524–536. [Google Scholar]
- Ying S., Simmen R.C.M. Soy isoflavone genistein upregulates epithelial adhesion molecule E-cadherin expression and attenuates beta-catenin signaling in mammary epithelial cells. Carcinogenesis. 2009;30:331–345. doi: 10.1093/carcin/bgn279. [DOI] [PubMed] [Google Scholar]
- Zhang X., Young H.A. PPAR and immune system--what do we know? Int. Immunopharmacol. 2002;2:1029–1044. doi: 10.1016/s1567-5769(02)00057-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C., Wu Y., Jiang Z., Zheng C., Wang L., Yang X., Ma X., Gao K., Hu Y. Dietary soy isoflavone attenuated growth performance and intestinal barrier functions in weaned piglets challenged with lipopolysaccharide. Int. Immunopharmacol. 2015;28:288–294. doi: 10.1016/j.intimp.2015.04.054. [DOI] [PubMed] [Google Scholar]
- Zhuang S., Zhong J., Bian Y., Fan Y., Chen Q., Liu P., Liu Z. Rhein ameliorates lipopolysaccharide-induced intestinal barrier injury via modulation of Nrf2 and MAPKs. Life Sci. 2019;216:168–175. doi: 10.1016/j.lfs.2018.11.048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.