Abstract
Broilers are an important reservoir of extended spectrum beta-lactamase and AmpC beta-lactamase (ESBL/pAmpC)-producing bacteria. In previous studies, a single supply of a competitive exclusion (CE) product before challenge with a high dose of ESBL/pAmpC-producing Escherichia coli led to reduced colonization, excretion, and transmission, but could not prevent colonization. The hypothesized mechanism is competition; therefore, in this study the effect of a prolonged supply of CE products on colonization, excretion, and transmission of ESBL-producing E. coli after challenge with a low dose at day 0 or day 5 was investigated. Day-old broilers (Ross 308) (n = 220) were housed in isolators. Two CE products, containing unselected fermented intestinal bacteria (CEP) or a selection of pre- and probiotics (SYN), were supplied in drinking water from day 0 to 14. At day 0 or 5, broilers were challenged with 0.5 mL with 101 or 102 cfu/mL E. coli encoding the beta-lactamase gene blaCTX-M-1 on an IncI plasmid (CTX-M-1-E. coli). Presence and concentration of CTX-M-1-E. coli were determined using cloacal swabs (days 0–14, 16, 19, and 21) and cecal content (day 21). Cox proportional hazard model and a mixed linear regression model were used to determine the effect of the intervention on colonization and excretion (log10 cfu/g). When challenged on the day of hatch, no effect of CEP was observed. When challenged at day 5, both CEP and SYN led to a prevention of colonization with CTX-M-1-E. coli in some isolators. In the remaining isolators, we observed reduced time until colonization (hazard ratio between 3.71 × 10−3 and 3.11), excretion (up to −1.60 log10 cfu/g), and cecal content (up to −2.80 log10 cfu/g), and a 1.5 to 3-fold reduction in transmission rate. Colonization after a low-dose challenge with ESBL-producing E. coli can be prevented by CE products. However, if at least 1 bird is colonized it spreads through the whole flock. Prolonged supply of CE products, provided shortly after hatch, may be applicable as an intervention to reduce the prevalence of ESBL/pAmpC-producing bacteria in the broiler production chain.
Key words: ESBL, pAmpC, antimicrobial resistance, intervention, competitive exclusion
Introduction
Plasmid-mediated extended spectrum beta-lactamase and AmpC beta-lactamase (ESBL/pAmpC)-producing bacteria are resistant to extended spectrum cephalosporins. ESBL/pAmpC-producing Escherichia coli are present in the environment, humans, and animals (Blaak et al., 2015). Although the prevalence has decreased in recent years in different animal divisions (Dorado-Garcia et al., 2016, Hesp et al., 2019, MARAN, 2019), 23.0% of the broilers at slaughter were positive for ESBL/pAmpC-producing E. coli in 2018 in the Netherlands (MARAN, 2019). High prevalence of ESBL/pAmpC-producing bacteria in poultry flocks (from 0.3 up to 100%) and poultry products (from 3.3 up to 94.5%) is also reported from several other European countries (Saliu et al., 2017), indicating broilers to be an important source of ESBL/pAmpC-producing bacteria. Although the contribution from poultry (meat) to human carriage of ESBL-producing E. coli seems less important than initially perceived (Mughini-Gras et al., 2019), all attempts to contribute to reducing emergence and spread of antibiotic resistance in humans and animals are important from a One Health perspective (World Health Organization, 2018). Moreover, direct contact with poultry (e.g., people working or living on a poultry farm) could be a transmission route of ESBL/pAmpC-producing bacteria (Dierikx et al., 2013b, Huijbers et al., 2014, Huijbers et al., 2015).
The broiler production chain has a pyramidal structure with a few purebred pedigree farms at the top and many broiler farms at the bottom, with multiplier and crossbreeding steps in between. ESBL/pAmpC-producing E. coli have been found in all levels of the production chain (Dierikx et al., 2013a, Apostolakos et al., 2019). Transmission occurs via several routes, vertically between different levels of the chain, horizontally within and between farms, and via the (farm) environment (Dame-Korevaar et al., 2019). Consequently, the introduction of ESBL/pAmpC-producing E. coli in a broiler flock can occur at different moments, for example in the hatchery, during transport or shortly after arrival at the farm, or during the fattening phase.
To reduce the prevalence of ESBL/pAmpC-producing E. coli in the broiler production chain, interventions targeted at different transmission routes are needed. Examples include reducing exposure of the flock to bacteria from the farm environment using hygiene barriers, or from the previous flock by cleaning and disinfection between production rounds. However, these interventions are not always sufficient in preventing colonization (Daehre et al., 2018). In addition, other types of interventions can be used to attempt to prevent colonization of resistant E. coli, such as supplying products via feed or water, like feed additives (Roth et al., 2017). Interventions applicable simultaneously at different levels of the production chain will most likely help control the spread of ESBL/pAmpC-producing E. coli in broilers, and consequently in meat products, as measures taken at the top of the pyramid can affect the presence of ESBL/pAmpC-producing E. coli at lower levels of the pyramid as well. Furthermore, the rapid colonization of young broilers, even after exposure to a low dose of ESBL/pAmpC-producing E. coli (Dame-Korevaar et al., 2019), shows that interventions should be implemented as soon as possible after hatching. Delayed colonization observed in conventional broilers which carried initial E. coli, compared to specific pathogen-free (SPF) broilers not carrying E. coli upon hatching (Dame-Korevaar et al., 2019), suggests that the gut microbiome plays an important role in susceptibility to colonization of ESBL/pAmpC-producing E. coli, and that this susceptibility may vary between development phases (Jurburg et al., 2019). Therefore, influencing the gut microbiome at an early age could potentially be a high-impact intervention, applicable at different levels of the broiler pyramid. This can be done using the concept of competitive exclusion (CE).
CE is based on early establishment of natural intestinal bacteria, to protect the bird from colonization with certain other bacteria (Nurmi et al., 1992). Different types of CE products, containing non-pathogenic bacterial cultures of single or mixed strains (Callaway et al., 2008), are available for poultry. The bacterial strains in these products can be defined, or consist of unselected intestinal bacteria from adult SPF chickens (e.g., Aviguard). Also, some products contain a selection of pre- and probiotics (SYN), the so-called synbiotics. These CE products reduce colonization of foodborne pathogens, such as Salmonella (Nakamura et al., 2002, Ferreira et al., 2003, Luoma et al., 2017, Markazi et al., 2018). The administration of a CE product to day-old broilers before challenge resulted in decreased intestinal and cecal colonization with resistant pathogenic E. coli (Hofacre et al., 2002). Other studies showed that, in the absence of antibiotics, a single oral supply of a CE product led to reduced cecal content (cfu/g) (Nuotio et al., 2013, Methner et al., 2019), excretion, and transmission (Ceccarelli et al., 2017) upon challenge with a high dose (105–108 cfu/mL) of ESBL/pAmpC-producing E. coli, but could not prevent colonization in the gut. However, under field circumstances the first colonized birds have likely been exposed to much lower numbers of ESBL/pAmpC-producing E. coli (Laube et al., 2013, Blaak et al., 2015), especially in a properly cleaned and disinfected poultry house. Exposure to a lower dose of ESBL/pAmpC-producing E. coli will reduce the risk of colonization (Dame-Korevaar et al., 2019), and the bacteria present in the CE products will most likely result in further reduction of this risk. In addition, a longer supply of a CE products might be more effective by supplying more of the competitive bacteria.
In this study, we investigated the effect of a prolonged supply of CE products in drinking water on time until colonization, excretion, and transmission of ESBL-producing E. coli after challenge with a low dose. In 3 transmission experiments with contact birds and orally inoculated seeder birds, the effect of 2 types of CE products (unselected fermented intestinal bacteria from SPF chickens [CEP] and a synbiotic selection of pre- and probiotics [SYN]) was investigated. Two scenarios of ESBL-producing E. coli introduction were studied: exposure of broilers to a low dose of ESBL-producing E. coli on the day of hatch (experiment 1) and during the first week of life (day 5, experiments 2 and 3).
Materials and methods
Ethics of Experimentation
Broilers were observed daily and clinical signs, abnormal behavior, and mortality were recorded. The study protocol was approved by the Dutch Central Authority for Scientific Procedures on Animals and the Animal Experiments Committee of Utrecht University (Utrecht, the Netherlands) under registration number AVD108002015314 and all procedures were performed in full compliance with all legislations.
Experimental Design
Three consecutive experiments were conducted (Table 1). In experiment 1 (n = 70 broilers), broilers were challenged on the day of hatch (day 0) with 0.5 mL with 101 or 102 cfu/mL CTX-M-1-E. coli and the intervention groups received a CE product in drinking water (day 0–14), derived from unselected fermented intestinal bacteria from SPF birds (CEP). In experiments 2 (n = 70 broilers) and 3 (n = 80 broilers), broilers were challenged at day 5 with 0.5 mL with 101 or 102 cfu/mL CTX-M-1-E. coli and the intervention groups received either CEP or a CE product based on synbiotics containing a selection of SYN.
Table 1.
Date, age of parent flock (weeks), day (0 or 5), and dose (non-inoculated, or 0.5 mL of 101 or 102 cfu/mL) of challenge and intervention (none (-), CEP, or SYN) for experiments 1 (n = 70 broilers), 2 (n = 70 broilers), and 3 (n = 80 broilers).
| Experiment | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| Date | 12 April–3 May 2017 | 24 May–14 June 2017 | 23 Oct–13 Nov 2017 | |||
| Parent flock1 | A, 54 wk | A, 60 wk | B, 57 wk | |||
| Day of challenge2 | Day 0 | Day 5 | Day 5 | |||
| Isolator | Challenge (cfu/mL)3 | Intervention4 | Challenge (cfu/mL)3 | Intervention4 | Challenge (cfu/mL)3 | Intervention4 |
| 1 | Non-inoculated (-) | - | Non-inoculated (saline solution) | - | Non-inoculated (saline solution) | - |
| 2 | 101 | None (-) | 101 | None (-) | 102 | None (-) |
| 3 | 101 | CEP | 101 | CEP | 102 | CEP |
| 4 | 101 | CEP | 101 | CEP | 102 | CEP |
| 5 | 102 | None (-) | 102 | None (-) | 102 | SYN |
| 6 | 102 | CEP | 102 | CEP | 102 | SYN |
| 7 | 102 | CEP | 102 | CEP | 102 | SYN |
| 8 | 102 | SYN | ||||
Abbreviations: CEP, competitive exclusion product; SYN, selection of pre- and probiotics.
In all 3 experiments, the eggs were disinfected with formaldehyde before incubation and in the hatcher before hatching.
Challenge with Escherichia coli E38.27 with blaCTX-M-1 on IncI1 plasmid.
Inoculated birds (n = 5 out of 10 in each isolator) received 0.5 mL of the mentioned challenge dose.
Intervention was implemented in all experiments from day 0, 4:00 pm until day 14, 4:00 pm. In experiment 1, supply of the intervention started immediately after challenge.
Birds, Housing, and Management
In all 3 experiments, 100 conventional broilers (Ross 308) were transported on the morning of the day of hatch (referred to as day 0 of age and day 0 of the experiment) to the animal facilities (Utrecht University, Utrecht, the Netherlands); they were individually tagged and weighed and randomly divided over the isolators (Table 1). In experiment 1, some of the broilers (n = 43, randomly selected) were placed temporally in 2 other isolators; 35 of these broilers were selected for the remainder of the experiment. Five of these 35 birds were moved to isolator 1; thereafter, the remaining birds were inoculated with CTX-M-1-E. coli (see the section E. coli Challenge below). One hour after inoculation, the inoculated (referred to as seeder) broilers were moved using transport boxes and added to the non-inoculated (referred to as contact) broilers in isolators 2 to 7 (5 seeder, 5 contact broilers per isolator). In experiments 2 and 3, upon arrival at day 0 all broilers were randomly distributed over isolators 1 to 7 (experiment 2) or 1 to 8 (experiment 3) (maximum 15 broilers per isolator). At day 5, just before the moment of inoculation, 10 broilers per isolator were selected for the remainder of the experiment, and randomly assigned to contact (n = 5) or seeder (n = 5) birds. The seeder broilers were inoculated. The surplus broilers not assigned as contacts or seeders in experiments 1, 2, and 3, including all broilers with signs of reduced health or development or low hatching weight, were euthanized using cervical dislocation and removed from the isolator. Before the start of each of the 3 experiments, samples were taken from the parent flock, incubators, hatchers, and research facilities to confirm the absence of ESBL/pAmpC-producing E. coli.
Broilers were housed in negative pressure high efficiency particulate air isolators, on paper linings with fine wood shavings. Standard broiler diet without any antibiotics or coccidiostats, radiated with 9 Gy, was available ad libitum. Feed and water were available from day 0, 4:00 pm. The intervention was supplied in drinking water (described below). A few broilers died or were euthanized before the end of the experiment due to causes unrelated to the experiment (8 in experiment 1, 2 in experiment 2, and 1 in experiment 3).
Intervention: CE Product
Composition
In this study, 2 CE products were used: 1) CE product (CEP) containing unselected, fermented intestinal bacteria, derived from SPF chickens and manufactured by fermentation (Aviguard; MSD Animal Health Nederland, Boxmeer, the Netherlands) (experiments 1, 2, and 3); and 2) a selection of a prebiotic compound and probiotic bacterial strains (SYN): fructo-oligosaccharides and Enterococcus faecium, Bifidobacterium animalis, Lactobacillus salivarius (PoultryStar sol; Biomin Holding GmbH, Getzersdorf, Austria) (experiment 3).
Supply
The CE products were supplied from the day of hatch (day 0), 4:00 pm, until day 14, 4:00 pm, twice a day, in drinking water. In experiment 1, supply of the intervention started immediately after challenge. Solutions with CEP or SYN in water were prepared in predilution directly before application with a dose according to recommendations of the manufacturer—that is 0.125 g CEP vs. 0.2 g SYN per 10 broilers—and added to the drinkers within the isolator. The amount of drinking water was restricted between day 0 and 14, based on the expected water consumption of 10 broilers in an isolator to ensure that all supplied CEP or SYN products would be consumed. Control groups received drinking water according to the same schedule, but without any intervention added.
E. coli Challenge
Broilers were challenged with E. coli strain E38.27, which carries the ESBL gene blaCTX-M-1 on an IncI1 plasmid, selected from healthy broilers and resistant to cefotaxime (Dierikx et al., 2010), using a 1 mL syringe without a needle with 0.5 mL of 101 or 102 cfu/mL. Serial dilutions of the E. coli strains were prepared on the day of challenge from fresh culture on heart infusion agar with 5% sheep blood (Becton Dickinson GmbH, Heidelberg, Germany) supplemented with cefotaxime (1 mg/L), after resuspending into saline solution. Bacterial dilutions were measured with the McFarland reader and retrospective colony counting.
From 1 h after inoculation onward, 5 contact birds were exposed to 5 seeder birds, either by moving the inoculated seeder birds to the isolators containing the contact birds (experiment 1) or by removing the temporal barrier between the inoculated seeder birds and the contact birds within the isolator (experiments 2 and 3). The unchallenged control birds were not inoculated (experiment 1) or received 0.5 mL physiological saline solution (experiments 2 and 3).
Cloacal and Cecal Samples
Samples were taken using sterile dry cotton swabs (MW100, Medical Wire & Equipment, England, during days 0 to 3, and Copan 155C, Copan Diagnostics Inc., Murrieta, CA, from day 4 onward). All birds were sampled just before inoculation to confirm absence of ESBL/pAmpC-producing bacteria (and additionally on days 1 and 3 in experiments 2 and 3), and from the moment of inoculation until day 7 twice a day (8:00 am and 4:00 pm), daily between days 8 and 14, and on days 16, 19, and 21 (8:00 am). On day 21, after the last sampling, post mortem examination was done within at maximum 30 min after euthanasia on each broiler. Broilers were weighed, sex was determined, broilers were checked for exterior and interior abnormalities, and ceca were collected and stored on ice for further analysis.
ESBL-producing E. coli Detection
All cloacal samples except the ones used for quantification of ESBL-producing E. coli and total E. coli (see below) were enriched in 3 mL Luria Bertani (LB) broth. After overnight incubation at 37°C, 10 μL broth was inoculated on MacConkey plates supplemented with 1 mg/L cefotaxime and incubated overnight at 37°C. E. coli colonies growing on the MacConkey plates supplemented with cefotaxime were referred to as CTX-M-1-E. coli. If visual assessment led to inconclusive results for the presence of E. coli, colonies were selected for further analysis using matrix-assisted laser desorption or ionization-time of flight mass spectrometry (Bruker Daltonik, Germany).
ESBL-producing E. coli and Total E. coli Quantification
Cloacal swabs obtained at 8:00 am were weighed before and after sampling to determine the amount of feces collected. The weight of the fecal material on the cloacal swab ranged from 0.01 to 0.43 g. At day 21, content from 1 of 2 ceca was collected. Samples were processed as previously described (Dame-Korevaar et al., 2019). Briefly, each cloacal swab was suspended in 3 mL LB broth. For the ceca, content from 1 of the 2 ceca was collected post mortem and 0.1 to 1.0 g was used to make a 10% dilution in PBS. Then, 200 μL of each suspension was used to prepare 10-fold dilution series, which were inoculated on MacConkey plates with and without 1 mg/L cefotaxime and incubated overnight at 37°C. Concentrations of ESBL-producing E. coli and total E. coli were determined semi-quantitatively (cfu/g feces), based on the highest consecutive dilution showing growth of typical E. coli colonies (Jett et al., 1997) and the weight of the feces on the swabs or the amount of cecal content collected, as previously described (Ceccarelli et al., 2017). The LB broth including the swab was also enriched overnight at 37°C. If no growth of E. coli colonies was observed in the dilution series, 10 μL of the overnight enrichment broth was inoculated on MacConkey plates supplemented with 1 mg/L cefotaxime and incubated overnight at 37°C. If colonies were detected, the concentration was assumed to be below the detection level of the dilution series and the concentration designated as such (see the section Statistical Analysis below).
E. coli colonies growing on MacConkey plates supplemented with cefotaxime were ESBL-producing E. coli, referred to here as CTX-M-1-E. coli. If visual assessment was inconclusive for the presence of E. coli, colonies were selected for further analysis using matrix-assisted laser desorption or ionization-time of flight mass spectrometry.
Statistical Analysis
Statistical analyses were performed in R, version 3.4.3 (RStudio Team, 2016), using packages “survival” (Cox proportional hazard regression) and “lme4” (mixed linear regression model).
Time Until Colonization
Individual broilers were considered colonized when 2 consecutive cloacal swabs tested positive for ESBL/pAmpC-producing E. coli. Time until colonization, using the first positive cloacal swab, was analyzed using Cox proportional hazard regression. Validity of the assumptions of proportional hazards was checked using Schoenfeld residuals, and these assumptions were met.
Excretion
Broilers negative for ESBL/pAmpC-producing E. coli in the dilution series but positive after overnight culturing were included in the analysis with excretion concentration 1 log10 cfu/mL LB, as the minimum detection level of the semi-quantitative method was 2 log10 cfu/mL LB. Results based on negative swab weight (or weight = 0 g) were excluded from the analysis. Moreover, samples negative for ESBL/pAmpC-producing E. coli after overnight culturing were excluded since the analysis was based on excreting broilers only. The effect of the challenge dose and the intervention on the ESBL-producing E. coli and total E. coli excretion (log10 cfu/g) was analyzed using a mixed linear regression model including the variables time, intervention, dose, contact or seeder bird, weight at hatch, weight at day 21, and the interaction between time and intervention. The variable sex was only included for experiments 1 and 2, as in experiment 3 only female birds were delivered by the hatchery. Random intercept was included, per bird, to adjust for clustered data in repeated measurements for the same bird. Weight at hatch and weight at day 21 were included as continuous variables, and the others as categorical variables. The best fitting model was obtained by backward selection, choosing the model with the lowest Akaike Information Criterion (AIC) value. Models with a difference in AIC of 2 or less were considered to be of equal fit and the most parsimonious model (lowest number of parameters) was chosen. Differences in ESBL-producing E. coli and total E. coli in cecal content (log10 cfu/g) between the control and intervention groups were tested using a linear regression model including the variables intervention, dose, contact or seeder bird, weight at hatch, weight at day 21, and sex. The best fitting model was obtained by backward selection, choosing the model with the lowest AIC value. Models with a difference in AIC of 2 or less were considered to be of equal fit and the most parsimonious model (lowest number of parameters) was chosen.
Transmission
The transmission coefficient (β) was estimated using the data of experiments 2 and 3 based on the stochastic susceptible infectious model (Velthuis et al., 2007, Dekker et al., 2013), in which the number of new cases is determined by transmission from infectious (I) birds to susceptible (S) birds for a total population of (N) birds. The expected number of new cases (C) in time interval Δt is calculated by . The force of infection (foi) was determined using different models. In model 1, direct transmission with mass action was assumed (), in which the force of infection was determined by the proportion of infectious birds (I-birds). In model 2, the cumulative time of excretion determined the force of infection (), in which is the cumulative sum of hours wherein all infectious birds were excreting up to the beginning of the interval. In model 3, the cumulative excretion determined the force of infection (), in which is the cumulative sum of excretion (log10 cfu/g feces) of all infectious birds. For all 3 models, different assumptions regarding the input data of I-birds were compared, by assuming that I-birds start excreting at the moment of the first positive cloaca swab (basic model) or half an interval before the first positive cloaca swab (alternative model).
Performance
Differences in performance (growth between day of hatch and day 21) between the control and intervention groups were tested using a linear regression model including the variables intervention, dose, contact or seeder bird, and sex. The best fitting model was obtained by backward selection, choosing the model with the lowest AIC value. Models with a difference in AIC of 2 or less were considered to be of equal fit and the most parsimonious model (lowest number of parameters) was chosen.
Results
Time Until Colonization
Experiment 1: CTX-M-1-E. coli Challenge with 101 or 102 cfu/mL on the Day of Hatch
All broilers were colonized with CTX-M-1-E. coli within 24 h after inoculation (Supplementary Table 1). There was no difference in the hazard ratio (HR) between control broilers and CEP broilers, nor between broilers challenged with dose 101 and 102. However, isolators 2, 6, and 7 had a higher HR than isolators 3, 4, and 5 (Table 2). Other variables, such as seeder or contact bird, weight on the day of hatch, weight at day 21, and sex, did not influence the time until colonization.
Table 2.
Hazard ratio (95% CI) of time until colonization for experiments 1 (n = 53), 2: dose 102 (n = 29), and 3 (n = 40); broilers were challenged with CTX-M-1-Escherichia coli at day 0 in experiment 1, and at day 5 in experiments 2 and 3.
| Experiment | Variable | HR2 (95% CI) | |
|---|---|---|---|
| 1 | Isolator1 | 2 (101 – none, reference) | 1 |
| 3 (101 – CEP) | 0.25 (0.09–0.72) | ||
| 4 (101 – CEP) | 0.24 (0.08–0.68) | ||
| 5 (102 – none) | 0.27 (0.09–0.76) | ||
| 6 (102 – CEP) | 0.74 (0.25–2.20) | ||
| 7 (102 – CEP) | 0.89 (0.29–2.71) | ||
| Seeder or contact bird | Seeder (reference) | 1 | |
| Contact | 0.67 (0.34–1.30) | ||
| Body weight at day 0 (hatch) | 0.99 (0.91–1.09) | ||
| Body weight at day 21 | 1.00 (1.00–1.01) | ||
| Sex | Male (reference) | 1 | |
| Female | 0.91 (0.43–1.91) | ||
| 2 | Isolator1 | 5 (102 – none, reference) | 1 |
| 6 (102 – CEP) | 0.08 (0.02–0.42) | ||
| 7 (102 – CEP) | 3.71 × 10−3 (2.71 × 10−4–0.05) | ||
| Seeder or contact bird | Seeder (reference) | 1 | |
| Contact | 1.09 (0.40–2.98) | ||
| Body weight at day 0 (hatch) | 0.93 (0.800–1.09) | ||
| Body weight at day 21 | 1.00 (0.99–1.00) | ||
| Sex | Male (reference) | 1 | |
| Female | 0.64 (0.25–1.60) | ||
| Total E. coli (cfu/g feces) day 5 | 1.06 (0.64–1.74) | ||
| 3 | Isolator1 | 2 (102 – none, reference) | 1 |
| 6 (102 – SYN) | 0.07 (0.01–0.39) | ||
| 7 (102 – SYN) | 1.29 (0.39–4.28) | ||
| 8 (102 – SYN) | 3.11 (0.97–10.05) | ||
| Seeder or contact bird | Seeder (reference) | 1 | |
| Contact | 0.43 (0.20–0.94) | ||
| Body weight at day 0 (hatch) | 1.03 (0.94–1.13) | ||
| Body weight at day 21 | 1.00 (0.99–1.00) | ||
| Total E. coli (cfu/g feces) day 5 | 1.18 (0.80–1.74) |
Abbreviations: CEP, competitive exclusion product; HR, hazard ratio; SYN, selection of pre- and probiotics.
Isolator number, dose level of challenge (101 or 102), and intervention (none, CEP, or SYN).
HR, indicating the ratio between the hazard of colonization with CTX-M-1-E. coli in the mentioned group and the reference group. A ratio of <1 indicates a smaller hazard, and a ratio of >1 indicates a higher hazard.
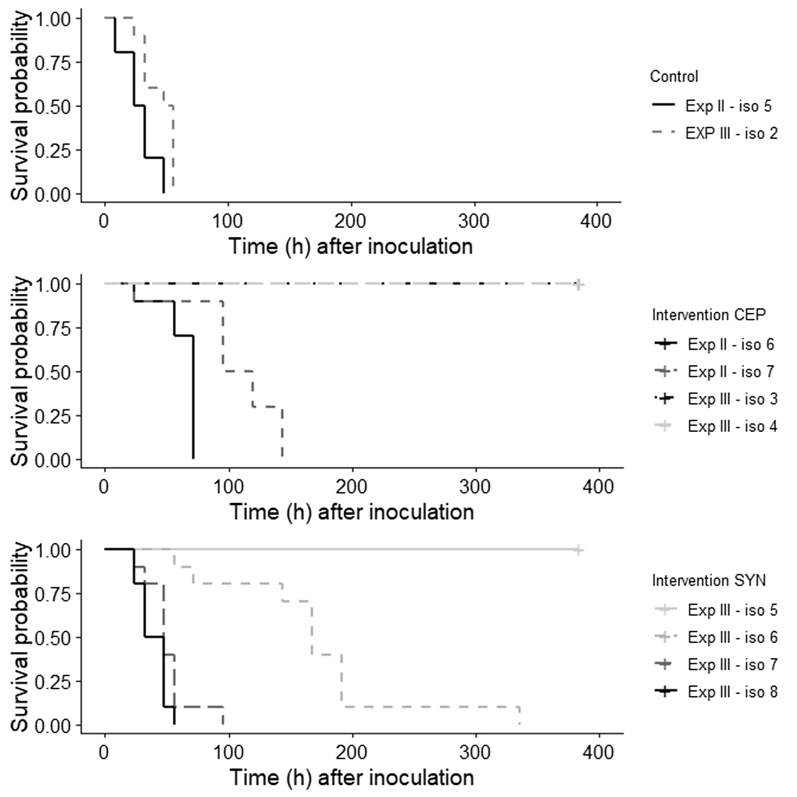
Experiment 2: CTX-M-1-E. coli Challenge with 101 or 102 cfu/mL at Day 5
Broilers challenged with 101 cfu/mL CTX-M-1-E. coli in both the control and the CEP groups were not colonized throughout the entire experiment. All broilers challenged with 102 cfu/mL CTX-M-1-E. coli were colonized within 48 (control) or 144 h (CEP) after inoculation (Figure 1 and Table 3). CEP broilers had a lower HR (HR isolator 6: 0.08, 95% CI 0.02–0.42 and isolator 7: 3.71 × 10−3, 95% CI 2.71 × 10−4 to 0.05) than the control isolator (Table 2). Factors seeder or contact bird, weight on the day of hatch, weight at day 21, sex, and the total E. coli excretion (log10 cfu/g feces) just before inoculation (day 5) did not influence the time until colonization.
Figure 1.
Survival curve of time until colonization of CTX-M-1-Escherichia coli for experiments 2 and 3, after challenge at day 5 with dose 102 cfu/mL. Abbreviations: CEP, competitive exclusion product; Exp, experiment; SYN, selection of pre- and probiotics.
Table 3.
Detection (+/−) and quantification (log10 cfu/g feces) of CTX-M-1-Escherichia coli in broilers in experiments 2 (dose 102 cfu/mL) and 3, determined at n hours p.i. at days 5 to 7 (8:00 am and 4:00 pm), 8 to 14, 16, 19, and 21 (8:00 am).
| Exp | Iso | Dose | Intervention | Bird ID | Seeder or contact | D5 8:00 | D5 4:00 | D6 8:00 | D6 4:00 | D7 8:00 | D7 4:00 | D8 8:00 | D9 8:00 | D10 8:00 | D11 8:00 | D12 8:00 | D13 8:00 | D14 8:00 | D16 8:00 | D19 8:00 | D21 8:00 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| hours p.i. | 0 | 8 | 24 | 32 | 48 | 56 | 72 | 96 | 120 | 144 | 168 | 192 | 216 | 264 | 336 | 384 | |||||
| 2 | 5 | 102 | None | 205 | Seeder | − | + | 3.63 | + | 5.48 | + | 5.78 | 3.57 | 6.52 | 6.63 | 7.57 | 5.44 | 6.40 | 3.88 | 5.57 | 5.25 |
| 5 | 102 | None | 212 | Seeder | − | − | 2.57 | + | 4.63 | + | 5.57 | 4.52 | 4.48 | 4.63 | 2.57 | 4.57 | 5.36 | 4.52 | 4.63 | 4.70 | |
| 5 | 102 | None | 275 | Seeder | − | − | 3.52 | + | 4.63 | + | 2.57 | 5.78 | 3.63 | 3.52 | 4.57 | 4.30 | 5.05 | 3.52 | 6.78 | 4.30 | |
| 5 | 102 | None | 289 | Seeder | − | − | 2.63 | + | 4.52 | + | 3.48 | 3.44 | 3.78 | 2.48 | 3.00 | 2.44 | 2.52 | 4.70 | 4.78 | 6.36 | |
| 5 | 102 | None | 296 | Seeder | + | − | − | + | 3.63 | + | 3.63 | 5.40 | 6.52 | 3.57 | 4.57 | 5.52 | 3.48 | 6.63 | 4.70 | 5.22 | |
| 5 | 102 | None | 226 | Contact | − | − | − | + | 2.48 | + | 3.57 | 5.88 | 5.70 | 4.63 | 7.88 | 4.36 | 4.27 | 4.57 | 4.48 | 5.12 | |
| 5 | 102 | None | 233 | Contact | − | − | − | − | 2.33 | + | 2.52 | 2.40 | 2.78 | 2.52 | 5.52 | 3.40 | 4.70 | 4.63 | 6.57 | 6.13 | |
| 5 | 102 | None | 240 | Contact | − | − | − | + | 2.70 | + | 3.52 | 2.70 | 2.48 | 2.70 | 3.27 | 2.63 | 2.57 | 3.57 | 4.57 | 4.27 | |
| 5 | 102 | None | 261 | Contact | − | − | 2.63 | − | 2.44 | + | 2.33 | 2.48 | 3.52 | 3.44 | 2.27 | 2.63 | 3.44 | 6.57 | 5.44 | 4.20 | |
| 5 | 102 | None | 282 | Contact | − | + | 2.63 | + | 4.30 | + | 5.57 | 7.44 | 6.48 | 4.70 | 4.78 | 5.10 | 3.63 | 4.78 | 4.44 | 7.52 | |
| 6 | 102 | CEP | 220 | Seeder | − | − | − | − | − | − | 2.88 | 2.48 | 4.57 | 3.70 | 5.57 | 4.78 | 2.52 | 4.63 | 4.57 | 3.63 | |
| 6 | 102 | CEP | 234 | Seeder | − | − | 2.27 | + | 2.40 | + | 3.36 | 4.40 | 5.52 | 5.78 | 3.78 | 3.30 | 3.40 | 5.52 | 4.48 | 5.40 | |
| 6 | 102 | CEP | 241 | Seeder | − | − | − | − | 2.44 | − | 2.48 | 2.40 | 3.70 | 4.63 | 5.63 | 4.48 | 2.57 | 2.63 | 2.52 | 3.40 | |
| 6 | 102 | CEP | 269 | Seeder | − | − | − | − | − | + | 2.44 | 2.57 | 2.63 | 2.52 | 3.63 | 2.70 | 3.44 | 2.48 | 2.57 | 2.48 | |
| 6 | 102 | CEP | 290 | Seeder | − | − | − | − | − | − | 2.44 | 2.36 | 2.57 | 2.48 | 3.52 | 2.52 | 2.63 | 2.48 | 3.63 | 3.33 | |
| 6 | 102 | CEP | 206 | Contact | − | − | − | − | − | − | 2.63 | 3.52 | 4.57 | 4.00 | 5.70 | 2.52 | −2 | ||||
| 6 | 102 | CEP | 227 | Contact | − | − | − | − | − | − | 2.52 | 2.52 | 2.57 | 3.88 | 3.63 | 3.48 | 4.52 | 3.57 | 4.52 | 4.27 | |
| 6 | 102 | CEP | 248 | Contact | − | − | − | − | − | − | 2.70 | 2.57 | 2.48 | 3.70 | 5.63 | 4.78 | 6.57 | 3.57 | 4.48 | 5.70 | |
| 6 | 102 | CEP | 255 | Contact | − | − | − | − | − | − | 2.63 | 2.40 | 3.40 | 3.63 | 2.57 | 3.95 | 3.22 | 3.57 | 3.48 | 3.18 | |
| 6 | 102 | CEP | 276 | Contact | − | + | − | − | − | + | 2.52 | 2.52 | 3.48 | 4.52 | 5.44 | 2.70 | 3.78 | 5.52 | 5.48 | 5.57 | |
| 7 | 102 | CEP | 207 | Seeder | − | − | 2.78 | + | 3.63 | + | 2.57 | 2.52 | 3.57 | 4.63 | 3.70 | 3.63 | 5.88 | 4.78 | 4.57 | 4.33 | |
| 7 | 102 | CEP | 242 | Seeder | − | + | − | − | − | − | − | − | 4.00 | 2.78 | 3.78 | 4.63 | 3.63 | 5.00 | 4.36 | 3.44 | |
| 7 | 102 | CEP | 277 | Seeder | − | − | − | − | − | − | − | − | − | 2.78 | 2.70 | 2.57 | 3.63 | 3.57 | 3.48 | 4.30 | |
| 7 | 102 | CEP | 291 | Seeder | − | − | − | − | − | − | − | − | − | 2.57 | 2.78 | 2.40 | 2.40 | 3.63 | 3.63 | 4.33 | |
| 7 | 102 | CEP | 298 | Seeder | − | − | − | − | − | − | − | − | − | 2.63 | 5.57 | 4.88 | 4.63 | 5.70 | 3.63 | 4.44 | |
| 7 | 102 | CEP | 214 | Contact | − | − | − | − | − | − | − | 3.44 | 3.63 | 4.63 | 4.63 | 4.36 | 3.57 | 3.48 | 4.57 | 4.27 | |
| 7 | 102 | CEP | 235 | Contact | − | − | − | − | − | − | − | 2.48 | 2.78 | 2.88 | 2.88 | 2.48 | 3.57 | 3.52 | 4.48 | 4.44 | |
| 7 | 102 | CEP | 256 | Contact | − | − | − | − | − | − | − | 2.48 | 2.63 | 2.52 | 3.63 | 3.52 | 2.30 | 4.52 | 4.44 | 5.25 | |
| 7 | 102 | CEP | 263 | Contact | − | − | − | − | − | − | − | 2.57 | 2.52 | 2.70 | 4.33 | 3.30 | 3.36 | 3.70 | 2.44 | 2.40 | |
| 7 | 102 | CEP | 284 | Contact | − | − | − | − | − | − | − | − | 2.63 | 3.48 | 3.57 | 5.57 | 2.70 | 3.78 | 3.88 | 3.25 | |
| 3 | 2 | 102 | None | 309 | Seeder | − | − | − | + | 2.30 | + | 2.52 | 2.52 | 4.40 | 4.48 | 3.36 | 4.27 | 4.25 | 3.57 | 3.22 | 5.27 |
| 2 | 102 | None | 333 | Seeder | − | − | − | + | 3.25 | + | 4.57 | 4.48 | 3.57 | 5.44 | 5.25 | 5.40 | 7.25 | 5.48 | 6.03 | 5.06 | |
| 2 | 102 | None | 341 | Seeder | − | − | 3.70 | + | 4.22 | + | 5.48 | 2.48 | 5.57 | 4.33 | 4.27 | 4.36 | 5.57 | 4.52 | 5.18 | 6.05 | |
| 2 | 102 | None | 349 | Seeder | − | − | − | + | 2.70 | + | 3.57 | 3.48 | 5.48 | 4.15 | 6.25 | 5.15 | 3.36 | 4.48 | 5.18 | 5.33 | |
| 2 | 102 | None | 373 | Seeder | − | − | − | − | − | + | 2.36 | 4.52 | 4.15 | 5.48 | 5.30 | 3.57 | 5.20 | 5.52 | 5.25 | 5.33 | |
| 2 | 102 | None | 317 | Contact | − | − | − | − | − | + | 3.57 | 4.40 | 4.70 | 7.30 | 4.33 | 5.52 | 4.63 | 4.63 | 5.12 | 6.06 | |
| 2 | 102 | None | 325 | Contact | − | − | − | − | 2.13 | + | 2.52 | 3.44 | 5.44 | 6.13 | 5.36 | 5.27 | 5.25 | 4.57 | 4.27 | 7.20 | |
| 2 | 102 | None | 357 | Contact | − | − | − | − | − | + | 3.48 | 4.57 | 4.57 | 5.33 | 6.18 | 7.20 | 6.33 | 4.88 | 5.36 | 6.33 | |
| 2 | 102 | None | 365 | Contact | − | − | − | − | − | + | 4.40 | 3.52 | 4.52 | 7.40 | 4.30 | 6.00 | 6.22 | 4.57 | 4.25 | 5.84 | |
| 2 | 102 | None | 381 | Contact | − | − | − | − | − | + | 2.57 | 4.52 | 4.70 | 4.22 | 5.18 | 4.57 | 4.27 | 5.63 | 4.44 | 5.40 | |
| 6 | 102 | SYN | 305 | Seeder | − | − | − | − | − | − | 2.33 | 2.70 | 2.40 | 2.13 | 5.15 | 5.33 | 6.40 | 3.52 | 4.36 | 5.48 | |
| 6 | 102 | SYN | 329 | Seeder | − | − | − | − | − | − | − | − | − | − | − | 2.48 | 2.27 | 2.57 | 3.06 | 5.40 | |
| 6 | 102 | SYN | 337 | Seeder | − | − | − | − | − | − | − | − | − | − | − | − | 2.48 | − | 2.33 | 4.48 | |
| 6 | 102 | SYN | 353 | Seeder | − | − | − | − | − | − | − | − | − | − | 2.01 | 2.12 | 2.36 | 2.52 | 4.20 | 4.57 | |
| 6 | 102 | SYN | 361 | Seeder | − | − | − | − | − | + | 2.36 | − | 2.52 | − | 2.57 | 2.25 | 2.13 | 2.78 | 4.15 | 5.36 | |
| 6 | 102 | SYN | 313 | Contact | − | − | − | − | − | − | − | − | − | 1.85 | 2.15 | 2.40 | 2.15 | 2.70 | 2.15 | 3.36 | |
| 6 | 102 | SYN | 321 | Contact | − | − | − | − | − | − | − | 2.52 | − | − | − | 3.00 | 2.06 | 4.00 | 4.13 | 3.48 | |
| 6 | 102 | SYN | 369 | Contact | − | − | − | − | − | − | − | − | − | − | − | 2.36 | 3.00 | 3.57 | 3.13 | 3.48 | |
| 6 | 102 | SYN | 377 | Contact | − | − | − | − | − | − | − | − | − | − | 2.57 | 2.40 | 3.18 | 2.63 | 2.48 | 4.30 | |
| 6 | 102 | SYN | 385 | Contact | − | − | − | − | − | − | − | − | − | − | 2.48 | 2.33 | 4.57 | 4.70 | 3.33 | 4.63 | |
| 7 | 102 | SYN | 314 | Seeder | − | − | 2.01 | + | 2.40 | + | 4.20 | 2.36 | 3.25 | 4.06 | 4.30 | 3.70 | 4.12 | +1 | 4.25 | 3.33 | |
| 7 | 102 | SYN | 338 | Seeder | − | − | − | + | 4.48 | + | 6.40 | 4.44 | 4.44 | 4.52 | 3.57 | 4.63 | 3.70 | 3.63 | 5.12 | 4.20 | |
| 7 | 102 | SYN | 354 | Seeder | − | − | − | − | − | + | − | 3.57 | 2.30 | 5.18 | 4.12 | 4.36 | 3.44 | 3.63 | 6.27 | 4.52 | |
| 7 | 102 | SYN | 370 | Seeder | − | − | − | − | − | + | 3.27 | 4.57 | 3.48 | 3.27 | 3.12 | 3.36 | 3.40 | − | 6.44 | 3.44 | |
| 7 | 102 | SYN | 394 | Seeder | − | − | − | − | 2.57 | + | 3.48 | 3.63 | 4.20 | 4.13 | 4.33 | 4.25 | 5.88 | 4.88 | 3.03 | 4.25 | |
| 7 | 102 | SYN | 322 | Contact | − | − | − | − | 3.63 | + | 3.48 | 3.57 | 4.30 | 4.30 | 5.52 | 5.30 | 4.52 | 4.78 | 5.20 | 5.52 | |
| 7 | 102 | SYN | 330 | Contact | − | − | − | − | 3.44 | + | 2.27 | 3.57 | 4.44 | 3.70 | 4.20 | 5.44 | 5.25 | 4.48 | 5.36 | 4.30 | |
| 7 | 102 | SYN | 362 | Contact | − | − | − | − | 2.33 | + | 2.48 | 2.78 | 5.05 | 5.22 | 3.33 | 6.27 | 4.70 | 3.70 | 3.90 | 4.15 | |
| 7 | 102 | SYN | 378 | Contact | − | − | − | − | − | + | 3.27 | 3.70 | 2.95 | 4.33 | 5.57 | 5.22 | 6.44 | 5.70 | 6.36 | 6.70 | |
| 7 | 102 | SYN | 386 | Contact | − | − | − | − | − | + | 3.27 | 3.44 | +1 | 5.18 | 4.05 | 4.30 | 4.44 | 3.70 | 4.12 | 5.44 | |
| 8 | 102 | SYN | 315 | Seeder | − | − | 2.52 | + | 4.57 | + | 3.33 | 3.52 | 5.48 | 2.91 | 3.85 | 3.40 | 3.18 | 2.44 | 3.00 | 2.78 | |
| 8 | 102 | SYN | 347 | Seeder | − | − | 2.57 | + | 2.52 | + | 3.44 | 4.25 | 4.57 | 3.33 | +1 | 3.52 | 3.52 | 2.40 | 2.44 | 4.57 | |
| 8 | 102 | SYN | 355 | Seeder | − | − | − | + | 5.36 | + | 5.44 | 6.12 | 4.27 | 3.22 | 4.30 | 3.10 | 4.12 | 2.52 | 3.40 | 3.15 | |
| 8 | 102 | SYN | 363 | Seeder | − | − | − | + | 4.36 | + | 3.48 | 6.40 | 4.88 | 3.70 | 4.63 | 4.48 | 3.63 | 2.78 | 2.44 | 3.52 | |
| 8 | 102 | SYN | 379 | Seeder | − | − | − | + | 4.25 | + | 3.70 | 4.10 | 3.63 | 4.12 | 3.33 | 4.18 | 4.48 | 2.63 | 2.27 | 3.25 | |
| 8 | 102 | SYN | 307 | Contact | − | − | − | − | − | + | 4.00 | 2.15 | 3.40 | 6.27 | 3.33 | 3.08 | 3.44 | 2.70 | +1 | 3.40 | |
| 8 | 102 | SYN | 323 | Contact | − | − | − | − | 2.48 | + | 2.30 | 2.99 | 3.48 | 3.48 | 4.30 | 4.27 | 3.57 | 3.88 | 3.52 | 4.36 | |
| 8 | 102 | SYN | 331 | Contact | − | − | − | − | 2.25 | + | 4.63 | 4.44 | 2.63 | 4.22 | 3.33 | 4.40 | 4.48 | − | 3.20 | 4.52 | |
| 8 | 102 | SYN | 387 | Contact | − | − | − | − | 2.40 | + | 2.78 | 3.63 | 4.44 | 4.27 | 3.36 | 3.27 | 2.52 | 2.70 | 3.13 | 5.57 | |
| 8 | 102 | SYN | 395 | Contact | − | − | − | − | 2.18 | + | 3.48 | 4.36 | 4.40 | 4.36 | 3.40 | 4.20 | 4.44 | 3.52 | 3.06 | 3.70 | |
Abbreviations: CEP, competitive exclusion product; Exp, experiment; Iso, isolator; p.i., post inoculation; SYN, selection of pre- and probiotics.
+ in quantification series are broilers excreting CTX-M-1-E. coli (i.e., growth of E. coli on MacConkey + cefotaxime), but excretion values were missing.
Chick died.
Experiment 3: CTX-M-1-E. coli Challenge with 102 cfu/mL at Day 5
Broilers treated with CEP were not colonized with CTX-M-1-E. coli during the experiment, whereas the broilers in 2 control isolators were colonized within 56 h after inoculation. The broilers in one of the SYN isolators (isolator 5) were not colonized, the broilers in the other 3 SYN isolators were all colonized within 336 h after inoculation (Figure 1 and Table 3). Although one of the SYN isolators showed a lower HR than the control isolator (isolator 6, HR 0.07, 95% CI 0.01–0.39), for the broilers in the other isolators there was no effect of SYN on time until colonization (HR isolator 7: 1.29, 95% CI 0.39–4.28 and HR isolator 8: 3.11, 95% CI 0.97–10.05) (Table 2). Weight on the day of hatch, weight at day 21, and total E. coli excretion just before inoculation (day 5) did not influence time until colonization. However, contact birds had a lower HR (HR 0.43, 95% CI 0.20–0.94) than seeder birds. The variable sex was not analyzed, as only female broilers were included in experiment 3.
Excretion
Experiment 1: Excretion of CTX-M-1-E. coli and Total E. coli
The effect of the CEP product on both total E. coli and CTX-M-1-E. coli excretion differed per time point. Female birds excreted slightly higher concentrations of CTX-M-1-E. coli (0.23, 95% CI 0.03–0.43 log10 cfu/g feces) than male birds, and broilers challenged with either 101 or 102 cfu/mL CTX-M-E. coli excreted slightly lower concentrations of total E. coli than non-inoculated broilers (−0.81, 95% CI −1.14 to −0.48 vs. −0.85, 95% CI −1.19 to −0.51 log10 cfu/g feces, Supplementary Table 2). Concentrations of CTX-M-1-E. coli in cecal content were lower in CEP broilers than control broilers (−0.71, 95% CI −1.06 to −0.37 log10 cfu/g cecal content) and higher in broilers receiving dose 102 than dose 101 (0.46, 95% CI 0.14–0.79 log10 cfu/g cecal content). Total E. coli concentrations in cecal content were slightly lower in CEP broilers than control broilers (−0.36, 95% CI −0.63 to −0.08 log10 cfu/g cecal content, Supplementary Table 3).
Experiment 2: Excretion of CTX-M-1-E. coli and Total E. coli
Broilers challenged with 101 cfu/mL CTX-M-1-E. coli did not excrete CTX-M-1-E. coli during the experiment. CEP broilers challenged with 102 cfu/mL excreted lower concentrations of CTX-M-1-E. coli (−0.89, 95% CI −1.33 to −0.45 log10 cfu/g feces) than control broilers. Female birds excreted slightly higher concentrations of CTX-M-1-E. coli (0.48, 95% CI 0.04–0.92 log10 cfu/g feces) than male birds. CEP broilers excreted lower or equal concentrations of E. coli than control broilers, except at day 1, but without a clear pattern (Supplementary Table 2). Mean concentrations of total E. coli and CTX-M-1-E. coli in cecal content were lower in CEP broilers than control broilers (−0.51, 95% CI −0.79 to −0.22, vs. −2.80, 95% CI −3.47 to −2.14 log10 cfu/g cecal content, Supplementary Table 3).
Experiment 3: Excretion of CTX-M-1-E. coli and Total E. coli
CEP broilers did not excrete CTX-M-1-E. coli. SYN broilers excreted lower concentrations of CTX-M-1-E. coli than control broilers from day 10 onward. Total E. coli excretion concentrations in CEP and SYN broilers were lower than or equal to the control broilers, except at day 1; however, the excretion per day was highly variable without a clear pattern (Supplementary Table 2). The concentrations of CTX-M-1-E. coli in cecal content of SYN broilers were lower (−1.13, 95% CI −1.94 to −0.33 log10/g cecal content) compared to the control broilers. Total E. coli concentrations were lower in CEP broilers than in control broilers (−1.50, 95% CI −1.76 to −1.24 log10/g cecal content, Supplementary Table 3).
Transmission
The transmission coefficients (βdirect, βtime, and βconcentration) were estimated using the data of experiments 2 and 3. These could not be estimated from experiment 1 because most broilers (seeder and contact) in the control and CEP isolators were colonized already at the first sampling moment (16 h) after inoculation. Also, estimation of the transmission coefficients in the CEP groups in experiment 3 was not possible, because the inoculation did not lead to colonization in the CEP groups.
Transmission coefficients (βdirect, βtime, and βconcentration), estimated using the assumptions in the alternative model (assuming that I-birds start excreting half an interval before the first positive cloaca swab, having slightly lower AIC values than the basic model), were lower in both intervention groups than in the control groups, based on model 2 (βtime: CEP: 0.19 day−2, 95% CI 0.04–0.87, SYN: 0.09 day−2, 95% CI 0.02–0.40, control: 0.27 day−2, 95% CI 0.13–0.49) and model 3 (βconcentration: CEP: 0.12 [cfu × day]−1, 95% CI 0.15–0.56, SYN: 0.14 [cfu × day]−1, 95% CI 0.02–0.63, control: 0.31 [cfu × day]−1, 95% CI 0.10–0.57). The transmission coefficients (βdirect, day−1) estimated based on model 1 were not different (βdirect: CEP: 2.57 day−1, 95% CI 0.51–11.47, SYN: 1.58 day−1, 95% CI 0.35–6.57, control: 2.19 day−1, 95% CI 1.09–3.91) (Table 4). The unit of β in model 2 is day−2 and can be interpreted as the number of new colonized broilers caused by a positive broiler per day, for each day this broiler has been excreting CTX-M-1-E. coli. The unit of β in model 3 is (cfu × day)−1 and can be interpreted as the number of new colonized broilers caused by a positive broiler per day, for each excreted unit of log10 CTX-M-1-E. coli per g of feces. In addition, a second alternative model was tested including the assumption that I-birds that were not colonized at 32 h after inoculation were S-birds. However, this assumption did not improve the fit of the model (data not shown).
Table 4.
Transmission coefficients (β, 95% CI) for experiments 2 and 3, using an SI model, for the basic model (assuming I-birds start excreting at the moment of the first positive cloaca swab) and the alternative model (assuming I-birds start excreting half an interval before the first positive cloaca swab).
| Transmission coefficient (β, 95% CI) | ||||||
|---|---|---|---|---|---|---|
| Basic model |
Alternative model |
|||||
| Model 1 |
Model 2 |
Model 3 |
Model 1 |
Model 2 |
Model 3 |
|
| (day−1) | (day−2) | (cfu × day)−1 | (day−1) | (day−2) | (cfu × day)−1 | |
| Control | 2.93 (1.38–5.40) | 0.40 (0.19–0.76) | 0.31 (0.10–0.57) | 2.19 (1.09–3.91) | 0.27 (0.13–0.49) | 0.31 (0.10–0.57) |
| CEP | 4.08 (0.76–19.43) | 0.30 (0.05–1.48) | 0.12 (0.15–0.56)∗ | 2.57 (0.51–11.47) | 0.19 (0.04–0.87) | 0.12 (0.15–0.56)∗ |
| SYN | 2.22 (0.46–9.96) | 0.12 (0.02–0.57)∗ | 0.14 (0.02–0.63)∗∗ | 1.58 (0.35–6.57) | 0.09 (0.02–0.40)∗ | 0.14 (0.02–0.63)∗∗ |
| AIC | 82.5 | 87.8 | 102.0 | 78.7 | 86.8 | 102.0 |
Expected number of cases (C) in model 1: , model 2: and model 3: In model 3, cumulative excretion (cfu/g feces) is independent of the number of I-birds and is therefore independent of the assumption regarding the start of excretion.
Statistically different transmission coefficients compared to the control group: ∗P < 0.05, ∗∗P < 0.10.
Abbreviations: AIC, Akaike Information Criterion; CEP, competitive exclusion product; I-birds, infectious birds; SI model, susceptible infectious model; SYN, selection of pre- and probiotics.
Performance
There was no effect of CEP on growth (experiments 1, 2, and 3). In experiment 3, SYN broilers had higher growth (from day of hatch until day 21) than control broilers (1021.1, 95% CI 914.1–1128.0 g vs. 914.8, 95% CI 866.5–963.1 g). However, this effect was mainly explained by the higher growth of broilers in one of the SYN isolators (isolator 7, mean growth 1070.0, 95% CI 884.9–1228.1 g).
Discussion
The supply of CE products to broilers during the first 2 wk of life resulted in an increased time until colonization and lower excretion of CTX-M-1-E. coli and even in the prevention of colonization of broilers challenged with a low dose of CTX-M-1-E. coli at day 5. Moreover, transmission rates of CTX-M-1-E. coli were lower in the broilers receiving one of the CE products (CEP or SYN) than in the control broilers. In contrast, the supply of CE products when challenged on the day of hatch did not affect colonization. Our results show that a prolonged supply of CE products can be a useful intervention to prevent or reduce colonization of ESBL/pAmpC-producing E. coli in a broiler flock, when exposure occurs after supply of CE products. These results are in line with earlier studies showing a reduction in transmission, colonization, and excretion of Salmonella (Nakamura et al., 2002, Ferreira et al., 2003, Luoma et al., 2017, Markazi et al., 2018), pathogenic E. coli (Hofacre et al., 2002), and ESBL/pAmpC-producing E. coli (Nuotio et al., 2013, Ceccarelli et al., 2017, Methner et al., 2019), when providing CE products before challenge. Moreover, in our study we were able to prevent colonization of CTX-M-1-E. coli, possibly as a result of the prolonged supply of CE products, whereas in earlier studies a single supply of CE products did not result in the prevention of colonization of a group of birds (Hofacre et al., 2002, Nuotio et al., 2013, Ceccarelli et al., 2017, Methner et al., 2019). In contrast to our study, in the studies of Nuotio et al. (2013) and Ceccarelli et al. (2017) broilers were exposed to high concentrations of ESBL-producing E. coli, whereas we used a low dose aiming to mimic the initial stages of colonization of a flock in the field. A prolonged supply of CE product followed by exposure to lower concentrations of EBSL-producing E. coli might give more potential for the bacteria in the CE products, and less potential for the ESBL/pAmpC-producing E. coli, to colonize.
Challenge with dose 101 at day 5 in experiment 2 did not result in colonization of CTX-M-1-E. coli in the control and intervention groups, although the results of experiment 1 and earlier studies showed that with this low dose young broilers could colonize (Dame-Korevaar et al., 2019). However, in this earlier study broilers were challenged at day 1, whereas we challenged them at day 5, simulating exposure to ESBL/pAmpC-producing E. coli during the first week at the farm. This age effect suggests that susceptibility to colonization is reduced with age (Chauvin et al., 2013, Braykov et al., 2016). Although we did not analyze microbiota composition in this study, it is likely that the gut microbiome composition might have played a role, as different successive stages in microbiome development (Jurburg et al., 2019) may also result in different stages of susceptibility to colonization with certain bacteria. Analysis of the microbiome would require experiments with intensive sampling of intestinal content for comparisons of the changes in microbiota composition in intervention and control groups, to facilitate understanding of the underlying mechanisms behind the differences in the observed time until colonization. However, due to the different factors influencing microbiota composition (Kers et al., 2018), many broilers would need to be tested to avoid spurious correlations.
The difference in the HR of colonization between CEP and SYN groups compared to the control groups might be caused by the composition of the products. Both products are aimed at establishing CE, but CEP contains natural, live, fermented intestinal microflora from SPF chickens, whereas SYN contains a prebiotic compound (fructo-oligosaccharides) and probiotic bacterial strains (E. faecium, B. animalis, and L. salivarius). In our study the total concentrations of E. coli at day 5, just before inoculation, did not influence the time until colonization. Therefore, the protective effects of the CE products might not be competition between the different E. coli strains (initially present, inoculated, and in the supplied intervention), but may do so between other (combinations of) supplied bacteria. Moreover, different mechanisms might have played a role: not only direct CE between specific bacteria, including competition for specific niches or nutrients (Callaway et al., 2008), but also more complex indirect host–microbe interactions, for example immune responses (Lawley and Walker, 2013). It is likely that the 2 CE products may have affected the gut microbiota composition in different ways, but to what extent and how this may have affected colonization of ESBL/pAmpC-E. coli in the intestinal tract cannot be elucidated with the data available from these experiments.
Some of the observed differences between isolators can also be a result of the so-called “cage effect”; animals housed together tend to show less variation in microbiota composition than a random group of animals, as described for mice (Laukens et al., 2016), which might result in differences in susceptibility to colonization between groups. Furthermore, other host and environmental factors can affect the microbiota composition and can influence experimental outcomes, as reviewed by Kers et al. (2018). Although we cannot exclude such effects completely, the experimental design was aimed to keep the impact of potential confounding factors to a minimum. All broilers originated from the same flock, were handled in the same way, and the isolators were intensively cleaned and disinfected before the start of the experiment.
The supply of CE products did not affect the time until colonization when provided at the same time as the ESBL-producing E. coli challenge (day of hatch, experiment 1). This is in line with earlier studies (Ceccarelli et al., 2017), showing that the effect of CE depends on the time of supply (Varmuzova et al., 2016), and indicates that the CE products need time to be established in the gut, before they can protect broilers from colonization with low doses of ESBL-producing E. coli that may be present at the farm, for example due to insufficient cleaning and disinfection, via parallel-housed flocks, or from the environment (Dame-Korevaar et al., 2019).
The prevention of colonization (experiments 2 and 3), and the quick colonization of one seeder bird in both isolators in experiment 2 followed by colonization of the remaining seeder birds and the contact birds suggest that the effect of CE upon low-dose exposure mainly lies in the prevention of colonization, rather than substantially affecting transmission. Nevertheless, transmission rates were lower in the intervention groups than in the control groups, according to model 2 and 3. We did not find this reduction when assuming direct transmission. Model 1 did have the lowest AIC value, but from biological reasoning environmental transmission should be a better model. ESBL/pAmpC-producing E. coli can survive in the environment for months (Merchant et al., 2012, Friese et al., 2013); therefore, the presence of ESBL/pAmpC-producing E. coli in the litter will facilitate transmission via the fecal–oral route, as described for Eimeria acervulina (Velkers et al., 2012). Thus, the accumulation of E. coli in the environment should be taken into account, as is done in model 2, with the force of infection based on excretion time of infectious broilers. We suggest using this model for generalization to larger populations, as it best describes the biological mechanisms of transmission of ESBL/pAmpC-producing E. coli. Model 3, with the force of infection based on excretion concentrations, did not improve the fit of the model. However, in both models including the environment CE products reduces the transmission coefficients.
The colonization of ESBL-producing E. coli in the broilers' intestinal tract as observed in our experiments is likely a result of both vertical and horizontal (via conjugation) transfer of the plasmids present in the inoculum E. coli to other E. coli strains, as was suggested in earlier experiments (Dame-Korevaar et al., 2019). This reflects the transmission dynamics of ESBL/pAmpC-producing E. coli in field situations (Huijbers et al., 2016, van Hoek et al., 2018), where horizontal gene transfer occurs naturally and is part of the transmission process.
In conclusion, CE products can prevent and reduce initial colonization, but even if only 1 bird is successfully colonized and starts to excrete ESBL/pAmpC-producing E. coli, the subsequent spread through the flock is inevitable. Therefore, additional interventions are needed to reduce transmission. CE products need time to get established in the gut, and therefore should be applied as soon as possible after hatch, before broilers are exposed to ESBL/pAmpC-producing E. coli. Further studies are recommended on the mechanisms behind the dynamical processes in the gut responsible for the CE effects, and to determine the best timing and type of bacterial composition manipulations to optimize these intervention strategies for practical use.
Acknowledgments
This work was funded by the 1 Health 4 Food Public Private Partnership project: Reduction of ESBLs: evaluation of ESBL interventions (grant number TKI-AF-14210). We thank Ineke Daemen and Mirlin Spaninks (Utrecht University) and Joop Testerink (Wageningen Bioveterinary Research) for their technical assistance in the microbial analysis, Kees Veldman (Wageningen Bioveterinary Research) for insightful discussions on microbial techniques, the animal caretakers and Nathan den Uil (Utrecht University) for their assistance during the animal trials, and the poultry facility for supplying the broilers.
Conflict of Interest Statement: Daniela Ceccarelli is currently employed by the Research Executive Agency. The authors have no conflict of interest to declare.
Footnotes
Supplementary data associated with this article can be found in the online version at http://doi.org/10.1016/j.psj.2020.04.025.
Supplementary data
References
- Apostolakos I., Mughini-Gras L., Fasolato L., Piccirillo A. Assessing the occurrence and transfer dynamics of ESBL/pAmpC-producing Escherichia coli across the broiler production pyramid. PLoS One. 2019;14:e0217174. doi: 10.1371/journal.pone.0217174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaak H., van Hoek A.H., Hamidjaja R.A., van der Plaats R.Q., Kerkhof-de Heer L., de Roda Husman A.M., Schets F.M. Distribution, numbers, and Diversity of ESBL-producing E. coli in the poultry farm environment. PLoS One. 2015;10:e0135402. doi: 10.1371/journal.pone.0135402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braykov N.P., Eisenberg J.N., Grossman M., Zhang L., Vasco K., Cevallos W., Munoz D., Acevedo A., Moser K.A., Marrs C.F., Foxman B., Trostle J., Trueba G., Levy K. Antibiotic resistance in animal and environmental samples associated with small-scale poultry farming in Northwestern Ecuador. mSphere. 2016;1:21. doi: 10.1128/mSphere.00021-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway T.R., Edrington T.S., Anderson R.C., Harvey R.B., Genovese K.J., Kennedy C.N., Venn D.W., Nisbet D.J. Probiotics, prebiotics and competitive exclusion for prophylaxis against bacterial disease. Anim. Health Res. Rev. 2008;9:217–225. doi: 10.1017/S1466252308001540. [DOI] [PubMed] [Google Scholar]
- Ceccarelli D., van Essen-Zandbergen A., Smid B., Veldman K.T., Boender G.J., Fischer E.A.J., Mevius D.J., van der Goot J.A. Competitive exclusion reduces transmission and excretion of extended-spectrum-beta-lactamase-producing Escherichia coli in broilers. Appl. Environ. Microbiol. 2017;83:3439. doi: 10.1128/AEM.03439-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvin C., Le Devendec L., Jouy E., Le Cornec M., Francart S., Marois-Crehan C., Kempf I. National prevalence of resistance to third-generation cephalosporins in Escherichia coli isolates from layer flocks in France. Antimicrob. Agents Chemother. 2013;57:6351–6353. doi: 10.1128/AAC.01460-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daehre K., Projahn M., Semmler T., Roesler U., Friese A. Extended-spectrum beta-lactamase-/AmpC beta-lactamase-producing Enterobacteriaceae in broiler farms: transmission dynamics at farm level. Microb. Drug Resist. 2018;24:511–518. doi: 10.1089/mdr.2017.0150. [DOI] [PubMed] [Google Scholar]
- Dame-Korevaar A., Fischer E.A.J., van der Goot J., Stegeman A., Mevius D. Transmission routes of ESBL/pAmpC producing bacteria in the broiler production pyramid, a literature review. Prev. Vet. Med. 2019;162:136–150. doi: 10.1016/j.prevetmed.2018.12.002. [DOI] [PubMed] [Google Scholar]
- Dekker N., Bouma A., Daemen I., Klinkenberg D., van Leengoed L., Wagenaar J.A., Stegeman A. Effect of spatial separation of pigs on spread of Streptococcus suis serotype 9. PLoS One. 2013;8:e61339. doi: 10.1371/journal.pone.0061339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierikx C., van Essen-Zandbergen A., Veldman K., Smith H., Mevius D. Increased detection of extended spectrum beta-lactamase producing Salmonella enterica and Escherichia coli isolates from poultry. Vet. Microbiol. 2010;145:273–278. doi: 10.1016/j.vetmic.2010.03.019. [DOI] [PubMed] [Google Scholar]
- Dierikx C.M., Van der Goot J.A., Smith H.E., Kant A., Mevius D.J. Presence of ESBL/AmpC -producing Escherichia coli in the broiler production pyramid: a descriptive study. PLoS ONE. 2013;8:e79005. doi: 10.1371/journal.pone.0079005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierikx C.M., van der Goot J., Fabri T., van Essen-Zandbergen A., Smith H., Mevius D. Extended-spectrum-beta-lactamase- and AmpC-beta-lactamase-producing Escherichia coli in Dutch broilers and broiler farmers. J. Antimicrob. Chemother. 2013;68:60–67. doi: 10.1093/jac/dks349. [DOI] [PubMed] [Google Scholar]
- Dorado-Garcia A., Mevius D.J., Jacobs J.J., Van Geijlswijk I.M., Mouton J.W., Wagenaar J.A., Heederik D.J. Quantitative assessment of antimicrobial resistance in livestock during the course of a nationwide antimicrobial use reduction in The Netherlands. J. Antimicrob. Chemother. 2016;71:3607–3619. doi: 10.1093/jac/dkw308. [DOI] [PubMed] [Google Scholar]
- Ferreira A.J., Ferreira C.S., Knobl T., Moreno A.M., Bacarro M.R., Chen M., Robach M., Mead G.C. Comparison of three commercial competitive-exclusion products for controlling Salmonella colonization of broilers in Brazil. J. Food Prot. 2003;66:490–492. doi: 10.4315/0362-028x-66.3.490. [DOI] [PubMed] [Google Scholar]
- Friese A., Schulz J., Laube H., von Salviati C., Hartung J., Roesler U. Faecal occurrence and emissions of livestock-associated methicillin-resistant Staphylococcus aureus (laMRSA) and ESbl/AmpC-producing E. coli from animal farms in Germany. Berl. Munch. Tierarztl. Wochenschr. 2013;126:175–180. [PubMed] [Google Scholar]
- Hesp A., Veldman K., van der Goot J., Mevius D., van Schaik G. Monitoring antimicrobial resistance trends in commensal Escherichia coli from livestock, The Netherlands, 1998 to 2016. Euro Surveill. 2019;24 doi: 10.2807/1560-7917.ES.2019.24.25.1800438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofacre C.L., Johnson A.C., Kelly B.J., Froyman R. Effect of a commercial competitive exclusion culture on reduction of colonization of an antibiotic-resistant pathogenic Escherichia coli in day-old broiler chickens. Avian Dis. 2002;46:198–202. doi: 10.1637/0005-2086(2002)046[0198:EOACCE]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Huijbers P.M., Graat E.A., van Hoek A.H., Veenman C., de Jong M.C., van Duijkeren E. Transmission dynamics of extended-spectrum beta-lactamase and AmpC beta-lactamase-producing Escherichia coli in a broiler flock without antibiotic use. Prev. Vet. Med. 2016;131:12–19. doi: 10.1016/j.prevetmed.2016.07.001. [DOI] [PubMed] [Google Scholar]
- Huijbers P.M., van Hoek A.H., Graat E.A., Haenen A.P., Florijn A., Hengeveld P.D., van Duijkeren E. Methicillin-resistant Staphylococcus aureus and extended-spectrum and AmpC beta-lactamase-producing Escherichia coli in broilers and in people living and/or working on organic broiler farms. Vet. Microbiol. 2015;176:120–125. doi: 10.1016/j.vetmic.2014.12.010. [DOI] [PubMed] [Google Scholar]
- Huijbers P.M., Graat E.A., Haenen A.P., van Santen M.G., van Essen-Zandbergen A., Mevius D.J., van Duijkeren E., van Hoek A.H. Extended-spectrum and AmpC beta-lactamase-producing Escherichia coli in broilers and people living and/or working on broiler farms: prevalence, risk factors and molecular characteristics. J. Antimicrob. Chemother. 2014;69:2669–2675. doi: 10.1093/jac/dku178. [DOI] [PubMed] [Google Scholar]
- Jett B.D., Hatter K.L., Huycke M.M., Gilmore M.S. Simplified agar plate method for quantifying viable bacteria. BioTechniques. 1997;23:648–650. doi: 10.2144/97234bm22. [DOI] [PubMed] [Google Scholar]
- Jurburg S.D., Brouwer M.S.M., Ceccarelli D., van der Goot J., Jansman A.J.M., Bossers A. Patterns of community assembly in the developing chicken microbiome reveal rapid primary succession. Microbiologyopen. 2019:e821. doi: 10.1002/mbo3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kers J.G., Velkers F.C., Fischer E.A.J., Hermes G.D.A., Stegeman J.A., Smidt H. Host and environmental factors affecting the intestinal microbiota in chickens. Front. Microbiol. 2018;9:235. doi: 10.3389/fmicb.2018.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laube H., Friese A., von Salviati C., Guerra B., Kasbohrer A., Kreienbrock L., Roesler U. Longitudinal monitoring of extended-spectrum-beta-lactamase/AmpC-producing Escherichia coli at German broiler chicken fattening farms. Appl. Environ. Microbiol. 2013;79:4815–4820. doi: 10.1128/AEM.00856-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laukens D., Brinkman B.M., Raes J., De Vos M., Vandenabeele P. Heterogeneity of the gut microbiome in mice: guidelines for optimizing experimental design. FEMS Microbiol. Rev. 2016;40:117–132. doi: 10.1093/femsre/fuv036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawley T.D., Walker A.W. Intestinal colonization resistance. Immunology. 2013;138:1–11. doi: 10.1111/j.1365-2567.2012.03616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luoma A., Markazi A., Shanmugasundaram R., Murugesan G.R., Mohnl M., Selvaraj R. Effect of synbiotic supplementation on layer production and cecal Salmonella load during a Salmonella challenge. Poult. Sci. 2017;96:4208–4216. doi: 10.3382/ps/pex251. [DOI] [PubMed] [Google Scholar]
- MARAN Monitoring of Antimicrobial Resistance and Antibiotic Usage in Animals in the Netherlands in 2018. Lelystad, The Netherlands. Accessed June 2020. 2019. https://www.wur.nl/upload_mm/a/7/9/89640bbc-53a2-40f0-ba4a-a9a34a7bf416_Nethmap%20Maran%202019.pdf
- Markazi A., Luoma A., Shanmugasundaram R., Mohnl M., Raj Murugesan G., Selvaraj R. Effects of drinking water synbiotic supplementation in laying hens challenged with Salmonella. Poult. Sci. 2018;97:3510–3518. doi: 10.3382/ps/pey234. [DOI] [PubMed] [Google Scholar]
- Merchant L.E., Rempel H., Forge T., Kannangara T., Bittman S., Delaquis P., Topp E., Ziebell K.A., Diarra M.S. Characterization of antibiotic-resistant and potentially pathogenic Escherichia coli from soil fertilized with litter of broiler chickens fed antimicrobial-supplemented diets. Can. J. Microbiol. 2012;58:1084–1098. doi: 10.1139/w2012-082. [DOI] [PubMed] [Google Scholar]
- Methner U., Friese A., Rosler U. Competitive exclusion: a tool to combat extended-spectrum beta-lactamase-producing Escherichia coli strains in chickens. Res. Vet. Sci. 2019;123:124–128. doi: 10.1016/j.rvsc.2019.01.003. [DOI] [PubMed] [Google Scholar]
- Mughini-Gras L., Dorado-Garcia A., van Duijkeren E., van den Bunt G., Dierikx C.M., Bonten M.J.M., Bootsma M.C.J., Schmitt H., Hald T., Evers E.G., de Koeijer A., van Pelt W., Franz E., Mevius D.J., Heederik D.J.J., ESBL Attribution Consortium Attributable sources of community-acquired carriage of Escherichia coli containing beta-lactam antibiotic resistance genes: a population-based modelling study. Lancet Planet. Health. 2019;3:e357–e369. doi: 10.1016/S2542-5196(19)30130-5. [DOI] [PubMed] [Google Scholar]
- Nakamura A., Ota Y., Mizukami A., Ito T., Ngwai Y.B., Adachi Y. Evaluation of Aviguard, a commercial competitive exclusion product for efficacy and after-effect on the antibody response of chicks to Salmonella. Poult. Sci. 2002;81:1653–1660. doi: 10.1093/ps/81.11.1653. [DOI] [PubMed] [Google Scholar]
- Nuotio L., Schneitz C., Nilsson O. Effect of competitive exclusion in reducing the occurrence of Escherichia coli producing extended-spectrum beta-lactamases in the ceca of broiler chicks. Poult. Sci. 2013;92:250–254. doi: 10.3382/ps.2012-02575. [DOI] [PubMed] [Google Scholar]
- Nurmi E., Nuotio L., Schneitz C. The competitive exclusion concept: development and future. Int. J. Food Microbiol. 1992;15:237–240. doi: 10.1016/0168-1605(92)90054-7. [DOI] [PubMed] [Google Scholar]
- Roth N., Mayrhofer S., Gierus M., Weingut C., Schwarz C., Doupovec B., Berrios R., Domig K.J. Effect of an organic acids based feed additive and enrofloxacin on the prevalence of antibiotic-resistant E. coli in cecum of broilers. Poult. Sci. 2017;96:4053–4060. doi: 10.3382/ps/pex232. [DOI] [PubMed] [Google Scholar]
- RStudio Team . Integrated Development for R; 2016. RStudio. [Google Scholar]
- Saliu E.M., Vahjen W., Zentek J. Types and prevalence of extended-spectrum beta-lactamase producing Enterobacteriaceae in poultry. Anim. Health Res. Rev. 2017:1–12. doi: 10.1017/S1466252317000020. [DOI] [PubMed] [Google Scholar]
- van Hoek A.H.A.M., Veenman C., Florijn A., Huijbers P.M.C., Graat E.A.M., de Greeff S., Dierikx C.M., van Duijkeren E. Longitudinal study of ESBL Escherichia coli carriage on an organic broiler farm. J. Antimicrob. Chemother. 2018;73:3298–3304. doi: 10.1093/jac/dky362. [DOI] [PubMed] [Google Scholar]
- Varmuzova K., Kubasova T., Davidova-Gerzova L., Sisak F., Havlickova H., Sebkova A., Faldynova M., Rychlik I. Composition of gut microbiota influences resistance of newly hatched chickens to Salmonella Enteritidis infection. Front. Microbiol. 2016;7:957. doi: 10.3389/fmicb.2016.00957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velkers F.C., Bouma A., Stegeman J.A., de Jong M.C. Oocyst output and transmission rates during successive infections with Eimeria acervulina in experimental broiler flocks. Vet. Parasitol. 2012;187:63–71. doi: 10.1016/j.vetpar.2011.12.032. [DOI] [PubMed] [Google Scholar]
- Velthuis A.G., Bouma A., Katsma W.E., Nodelijk G., De Jong M.C. Design and analysis of small-scale transmission experiments with animals. Epidemiol. Infect. 2007;135:202–217. doi: 10.1017/S095026880600673X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization Fact Sheet Antimicrobial Resistance Number 194. Accessed Jan. 2019. 2018. https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.