Abstract
Long photoperiods are used in the broiler industry to maximize animal performance, though the impact on meat quality remains poorly understood. The current study evaluated the impact of photoperiod on functional/physicochemical properties and oxidative stability of meat through broiler processing. Ross 308 broilers (n = 432) were randomly assigned to 4 photoperiod treatments (hours in L = light, D = dark): 20L:4D, 18L:6D, 16L:8D, or 12L:12D with 6 pens per treatment. At 42 D of age, 2 broilers per pen (n = 12 per treatment) were harvested under standard conditions. Broiler tenderloin (M. Pectoralis minor) and leg muscles were removed at 1 D postmortem and frozen/stored at −40°C. After 24 h thawing at 2°C, the samples were deboned, ground, and formed into patties in 3 independent batches. Photoperiod had no impact on pH, water-holding capacity, textural profile, meat emulsion activity index, and thiol content (P > 0.05). The patties from 12L:12D and 16L:8D had lower CIE b∗ (yellowness) values than 18L:6D and 20L:4D (P < 0.05), whereas 12L:12D had lower chroma (color intensity) values than other treatments (P < 0.05). The meat from 20L:4D exhibited lower sarcoplasmic protein solubility than other treatments (P < 0.05), whereas both 20L:4D and 18L:6D exhibited lower total protein solubility than 12L:12D (P < 0.05). Higher transmission values (indication of protein denaturation) were observed in 20L:4D than in other treatments (P < 0.05), whereas 12L:12D also maintained lower values than both 18L:6D and 16L:8D (P < 0.05). There was an interaction (P < 0.05) between photoperiod and display storage on 2-thiobarbituric acid reactive substances values, where the patties from 12L:12D maintained less lipid oxidation compared with the patties from other treatments. Results of this study suggest photoperiod has limited impact on meat quality attributes, though rearing broilers with a 12L:12D lighting schedule may be beneficial in reducing protein denaturation and improving lipid stability.
Key words: broiler, oxidative stability, photoperiod, processed meat quality, protein functionality
Introduction
It has been well established that broiler growth rate and economic efficiency can be controlled to some extent by manipulating photoperiod (Olanrewaju et al., 2019). Lighting regimes (hours in light = L, dark = D) with continuous (24L:0D) or near-continuous (23L:1D) photoperiods have been demonstrated to improve broiler performance attributes such as growth rate, feed consumption, and feed conversion, especially during the starter phase (Classen et al., 1991, Lien et al., 2007, Lien et al., 2009). Moreover, there is substantial evidence that long photoperiod lighting schedules can improve breast meat yields at the expense of thigh, drum, and wing yields (Downs et al., 2006, Lien et al., 2007, Lien et al., 2009). Increased deposition of breast muscle at the expense of leg muscle has been demonstrated to increase incidence of leg abnormalities and impair walking ability (Classen and Riddell, 1989, Classen et al., 1991, Sanotra et al., 2002). These detriments are thought to be the product of the genetic selection for high degree of weight gain before skeletal maturity coupled with inferior bone deposition through disruptions to the normal circadian rhythm (Olanrewaju et al., 2006). Thus, there is an interest in the broiler industry to develop a photoperiod program that allows birds to maintain the physiological diurnal rhythm for improving welfare without affecting meat production and quality.
Information regarding the photoperiod effects on broiler meat quality is currently limited, although there are a couple of studies conducted in pigs. It has been reported that the raw hams from pigs of a longer photoperiod group (14L:10D) had a higher saturation degree of subcutaneous fat than pigs of a shorter photoperiod group (8L:16D) (Sardi et al., 2012). Martelli et al. (2015) also reported that the hams from the longer photoperiod group had a lower weight loss during the dry-curing period than the shorter photoperiod counterpart. With respect to broiler meat quality associated with photoperiod, the study by Li et al. (2010) demonstrated that the breast muscle (M. Pectoralis major) from broilers reared under a 23L:1D lighting schedule had reduced percent meat protein and increased lipid oxidation compared to 12L:12D. The finding of increased lipid oxidation with longer photoperiod in M. Pectoralis major muscles was corroborated by our parallel study published by Tuell et al. (2020). While most measures of carcass and meat quality were unaffected by photoperiod regime, fillets from 20L:4D appeared lighter with increased discoloration compared with shorter photoperiod groups, attributed to alteration of muscle metabolites related to oxidative stress (e.g., oxidized glutathione). It has been established that broiler meat with higher CIE L∗ values has inferior water-holding capacity (WHC) and emulsification capacity when ground (Qiao et al., 2001), but currently, there is little to no published literature available regarding the impacts of photoperiod on processed broiler meat functional/physicochemical properties and oxidative stability. Thus, the objectives of this study were to determine the impacts of photoperiod on processing functional properties of ground raw meat and physicochemical characteristics and oxidative stability of meat patties of broilers. Results of this study would provide the broiler industry with practical knowledge regarding the impact of photoperiod regime and processing effects on meat quality.
Materials and methods
Animal Handling
Animal use and procedures were approved by the Purdue Animal Care and Use Committee (1712001657).
Photoperiod Treatments
Four hundred thirty-two 1-day-old chicks (Ross 308 broilers) were weighted in 18 bird-groups and assigned to 24 pens (110 cm × 110 cm per pen) with equal weight distribution among the pens. The pens were randomly assigned to 4 temperature and lighting–controlled rooms at the Poultry Research Farm of Purdue University. The rooms were assigned to one of 4 photoperiod treatments (n = 6): 20L:4D, 18L:6D, 16L:8D, and 12L:12D, started at day 14. The lighting regimen was constant at 30 L× for 24L:0D at day 1, reduced to 23L:1D from day 2 to 7, adjusted in gradual increments to reach the respective regimes at day 14, and then maintained at each respective regime until 42 D of age.
The brooding temperature was 34°C for the first 3 D, gradually reduced as the birds progressed in age until 21°C to 24°C was reached and then maintained until the end of this study. All birds received a starter diet with 23.43% CP and 3,050 kcal ME/kg from day 1 to 14; a grower diet with 22.81% CP and 3,150 kcal ME/kg from day 15 to 28; and then a finisher diet with 19.17% CP and 3,200 kcal ME/kg from day 29 to 42. Each pen was equipped with 1 basic feed dispenser made of UV-resistant plastic and 1 water line. Throughout the entire experimental period, the birds had free access to feed and water.
Slaughter and Meat Processing
At 42 D of age, 2 broilers per pen (n = 6; 12 birds per treatment) were randomly selected and transported approximately 30 min to a harvest facility where birds were electrically stunned and slaughtered under standard procedures. Carcasses were air-chilled in a 2°C carcass cooler for 24 h. After chilling, tenderloins (M. Pectoralis minor), thigh, and drum muscles were collected from both sides of each carcass, and skin and visible connective tissue were removed. Samples were vacuum-packaged in nylon/polyethylene pouches (Bunzl Processor, Riverside, MO) and frozen/stored at −40°C. Before grinding and patty manufacture, meat was thawed for 24 h at 2°C. Thawed meat was deboned, cubed, ground through an 8-mm plate (M-12-FS, Torrey, Monterey, NL, Mexico), and mixed uniformly. Ground meat and patties were manufactured in 3 independent batches, and proportions of tenderloin, thigh, and drum muscles were kept constant across treatments and batches. A total of 12 patties (approximately 100 g each) per treatment was manufactured per batch using a round-shape patty maker (10 cm diameter × 3 cm depth). Remaining ground raw meat was used to analyze pH and functional properties as described in the subsequent sections. Three patties were immediately used for cooking loss and texture profile analysis. Remaining patties were displayed for either 0 D, 2 D, or 4 D at 2°C on foam trays with oxygen-permeable polyvinylchloride film overwrap (0.5 mil; Reynolds Food Service Packaging, Richmond, VA) with soaking pad underneath, with 3 patties randomly assigned to each display duration. Freezing/thawing loss was measured on 0-day displayed patties, while display weight loss was measured on both day 2 and day 4 displayed patties. Instrumental color attributes were measured on patties displayed for the full 4 D of display storage. Light during the display period was provided using a fluorescent bulb at 1,850 L× intensity. At each respective display storage duration, 3 patties each were frozen/stored at −40°C for later determination of oxidative stability.
Functional Properties of Ground Raw Meat
pH Measurement
To determine pH, 3 g of ground raw meat was homogenized with 27 mL of distilled water at 6,000 rpm for 60 s using an Ultra Turrax homogenizer (Ultra-Turrax T25; Janke & Kunkel IKA-Labortechnik, Staufen, Germany). The pH value of ground raw meat was determined using an electronic pH meter (Sartorius Basic Meter PB-11, Sartorius AG, Germany) calibrated with pH 4 and 7 buffers. Measurements were performed in triplicate per batch. The mean value of replications was used for statistical analysis.
Protein Solubility
Protein solubility was determined in triplicate per batch for sarcoplasmic protein, myofibrillar protein, and total protein solubility in accordance with the method described by Kim et al. (2017) with the following modifications. For the assay, 1 g sample was homogenized with either 10 mL of 0.025 mol potassium phosphate buffer (pH 7.2) for sarcoplasmic protein or 10 mL of 0.55 mol potassium iodide in 0.05 mol potassium phosphate buffer (pH 7.2) for total protein solubility. The homogenate was held at 4°C for a period of 20 h before centrifugation at 2,600 g. To determine myofibrillar protein, the resulting pellet from determining sarcoplasmic protein solubility was homogenized with 10 mL of total protein extraction buffer and treated in the same manner as previously described. Protein concentration of the supernatant was determined using the biuret assay with the BSA standard curve. Protein solubility was expressed as mg soluble protein per g sample.
Emulsion Activity Index
The emulsion activity index (EAI) of the ground raw meat was determined in quadruplicate as per the method described by Chan et al. (2011) with modifications described by Bowker and Zhuang (2016). Sarcoplasmic and myofibrillar proteins were isolated in accordance with the protocol, and protein concentration was adjusted to 1.5 mg/mL using extraction buffer. Protein extracts were mixed with corn oil in a 3:1 ratio using an Ultra Turrax homogenizer (Ultra-Turrax T25; Janke & Kunkel IKA-Labortechnik, Staufen, Germany) at 14,000 rpm. A dilution consisting of 35 μL of the emulsified layer with 3.5 mL of 0.1% (w/v) sodium dodecyl sulfate buffer was prepared, and absorbance was read at 500 nm using a spectrophotometer (Epoch, BioTek Instruments, Inc., Winooski, VT). The EAI was calculated as: EAI = 2.33∗Abs500nm.
Transmission Value
Transmission value was determined in duplicate per batch in accordance with the method described by Ockerman and Cahill (1968) with modifications described by Kim et al. (2010). In brief, 5.0 g sample was homogenized in 10 mL distilled water, after which the homogenate was gently rocked for 1 h at 4°C. After this storage, tubes were centrifuged at 1,000 g for 10 min at 4°C, and the resulting supernatant was filtered through a Whatman #1 filter paper. One milliliter of the filtrate was mixed with 5 mL of 0.1 mol citric acid in 0.2 mol sodium phosphate buffer (pH 4.6). After 30 min incubation, percent turbidity at 600 nm was measured using a spectrophotometer (Epoch, BioTek Instruments, Inc., Winooski, VT). Higher transmission values indicate less soluble protein and higher protein denaturation.
Quality Attributes and Oxidative Stability of Manufactured Patties
Moisture Content
Moisture content (934.01) of manufactured patties was determined in triplicate in accordance with AOAC guidelines (AOAC, 2006).
Water-Holding Capacity
The WHC of patties was determined by freezing/thawing loss (%), cooking loss (%), and display weight losses (%). Freezing/thawing loss was calculated in triplicate as the percent difference between weights of 0 D displayed patties before frozen storage at −40°C and later thawing for 24 h at 2°C. Cooking loss was determined in triplicate by cooking fresh patties on an electric griddle set at 135°C until internal temperature reached 41°C, after which patties were flipped and cooked until internal temperature reached 71°C. Temperature increase of patties was monitored using a data logger (OctTemp 2,000; MadgeTech, Inc., Warner, NH) connected to a type T thermocouple (Omega Engineering, Stamford, CT) inserted into the geometric center of each patty. Cooking loss was determined as the percent difference between initial weight and weight after cooking. Display losses were calculated as the percent difference between patty weights before and after 2 or 4 D of display storage, respectively, with 3 patties per display duration.
Texture Profile Analysis
Texture profile analysis was determined as per the method described by Bourne (1978) with modification. Texture profile attributes were determined in triplicate from cooked samples after overnight storage at 4°C using a TA-XT Plus Texture Analyzer (Stable Micro System Ltd., Surrey, UK). Cores (n = 4 per patty, approximately 2 cm diameter each) were taken from each patty and used for a twice compression cycle test (5 mm/s test speed, 10 mm distance). Values for hardness (g), resilience (%), cohesion, springiness (%), gumminess, and chewiness were determined, and mean values from the replicates were used for statistical analysis.
Instrumental Color Attributes
Instrumental color attributes (CIE L∗ [lightness], CIE a∗ [redness], and CIE b∗ [yellowness]) were measured daily on 3 randomly selected locations per patty during the display storage. Values were recorded using a CR-400 Chroma Meter (Konica Minolta, Chiyoda, Tokyo, Japan) with CIE standard illuminant D65. Values for hue angle (indication of discoloration) and chroma (color saturation) were calculated in accordance with American Meat Science Association color measurement guidelines. The hue angle was calculated as hue angle = tan−1(CIE b∗/CIE a∗) and chroma as chroma = (AMSA, 2012). Samples used for color measurements were then frozen/stored and considered as a 4-day-displayed biochemical sample.
Lipid Oxidation
Lipid oxidation was determined in duplicate for each display time point per batch using the 2-thiobarbituric acid reactive substances (TBARS) assay described by Buege and Aust (1978) with the modifications described by Kim et al. (2017). Absorbance was measured at 531 nm using a microplate spectrophotometer (Epoch, Biotek Instruments, Inc., Winooski, VT). Values for TBARS were expressed as mg malondialdehyde (MDA) per kg sample.
Protein Oxidation
Protein oxidation was determined in duplicate by the loss of thiol groups using Ellman's 5, 5′-Dithiobis (2-nitrobenzoic acid) assay following the method described by Vossen and De Smet (2015) for each display time point per batch. Absorbance was measured at 412 nm using a microplate spectrophotometer (Epoch, Biotek Instruments, Inc., Winooski, VT). Concentration of thiol groups was determined by the formula of Lambert–Beer (ε412 = 14,000 M−1 cm−1), and the result was expressed in nmol thiol/mg protein. Protein concentration was determined using BSA to create the standard curve.
Statistical Analysis
Most data were analyzed in a completely randomized block design with photoperiod treatments (20L:4D, 18L:6D, 16L:8D, and 12L:12D) as the fixed effect. Batch (n = 3) was considered as the experimental unit. Batches and their interactions with the main effects were considered as random effects. A model of the fixed effect was analyzed using the PROC MIXED procedure of SAS 9.4 (SAS Institute Inc., Cary, NC). Data including display period were analyzed as a split plot, with photoperiod treatment as a whole plot and display duration as a subplot. A model including the main effects and their interaction was analyzed for these attributes, with batches and their interactions with the main effects as random effects. The PDIFF option of SAS was used to determine least significant differences for separation of least square means (P < 0.05). Trends in the data were defined as (0.05 ≤ P < 0.10).
Results and discussion
Functional Properties of Ground Raw Meat
Photoperiod treatments had no meaningful impact on pH of the ground raw meat (P > 0.05; Table 1). There were, however, differences in sarcoplasmic and total protein solubility, as well as transmission value, owing to photoperiod effects (P < 0.05). Ground raw meat from 20L:4D exhibited lower sarcoplasmic protein solubility than other photoperiod treatments (P < 0.05), whereas no differences were observed among 18L:6D, 16L:8D, and 12L:12D (P > 0.05). Similarly, meat from 20L:4D had lower total protein solubility than that of 12L:12D (P < 0.05) but was not different from both 18L:6D and 16L:8D (P > 0.05). Ground raw meat from 12L:12D had the highest total protein solubility, though there were no differences between 12L:12D and 16L:8D (P > 0.05). Despite treatment effects for total and sarcoplasmic protein solubility, no differences in myofibrillar protein solubility were observed (P > 0.05). Li et al. (2010) found that longer photoperiods (23L:1D and 20L:4D) reduced percent meat protein than 12L:12D. Although the CP content of the ground broiler meat was not determined in the present study, it has been well established that both protein content and extractability heavily influence processed meat quality (Carballo et al., 1995, Pietrasik, 1999). During thermal processing, a protein gel matrix forms in meat, and its strength is highly dependent on myofibrillar proteins (Yasui et al., 1980, Asghar et al., 1985). In the present study, photoperiod influenced the sarcoplasmic fraction (and hence total protein solubility) rather than myofibrillar protein, which may indicate that the tested photoperiods had no considerable alteration to processed meat quality. Supporting this, the EAI was not affected by tested photoperiods, with no treatment effects on both myofibrillar and sarcoplasmic fractions (P > 0.05). In addition, the EAI of ground broiler breast meat is positively correlated with pH and WHC (Qiao et al., 2001), where no differences were observed in the present study among these attributes because of photoperiod effect.
Table 1.
Effect of photoperiod on pH, protein solubility, emulsion activity index, and transmission value (protein denaturation) of ground raw broiler meat.
| Treatment | pH | Protein solubility1 |
Emulsion activity index2 |
Transmission value (%) | |||
|---|---|---|---|---|---|---|---|
| Myofibrillar | Sarcoplasmic | Total | Myofibrillar | Sarcoplasmic | |||
| 20L:4D | 6.05 | 126.2 | 52.6b | 175.8b,c | 0.54 | 0.12 | 30.7a |
| 18L:6D | 6.10 | 130.9 | 67.3a | 167.5c | 0.56 | 0.13 | 24.6b |
| 16L:8D | 6.04 | 130.4 | 60.8a | 184.2a,b | 0.57 | 0.12 | 23.9b |
| 12L:12D | 6.12 | 120.9 | 68.1a | 189.1a | 0.61 | 0.12 | 16.3c |
| SEM | 0.04 | 6.2 | 2.3 | 3.8 | 0.10 | 0.01 | 2.1 |
| Significance of P-value | 0.254 | 0.626 | 0.005 | 0.005 | 0.422 | 0.413 | 0.006 |
a-cLeast square means lacking a common superscript within a column are different because of photoperiod effect (P < 0.05).
Protein solubility is expressed as mg soluble protein per g sample.
Emulsion activity index is expressed as 2.33∗Abs500nm.
Transmission value has been used as an indication of protein denaturation in meat quality analyses (den Hertog-Meischke et al., 1997). In the present study, transmission value was increased with longer photoperiod, which was in the order 30.7% (20L:4D), 24.6% (18L:6D), 23.9% (16L:8D), and 16.3% (12L:12D). Significant differences were found between 20L:4D and other treatments (P < 0.05), as well as between 12L:12D and other treatments (P < 0.05), whereas no difference was found between 18L:6D and 16L:8D groups (P > 0.05). The photoperiod-induced alterations of transmission value were paralleled with the identified changes of sarcoplasmic and total protein solubility. These may indicate that longer photoperiod may impair meat protein functionality, as denatured proteins have a lower solubility (Ockerman and Cahill, 1968) with an increased transmission value (den Hertog-Mejschke et al., 1997). It has also been established that heavily denatured proteins impair WHC (Sammel et al., 2002). Given that WHC of the patties was unaffected by photoperiod despite higher denatured proteins, it is possible that protein denaturation occurred primarily in the sarcoplasmic fraction. To support the hypothesis, Van Laack and Lane (2000) reported that protein denaturation has no impact on chicken M. Pectoralis profundus myofibrillar protein solubility. It should be noted that a more efficient chilling regime (i.e., water-immersion chilling) than the air chilling in the present study could potentially mitigate photoperiod-associated protein denaturation, although this postulation would need further study.
Quality Attributes of Manufactured Patties
There was no photoperiod treatment effect on moisture content or any WHC attribute, including freezing/thawing loss, cooking loss, and display weight losses (P > 0.05; Table 2). Similarly, photoperiod did not impact the textural profile of patties (P > 0.05; Table 3).
Table 2.
Effect of photoperiod on moisture content (%), freezing/thawing loss (%), cooking loss (%), and display weight loss (%) of ground broiler meat patties.
| Treatment | Moisture content (%) | Freezing/thawing loss (%) | Cooking loss (%) | Display weight loss (%) |
|
|---|---|---|---|---|---|
| 2 D | 4 D | ||||
| 20L:4D | 73.6 | 4.7 | 25.0 | 1.4 | 2.2 |
| 18L:6D | 74.1 | 4.6 | 24.6 | 1.2 | 1.7 |
| 16L:8D | 74.2 | 4.9 | 25.3 | 1.2 | 1.9 |
| 12L:12D | 73.4 | 3.9 | 24.6 | 1.0 | 1.8 |
| SEM | 0.5 | 0.9 | 2.4 | 0.2 | 0.4 |
| Significance of P-value | 0.676 | 0.686 | 0.955 | 0.134 | 0.218 |
Table 3.
Effect of photoperiod on textural profile of ground broiler meat patties.
| Treatment | Hardness (g) | Resilience (%) | Cohesion | Springiness (%) | Gumminess | Chewiness |
|---|---|---|---|---|---|---|
| 20L:4D | 5,580 | 24.7 | 0.498 | 68.7 | 2,800 | 1,920 |
| 18L:6D | 5,220 | 24.9 | 0.497 | 69.4 | 2,600 | 1,820 |
| 16L:8D | 5,410 | 24.6 | 0.498 | 67.8 | 2,700 | 1,840 |
| 12L:12D | 5,150 | 25.2 | 0.491 | 69.2 | 2,550 | 1,790 |
| SEM | 375 | 0.7 | 0.011 | 3.0 | 241 | 204 |
| Significance of P-value | 0.698 | 0.927 | 0.947 | 0.877 | 0.819 | 0.948 |
Photoperiod treatments, however, exhibited some impact on instrumental color attributes of patties during retail display (Table 4). There was a strong trend (P = 0.053) for patties from 20L:4D to have higher CIE L∗ values than those from other treatments. This finding agrees with the increased protein denaturation and lower sarcoplasmic protein solubility observed in 20L:4D, as previously discussed. This could be attributed to a more open muscle structure allowing moisture migration to the meat surface, thus increasing reflectance (MacDougall, 1982, Kim et al., 2010). It would be reasonable to postulate that this would likely impair WHC, as several studies have corroborated that higher L∗ poultry meat exhibits poorer WHC (Barbut, 1993, Qiao et al., 2001). These differences may arise from the meat having been ground, as this could mitigate the previously mentioned more open muscle structure associated with protein denaturing conditions. Alternatively, the parallel study by Tuell et al. (2020) observed a trend (P = 0.07) of greater moisture loss in 20L:4D carcasses during carcass chilling compared with shorter photoperiod groups, whereas no measures of WHC in the intact M. Pectoralis major muscles were affected (P > 0.05). As such, it is reasonable to postulate photoperiod-associated effects on WHC would be most apparent early postmortem during carcass chilling, contributing to minimal differences in WHC of both intact and ground broiler meat across treatments despite exhibiting a lighter color.
Table 4.
Effect of photoperiod on instrumental color attributes (CIE L∗ [lightness], CIE a∗ [redness], CIE b∗ [yellowness], hue angle [discoloration], and chroma [color intensity]) and thiol content of ground broiler meat patties during display storage.
| Treatment | Instrumental color attribute |
Thiol content1 | ||||
|---|---|---|---|---|---|---|
| CIE L∗ | CIE a∗ | CIE b∗ | Hue angle | Chroma | ||
| Photoperiod effect (P) | ||||||
| 20L:4D | 56.5 | 8.8 | 11.0a | 51.3 | 14.1a | 22.6 |
| 18L:6D | 55.4 | 9.0 | 10.8a | 50.1 | 14.1a | 23.0 |
| 16L:8D | 54.6 | 9.1 | 10.2b | 47.9 | 13.7a | 22.7 |
| 12L:12D | 55.1 | 8.3 | 9.8b | 49.6 | 12.9b | 22.2 |
| SEM | 0.8 | 0.4 | 0.3 | 1.0 | 0.4 | 0.6 |
| Display period effect (D) | ||||||
| 0 D | 55.5 | 10.4x | 12.8x | 50.9 | 16.6x | 24.7x |
| 1 D | 55.6 | 8.8y | 10.4y | 49.6 | 13.6y | - |
| 2 D | 55.1 | 8.6y,z | 9.6y | 48.3 | 12.9y | 22.6x,y |
| 3 D | 55.2 | 8.2z | 9.6y | 49.3 | 12.7y | - |
| 4 D | 55.5 | 8.1z | 9.8y | 50.7 | 12.7y | 20.5y |
| SEM | 0.8 | 0.4 | 0.5 | 1.1 | 0.5 | 0.7 |
| Significance of P-value | ||||||
| P | 0.053 | 0.128 | 0.009 | 0.150 | 0.006 | 0.490 |
| D | 0.869 | <0.001 | 0.003 | 0.399 | <0.001 | 0.023 |
| P × D | 0.889 | 0.102 | 0.867 | 0.801 | 0.466 | 0.437 |
a,bLeast square means lacking a common superscript within a column are different due to photoperiod effect (P < 0.05).
x-zLeast square means lacking a common superscript within a column are different due to display period effect (P < 0.05).
Thiol content is expressed as nM thiol groups per mg protein.
There were no interaction effects of photoperiod treatment and display storage duration on color attributes (P > 0.05. Table 4). However, patties from shorter photoperiod treatments (12L:12D and 16L:8D) had lower CIE b∗ values than those from both 18L:6D and 20L:4D (P < 0.05), whereas there were no differences between 12L:12D and 16L:8D and between 18L:6D and 20L:4D (P > 0.05). According to Du et al. (2000), CIE b∗ values may be correlated with the antioxidative capacity of the muscle tissue. In that study, the meat from White Leghorn hens fed a diet with antioxidant conjugated linoleic acid had significantly lower CIE b∗ values with a lower lipid oxidation than controls fed a regular diet. However, hue angle (indication of discoloration) was not affected by treatment in the present study (P > 0.05). It is possible hue angle may not be the optimal indicator of processed chicken meat discoloration with relatively short display storage duration, as hue angle did not show any meaningful change with display (P > 0.05). Lower CIE b∗ values in 12L:12D, however, influenced chroma values (color saturation), where patties from 12L:12D were found to have less intense color compared with other treatments (P < 0.05). No differences between 16L:8D and the longer photoperiod treatments (20L:4D and 18L:6D) were shown (P > 0.05), likely attributable to 16L:8D having numerically higher CIE a∗ and CIE b∗ values than 12L:12D.
As expected, CIE a∗ and chroma values decreased during the display period (P < 0.05), irrespective of the photoperiod treatment, especially apparent from 0 to 1 D of display. These findings are well supported in the literature, where chicken meat color stability has been shown to worsen with display storage (Yang and Chen, 1993, Allen et al., 1997, Allen et al., 1998). Interestingly, CIE b∗ values also decreased with display, with a higher value observed at 0 D of display than all other display dates (P < 0.05). A study by Yang and Chen (1993) demonstrated a similar phenomenon, where CIE b∗ values of raw ground broiler meat decreased after day 0 of refrigerated storage before increasing much later in the storage period (approximately 24 D of refrigerated storage).
Oxidative Stability of Manufactured Patties
No interaction of photoperiod and display storage was observed for protein oxidation (P > 0.05; Table 4). However, thiol content was decreased by display duration (P < 0.05), indicating a higher degree of protein oxidation, regardless of photoperiod treatment (P > 0.05). Patties displayed 0 D had higher thiol content than those at 4 D of display (P < 0.05), whereas no differences in thiol content of patties between either day 0 and day 2, or between day 2 and day 4, of display storage were found (P > 0.05). A previous study has corroborated the relationship between storage duration and loss of sulfhydryl groups in chicken meat (Khan et al., 1963). Tuell et al. (2020) found an interaction in thiol contents of intact M. Pectoralis major muscles from day 1 to day 7 of aerobic display, where a detectable loss of thiol groups was observed in the 20L:4D group only (P < 0.05), whereas thiol contents of shorter photoperiod groups were unaffected (P > 0.05).
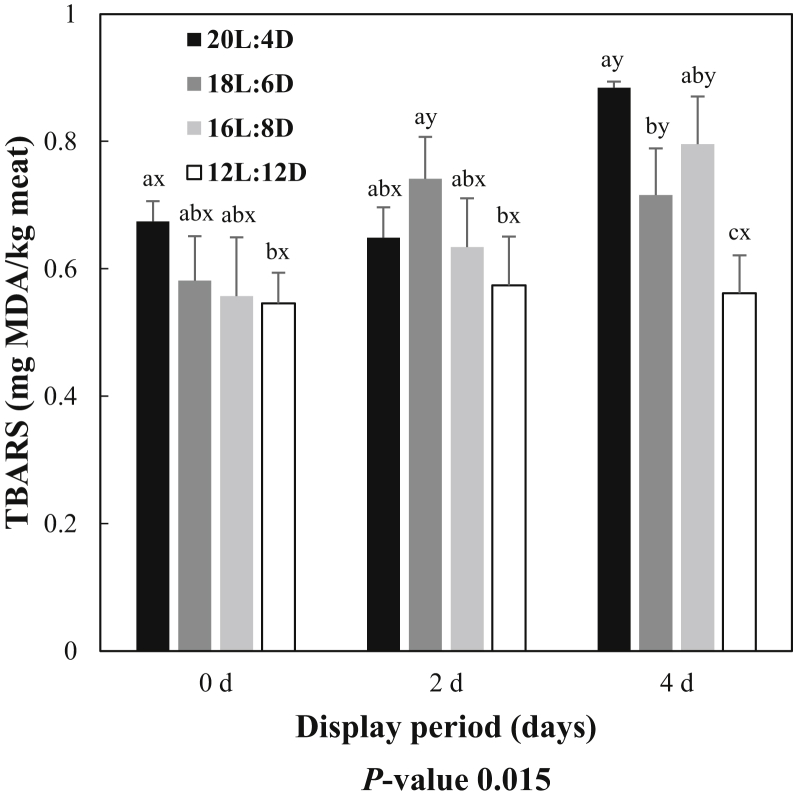
A significant interaction between photoperiod and display storage duration was found for lipid oxidation as assessed by TBARS values (P < 0.05; Figure. 1). On day 0 of display, patties from 20L:4D had higher content of MDA than patties from 12L:12D (P < 0.05), but neither 20L:4D nor 12L:12D were different from 16L:8D or 18L:6D (P > 0.05). At display day 2, a significant increase in MDA content was observed in 18L:6D (P < 0.05) but not in other treatments compared with the level at day 0 of the respective treatment (P > 0.05). Consequently, 18L:6D had higher MDA content on day 2 than 12L:12D (P < 0.05) but was not different from 20L:4D and 16L:8D (P > 0.05). On display day 4, MDA contents for both 20L:4D and 16L:8D increased from its levels observed on both day 0 and day 2 (P < 0.05). Interestingly, TBARS values did not increase in patties from the 12L:12D treatment during the display duration (P > 0.05). As a result, 12L:12D maintained at lower level of MDA at day 4 than all other treatments (P < 0.05). Patties from 20L:4D had higher MDA content than 18L:6D at day 4 (P < 0.05) but was not different from 16L:8D (P > 0.05). There was no difference of MDA content at day 4 between the 18L:6D and 16L:8D (P > 0.05). These results indicate that using a 12L:12D photoperiod regime can improve lipid stability. Although its mechanisms were not tested in this study, it could be related to the decrease of MDA contents in both live broiler serum (Guob et al., 2010) and fillet muscles (Li et al., 2010, Tuell et al., 2020) associated with shorter photoperiod regimes. This hypothesis is supported by the findings of longer photoperiod promoting the release of free radicals with lowering immune function (Abbas et al., 2008, Guob et al., 2010). Furthermore, protein denaturation, as identified in the present study, promotes lipid oxidation in meat products (Love and Pearson, 1971, Li and King, 1996). The current and previous results suggest that 12L:12D lighting program causes less protein denaturation and maintains lipid stability in ground broiler meat during display, at least up to 4 D of aerobic storage.
Figure 1.
Interaction of photoperiod treatment and display storage duration on 2-thiobarbituric acid reactive substances (TBARS) values of ground broiler meat patties. TBARS values are expressed as mg malondialdehyde (MDA) per kg meat. Results are displayed as least square means +SEM. a-cLeast square means lacking a common superscript differ because of photoperiod treatment within the respective display storage duration (P < 0.05). x,yLeast square means lacking a common superscript differ because of display storage duration within same photoperiod treatment (P < 0.05).
Conclusions
Results of the present study suggest that photoperiod can influence protein functionality and oxidative stability of processed broiler meat products. The 20L:4D photoperiod regime traditionally used for reaching maximized growth potential in broilers appeared to reduce total and sarcoplasmic protein solubility and increase protein denaturation. Patties from longer photoperiods (20L:4D and 18L:6D) were more yellow in color. Lipid oxidation but not protein oxidation was affected by photoperiod during simulated retail display storage, where the patties from 12L:12D treatment showed the least accumulation of MDA compared with patties from other longer photoperiod groups at the end of display storage. These data suggest that light management is an important factor affecting broiler meat quality. Specifically, the 12L:12D photoperiod regime may be beneficial in producing broiler meat products with less protein denaturation and lipid oxidation.
Acknowledgments
Appreciation is extended to the members of the Meat Science and Muscle Biology Laboratory of Purdue University for assistance with sample and data collections. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement of the USDA. The USDA is an equal opportunity provider and employer.
Conflicts of Interest Statement: The authors declare no conflicts of interest.
References
- Allen C.D., Fletcher D.L., Northcutt J.K., Russell S.M. The relationship of broiler breast color to meat quality and shelf-life. Poult. Sci. 1998;77:361–366. doi: 10.1093/ps/77.2.361. [DOI] [PubMed] [Google Scholar]
- Abbas O., Alm El-Dein A.K., Desoky A.A., Galal M.A.A. The effects of photoperiod programs on broiler chicken performance and immune Response. Int. J. Poult. Sci. 2008;7:665–671. [Google Scholar]
- Allen C., Russell S., Fletcher D. The relationship of broiler breast meat color and pH to shelf-life and odor development. Poult. Sci. 1997;76:1042–1046. doi: 10.1093/ps/76.7.1042. [DOI] [PubMed] [Google Scholar]
- AMSA . American Meat Science Association; IL, USA: 2012. Meat Color Measurement Guidelines; pp. 1–17. [Google Scholar]
- AOAC . 18th ed. Association of Official Analytical Chemists; Gaithersbug, MD, USA: 2006. Official Methods of Analysis of AOAC. [Google Scholar]
- Asghar A., Samejima K., Yasui T., Henrickson R.L. Functionality of muscle proteins in gelation mechanisms of structured meat products. C R. C Crit. Rev. Food Sci. Nutr. 1985;22:27–106. doi: 10.1080/10408398509527408. [DOI] [PubMed] [Google Scholar]
- Barbut S. Colour measurements for evaluating the pale soft exudative (PSE) occurrence in Turkey meat. Food Res. Int. 1993;26:39–43. [Google Scholar]
- Bourne M.C. Texture profile Analysis. Food Technol. 1978;32:62–66. [Google Scholar]
- Bowker B., Zhuang H. Impact of white striping on functionality attributes of broiler breast meat. Poult. Sci. 2016;95:1957–1965. doi: 10.3382/ps/pew115. [DOI] [PubMed] [Google Scholar]
- Buege J.A., Aust S.D. [30] Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- Carballo J., Mota N., Barreto G., Colmenero F.J. Binding properties and colour of Bologna sausage made with varying fat levels, protein levels and cooking temperatures. Meat Sci. 1995;41:301–313. doi: 10.1016/0309-1740(95)00001-2. [DOI] [PubMed] [Google Scholar]
- Chan J.T.Y., Omana D.A., Betti M. Functional and rheological properties of proteins in frozen Turkey breast meat with different ultimate pH. Poult. Sci. 2011;90:1112–1123. doi: 10.3382/ps.2010-01185. [DOI] [PubMed] [Google Scholar]
- Classen H.L., Riddell C. Photoperiodic effects on performance and leg abnormalities in broiler chickens. Poult. Sci. 1989;68:873–879. doi: 10.3382/ps.0680873. [DOI] [PubMed] [Google Scholar]
- Classen H.L., Riddell C., Robinson F.E. Effects of increasing photoperiod length on performance and health of broiler chickens. Br. Poult. Sci. 1991;32:21–29. doi: 10.1080/00071669108417324. [DOI] [PubMed] [Google Scholar]
- den Hertog-Meischke M.J., Klont R.E., Smulders F.J., van Logtestijn J.G. Variation in post-mortem rate of glycolysis does not necessarily affect drip loss of non-stimulated veal. Meat Sci. 1997;47:323–329. doi: 10.1016/s0309-1740(97)00064-8. [DOI] [PubMed] [Google Scholar]
- Downs K.M., Lien R.J., Hess J.B., Bilgili S.F., Dozier W.A. The effects of photoperiod length, light intensity, and feed Energy on growth Responses and meat yield of broilers. J. Appl. Poult. Res. 2006;15:406–416. [Google Scholar]
- Du M., Ahn D.U., Nam K.C., Sell J.L. Influence of dietary conjugated linoleic acid on volatile profiles, color and lipid oxidation of irradiated raw chicken meat. Meat Sci. 2000;56:387–395. doi: 10.1016/s0309-1740(00)00067-x. [DOI] [PubMed] [Google Scholar]
- Guob Y.L., Li W.B., Chen J.L. Influence of nutrient density and lighting regime in broiler chickens: effect on antioxidant status and immune function. Br. Poult. Sci. 2010;51:222–228. doi: 10.1080/00071661003746503. [DOI] [PubMed] [Google Scholar]
- Khan A.W., Berg L., Lentz C.P. Effects of frozen storage on chicken muscle proteins. J. Food Sci. 1963;28:425–430. [Google Scholar]
- Kim H.-W., Kim J.-H., Yan F., Cheng H., Kim Y.H.B. Effects of heat stress and probiotic supplementation on protein functionality and oxidative stability of ground chicken leg meat during display storage. J. Sci. Food Agric. 2017;97:5343–5351. doi: 10.1002/jsfa.8423. [DOI] [PubMed] [Google Scholar]
- Kim Y.H., Lonergan S.M., Huff-Lonergan E. Protein denaturing conditions in beef deep semimembranosus muscle results in limited μ-calpain activation and protein degradation. Meat Sci. 2010;86:883–887. doi: 10.1016/j.meatsci.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Li W., Guo Y., Chen J., Wang R., He Y., Su D. Influence of lighting schedule and nutrient density in broiler chickens: effect on growth performance, carcass Traits and meat quality. Asian-australas. J. Anim. Sci. 2010;23:1510–1518. [Google Scholar]
- Li S.J., King A.J. Lipid oxidation and Myosin denaturation in dark chicken meat. J. Agric. Food Chem. 1996;44:3080–3084. [Google Scholar]
- Lien R.J., Hess J.B., McKee S.R., Bilgili S.F., Townsend J.C. Effect of light intensity and photoperiod on live performance, Heterophil-to-Lymphocyte ratio, and processing yields of broilers. Poult. Sci. 2007;86:1287–1293. doi: 10.1093/ps/86.7.1287. [DOI] [PubMed] [Google Scholar]
- Lien R.J., Hooie L.B., Hess J.B. Influence of long-bright and increasing-dim photoperiods on live and processing performance of two broiler strains. Poult. Sci. 2009;88:896–903. doi: 10.3382/ps.2008-00309. [DOI] [PubMed] [Google Scholar]
- Love J.D., Pearson A.M. Lipid oxidation in meat and meat products-A review. J. Am. Oil Chem. Soc. 1971;48:547–549. [Google Scholar]
- MacDougall D.B. Changes in the colour and opacity of meat. Food Chem. 1982;9:75–88. [Google Scholar]
- Martelli G., Nannoni E., Grandi M., Bonaldo A., Zaghini G., Vitali M., Biagi G., Sardi L. Growth parameters, behavior, and meat and ham quality of heavy pigs subjected to photoperiods of different duration. J. Anim. Sci. 2015;93:758–766. doi: 10.2527/jas.2014-7906. [DOI] [PubMed] [Google Scholar]
- Ockerman H.W., Cahill V.R. Water extractability of muscle protein and factors which affect this procedure as a method of determining pork quality. J. Anim. Sci. 1968;27:31–38. [Google Scholar]
- Olanrewaju H.A., Miller W.W., Maslin W.R., Collier S.D., Purswell J.L., Branton S.L. Interactive effects of light-sources, photoperiod, and strains on growth performance, carcass characteristics, and health indices of broilers grown to heavy weights. Poult. Sci. 2019;98:6232–6240. doi: 10.3382/ps/pez476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olanrewaju H.A., Thaxton J.P., Dozier W.A., Joseph Purswell, Roush W.B., Branton S.L. A review of lighting programs for broiler production. Int. J. Poult. Sci. 2006;5(4):301–308. [Google Scholar]
- Pietrasik Z. Effect of content of protein, fat and modified starch on binding textural characteristics, and colour of comminuted scalded sausages. Meat Sci. 1999;51:17–25. doi: 10.1016/s0309-1740(98)00068-0. [DOI] [PubMed] [Google Scholar]
- Qiao M., Fletcher D.L., Smith D.P., Northcutt J.K. The effect of broiler breast meat color on pH, moisture, water-holding capacity, and emulsification capacity. Poult. Sci. 2001;80:676–680. doi: 10.1093/ps/80.5.676. [DOI] [PubMed] [Google Scholar]
- Sammel L.M., Hunt M.C., Kropf D.H., Hachmeister K.A., Kastner C.L., Johnson D.E. Influence of Chemical characteristics of beef inside and outside semimembranosus on color Traits. J. Food Sci. 2002;67:1323–1330. [Google Scholar]
- Sanotra G.S., Lund J.D., Vestergaard K.S. Influence of light-dark schedules and stocking density on behaviour, risk of leg problems and occurrence of chronic fear in broilers. Br. Poult. Sci. 2002;43:344–354. doi: 10.1080/000716601201036023611. [DOI] [PubMed] [Google Scholar]
- Sardi L., Nannoni E., Grandi M., Vignola G., Zaghini G., Martelli G. Meat and ham quality of Italian heavy pigs subjected to different illumination regimes. Berl. Munch. Tierarztl. Wochenschr. 2012;125:463–468. [PubMed] [Google Scholar]
- Tuell J.R., Park J.-Y., Wang W., Cooper B., Sobreira T., Cheng H.-W., Kim Y.H.B. Effects of photoperiod regime on meat quality, oxidative stability, and metabolites of postmortem broiler fillet (M. Pectoralis major) muscles. Foods. 2020;9:215. doi: 10.3390/foods9020215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Laack R.L., Lane J.L. Denaturation of myofibrillar proteins from chickens as affected by pH, temperature, and adenosine triphosphate concentration. Poult. Sci. 2000;79:105–109. doi: 10.1093/ps/79.1.105. [DOI] [PubMed] [Google Scholar]
- Vossen E., De Smet S. Protein oxidation and protein Nitration influenced by sodium nitrite in two different meat model Systems. J. Agric. Food Chem. 2015;63:2550–2556. doi: 10.1021/jf505775u. [DOI] [PubMed] [Google Scholar]
- Yang C.C., Chen T.C. Effects of refrigerated storage, pH Adjustment, and Marinade on color of raw and Microwave cooked chicken meat. Poult. Sci. 1993;72:355–362. [Google Scholar]
- Yasui T., Ishioroshi M., Samejima K. Heat-induced gelation of myosin in the presence of actin. J. Food Biochem. 1980;4:61–78. [Google Scholar]