Abstract
The objective of this study was to investigate the effects of dietary 3 kinds of sweeteners supplementation on growth performance, serum biochemicals, and jejunal physiological functions of broiler chickens for 21 D. A total of one hundred ninety-two 1-day-old male Ross 308 broiler chicks were randomly divided into 4 treatments with 6 replicates for each treatment. The treatments were basal diet (CON), a basal diet supplemented with 250 mg/kg stevioside (STE), a basal diet supplemented with 100 mg/kg sucralose (SUC), and a basal diet supplemented with 600 mg/kg saccharin sodium (SAC). All birds were housed in 3-level battery cages. The results showed that dietary STE supplementation increased (P < 0.05) growth performance, serum total protein, serum albumin, and jejunal antioxidant capacity of broiler chickens. Both SUC and SAC supplementation decreased (P < 0.05) serum total protein and albumin. Dietary SAC supplementation impaired the intestinal integrity, permeability, and mucus layer of the jejunum in broiler chickens. In addition, SAC supplementation elevated (P < 0.05) the transcription expression level of jejunal bitter taste receptors and induced excessive jejunal apoptosis. Our data suggest that STE could be potentially applied as a growth-promoting and antioxidant feed additive in broiler chickens. Whereas, dietary supplementation with high level SAC has side-effects on the jejunal physiological functions of broiler chickens.
Key words: sweetener, broiler, stevioside, sucralose, saccharin sodium
Introduction
Nowadays, high-potency sweeteners have been widely used in the animal feed for mammals, including pig and ruminants (Buerge et al., 2011, Moran et al., 2014, Ma et al., 2017). Owing to the high sweetness and low-calorie, some sweeteners are extensively used to improve the palatability of the feed (Figueroa et al., 2019). Sweet taste is also correlated with feed intake. A large number of studies have shown that dietary supplementation with sweeteners could promote the feed intake and thereby improve growth performance in livestock, including piglets (Sterk et al., 2008, Zhang et al., 2019), cattle (McMeniman et al., 2006), and goat (Han et al., 2019). Nevertheless, data are lacking about the impact of dietary supplementation with sweeteners on the feed intake and body weight (BW) gain of broiler chickens. It is worthwhile to investigate the effects of sweeteners supplementation on the growth performance of broiler chickens.
Taste is mediated by taste receptors (Lee et al., 2017). It is well established that, in mammals, sweet substances could bind with the sweet taste receptors on the taste buds and induce the sweet signal transduction (Damak et al., 2003). Sweet taste receptors in mammals are consist of taste receptor family 1 member 2 (T1R2) and taste receptor family 1 member 3 (Chaudhari and Roper, 2010). However, T1R2 is missing in chickens, which could be the reason that chickens are insensitive to sweet substances (Ganchrow et al., 1990, Shi and Zhang, 2006, Cheled-Shoval et al., 2017). Unlike chickens, some bird, like the hummingbird, has evolved to adapt the umami taste receptors to respond to the sweetness (Baldwin et al., 2014). In addition, a study done by Milner (1969) showed that Japanese quail prefers sucrose solution rather than normal water because of palatability. Whether the umami taste receptors of broiler chickens could perceive sweetness has not been investigated yet.
Interestingly, an increasing number of studies in mammals have suggested that sweeteners not only induce the sense of sweet taste but also exert additional biological functions in the gastrointestinal tract (Brown and Rother, 2012, Meyer-Gerspach et al., 2018, Hunter et al., 2019). Stevioside (STE) has been proven to exhibit anti-inflammatory activity in intestinal cells (Boonkaewwan et al., 2008). Sucralose (SUC) could reduce the beneficial bacteria in the gastrointestinal tract of rats (Schiffman and Rother, 2013). Consumption of saccharin sodium (SAC) has been suggested to be able to disrupt monolayer integrity using a human Caco-2 cell model in vitro (Santos et al., 2018). However, few studies have been focused on the physiological relationship between sweeteners and gastrointestinal tract in chickens (Kimmich et al., 1989). Understanding the biological functions of sweeteners on the gastrointestinal tract could be helpful for exploring new feed additives for broiler chickens.
Based on the findings above, we hypothesized that dietary supplementation with sweeteners might have physiological functions on the gastrointestinal tract of broiler chickens. Three sweeteners (STE, SUC, and SAC) that commonly used in our daily life were selected in this study. The present study was conducted to evaluate the effects of dietary sweeteners supplementation on growth performance, serum biochemicals, and jejunal physiological functions of broiler chickens.
Materials and methods
Animals and Treatment
The experiments were performed in accordance with the Animal Care and Use Committee of Nanjing Agricultural University, Nanjing, China (PZ2019088). A total of one hundred ninty-two 1-day-old male Ross 308 broiler chicks with similar original weights (41.45 ± 0.15 g) were purchased from a commercial hatchery. Broiler chicks were randomly assigned into 4 treatments with 6 replicates (cages) for each treatment and 8 birds per replicate. The whole experiment lasted for 21 D. The basal diet used in this study was according to the Nutrient Requirements of Poultry (Table 1) (Council, 1994). The 4 treatments were as follows: (1) broiler chickens fed a basal diet (CON); (2) broiler chickens fed a basal diet supplemented with 250 mg/kg STE; (3) broiler chickens fed a basal diet supplemented with 100 mg/kg SUC; (4) broiler chickens fed a basal diet supplemented with 600 mg/kg SAC. The supplemental level of STE was chosen according to our previous study (Jiang et al., 2019b). The supplemental levels of SUC and SAC were chosen according to a previous study to obtain equivalent sweetness with the amount of STE (Keast et al., 2004). Stevioside was purchased from Macklin (Shanghai, China). Sucralose and SAC were purchased from Aladdin (Shanghai, China). Any purity of these 3 sweeteners used in the present experiment is more than 98%. All birds were housed in 3-level battery cages (dimension of each cage: 120 × 60 × 50 cm) in the animal house of the Nanjing Agricultural University with temperature control and continuous light. All broilers had ad libitum access to mash feed and water. The temperature of the room was maintained at 32 to 34°C for 7 D, and it was then gradually decreased by 1°C every 2 D until 26°C was reached. Furthermore, all broilers were inoculated with a Newcastle disease vaccine on seventh day and with an inactivated infectious bursal disease vaccine on 14th D. At day 14 and 21 of the experiment, all birds were weighed after fasting for 12 h to determine BW and average daily gain (ADG). The feed consumption by the broilers in a replicate (cage) was recorded to calculate average daily feed intake (ADFI). The spilled feed were collected and weighted to correct the final data of ADFI. Feed conversion ratio was defined as ADFI: ADG.
Table 1.
Ingredient composition and calculation of ingredients of the basal diet for broiler chickens.
| Items | 1 to 21 D |
|---|---|
| Ingredient (%) | |
| Corn | 53.28 |
| Soybean meal | 38.57 |
| Soybean oil | 3.70 |
| Dicalcium phosphate | 1.98 |
| Mineral premix | 0.50 |
| Vitamin premix | 0.10 |
| Limestone | 1.05 |
| Choline chloride (50%) | 0.30 |
| Salt | 0.35 |
| Methionine | 0.17 |
| Total | 100 |
| Calculation of nutrients | |
| Metabolizable energy, kcal/kg | 2,953 |
| Crude protein, % | 21.57 |
| Lysine, % | 1.15 |
| Methionine, % | 0.49 |
| Calcium, % | 1.05 |
| Available phosphorus, % | 0.45 |
Provided per kilogram of diet: vitamin A, 12,500 IU; vitamin D3, 2,500 IU; vitamin E, 80 IU; vitamin K, 2.65 mg; vitamin B1, 2 mg; vitamin B2, 6 mg; nicotinic acid, 50 mg; pantothenic acid, 20 mg; vitamin B6, 4 mg; folic acid, 1.25 mg; vitamin B12, 0.025 mg; biotin, 0.0325 mg; folic acid, 1.25 mg; pantothenic acid, 12 mg; niacin, 50 mg; Fe, 80 mg; Zn, 75 mg; Mn, 100 mg; Cu, 8 mg; I, 0.35 mg; Co, 0.2 mg; and Se, 0.15 mg.
Sample Collection
At day 21 of the experiment, all birds were weighed, and 1 bird was selected from each replicate (6 broilers per treatment) with a BW close to the average BW in the respective cage. Blood samples were collected from the wing vein. Serum was then obtained after centrifugation at 3,000g for 15 min at 4°C, and it was stored at −20°C for biochemical assays. The birds were euthanized after blood collection, and the liver, thymus, bursa of fabricius, and breast muscle were carefully separated and weighed. The jejunum was then gingerly taken out. Section of approximately 2 cm in length was cut-off from the middle of each jejunum, and it was washed gently using phosphate buffer saline (pH 7.4) and promptly fixed in 4% paraformaldehyde. The jejunal mucosae were gently scraped by a glass microscope slide from the rest of jejunum. The mucosae were stored at −80°C for the analysis of oxidative status and gene expression.
Measurement of Serum Biochemical Indexes and Diamine Oxidase Activity
Total protein (TP), albumin (ALB), total bilirubin, direct bilirubin, and uric acid were measured by using a commercial kit (NovaTech Co., Ltd., Shandong, China) and an automatic clinical biochemistry analyzer (NVAS6805, NovaTech Co., Ltd.). The activity of serum diamine oxidase (DAO) was measured using a commercial reagent kit (Jin Yibai Biological Technology, Nanjing, China). The whole experimental procedure was strictly performed according to the manufacturer's instructions.
Intestinal Morphology Analysis
After fixation in 4% paraformaldehyde for 24 h, the jejunal sections were soaked through a graded series of ethanol and xylene and embedded in paraffin. The jejuna were sectioned at 5 μm with a Lecia RM2235 microtome (Leica Biosystems Inc., Buffalo Grove, IL). The sections were deparaffinized with xylene and rehydrated through a graded dilution of ethanol. Hematoxylin and eosin staining and Alcian blue-periodic acid Schiff staining were performed, respectively. The images of jejuna were acquired using an Olympus simon-01 microscope (Olympus Optical Co., Ltd., Beijing, China). The values of villus height (VH), crypt depth (CD), and the number of jejunal goblet cells were measured 5 times from different villus and crypts per section from each broiler using the Image-Pro Plus software 6.0 (Media Cybernetics, Inc., Rockville, MD).
Terminal Deoxynucleotidyl Transferase-Mediated Deoxyuridine Triphosphate Nick-End Labeling Assay
Terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL) assay was used to determine the jejunal apoptosis. The whole experiment was performed using a commercial TUNEL BrightRed Apoptosis Detection Kit (A113, Vazyme Biotech, Nanjing, China) according to the manufacturer's instructions. First, the jejunal sections were deparaffinized, rehydrated, then incubated with Proteinase K (20 mg/ml) at room temperature for 20 min. Second, the sections were incubated with the terminal deoxynucleotidyl transferase enzyme with BrightRed Labeling Mix at 37°C for 60 min in the dark. Finally, the sections were stained with 4′,6-diamidino-2-phenylindole staining solution (C1005, Beyotime Biotechnology, Shanghai, China) for 5 min in the dark. To ensure there was no nonspecific reaction, the negative control was performed without incubation of the TdT enzyme. The fluorescent images were acquired using a LSM 700 confocal laser scanning microscope (Carl Zeiss, Oberkochen, Germany).
Determination of Jejunal Oxidative Status
The amount of 0.2 g frozen jejunal mucosae was precisely weighed and homogenized in 2 mL of ice-cold saline. After being centrifuged at 12,000 g for 10 min at 4°C, the supernatants were separated to measure the oxidative status in the jejunal mucosae. A bicinchoninic acid protein assay kit (P0010, Beyotime Biotechnology) was used to measure the protein content. Catalase activity, superoxide dismutase activity, and glutathione peroxidase activity and malondialdehyde (MDA) content in the jejunal mucosae were assessed using commercial reagent kits (S0051, S0101, S0056, and S0131, Beyotime Biotechnology). All experimental procedures were performed according to the manufacturer's instructions. The final results were normalized to protein concentration in each sample.
Total RNA Extraction and mRNA Quantification
The total RNA of jejunal mucosae was extracted using the RNAiso Plus (9109, Takara Bio Inc., Dalian, China). The concentration and quality of total RNA was identified by a ND-2000 micro spectrophotometer (Thermo Scientific, Wilmington, USA). Afterward, the RNA was reverse-transcribed into complementary DNA using a HiScript Ⅱ first Strand cDNA Synthesis Kit with gDNA wiper (R323-01, Vazyme Biotech). The gDNA wiper was added to remove the DNA, and a total of 1 μg of RNA was reverse-transcribed to complementary DNA. Complementary DNA was diluted 10 × before real-time PCR. Real-time PCR was performed using the ChamQ SYBR qPCR Master Mix (Q311-02, Vazyme Biotech) on the QuantStudio 5 Real-Time PCR System (Thermo Scientific). The β-actin gene was selected to be the housekeeping gene to normalize the expression of the other target genes. The primers were synthesized by Sangon Biotech (Shanghai, China), and the primer sequences were shown in Table 2. All genes were assayed 3 times. The reaction program was set as follows: 95°C for 30 s, 40 cycles of 95°C for 5 s, followed by 60°C for 30 s. The amplification of a single product was verified by the melting curve. Relative gene expression levels were analyzed by the 2−ΔΔCt method after normalization against β-actin.
Table 2.
Primer sequences used for RT-qPCR in this study.
| Gene | Primer sequence (5′ → 3′) | Amplicon size (bp) | GeneBank accession number |
|---|---|---|---|
| ggTAS1R1 | Forward: GTGTCATCCCCACAACCAA | 137 | XM_015297004.2 |
| Reverse: CACCACTGCCTCAAAGAAGG | |||
| ggTAS1R3 | Forward: CATTACCGTCTTCGCCACTC | 143 | XM_025142692.1 |
| Reverse: CTCTGTTCAAATCGGGCTTC | |||
| ggTAS2R1 | Forward: TGCCAGTCTCATACCCTGGA | 100 | AB249766.1 |
| Reverse: GAAGTTGCTGTGTGCGTTGT | |||
| ggTAS2R2 | Forward: TCAACGGGGAACTGTGGAGA | 174 | XM_004938927.3 |
| Reverse: GCATTGCATCTTCTTGGTGTGT | |||
| ggTAS2R7 | Forward: TGTGGCTGCGTCTTGTATGG | 104 | NM_001080719.1 |
| Reverse: TGGCGCACAGATACCAAAAC | |||
| CLDN1 | Forward: CATACTCCTGGGTCTGGTTGGT | 100 | NM_001013611.2 |
| Reverse: GACAGCCATCCGCATCTTCT | |||
| CLDN2 | Forward: CTGCTCACCCTCATTGGA | 140 | NM_001277622.1 |
| Reverse: AACTCACTCTTGGGCTTCTG | |||
| ZO-1 | Forward: CTTCAGGTGTTTCTCTTCCTCCTC | 131 | XM_015278975.2 |
| Reverse: CTGTGGTTTCATGGCTGGATC | |||
| OCLN | Forward: ACGGCAGCACCTACCTCAA | 123 | NM_205128.1 |
| Reverse: GGGCGAAGAAGCAGATGAG | |||
| MUC2 | Forward: CCCTGGAAGTAGAGGTGACTG | 143 | XM_001234581.3 |
| Reverse: TGACAAGCCATTGAAGGACA | |||
| β-actin | Forward: TGTTACCAACACCCACACCC | 110 | NM_205518.1 |
| Reverse: TCCTGAGTCAAGCGCCAAAA |
Abbreviations: CLDN1, claudin 1; CLDN2, claudin 2; ggTAS1R1, Gallus gallus taste receptor family 1 member 1; ggTAS1R3, Gallus gallus taste receptor family 1 member 3; ggTAS2R1, Gallus gallus taste receptor family 2 member 1; ggTAS2R2, Gallus gallus taste receptor family 2 member 2; ggTAS2R7, Gallus gallus taste receptor family 2 member 7; MUC2, mucin 2; OCLN, occludin; ZO-1, zonula occludens-1.
Statistical Analysis
Data were statistically analyzed by one-way ANOVA with multiple comparisons among groups tested by Tukey's post hoc tests, using GraphPad Prism 7. The Shapiro–Wilk test was used to assess the normality distribution of the data. Data were presented as the mean and standard error of the mean. Differences were considered to be statistically significant at P < 0.05.
Results
Growth Performance and Relative Organ Weight
The data of growth performance are shown in Table 3. At 14 D, the STE group had higher (P < 0.05) BW compared with the control and SUC groups. At 21 D, dietary supplementation with STE increased (P < 0.05) BW of broiler chickens compared with the control group. In addition, dietary supplementation with STE increased ADFI and significantly differed from control only from 1 to 14 D (P < 0.05), while supplementation with STE significantly increased (P < 0.05) ADG compared with the control and SUC groups from 1 to 14 D. There were no differences (P > 0.05) in ADFI and ADG among any groups during 15 to 21 D. In general, from 1 to 21 D, dietary STE supplementation elevated (P < 0.05) ADFI and ADG of broiler chickens compared with the control group. There were no significant differences (P > 0.05) in feed conversion ratio among any groups during the whole experimental period. As shown in Table 4, there were no differences (P > 0.05) in the relative organ weight of liver, thymus, bursa of fabricius, and breast muscle among any groups.
Table 3.
Effects of supplementation with sweeteners on the growth performance of broiler chickens.
| Items1 | Treatments |
SEM | P-value | |||
|---|---|---|---|---|---|---|
| CON | STE | SUC | SAC | |||
| BW (g) | ||||||
| 14 D | 360.15b | 405.70a | 360.33b | 381.31a,b | 10.48 | 0.027 |
| 21 D | 638.06b | 717.12a | 653.30a,b | 668.38a,b | 17.84 | 0.045 |
| ADFI (g/D) | ||||||
| 1–14 D | 39.20b | 45.35a | 42.98a,b | 40.49a,b | 1.20 | 0.017 |
| 15–21 D | 58.35 | 63.26 | 62.34 | 65.05 | 3.06 | 0.570 |
| 1–21 D | 47.46b | 53.44a | 52.07a,b | 49.58a,b | 1.25 | 0.031 |
| ADG (g/D) | ||||||
| 1–14 D | 24.49b | 28.02a | 24.56b | 26.13a,b | 0.81 | 0.029 |
| 15–21 D | 39.70 | 44.49 | 44.01 | 38.86 | 1.99 | 0.153 |
| 1–21 D | 29.82b | 33.79a | 31.37a,b | 30.58a,b | 0.90 | 0.046 |
| FCR (g:g) | ||||||
| 1–14 D | 1.61 | 1.62 | 1.65 | 1.65 | 0.07 | 0.951 |
| 15–21 D | 1.48 | 1.43 | 1.51 | 1.58 | 0.08 | 0.684 |
| 1–21 D | 1.60 | 1.59 | 1.66 | 1.62 | 0.05 | 0.768 |
a,bMeans with different letters within a row are significantly different (P < 0.05).
n = 6.
Abbreviations: CON, broiler chickens fed a basal diet; SAC, broiler chickens fed a basal diet supplemented with 600 mg/kg saccharin sodium; SEM, standard error of means; STE, broiler chickens fed a basal diet supplemented with 250 mg/kg stevioside; SUC, broiler chickens fed a basal diet supplemented with 100 mg/kg sucralose.
BW: body weight; ADFI: average daily feed intake; ADG: average daily gain; FCR: feed conversion ratio.
Table 4.
Effects of supplementation with sweeteners on the relative organ weight of broiler chickens at 21 D.
| Items | Treatments |
SEM | P-value | |||
|---|---|---|---|---|---|---|
| CON | STE | SUC | SAC | |||
| Liver (g/kg) | 16.99 | 18.56 | 17.79 | 17.65 | 0.58 | 0.324 |
| Thymus (g/kg) | 1.68 | 1.61 | 1.84 | 1.61 | 0.15 | 0.683 |
| Bursa of Fabricius (g/kg) | 1.57 | 1.45 | 1.68 | 1.75 | 0.11 | 0.517 |
| Breast muscle (%) | 20.14 | 19.30 | 19.28 | 18.38 | 0.40 | 0.054 |
n = 6.
Abbreviations: CON, broiler chickens fed a basal diet; SAC, broiler chickens fed a basal diet supplemented with 600 mg/kg saccharin sodium; SEM, standard error of means; STE, broiler chickens fed a basal diet supplemented with 250 mg/kg stevioside; SUC, broiler chickens fed a basal diet supplemented with 100 mg/kg sucralose.
Serum Biochemical Indexes
The effects of sweeteners supplementation on serum biochemical indexes of broilers at 21 D are shown in Table 5. Daily STE supplementation significantly increased (P < 0.001) the concentration of TP and ALB compared with the other 3 groups. Both SUC and SAC supplementation reduced (P < 0.001) the level of serum ALB compared with the control group. Moreover, the supplementation of sweeteners did not alter the concentration of total bilirubin, direct bilirubin, and uric acid in the serum (P > 0.05).
Table 5.
Effects of supplementation with sweeteners on the serum biochemical indexes of broiler chickens at 21 D.
| Items1 | Treatments |
SEM | P-value | |||
|---|---|---|---|---|---|---|
| CON | STE | SUC | SAC | |||
| TP (g/L) | 18.00b | 25.11a | 13.70b | 15.44b | 1.32 | <0.001 |
| ALB (g/L) | 14.50b | 18.38a | 10.75c | 11.25c | 0.66 | <0.001 |
| TBIL (μmol/L) | 37.97 | 32.74 | 31.80 | 36.79 | 3.62 | 0.603 |
| DBIL(μmol/L) | 7.98 | 7.06 | 8.44 | 10.08 | 0.93 | 0.188 |
| UA (μmol/L) | 333.61 | 251.75 | 324.88 | 407.02 | 47.26 | 0.251 |
a-cMeans with different letters within a row are significantly different (P < 0.05).
n = 6.
Abbreviations: CON, broiler chickens fed a basal diet; SAC, broiler chickens fed a basal diet supplemented with 600 mg/kg saccharin sodium; SEM, standard error of means; STE, broiler chickens fed a basal diet supplemented with 250 mg/kg stevioside; SUC, broiler chickens fed a basal diet supplemented with 100 mg/kg sucralose.
TP: Total protein; ALB: albumin; TBIL: total bilirubin; DBIL: direct bilirubin; UA: uric acid.
Intestinal Morphology and Permeability
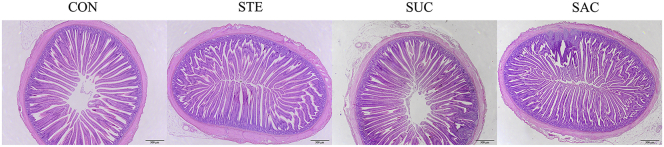
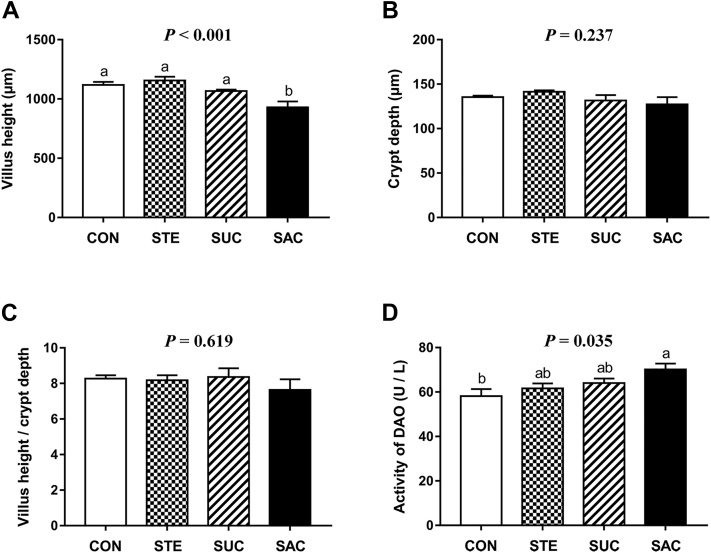
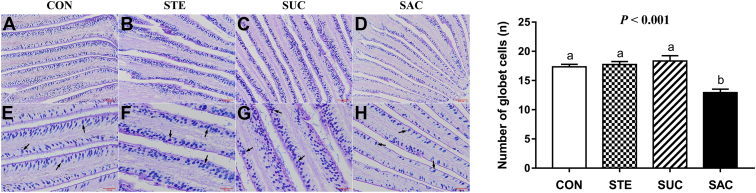
To observe the effects of sweeteners supplementation on the jejunal morphology of broilers, hematoxylin and eosin staining was performed (Figure 1). The data of jejunal VH and CD are shown in Figure 2. Saccharin sodium supplementation markedly decreased (P < 0.001) the VH in the jejunum of broilers compared with the other 3 groups (Figure 2A). There were no differences (P > 0.05) in the CD and VH/CD of the jejunum among any groups (Figure 2B and 2C). Furthermore, to evaluate the jejunal permeability, the activity of DAO in the serum of broilers was assessed (Figure 2D). The data showed that dietary supplementation with SAC significantly elevated (P < 0.05) the serum DAO activity compared with the CON group. The jejunal goblet cells indicated by arrows were observed using and Alcian blue-periodic acid Schiff staining (Figure 3). Statistical results showed that dietary supplementation with SAC notably reduced (P < 0.001) the number of jejunal goblet cells compared with other 3 groups.
Figure 1.
Representative images of H&E staining on the intestinal morphology of broiler chickens. Scale bar = 500 µm. Abbreviations: CON, broiler chickens fed a basal diet; H&E, hematoxylin and eosin staining; STE, broiler chickens fed a basal diet supplemented with 250 mg/kg stevioside; SUC, broiler chickens fed a basal diet supplemented with 100 mg/kg sucralose; SAC, broiler chickens fed a basal diet supplemented with 600 mg/kg saccharin sodium.
Figure 2.
Effects of supplementation with sweeteners on the intestinal integrity and permeability of broiler chickens. (A) Villus height of the jejunum. (B) Crypt depth of the jejunum. (C) Villus height/crypt depth of the jejunum. (D) Activity of serum diamine oxidase (DAO). Data are presented as mean value ± SEM (n = 6). Values without the same letter (a, b) represent statistically significant differences (P < 0.05). Abbreviations: CON, broiler chickens fed a basal diet; STE, broiler chickens fed a basal diet supplemented with 250 mg/kg stevioside; SUC, broiler chickens fed a basal diet supplemented with 100 mg/kg sucralose; SAC, broiler chickens fed a basal diet supplemented with 600 mg/kg saccharin sodium.
Figure 3.
Representative images of AB-PAS staining on the jejunum of broiler chickens. (A–D) AB-PAS staining on the jejunum. Scale bar = 100 μm. (E–H) Enlargement of AB-PAS staining on the jejunum. Scale bar = 50 μm. The histogram represents the number of jejunum goblet cells in different treatment groups. Data are presented as mean value ± SEM (n = 6). Values without the same letter (a, b) represent statistically significant differences (P < 0.05). Abbreviations: AB-PAS, Alcian blue-periodic acid Schiff staining; CON, broiler chickens fed a basal diet; STE, broiler chickens fed a basal diet supplemented with 250 mg/kg stevioside; SUC, broiler chickens fed a basal diet supplemented with 100 mg/kg sucralose; SAC, broiler chickens fed a basal diet supplemented with 600 mg/kg saccharin sodium.
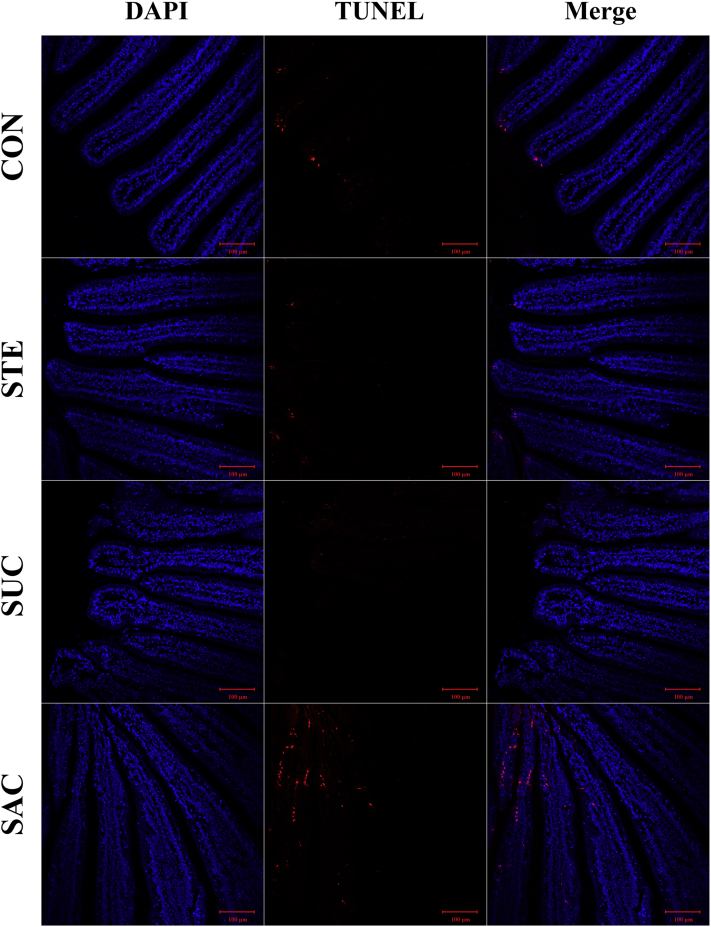
TUNEL Assay
To estimate the effects of sweeteners supplementation on the jejunal apoptosis of broilers, TUNEL assay was performed (Figure 4). As shown by TUNEL assay, the apoptotic cells were distributed mainly in the apical region of the jejunal villus. Daily supplementation of SAC obviously increased the number of apoptotic cells in the jejunal villus. While STE and SUC supplementation had no influences on the jejunal apoptosis.
Figure 4.
Representative images of TUNEL assay on the jejunal sections of broiler chickens by immunofluorescence. The blue color represents the total cell nuclei, and the red color represents the apoptotic cells in the jejunum. Scale bar = 100 μm. Abbreviations: CON, broiler chickens fed a basal diet; STE, broiler chickens fed a basal diet supplemented with 250 mg/kg stevioside; SUC, broiler chickens fed a basal diet supplemented with 100 mg/kg sucralose; SAC, broiler chickens fed a basal diet supplemented with 600 mg/kg saccharin sodium.
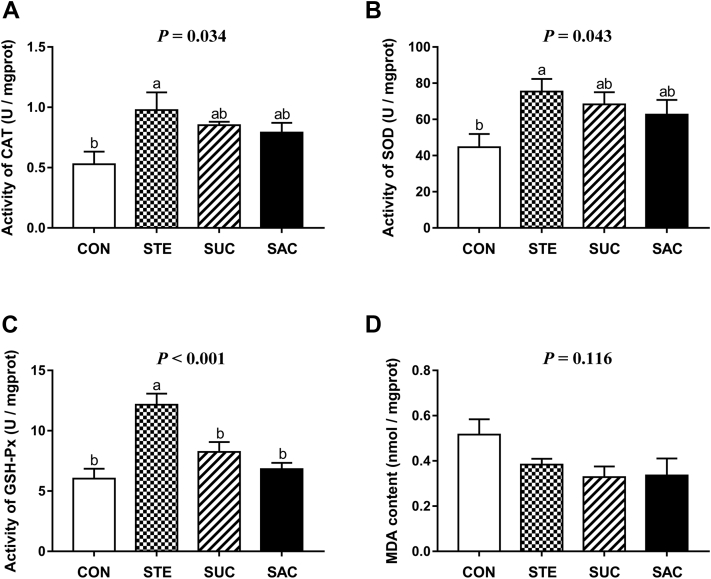
Oxidative Status of the Jejunal Mucosa
As shown in Figure 5, STE supplementation significantly increased (P < 0.05) the activities of catalase, superoxide dismutase, and glutathione peroxidase in the jejunal mucosa compared with the control group. There was no effect of either SUC or SAC supplementation on the oxidative status of the jejunal mucosa (P > 0.05). In addition, supplementation with sweeteners did not affect the content of MDA in the jejunal mucosa (P > 0.05).
Figure 5.
Effects of supplementation with sweeteners on the intestinal oxidative status of broiler chickens. (A) Activity of jejunal CAT. (B) Activity of jejunal SOD. (C) Activity of jejunal GSH-Px. (D) Content of jejunal MDA. Data are presented as mean value ± SEM (n = 6). Values without the same letter (a, b) represent statistically significant differences (P < 0.05). Abbreviations: CAT, catalase; SOD, superoxide dismutase; GSH-Px, glutathione peroxidase; MDA, malondialdehyde; CON, broiler chickens fed a basal diet; STE, broiler chickens fed a basal diet supplemented with 250 mg/kg stevioside; SUC, broiler chickens fed a basal diet supplemented with 100 mg/kg sucralose; SAC, broiler chickens fed a basal diet supplemented with 600 mg/kg saccharin sodium.
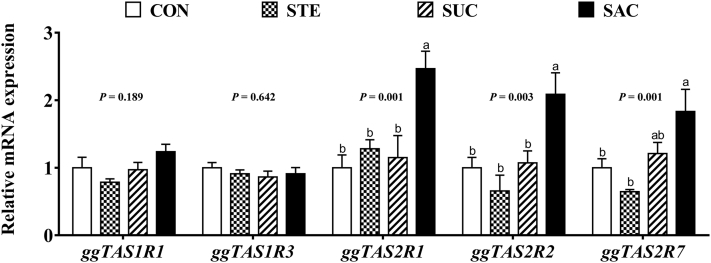
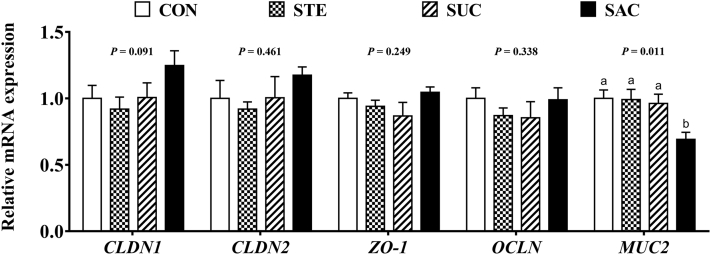
Jejunal Gene Expression
Real-time PCR was used to determine the effects of sweeteners supplementation on the gene expression of taste receptors and tight-junction–related genes. There were no differences (P > 0.05) on the gene expression of ggTAS1R1 and ggTAS1R3 in the jejunum among any groups (Figure 6). Saccharin sodium supplementation significantly increased (P < 0.05) the gene expression of ggTAS2R1, ggTAS2R2, and ggTAS2R7 compared with the control group (Figure 6). In addition, sweeteners supplementation had no effect (P > 0.05) on the transcription level of claudin 1, claudin 2, zonula occludens-1, and occludin in the jejunum (Figure 7). Dietary supplementation with SAC significantly decreased (P < 0.05) the mRNA expression level of jejunal mucin 2 (MUC2) (Figure 7).
Figure 6.
Effects of supplementation with sweeteners on jejunal mRNA expression of taste receptors in broiler chickens. Data are presented as mean value ± SEM (n = 6). Values without the same letter (a, b) represent statistically significant differences (P < 0.05). Abbreviations: CON, broiler chickens fed a basal diet; STE, broiler chickens fed a basal diet supplemented with 250 mg/kg stevioside; SUC, broiler chickens fed a basal diet supplemented with 100 mg/kg sucralose; SAC, broiler chickens fed a basal diet supplemented with 600 mg/kg saccharin sodium; ggTAS1R1, Gallus gallus taste receptor family 1 member 1; ggTAS1R3, G. gallus taste receptor family 1 member 3; ggTAS2R1, G. gallus taste receptor family 2 member 1; ggTAS2R2, G. gallus taste receptor family 2 member 2; ggTAS2R7, G. gallus taste receptor family 2 member 7.
Figure 7.
Effects of supplementation with sweeteners on jejunal mRNA expression of tight junction genes in broiler chickens. Data are presented as mean value ± SEM (n = 6). Values without the same letter (a, b) represent statistically significant differences (P < 0.05). Abbreviations: CON, broiler chickens fed a basal diet; STE, broiler chickens fed a basal diet supplemented with 250 mg/kg stevioside; SUC, broiler chickens fed a basal diet supplemented with 100 mg/kg sucralose; SAC, broiler chickens fed a basal diet supplemented with 600 mg/kg saccharin sodium; CLDN1, claudin 1; CLDN2, claudin 2; ZO-1, zonula occludens-1; OCLN, occludin; MUC2, mucin 2.
Discussion
Mammals are sensitive to sweet taste because of advanced evolution of sweet taste receptors on the taste buds (Ahn et al., 2016). Unlike mammals, chickens are lacking T1R2, one of the taste receptor family gene responding to sweeteners in mammals (Shi and Zhang, 2006). This leads to the insensitivity to sweet substances of chickens (Shi and Zhang, 2006). Consistently, in the present study, broiler chickens did not show specific response to 2 artificial sweeteners (SUC and SAC). Dietary supplementation with SUC and SAC had no influence on the growth performance of broiler chickens. However, STE supplementation surprisingly promoted the feed intake, which, in turn, increased the growth performance of broiler chickens at an early age. In accordance with our results, a previous study has also shown that STE supplementation could increase the average BW gain of broiler chickens at 0–2 wks of age (Atteh et al., 2008). In addition, STE could be hydrolyzed to steviol by the intestinal microflora (Renwick and Tarka, 2008). A recent study has shown that dietary supplementation with STE could alter the microflora distribution in the cecum of broiler chickens (Wu et al., 2019). The gut microbiota plays an important role in producing short-chain fatty acids and neuropeptides, which could affect the feed intake of animals (Cryan et al., 2019, Metzler-Zebeli et al., 2019a, Metzler-Zebeli et al., 2019b). Because chickens are insensitive to sweet taste, promoted feed intake induced by STE supplementation might be associated with orexigenic neuropeptides secreted by the gut microbiota or the hypothalamus. This hypothesis requires further investigation and validation.
The serum biochemical parameters represent the physiological status of animals (Zhang et al., 2019). Stevioside has been proven to exert immunomodulatory activity in vitro (Boonkaewwan et al., 2006, Sehar et al., 2008). In chickens, it has been suggested that STE supplementation could increase the concentration level of IgA and IgG (Wu et al., 2019). Identically, elevated serum concentration levels of TP and ALB induced by STE supplementation suggested that STE could potentially enhance the immunity and protein synthesis of broiler chickens. This result was in agreement with the increase of growth performance. Moreover, the present data showed that dietary supplementation with SUC and SAC negatively altered the protein synthesis of broiler chickens. Consistent with our results, daily administration of SAC also decreased serum TP and ALB contents in rats (Abdelaziz and Ashour, 2011). Therefore, our data suggest that dietary STE supplementation could enhance the protein synthesis, whereas SUC and SAC supplementation could reduce it in broiler chickens.
Intestinal integrity plays some functionally significant roles in preventing pathogens invasion in broiler chickens. Dietary supplementation with SAC impaired the intestinal morphology, as indicated by the decreased VH in the jejunum. Similarly, a previous study has also shown that SAC could disrupt the barrier function of intestinal epithelial cells in vitro (Santos et al., 2018). The structural damage of the jejunal villus suggested that the ability of nutrients absorption was reduced by SAC supplementation in broiler chickens. Decreased VH also represented a fragile physical barrier in the jejunum, which might result in increased infection rate of pathogenic bacteria and thereby damage the gut health (Shao et al., 2013). In addition, DAO is mainly generated by the intestinal mucosae, and it can be released into the blood once the mucosal barrier is damaged. The activity of DAO in the serum reflects the intestinal permeability (Gilani et al., 2017). In the present study, increased serum DAO activity suggested that supplementation with SAC could impair the intestinal permeability, which was consistent with the damaged jejunal morphology. Furthermore, goblet cells are mainly responsible of secreting mucus in the gut. Lower goblet cell density was observed in the SAC supplemented group, and this could result in decreased secretion of intestinal mucus. Intestinal mucus is essential for efficient nutritional uptake, and it contains many immunomodulatory molecules (Johansson and Hansson, 2016). The intestinal mucus layer also has protective effect on the gut of broiler chickens (Hermans et al., 2010). The loss of production in intestinal mucus might lead to intestinal mucosal damage in broiler chickens. Moreover, MUC2, the main gel-forming mucin gene, is secreted from goblet cells and controls the formation of mucus layer (Johansson and Hansson, 2016). Several studies have indicated that lack of MUC2 could result in less mucus, which increases the development of inflammation in mice (Velcich et al., 2002, Johansson et al., 2008). Our finding of decreased transcription level of jejunal MUC2 also suggested that the mucus secretion of jejunum was damaged by supplementation with SAC. This result was in accordance with lower goblet cells density and impaired intestinal permeability in the jejunum. Collectively, our results have shown that dietary supplementation with SAC could impair intestinal integrity, permeability, and mucus layer of broiler chickens.
Emerging evidence has shown that high-potency sweeteners could induce oxidative stress in mammals (Simintzi et al., 2007, Erbaş et al., 2018, Iyaswamy et al., 2018). To evaluate whether sweeteners used in the present study could cause oxidative stress in the jejunum of broiler chickens, the antioxidant capacity and MDA content in the jejunal mucosae were determined. The results suggested that those 3 sweeteners did not induce oxidative stress in the jejunal mucosae. Nevertheless, our recent study has demonstrated that dietary supplementation with STE is able to alleviate lipopolysaccharide-induced intestinal mucosal damage through antioxidant and anti-inflammatory effects in broiler chickens (Jiang et al., 2019b). In agreement, STE could also enhance the antioxidant capacity of jejunal mucosae in the present study. Thus, our data suggest that dietary supplementation with sweeteners had no harm to the oxidative status of jejunal mucosae.
Taste receptors have been indicated to exist in many other organs beyond taste buds in mammals, including the gastrointestinal tract (Behrens and Meyerhof, 2011). Similarly, a previous study has demonstrated that umami taste receptors and bitter taste receptors are expressed in the gut of chickens (Cheled-Shoval et al., 2015). Although hummingbird has adapted the umami taste receptors to sense the sweetness, our data showed that the jejunal umami taste receptors (ggTAS1R1 and ggTAS1R3) had no response to the sweeteners in broiler chickens. Surprisingly, SAC supplementation remarkably increased the transcription expression level of all 3 bitter taste receptors (ggTAS2R1, ggTAS2R2, and ggTAS2R7). Several previous studies have reported that SAC is capable of binding the bitter taste receptors at high concentration (Behrens et al., 2017, Kuhn et al., 2004). A previous study has also shown that high-dose SAC treatment could damage testicular functions via activating testicular bitter taste receptors, whereas low-dose and middle-dose SAC treatments could not activate the bitter taste receptors and have no adverse effects on the testicular functions in mice (Gong et al., 2016). Therefore, in the present study, there is a possibility that the activation of jejunal bitter taste receptors was because of the high supplemented level of SAC in the feed.
In addition, our previous study has shown that bitter taste receptors exert biological functions in the jejunum (Jiang et al., 2019a). The activation of bitter taste receptors could increase cytosolic Ca2+ concentration (Freund et al., 2018). Excessive Ca2+ concentration in the jejunum is possible to induce apoptosis via calpain/caspase-dependent mechanism. Despite the fact that cell apoptosis is vital for the turnover and homeostasis of intestinal epithelium, the intestinal mucosal damage of broiler chickens is likely associated with excessive cell apoptosis in the intestines (Gunther et al., 2013). In the present study, obviously amplified apoptotic rate was observed in the jejunal epithelial cells of SAC supplemented group. Similarly, a recent study has shown that daily administration of SAC increases ovarian apoptosis in female rats (Ngekure et al., 2019). Another study has also reported that treatment with a complex of SAC and acesulfame K increases the expression levels of proapoptotic proteins in human colonic cell line (Bua et al., 2019). Hence, the adverse effects on the jejunal function caused by supplementation with high level SAC might be associated with excessive apoptosis induced by the upregulation of bitter taste receptors.
In summary, dietary supplementation with STE increased the growth performance of broiler chickens and improved antioxidant capacity of jejunum mucosa. Dietary supplementation with SUC had no effects on either growth performance or jejunal physiological functions but decreased the serum protein synthesis of broiler chickens. Saccharin sodium supplementation impaired the intestinal integrity, permeability, and mucus layer of the jejunum in broiler chickens. In addition, SAC supplementation enhanced the jejunal apoptosis, which was associated with the activation of jejunal bitter taste receptors. Our results suggest that STE has the potential to be used as a growth-promoting and antioxidant feed additive in broiler chickens. Whereas, dietary supplementation with high level SAC could impair the intestinal physiological functions of broiler chickens.
Acknowledgments
This study was supported by Innovation and Entrepreneurship Training Program of Nanjing Agricultural University (201910307029Z) and Jiangsu Agriculture Science and Technology Innovation Fund (JASTIF), China, (CX(18)2002).
Conflict of Interest Statement: The authors declare that there are no conflicts of interest.
References
- Abdelaziz I., Ashour A.E.R.A. Effect of saccharin on albino rats’ blood indices and the therapeutic action of vitamins C and E. Hum. Exp. Toxicol. 2011;30:129–137. doi: 10.1177/0960327110368695. [DOI] [PubMed] [Google Scholar]
- Ahn S.R., An J.H., Song H.S., Park J.W., Lee S.H., Kim J.H., Jang J., Park T.H. Duplex Bioelectronic Tongue for sensing umami and sweet tastes based on human taste receptor Nanovesicles. ACS Nano. 2016;10:7287–7296. doi: 10.1021/acsnano.6b02547. [DOI] [PubMed] [Google Scholar]
- Atteh J.O., Onagbesan O.M., Tona K., Decuypere E., Geuns J.M.C., Buyse J. Evaluation of supplementary stevia (Stevia rebaudiana, bertoni) leaves and stevioside in broiler diets: effects on feed intake, nutrient metabolism, blood parameters and growth performance. J. Anim. Physiol. Anim. Nutr. 2008;92:640–649. doi: 10.1111/j.1439-0396.2007.00760.x. [DOI] [PubMed] [Google Scholar]
- Baldwin M.W., Toda Y., Nakagita T., O'Connell M.J., Klasing K.C., Misaka T., Edwards S.V., Liberles S.D. Sensory biology. Evolution of sweet taste perception in hummingbirds by transformation of the ancestral umami receptor. Science. 2014;345:929–933. doi: 10.1126/science.1255097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens M., Blank K., Meyerhof W. Blends of non-caloric sweeteners saccharin and Cyclamate show reduced off-taste due to TAS2R bitter receptor inhibition. Cell Chem. Biol. 2017;24:1199–1204.e1192. doi: 10.1016/j.chembiol.2017.08.004. [DOI] [PubMed] [Google Scholar]
- Behrens M., Meyerhof W. Gustatory and extragustatory functions of mammalian taste receptors. Physiol. Behav. 2011;105:4–13. doi: 10.1016/j.physbeh.2011.02.010. [DOI] [PubMed] [Google Scholar]
- Boonkaewwan C., Ao M., Toskulkao C., Rao M.C. Specific immunomodulatory and secretory activities of stevioside and steviol in intestinal cells. J. Agric. Food Chem. 2008;56:3777–3784. doi: 10.1021/jf072681o. [DOI] [PubMed] [Google Scholar]
- Boonkaewwan C., Toskulkao C., Vongsakul M. Anti-inflammatory and immunomodulatory activities of stevioside and its Metabolite steviol on THP-1 cells. J. Agric. Food Chem. 2006;54:785–789. doi: 10.1021/jf0523465. [DOI] [PubMed] [Google Scholar]
- Brown R.J., Rother K.I. Non-nutritive sweeteners and their role in the gastrointestinal tract. J. Clin. Endocrinol. Metab. 2012;97:2597–2605. doi: 10.1210/jc.2012-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bua S., Lomelino C., Murray A.B., Osman S.M., Alothman Z.A., Bozdag M., Abdel-Aziz H.A., Eldehna W.M., McKenna R., Nocentini A., Supuran C.T. “A sweet Combination”: developing saccharin and acesulfame K Structures for Selectively targeting the Tumor-associated Carbonic Anhydrases IX and XII. J. Med. Chem. 2020;63:321–333. doi: 10.1021/acs.jmedchem.9b01669. [DOI] [PubMed] [Google Scholar]
- Buerge I.J., Keller M., Buser H.-R., Müller M.D., Poiger T. Saccharin and other artificial sweeteners in Soils: Estimated Inputs from Agriculture and Households, degradation, and Leaching to Groundwater. Environ. Sci. Technology. 2011;45:615–621. doi: 10.1021/es1031272. [DOI] [PubMed] [Google Scholar]
- Chaudhari N., Roper S.D. The cell biology of taste. J. Cell Biol. 2010;190:285–296. doi: 10.1083/jcb.201003144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheled-Shoval S.L., Druyan S., Uni Z. Bitter, sweet and umami taste receptors and downstream signaling effectors: expression in embryonic and growing chicken gastrointestinal tract. Poult. Sci. 2015;94:1928–1941. doi: 10.3382/ps/pev152. [DOI] [PubMed] [Google Scholar]
- Cheled-Shoval S.L., Reicher N., Niv M.Y., Uni Z. Detecting thresholds for bitter, umami, and sweet tastants in broiler chicken using a 2-choice test method. Poult. Sci. 2017;96:2206–2218. doi: 10.3382/ps/pex003. [DOI] [PubMed] [Google Scholar]
- Council N.R. The National Academies Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Cryan J.F., O'Riordan K.J., Cowan C.S.M., Sandhu K.V., Bastiaanssen T.F.S., Boehme M., Codagnone M.G., Cussotto S., Fulling C., Golubeva A.V., Guzzetta K.E., Jaggar M., Long-Smith C.M., Lyte J.M., Martin J.A., Molinero-Perez A., Moloney G., Morelli E., Morillas E., O'Connor R., Cruz-Pereira J.S., Peterson V.L., Rea K., Ritz N.L., Sherwin E., Spichak S., Teichman E.M., Wouw M.v. d., Ventura-Silva A.P., Wallace-Fitzsimons S.E., Hyland N., Clarke G., Dinan T.G. The microbiota-gut-brain Axis. Physiol. Rev. 2019;99:1877–2013. doi: 10.1152/physrev.00018.2018. [DOI] [PubMed] [Google Scholar]
- Damak S., Rong M., Yasumatsu K., Kokrashvili Z., Varadarajan V., Zou S., Jiang P., Ninomiya Y., Margolskee R.F. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science. 2003;301:850–853. doi: 10.1126/science.1087155. [DOI] [PubMed] [Google Scholar]
- Erbaş O., Erdoğan M.A., Khalilnezhad A., Solmaz V., Gürkan F.T., Yiğittürk G., Eroglu H.A., Taskiran D. Evaluation of long-term effects of artificial sweeteners on rat brain: a biochemical, behavioral, and histological study. J. Biochem. Mol. Toxicol. 2018;32:e22053. doi: 10.1002/jbt.22053. [DOI] [PubMed] [Google Scholar]
- Figueroa J., Frías D., Solà-Oriol D., Tadich T., Franco-Rosselló R., Nuñez V., Dwyer D.M. Palatability in pigs, the pleasure of consumption1. J. Anim. Sci. 2019;97:2165–2174. doi: 10.1093/jas/skz085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund J.R., Mansfield C.J., Doghramji L.J., Adappa N.D., Palmer J.N., Kennedy D.W., Reed D.R., Jiang P., Lee R.J. Activation of airway epithelial bitter taste receptors by Pseudomonas aeruginosa quinolones modulates calcium, cyclic-AMP, and nitric oxide signaling. J. Biol. Chem. 2018;293:9824–9840. doi: 10.1074/jbc.RA117.001005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganchrow J.R., Steiner J.E., Bartana A. Behavioral reactions to gustatory stimuli in young chicks (Gallus gallus domesticus) Developmental Psychobiology. 1990;23:103–117. doi: 10.1002/dev.420230202. [DOI] [PubMed] [Google Scholar]
- Gilani S., Howarth G.S., Kitessa S.M., Tran C.D., Forder R.E.A., Hughes R.J. Intestinal permeability induced by lipopolysaccharide and measured by lactulose, rhamnose and mannitol sugars in chickens. Animal. 2017;11:1174–1179. doi: 10.1017/S1751731116002470. [DOI] [PubMed] [Google Scholar]
- Gong T., Wei Q.W., Mao D.G., Nagaoka K., Watanabe G., Taya K., Shi F.X. Effects of daily Exposure to saccharin and sucrose on testicular biologic functions in mice. Biol. Reprod. 2016;95:116. doi: 10.1095/biolreprod.116.140889. [DOI] [PubMed] [Google Scholar]
- Gunther C., Neumann H., Neurath M.F., Becker C. Apoptosis, necrosis and necroptosis: cell death regulation in the intestinal epithelium. Gut. 2013;62:1062–1071. doi: 10.1136/gutjnl-2011-301364. [DOI] [PubMed] [Google Scholar]
- Han X., Chen C., Zhang X., Wei Y., Tang S., Wang J., Tan Z., Xu L. Effects of dietary stevioside supplementation on feed intake, digestion, ruminal Fermentation, and blood metabolites of goats. Animals. 2019;9:32. doi: 10.3390/ani9020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans D., Martel A., Van Deun K., Verlinden M., Van Immerseel F., Garmyn A., Messens W., Heyndrickx M., Haesebrouck F., Pasmans F. Intestinal mucus protects Campylobacter jejuni in the ceca of colonized broiler chickens against the bactericidal effects of medium-chain fatty acids. Poult. Sci. 2010;89:1144–1155. doi: 10.3382/ps.2010-00717. [DOI] [PubMed] [Google Scholar]
- Hunter S.R., Reister E.J., Cheon E., Mattes R.D. Low calorie sweeteners differ in their physiological effects in humans. Nutrients. 2019;11:2717. doi: 10.3390/nu11112717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyaswamy A., Kammella A.K., Thavasimuthu C., Wankupar W., Dapkupar W., Shanmugam S., Rajan R., Rathinasamy S. Oxidative stress evoked damages leading to attenuated memory and inhibition of NMDAR–CaMKII–ERK/CREB signalling on consumption of aspartame in rat model. J. Food Drug Anal. 2018;26:903–916. doi: 10.1016/j.jfda.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J., Lv Z., Qi L., Enayatullah H., Wei Q., Yu D., Shi F. Denatonium as a bitter taste receptor agonist damages jejunal epithelial cells of yellow-feathered chickens via inducing apoptosis. Animal. 2019:1–11. doi: 10.1017/S1751731119002994. [DOI] [PubMed] [Google Scholar]
- Jiang J., Qi L., Lv Z., Jin S., Wei X., Shi F. Dietary stevioside supplementation alleviates lipopolysaccharide-induced intestinal mucosal damage through anti-inflammatory and antioxidant effects in broiler chickens. Antioxidants. 2019;8:575. doi: 10.3390/antiox8120575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M.E.V., Hansson G.C. Immunological aspects of intestinal mucus and mucins. Nat. Rev. Immunol. 2016;16:639–649. doi: 10.1038/nri.2016.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M.E.V., Phillipson M., Petersson J., Velcich A., Holm L., Hansson G.C. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl. Acad. Sci. U S A. 2008;105:15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keast R.S.J., Canty T.M., Breslin P.A.S. Oral Zinc Sulfate solutions Inhibit sweet taste perception. Chem. Senses. 2004;29:513–521. doi: 10.1093/chemse/bjh053. [DOI] [PubMed] [Google Scholar]
- Kimmich G.A., Randles J., Anderson R.L. Effect of saccharin on the ATP-induced increase in Na+ permeability in isolated chicken intestinal epithelial cells. Food Chem. Toxicol. 1989;27:143–149. doi: 10.1016/0278-6915(89)90062-8. [DOI] [PubMed] [Google Scholar]
- Kuhn C., Bufe B., Winnig M., Hofmann T., Frank O., Behrens M., Lewtschenko T., Slack J.P., Ward C.D., Meyerhof W. Bitter taste receptors for saccharin and acesulfame K. J. Neurosci. 2004;24:10260–10265. doi: 10.1523/JNEUROSCI.1225-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H., Macpherson L.J., Parada C.A., Zuker C.S., Ryba N.J.P. Rewiring the taste system. Nature. 2017;548:330–333. doi: 10.1038/nature23299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L., Liu Y., Xu J., Sun H., Chen H., Yao Y., Zhang P., Shen F., Alder A.C. Mass loading of typical artificial sweeteners in a pig farm and their dissipation and uptake by plants in neighboring farmland. Sci. Total Environ. 2017;605-606:735–744. doi: 10.1016/j.scitotenv.2017.06.027. [DOI] [PubMed] [Google Scholar]
- McMeniman J.P., Rivera J.D., Schlegel P., Rounds W., Galyean M.L. Effects of an artificial sweetener on health, performance, and dietary preference of feedlot cattle1. J. Anim. Sci. 2006;84:2491–2500. doi: 10.2527/jas.2006-098. [DOI] [PubMed] [Google Scholar]
- Metzler-Zebeli B.U., Siegerstetter S.-C., Magowan E., Lawlor P.G., O′Connell N.E., Zebeli Q. Fecal microbiota Transplant from highly feed efficient donors affects cecal Physiology and microbiota in low- and high-feed efficient chickens. Front. Microbiol. 2019;10 doi: 10.3389/fmicb.2019.01576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler-Zebeli B.U., Siegerstetter S.-C., Magowan E., Lawlor P.G., Petri R.M., O'Connell N.E., Zebeli Q. Feed Restriction Modifies intestinal microbiota-Host mucosal Networking in chickens divergent in Residual feed intake. mSystems. 2019;4 doi: 10.1128/mSystems.00261-18. e00261-e00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Gerspach A.C., Biesiekierski J.R., Deloose E., Clevers E., Rotondo A., Rehfeld J.F., Depoortere I., Van Oudenhove L., Tack J. Effects of caloric and noncaloric sweeteners on antroduodenal motility, gastrointestinal hormone secretion and appetite-related sensations in healthy subjects. Am. J. Clin. Nutr. 2018;107:707–716. doi: 10.1093/ajcn/nqy004. [DOI] [PubMed] [Google Scholar]
- Milner H.J.S. Preference for sucrose solutions by Japanese quail (Coturnix coturnix japonica) in two-Bottle drinking tests. Am. Midland Naturalist. 1969;81:575–578. [Google Scholar]
- Moran A.W., Al-Rammahi M., Zhang C., Bravo D., Calsamiglia S., Shirazi-Beechey S.P. Sweet taste receptor expression in ruminant intestine and its activation by artificial sweeteners to regulate glucose absorption. J. Dairy Sci. 2014;97:4955–4972. doi: 10.3168/jds.2014-8004. [DOI] [PubMed] [Google Scholar]
- Ngekure M.X.K., Jiang J., Enayatullah H., Ennab W., Mustafa S., Rodeni S., Wei Q., Shi F. Sweet taste receptor agonists alter ovarian functions and ovarian cycles in aged mice. Reprod. Biol. 2019;19:230–236. doi: 10.1016/j.repbio.2019.07.007. [DOI] [PubMed] [Google Scholar]
- Renwick A.G., Tarka S.M. Microbial hydrolysis of steviol glycosides. Food Chem. Toxicol. 2008;46(Suppl 7):S70–S74. doi: 10.1016/j.fct.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Santos P.S., Caria C.R.P., Gotardo E.M.F., Ribeiro M.L., Pedrazzoli J., Gambero A. Artificial sweetener saccharin disrupts intestinal epithelial cells' barrier function in vitro. Food Funct. 2018;9:3815–3822. doi: 10.1039/c8fo00883c. [DOI] [PubMed] [Google Scholar]
- Schiffman S.S., Rother K.I. Sucralose, a synthetic organochlorine sweetener: overview of biological issues. J. Toxicol. Environ. Health B Crit. Rev. 2013;16:399–451. doi: 10.1080/10937404.2013.842523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehar I., Kaul A., Bani S., Pal H.C., Saxena A.K. Immune up regulatory response of a non-caloric natural sweetener, stevioside. Chemico-Biological Interactions. 2008;173:115–121. doi: 10.1016/j.cbi.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Shao Y., Guo Y., Wang Z. β-1,3/1,6-Glucan alleviated intestinal mucosal barrier impairment of broiler chickens challenged with Salmonellaenterica serovar Typhimurium. Poult. Sci. 2013;92:1764–1773. doi: 10.3382/ps.2013-03029. [DOI] [PubMed] [Google Scholar]
- Shi P., Zhang J. Contrasting modes of evolution between vertebrate sweet/umami receptor genes and bitter receptor genes. Mol. Biol. Evol. 2006;23:292–300. doi: 10.1093/molbev/msj028. [DOI] [PubMed] [Google Scholar]
- Simintzi I., Schulpis K.H., Angelogianni P., Liapi C., Tsakiris S. The effect of aspartame metabolites on the suckling rat frontal cortex acetylcholinesterase. An in vitro study. Food Chem. Toxicol. 2007;45:2397–2401. doi: 10.1016/j.fct.2007.06.016. [DOI] [PubMed] [Google Scholar]
- Sterk A., Schlegel P., Mul A.J., Ubbink-Blanksma M., Bruininx E.M.A.M. Effects of sweeteners on individual feed intake characteristics and performance in group-housed weanling pigs1. J. Anim. Sci. 2008;86:2990–2997. doi: 10.2527/jas.2007-0591. [DOI] [PubMed] [Google Scholar]
- Velcich A., Yang W., Heyer J., Fragale A., Nicholas C., Viani S., Kucherlapati R., Lipkin M., Yang K., Augenlicht L. Colorectal Cancer in mice Genetically deficient in the mucin Muc2. Science. 2002;295:1726–1729. doi: 10.1126/science.1069094. [DOI] [PubMed] [Google Scholar]
- Wu X.Z., Yang P.L., Dai S.F., Wen Z.G. Effect of dietary stevioside supplementation on growth performance, nutrient digestibility, serum parameters, and intestinal microflora in broilers. Food Funct. 2019;10:2340–2346. doi: 10.1039/c8fo01883a. [DOI] [PubMed] [Google Scholar]
- Zhang C., Chen K.K., Zhao X.H., Wang C., Geng Z.Y. Effect of l-theanine on the growth performance, immune function, and jejunum morphology and antioxidant status of ducks. Animal. 2019;13:1145–1153. doi: 10.1017/S1751731118002884. [DOI] [PubMed] [Google Scholar]
- Zhang W., He H., Gong L., Lai W., Dong B., Zhang L. Effects of sweetener sucralose on diet preference, growth performance and hematological and biochemical parameters of weaned piglets. Asian-australas J. Anim. Sci. 2020;33:802–811. doi: 10.5713/ajas.18.0863. [DOI] [PMC free article] [PubMed] [Google Scholar]