Abstract
Newcastle disease, which is a highly contagious and fatal disease caused by the Newcastle disease virus (NDV), has harmed the poultry industry for decades. The administration of effective vaccines can control most outbreaks and epidemics of Newcastle disease in the world. However, vaccination failures of live attenuated vaccines becasue of storage and transportation problems have been reported. Hence, thermostable live vaccine strains, such as V4 and I-2 strains, are being used and welcomed in tropical regions such as Africa and Southeast Asia. In this study, a thermostable, attenuated vaccine candidate strain NDV/rHR09 was generated using the genotype VIII heat-resistant virulent NDV strain HR09 by the reverse genetics system. The results of the determination of the mean death time and intracerebral pathogenicity index indicated that NDV/rHR09 is lentogenic even after 15 serial passages in embryonated chicken eggs. The thermostability assessment showed that the NDV/rHR09 strain exhibited hemagglutination activity and infectivity when exposed to 56°C for 60 min. Compared with the commercially available La Sota and V4 vaccines, the NDV/rHR09 induced higher antibody titers in specific pathogen-free chickens. In addition, NDV/rHR09 conferred complete protection against virulent genotype VII NDV challenge and virus shedding from vaccinated chickens. These results suggest that NDV/rHR09 is a promising thermostable vaccine candidate strain.
Key words: Newcastle disease virus, thermostability, genotype VIII, vaccine candidate
Introduction
Newcastle disease (ND), which is caused by virulent Newcastle disease virus (NDV), is one of the most devastating poultry infectious diseases because it has led to severe economic losses for the poultry industry worldwide. The current effective strategy for the prevention and control of ND in poultry mainly depends on vaccination. However, almost all commercially available ND vaccines require refrigeration and begin to deteriorate rapidly after 1–2 h at room temperature (approximately 25°C). Heat damage is believed to account for many vaccination failures in developing countries (Mahmood et al., 2014). But maintaining an adequate supply in refrigerated facilities is difficult in many rural areas of developing countries without reliable electrical supplies. Fortunately, the thermostable V4 and I-2 vaccine strains have been widely administered for the vaccination of rural chicken flocks in many developing countries (Alders, 2014). However, the V4 and I-2 vaccines are phylogenetically the same genotype as viruses isolated in the 1960s and 1990s, respectively, which are phylogenetically divergent from current outbreak viruses. These vaccines are effective in protecting against clinical disease and mortality from a virulent NDV challenge but do not prevent viral replication (Susta et al., 2015). The sequence divergence exists between circulating and vaccine strains that might be responsible for the incomplete protection in vaccinated poultry flocks. Therefore, the development of novel NDV vaccines that are closely related to prevalent strains and heat stable is urgently needed.
The NDV strains have been categorized into virulent (velogenic), intermediate (mesogenic), and avirulent (lentogenic) strains on the basis of their pathogenicity in chickens. The NDV is a single strand, enveloped RNA virus that encodes 6 structural proteins: nucleoprotein, phosphoprotein (P), matrix, fusion (F), hemagglutinin-neuraminidase (HN) protein, and the large RNA polymerase (L). The two surface glycoproteins, HN and F, are viral neutralization antigens. The HN protein is responsible for virus attachment and release. The F protein is synthesized as an inactive precursor that is cleaved into 2 biologically active F1 and F2 subunits by host cell proteases. Cleavage of F protein is required for virus entry and cell-to-cell fusion (Panda et al., 2004). The sequence of the F protein in virulent and intermediate strains of NDV typically contains the polybasic cleavage sites 112(R/K)RQ(R/K)R↓F117, which can be cleaved by ubiquitous furin-like proteases present in a wide range of host cells and tissues (Romer-Oberdorfer et al., 2003). In contrast, the F protein cleavage site in avirulent NDV strains typically have basic residues at the 112 and 115 positions in the cleavage site 112(G/E) (K/R)Q(G/E)R↓L117 and depends on a limited trypsin-like protease that is restricted to respiratory and intestinal tracts for cleavage (Yadav et al., 2018). Thus, the F protein cleavage site has been characterized as a major determinant of NDV virulence (Peeters et al., 1999, Xu et al., 2019). However, some intermediate and virulent strains of NDV have the same amino acid sequences at their F protein cleavage site but differ in virulence, indicating that additional viral factors also contribute to the virulence of NDV (Tan et al., 2008).
The thermostable NDV strain HR09 was isolated from chickens, and 15,192 nucleotides genome has been sequenced completely. Compared with the La Sota and V4 vaccine strains, the HR09 has a closer phylogenetic distance and higher amino acid sequence identity to F and HN proteins of the prevalent genotype VII NDV (Cao et al., 2017). In this study, a recombinant HR09 virus (NDV/rHR09) in which the virulent F protein cleavage site motif “RRQKR↓F″ was modified to an avirulent motif “GRQGR↓L″ using a reverse genetics system. Furthermore, the biological properties and thermostability of NDV/rHR09 were analyzed, and its potentiality as a vaccine candidate was evaluated with regard to its immunogenicity and protective efficacy against infection of currently prevalent genotype VII NDV strains in specific pathogen-free (SPF) chickens.
Materials and methods
Virus, cells, and plasmid
Newcastle disease virus strain HR09 was isolated from chickens in China by our laboratory and identified as a genotype VIII virus (Cao et al., 2017). The virus was plaque purified on chicken embryo fibroblast (CEF) cells and propagated in 9-day-old SPF chicken embryos (Beijing Boehringer Ingelheim Vital Bio, Beijing, China). Vaccine strains of La Sota and V4 and velogenic genotype VII strain ZJ1 were provided by the animal infectious disease laboratory of Yangzhou University. BSR T7/5 cells stably expressing T7 RNA polymerase were provided from Harbin Veterinary Institute and maintained in Dulbecco's modified eagle medium (Gibco) supplemented with 10% fetal bovine serum), 100 U/mL of penicillin, and 100 μg/mL of streptomycin. The TVT7R (0.0) plasmid was a gift from Professor L. Andrew Ball (the University of Alabama, USA).
Construction of NDV Reverse Genetic and Modification of the F Protein Cleavage Site
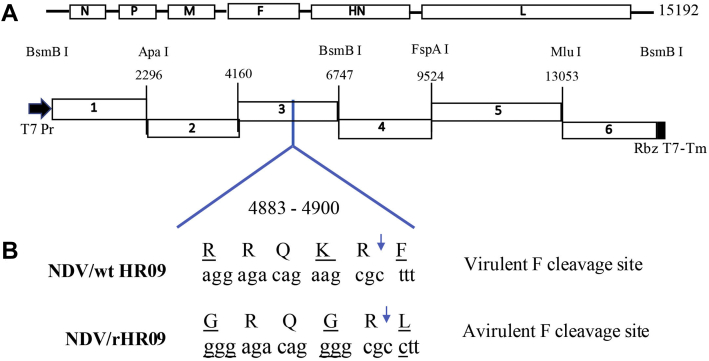
Viral RNAs from 10-day-old SPF chicken embryos infected with HR09 were extracted using an Ultrapure RNA Kit (CoWin Bio, Beijing, China). The cDNA was generated using Moloney murine leukemia virus reverse transcriptase (TransGen Biotech, Beijing, China). A full-length cDNA of 15,192-nucleotide-long genome of HR09 was constructed in plasmid TVT7R (0.0) (Johnson et al., 2000) from 6 fragments that were generated by PCR using Q5 High-Fidelity DNA Polymerase (New England Biolabs, Ipswich, MA). To facilitate construction, a total of 6 restriction sites were introduced into the full-length NDV cDNA during PCR as follows: an Apa I site was introduced into the P open reading frame (ORF); a BsmB I site was introduced into the HN ORF; a FspA I site was added to the L ORF; an Mlu I site was created in the L ORF, resulting in one nucleotide change (G13048A) in the region that is considered a molecular marker; and 2 BsmB I sites were created at the head and tail of the full-length cDNA. The six fragments were digested and inserted into the TVT7R (0.0) plasmid between the T7 promoter and the hepatitis delta virus antigenome ribozyme sequence (Figure 1A). The F protein cleavage site was mutated from 112RRQKRF117 to 112GRQGRL117 by overlapping PCR. This involved 3 amino acid substitutions at position 112, 115, and 117 associated with the F protein (Figure 1B). The constructed full-length cDNA clone with the mutated F gene was named NDV/rHR09.
Figure 1.
Generation of a full-length antigenomic cDNA of the NDV HR09 strain and modification of its F protein cleavage site. (A) Construction of the full-length antigenomic cDNA of the HR09 strain. Six cDNA fragments were generated by reverse transcription-polymerase chain reaction that inserted six unique restriction sites (the names and positions are indicated). The full-length cDNA fragments were inserted into the TVT7R (0.0) plasmid and flanked at the upstream side by a T7 RNA polymerase promoter (Pr) and at the downstream side by the hepatitis delta virus ribozyme sequence (Rbz) followed by a T7 terminator (Tm). (B) Mutation of the F protein cleavage site. The mutated amino acid residues and nucleotides are indicated in bold. The arrows show where F protein cleavage occurs. Abbreviations: F, fusion; NDV, Newcastle disease virus.
Rescue of the Newcastle virus was performed with 3 support plasmids, nucleoprotein, P, and L, in 6-well cell culture plates, as described previously (Hu et al., 2011). Briefly, the full-length cDNA plasmids with 3 support plasmids were cotransfected into BSR cells. At 12 h after transfection, a final concentration of 10% allantoic fluid was added to the BSR cells. The mixture of transfected cells and supernatant was collected and inoculated into 10-day-old SPF chicken embryos at 60 h after transfection, and successful virus recovery was identified by harvesting allantoic fluid at 96 h post-infection (hpi) with a positive hemagglutination (HA) test. The modified F protein cleavage sites were amplified by reverse transcription-polymerase chain reaction and confirmed by Sanger sequencing.
Characterization of the Rescued Virus
The HA assay was performed using 1% chicken red blood cells at 37°C in 96-well microtiter plates. The growth kinetics of the virus were evaluated in 10-day-old SPF chicken embryos. Briefly, the virus was propagated in 10-day-old SPF chicken embryos by allantoic sac inoculation at 103 50% egg infectious dose (EID50). The allantoic fluid was collected at 24, 48, 72, 96, 120, and 144 hpi, and EID50 values were calculated by the method of Reed and Muench.
The pathogenicity stability of the NDV was examined by passage in SPF chicken embryos. Briefly, the NDV/rHR09 virus was propagated in 10-day-old SPF chicken embryos by allantoic sac inoculation. At 3 D after incubation, the allantoic fluids were harvested and titrated for HA assay, and then, positive allantoic fluids were diluted 103 times for inoculation into SPF chicken embryos. After 15 passages, the pathogenicity of NDV was determined by the mean death time (MDT) test in 10-day-old SPF chicken embryos and the intracerebral pathogenicity index (ICPI) test in 1-day-old SPF chickens, as previously described (Alexander, 2000).
Thermostability test was determined by heat treatment at 56°C, as previously described (Wen et al., 2016). Briefly, the NDV strains were submerged into a water bath and incubated at 56°C for different time periods. After heat treatment, samples were chilled rapidly using an ice-cold water bath and then performed the HA assay and EID50 assay.
Immunization and Challenge Studies in SPF Chickens
A total of 60 1-day-old SPF chickens hatched in a fully automatic chicken egg incubator (Shandong Keyu Incubation Equipment Co., Ltd., Dezhou, China) were randomly divided into 5 equal groups in isolators (34°C, relative humidity 60%). One week later, birds in groups 1 and 2 were inoculated with 200 μL of PBS via the muscle injection route and served as an unvaccinated control. Each bird in groups 3, 4, and 5 was inoculated intramuscularly with 106 EID50 La Sota, NDV/rHR09, and V4, respectively. Three week postinoculation (wpi), all birds except those in group 1 were subjected to intranasal and eye drop–mediated challenge with 105 EID50 virulent genotype VII ZJ1 virus. After the challenge, all birds were monitored daily for overt clinical signs of disease and mortality for 14 D postchallenge (dpc). Oropharyngeal swabs and cloacal swabs samples were collected at 2, 4, and 6 dpc for virus isolation and identification.
Assessment of Antibody Levels
To evaluate the humoral immune response, antibody levels in vaccinated chickens were determined by hemagglutination inhibition (HI) test and virus neutralization (VN) assay using genotype VII NDV as an antigen. The serum samples from different vaccinated groups, taken at day 3 wpi, were subjected to HI assay using 1% chicken red blood cells in accordance with the standard method (Alexander, 2000). The antibody titer was measured by VN assay with modifications (Chumbe et al., 2017). Briefly, monolayer cultures of CEF cells were seeded at 80–90% confluence in 96-well plates. The serum samples were serially diluted 2 times (starting at 1:10) in serum-free Dulbecco's modified eagle medium. Diluted serum samples were mixed with an equal volume of diluent containing 50% neutralization of 100 TCID50 ZJ1 viruses and incubated at 37°C for 1 h. Each dilution was evaluated in 3 duplicate samples. A total of 100 μL of both serum and virus mixture was inoculated into CEF cells in 96-well microtiter plates at 37°C in a 5% CO2 atmosphere. The cell supernatant was harvested at 48 hpi and titrated by HA test. The VN titer was determined as the highest dilution of serum sample that inhibited CEF cells infected by virus. The geometric mean titers of HI and VN antibodies from each vaccinated group were evaluated and presented as the log2 value plus SD.
Virus Shedding Analysis
Virus shedding was determined by collecting oropharyngeal and cloacal swabs at 2, 4, and 6 dpc. The swab samples were submerged in 800 μL of PBS complemented with 4 kinds of antibiotics (penicillin 200 IU/mL; streptomycin 2 mg/mL; gentamicin 50 μg/mL; and nystatin 1000 IU/mL). The swab samples were clarified by centrifugation at 6,000 rpm for 10 min at 4°C. The presence of a virus was determined by inoculating clarified swab samples into 10-day-old SPF chicken embryos for 3 D and subsequently evaluating them by HA assay. The total viral RNA was extracted by using an Ultrapure RNA Kit (CWbio Bio, Beijing, China) as recommended by the manufacturer. Viral cDNA was synthetized using murine leukemia virus reverse transcriptase (TransGen Biotech, Beijing China) and 6 random hexamers as per the manufacturer's instructions. The amount of viral cDNA was measured by a quantitative real-time PCR assay using the relative standard curve method (Farkas et al., 2009).
Ethics Statements
All animal experiments were performed in isolators (Suzhou Fengshi Laboratory Animal Equipment Co., Ltd., Suzhou, China), approved by the Jiangsu Administrative Committee for Laboratory Animals, and complied with the guidelines of the Jiangsu Laboratory Animal Welfare and Ethics of Jiangsu Administrative Committee. All experiments involving the virulent NDV were performed in biosecurity level 3 facilities.
Statistical Analysis
All data in this study are expressed as the mean ± SD and were analyzed by Student's t-test or one-way ANOVA using GraphPad Prism 5 software. Serology titers and virus shedding are presented as the geometric mean (±) SD. A P-value of less than 0.05 was considered statistically significant (∗).
Results
Comparison of the Amino Acid Sequences of the F and HN Proteins
The amino acid sequences of the F and HN proteins of the vaccine viruses and the prevalent NDV ZJ1 were compared by ClustalW method alignment (Table 1). In comparison with ZJ1, the amino acid similarity of F protein in HR09 (92.8%) was higher than both V4 (90.3%) and La Sota (87.9%). The results of amino acid similarity in HN protein were similar with F protein. This comparison reveals that the HR09 is more closely related to the prevalent NDV ZJ1 than both the V4 and La Sota. This comparison reveals that the HR09 is more closely related to the prevalent NDV ZJ1 than both the V4 and La Sota.
Table 1.
Amino acid similarity of F and HN proteins between the vaccine strains and the prevalent NDV challenge ZJ1 strain.
| Vaccine | Amino acid similarity with challenge virus (%) |
|
|---|---|---|
| F | HN | |
| V4 | 90.3 | 90.0 |
| La Sota | 87.9 | 88.6 |
| NDV/rHR09 | 92.8 | 94.1 |
Abbreviations: F, fusion; HN, hemagglutinin-neuraminidase; NDV, Newcastle disease virus.
Generation and Identification of the NDV/rHR09 Viruses
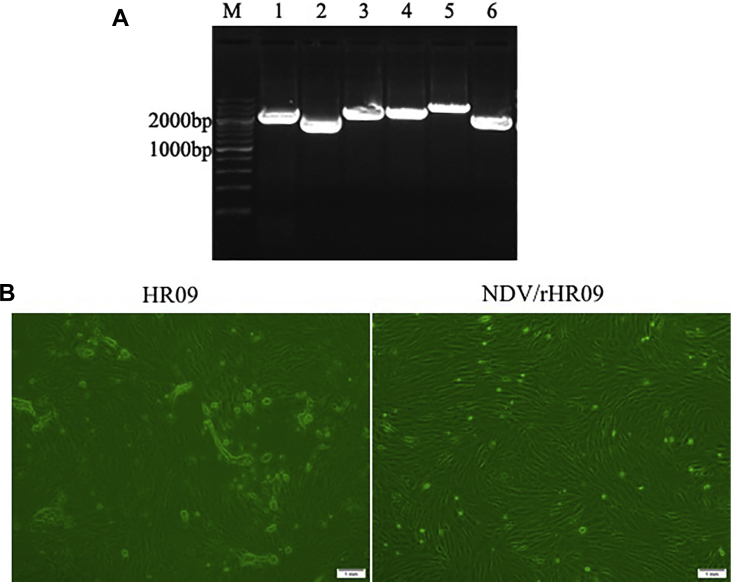
The full-length clone NDV/rHR09 was modified by overlapping PCR method to mutate the 112RRQKR↓F117 to 112GRQGR↓L117. This involved 3 amino acid substitutions at position 112, 115, and 117 relative to F protein (Figure 1), and the NDV/rHR09 was recovered successfully from the full-length cDNA. Viral RNAs were extracted and analyzed by reverse transcription-polymerase chain reaction, using 6 pairs of primers covering the full-length sequence. The lengths of the PCR products were correct (Figure 2A) and further confirmed by sequence analysis.
Figure 2.
(A) Reverse transcription-polymerase chain reaction analysis of six fragments of the complete NDV genome. (M) 200 bp DNA ladder. (B) Infection of CEF cells by the NDV. CEF cells were infected with 105 EID50 HR09 and NDV/rHR09 viruses. At 36 hpi, the CPE induced by NDV strains was observed on an inverted microscope, the scale bare = 1 mm. Abbreviations: CEF, chicken embryo fibroblast; EID50, 50% egg infectious dose; NDV, Newcastle disease virus.
To evaluate the difference in pathogenicity between parental HR09 and the generated NDV/rHR09, both HR09 and NDV/rHR09 were inoculated into CEF cells and examined the cytopathic effect (CPE). At 36 hpi, the CEF cells infected with NDV/rHR09 showed normal morphology, whereas a typical CPE was observed on HR09 infected cells (Figure 2B). Therefore, the pathogenicity tests in CEF suggested that the generated NDV/rHR09 was avirulent.
Biological Characterization of the NDV Strains
The pathogenicity of the NDV/rHR09 was evaluated by a standard pathogenicity assay (Table 2). The MDT and ICPI values of the HR09 were 58 h and 1.80, respectively, which indicates that the HR09 is a velogenic strain. However, the MDT value of the NDV/rHR09 was 120 h, which is higher than that of the La Sota (105 h) but lower than that of the V4 (154 h). The ICPI value of the NDV/rHR09 virus (0.06) was less than that of the La Sota (0.38) but more than that of the V4 (0.00). These results indicate that the NDV/rHR09 is avirulent and more attenuated than the La Sota.
Table 2.
NDV pathogenicity and stability assays.
| Parental |
NDV/rHR09 |
V4 | La Sota | |||
|---|---|---|---|---|---|---|
| HR09 | Passage 5C | Passage 10 | Passage 15 | |||
| MDTa | 58 | 120 | 110 | 120 | 154 | 105 |
| ICPIb | 1.80 | 0.06 | 0.06 | 0.07 | 0.00 | 0.38 |
Abbreviation: NDV, Newcastle disease virus.
MDT = mean death time (h) assay in 10-day-old SPF chicken embryos.
ICPI = intracerebral pathogenicity index assay in 1-day-old SPF chickens.
Passage 5 = virus was passaged in 10-day-old SPF chicken embryos for 5 times.
To determine the stability of the pathogenicity of the NDV/rHR09, MDT and ICPI tests were evaluated after being serially passaged for 15 generations in 10-day-old SPF chicken embryos. The values of MDT and ICPI as shown in Table 2 of NDV/rHR09 were largely different with HR09. The pathogenicity assay results showed that the NDV/rHR09 was phenotypically stable during extensive passaging in vivo and did not revert to a virulent virus.
Thermostability and Growth Kinetics
As shown in Table 3, the HA and embryonated egg infectivity titer of the NDV/rHR09 were 7 log2 and 2.2 log10 EID50/0.2 mL after heat treatment at 56°C for 60 min. These results indicate that NDV/rHR09 is heat resistant and is a thermostable NDV strain.
Table 3.
HA and embryonated egg infectivity titer of vaccine strains after heat treatment at 56°C.
| Virus | Parameter | Heat treatment time |
||
|---|---|---|---|---|
| 0 min | 30 min | 60 min | ||
| NDV/rHR09 | HA titer (log2)a | 10 | 9 | 7 |
| Infectivity titer (log10)b | 8.5 | 4.2 | 2.2 | |
| V4 | HA titer | 10 | 10 | 10 |
| Infectivity titer | 9.5 | 8.2 | 6.8 | |
| La Sota | HA titer | 11 | 0 | 0 |
| Infectivity titer | 8.7 | 0 | 0 | |
Abbreviations: HA, hemagglutination assay; NDV, Newcastle disease virus.
50% embryonated egg infectious dose (EID50/0.2 mL).
The growth kinetics of the NDV/rHR09 were measured in 10-day-old SPF chicken embryos inoculated with 103-fold diluted virus. Then, the allantoic fluid was collected daily, and viral titers were measured by EID50 (Figure 3). The result showed that the NDV/rHR09 viral titers increased gradually, reaching a peak titer of 109 EID50/0.2 mL at 96 hpi. Thus, the NDV/rHR09 replicates efficiently in SPF chicken embryos and produces a high yield.
Figure 3.
The growth kinetics of NDV/rHR09 virus were determined with EID50 assay in 10-day-old SPF chicken embryos. Abbreviations: EID50, 50% egg infectious dose; NDV, Newcastle disease virus; SPF, specific pathogen-free.
Antibody Response and Survival Rate
To evaluate the protective immunity, 1-wk-old SPF chicks were immunized with La Sota, V4, and NDV/rHR09 via muscle injection. The serological cross-reaction was evaluated by collecting serum samples for HI and VN assays using genotype VII NDV. The results showed that NDV/rHR09 induced a HI geometric mean antibody titer of 7 log2 at 3 wpi higher than that of both La Sota (5 log2) and V4 (2 log2) (Figure 4A). The titer of VN antibodies was detected in CEF cells, and the result was similar to that of the HI assay (Figure 4B).
Figure 4.
The HI (A) and VN (B) antibody titers (log2) were measured using genotype VII NDV antigen at weekly intervals in all SPF chicken groups. Each sample was tested in duplication well, and HI and VN titers ± SD were shown. Abbreviations: HI, hemagglutination inhibition; NDV, Newcastle disease virus; SPF, specific pathogen-free; VN, virus neutralization
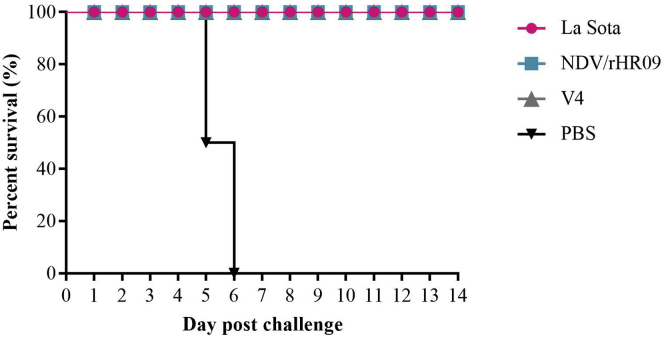
The morbidity and mortality were monitored for 14 D, and survival rates were assessed after challenging (Figure 5). We found that vaccinated chickens showed no clinical symptoms and a 100% survival rate at 14 dpc; however, the chickens in the control group all died.
Figure 5.
The survival rate (%) over 2 wk after genotype VII NDV challenge in all SPF chicken groups (n = 12 per group). Abbreviations: NDV, Newcastle disease virus; SPF, specific pathogen-free.
Reduction in Virulent NDV Shedding
To better understand the protective efficacy after infection with genotype VII NDV, virus shedding was examined in all 4 groups at 2, 4, and 6 dpc. As shown in Table 4, almost 100% of the samples from both oropharyngeal and cloacal swabs were positive in the PBS group at 4 dpc. The positive rates in the vaccinated groups were largely decreased, particularly in NDV/rHR09 were 33.3% and 16.7% compared with La Sota (50.0% and 33.3%) and V4 (75.0% and 50%) in oropharyngeal and cloacal swabs, respectively.
Table 4.
Frequency of isolation of challenge virus in SPF chickens.
| Group | Postchallenge samples (positive number/total) |
|||||
|---|---|---|---|---|---|---|
| D 2 pc |
D 4pc |
D 6 pc |
||||
| Oa | Cb | O | C | O | C | |
| La Sota | 2/12(16.7%) | 0/12(0%) | 6/12(50.0%) | 4/12(33.3%) | 0/12(0%) | 0/12(0%) |
| NDV/rHR09 | 0/12(0%) | 0/12(0%) | 4/12(33.3%) | 2/12(16.7%) | 1/12(8.3%) | 0/12(0%) |
| V4 | 0/12(0%) | 0/12(0%) | 9/12(75.0%) | 6/12(50%) | 0/12 (0%) | 1/12 (8.3%) |
| PBS | 2/12 (16.7%) | 1/12 (8.3%) | 12/12 (100%) | 11/12 (91.7%) | NS | NS |
Abbreviations: pc, post challenge; SPF, specific pathogen-free; NS, no survivors.
O = oropharyngeal swabs.
C = cloacal swabs.
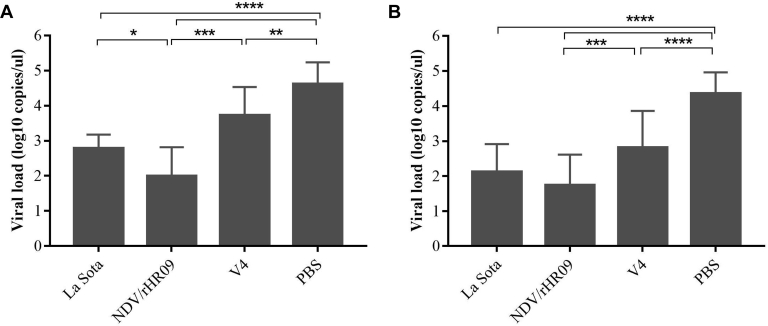
The virus shedding in oropharyngeal and cloacal swabs at 4 dpc was further detected by real-time quantitative PCR. The PBS-vaccinated group had significantly higher (P < 0.001) virus shedding in the oropharyngeal and cloacal swabs samples than the other vaccine groups (Figures 6A, 6B). In addition, we found that the number of virus copies in the NDV/rHR09-vaccinated group was lower (P < 0.05) than that in the La Sota- and V4-vaccinated group.
Figure 6.
Virus shedding (log10) in the (A) oropharyngeal and (B) cloaca swabs at 4 dpc in all SPF chicken groups. Asterisks indicate statistically significant difference (∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001). Abbreviations: dpc, d postchallenge; SPF, specific pathogen-free.
Discussion
Thermostable vaccines play an important role in controlling ND in the poultry of villages in many developing countries (Bensink and Spradbrow, 1999, Foster et al., 1999). In this study, we developed a reverse genetics system for the thermostable genotype VIII NDV HR09 strain and generated an attenuated strain (NDV/rHR09) by changing its cleavage site of F protein. As expected, the newly generated NDV/rHR09 was highly attenuated (Table 2). To our knowledge, this is the first report of the generation of an attenuated thermostable NDV strain by a reverse genetics system. The NDV/rHR09 induced a higher level of HI and VN antibody titers (Figure 4), than La Sota and V4.
Most commercial ND vaccines are attenuated vaccine viruses formulated with strains isolated in the 1940s and 1960s (Dimitrov et al., 2017). Although the current ND vaccines offer substantial protection against clinical disease, they fail to completely prevent infection or shedding (Kapczynski and King, 2005, Susta et al., 2015). The La Sota and V4 vaccine strains, which belong to genotypes II and I, respectively, have reduced amino acid similarity compared with the prevalent genotype VII NDV strains (Table 1), probably resulting in an antigenic mismatch situation. In our study, the analysis of virus shedding after challenge showed that NDV/rHR09 could reduce virus shedding more significantly than both the La Sota and V4 in SPF chickens (Figure 6). These results are consistent with those of previous reports showing that when ND vaccine strains are antigenically homologous, they induce a greater humoral immune response to the challenge virus than heterologous vaccines (Xiao et al., 2012, Sedeik et al., 2019). More recently, the virulent genotype VIII NDV isolated from game fowl with clinic signs re-emerged in China after 13 yr, and the NDV/rHR09 vaccine contributed to decreasing the risk that this genotype VIII of NDV may spread to commercial chickens from game fowl (Wei et al., 2019).
In summary, the results of this study showed that the generated NDV/rHR09 virus is attenuated and thermostable. NDV/rHR09 delivered via intramuscular immunization induced higher levels of HI and VN antibodies and reduced virus shedding than La Sota and V4 strains in SPF chickens. Therefore, the novel attenuated and thermostable NDV/rHR09 has some advantages and may be useful as a potential vaccine candidate to control infection of the prevalent genotype VII NDV.
Acknowledgments
This work was supported by the National Key Research and Development Project of China (2016YFD0501604, 2016YFD0501609), the Earmarked Fund for Modern Agroindustry Technology Research System (CARS-40-K16), A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and The “High-end Talent Support Program” of Yangzhou University (2016).
Conflict of Interest: The authors have no potential conflict of interests with this manuscript.
Contributor Information
Yongzhong Cao, Email: 493787740@qq.com.
Yantao Wu, Email: yzdxcyz@163.com.
References
- Alders R.G. Making Newcastle disease vaccines available at village level. Vet. Rec. 2014;174:502–503. doi: 10.1136/vr.g3209. [DOI] [PubMed] [Google Scholar]
- Alexander D.J. Newcastle disease and other avian paramyxoviruses. Rev. Sci. Tech. Oie. 2000;19:443–462. doi: 10.20506/rst.19.2.1231. [DOI] [PubMed] [Google Scholar]
- Bensink Z., Spradbrow P. Newcastle disease virus strain I-2 - a prospective thermostable vaccine for use in developing countries. Vet. Microbiol. 1999;68:131–139. doi: 10.1016/s0378-1135(99)00069-3. [DOI] [PubMed] [Google Scholar]
- Cao Y., Liu Q., Zhang X., Hu H., Wu Y. Complete genome sequence of heat-resistant newcastle disease virus strain HR09. Genome Announc. 2017;5 doi: 10.1128/genomeA.01149-17. e01149-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumbe A., Izquierdo-Lara R., Calderon K., Fernandez-Diaz M., Vakharia V.N. Development of a novel Newcastle disease virus (NDV) neutralization test based on recombinant NDV expressing enhanced green fluorescent protein. Virol. J. 2017;14:232–242. doi: 10.1186/s12985-017-0900-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov K.M., Afonso C.L., Yu Q., Miller P.J. Newcastle disease vaccines-A solved problem or a continuous challenge? Vet. Microbiol. 2017;206:126–136. doi: 10.1016/j.vetmic.2016.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas T., Szekely E., Belak S., Kiss I. Real-time PCR-based Pathotyping of newcastle disease virus by Use of TaqMan minor Groove Binder Probes. J. Clin. Microbiol. 2009;47:2114–2123. doi: 10.1128/JCM.01652-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster H.A., Chitukuro H.R., Tuppa E., Mwanjala T., Kusila C. Thermostable newcastle disease vaccines in Tanzania. Vet. Microbiol. 1999;68:127–130. doi: 10.1016/s0378-1135(99)00068-1. [DOI] [PubMed] [Google Scholar]
- Hu Z., Hu S., Meng C., Wang X., Zhu J., Liu X. Generation of a genotype VII Newcastle disease virus vaccine candidate with high yield in embryonated chicken eggs. Avian Dis. 2011;55:391–397. doi: 10.1637/9633-122410-Reg.1. [DOI] [PubMed] [Google Scholar]
- Johnson K.N., Zeddam J.L., Ball L.A. Characterization and construction of functional cDNA clones of pariacoto virus, the first Alphanodavirus isolated outside Australasia. J. Virol. 2000;74:5123–5132. doi: 10.1128/jvi.74.11.5123-5132.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapczynski D.R., King D.J. Protection of chickens against overt clinical disease and determination of viral shedding following vaccination with commercially available Newcastle disease virus vaccines upon challenge with highly virulent virus from the California 2002 exotic Newcastle disease outbreak. Vaccine. 2005;23:3424–3433. doi: 10.1016/j.vaccine.2005.01.140. [DOI] [PubMed] [Google Scholar]
- Mahmood M.S., Siddique F., Hussain I., Ahmad S.I., Rafique A. Thermostable vaccines for Newcastle disease: a review. World Poultry Sci. J. 2014;70:829–838. [Google Scholar]
- Panda A., Huang Z.H., Elankumaran S., Rockernann D.D., Samal S.K. Role of fusion protein cleavage site in the virulence of Newcastle disease virus. Microb. Pathogenesis. 2004;36:1–10. doi: 10.1016/j.micpath.2003.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters B.P.H., de Leeuw O.S., Koch G., Gielkens A.L.J. Rescue of Newcastle disease virus from cloned cDNA: Evidence that cleavability of the fusion protein is a major determinant for virulence. J. Virol. 1999;73:5001–5009. doi: 10.1128/jvi.73.6.5001-5009.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer-Oberdorfer A., Werner O., Veits J., Mebatsion T., Mettenleiter T.C. Contribution of the length of the HN protein and the sequence of the F protein cleavage site to Newcastle disease virus pathogenicity. J. Gen. Virol. 2003;84:3121–3129. doi: 10.1099/vir.0.19416-0. [DOI] [PubMed] [Google Scholar]
- Sedeik M.E., Elbestawy A.R., El-Shall N.A., Abd El-Hack M.E., Saadeldin I.M., Swelum A.A. Comparative efficacy of commercial inactivated Newcastle disease virus vaccines against Newcastle disease virus genotype VII in broiler chickens. Poult. Sci. 2019;98:2000–2007. doi: 10.3382/ps/pey559. [DOI] [PubMed] [Google Scholar]
- Susta L., Jones M.E.B., Cattoli G., Cardenas-Garcia S., Miller P.J., Brown C.C., Afonso C.L. Pathologic characterization of genotypes XIV and XVII newcastle disease viruses and efficacy of Classical vaccination on specific pathogen-free birds. Vet. Pathol. 2015;52:120–131. doi: 10.1177/0300985814521247. [DOI] [PubMed] [Google Scholar]
- Tan L.T., Xu H.Y., Wang Y.L., Qin Z.M., Sun L., Liu W.J., Cui Z.Z. Molecular characterization of three new virulent Newcastle disease virus variants isolated in China. J. Clin. Microbiol. 2008;46:750–753. doi: 10.1128/JCM.01587-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei T.C., Deng Q.M., Zhai G.S., He C.W., Li H.Q., Zhang Y.Q., Zeng R.L., Mo M.L., Huang T., Wei P. Re-emergence of a genotype VIII virulent Newcastle disease virus isolated from Chinese game fowl after 13 years. Transbound Emerg. Dis. 2019;66:1077–1084. doi: 10.1111/tbed.13129. [DOI] [PubMed] [Google Scholar]
- Wen G., Hu X., Zhao K., Wang H., Zhang Z., Zhang T., Yang J., Luo Q., Zhang R., Pan Z., Shao H., Yu Q. Molecular basis for the thermostability of Newcastle disease virus. Sci. Rep. 2016;6:22492. doi: 10.1038/srep22492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S., Nayak B., Samuel A., Paldurai A., Kanabagattebasavarajappa M., Prajitno T.Y., Bharoto E.E., Collins P.L., Samal S.K. Generation by reverse genetics of an effective, stable, live-attenuated newcastle disease virus vaccine based on a currently circulating, highly virulent Indonesian strain. PLoS One. 2012;7:e52751. doi: 10.1371/journal.pone.0052751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D.W., Li C.F., Liu G.Q., Chen Z.Y., Jia R.Y. Generation and evaluation of a recombinant goose origin Newcastle disease virus expressing Cap protein of goose origin avastrovirus as a bivalent vaccine in goslings. Poult. Sci. 2019;98:4426–4432. doi: 10.3382/ps/pez255. [DOI] [PubMed] [Google Scholar]
- Yadav K., Pathak D.C., Saikia D.P., Debnath A., Ramakrishnan S., Dey S., Chellappa M.M. Generation and evaluation of a recombinant Newcastle disease virus strain R2B with an altered fusion protein cleavage site as a vaccine candidate. Microb. Pathogenesis. 2018;118:230–237. doi: 10.1016/j.micpath.2018.03.038. [DOI] [PubMed] [Google Scholar]