Abstract
Less evidence is available currently to reveal whether the immune system and productivity of laying hens change under long periods of ammonia exposure in hot climate. The present study was conducted to determine the effects of chronic exposure to high temperature and ammonia concentrations on health, immune response, and reproductive hormones of commercial laying hens. A total of five hundred and seventy six 20-week-old laying hens (Hy-Line Brown) were used in this study. Birds were housed in cages (4 birds per cage) and received 16-wk treatments in 6 artificial environmental chambers. Hens were allocated to 6 treatments: treatment 1 (T1, 20°C, ≤5 ppm, control group), treatment 2 (T2, 20°C, 20 ppm), treatment 3 (T3, 20°C, 45 ppm), treatment 4 (T4, 35°C, ≤5 ppm), treatment 5 (T5, 35°C, 20 ppm), and treatment 6 (T6, 35°C, 45 ppm). Blood samples were collected at 22, 26, 30, 34, and 38 wk of age and plasma IgG, IgM, IgA, corticosterone (CORT), total antioxidant capacity (T-AOC), luteinizing hormone (LH), estradiol (E2), and follicular stimulating hormone (FSH) were measured. The results of this study showed that high ambient temperature and excessive ammonia increased the concentration of IgG but decreased the concentration of IgA, T-AOC, LH, FSH, and E2 of hens compared with those of the control birds. From the age of 34 wk, significantly increased concentrations of IgG were observed in hens exposed to moderate and high levels of ammonia. CORT level showed marked differences between the treatments only at the age of 26 wk. In addition, LH and E2 of hens demonstrated significant differences among the treatments in the middle and later stages of the experiment, while FSH levels of the control birds were significantly higher than the others at the age of 38 wk. Excessive ammonia in high temperature was a physiological stress factor that had a negative effect, which inhibited immune function and impacted the reproductive hormones.
Key words: heat stress, ammonia, blood parameter, immunity, laying hen
Introduction
Aerial ammonia is recognized as one of the most noxious odors in poultry operations, and its negative effect on the environment has been documented by numerous studies (Koerkamp and Bleijenberg, 1998, Koerkamp et al., 1998, Wheeler et al., 2000). Poultry production industry, as the largest contributor to ammonia emissions of all animal husbandry operations, has undergone considerable surveillance from public and regulatory agencies due to their environmental impacts (Hale et al., 2010, Lin et al., 2017). Meanwhile, poultry companies have to be concerned not only for worker health, but also for poultry health because elevated concentrations of atmospheric ammonia have an adverse impact on bird physiology and productivity (Carlile, 1984, Ritz et al., 2006, Almuhanna, 2011a, Almuhanna et al., 2011b; Kearney et al., 2014, Nemer et al., 2015). Ammonia is known as an irritant alkaline air contaminant, with occupational limits set at 50 ppm (OSHA, 2012) for the 8-h permissible exposure limit or 25 ppm (NIOSH, 2016), and a level of 300 ppm is considered to be immediately dangerous to human life and health (Wheeler et al., 2000).
Exposure of poultry to ammonia has led to significant impacts on health and growth performance. Excessive ammonia has been a physiological stress factor that reduced feed intake and stunt growth (Charles and Payne, 1966, Deaton et al., 1984, Beker et al., 2004, Miles et al., 2004, Miles et al., 2006, Wei et al., 2014), decreased egg production significantly with 7-wk exposure to ammonia at an ammonia concentration of 102 ppm (Charles and Payne, 1966), and adversely affected egg quality (Cotterill and Nordskog, 1954). Moreover, it damaged the respiratory tract and lung atrial wall (Nagaraja et al., 1983, Kristensen and Wathes, 2000), and increased the incidence of diseases and secondary infections such as Newcastle disease, airsacculitis, and the prevalence of Mycoplasma gallisepticum (Anderson et al., 1964, Sato et al., 1973, Oyetunde et al., 1978). Controversial results indicated that excessive ammonia exposure lowered the immunological response of birds, which depended on bird age as well as ammonia level and duration. For broiler chickens, the hemagglutination inhibition antibody titer of Newcastle disease virus was significantly lower when exposed to 26 and 52 ppm ammonia compared to the 0 ppm treatment group for 21 D (Wang et al., 2010). On the contrary, average body weights, air sac scores, lung weights, relative bursa of Fabricius weights, and carcass grades were not significantly different among the birds exposed to ammonia concentration at 0, 25, or 50 ppm for 49 D (Caveny et al., 1981).
Previous studies have reported that the ammonia level inside poultry houses was affected by multiple factors, such as housing system, ventilation, manure management, bird density (Tasistro et al., 2007, David et al., 2015, Zhao et al., 2015), and components of the aerial environment including temperature, humidity, dust, and pathogens. All these may interact with ammonia (Koon et al., 1963, Dennis and Gee, 1973, Feddes et al., 1985, Kristensen and Wathes, 2000). Wathes et al. (1997) reported that the mean ammonia concentration of cage houses was as high as 24.2 ppm in summer. Miles et al. (2011) found that the maximum ammonia level was up to 7 times greater at 40.6°C vs. 18.3°C. A study carried out in floor systems revealed that most houses exceeded concentrations of 25 ppm ammonia, and in some areas went up to 80 ppm (David et al., 2015). All these studies indicated that high temperature was an important factor resulting in excessive ammonia. Additionally, both acute and chronic heat stress (≥35°C) have been documented to have an adverse influence on egg production and feed intake of laying hens (Deaton et al., 1981, Mahmoud et al., 1996, Mashaly et al., 2004). In terms of physiological health, previous studies have shown that heat stress impaired the activity of antioxidant enzymes and the redox system of the bird (Altan et al., 2003, Lin et al., 2008). Heat stress also resulted in the disruption of hormones responsible for ovulation and a decrease in responsiveness of granulosa cells to luteinizing hormone (LH) (Donoghue et al., 1989, Novero et al., 1991).
Various parameters have been used to evaluate the health and immune response in birds. Immunoglobulins G, M, and A are the 3 major classical Ig secreted by immune B-cells for the host against non-self antigens (Mestecky et al., 1999, Suzuki et al., 2007, Gomes et al., 2014). Total antioxidant capacity (T-AOC) was considered as an important indicator of animal health (Aslan et al., 2005, Gao et al., 2013, Zeng et al., 2014).
Laying hens live longer than broilers in poultry houses and may develop different disorders when suffering from extended periods of ammonia exposure (Beker et al., 2004). Although there are a multitude of researches on the adverse effects of ammonia on the health and performance of poultry, currently less evidence is available to reveal whether the immune system and productivity of laying hens change under long periods of ammonia exposure in hot climate. Therefore, this study was carried out to evaluate the effects of heat stress and ammonia on blood parameters and the interaction between them.
Materials and methods
Experimental Chambers
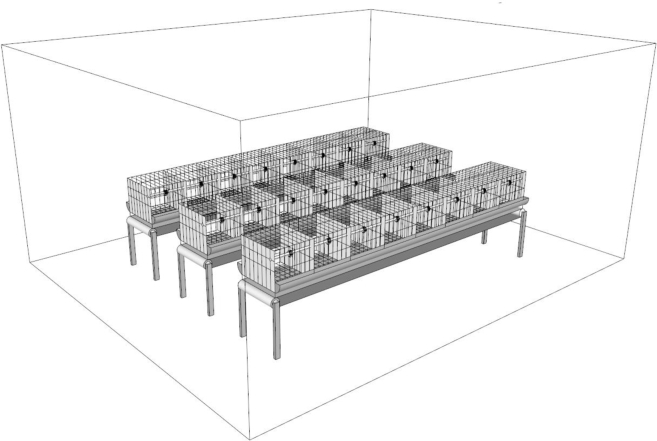
Six artificial environmental chambers (each 24.8 m2, 4.5 × 5.5 m) in the College of Animal Science and Technology (Hebei Agricultural University, China) were used for the experiment. Cages (50 × 40 × 40 cm, L × W × H) were divided into 3 groups (8 cages per group) and were set evenly in the chamber for hen rearing (Figure 1). The chambers were computer programmed to keep the environmental conditions as required. Sensors installed in these chambers were used to monitor temperature, humidity, ammonia, and carbon dioxide concentration every 10 s, which were then displayed on the computer screen for observation.
Figure 1.
Schematic diagram of cages arrangement in each experimental chamber.
The supply system of ammonia gas has been studied in detail earlier (Kristensen et al., 2000) in order to maintain the desired ammonia concentrations. Compressed anhydrous ammonia was stored in a cylinder and its flow rate was adjusted by a flow meter. This design enabled each chamber to be filled with gaseous ammonia independently of the others.
Light emitting diode lamps were fixed on the ceiling of the chamber and could be adjusted to provide the required light intensity. Temperature was adjusted automatically by an air conditioner, and air inlet and outlet were trepanned for mechanical ventilation. Feeder and drinker were provided in each chamber and video cameras were fitted.
Bird Management and Experimental Design
A total of 576 Hy-Line Brown hens acquired from a commercial farm were used for this study. The study proposal was approved by the Laboratory Animal Ethical Committee of China Agricultural University. Birds were acclimatized for 2 wk in the chambers at the age of 20 wk before treatment. There were a total of 6 treatments of 3 ammonia concentration exposures (≤5, 20, and 45 ppm) under 2 temperatures (20 ± 2°C, 35 ± 2°C) as shown in Table 1. T1 was the control group. Birds were randomly assigned to 6 groups and were transferred to the cages (4 birds per cage). Each treatment had 3 replications with 32 birds per replicate.
Table 1.
Experiment treatments.
T, temperature; AC, ammonia concentration.
T1–T6 = treatment 1 to treatment 6.
All birds were provided with a corn-soybean basal diet; feed and water were provided ad libitum. Ambient relative humidity was maintained at 40 to 60% and carbon dioxide level was kept below 1,000 ppm during the experimental period.
The duration of light exposure during the growing period from 20 wk of age was 13.5 h (4:00–17:30) per day for hens in the first week and then stepped up gradually per week until it reached 16 h (4:00–20:00) at 31 wk of age. From then on, permanent illumination of 16 h was employed from 32 to 38 wk of age. Besides, light intensity of 30 lx was equalized at bird head level following the Hy-Line Brown Layers Guide Manual (Hy-Line International; http://www.hyline.com).
Sample Preparation and Collection
For each sampling, 4 birds were randomly selected from each replicate and marked with tags for the following operation. Wing venipuncture was performed for blood collection after fasting for 12 h starting at week 22, 26, 30, 34, and 38. Plasma was prepared after centrifugation and stored at −20°C.
Measured Contents and Methods
The concentrations of plasma Ig (IgG, IgA, and IgM), corticosterone (CORT), T-AOC, and reproductive hormones (LH, estradiol [E2], and follicular stimulating hormone [FSH]) were detected using commercially available ELISA kits (Jianglai Biological Technology Co. Ltd., Shanghai, China).
Statistical Analysis
All statistical analyses were performed using linear mixed models parameterized with SPSS (IBM SPSS Statistics 25.0; IBM Corporation, Armonk, NY, USA). The data were analyzed with fixed effects of temperature, ammonia concentration, week of age, and the random effect of replicate. The model equation was as follows:
where AC = ammonia concentration; Yijku = parameters investigated; μ = model constant; Ti = effect of temperature (i = 1–2); ACj = effect of ammonia concentration (j = 1–3); WOAk = effect of age (k = 1–5); Ru = effect of replicate (u = 1–3); T × ACij = effect of interaction between temperature and ammonia concentration; T × WOAik = effect of interaction between temperature and week of age; AC × WOAjk = effect of interaction between ammonia concentration and week of age; and εijk = the residual error term.
Effects in the statistical model were tested simultaneously and they were removed from the original model when they were not significant. When the effect was statistically different (P < 0.05), further analysis was needed. Duncan's multiple range (least significant difference) test was applied for post-hoc group comparisons.
Results
Statistical analysis showed that for all the blood parameters, the effect of replicate was not significant and it was excluded from the original model.
Plasma Ig Concentrations
As shown in Table 2, temperature, ammonia concentration, and bird age had significant effects on IgG and IgA (P < 0.05). However, IgM was not affected by temperature, ammonia concentration, and bird age (P > 0.05). The concentration of IgG was increased with increasing temperature. However, the concentration of IgA was decreased under 35°C treatment. Compared with the treatment of low ammonia concentration (≤5 ppm), the level of IgG was significantly increased under ammonia concentrations of 20 and 45 ppm (P < 0.05), while the concentrations of IgA were significantly decreased (P < 0.05). Furthermore, IgG increased steadily with age (P < 0.05).
Table 2.
Plasma Ig concentrations of laying hens at 22, 26, 30, 34, and 38 WOA exposed to different ammonia concentrations under 20°C and 35°C.
| Item1 | IgG (μg/mL) | IgM (μg/mL) | IgA (μg/mL) |
|---|---|---|---|
| T (°C) | |||
| 20 | 1,467.53 ± 25.32a | 465.74 ± 4.91 | 217.18 ± 2.64a |
| 35 | 1,522.54 ± 27.14b | 476.44 ± 5.00 | 202.31 ± 3.27b |
| AC (ppm) | |||
| ≤5 | 1,350.32 ± 20.48c | 469.71 ± 5.31 | 224.09 ± 3.52a |
| 20 | 1,512.02 ± 29.85b | 468.58 ± 5.64 | 201.68 ± 3.29b |
| 45 | 1,622.76 ± 38.75a | 474.97 ± 7.17 | 203.47 ± 3.94b |
| WOA | |||
| 22 | 1,274.80 ± 23.54c | 483.02 ± 7.98 | 216.74 ± 3.90a |
| 26 | 1,308.15 ± 23.99c | 481.26 ± 7.53 | 211.66 ± 4.50a,b |
| 30 | 1,342.49 ± 23.80c | 469.55 ± 8.44 | 198.53 ± 4.00b |
| 34 | 1,674.38 ± 30.10b | 465.32 ± 7.24 | 204.68 ± 5.47a,b |
| 38 | 1,875.34 ± 46.28a | 456.29 ± 7.78 | 217.13 ± 5.50a |
| P-value2 | |||
| T | <0.05 | NS | <0.05 |
| AC | <0.05 | NS | <0.05 |
| WOA | <0.05 | NS | <0.05 |
| T × AC | NS | <0.05 | <0.05 |
| T × WOA | NS | NS | <0.05 |
| AC × WOA | <0.05 | <0.05 | <0.05 |
a–cMeans within main effects without a common letter differ (P < 0.05).
T, temperature; AC, ammonia concentration; WOA, week of age.
NS, no significance.
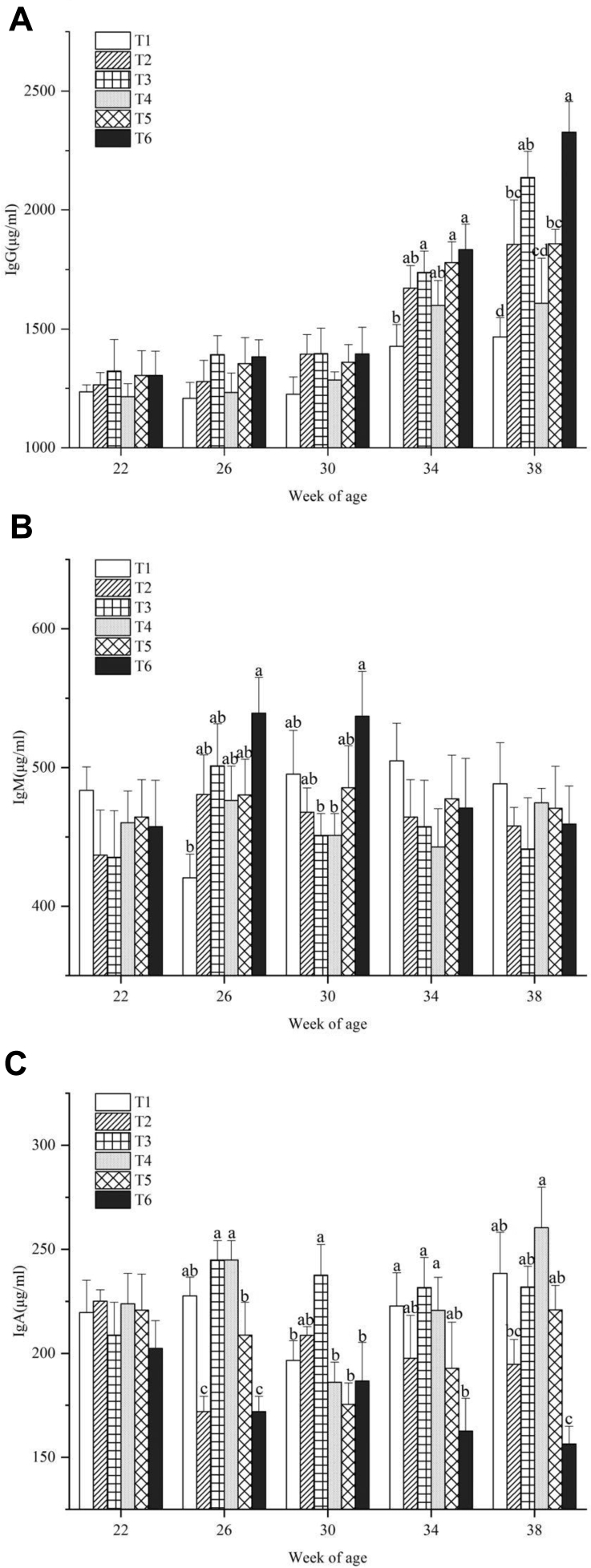
Less change was observed in the plasma concentration of IgG and no significant difference was found among the 6 treatments from 22 to 30 wk of age (Figure 2). A significant increase of IgG occurred in all treatments at 34 wk of age (P < 0.05), and this kept increasing until 38 wk of age. Exposure to high temperature and ammonia resulted in a significant elevation in IgG (P < 0.05). At 34 wk of age, plasma IgG concentrations of hens in T6, T5, and T3 were significantly higher than those of T1 (P < 0.05), and the differences increased at 38 wk of age (P < 0.05). IgM and IgA levels of birds varied from 22 to 38 wk of age. Compared to other treatments, the IgA level in T6 was significantly lower at week 38 (P < 0.05).
Figure 2.
(A) Plasma IgG, (B) IgM, and (C) IgA concentrations of laying hens exposed to different temperatures and ammonia concentrations at 22, 26, 30, 34, and 38 wk of age. Data represent mean ± SE. a–dValues marked with different letters are significantly different (P < 0.05).
Plasma CORT and T-AOC Levels
Table 3 shows that CORT level was affected by temperature and week of age (P < 0.05) significantly and a 2-way interaction was found between them. All factors significantly affected the levels of T-AOC (P < 0.05). Levels of CORT and T-AOC were significantly decreased with increased temperature (P < 0.05). Furthermore, the concentrations of T-AOC were significantly decreased with increased ammonia concentrations (P < 0.05). T-AOC increased with age and significant differences were found (P < 0.05). Levels of CORT peaked (73.06 ng/mL) at 26 wk of age (P < 0.05), compared to that at 22 wk of age (63.37 ng/mL), and decreased (62.51 ng/mL) until 38 wk of age.
Table 3.
Plasma CORT and T-AOC levels of laying hens at 22, 26, 30, 34, and 38 WOA exposed to different ammonia concentrations under 20°C and 35°C.
| Item1 | CORT (ng/mL) | T-AOC (U/mL) |
|---|---|---|
| T (°C) | ||
| 20 | 68.09 ± 1.03a | 9.11 ± 0.32a |
| 35 | 66.56 ± 0.86b | 8.15 ± 0.27b |
| AC (ppm) | ||
| ≤5 | 65.90 ± 0.94 | 9.94 ± 0.47a |
| 20 | 65.14 ± 1.05 | 8.53 ± 0.33b |
| 45 | 68.66 ± 1.45 | 7.41 ± 0.22c |
| WOA | ||
| 22 | 63.37 ± 1.36b | 5.87 ± 0.12c |
| 26 | 73.06 ± 1.56a | 6.04 ± 0.13c |
| 30 | 69.84 ± 1.31a | 6.58 ± 0.19c |
| 34 | 64.05 ± 1.59b | 11.17 ± 0.32b |
| 38 | 62.51 ± 1.34b | 13.67 ± 0.49a |
| P-value2 | ||
| T | <0.05 | <0.05 |
| AC | NS | <0.05 |
| WOA | <0.05 | <0.05 |
| T × AC | NS | <0.05 |
| T × WOA | NS | <0.05 |
| AC × WOA | <0.05 | <0.05 |
a–cMeans within main effects without a common letter differ (P < 0.05).
Abbreviations: CORT, corticosterone; T-AOC, total antioxidant capacity.
T, temperature; AC, ammonia concentration; WOA, week of age.
NS, no significance.
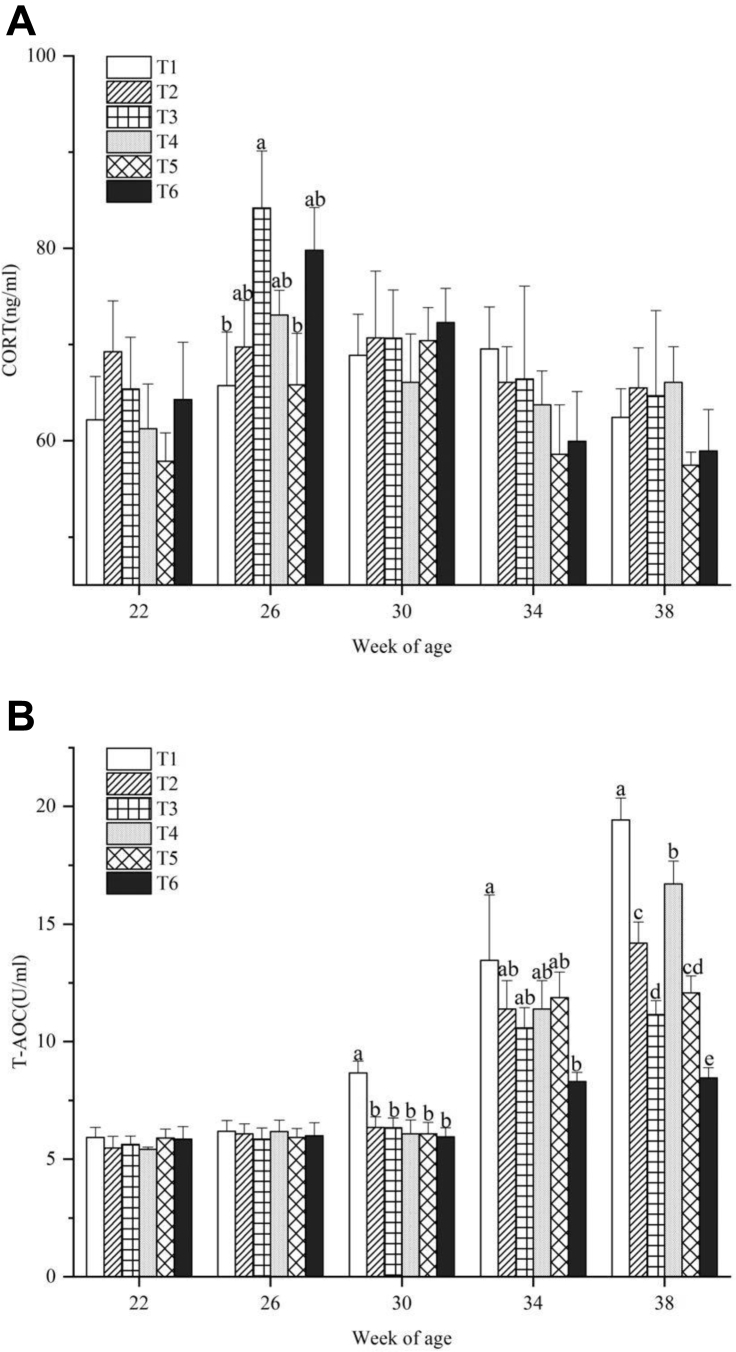
Blood CORT and T-AOC concentrations of the laying hens exposed to different ammonia concentrations and temperatures are shown in Figure 3. An increase in CORT was observed after 4 wk of treatment at the age of 26 wk. Then, CORT levels decreased at 30 wk of age and this was maintained until the end of the experiment at 38 wk. Significant differences among the treatments were found only at 26 wk of age (P < 0.05). Birds exposed to high ammonia concentrations had significantly higher CORT levels than birds in T1 (P < 0.05). Less change was observed in plasma T-AOC at 22 and 26 wk of age. Then, plasma T-AOC of T1 increased and was significantly higher compared to the other groups at 30 wk of age. At the age of 34 wk, the T-AOC levels of birds in all treatments increased significantly and at 38 wk they reached their maximum. From 30 wk of age, the plasma T-AOC level of birds in T1 was significantly higher (P < 0.05) compared to the high temperature and ammonia treatments and the difference increased significantly with age.
Figure 3.
(A) Plasma corticosterone (CORT) and (B) total antioxidant capacity (T-AOC) levels of laying hens exposed to different temperatures and ammonia concentrations at 22, 26, 30, 34, and 38 wk of age. Data represent mean ± SE. a–eValues marked with different letters are significantly different (P < 0.05).
Plasma LH, E2, and FSH
As presented in Table 4, temperature, ammonia concentration, and age significantly affected the levels of LH and E2 (P < 0.05). Temperature and ammonia concentration had a significant influence on FSH. Moreover, LH, E2, and FSH levels were significantly decreased with higher temperature (P < 0.05). High ammonia concentrations of 45 ppm significantly decreased the levels of these 3 hormones compared to those at low ammonia concentration (≤5 ppm) (P < 0.05).
Table 4.
Plasma LH, FSH, and E2 levels of laying hens at 22, 26, 30, 34, and 38 WOA exposed to different ammonia concentrations under 20°C and 35°C.
| Item1 | LH (pg/mL) | FSH (mIU/mL) | E2 (pg/mL) |
|---|---|---|---|
| T (°C) | |||
| 20 | 53.89 ± 0.69a | 8.34 ± 0.16a | 94.64 ± 1.49a |
| 35 | 45.48 ± 0.80b | 7.68 ± 0.14b | 81.95 ± 1.30b |
| AC (ppm) | |||
| ≤5 | 51.99 ± 1.02a | 8.55 ± 0.20a | 99.32 ± 0.78a |
| 20 | 49.88 ± 0.90a,b | 7.87 ± 0.17b | 86.64 ± 1.50b |
| 45 | 47.19 ± 1.02b | 7.61 ± 0.17b | 78.92 ± 1.60c |
| WOA | |||
| 22 | 49.37 ± 0.95b | 7.80 ± 0.23 | 84.09 ± 1.74c |
| 26 | 55.49 ± 0.96a | 8.15 ± 0.22 | 85.08 ± 1.89b,c |
| 30 | 49.62 ± 1.37b | 7.63 ± 0.22 | 91.38 ± 2.29a,b |
| 34 | 48.38 ± 1.37b,c | 8.17 ± 0.23 | 93.53 ± 2.64a |
| 38 | 45.58 ± 1.40c | 8.31 ± 0.27 | 87.39 ± 2.76a,b,c |
| P-value2 | |||
| T | <0.05 | <0.05 | <0.05 |
| AC | <0.05 | <0.05 | <0.05 |
| WOA | <0.05 | NS | <0.05 |
| T × AC | <0.05 | <0.05 | NS |
| T × WOA | <0.05 | NS | <0.05 |
| AC × WOA | <0.05 | NS | NS |
a–cMeans within main effects without a common letter differ (P < 0.05).
Abbreviations: E2, estradiol; FSH, follicular stimulating hormone; LH, luteinizing hormone.
T, temperature; AC, ammonia concentration; WOA, week of age.
NS, no significance.
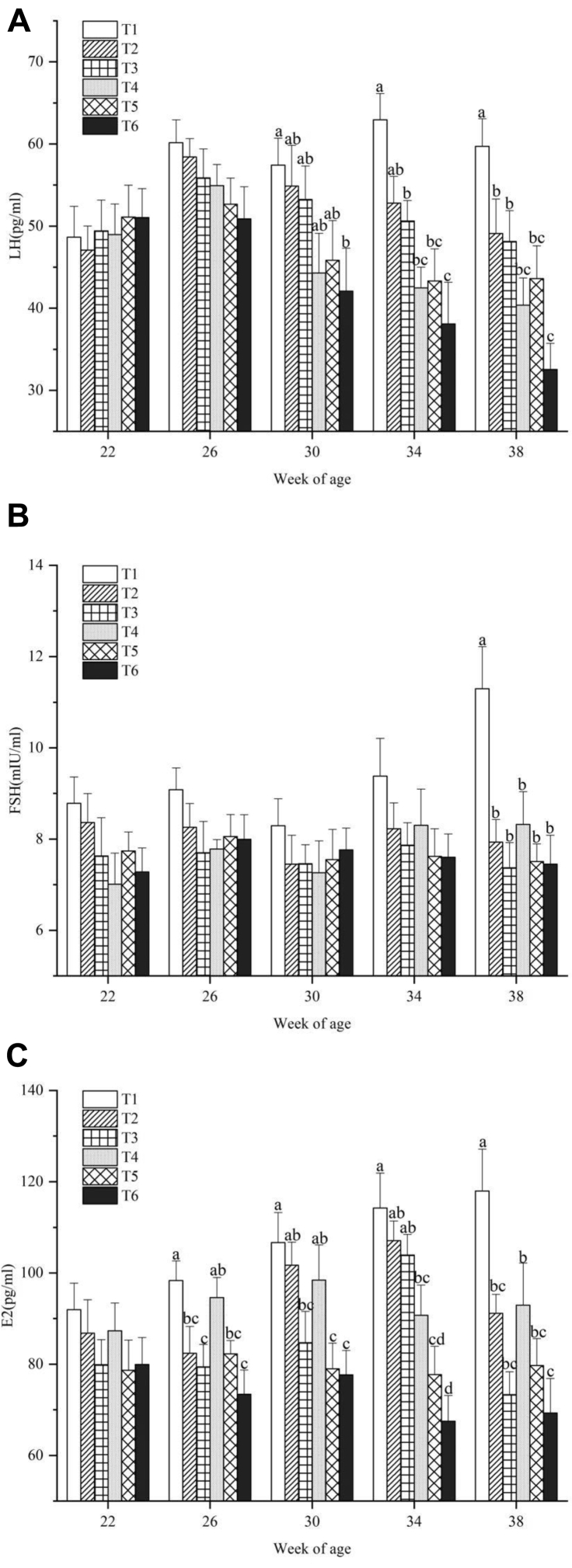
The change of plasma LH, FSH, and E2 concentrations in different treatments with age of birds is shown in Figure 4: A (LH), B (FSH), and C (E2). Significant differences of LH levels between T1 and T6 were observed at 30, 34, and 38 wk of age (P < 0.05). Hens treated with high temperature and ammonia concentration presented decreased LH levels. Plasma FSH of birds in T2 to T6 remained constant from 22 to 38 wk of age. In contrast, the FSH levels of birds in the control group increased with age. As a result, birds in the control group had a significantly higher level of FSH than birds subjected to high temperature and ammonia treatments at the age of 38 wk (P < 0.05). E2 levels showed a similar trend to LH. At each sampling week, T1 had the highest level of E2 in all treatments while T6 had the lowest level of E2.
Figure 4.
(A) Plasma luteinizing hormone (LH), (B) follicular stimulating hormone (FSH), and (C) estradiol (E2) levels of laying hens exposed to different temperatures and ammonia concentrations at 22, 26, 30, 34, and 38 wk of age. Data represent mean ± SE. a–dValues marked with different letters are significantly different (P < 0.05).
Discussion
The effect of temperature and ammonia on immune parameters and reproductive-related hormones of laying hens was investigated in this study. The results showed that the blood parameters of laying hens were affected by high temperature, ammonia concentration, and age, and there was an interaction between them.
Heat stress and ammonia exposure caused a significant increase in plasma levels of IgG at the age of 34 and 38 wk of laying hens. Previous studies have indicated that stressors, such as heat and ammonia, are able to elevate IgG levels (Nasrin et al., 2013, Honda et al., 2015, Wu et al., 2017), consistent with the results of this study. On the other hand, increased levels of IgM were observed at the age of 22 wk and it decreased from 26 wk in high temperature and ammonia treatments in this study. Honda et al. (2015) reported that birds exposed to 19-D heat stress of 38°C had higher plasma IgM levels. Meanwhile, Wu et al. (2017) indicated that plasma IgM level reduced after 45 wk of 30-ppm ammonia pollution. The early increase of IgM at the beginning of egg laying may have been caused by high temperature and late reduction was due to ammonia exposure. As the experiment went on, ammonia concentration had a greater effect on IgM than that of high temperature at peak egg production. The decrease of IgA caused by ammonia and heat stress may be explained by the result reported by Nasrin et al. (2013), who showed that heat stress and Newcastle disease virus resulted in increased IgA concentrations in the trachea, cecal tonsils, and Harderian gland. In other words, the concentration of IgA in stressed birds increased in lymphoid organs, where immune system activation occurs, but not in the peripheral blood. Therefore, the different changes of Ig (IgG, IgM, and IgA) may be determined by the type of stress and its intensity and duration. In addition, different types of stress had different effects on different periods of egg laying. Overall, the present study showed that heat and ammonia stress in the early laying period of laying hens impacts the immune system and activity for a long period of time.
Corticosterone is a common hormone that is released in many stress situations by the hypothalamus–pituitary–adrenal axis. It is a glucocorticoid that has been reported to dysregulate immune responses and is potentially harmful to the health of animals if it remains at a high level for a long period of time (Post et al., 2003). Shini et al. (2009) demonstrated that oral CORT treatment affected the physiology of hens, reduced performance, and may model the effects of production stressors. Negative correlation between plasma CORT levels and immune response under heat stress has been reported in birds (Elenkov et al., 2000, Quinteiro et al., 2010, Calefi et al., 2014). In our study, high temperature and ammonia exposure significantly increased the plasma level of CORT after 4-wk treatment, which indicated that both were stressors during the growth of laying hens. However, there were no statistically significant changes of measured CORT level in laying hens from 30 wk of age, which suggested that hens may have the capability to adapt to moderate and high levels of chronic ammonia stimulation and heat stress after a certain period of time. Therefore, CORT was not considered to be a stress indicator during prolonged exposure of high temperatures and ammonia from the age of 30 wk until the end of the experiment. The SEMs of CORT were relatively large, which may indicate that CORT varied widely among individuals. Oxidative stress was part of the stress response of birds to heat exposure and other stressors (Droge, 2002, Lin et al., 2006). Devi et al. (2000) and Thomas et al. (2000) reported that increased activities of antioxidant enzymes were considered to be a protective response against oxidative stress. In the present study, the T-AOC of layer hens in the control group was significantly higher than that of other treatments at 30 wk of age. It indicated that stress suppressed the T-AOC release of birds under chronic treatment. This result is in agreement with a research in ducks which mainly involved acute stress of 28 D and a lower concentration of T-AOC was found under 34°C treatment compared to the control group of 23°C (Ma et al., 2014). Earlier and current studies indicate that the antioxidant systems in the plasma of ammonia and heat-stressed birds are compromised as compared with those of the control birds.
Lin et al., 2004a, Lin et al., 2004b reported that broilers fed with a diet supplemented with 30 mg CORT/kg showed an increased nonenzymatic antioxidant such as T-AOC during a 14-D treatment, revealing that CORT can be an inducer of oxidative stress. According to previous and ongoing studies, ammonia and heat stress may cause oxidative stress in hens. Meanwhile, the possible influence of CORT should not be excluded in the induction of oxidative stress by chronic heat and ammonia exposure.
Levels of LH and hypothalamic gonadotropin-releasing hormone-I were negatively influenced by heat stress of 35°C (Donoghue et al., 1989, Novero et al., 1991), and additionally, a reduction of progesterone was found (Novero, et al., 1991). In this study, except the control group, the LH levels of all other treatments showed a declining trend from 26 wk of age, which suggested that chronic exposure to high temperature and ammonia may affect the LH level by influencing the function of hypophysis. Here, FSH increased steadily in the control birds to 11.30 mIU/mL, which was significantly higher compared to birds with other treatments where FSH was maintained around 7 to 8 mIU/mL. This suggested that there was no significant effect of heat stress and ammonia on hypophysis function of secreting FSH at the early and middle stages of treatments from 22 to 34 wk of age, which agreed with the studies of Rozenboim et al. (2007) in laying hens treated at temperatures of 42 (±3)°C for 15 D. However, at 38 wk of age, the control birds had a markedly higher (29.7–34.7%) FSH level than the birds in other treatments, which may be explained by the inhibitory effect of ammonia and temperature on the secretion of FSH that only emerged over a long period of accumulation. Rozenboim et al. (2007) also reported a decrease of E2 in heat-stressed hens with a 42°C treatment of 15 D and a small increase that was observed in the control group. Under long-term treatment of heat stress and ammonia, the plasma level of E2 in T6 was significantly lower than the control group, which may have been caused by depressing ovarian functions due to the decreased secretion of LH and FSH (Youngren et al., 1991, You et al., 1995, Rozenboim et al., 2007). Another possible mechanism for the reduction of ovarian function might be that heat stress can cause a reduction in blood flow to the ovary of cattle (Wolfenson et al., 1981, Wolfenson et al., 1997), which may be another explanation for the result of our study. All these results of the 3 reproductive hormones may have an influence on ovarian function, and then may result in a decrease in reproductive performance or reproductive failure.
This study indicated that heat stress and high concentration of ammonia were negative factors for the immune system and increased the incidence of diseases. Based on the changes of IgG, ammonia concentration and temperature should be more strictly controlled during peak egg production. In addition, according to the indication of Ig, a normal temperature at the beginning of the laying process should be maintained, while ammonia concentration should be focused on during peak egg production. The difference of CORT concentration in 6 treatments indicated that high temperature and ammonia concentration would increase the stress of laying hens, which is not conducive to the health of laying hens. Only in the middle and late stages of this experiment, the blood T-AOC concentrations of laying hens showed significant differences, which indicated that under heat stress, the hen's antioxidant capacity decreased significantly with ammonia concentration increasing after long periods of treatment. The reproductive hormone data presented in this study suggested that potential reproductive failure associated with high environmental temperature and ammonia concentration might be caused directly by depressing ovarian functions or decreasing the related hormones secreted by hypophysis.
Acknowledgments
This study was funded by the National Key R&D Program of China (grant number 2017YFE0122200) and the China Agricultural Research System (CARS-40). The authors also thank the staff of the College of Animal Science and Technology, Hebei Agricultural University.
Conflict of Interest Statement: The authors did not provide a conflict of interest statement.
References
- Almuhanna E.A. Characteristics of air contaminants in naturally and mechanically ventilated poultry houses in Al-Ahsa, Saudi Arabia. Trans. ASABE. 2011;54:1433–1443. [Google Scholar]
- Almuhanna E.A., Ahmed A.S., Alyousif Y. Effect of air contaminants on poultry immunological and production performance. Int. J. Poult. Sci. 2011;10:461–470. [Google Scholar]
- Altan O., Pabuccuoglu A., Altan A., Konyalioglu S., Bayraktar H. Effect of heat stress on oxidative stress, lipid peroxidation and some stress parameters in broilers. Br. Poult. Sci. 2003;44:545–550. doi: 10.1080/00071660310001618334. [DOI] [PubMed] [Google Scholar]
- Anderson D.P., Beard C.W., Hanson R.P. The adverse effects of ammonia on chickens including resistance to infection with Newcastle disease virus. Avian Dis. 1964;8:369–379. [PubMed] [Google Scholar]
- Aslan R., Dundar Y., Eryavuz A., Bulbul A., Kucukkurt I., Fidan A.F., Akinci Z. Effects of various quantities of Yucca schidigera powder (Deodorase) added to diets on the performance, some hematological and biochemical blood parameters, and total antioxidant capacity of laying hens. Rev. Med. Vet. (Toulouse) 2005;156:350–355. [Google Scholar]
- Beker A., Vanhooser S.L., Swartlzander J.H., Teeter R.G. Atmospheric ammonia concentration effects on broiler growth and performance. J. Appl. Poult. Res. 2004;13:5–9. [Google Scholar]
- Calefi A.S., Honda B.T.B., Costola-de-Souza C., de Siqueira A., Namazu L.B., Quinteiro W.M., Fonseca J.G.D., Aloia T.P.A., Piantino-Ferreira A.J., Palermo-Neto J. Effects of long-term heat stress in an experimental model of avian necrotic enteritis. Poult. Sci. 2014;93:1344–1353. doi: 10.3382/ps.2013-03829. [DOI] [PubMed] [Google Scholar]
- Carlile F.S. Ammonia in poultry houses: a Literature-review. World Poult. Sci. J. 1984;40:99–113. [Google Scholar]
- Caveny D.D., Quarles C.L., Greathouse G.A. Atmospheric ammonia and broiler cockerel performance. Poult. Sci. 1981;60:513–516. [Google Scholar]
- Charles D.R., Payne C.G. The influence of graded levels of atmospheric ammonia on chickens: I. Effects on respiration and on the performance of broilers and replacement growing stock. Br. Poult. Sci. 1966;7:177–187. doi: 10.1080/00071668608415622. [DOI] [PubMed] [Google Scholar]
- Cotterill O.J., Nordskog A.W. Influence of ammonia on egg white quality. Poult. Sci. 1954;33:432–434. [Google Scholar]
- David B., Mejdell C., Michel V., Lund V., Moe R. Air quality in alternative housing systems may have an impact on laying hen welfare. Part II—Ammonia. Animals. 2015;5:886–896. doi: 10.3390/ani5030389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deaton J.W., Reece F.N., Lott B.D. Effect of atmospheric ammonia on pullets at point of lay. Poult. Sci. 1984;63:384–385. doi: 10.3382/ps.0630384. [DOI] [PubMed] [Google Scholar]
- Deaton J.W., Reece F.N., Mcnaughton J.L., Lott B.D. Effect of differing temperature cycles on eggshell quality and layer performance. Poult. Sci. 1981;60:733–737. [Google Scholar]
- Dennis C., Gee J.M. The microbial flora of broiler-house litter and dust. J. Gen. Microbiol. 1973;78:101–107. [Google Scholar]
- Devi G.S., Prasad M.H., Saraswathi I., Raghu D., Rao D.N., Reddy P.P. Free radicals antioxidant enzymes and lipid peroxidation in different types of leukemias. Clin. Chim. Acta. 2000;293:53–62. doi: 10.1016/s0009-8981(99)00222-3. [DOI] [PubMed] [Google Scholar]
- Donoghue D., Krueger B.F., Hargis B.M., Miller A.M., El Halawani M.E. Thermal stress reduces serum luteinizing hormone and bioassayable hypothalamic content of luteinizing hormone releasing hormone in the hen. Biol. Reprod. 1989;41:419–424. doi: 10.1095/biolreprod41.3.419. [DOI] [PubMed] [Google Scholar]
- Droge W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Elenkov I.J., Wilder R.L., Chrousos G.P., Vizi E.S. The sympathetic nerve - an integrative interface between two supersystems: the brain and the immune system. Pharmacol. Rev. 2000;52:595–638. [PubMed] [Google Scholar]
- Feddes J.J.R., Mcquitty J.B., Clark P.C. Laying hen heat and moisture production under commercial conditions. Can. Agr. Eng. 1985;27:21–29. [Google Scholar]
- Gao Y.Y., Xie Q.M., Ma J.Y., Zhang X.B., Zhu J.M., Shu D.M., Sun B.L., Jin L., Bi Y.Z. Supplementation of xanthophylls increased antioxidant capacity and decreased lipid peroxidation in hens and chicks. Br. J. Nutr. 2013;109:977–983. doi: 10.1017/S0007114512002784. [DOI] [PubMed] [Google Scholar]
- Gomes A.V.S., Quinteiro W.M., Ribeiro A., Ferraz-de-Paula V., Pinheiro M.L., Baskeville E., Akamine A.T., Astolfi-Ferreira C.S., Ferreira A.J.P., Palermo-Neto J. Overcrowding stress decreases macrophage activity and increases Salmonella Enteritidis invasion in broiler chickens. Avian Pathol. 2014;43:82–90. doi: 10.1080/03079457.2013.874006. [DOI] [PubMed] [Google Scholar]
- Hale B.D., Fairchild B., Worley J., Harper L., Ritz C., Czarick M., Rathbun S.L., Irvin E.A., Naeher L.P. Comparison of ammonia measurement methods inside and outside tunnel-ventilated broiler houses. J. Appl. Poult. Res. 2010;19:245–262. [Google Scholar]
- Honda B.T.B., Calefi A.S., Costola-de-Souza C., Quinteiro W.M., Fonseca J.G.D., de Paula V.F., Palermo-Neto J. Effects of heat stress on peripheral T and B lymphocyte profiles and IgG and IgM serum levels in broiler chickens vaccinated for Newcastle disease virus. Poult. Sci. 2015;94:2375–2381. doi: 10.3382/ps/pev192. [DOI] [PubMed] [Google Scholar]
- Kearney G.D., Shaw R., Prentice M., Tutor-Marcom R. Evaluation of respiratory symptoms and respiratory protection behavior among poultry workers in small farming operations. J. Agromed. 2014;19:162–170. doi: 10.1080/1059924X.2014.886536. [DOI] [PubMed] [Google Scholar]
- Koerkamp P.W.G.G., Bleijenberg R. Effect of type of aviary, manure and litter handling on the emission kinetics of ammonia from layer houses. Br. Poult. Sci. 1998;39:379–392. doi: 10.1080/00071669888935. [DOI] [PubMed] [Google Scholar]
- Koerkamp P.W.G.G., Speelman L., Metz J.H.M. Litter composition and ammonia emission in aviary houses for laying hens. Part 1: performance of a litter drying system. J. Agric. Eng. Res. 1998;70:375–382. [Google Scholar]
- Koon J., Howes J.R., Grub W., Rollo C.A. Poultry dust: Origin and composition. Agric. Eng. 1963;44:608–609. [Google Scholar]
- Kristensen H.H., Wathes C.M. Ammonia and poultry welfare: a review. World Poult. Sci. J. 2000;56:235–245. [Google Scholar]
- Kristensen H.H., Burgess L.R., Demmers T.G.H., Wathes C.M. The preferences of laying hens for different concentrations of atmospheric ammonia. Appl. Anim. Behav. Sci. 2000;68:307–318. doi: 10.1016/s0168-1591(00)00110-6. [DOI] [PubMed] [Google Scholar]
- Lin H., De Vos D., Decuypere E., Buyse J. Dynamic changes in parameters of redox balance after mild heat stress in aged laying hens (Gallus gallus domesticus) Comp. Biochem. Phys. C. 2008;147:30–35. doi: 10.1016/j.cbpc.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Lin H., Deculypere E., Buyse J. Oxidative stress induced by corticosterone administration in broiler chickens (Gallus gallus domesticus): 1. Chronic exposure. Comp. Biochem. Phys. B. 2004;139:737–744. doi: 10.1016/j.cbpc.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Lin H., Deculypere E., Buyse J. Oxidative stress induced by corticosterone administration in broiler chickens (Gallus gallus domesticus): 2. Short-term effect. Comp. Biochem. Phys. B. 2004;139:745–751. doi: 10.1016/j.cbpc.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Lin H., Deculypere E., Buyse J. Acute heat stress induces oxidative stress in broiler chickens. Comp. Biochem. Phys. A. 2006;144:11–17. doi: 10.1016/j.cbpa.2006.01.032. [DOI] [PubMed] [Google Scholar]
- Lin X.J., Zhang R.H., Jiang S.M., El-Mashad H., Xin H.W. Emissions of ammonia, carbon dioxide and particulate matter from cage-free layer houses in California. Atmos. Environ. 2017;152:246–255. [Google Scholar]
- Ma X.Y., Lin Y.C., Zhang H.X., Chen W., Wang S., Ruan D., Jiang Z.Y. Heat stress impairs the nutritional metabolism and reduces the productivity of egg-laying ducks. Anim. Reprod. Sci. 2014;145:182–190. doi: 10.1016/j.anireprosci.2014.01.002. [DOI] [PubMed] [Google Scholar]
- Mahmoud K.Z., Beck M.M., Scheideler S.E., Forman M.F., Anderson K.P., Kachman S.D. Acute high environmental temperature and calcium-estrogen relationships in the hen. Poult. Sci. 1996;75:1555–1562. doi: 10.3382/ps.0751555. [DOI] [PubMed] [Google Scholar]
- Mashaly M.M., Hendricks G.L., Kalama M.A., Gehad A.E., Abbas A.O., Patterson P.H. Effect of heat stress on production parameters and immune responses of commercial laying hens. Poult. Sci. 2004;83:889–894. doi: 10.1093/ps/83.6.889. [DOI] [PubMed] [Google Scholar]
- Mestecky J., Russell M.W., Elson C.O. Intestinal IgA: novel views on its function in the defence of the largest mucosal surface. Gut. 1999;44:2–5. doi: 10.1136/gut.44.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles D.M., Rowe D.E., Cathcart T.C. High litter moisture content suppresses litter ammonia volatilization. Poult. Sci. 2011;90:1397–1405. doi: 10.3382/ps.2010-01114. [DOI] [PubMed] [Google Scholar]
- Miles D.M., Branton S.L., Lott B.D. Atmospheric ammonia is detrimental to the performance of modern commercial broilers. Poult. Sci. 2004;83:1650–1654. doi: 10.1093/ps/83.10.1650. [DOI] [PubMed] [Google Scholar]
- Miles D.M., Miller W.W., Branton S.L., Maslin W.R., Lott B.D. Ocular responses to ammonia in broiler chickens. Avian Dis. 2006;50:45–49. doi: 10.1637/7386-052405R.1. [DOI] [PubMed] [Google Scholar]
- Nagaraja K.V., Emery D.A., Jordan K.A., Newman J.A., Pomeroy B.S. Scanning electron microscopic studies of adverse effects of ammonia on tracheal tissues of turkeys. Am. J. Vet. Res. 1983;44:1530–1536. [PubMed] [Google Scholar]
- Nasrin M., Khan M.Z.I., Siddiqi M.N.H., Masum M.A. Mobilization of immunoglobulin (Ig)-containing plasma cells in Harderian gland, cecal tonsil and trachea of broilers vaccinated with Newcastle Disease Vaccine. Tissue and Cell. 2013;45:191–197. doi: 10.1016/j.tice.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Nemer M., Sikkeland L.I.B., Kasem M., Kristensen P., Nijem K., Bjertness E., Skare O., Bakke B., Kongerud J., Skogstad M. Airway inflammation and ammonia exposure among female Palestinian hairdressers: a cross-sectional study. Occup. Environ. Med. 2015;72:428–434. doi: 10.1136/oemed-2014-102437. [DOI] [PubMed] [Google Scholar]
- The National Institute of Occupational Safety and Health (NIOSH). NIOSH pocket guide to chemical hazards - Ammonia. 2016. www.cdc.gov/niosh/npg/npgd0028.html
- Novero R.P., Beck M.M., Gleaves E.W., Johnson A.L., Deshazer J.A. Plasma progesterone, luteinizing hormone concentrations and granulose cell responsiveness in heat-stressed hens. Poult. Sci. 1991;70:2335–2339. doi: 10.3382/ps.0702335. [DOI] [PubMed] [Google Scholar]
- The Occupational Safety and Health Administration (OSHA). Chemical sampling information - Ammonia. 2012. https://www.osha.gov/dts/chemicalsampling/data/CH218300.html
- Oyetunde O.O.F., Thomson R.G., Carlson H.C. Aerosol exposure of ammonia, dust and Escherichia coli in broiler chickens. Can. Vet. J. 1978;19:187–193. [PMC free article] [PubMed] [Google Scholar]
- Post J., Rebel J.M.J., ter Huurne A.A.H.M. Physiological effects of elevated plasma corticosterone concentrations in broiler chickens. An alternative means by which to assess the physiological effects of stress. Poult. Sci. 2003;82:1313–1318. doi: 10.1093/ps/82.8.1313. [DOI] [PubMed] [Google Scholar]
- Quinteiro W.M., Ribeiro A., Ferraz-de-Paula V., Pinheiro M.L., Sakai M., Sa L.R.M., Ferreira A.J.P., Palermo-Neto J. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult. Sci. 2010;89:1905–1914. doi: 10.3382/ps.2010-00812. [DOI] [PubMed] [Google Scholar]
- Ritz C.W., Mitchell B.W., Fairchild B.D., Czarick M., Worley J.W. Improving in-house air quality in broiler production facilities using an electrostatic space charge system. J. Appl. Poult. Res. 2006;15:333–340. [Google Scholar]
- Rozenboim I., Tako E., Gal-Garber O., Proudman J.A., Uni Z. The effect of heat stress on ovarian function of laying hens. Poult. Sci. 2007;86:1760–1765. doi: 10.1093/ps/86.8.1760. [DOI] [PubMed] [Google Scholar]
- Sato S., Shoya S., Kobayashi H. Effect of ammonia on Mycoplasma-gallisepticum infection in chickens. Natl. Inst. Anim. Health Q. (Tokyo) 1973;13:45–53. [PubMed] [Google Scholar]
- Shini S., Shini A., Huff G.R. Effects of chronic and repeated corticosterone administration in rearing chickens on physiology, the onset of lay and egg production of hens. Physiol. Behav. 2009;98:73–77. doi: 10.1016/j.physbeh.2009.04.012. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Ha S.A., Tsuji M., Fagarasan S. Intestinal IgA synthesis: a primitive form of adaptive immunity that regulates microbial communities in the gut. Semin. Immunol. 2007;19:127–135. doi: 10.1016/j.smim.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Tasistro A.S., Ritz C.W., Kissel D.E. Ammonia emissions from broiler litter: response to bedding materials and acidifiers. Br. Poult. Sci. 2007;48:399–405. doi: 10.1080/00071660701473865. [DOI] [PubMed] [Google Scholar]
- Thomas M.J. The role of free radicals and antioxidants. Nutrition. 2000;16:716–718. doi: 10.1016/s0899-9007(00)00343-9. [DOI] [PubMed] [Google Scholar]
- Wang Y.M., Meng Q.P., Guo Y.M., Wang Y.Z., Wang Z., Yao Z.L., Shan T.Z. Effect of atmospheric ammonia on growth performance and immunological response of broiler chickens. J. Anim. Vet. Adv. 2010;9:2802–2806. [Google Scholar]
- Wathes C.M., Holden M.R., Sneath R.W., White R.P., Phillips V.R. Concentrations and emission rates of aerial ammonia, nitrous oxide, methane, carbon dioxide, dust and endotoxin in UK broiler and layer houses. Br. Poult. Sci. 1997;38:14–28. doi: 10.1080/00071669708417936. [DOI] [PubMed] [Google Scholar]
- Wei F.X., Hu X.F., Sa R.N., Liu F.Z., Li S.Y., Sun Q.Y. Antioxidant capacity and meat quality of broilers exposed to different ambient humidity and ammonia concentrations. Gen. Mol. Res. 2014;13:3117–3127. doi: 10.4238/2014.April.17.8. [DOI] [PubMed] [Google Scholar]
- Wheeler E.F., Weiss R.W.J., Weidenboerner E. Evaluation of instrumentation for measuring aerial ammonia in poultry houses. J. Appl. Poult. Res. 2000;9:443–452. [Google Scholar]
- Wolfenson D., Lew B.J., Thatcher W.W., Graber Y., Meidan R. Seasonal and acute heat stress effects on steroid production by dominant follicles in cows. Anim. Reprod. Sci. 1997;47:9–19. doi: 10.1016/s0378-4320(96)01638-7. [DOI] [PubMed] [Google Scholar]
- Wolfenson D., Frei Y.F., Snapir N., Berman A. Heat-stress effects on capillary blood-flow and its redistribution in the laying hen. Pflug. Arch. Eur. J. Phy. 1981;390:86–93. doi: 10.1007/BF00582717. [DOI] [PubMed] [Google Scholar]
- Wu Y.N., Yan F.F., Hu J.Y., Chen H., Tucker C.M., Green A.R., Cheng H.W. The effect of chronic ammonia exposure on acute-phase proteins, immunoglobulin, and cytokines in laying hens. Poult. Sci. 2017;96:1524–1530. doi: 10.3382/ps/pew454. [DOI] [PubMed] [Google Scholar]
- Youngren O.M., Elhalawani M.E., Silsby J.L., Phillips R.E. Intracranial prolactin perfusion induces incubation behavior in Turkey hens. Biol. Reprod. 1991;44:425–431. doi: 10.1095/biolreprod44.3.425. [DOI] [PubMed] [Google Scholar]
- You S., Foster L.K., Silsby J.L., El Halawani M.E., Foster D.N. Sequence analysis of the Turkey LH subunit and its regulation by gonadotropin-releasing hormone and prolactin in cultured pituitary cells. J. Mol. Endocrinol. 1995;14:117–129. doi: 10.1677/jme.0.0140117. [DOI] [PubMed] [Google Scholar]
- Zeng T., Li J.J., Wang D.Q., Li G.Q., Wang G.L., Lu L.Z. Effects of heat stress on antioxidant defense system, inflammatory injury, and heat shock proteins of Muscovy and Pekin ducks: evidence for differential thermal sensitivities. Cell Stress and Chaperones. 2014;19:895–901. doi: 10.1007/s12192-014-0514-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Shepherd T.A., Li H., Xin H. Environmental assessment of three egg production systems - Part I: Monitoring system and indoor air quality. Poult. Sci. 2015;94:518–533. doi: 10.3382/ps/peu076. [DOI] [PMC free article] [PubMed] [Google Scholar]