Abstract
Salmonellosis in broilers is not merely a significant disease with high economic costs in the poultry industry but also the foodborne disease with the impact on public health by cross-contamination. This study was to investigate the prebiotic ability of trehalose supplementing in diets (0, 1, 3, and 5%, w/w) against Salmonella by using S. Typhimurium (ST)-inoculated broilers. The improvements (P < 0.05) of feed conversion ratio (FCR) were observed with 5% trehalose supplementation in ST-inoculated broilers' diets. An addition of 3 or 5% trehalose in diets increased (P < 0.05) the abundance of lactobacilli in the duodenum and jejunum but decreased (P < 0.05) the growth of ST in the cecum. The adverse effects on serum levels of aspartate aminotransferase, triglyceride, and albumin and globulin ratio in ST-inoculated broilers were noticed and counteracted by supplementing 3 or 5% trehalose in diets (P < 0.05). Besides, the inclusion of trehalose in diets alleviated the intestinal damages and maintained the integrity of cecal epithelial cells after ST challenge under an haematoxylin and eosin-staining observation. Supplementing trehalose further showed the inhibitions of toll-like receptor 4-mediated nuclear factor-kappa-B pathway, including the downregulation (P < 0.05) of proinflammatory cytokine genes, such as interleukin 1 beta and lipopolysaccharide-induced tumor necrosis factor-alpha factor and the upregulation (P < 0.05) of interleukin 10 and interferon-alpha in ST-inoculated broilers. Overall, supplementing trehalose alleviated the adverse effects from ST challenge on FCR, serum biochemistry, the damage, and inflammation in the liver and cecum. Those improvements on ST challenged broilers also contributed to the overgrowth of lactobacilli, the decrement of ST, and anti-inflammatory effects in affected broilers. Trehalose, therefore, could be a promising prebiotic against salmonellosis to benefit broiler production and promote food safety in the poultry industry.
Key words: anti-inflammation, broilers, lactobacillus, Salmonella Typhimurium, trehalose
Introduction
In many countries, poultry meat and poultry products are one of the most important sources of protein and essential nutrients for human consumption. Following the rise of customers' attention to foodborne pathogens and antimicrobial resistance issues, food safety is valued and emphasized by governments and societies. Salmonella is an enteric pathogen that can infect the poultry and human beings and is one of the top 5 pathogens that cause illnesses from food consumption in the United States (CDC, 2017). The Salmonella contamination in poultry is one of the most common sources for human infection. The use of antimicrobials could reduce the number of bacteria excreted by infected poultry but does not cure salmonellosis, namely, eliminating Salmonella thoroughly from affected poultry. Besides, infected poultry are asymptomatic and ignored (Gast, 2008). These characteristics and the nature of ubiquity arise the difficulty to control Salmonella in poultry. Owing to its ineradicable property, Salmonella possesses higher opportunities to contaminate foods, drinking water, carcasses, and equipment in the entire food chains. For example, the evisceration during the operating chain of slaughter regularly has the case of digestive tract rupture, resulting in the cross-contamination between Salmonella and the carcasses. Center for Disease Control and Prevention estimated that Salmonella causes about 1.2 million illnesses, 23,000 hospitalizations, and 450 deaths in the United States every year (Scallan et al., 2011). Salmonella Typhimurium (ST) and Salmonella Enteritidis were the most common serotypes in the United States (CDC, 2014). It is the emerging challenge for the poultry industry to prevent and control this significant disease.
Antimicrobial growth promoters (AGP) have been used in the poultry husbandry over 50 y for preventing microbial diseases and improving growth performance. Based on the concern that an improper use of AGP may promote the bacteria in animals to develop the antimicrobial resistance, the European Commission commenced the ban of supplementing AGP in diets since 2006 and simultaneously reduced the utilization of antibiotics for therapeutics (Kostrzynska and Bachand, 2006, Ivey et al., 2013). As a result, the well-controlled bacterial infections in the past were recurred and caused economic losses in the industry. Therefore, the industry must seek an alternative that provides a promising efficacy on the prevention and control of enteric diseases, such as salmonellosis.
Prebiotic is defined as “Non-digestible food that beneficially affects the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon, thus improves host health” (Gibson and Roberfroid, 1995). It is also indicated as a group of dietary fibers or oligosaccharides exerting biological effects on hosts by a selective stimulation of growth or bioactivity of beneficial microorganisms either present or therapeutically introduced to the intestines (Tomasik and Tomasik, 2003). Feeding fructooligosaccharides (FOS) in the chicken diet has demonstrated to promote a change in the intestinal gut microflora and, in some cases, reduced susceptibility of bacterial colonization (Kim et al., 2011). In addition, inulin and oligofructose are considered as mainly soluble and fermentable dietary fibers, which have resistance to the hydrolysis caused by human alimentary enzymes and further being fermented by bifidobacteria and lactobacilli in the colon (Figueroa-González et al., 2011). Trehalose is a disaccharide consisting of 2 glucose units joined by a 1-1 alpha bond and also known as mycose or tremalose. It is usually found in animals, plants, and microorganisms. It has been widely used in cosmetics, food, and pharmaceuticals because of its unique chemical and physical properties. The activity of trehalase, an enzyme to digest trehalose, in broilers has been demonstrated not to be detected after a 21-day-old birth (Chotinsky et al., 2001). Chen et al. (2007) reported that trehalose significantly increases the growth of bacteriocin-producing lactic acid bacteria, particularly for Lactococcus lactis spp. C101910, and Lactococcus sp. GM005 compared with the FOS treatment. The production of bacteriocin from Lactobacillus animalis, Enterococcus durans L28-1, Lactococcus lactis spp. C101910, and Lactococcus sp. GM005 were higher in cultured media supplemented with trehalose than those with dextrose, FOS, or raffinose.
Based on the pieces of evidence mentioned above, trehalose may exert antibacterial or prebiotic activities contributing to the reduction of Salmonella in broilers. This study addressed the beneficial effects of trehalose on broilers against Salmonella by (1) evaluating the growth performance and serum biochemistry in the Salmonella-infected broilers; (2) investigating the population of total lactobacilli and ST in each section of the intestine of Salmonella-infected broilers supplemented with trehalose; and (3) examining the expression of inflammatory-related cytokine genes and histopathological changes in the cecum of Salmonella-infected broilers supplemented with trehalose.
Materials and methods
Inoculum Preparation
The preparation of ST inoculum was based on the previous study (Marcq et al., 2011) and described briefly as follows: ST (ATCC 23566 strain) was incubated in brain–heart infusion broth (BD, Franklin Lakes, NJ) for 18 h at 37°C. On the challenge day, ST was subcultured for 3 times to reach a high bioactivity, and the bacterial inoculum was prepared at the concentration of 3.5 × 108 colony-forming unit (CFU)/mL in sterile phosphate-buffered saline (PBS). Lactobacillus johnsonii (ATCC 17474 strain) kindly provided from Dr. Ming-Ju Chen (Department of Animal Science and Technology, National Taiwan University [NTU]) was cultured in De Man, Rogosa, and Sharpe broth (Neogen, Lansing, MI) for 17 h at 37°C for qPCR analysis.
Treatments for Animals
The commercial meat-type broiler, Arbor Acres plus, was used in this study. Total of 75 male 1-day-old chickens was obtained from a local breeder farm (Ju-Ling Farming Co., Ltd., ILan County, Taiwan) and screened as Salmonella-free before the treatments. The trial was conducted under the permission of NTU Institution Animal Care and Use Committee (IACUC No. 103-EL-008), and the diets of 3 feeding periods were formulated based on the commercial guideline (Supplementary Tables 1–3) with the water ad libitum. A trehalose product (Treh) with a purity of at least 98% trehalose dihydrate (C12H22O11 · 2H2O) was kindly offered from Hayashibara Co., Ltd. (Okayama, Japan). All 1-day-old male chicks were randomly assigned to each following group with its corresponding diet design throughout the whole experimental period of 35 D: (1) Control: diet with 0% Treh, (2) 0% Treh + ST: diet with 0% Treh, (3) 1% Treh + ST: diet with 1% Treh, (4) 3% Treh + ST: diet with 3% Treh, and (5) 5% Treh + ST: diet with 5% Treh. Owing to the interference of intestinal maternal immunity in broilers younger than 21 D and optimal observation period of the Salmonella response to antibacterial preparations within 1 to 2 wk after inoculation (Marcq et al., 2011), except the control group (n = 15, inoculated with 1 mL of sterile PBS), 60 broilers from other 4 groups (15 broilers per group) were individually oral-challenged with 1 mL of ST inoculum with the concentration of 3.5 × 108 CFU/mL on the day 28. Broilers in each group (n = 15) were separately reared in isolated floor pens by using wood racks and iron nets (5 broilers per pen); meanwhile, all procedures were conducted with biosecurity to avoid the potential cross-contamination of groups. All broilers were housed at the temperature of 22°C to 30°C with the relative humidity of 60 to 70% and dark-light cycle following 1/23 h for 0- to 1-wk old age and 4/20 h for broilers older than 1-wk age according to the commercial guideline. At the end of the trial, all broilers were sacrificed, and meanwhile, their blood and tissue samples (duodenum, jejunum, ileum, and cecum) were collected for further analyses.
Sample Collection
During the experimental period, the feed intakes (n = 3 per group) and body weight (n = 15 or 14 per group) recorded as pen basis and individually, respectively, once a week to analyze their growth performance. The daily body weight gain, daily feed intake, and feed conversion ratio (n = 3) were calculated on a broiler per pen in each group. Fecal samples on day 35 were collected directly from the cloaca of 9 chickens (3 birds per pen, randomly) in each group. Blood from each chicken (n = 15 per group) was obtained via an alar cardinal vein puncture and then placed at room temperature for 1 h to allow clotting. Then, the sera were collected by centrifugation at 3,000 × g, 4°C for 15 min (Centrifuge, 3700, Kubota Co., Tokyo, Japan) and then stored at −20°C for further analyses. Chymus in the middle section of duodenum, jejunum, ileum, and cecum were collected from 8 broilers randomly collected from each group for microbial analyses. The same cecal tissues (n = 8 per group) after collecting chymus were washed with PBS and performed for the analyses of inflammatory-related cytokines.
Identification and Quantification of Bacteria by qPCR
Bacterial DNA from intestinal chymus (n = 8) in duodenum, jejunum, ileum, and cecum of broilers, as well as fecal samples (n = 9) were extracted using Favorprep DNA kit (Favorgen Biotech Co., Ping-Tung, Taiwan). The 2-step reactions of qPCR were applied by SensiFAST HRM Kit (Bioline Reagents Ltd., London, UK). The 40-cycle reactions were performed with 3 min of polymerase activation at 95°C and followed by 5 s of denaturation at 95°C and 30 s of annealing/extension with different temperature settings (64°C for ST and 63°C for Lactobacillus spp.). The primers for fliC of ST and 16S-23S rRNA gene of Lactobacillus spp. were F: 5′-TGCAGAAAATTGATGCTGCT-3′; R: 5′-TTGCCCAGGTTGGTAATAGC-3′ (Lee et al., 2009); and F: 5′-TGGATGCCTTGGCACTAGGA-3′; R: 5′-AAATCTCCGGATCAAAGCTTACTTA-3′ (Haarman and Knol, 2006), respectively. The standard curves of qPCR were established by using the reference strain of ST (ATCC 23566), ranging from 104 to 108 CFU/g, and the strain of L. johnsonii (ATCC 17474), ranging 109 to 1012 CFU/g.
Serum Biochemistry
The serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), triglyceride (TG), total cholesterol, total protein, and albumin in each chicken of groups (n = 15 for Control, 0% Treh + ST, and 1% Treh + ST groups; n = 14 for 3% Treh + ST and 5% Treh + ST groups) were evaluated by using an ARKARY SPOTCHEM SP-4410 automatic dry chemistry analyzer (AKARAY, Kyoto, Japan) with corresponding kits. Because albumin and globulin constitute 2 major components of serum proteins and play critical roles in inflammation, globulin was calculated as subtraction of albumin value from that of the total protein (Salem et al., 2018).
Analyses of Inflammatory-Related Cytokine Gene Expression
Approximate 0.3 g of cecal tissue in the same collected tissue samples (n = 8 per group) in identification and quantification of bacteria from each group was homogenized in liquid nitrogen mixing with 1 mL TRIsure (Bioline Reagents Ltd.). The cDNA of sampled tissue was further synthesized by ImProm-II Reverse Transcriptase (Promega, Madison, WI). Differences on the expressions of inflammatory-related cytokine genes including nuclear factor-kappa-B-inhibitor alpha (NFκB1A), interleukin-1 beta (IL-1β), interleukin-6 (IL-6), interleukin-12 beta (IL-12β), interferon gamma (IFNγ), lipopolysaccharide (LPS)-induced tumor necrosis factor-alpha factor (LITAF), interleukin 10 (IL-10), and interferon alpha (IFNα) were investigated. Primers designed for each tested gene and sequences of amplicons were shown below: NFκB1A, accession no.: NM_001001472, F: 5′-GCAGATACTGCCCGAAAGTG-3′ and R: 5′-TGTCAGCTGTCTTCCTCCAA-3′; IL-1β, accession no.: Y15006, F: 5′-TGGGCATCAAGGGCTACA-3′ and R: 5′-TCGGGTTGGTTGGTGATG-3′; IL-6, accession no.: NM_204628, F: 5′-AAATCCCTCCTCGCCAATCT-3′ and R: 5′-CCCTCACGGTCTTCTCCATAAA-3′; IL-12β, accession no.: NM_213571, F: 5′-CTGTGGCTCGCACTGATAAA-3′ and R: 5′-GGTGCTCTTCGGCAAATGG-3′; IFNγ, accession no.: NM_205149, F: 5′-AGCTGACGGTGGACCTATTATT-3′ and R: 5′-GGCTTTGCGCTGGATTC-3′; LITAF, accession no.: NM_204267, F: 5′-GGAATGAACCCTCCGCAGTA-3′ and R: 5′-CTGAACTGGGCGGTCATAGA-3′; IL-10, accession no.: AJ621614, F: 5′-AATCACGGGCTGACTTTCAC-3′ and R: 5′-AACTCCCCCATGGCTTTGTA-3′; IFNα, accession no.: AB021154, F: 5′-GACATCCTTCAGCATCTCTTCA-3′ and R: 5′-AGGCGCTGTAATCGTTGTCT-3′; glyceraldehyde 3-phosphate dehydrogenase (GAPDH), accession no. K01458, F: 5′-GGTGGTGCTAAGCGTGTTAT-3′ and R: 5′-ACCTCTGTCATCTCTCCACA-3′. The sizes of PCR products were as follows: 109 bp for NFKBIA, 244 bp for IL-1β, 106 bp for IL-6, 84 bp for IL-12β, 259 bp for IFNγ, 114 bp for LITAF, 64 bp for IL-10, 238 bp for IFNα, and 264 bp for glyceraldehyde 3-phosphate dehydrogenase. The reaction mixture of qPCR was prepared by 2 μL of cDNA (25 ng/μL), 5 μL of SYBR Green (Fast SYBR Green Master Mix, ThermoFisher Scientific, Inc., Waltham, MA), 2.4 μL of water, and 0.6 μL of primers. Glyceraldehyde 3-phosphate dehydrogenase was used as an internal control. The values of genes in each group were expressed relatively to the mean of the control group, which was set to 1.0.
Cecal Histopathology and Staining (Haematoxylin and Eosin Stain)
Owing to the tissue integrity, the cecum tissues in the rest of broilers of each group after picked up for identification and quantification of bacteria (n = 7 for Control, 0% Treh + ST, and 1% Treh + ST groups; n = 6 for 3% Treh + ST and 5% Treh + ST groups) were placed in 10% formalin (Sigma-Aldrich, St. Louis, MO) up to 24 h, and all samples were fixed in a new neutral-buffered formalin solution again. Subsequently, they were dehydrated in graded (30, 50, 75, 95, and 99%) alcohol (ECHO chemical, Taipei, Taiwan), cleared in xylene (Merck Millipore, Darmstadf, Germany) for 5 h, and the second replacement of new xylene was kept for 12 h, then embedded in paraffin wax (Leica Microsystem, Singapore) for 24 h by embedding plate (Electron Microscopy Science, Hatfield) and plastic ware (Simport, Beloeil, Canada) under the hybridization oven (RH-800; YIH DER, Taipei, Taiwan) and Digital Dry Bath incubator (Genepure Technology, Taipei, Taiwan) under 63°C. The well-fixed samples in paraffin wax were then sliced into 3 μm thick by using. Moreover, the paraffin-embedded sections were put in a water bath at 45°C for spreading out and dried by an electronic heating plate under 35°C for 4 h. Regarding the staining processes, all slides were deparaffinized in xylene for 20 min, then rehydrated in graded alcohol and stained with hematoxylin (Merck Millipore Co., Darmstadt, Hesse, Germany) and eosin (Merck Millipore Co.) in an optimal period (20 s for hematoxylin, 20 min for eosin). Photomicrographs under haematoxylin and eosin stainings were taken under a Zeiss Axioskop microscope AxioCam ERc 5s camera system with AxioVision Release 4.8.2 (06-2010) software (Carl Zeiss Microscopy, LLC, Thornwood, NY).
Statistical Analysis
The experiment was conducted by a completely random design. A significant difference was identified at the 0.05 of probability level. When a significant difference among groups was detected by using the one-way analysis of variance, differences between treatments were tested by using the Least Significant Difference test. All statistical analyses were performed by using SAS software (SAS Institute Inc., Cary, NC, 2002).
Results
Effects of Trehalose on the Growth Performance and the Abundance of ST/Lactobacilli in Small Intestines of ST-Inoculated Broilers
The body weights of broilers during on day 28 were not (P > 0.05) affected by Treh supplementation. Even though broilers were inoculated with ST, the body weight at the end of the trial (on day 35) was not (P > 0.05) different among groups (Table 1). However, the daily feed intake and feed conversion ratio (FCR) were significantly decreased and affected (P < 0.05). No (P > 0.05) differences on daily weight gain and FCR between groups were noticed before the inoculation of ST performed on day 28. After the ST challenge, the daily weight gains of groups were not (P > 0.05) influenced till day 35. At that day, the daily feed intake and FCR were decreased and improved (P < 0.05) in the group supplemented with 5% Treh compared with other ST-inoculated groups. As a result, 5% Treh in the diet alleviated the adverse effects of ST challenge on daily feed intake and FCR of affected broilers. To investigate the effects of Treh on the intestinal microbiota of ST-affected broilers, the abundance of ST and lactobacilli in the small intestines (duodenum, jejunum, and ileum), cecum, and feces were evaluated by using qPCR. The ST was not found in the small intestines but detected in the cecum and/or feces in the infected groups (Table 2). The amount of ST was notably reduced (P < 0.05) in the cecum with the concentration of 1 or 3% Treh in the ST-inoculated groups. As for results of feces, no (P > 0.05) significant difference on ST counts was noted, except in 3% Treh + ST group compared with the ST-inoculated broilers. For the abundance of lactobacilli in small intestines, the ST-inoculated broilers without supplementing trehalose (0% Treh + ST group) significantly represented less (P < 0.05) amounts of lactobacilli in duodenum, jejunum, and ileum, but no (P > 0.05) influence was observed in the cecum and feces compared with the control group (Table 3). The Treh addition in diets with the concentration of 3 and 5% both significantly increased (P < 0.05) the abundance of lactobacilli in the small intestines and cecum of ST-inoculated broilers.
Table 1.
Effects of trehalose (Treh) on daily weight gain, daily feed intake, feed conversion ratio (FCR), and mortality of broilers during the periods of preinoculation and postinoculation of Salmonella Typhimurium (ST).
| Treatment | Control | 0% Treh + ST | 1% Treh + ST | 3% Treh + ST | 5% Treh + ST | SEM | P value |
|---|---|---|---|---|---|---|---|
| Body weight (BW, g/broiler/pen) | |||||||
| Day 1 | 40.6a | 39.6a | 40.8a | 40.7a | 40.6a | 0.36 | 0.769 |
| Day 28 | 1511.7a | 1495.8a | 1493.4a | 1480.1a | 1413.6a | 19.63 | 0.442 |
| Day 35 | 2134.2a | 2097.8a | 2131.0a | 2102.0a | 2008.4a | 25.66 | 0.421 |
| Daily BW gain (DBWG, g/broiler/pen) | |||||||
| Day 1–28 | 52.2a | 50.9a | 51.3a | 51.9a | 49.1a | 0.70 | 0.445 |
| Day 29–35 | 91.5a | 88.2a | 90.7a | 92.7a | 84.4a | 4.34 | 0.966 |
| Daily feed intake (g/broiler/pen) | |||||||
| Day 1–28 | 74.2a | 72.5a | 74.4a | 74.8a | 71.2a | 1.42 | 0.197 |
| Day 29–35 | 157.0b | 194.8a | 194.9a | 192.9a | 154.4b | 7.70 | 0.005 |
| Feed conversion ratio (g feed/g BW) | |||||||
| Day 1–28 | 1.44a | 1.41a | 1.41a | 1.46a | 1.46a | 0.02 | 0.466 |
| Day 29–35 | 1.65b | 2.23a | 2.20a | 2.11a | 1.83b | 0.08 | 0.002 |
| Number of dead broiler in the trial | |||||||
| 0 | 0 | 0 | 1 | 1 | |||
a,bMean value in the same row without the common letter was significantly different (P < 0.05).
The data were given as mean (n = 15 for BW in all groups during the experimental period, except n = 14 for day 28 and day 35 BW of both 3% Treh + ST and 5% Treh + ST groups because of the broiler death; n = 3 for DBWG, feed intake, and feed conversion ratio).
Table 2.
Effects of trehalose (Treh) on the abundance of Salmonella Typhimurium (ST) in the small intestines, cecum, and feces of broilers orally challenged with or without ST on day 35 (Unit: log CFU/g).
| Treatment | Control | 0% Treh + ST | 1% Treh + ST | 3% Treh + ST | 5% Treh + ST | SEM | P value |
|---|---|---|---|---|---|---|---|
| Duodenum | ND | ND | ND | ND | ND | ||
| Jejunum | ND | ND | ND | ND | ND | ||
| Ileum | ND | ND | ND | ND | ND | ||
| Cecum | ND | 5.12a | ND | ND | 4.86b | 0.10 | <0.000 |
| Feces | ND | 4.97a | 4.77a | ND | 4.66a | 0.08 | <0.000 |
a,bMean value in the same row without the common letter was significantly different (P < 0.05).
The data were given as mean (n = 8 for duodenum, jejunum, ileum, and cecum; n = 9 for feces).
ND, not detectable indicated the value was less than 104 CFU/g.
Table 3.
Effects of trehalose (Treh) on the abundance of lactobacilli in the small intestines, cecum, and feces of broilers orally challenged with or without Salmonella Typhimurium (ST) on day 35 (Unit: log CFU/g).
| Treatment | Control | 0% Treh + ST | 1% Treh + ST | 3% Treh + ST | 5% Treh + ST | SEM | P value |
|---|---|---|---|---|---|---|---|
| Duodenum | 10.86b | 9.82c | 11.15a,b | 11.91a | 10.93b | 0.16 | 0.003 |
| Jejunum | 11.54b | 10.49c | 11.30b | 12.48a | 11.21b | 0.12 | 0.000 |
| Ileum | 12.86a | 11.61b | 11.70b | 12.54a | 12.09b | 0.12 | 0.023 |
| Cecum | 10.29b,c | 9.62c | 10.91a,b | 11.67a | 10.64a,b | 0.17 | 0.008 |
| Feces | 12.73a | 12.59a | 12.87a | 12.50a | 12.49a | 0.09 | 0.936 |
a–cMean value in the same row without the common letter was significantly different (P < 0.05).
The data were given as mean (n = 8 for duodenum, jejunum, ileum, and cecum; n = 9 for feces).
Effects of Trehalose on Serum Biochemistry and Cecal Histopathology of ST-Inoculated Broilers
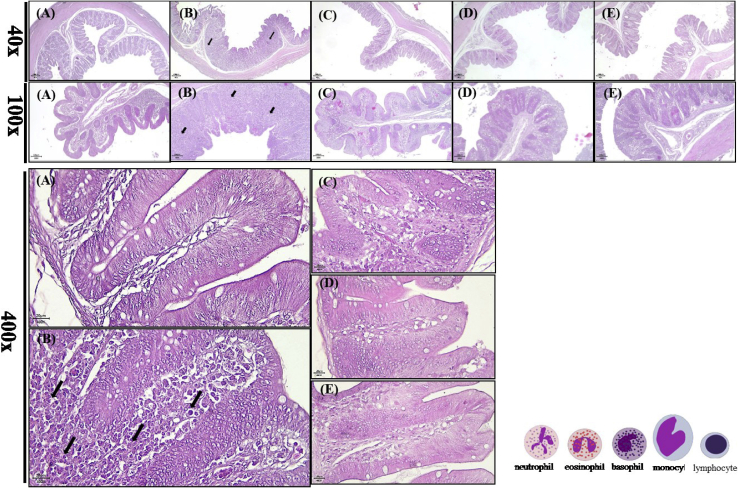
For investigating physiological and immunological statuses of ST-inoculated broilers with different concentrations (0, 1, 3, and 5%) of Treh, serum biochemistry (Table 4) and cecal histopathology (Figure 1) were also evaluated. The challenge of ST increased the level of serum AST and ALT in broilers compared with the control broilers. The serum level of ALT was significantly increased (P < 0.05), indicating the serum ALT is more specific for the screening of ST infestation compared with AST. Treh supplementation reduced (P < 0.05) the level of AST and ALT in ST-inoculated broilers. The ST inoculation also decreased (P < 0.05) serum triglyceride (TG) in challenged broilers compared with the control broilers. However, the addition of 3 or 5% of trehalose in diets significantly restored (P < 0.05) the level of TG in ST-challenged broilers. Meanwhile, the values of TG in 3 or 5% trehalose supplemented groups were still significantly below (P < 0.05) the level in the control group. In contrast to AST, ALT, and TG levels in sera, serum total cholesterol, total protein, albumin, and globulin were not (P > 0.05) affected among groups. Lastly, the inoculation of ST significantly reduced (P < 0.05) albumin/globulin ratio (A/G) in affected broilers, and an addition of 3 or 5% of Treh in diets significantly counteracted (P < 0.05) the decrement of A/G ratio resulted from the ST inoculation. Based on the result of histopathological examinations (Figure 1), the infiltration of immune cells (black arrow) in cecal tissues of ST-inoculated broilers were observed. The ST inoculation resulted in the infiltration of mononuclear cells and thinner intestinal epithelial cell around the lamina propria (Figure 1B) compared with the control group (Figure 1A).
Table 4.
Effects of trehalose (Treh) on serum biochemistry of broilers orally challenged with or without Salmonella Typhimurium (ST).
| Treatment | Control | 0% Treh + ST | 1% Treh + ST | 3% Treh + ST | 5% Treh + ST | SEM | P value |
|---|---|---|---|---|---|---|---|
| AST (IU/L) | 202.25a | 251.23a | 215.29a | 222.50a | 233.55a | 6.26 | 0.157 |
| ALT (IU/L) | 5.58b,c | 12.31a | 6.54b | 7.78b | 3.31c | 0.47 | <0.000 |
| TG (mg/dL) | 88.33a | 23.15c | 27.67c | 51.50b | 63.31b | 2.78 | <0.000 |
| TC (mg/dL) | 132.4a | 137.21a | 134.79a | 146.18a | 151.08a | 3.67 | 0.454 |
| Total protein (g/dL) | 3.08a | 3.57a | 3.39a | 3.48a | 3.68a | 0.09 | 0.288 |
| Albumin (g/dL) | 1.34a | 1.44a | 1.36a | 1.48a | 1.54a | 0.04 | 0.502 |
| Globulin (g/dL) | 1.74a | 2.14a | 2.04a | 2.00a | 2.15a | 0.05 | 0.128 |
| A/G ratio | 0.78a | 0.65c | 0.68b,c | 0.74a,b | 0.74a,b | 0.01 | 0.009 |
a–cMean value in the same row without the common letter was significantly different (P < 0.05).
The data were given as mean (n = 15 for Control, 0% Treh + ST, and 1% Treh + ST groups; n = 14 for 3% Treh + ST and 5% Treh + ST groups).
Abbreviations: Treh, Trehalose; AST, aspartate aminotransferase; ALT, alanine transaminase; TG, triglyceride; TC, total cholesterol; A/G ratio, albumin and globulin ratio.
Figure 1.
Histopathological examinations of cecal tissues from broilers with or without the challenge of Salmonella Typhimurium (ST) by haematoxylin and eosin staining. (A) Control group; (B) 0% Treh + ST group; (C) 1% Treh + ST group; (D) 3% Treh + ST group; (E) 5% Treh + ST group. The scales were shown in 20 μm (400×), 100 μm (100×), and 200 μm (40×). The infiltration of immune cells was indicated by the black arrow.
Anti-Inflammatory Effects of Trehalose on Cecal Tissues of ST-Inoculated Broilers
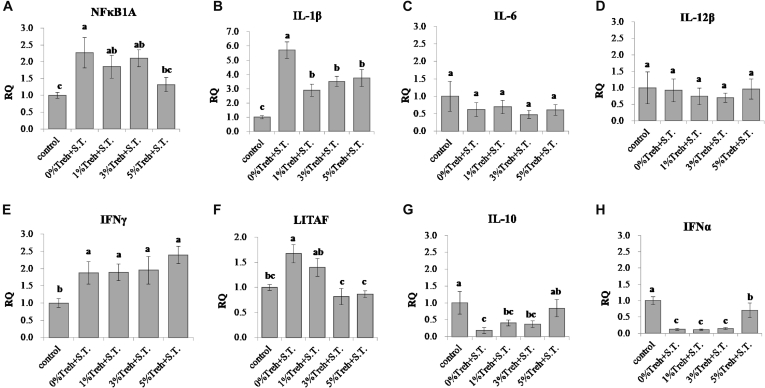
For further studying anti-inflammatory effects of Treh on cecal histopathology observed in ST-inoculated broilers, expressions of inflammatory- and anti-inflammatory-related cytokine genes, including NFκB1A, IL-1β, IL-6, IL-12β, IFNγ, LITAF, IL-10, and IFNα, were evaluated by qPCR. As a result, the expression of NFκB1A was upregulated (P < 0.05) in ST-inoculated group, and the supplementation with a high dose (5%) of Treh significantly downregulated (P < 0.05) its expression to the level as that in the control group (Figure 2A). Furthermore, the genes associated with proinflammatory cytokines (IL-6, IL-12β, IL-1β, and LITAF) in cecal tissues were examined as well (Figures 2B–2F). The expression of IL-6 and IL-12β genes were not (P > 0.05) different among groups. Interleukin-1 beta and LITAF, which is a novel transcriptional factor activating TNFα (Tang et al. 2005), were upregulated (P < 0.05) in ST-inoculated broilers but downregulated by Treh-supplemented groups, notably for the concentrations of 3 and 5%. Those findings demonstrated that Treh counteracted inflammatory responses resulted from ST inoculation and coincided with the histopathological results noted in the cecal tissues. Nevertheless, the Treh addition promoted the expression of IFNγ gene similar to the effect of ST inoculation, suggesting the modulation of anti-inflammation by trehalose was not associated with the IFNγ gene. Regarding anti-inflammatory cytokine genes, such as IL-10 and IFNα, they were both significantly downregulated (P < 0.05) after ST challenge, but a high dose (5%) of Treh in diets promoted their expressions with statistical significances in the ST-inoculated group (Figures 2G, 2H).
Figure 2.
Effects of trehalose (Treh) on the expression of inflammatory-related cytokine genes in cecal tissues of broilers with the challenge of Salmonella Typhimurium (ST). (A) NFκB1A; (B) IL-1β; (C) IL-6; (D) IL-12β; (E) IFNγ; (F) LITAF; (G) IL-10; (H) IFNα. The data were presented by mean of fold change ± SEM (n = 8). Mean value without the common letter on data bar in each figure indicated that the difference was significant (P < 0.05). Abbreviations: IFNα, interferon alpha; IFNγ, interferon gamma; IL-1β, interleukin-1 beta; IL-6, interleukin-6, IL-10, interleukin 10;IL-12β, interleukin-12 beta; LITAF, lipopolysaccharide-induced tumor necrosis factor-alpha factor; NFκB1A, nuclear factor-kappa-B-inhibitor alpha.
Discussion
In this trial, none of the clinical symptoms of salmonellosis was observed in ST-inoculated broilers. This result was similar to the observations of previous studies (Chalghoumi et al., 2009, Marcq et al., 2011, Faber et al., 2012). In addition to improving the growth performance in Salmonella-affected broilers, modulations of intestinal microbiota by supplementing prebiotics have been reported (Gómez et al., 2013). Several studies showed that the supplementation of prebiotics, including isomalto-oligosaccharides, mannan-oligosaccharides, FOS, and fructans in diets reduced the level of intestinal pathogens, such as E. coli, Salmonella spp., and Clostridium perfringens in birds but increased the amount of intestinal lactobacilli (Spring et al., 2000, Thitaram et al., 2005, Dahiya et al., 2006, Kim et al., 2011). Another study using galactoglucomannan oligosaccharide-arabinoxylan (GGMO-AX) complex as a prebiotic in diets for ST-inoculated broilers demonstrated that the supplementation of GGMO-AX, and Safmannan increased the number of ileal lactobacilli of ST-inoculated birds (Faber et al., 2012). In addition, the supplementation of GGMO-AX increased the total bifidobacteria in ceca and alleviated the level of IFN-γ and IL-1β, suggesting that GGMO-AX exerted prebiotic-like effects and decreased the virulence of ST by decreasing cytokines in the intestines. Regarding prebiotic-like effects, Chen et al. (2007) reported that trehalose could increase growth of bacteriocin-producing lactic acid bacteria, particularly for Lactocossus lactis spp. C101910 and Lactococcus sp. GM005, as well as produce bacteriocin from Lactobacillus animalis, E. durans L28-1, Lactococcus lactis spp. C101910, and Lactococcus sp. GM005. In the current study, the addition of 3% Treh either reduced the amount of ST or increased the abundance of lactobacilli in the small intestines, ceca, and feces, indicating that trehalose supplementation could act as a prebiotic to selectively promote the growth of beneficial bacteria and inhibit the multiplication of pathogens in broiler intestines.
Salmonella infection caused not only the inflammation in the intestines but also damages in the livers of hosts (Rescigno et al., 2001, Salcedo et al., 2001). The serum ALT level was considered more specific for liver damage compared with serum AST (Harr, 2002). A recent study also found that the serum AST level in layers was increased after the Salmonella inoculation (Garcia et al., 2013). Therefore, serum AST and ALT levels were analyzed to depict the serum profile of ST-inoculated broilers. The results showed that both serum AST and ALT levels were elevated when broilers were challenged with ST. Nevertheless, the alteration of serum ALT levels was more significant for liver damage in ST-affected broilers. The low serum TG level in the ST-inoculated group may result from the failure or damage of liver function (Garcia et al., 2013). Moreover, the decreased serum A/G ratio indicated the inflammation occurring in ST-affected broilers (Lumeij, 2008). To sum up, the levels of serum ALT, TG, and A/G were altered following the ST inoculation, suggesting that the inflammation and/or damage in the liver and intestines was in progress at the time point of evaluation. The Treh supplementation, particularly with the concentration of 3 or 5%, significantly reduced the adverse effects from the ST challenge in broilers. Moreover, the main defense mechanism in the host against the infection of Salmonella spp. was through the neutrophils and mononuclear cells (Kaur and Jain, 2012). Deriu et al. (2013) indicated that the mild inflammatory response is regularly observed after 7 D of ST inoculation. The supplementation of 1, 3, and 5% of Treh in diets reduced the infiltrations of immune cells and maintained the integrity of intestinal epithelial cells in the cecum of broilers after the inoculation of ST (Figures 1C–1E).
Several studies demonstrated that the NFκB activation could be stimulated by LPS (Iwasaki and Medzhitov, 2010, Ziglam et al., 2013), further promoting the secretions of proinflammatory cytokines. Based on the results (Figure 2), the Treh addition in diets of ST-inoculated groups suppressed the expressions of inflammatory-related cytokine genes and simultaneously promoted the gene expressions of anti-inflammatory cytokines. According to the literature, IL-10 could suppress the production of proinflammatory cytokine by antigen-presenting cells and T cells, and IFNα promoted epithelial regeneration and induced IL-10-producing cells secreted by dendritic cells (Neurath, 2014). Another study reported that the Lactobacillus rhamnosus could upregulate the mRNA expression of IL-10 receptor which is associated with signal transducer and activator in suppressors of cytokine signaling family (Mirpuri et al., 2012). Hence, it is speculated that the mechanism of Treh supplementation to alleviate inflammatory effects from ST inoculation may result from the promotion of growth of lactobacilli (Table 3) and upregulation of the expression of anti-inflammatory cytokines (IL-10 and IFNα) (Figures 2G, 2H), thus suppressing the proinflammatory cytokines (Figures 2A–2F). Besides, the reduced abundance of ST in Treh-supplemented groups may contribute to less amount of LPS in the cecum, not sufficient to activate the TLR4-NFκB pathway for the downstream inflammatory responses (Table 2).
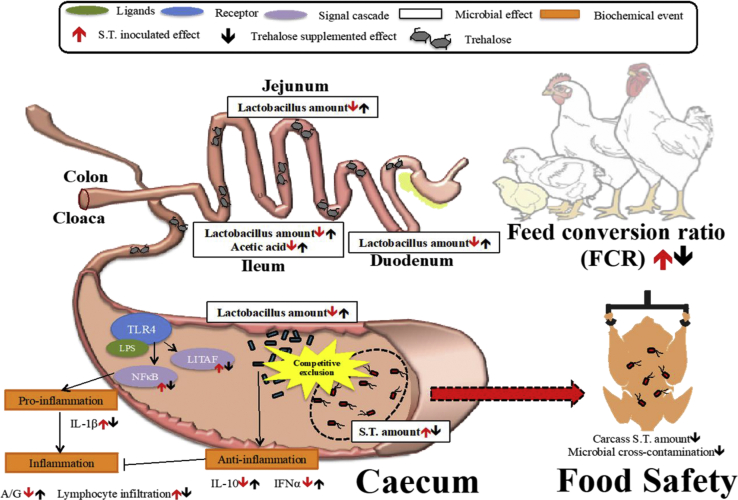
According to the results in the present study (Figure 3), supplementing 5% Treh in ST-inoculated model alleviated the adverse effects of ST challenge on daily feed intake and FCR of affected broilers. Furthermore, a trehalose addition increased the abundance of lactobacilli but suppressed the growth of ST in the cecum. Treh supplementation also counteracted the damage and inflammation in the liver and cecum caused by the ST. The anti-inflammatory effects of Treh supplementation were demonstrated by the results of serum biochemistry and cecal histopathology. Those effects further associated with the modulations of inflammatory-related cytokines (IL-10, IFNα, and LPS upregulated NFκB-related pathway). Therefore, Treh could act as a promising candidate for prebiotic applied for the commercial broiler farms against the adverse effects on growth performance and inflammation caused by the ST infection. Moreover, Salmonella pathogen regularly causes human illnesses resulted from contaminations to food from poultry side, eliciting the critical concern on public health. A poor management of Salmonella not merely promotes consumers not to consume poultry products but also contributes economic losses to the industry. In addition to the benefit from disease prevention, the reduced abundance of ST in the cecum of broilers decreases the possibility of cross-contamination during slaughtering. The utilization of trehalose as the feed additives suggests not only to advantage economic production but also to promote food safety in the industry.
Figure 3.
Beneficial effects of trehalose supplementation against the S. Typhimurium (ST) infection in the cecum of broilers. Abbreviations: A/G ratio, albumin/globulin ratio; IFNα, interferon alpha; IL-1β, interleukin-1 beta; IL-10, interleukin 10.
Acknowledgments
The authors acknowledge the funding of this research by the Ministry of Science and Technology, Taiwan (Project: MOST 105-2313-B-002-031) and Council of Agriculture, Executive Yuan, Taiwan (Project: 107AS-1.2.1-AD-U5, 107AS-15.4.1-ST-a5, 108AS-14.1.1-ST-a2, and 108AS-2.2.2-AD-U1). This study was approved by the NTU Institution Animal Care and Use Committee (IACUC No. 103-EL-008).
Conflict of Interest Statement: The authors declare no conflict of interest.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.psj.2020.03.053.
Supplementary data
References
- Center for Disease Control and Prevention (CDC) 2017. Surveillance for Foodborne Disease Outbreaks, United States, 2015, Annual Report. U.S. Department of Health and Human Services, CDC, Atlanta, GA. https://www.cdc.gov/foodsafety/pdfs/2015FoodBorneOutbreaks_508.pdf
- Center for Disease Control and Prevention (CDC). 2014 Foodborne Diseases Active Surveillance Network (FoodNet): FoodNet Surveillance Report for 2012 (Final Report). U.S. Department of Health and Human Services, CDC, Atlanta, GA. https://www.cdc.gov/foodnet/PDFs/2012_annual_report_508c.pdf
- Chalghoumi R., Marcq C., Thewis A., Portetelle D., Beckers Y. Effects of feed supplementation with specific hen egg yolk antibody (immunoglobin Y) on Salmonella species cecal colonization and growth performances of challenged broiler chickens. Poult. Sci. 2009;88:2081–2092. doi: 10.3382/ps.2009-00173. [DOI] [PubMed] [Google Scholar]
- Chen Y.S., Srionnual S., Onda T., Yanagida F. Effects of prebiotic oligosaccharides and trehalose on growth and production of bacteriocins by lactic acid bacteria. Lett. Appl. Microbiol. 2007;45:190–193. doi: 10.1111/j.1472-765X.2007.02167.x. [DOI] [PubMed] [Google Scholar]
- Chotinsky D., Toncheva E., Profirov Y. Development of disaccharidase activity in the small intestine of broiler chickens. Br. Poult. Sci. 2001;42:389–393. doi: 10.1080/00071660120055386. [DOI] [PubMed] [Google Scholar]
- Dahiya J.P., Wilkie D.C., Van Kessel A.G., Drew M.D. Potential strategies for controlling necrotic enteritis in broiler chickens in post-antibiotic era. Anim. Feed Sci. Technol. 2006;129:60–88. [Google Scholar]
- Deriu E., Liu J.Z., Pezeshki M., Edwards R.A., Ochoa R.J., Contreras H., Libby S.J., Fang F.C., Raffatellu M. Probiotic bacteria reduce Salmonella Typhimurium intestinal colonization by competing for iron. Cell Host Microbe. 2013;14:26–37. doi: 10.1016/j.chom.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber T.A., Dilger R.N., Iakiviak M., Hopkins A.C., Price N.P., Fahey G.C. Ingestion of a novel galactoglucomannan oligosaccharide-arabinoxylan (GGMO-AX) complex affected growth performance and fermentative and immunological characteristics of broiler chicks challenged with Salmonella Typhimurium. Poult. Sci. 2012;91:2241–2254. doi: 10.3382/ps.2012-02189. [DOI] [PubMed] [Google Scholar]
- Figueroa-González I., Quijano G., Ramirez G., Cruz-Guerrero A. Probiotics and prebiotics-perspectives and challenges. J. Sci. Food Agric. 2011;91:1341–1348. doi: 10.1002/jsfa.4367. [DOI] [PubMed] [Google Scholar]
- Garcia K.O., Berchieri A., Jr., Santana A.M., Alarcon M.F.F., Freitas Neto O.C., Fagliari J.J. Experimental infection of commercial layers with wild or attenuated Salmonella Gallinarum mutant strains: anatomic pathology, total blood cell count and serum protein levels. Rev. Bras. Cienc. Avic. 2013;15:91–104. [Google Scholar]
- Gast R.K. Paratyphoid infections. In: Saif Y.M., Fadly A.M., Glisson J.R., McDougald L.R., Nolan L.K., Swayne D.E., editors. Diseases of Poultry. 12th ed. Blackwell Publishing Professionals; Ames, IA: 2008. pp. 636–665. [Google Scholar]
- Gibson G.R., Roberfroid M.B. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J. Nutr. 1995;125:1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- Gómez B., Míguez B., Yáñez R., Alonso J.L. Manufacture and properties of glucomannans and glucomannooligosaccharides derived from konjac and other sources. J. Agric. Food Chem. 2017;65:2019–2031. doi: 10.1021/acs.jafc.6b05409. [DOI] [PubMed] [Google Scholar]
- Haarman M., Knol J. Quantitative real-time PCR analysis of fecal Lactobacillus species in infants receiving a prebiotic infant formula. Appl. Environ. Microbiol. 2006;72:2359–2365. doi: 10.1128/AEM.72.4.2359-2365.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harr K.E. Clinical chemistry of companion avian species: a review. Vet. Clin. Pathol. 2002;31:140–151. doi: 10.1111/j.1939-165x.2002.tb00295.x. [DOI] [PubMed] [Google Scholar]
- Ivey M., Massel M., Phister T.G. Microbial interactions in food fermentations. Annu. Rev. Food Sci. Technol. 2013;4:141–162. doi: 10.1146/annurev-food-022811-101219. [DOI] [PubMed] [Google Scholar]
- Iwasaki A., Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327:291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.H., Jung B.Y., Rayamahij N., Lee H.S., Jeon W.J., Choi K.S., Kweon C.H., Yoo H.S. A multiplex real-time PCR for differential detection and quantification of Salmonella spp., Salmonella enterica serovar Typhimurium and Enteritidis in meats. J. Vet. Sci. 2009;10:43–51. doi: 10.4142/jvs.2009.10.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeij J.T. Avian clinical biochemistry. In: Kaneko J.J., Harvey J.W., Bruss M.L., editors. Clinical Biochemistry of Domestic Animals. 6th ed. Academic Press; New York, NY: 2008. pp. 838–872. [Google Scholar]
- Kaur J., Jain S.K. Role of antigens and virulence factors of Salmonella enterica serovar Typhi in its pathogenesis. Microbiol. Res. 2012;167:199–210. doi: 10.1016/j.micres.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Kim G.B., Seo Y.M., Kim C.H., Paik I.K. Effect of dietary prebiotic supplementation on the performance, intestinal microflora, and immune response of broilers. Poult. Sci. 2011;90:75–82. doi: 10.3382/ps.2010-00732. [DOI] [PubMed] [Google Scholar]
- Kostrzynska M., Bachand A. Use of microbial antagonism to reduce pathogen levels on produce and meat products: a review. Can. J. Microbiol. 2006;52:1017–1026. doi: 10.1139/w06-058. [DOI] [PubMed] [Google Scholar]
- Marcq C., Cox E., Szalo I.M., Thewis A., Beckers Y. Salmonella Typhimurium oral challenge model in mature broilers: bacteriological, immunological, and growth performance aspects. Poult. Sci. 2011;90:59–67. doi: 10.3382/ps.2010-01017. [DOI] [PubMed] [Google Scholar]
- Mirpuri J., Sotnikov I., Myers L., Denning T.L., Yarovinsky F., Parkos C.A., Denning P.W., Louis N.A. Lactobacillus rhamnosus (LGG) regulates IL-10 signaling in the developing murine colon through upregulation of the IL-10R2 receptor subunit. PLoS One. 2012;7:e51955. doi: 10.1371/journal.pone.0051955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurath M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014;14:329–342. doi: 10.1038/nri3661. [DOI] [PubMed] [Google Scholar]
- Rescigno M., Urbano M., Valzasina B., Francolini M., Rotta G., Bonasio R., Granucci F., Kraehenbuhl J.P., Ricciardi-Castagnoli P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- Salcedo S.P., Noursadeghi M., Cohen J., Holden D.W. Intracellular replication of Salmonella Typhimurium strains in specific subsets of splenic macrophages in vivo. Cell Microbiol. 2001;3:587–597. doi: 10.1046/j.1462-5822.2001.00137.x. [DOI] [PubMed] [Google Scholar]
- Salem R., El-Habashi N., Fadl S.E., Sakr O.A., Elbialy Z.I. Effect of probiotic supplement on aflatoxicosis and gene expression in the liver of broiler chicken. Environ. Toxicol. Pharmacol. 2018;60:118–127. doi: 10.1016/j.etap.2018.04.015. [DOI] [PubMed] [Google Scholar]
- Scallan E., Hoekstra R.M., Angulo F.J., Tauxe R.V., Widdowson M.A., Roy S.L., Jones J.L., Griffin P.M. Foodborne illness acquired in the United States–major pathogens. Emerg. Infect. Dis. 2011;17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring P., Wenk C., Dawson K.A., Newman K.E. The effects of dietary mannanoligosaccharides on cecal parameters and the concentrations of enteric bacteria in the ceca of Salmonella-challenged broiler chicks. Poult. Sci. 2000;79:205–211. doi: 10.1093/ps/79.2.205. [DOI] [PubMed] [Google Scholar]
- Tang X., Marciano D.L., Leeman S.E., Amar S. LPS induces the interaction of a transcription factor, LPS-induced TNF- alpha factor, and STAT6(B) with effects on multiple cytokines. Proc. Natl. Acad. Sci. U. S. A. 2005;102:5132–5137. doi: 10.1073/pnas.0501159102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thitaram S.N., Chung C.H., Day D.F., Hinton A., Bailey J.S., Siragusa G.R. Isomaltooligosaccharide increases cecal Bifidobacterium population in young broiler chickens. Poult. Sci. 2005;84:998–1003. doi: 10.1093/ps/84.7.998. [DOI] [PubMed] [Google Scholar]
- Tomasik P.J., Tomasik P. Probiotics and prebiotics. Cereal Chem. 2003;80:113–117. [Google Scholar]
- Ziglam H.M., Daniels I., Finch R.G. Immunomodulating activity of rifampicin. J. Chemother. 2013;16:357–361. doi: 10.1179/joc.2004.16.4.357. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.