Abstract
Soybean oligosaccharides have been previously shown to be associated with the production of major odor-causing compounds in broilers, although little is known about the role of stachyose and raffinose, which are key components of soybean oligosaccharide, in broiler cecal microbiota and odor compound production. To this end, soybean oligosaccharide, stachyose, and raffinose were added to the birds' diets to investigate their effects on odor compound production and the microbial community characteristics of the cecum in broilers. A total of 300 one-day-old Arbor Acre broilers with similar initial live weight were randomly allocated into 5 dietary groups with 6 replicates of 10 birds. The diets included soybean meal (positive control), soybean meal-free (negative control), 0.6% soybean oligosaccharide, 0.6% stachyose, or 0.6% raffinose. After a 49-D feeding period, both ceca were aseptically removed postmortem, and the contents were collected and analyzed for skatole, indole, volatile fatty acids, and lactic acid by using high performance liquid chromatography. Bacterial communities were detected by using a high-throughput sequencing platform based on IlluminaMiSeq 2500. Levels of skatole and indole tended to be lower in the dietary supplementation of oligosaccharides. The lowest levels of skatole and indole were observed in the stachyose group (P < 0.05), while the highest levels were found in the negative control group (P < 0.05). Concentrations of acetic acid and propionic acid in the stachyose group were increased (P < 0.05) while those of butyric acid and lactic acid were decreased (P < 0.05) compared with the soybean oligosaccharide and raffinose groups. Firmicutes and Bacteroidetes were prevalent in all groups, the proportion of Bacteroidetes was slightly decreased in the stachyose group, and Verrucomicrobia was abundant in the raffinose group (P > 0.05). Bacterial genera Alistipes and Parabacteroides were comparably abundant in the stachyose group, while Bacteroides, Lactobacillus, and Akkermansia were more abundant in the negative control, stachyose, and raffinose groups, respectively. Collectively, these findings demonstrated that dietary oligosaccharide supplementation significantly reduced odor compound production by modulating the cecal microbial community. Compared with soybean oligosaccharide and raffinose, the addition of stachyose into diets may help improve gut fermentation and minimize odor compound generation in broilers.
Key words: skatole, indole, cecal microbiota, oligosaccharide, broiler
Introduction
A difficult persistent situation occurs between the increasing global population, which is estimated to comprise approximately 9.6 billion individuals by 2050, and the demand for animal protein (Borda-Molina et al., 2018). Correspondingly, intensive poultry production systems have proven economically effective to match the demands of a growing world population. The extreme growth in intensive broiler farming leads to not only high chicken meat yield but also associated adverse environmental problems, such as odor production (Benoît et al., 2001). Strict environmental protection regulations enacted by governments restrict the expansion of broiler production and set limits on the emissions of offensive odors.
The source of odor is mainly due to anaerobic bacterial fermentation of nutrients in the feed in the gut. Many types of odorous compounds have been identified and classified into 4 main groups: 1) sulfur compounds, 2) phenolic and indolic compounds, 3) volatile fatty acids (VFAs), 4) ammonia and amines (Mackie et al., 1998). Among these compounds, indole and skatole (3-methylindole), which are produced from anoxic metabolism of L-tryptophan by microbial degradation, are well-known foul-smelling fecal odorants in mammalian and avian feces (Benoît et al., 2001, Trabue et al., 2010, Tesso et al., 2019). Acetic acid, propionic acid, and butyric acid are the most common VFAs produced from the microbial conversion of dietary residues. A few strategies directed at reducing odorants arising from livestock and poultry breeding have been evaluated, including the modification of diets (Cho et al., 2015, Sharma et al., 2015, Recharla et al., 2017) and addition of oligosaccharides (Rideout et al., 2004, Yang et al., 2016), enzymes (O'Shea et al., 2014, Sharma et al., 2016), organic acids (Claus et al., 2003, Øverland et al., 2008), probiotics, or minerals (Armstrong et al., 2000, Borowski et al., 2017, Tesso et al., 2019).
Our previous studies have demonstrated that the addition of soybean oligosaccharides decreased the concentrations of indole and skatole in vitro (Liu et al., 2018c), which may be caused by a decrease in the L-tryptophan degradation rate. Dietary soybean oligosaccharide supplementation also decreased the concentrations of indole and skatole and increased total VFAs in the excreta of broilers in vivo (Yang et al., 2016). Moreover, soybean oligosaccharide supplementation can foment the growth of lactic acid bacteria and change the bacterial community structure of broiler cecal contents, which is beneficial for uncultured Lachnospiraceae bacterium and Bacteroides sp. and contribute to reducing the excreta odor of the broilers (Yang et al., 2016). As the main components of soybean oligosaccharides, stachyose and raffinose were linked to the prevention and inhibition of intestinal pathogenic bacteria colonization and promotion of gut development and health (Ishizuka et al., 2009, Altamimi et al., 2016, Liu et al., 2018b, Liu et al., 2019). Therefore, this study was designed to investigate the effects of soybean oligosaccharide, stachyose, and raffinose in diets of broilers on odor compound production and cecal microbiota.
Materials and methods
Animals
A total of 300 one-day-old Arbor Acre commercial broiler chicks with similar initial live weights were used in this study. The chicks were randomly allocated into 5 treatments with 6 replicates of 10 birds (5 males and 5 females) each and housed in 3-layer cages equipped with a separate feeder and a nipple drinker under a constant 24-h light condition. The room temperature was maintained at 33°C for the first 3 D, after which the temperature was gradually reduced by 3°C each week until reaching 25°C, which was maintained until the end of the 49-D experiment. All procedures involving animals were approved by the Animal Care and Use Committee of Shenyang Agricultural University.
Diets
Diets (in mash form) for the broilers were formulated in accordance with the Arbor Acres management guide (Beijing Arbor Acres Poultry Breeding Co., Ltd.) and shown in Table 1. Soybean oligosaccharide, stachyose, and raffinose products were obtained from Guangzhou YiBaoLai Biotechnology Co., Ltd. (Guangzhou, China) and contained 997.3 g/kg, 853.3 g/kg, and 630.4 g/kg of total sugar (measured values), respectively. Five groups were designated: a group fed a typical corn-soybean meal diet (positive control), a group fed a soybean meal-free diet (negative control), a group each fed 0.6% soybean oligosaccharide, 0.6% stachyose, or 0.6% raffinose as a supplement to the negative control diet on a total sugar basis. The diets were fed in a unlimited manner, and all broilers were given free access to water from nipple drinkers for the experimental period of 49 D.
Table 1.
Feed ingredients and nutrient composition of experiment diets (air dry basis, g/kg).
| Feed ingredients | Corn-soybean meal diets |
Soybean meal-free diets |
||
|---|---|---|---|---|
| Starter (1–21 D post-hatch) | Finisher (22–49 D post-hatch) | Starter (1–21 D post-hatch) | Finisher (22–49 D post-hatch) | |
| Corn | 468 | 511 | 414 | 473 |
| Soybean meal | 233 | 169 | 0.0 | 0.0 |
| Corn umbilicus pulp | 80 | 50 | 234 | 160 |
| Corn protein meal | 60 | 60 | 187 | 152 |
| Soybean oil | 37 | 45 | 35 | 43 |
| Rice bran | 50 | 80 | 50 | 80 |
| Corn DDGS | 30 | 50 | 30 | 50 |
| Limestone | 12.5 | 11.8 | 12.0 | 11.4 |
| Calcium hydrophosphate | 13.5 | 10.0 | 14.2 | 10.5 |
| Sodium chloride | 3.0 | 3.0 | 2.6 | 2.7 |
| DL-methionine | 3.0 | 2.5 | 2.4 | 2.0 |
| L-lysine | 4.9 | 4.6 | 10.0 | 8.4 |
| L-threonine | 1.6 | 1.2 | 2.2 | 1.7 |
| L-arginine | 1.5 | 0.8 | 4.5 | 3.0 |
| Choline chlotide | 0.5 | 0.5 | 0.5 | 0.5 |
| Vitamin mineral premix1 | 1.5 | 1.5 | 1.5 | 1.5 |
| Phytase | 0.1 | 0.1 | 0.1 | 0.1 |
| Nutrient composition2 | ||||
| Metabolizable energy (MJ/kg) | 12.76 | 13.21 | 12.76 | 13.21 |
| Dry matter | 903.5 | 906.3 | 901.8 | 899.6 |
| Crude protein | 216.5 | 196.7 | 218.2 | 197.9 |
| Calcium | 9.6 | 8.1 | 9.5 | 7.8 |
| Total phosphorus | 6.8 | 6.2 | 6.9 | 6.3 |
| Available phosphorus | 3.6 | 3.0 | 3.6 | 3.0 |
| Lysine | 15.1 | 13.6 | 14.6 | 14.2 |
| Methionie | 6.4 | 5.9 | 6.3 | 5.7 |
| Methionie + cystine | 9.5 | 7.9 | 9.3 | 8.3 |
Provided per kg diet: VA, 22,500 IU; VB1, 2.5 mg; VB2, 1.5 mg; VB6, 20 mg; VD3, 5,500 IU; VE3, 35 IU; antioxidant, 0.25 mg; pantothenic acid, 5 mg; folic acid, 2 mg; niacin 75 mg; biotin 0.12 mg; Mn, 60 mg; Fe, 44 mg; Zn, 76.5 mg; Cu, 6.8 mg; and K, 10 mg.
The metabolizable energy and available phosphorus were calculated values, while the others were determined based on triplicate assays.
Sample Collection
Cecal digesta samples of broilers from each replicate (male and female in half per treatment) for skatole, indole, VFAs, and microbial examination were collected on the day of slaughter (49 D). For each broiler chicken, both ceca were taken out, and then the cecal digesta was collected in a tube and stored at −80°C until further analysis.
Skatole and Indole Analysis
The concentrations of skatole and indole in cecal digesta were quantitatively measured by high-performance liquid chromatography (HPLC) method as described before (Yang et al., 2016). Briefly, 1 g of cecal digesta and 2 mL of distilled water were added to a 5-mL centrifuge tube, which was homogenized by a tissue homogenizer for 15 s before centrifuging at 3,000 × g for 10 min. Then, 1 mL of supernatant liquid and 2 mL of methyl alcohol were added to a 5-mL centrifuge tube, mixed by a vortex mixer, and then kept at −20°C for 30 min to precipitate particles. After centrifuging at 3,000 × g for 10 min, 1 mL of supernatant liquid was transferred into a 1.5-mL centrifuge tube and then centrifuged at 15,000 × g for 30 min. The collected supernatant liquid was passed through a 0.45-μm filter membrane for the analysis of skatole and indole using an HPLC (1100; Agilent, Wilmington, DE) equipped with a fluorescence detector and a chromatographic column (250 mm × 4.6 mm × 5 μm; Dikma, Beijing, China). The mobile phase was the mixture of acetonitrile and distilled water at a ratio of 60:40 (v/v) with a flow rate of 1.0 mL/min. The excitation wavelength and emission wavelength were 263 nm and 358 nm, respectively, and the sample injection volume was 20 μL.
VFA and Lactic Acid Analyses
VFA and lactic acid analyses were performed by using an HPLC system (1100; Agilent) equipped with a photodiode array detector and a chromatographic column (250 mm × 4.6 mm × 5 μm; Dikma). Briefly, 1 g of cecal digesta and 2 mL of distilled water were mixed, homogenized, and centrifuged at 3,000 × g for 10 min. Then, 1 mL of the supernatant liquid was taken into a 2-mL centrifuge tube containing 0.2 mL of 25% metaphosphoric acid and centrifuged at 15,000 × g for 10 min. The supernatant liquid was collected and filtered through a 0.45-μm filter membrane to determine the concentrations of VFAs and lactic acid. The mobile phase was phosphate buffer (pH = 2.5) and methyl alcohol at a ratio of 95:5 (v/v) with a flow rate of 1.0 mL/min, and the sample injection volume was 10 μL.
DNA Extraction and PCR Amplification
Subsequently, 200 mg of cecal digesta was weighed and put into a 2-mL sterile centrifuge tube. Total genomic DNA from the cecal digesta samples was extracted using the cetyltrimethylammonium bromide method as previously described (Yang et al., 2016). Bacterial 16S ribosomal DNA genes containing V3-V4 of the variable region were amplified using the 341F and 805R primer pair (F: CCCTACACGACGCTCTTCCGATCTG, R: GACTGGAGTTCCTTGGCACCCGAGAATTCCA). The amplification conditions of the PCR were followed: one cycle at 95°C for 3 min; 30 cycles of 30 s at 95°C, 30 s at 50°C, and 60 s at 72°C; and one cycle at 72°C for 7 min.
Bioinformatic Analysis
The cecal microbiota was detected using a second-generation high-throughput sequencing platform based on IlluminaMiSeq 2500.
After sequencing, the primer connector sequences of the raw sequenced reads were discarded, and the paired reads were merged one sample read according to barcode label. Whole sample reads were filtered for quality control and processed to remove nonamplification sequences and chimeric sequences using the Usearch and Uchime software packages (drive5.com; Edgar, 2010; Edgar et al., 2011), respectively, and assembled to obtain operational sequences using the Uclust algorithm (Edgar, 2010). Sets of sequences with ≥97% identity were defined as an operational taxonomic unit. We used Ribosomal Database Project classifier software and the Greengenes database (Wang et al., 2007) for species annotation and statistical analyses of the species composition for each sample at the phylum and genus levels. The Shannon, Chao1, and Simpson indices were used to estimate alpha diversity (Qiao et al., 2018).
Statistical Analysis
Data were analyzed using a one-way ANOVA via the software SPSS 17.0 (SPSS Inc., Chicago, IL), and significant differences among groups were compared using Duncan's multiple comparison tests. Probability values less than 0.05 (P < 0.05) were considered for all measured variables.
Results
Odorous Compound Production
As shown in Table 2, the concentration of indole in the negative control group was higher than that in the positive control, soybean oligosaccharide, and raffinose groups (P < 0.05), and the lowest indole concentration was observed in the stachyose group. The indole levels in the soybean oligosaccharide, stachyose, and raffinose groups were lower than those in the negative control group by 9, 15, and 9% (P < 0.05), respectively. Similarly, the concentration of skatole in the negative control group was higher than that in the other groups (P < 0.05) and that in the positive control, soybean oligosaccharide and stachyose were lower than those in the raffinose group (P < 0.05). The concentration of skatole in the soybean oligosaccharide, stachyose, and raffinose groups were decreased by 42, 45, and 14%, respectively, compared to the negative control group.
Table 2.
Effects of soybean oligosaccharides, stachyose, and raffinose on the concentrations of skatole and indole in the cecum of broilers (μg/g).
| Groups | Skatole | Indole |
|---|---|---|
| Positive control | 1.1c | 1.8b |
| Negative control | 1.9a | 2.0a |
| Soybean oligosaccharide | 1.1c | 1.8b |
| Stachyose | 1.1c | 1.7c |
| Raffinose | 1.7b | 1.8b |
| SEM | 0.07 | 0.02 |
a-cFigures with different superscripts within the same column are significantly different (P < 0.05).
VFA and Lactic Acid Production
As shown in Table 3, the concentrations of acetic acid and propionic acid in the stachyose group were higher than those in the other groups (P < 0.05). The highest concentrations of butyric acid and lactic acid were observed in the positive control group (P < 0.05). Compared with the negative control, the concentrations of acetic acid in the soybean oligosaccharide, stachyose, and raffinose groups were increased by 114, 180, and 68%, respectively; propionic acid were increased by 551, 783, and 381%, respectively; butyric acid were increased by 68, 33, and 81%; and lactic acid were decreased by 21, 35, and 68%, respectively.
Table 3.
Effects of soybean oligosaccharides, stachyose, and raffinose on the concentrations of volatile fatty acids and lactic acid in the cecum of broilers (mg/g).
| Groups | Acetic acid | Propionic acid | Butyric acid | Latic acid |
|---|---|---|---|---|
| Positive control | 1.4d | 0.5d | 1.2a | 0.8a |
| Negative control | 1.2d | 0.3d | 0.4d | 0.7a,b |
| Soybean oligosaccharide | 2.6b | 2.0b | 0.7b | 0.5b |
| Stachyose | 3.4a | 2.7a | 0.6c | 0.3c |
| Raffinose | 2.1c | 1.5c | 1.8b | 0.7a,b |
| SEM | 0.15 | 0.19 | 1.15 | 0.04 |
a-dFigures with different superscripts within the same column are significantly different (P < 0.05).
Cecal Microbiota
The microbial 16S rDNA V3-V4 region of 20 cecal digesta samples in 5 treatments were sequenced base on IlluminaMiSeq sequencing technology. Operational taxonomic units were classified according the similarity of 97%. The raw sequence range of each sample was 45,624 to 85,230 reads, the sequence length was 400 to 440 bp after quality control (removal of the barcode, primer, and low-quality sequences), and the range of filtered sequences was 40,330 to 77,896 reads. There was no difference in the number of raw sequences, chimeras, and filtered sequences among groups (P > 0.05).
Cecal Microbial Alpha Diversity Analysis
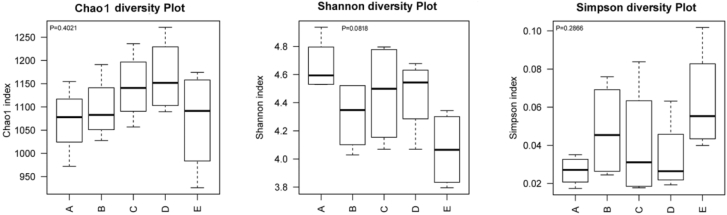
The microbial complexity in the cecum was estimated on the basis of alpha-diversity indices (Chao1 index, Simpson and Shannon index). The Chao1 index was used to estimate species richness, and index of Shannon and Simpson was used to indicate species diversity. There was no difference in the Chao1 and Simpson indices among groups (P > 0.05), although the Shannon index of the positive control was higher than that of the raffinose group (P < 0.05) (Figure 1).
Figure 1.
Effects of soybean oligosaccharides, stachyose, and raffinose on the cecal microbiota diversity and richness in broilers. A, B, C, D, and E are the positive control, negative control, soybean oligosaccharide, stachyose, and raffinose groups, respectively.
Microbial Species Annotation
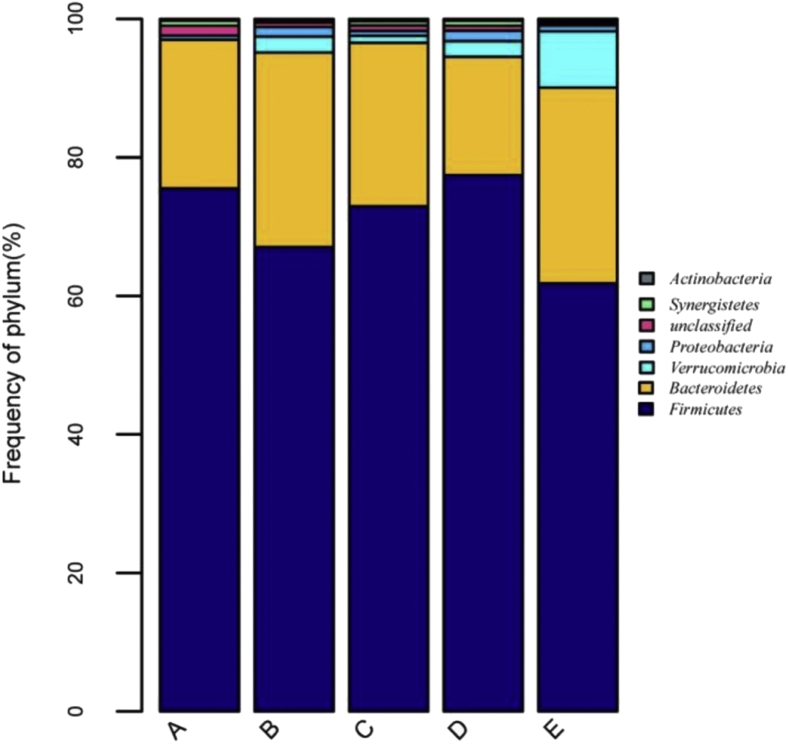
We annotated the microbial species found in this study. A total of 13 phyla and 66 genera were obtained from the 20 broiler cecal samples. There were 7 phyla with proportions greater than 0.1% (Figure 2): Firmicutes, Bacteroidetes, Verrucomicrobia, Proteobacteria, unclassified, Synergistetes, and Actinobacteria. Firmicutes was the dominant phylum in the cecal microbiota, accounting for 71% of the overall bacteria community, although no significant difference (P > 0.05) was observed among groups. Bacteroidetes was the second most abundant (24%) phylum in the cecum, and the average abundance value of both Firmicutes and Bacteroidetes was over 94.6% in all groups. The number of Verrucomicrobia (8.87%) was the highest in the raffinose group, and the number of Proteobacteria (1.38%) in the stachyose group was higher than that of soybean oligosaccharide (0.72%) and raffinose (0.86%) groups. Interestingly, although no Verrucomicrobia was found in the positive control, the unclassified bacteria were overrepresented (1.44%) compared with the other groups.
Figure 2.
Relative abundance of bacterial phylum in the cecal digesta of broilers. A, B, C, D, and E are the positive control, negative control, soybean oligosaccharide, stachyose, and raffinose groups, respectively.
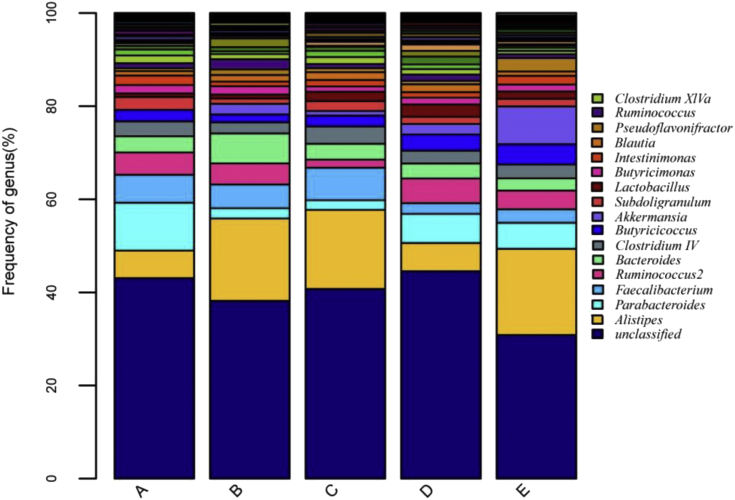
Figure 3 shows the structure of the bacterial community of all groups in the broiler cecal samples at the genus level. The sequences were more abundant by an unclassified genus with average relative abundance >40% among groups, and the 16 major classified genera across all groups were the Alistipes, Parabacteroides, Faecalibacterium, Ruminococcus2, Bacteroides, Clostridium IV, Butyricicoccus, Akkermansia, Subdoligranulum, Lactobacillus, Butyricimonas, Intestinimonas, Blautia, Pseudoflavonifractor, Rumonococcus, and Clostridium XIVa, with relative abundance >1%. The results showed that Alistipes was more abundant in the negative control, soybean oligosaccharide, and raffinose groups, whereas Parabacteroides was more abundant in the positive control. Alistipes and Parabacteroides were comparably abundant in the stachyose group (P > 0.05). The highest proportion of Bacteroides was observed in the negative control group, and that of Lactobacillus in the stachyose group, although these were not different from the other groups (P > 0.05). Finally, Ruminococcus and Ruminococcus2 were decreased in the soybean oligosaccharide group and Akkermansia and Pseudoflavonifractor were increased in the raffinose group.
Figure 3.
Cecal microbial composition of bacterial genera in broilers. A, B, C, D, and E are the positive control, negative control, soybean oligosaccharide, stachyose, and raffinose groups, respectively.
Discussion
Many attempts have been made to understand the process of odorous compound production and reduce odor emission from livestock and poultry system. Our previous studies have demonstrated that the older the broiler, the higher the odorous compound concentrations (Zhang, 2016). The skatole and indole concentrations in the digesta of intestinal segments from broilers varied and followed the order of the cecum > rectum > ileum (Yang et al., 2019). In addition, the concentrations of skatole and indole in the cecum were significantly higher than those in the rectum and excreta, and the concentrations of odorous compounds (skatole and indole) in the cecum showed positive correlations with those in the excreta (Wang et al., 2016). Therefore, the cecal digesta of broilers were sampled to determine the levels of cecal odorous compounds at the end of feeding trial (49 D old) in this study.
Indole and skatole result from a multistep degradation of L-tryptophan by microbial activity, which are considered to be the contributors to chicken excreta malodor (Roager and Licht, 2018). Skatole is a malodorous compound that contributes to the characteristic smell of animal fecal material (Liu et al., 2018a). Our previous studies have demonstrated that the in vitro addition of soybean oligosaccharide decreased the concentrations of skatole and indole in broiler cecal and rectal microbiota fermentation broth (Liu et al., 2018c). The inclusion of 3.5 to 5.0 g/kg of soybean oligosaccharide to the diet had a decreasing effect on the excreta skatole of broilers (Li, 2018), and except for soybean oligosaccharides, the addition of stachyose, raffinose, and saccharose also decreased the concentrations of skatole in broiler cecal microbial broth (Yang et al., 2017). The results of the study by Myint et al. (2018) suggested that feeding bean husk as a dietary fiber supplement (containing 0.1% stachyose and 0.1% raffinose in carbohydrate) exhibited lower skatole and indole levels in rat cecum. Similarly, the present study indicated that dietary supplementation to a soybean meal–free diet with soybean oligosaccharide, stachyose, and raffinose significantly reduced skatole and indole levels in broiler cecal digesta.
Oligosaccharides are a constituent of dietary fiber that cannot be broken down by the host's digestive enzymes in the small intestine but can be fermented to a large extent by microorganisms in the lower gastrointestinal tract into VFAs, such as acetic acid, propionic acid, and butyric acid (Bedford and Gong, 2018). Soybean oligosaccharides have been demonstrated to contribute to the growth of beneficial bacteria and improve the intestinal microenvironment, thus resulting in the change of VFAs concentrations. Zhou et al. (2012) found that the in vitro addition of 2% soybean oligosaccharides into the fermentation broth of pig cecal digesta increased the concentrations of acetic acid, propionic acid, and butyric acid. Lan et al. (2007) also found that soybean oligosaccharides increased the concentrations of acetic acid, propionic acid, and butyric acid in vitro fermentation of broiler cecal contents. Our previous studies have demonstrated that the addition of soybean oligosaccharides increased the acetic acid level in vitro and total VFA level in vivo (Yang et al., 2016, Liu et al., 2018c). Here, we found that dietary oligosaccharide supplementation can increase cecal VFAs and decrease lactic acid concentration in broilers, and among oligosaccharides, stachyose was superior to soybean oligosaccharides and raffinose, implying that stachyose may be the key functional component of soybean oligosaccharide.
It is worth mentioning that our preliminary study of the project showed that the growth of broilers at 1 to 49 D of age in the positive control group was the best (owing to the dependence of broiler chickens on soybean meal), followed by the stachyose group, although this was not different from the negative control and the soybean oligosaccharide group. The worst growth of broilers was observed in the raffinose group (unpublished data). These data mean that the birds fed stachyose or soybean oligosaccharides grew well compared with those in the negative control group and had no effect on odorous metabolites.
The gut microbiota of the host plays important roles in absorbing nutrients, enhancing growth and metabolism, protecting against harmful bacteria, and modulating the immune system (Borda-Molina et al., 2018, Rowland et al., 2018). Thus, it is essential to elucidate the link between gut microbiota and odor compound production to understand the internal mechanism. Soybean oligosaccharides have been previously shown to modulate the structure and biomass of broiler cecal microbiota fermentation broth in vitro, which decreased the microbiota richness but not microbiota diversity, thus resulting in lower L-tryptophan degradation rate and skatole and indole levels (Liu et al., 2018b). Yang et al. (2016) found that supplementation of soybean oligosaccharides into diets increased the Shannon Wiener index and richness of the microbiota, increased the total VFAs, and decreased skatole and indole levels in the cecal digesta of broilers. Zhou et al. (2014) also suggested that the addition of soybean oligosaccharide could improve the balance of cecal microbial community and increase the diversity of intestinal microbiota. In this study, we found that dietary soybean oligosaccharide and stachyose supplementation could modulate the balance of intestinal microbiota to maintain the diversity and richness of cecal microbiota, while raffinose supplementation decreased the diversity of cecal microbiota in broilers.
The cecum is highly dominated by not-yet-characterized bacteria and exhibits the highest concentrations of VFAs (Rowland et al., 2018). Generally, the normal cecal microbiota of chicken are mainly colonized by the phyla Firmicutes, Bacteroides, and Proteobacteria (Borda-Molina et al., 2018). Consistent with this, the present study also showed that Firmicutes and Bacteroides constituted the majority of the cecal bacteria, which were associated with carbohydrate and protein metabolism. In addition, the relative abundance of Bacteroides in the stachyose group was lower than that in the other groups, indicative of its role in the process of deamination and decarboxylation of tryptophan to produce malodorous compounds, such as skatole (Cho et al., 2015). Conversely, the higher Proteobacteria abundance in the stachyose group than the soybean oligosaccharide and raffinose groups may account for the relatively lower skatole and indole levels. Noticeably, raffinose may serve as a preferential fermentation substrate for Verrucomicrobia, which was abundant in the raffinose group.
At the genus level, our study showed that Alistipes, Parabacteroides, and Faecalibacterium were the main groups in the cecum, which was different from other studies in which the cecum was dominated by Acinetobacter, Bacteroides, Streptococcus, Clostridium, and Lactobacillus (Borda-Molina et al., 2018), although they were similar to the study by Xiao et al. (2017) in which it was indicated that Alistipes, Ruminococcus, and Faecalibacterium were the dominant bacteria genera in the cecum. Alistipes and Bacteroides can metabolize tryptophan to indole-3-lactate and then to indole and skatole in animal faces (Cho et al., 2015), which is consistent with the observation in this study that the higher abundance of Alistipes and Bacteroides in the negative control was along with the higher concentrations of skatole and indole, although it was completely opposite to the findings in the stachyose group. Lactobacillus, a widely used probiotic, inhibits the growth and colonization of pathogenic bacteria via the production of organic acids (such as lactic acid) or bacteriocins (Caly et al., 2015). Nevertheless, in this study, the abundance of Lactobacillus in the stachyose group did not increase the lactic acid levels, although the concentration of lactic acid in the stachyose group was lower than that in other groups. Presumably, lactic acid may be a fermentation substrate to be used and converted into propionate or butyrate by other bacteria, thus leading to lower levels in the cecal digesta (Rowland et al., 2018). The abundance of Akkermansia, which is a genus in the phylum Verrucomicrobia, in the raffinose group further suggested that it was related to the degradation of raffinose. In general, our findings clearly indicate an evident change in bacterial community, microbial fermentation, and odor compound production of the cecum in broilers after dietary oligosaccharide supplementation.
In conclusion, the results from this study indicate that the addition of oligosaccharides into diets affects odor compound production and the bacterial community composition. Supplementation of stachyose rather than soybean oligosaccharide or raffinose reduced odor compound production and increased acetic acid and propionic acid levels of the cecum in broilers. The microbiome analysis revealed that the bacterial community is related to the production of skatole, indole, VFAs, and lactic acid. Compared with soybean oligosaccharides and raffinose, stachyose can preferably improve cecal fermentation and help minimize odor compound generation from broiler cecum. We suggest that the results of this study might contribute to the reduction of odor compounds in broilers.
Acknowledgments
The authors thank the National Natural Science of China (No. 31772618) for supporting this study.
Conflict of Interest Statement: The authors declare that they have no competing interests.
References
- Altamimi M., Abdelhay O., Rastall R.A. Effect of oligosaccharides on the adhesion of gut bacteria to human HT-29 cells. Anaerobe. 2016;39:136–142. doi: 10.1016/j.anaerobe.2016.03.010. [DOI] [PubMed] [Google Scholar]
- Armstrong T.A., Williams C.M., Spears J.W., Schiffman S.S. High dietary copper improves odor characteristics of swine waste. J. Anim. Sci. 2000;78:859–864. doi: 10.2527/2000.784859x. [DOI] [PubMed] [Google Scholar]
- Bedford A., Gong J. Implications of butyrate and its derivatives for gut health and animal production. Anim. Nutr. 2018;4:151–159. doi: 10.1016/j.aninu.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoît D., Gariépy C., Houde A. Review of microbiological and biochemical effects of skatole on animal production. Livest. Prod. Sci. 2001;71:193–200. [Google Scholar]
- Borda-Molina D., Seifert J., Camarinha-Silva A. Current perspectives of the chicken gastrointestinal tract and its microbiome. Comput. Struct. Biotechnol. J. 2018;16:131–139. doi: 10.1016/j.csbj.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowski S., Matusiak K., Powatowski S., Pielech-Przybylska K., Makowski K., Nowak A., Rosowski M., Komorowski P., Gutarowska B. A novel microbial-mineral preparation for the removal of offensive odors from poultry manure. Int. Biodeterior. Biodegrad. 2017;119:299–308. [Google Scholar]
- Caly D.L., D'Inca R., Auclair E., Drider D. Alternatives to antibiotics to prevent necrotic enteritis in broiler chickens: a microbiologist's perspective. Front. Microbiol. 2015;6:1336. doi: 10.3389/fmicb.2015.01336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S., Hwang O., Park S. Effect of dietary protein levels on composition of odorous compounds and bacterial ecology in pig manure. Asian-Australas J. Anim. Sci. 2015;28:1362–1370. doi: 10.5713/ajas.15.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus R., Lösel D., Lacorn M., Mentschel J., Schenkel H. Effects of butyrate on apoptosis in the pig colon and its consequences for skatole formation and tissue accumulation1. J. Anim. Sci. 2003;81:239–248. doi: 10.2527/2003.811239x. [DOI] [PubMed] [Google Scholar]
- Edgar R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- Edgar R.C., Haas B.J., Clemente J.C., Quince C., Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27:2194–2200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka S., Iwama A., Dinoto A., Suksomcheep A., Maeta K., Kasai T., Hara H., Yokota A. Synbiotic promotion of epithelial proliferation by orally ingested encapsulated Bifidobacterium breve and raffinose in the small intestine of rats. Mol. Nutr. Food Res. 2009;53 Suppl 1:S62–S67. doi: 10.1002/mnfr.200800041. [DOI] [PubMed] [Google Scholar]
- Lan Y., Williams B.A., Verstegen M.A., Patterson R., Tamminga S. Soy oligosaccharides in vitro fermentation characteristics and its effect on caecal microorganisms of young broiler chickens. Anim. Feed Sci. Tech. 2007;133:286–297. [Google Scholar]
- Li X. Shenyang Agr. Univ.; Shenyang, China: 2018. Effects of Soybean Oligosaccharides on Growth, Immune, Skatole Concentrations in Excreta and Cecal Microflora Structure in Broilers. MD Diss. [Google Scholar]
- Liu D., Wei Y., Liu X., Zhou Y., Jiang L., Yin J., Wang F., Hu Y., Nanjaraj Urs A.N., Liu Y., Ang E.L., Zhao S., Zhao H., Zhang Y. Indole acetate decarboxylase is a glycyl radical enzyme catalysing the formation of malodorant skatole. Nat. Commun. 2018;9:4224. doi: 10.1038/s41467-018-06627-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G., Bei J., Liang L., Yu G., Li L., Li Q. Stachyose improves inflammation through modulating gut microbiota of high-fat diet/streptozotocin-induced type 2 diabetes in rats. Mol. Nutr. Food Res. 2018;62:e1700954. doi: 10.1002/mnfr.201700954. [DOI] [PubMed] [Google Scholar]
- Liu H.Y., Hou R., Yang G.Q., Zhao F., Dong W.G. In vitro effects of inulin and soya bean oligosaccharide on skatole production and the intestinal microbiota in broilers. J. Anim. Physiol. Anim. Nutr. 2018;102:706–716. doi: 10.1111/jpn.12830. [DOI] [PubMed] [Google Scholar]
- Liu Y., Li T., Alim A., Ren D., Zhao Y., Yang X. Regulatory effects of stachyose on colonic and hepatic inflammation, gut microbiota dysbiosis, and peripheral CD4(+) T cell distribution abnormality in high-fat diet-fed mice. J. Agric. Food Chem. 2019;67:11665–11674. doi: 10.1021/acs.jafc.9b04731. [DOI] [PubMed] [Google Scholar]
- Mackie R.I., Stroot P., Varel V. Biochemical identification and biological origin in key odor components in livestock waste. J. Anim. Sci. 1998;76:1331–1342. doi: 10.2527/1998.7651331x. [DOI] [PubMed] [Google Scholar]
- Myint H., Kishi H., Iwahashi Y., Saburi W., Koike S., Kobayashi Y. Functional modulation of caecal fermentation and microbiota in rat by feeding bean husk as a dietary fibre supplement. Benef. Microbes. 2018;9:963–974. doi: 10.3920/BM2017.0174. [DOI] [PubMed] [Google Scholar]
- O'Shea M.A.P., Solan P., Curran T., Varley P.F., Walsh A.M., Doherty J. The effect of protease and xylanase enzymes on growth performance, nutrient digestibility, and manure odour in grower–finisher pigs. Anim. Feed. Sci. Tech. 2014;189:88–97. [Google Scholar]
- Øverland N., Skjerve E., Sørum H. Organic acids in diets for entire male pigs: effect on skatole level, microbiota in digesta, and growth performance. Livest. Sci. 2008;115:169–178. [Google Scholar]
- Qiao H., Song Y., Shi H., Bian C. Fermented Astragalus in diet altered the composition of fecal microbiota in broiler chickens. AMB Express. 2018;8:151. doi: 10.1186/s13568-018-0682-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recharla N., Kim K., Park J., Jeong J., Jeong Y., Lee H., Hwang O., Ryu J., Baek Y., Oh Y., Park S. Effects of amino acid composition in pig diet on odorous compounds and microbial characteristics of swine excreta. J. Anim. Sci. Tech. 2017;59:28. doi: 10.1186/s40781-017-0153-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rideout T.C., Fan M.Z., Cant J.P., Wagner-Riddle C., Stonehouse P. Excretion of major odor-causing and acidifying compounds in response to dietary supplementation of chicory inulin in growing pigs. J. Anim. Sci. 2004;82:1678–1684. doi: 10.2527/2004.8261678x. [DOI] [PubMed] [Google Scholar]
- Roager H.M., Licht T.R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 2018;9:3294. doi: 10.1038/s41467-018-05470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland I., Gibson G., Heinken A., Scott K., Swann J., Thiele I., Tuohy K. Gut microbiota functions: metabolism of nutrients and other food components. Eur. J. Nutr. 2018;57:1–24. doi: 10.1007/s00394-017-1445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N.K., Choct M., Wu S.B., Smillie R., Swick R.A. Dietary composition affects odour emissions from meat chickens. Anim. Nutr. 2015;1:24–29. doi: 10.1016/j.aninu.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N.K., Choct M., Wu S.B., Smillie R., Morgan N., Omar A.S., Sharma N., Swick R.A. Performance, litter quality and gaseous odour emissions of broilers fed phytase supplemented diets. Anim. Nutr. 2016;2:288–295. doi: 10.1016/j.aninu.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesso T.A., Zheng A., Cai H., Liu G. Isolation and characterization of two Acinetobacter species able to degrade 3-methylindole. PLoS One. 2019;14:e0211275. doi: 10.1371/journal.pone.0211275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabue S.L., Scoggin K., Li H., Burns R., Xin H., Hatfield J. Speciation of volatile organic compounds from poultry production. Atmos. Environ. 2010;44:3538–3546. [Google Scholar]
- Wang Q., Garrity G.M., Tiedje J.M., Cole J.R. Naïve bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Guan Q.N., Yang G.Q., Liu H.Y., Ning Z.L., Dong W.G. Effects of dietary inulin supplementation on growth performance, concentrations of the major odor-causing compounds in excreta and intestinal digesta of broilers. Chin. J. Anim. Nutr. 2016;28:3875–3884. doi: 10.3382/ps/pew124. [DOI] [PubMed] [Google Scholar]
- Xiao Y., Xiang Y., Zhou W., Chen J., Li K., Yang H. Microbial community mapping in intestinal tract of broiler chicken. Poult. Sci. 2017;96:1387–1393. doi: 10.3382/ps/pew372. [DOI] [PubMed] [Google Scholar]
- Yang G.Q., Yin Y., Liu H.Y., Liu G.H. Effects of dietary oligosaccharide supplementation on growth performance, concentrations of the major odor-causing compounds in excreta, and the cecal microflora of broilers. Poult. Sci. 2016;95:2342–2351. doi: 10.3382/ps/pew124. [DOI] [PubMed] [Google Scholar]
- Yang G., Yang H., Liu J., Liu H., Dong W., Zhu X. Effects of soybean oligosaccharide and its functional components on skatole production and microbiota composition of broilers cecal contents in vitro. Chin. J. Anim. Nutr. 2017;29:4058–4068. [Google Scholar]
- Yang G., Zhang P., Liu H., Zhu X., Dong W. Spatial variations in intestinal skatole production and microbial composition in broilers. Anim. Sci. J. 2019;90:412–422. doi: 10.1111/asj.13164. [DOI] [PubMed] [Google Scholar]
- Zhang P. Shenyang Agr. Univ.; Shenyang, China: 2016. Research on the Basic Laws of Skatole Production and its Variation with Intestinal Microbial Components in Broilers. MD Diss. [Google Scholar]
- Zhou X.L., Kong X.F., Yang X.J., Yin Y.L. Soybean oligosaccharides alter colon short-chain fatty acid production and microbial population in vitro. J. Anim. Sci. 2012;90 Suppl 4:37–39. doi: 10.2527/jas.50269. [DOI] [PubMed] [Google Scholar]
- Zhou X.L., Kong X.F., Lian G.Q., Blachier F., Geng M.M., Yin Y.L. Dietary supplementation with soybean oligosaccharides increases short-chain fatty acids but decreases protein-derived catabolites in the intestinal luminal content of weaned Huanjiang mini-piglets. Nutr. Res. 2014;34:780–788. doi: 10.1016/j.nutres.2014.08.008. [DOI] [PubMed] [Google Scholar]