Abstract
Nighttime light pollution is linked to metabolic and cognitive dysfunction. Many patients with autism spectrum disorders (ASD) show disturbances in their sleep/wake cycle, and may be particularly vulnerable to the impact of circadian disruptors. In this study, we examined the impact of exposure to dim light at night (DLaN, 5 lx) in a model of ASD: the contactin associated protein-like 2 knock out (Cntnap2 KO) mice. DLaN was sufficient to disrupt locomotor activity rhythms, exacerbate the excessive grooming and diminish the social preference in Cntnap2 mutant mice. On a molecular level, DLaN altered the phase and amplitude of PER2:LUC rhythms in a tissue-specific manner in vitro. Daily treatment with melatonin reduced the excessive grooming of the mutant mice to wild-type levels and improved activity rhythms. Our findings suggest that common circadian disruptors such as light at night should be considered in the management of ASD.
Keywords: Autism spectrum disorder, Circadian, Cntnap2, Light pollution, Melatonin, Mice
1. Introduction
There is a growing concern that nighttime light pollution may be negatively impacting human health, as much of the world’s urban populations do not experience true night due to the widespread use of artificial lighting (Falchi et al., 2016). Previous studies have shown that night time light exposure leads to cognitive, skeletal motor, and metabolic dysfunction in laboratory animals (Bedrosian et al., 2016; Bedrosian et al., 2011; Borniger et al., 2014; Fonken et al., 2013; Fonken et al., 2012; Fonken and Nelson, 2011; Lucassen et al., 2016; Walker 2nd et al., 2019). The deleterious effects of night time light pollution also affect animals in the wild (Dominoni et al., 2013; Raap et al., 2015; Stevenson et al., 2015). Despite all of the social and economic benefits of artificial lighting, we may be paying a price for inappropriate light exposure (Czeisler, 2013; Roenneberg and Merrow, 2016; Rybnikova et al., 2016). One of the major factors leading to disrupted sleep is an inappropriate photic environment caused by nighttime exposure to electric lights and light-emitting devices as well as diminished exposure to daylight while indoors.
A significant proportion of individuals with neurodevelopmental disorders such as autism spectrum disorders (ASD) experience disruptions to their daily sleep/wake cycles. Among the most common complaints is delayed bedtime with frequent nocturnal awakenings (Devnani and Hegde, 2015; Elrod and Hood, 2015; Mazurek and Sohl, 2016; Robinson-Shelton and Malow, 2016; Veatch et al., 2015). Perhaps because of this disrupted temporal pattern of sleep, these patients are exposed to more light via electronic screens during the night (Engelhardt et al., 2013; Mazurek et al., 2016). This nocturnal light exposure by itself has been shown to delay sleep in healthy young people (Chang et al., 2015; Gronli et al., 2016; Wood et al., 2013). Melatonin as well as conventional sleeping aids are commonly used as counter-measures to help sleep onset in patients with neurodevelopmental disorders (Goldman et al., 2014; Kawabe et al., 2014; Malow et al., 2012; Rossignol and Frye, 2014; Schwichtenberg and Malow, 2015). Animal models are helpful to better understand the mechanisms underlying circadian and sleep disorders as well as in developing strategies for disease management focused on restoring the sleep/wake cycle.
In this study, we set out to test the possibility that mice with a genetic risk factor associated with ASD are more vulnerable to the detrimental effects of nighttime light exposure. Rare and common variants of the contactin associated protein-like 2 (Cntnap2) gene are associated with ASD, intellectual disabilities, and seizures in patients (Arking et al., 2008; Bakkaloglu et al., 2008; Nord et al., 2011; O’Roak et al., 2011; Strauss et al., 2006) as well as in mouse models (Peñagarikano et al., 2011; Poliak et al., 1999). We examined the impact of nighttime light exposure by housing the mutant animals and their wild-type (WT) controls under conditions that mimic experiencing very dim LED-generated light in the bedroom at night. We describe the deleterious effect of dim light at night (DLaN) on daily rhythms of activity, sleep, and on the molecular circadian clock as measured by PERIOD2-driven bioluminescence. In addition, we examined the impact of DLaN on repetitive behavior and sociability in Cntnap2 mutant mice and examined the persistence of the effects of DLaN after returning the mice to a normal lighting cycle. Finally, we tested the ability of melatonin to counteract the effects of DLaN on repetitive behavior and circadian disruption.
2. Materials and methods
2.1. Animals
All animal procedures were performed in accordance with the UCLA animal care committee’s regulations. Cntnap2tm2Pele mutant mice backcrossed to the C57BL/6J background strain were acquired (B6.129(Cg)-Cntnap2tm2Pele/J, https://www.jax.org/strain/017482, (Poliak et al., 1999). Cntnap2 null mutant (KO) and wild-type (WT) mice were obtained from heterozygous breeding pairs. Weaned mice were genotyped (TransnetYX, Cordova, TN) and group-housed prior to experimentation. We limited our studies to mice between the ages of 2 and 4 months as the circadian system is mature at this age and it is prior to the development of epilepsy in the mutant mice (Peñagarikano et al., 2011). Only male animals were used in this study as it has previously been determined that the C57BL/6J strain of mice exhibit subtle sex differences in circadian rhythms of locomotor activity (Kuljis et al., 2013).
2.2. Home cage lighting manipulations
Mice were housed in light-tight ventilated cabinets in temperature- and humidity-controlled conditions, with free access to food and water. Young adult (2–3 mo old) Cntnap2 KO and age-matched WT littermate controls were first entrained to a normal lighting cycle: 12 h light: 12 h dark (LD). Light intensity during the day was 300 lx as measured at the base of the cage, and 0 lx during the night. Following entrainment to normal LD, mice were singly housed under 1 of 2 lighting conditions for 2 weeks: LD, or dim light at night (DLaN, nighttime dim light 5 lx). The spectral properties of the DLaN are shown in Fig. S1. The melanoptic light intensity in the cage was 2.8 lx. The body weights of the mice are shown in Fig. S3.
2.3. Cage activity
Home cage activity (Loh et al., 2013; Wang et al., 2017) was monitored using a top-mounted passive infra-red (PIR) motion detector reporting to a VitalView data recording system (Mini Mitter, Bend, OR). Detected movements were recorded in 3 min bins, and 10 days of data were averaged for analysis using the El Temps chronobiology program (A. Diez-Noguera, Barcelona, Spain; http://www.el-temps.com/principal.html). Cage activity was determined by averaging 10 days of PIR-detected motion (movements/h), and the relative distribution of activity during the day versus the night was determined (day activity, %). The strength of the rhythm was determined from the amplitude of the χ2 periodogram at 24 h, to produce the rhythm power (%V) normalized to the number of data points examined. The periodogram analysis uses a χ2 test with a threshold of 0.001 significance, from which the amplitude of the periodicities is determined at the circadian harmonic to obtain the rhythm power. Precision was determined using the Clocklab program (Actimetrics, Wilmette, IL; http://actimetrics.com/products/clocklab/) by first determining the daily onset of activity over 10 days, and then determining the deviation from the best-fit line drawn through the 10 onset times. To produce the representative waveforms, hourly running averages of the same series of activity data was plotted against time of day (Zeitgeber Time, ZT, where ZT 0 indicates the time of lights-on).
2.4. Monitoring of immobility-defined sleep behavior
Immobility-defined sleep was determined as described previously (Whittaker et al., 2018). Singly housed Cntnap2 KO and WT littermate controls (2–3 mo old) were first entrained to a normal lighting cycle (LD 12:12, 7 days). Mice were then kept in the LD or exposed to DLaN for 2 weeks prior to 2 days of measurement of sleep behavior. Mice were housed in see-through plastic cages containing bedding (without the addition of nesting material). A side-on view of each cage was obtained, with minimal occlusion by the food bin or water bottle, both of which were top-mounted. Cages were top-lit using IR-LED lights. Video capture was accomplished using surveillance cameras with visible light filters (Gadspot Inc., City of Industry, CA) connected to a video-capture card (Adlink Technology Inc., Irvine, CA) on a Dell Optiplex computer system. ANY-maze software (Stoelting Co., Wood Dale, IL) was used for automated tracking of mouse immobility.
Immobility was registered when 95% of the area of the animal stayed immobile for more than 40 s, as was previously determined to have 99% correlation with simultaneous EEG/EMG defined sleep (Fisher et al., 2016). Continuous tracking of the mice was performed for a minimum of 5 sleep-wake cycles, with randomized visits (1–2 times/day) by the experimenter to confirm mouse health and video recording. The 3rd and 4th sleep-wake cycles were averaged for further analysis. Immobility-defined sleep data were exported in 1 min bins, and total sleep time was determined by summing the immobility durations in the rest phase (ZT 0–12) or active phase (ZT 12–24). An average waveform of hourly immobile-sleep over the two sleep-wake cycles was produced. Variability of sleep onset and awake time was determined using Clocklab to draw the best-fit line over the sleep cycles, and the differences between sleep offset and best-fit regression of each sleep cycle were averaged.
2.5. Recordings of neurons in suprachiasmatic nucleus (SCN)
Male Cntnap2 mutant mice and WT littermates (3–4 mo) entrained on a 12:12 LD or DL cycle were anesthetized in an isoflurane chamber at ZT 4.5 and ZT 16.5 for ZT 6–8 and ZT 18–20 recordings respectively. Anesthetized mice were decapitated, brains extracted, and brains incubated in ice-cold slice solution for 5 min. Coronal slices (300 μm) of mid-SCN were collected in slice solution (in mM: 26 NaHCO3, 1.25 NaH2PO4, 10 glucose, 125 NaCl, 3 KCl, 5 MgCl2, 1 CaCl2) using a vibratome, and then incubated for 1 h at 34 °C in artificial cerebrospinal fluid (ACSF, in mM; 26 NaHCO3, 1.25 NaH2PO4, 10 glucose, 125 NaCl, 3 KCl, 2 MgCl2, 2 CaCl2) while continually being aerated with 95% O2/5% CO2. Slices were placed in a recording chamber (PH-1, Warner Instruments, Hamden, CT) attached to the stage of a fixed stage upright DIC microscope (OLYMPUS, Tokyo, Japan), and superfused continuously (2 ml/min) with ACSF aerated with 95% O2/5% CO2 at 34 °C. Two sets of experiments were carried out to measure spontaneous firing rate (SFR) from SCN neurons. First, extracellular single-unit recordings from the SCN were taken with recording electrodes. These micropipettes (4–7 MΩ) were pulled from borosilicate tubing (Sutter Instrument) and filled with 3 M NaCl, pH 7.4. Second, cell-attached SCN recordings were made using electrode micropipettes (3–7MΩ) pulled from glass capillaries and recording electrodes were filled with ACSF. Recordings were obtained with the AXOPATCH 200B amplifier (Molecular Devices, Sunnyvale, CA) and monitored on-line with pCLAMP (Ver 10, Molecular Devices). The daytime SFR measured from WT mice was virtually identical between these two techniques (3.0 ± 0.2 Hz extracellular vs 3.0 ± 0.4 Hz cell-attached).
2.6. In vitro bioluminescence
In vitro measurements of bioluminescence were performed as previously described (Whittaker et al., 2018). Cntnap2 KO and WT PER2:LUC male mice were subjected to control LD or DLaN for 2 weeks prior to sampling. Mice were sacrificed after anesthesia (isoflurane) between ZT 10 and 11, and 1–2 mm3 explants were immediately dissected in ice-cold Hanks’ balanced salt solution (HBSS; Sigma) supplemented with 4.5 mM NaHC03, 10 mM HEPES and 100 U/ml penicillin-streptomycin. Brains were incubated in ice-cold slice solution (in mM: 26 NaHCO3, 1.25 NaH2PO4, 10 glucose, 125 NaCl, 3 KCl, 5 MgCl2, 1 CaCl2) aerated with 95% O2/5% CO2 for 5 min, and 300 μm coronal sections were collected using a vibratome and further microdissected in HBSS under a 10× dissecting microscope. The SCN was cut away from the rest of the section using two cuts with a surgical scalpel (No. 21 blade, Fisher Sci.). All explants were individually transferred to Millicell membranes (0.4 μm, PICMORG50, Millipore, Bedford, MA) resting on 1.2 ml of recording media: (1× DMEM (Sigma), 1× B27 supplement (Gibco), 4.5 mM NaHCO3, 10 mM HEPES, 40 mM Glutamax (Gibco), 4.5 mg/ml d-glucose, 25 U/ml penicillin, 25 U/ml streptomycin, 0.1 mM sodium salt monohydrate luciferin (Biosynth, Staad, Switzerland)) in a 35 mm dish sealed with autoclaved vacuum grease (Dow Corning, Midland, MI). SCN, hippocampus, and liver explants were inserted into the Lumicycle photometer (Actimetrics, Wilmette, IL), incubated at 37 °C, and bioluminescence was continuously monitored for 7 consecutive days. Raw bioluminescence values were normalized by baseline subtraction (24 h running average) and smoothed with 2 h windows to prepare the representative bioluminescence traces. The phase and amplitude of each explant were determined as previously described (Whittaker et al., 2018). Period was determined using the sine-wave fit function in Lumicycle Analysis (Actimetrics).
2.7. Behavioral tests
During the 14th day of LD or DLaN treatment, mice were placed in a novel arena for 10 min, and their grooming and exploration behavior was recorded. Following habituation to the arena, mice were tested using the three chamber sociability protocol (Yang et al., 2011). In this test, mice are freely mobile in an arena with three chambers where the central chamber remains empty. In each of the flanking chambers, an up-turned metal-grid pencil cup is placed: one remains empty as the novel object (O), and an age- and sex-matched WT stranger mouse (S1) is placed in the second up-turned cup. The stranger mice were previously habituated to the cups for 3 × 15 min sessions. To test for social preference, the mice were presented with the choice of the object (O) or the stranger mouse (S1). Social preference was determined by comparing the dwell time of the tested mouse in the two chambers, and a social preference index was calculated using the following: (S1 − O)/(S1 + O), where a higher value indicates greater social preference. The time that the tested mouse spent investigating the object and the stranger mouse was also determined.
Grooming and investigating behaviors were manually scored and averaged post-hoc by two independent observers who were blind to the treatment and genotype conditions.
All three chamber tests were performed under dim red light (< 2 lx at arena level) during the animals’ night between 2 and 4 h after lights-off (ZT 14–16). Video recordings of each arena were captured using surveillance cameras, supplemented with infra-red lighting, connected to a video-capture card (Adlink Technology Inc., Irvine, CA) on a Dell Optiplex computer system. Automated tracking of the mice was achieved using ANY-maze software (Stoelting, Wood Dale, IL), which allowed us to determine distance traveled and chamber dwell time.
2.8. Drug treatments
WT and Cntnap2 KO mice were randomly distributed into one of two groups and housed under DLaN conditions. One group of mice was treated daily with melatonin (3.0 mg/kg) suspended in a small amount of condensed milk (10 mg condensed milk/g body weight). The second group of mice was treated daily with the suspension vehicle (10 mg condensed milk/g body weight). Both groups were first habituated to the ceramic food bowls containing condensed milk for 3 days prior to the start of the light and drug treatment. All mice in the study learnt to find and consume the condensed milk within the food bowls within 15 min of presentation. All food bowls were weighed at 15 min post-presentation to confirm that the treatment was fully consumed throughout the duration of the drug treatment. Mice in both groups were given free access to the treatments at ZT 11.5 (30 min prior to lights off) daily from the first day of the DLaN treatment. The total treatment duration was 2 weeks to match the duration of DLaN.
2.9. Morphometrical and Histoanatomical analyses of the SCN
Structural analysis of the SCN was performed as previously described (Lee et al., 2018). WT and Cntnap2 KO mice (males, 3–4 mo) were anesthetized with isoflurane at a specific time during the night (ZT 14) and transcardially perfused with phosphate buffered saline (PBS, 0.1 M, pH 7.4) containing 4% (w/v) paraformaldehyde (PFA, Electron Microscopy Sciences, Hatfield, PA). The brains were rapidly dissected out, post-fixed overnight in 4% PFA at 4 °C, and cryoprotected in 15% sucrose. Coronal sections were obtained using a cryostat (Leica, Buffalo Grove, IL), collected sequentially and then processed for Nissl staining using cresyl violet (Sigma) or immunofluorescence. The histomorpho-logical and stereological assessments were performed by two independent observers “masked” to the treatment and genotype.
2.9.1. Nissl staining
Coronal brain sections (20–30 μm) containing the entire left and right SCN were stained with 1% cresyl violet solution (Sigma). Photographs were acquired on a Zeiss Axioskop equipped with a Zeiss colour Axiocam using a 10× objective, to include both left and right SCN, and the AxioVision software (Zeiss, Pleasanton, CA) and then, used to estimate the area, height and width of the SCN. Measurements (in μm) were obtained using the AxioVision. For each animal, the three measurements were performed in consecutive slices of the SCN. Those from the 2 most central sections (largest area), the 2 sections anterior and 3 posterior to these were summed (a total of 7 consecutive sections). No significant differences between the measurements in the left or right SCN were found so the results were averaged.
2.9.2. Immunofluorescence
Free-floating coronal sections (30 μm) containing the entire SCN were blocked for 1 h at RT in carrier solution (1% BSA, 0.3% Triton X-100, PBS) containing 10% normal donkey serum and then incubated with gentle shacking for 2 h at 37 °C with the primary antibodies against arginine vasopressin (AVP; 1:500, guinea pig polyclonal, Peninsula Lab, San Carlos, CA) and vasoactive intestinal peptide (VIP; 1:1000, rabbit polyclonal, ImmunoStar, Hudson, WI) diluted in the carrier solution containing 5% normal donkey serum, followed by incubation in the appropriate secondary antibody conjugated to Cy3 or AlexaFluor 488 (Jackson ImmunoResearch Laboratories, Bar Harbor, ME). Sections were mounted and coverslips applied onto a drop of Vectashield mounting medium containing DAPI (4′−6-diamidino-2-phenylinodole; Vector Laboratories, Burlingame, CA). Stereological analysis was performed using a Zeiss AxioImager M2 microscope (Zeiss, Pleasanton, CA) equipped with a motorized stage controlled by the StereoInvestigator software (MicroBrightField Biosciences, Williston, VT). Due to the SCN’s small area and the low number of VIP+ and AVP + neurons in the SCN, stereological parameters were designed to cover the entire area of interest. All immunopositive cell bodies were counted directly using a 40× objective. Images were acquired on a Zeiss AxioImager M2 microscope equipped with an AxioCam MRm and the ApoTome imaging system, using a 10× objective and the Zeiss Zen digital imaging software.
2.10. Statistics
To determine the impact of DLaN on waveform, we used a repeated measures two-way analysis of variance (ANOVA) with time and treatment as factors. To determine the impact of DLaN on other behavioral measures, we used a two-way ANOVA with genotype and treatment as factors. Post hoc Holm-Sidak pairwise comparisons were applied after the ANOVA. A t-test was used to compare Nissl measurements and cell counts in WT and Cntnap2 KO mice. Values were reported as the mean ± standard error of the mean (SEM). Differences were deemed significant if P < 0.05.
3. Results
3.1. Nighttime light exposure alters daily rhythms of cage activity in WT and Cntnap2 KO mice
To determine whether DLaN altered the daily rhythms in locomotor activity, separate cohorts of KO and WT mice were housed under either control conditions (LD) or DLaN for 2 weeks. We first examined the impact of DLaN on the average waveform (1 h bins) of each genotype using a 2-way ANOVA for repeated measures (Fig. 1A, B). Both Cntnap2 KO (n = 10) and WT controls (n = 10) exhibited clear rhythms in activity under both lighting conditions. The Cntnap2 KO activity levels significantly varied with time but not treatment; while in WT mice, there were significant effects of both time and treatment. Post-hoc analysis by Multiple Comparison Procedures (Holm-Sidak method) indicated significant reductions in activity caused by DLaN exposure at a number of phases during the night in both genotypes (Fig. 1A, B). To provide another measure, DLaN equally reduced the peak/trough amplitude of the WT (63.9 ± 4.0–9.2 ± 1.0) and Cntnap2 KO (61.8 ± 7.5–12.0 ± 2.9). Quantitative analysis of all the activity rhythms (2-way ANOVA; Table 1) found that, under normal lighting conditions, Cntnap2 KO mice exhibited reduced rhythm strength (Fig. 1D), increased daytime activity (Fig. 1E) and decreased precision in the daily onset of activity (Fig. 1F) compared to WT controls. DLaN significantly reduced cage activity (Fig. 1C) and rhythm power (Fig. 1D), while increasing the amount of activity during the day (Fig. 1E) and the cycle-to-cycle variation (Fig. 1F) in both genotypes. After return to the original LD cycle, both genotypes of mice exhibited partial recovery of their activity rhythms (Fig.S 4). These results indicate that the Cntnap2 KO mice exhibit some deficits in the generation of diurnal rhythms in cage activity and that DLaN negatively affected most of the locomotor activity parameters in both genotypes.
Fig. 1.
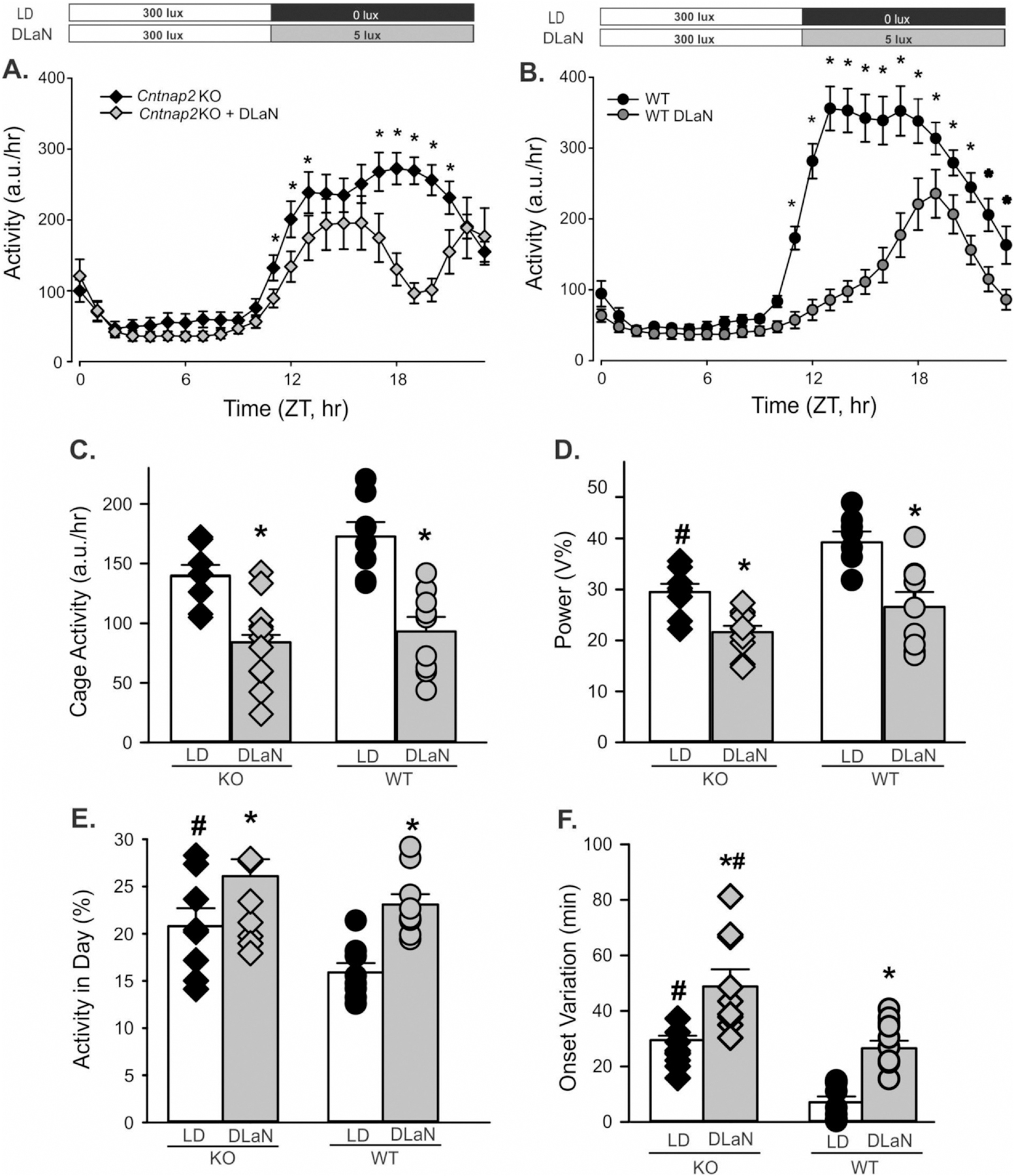
Rhythms in cage activity are altered by dim light at night (DLaN). Waveforms of daily rhythms in cage activity under LD and DLaN show the activity-depressing effect of DLaN (5 lx) in both Cntnap2 KO (A) and WT (B) mice. The mean ± SEM is shown at each time point. For this and all other figures, the mutants are shown as diamonds while the WT values are shown as circles. For this and all other figures, black symbols are from mice in normal LD while grey symbols are from mice in DLaN. The activity waveform (1 h bins) of each genotype was analyzed using a 2-way ANOVA for repeated measures with genotype and time as factors. Both Cntnap2 KO (n = 10) and WT controls (n = 10) exhibited clear rhythms in activity under both lighting conditions. Overall, the Cntnap2 KO exhibited significant effects of time (F(23, 383) = 23.491, P < 0.001) but not treatment (F(1, 383) = 3.353, P = 0.089). The WT mice exhibited significant effects of both time (F(23, 383) = 79.375, P = 0.001) and treatment (F(1, 383) = 25.744, P < 0.001). Post-hoc analysis by Multiple Comparison Procedures (Holm-Sidak method) indicated significant reductions in activity caused by DLaN exposure at a number of phases during the night in both genotypes. Significant (P < 0.05) differences between the genotypes are indicated with an asterisk over the untreated values. Histograms (C–F) show means ± SEM with the values from individual animals overlaid. The locomotor activity parameters were analyzed using a 2-way ANOVA with genotype and treatment as factors. Significant (P < 0.05) differences as a result of treatment are indicated with an asterisk (*) over the treated values while the number sign (#) indicates a genotypic difference. (C) Cage activity was reduced by treatment (F(1, 35) = 37.428, P < 0.001) but there were no effects of genotype (F(1,35) = 3.716, P = 0.063). (D) The power of the rhythms (% variation) was reduced by treatment (F(1, 35) = 26.780, P < 0.001) as well as genotype (F(1,35) = 13.789, P < 0.001). (E) The amount of activity during the day (%) was increased by the treatment (F(1, 35) = 15.346, P < 0.001) as well as genotype (F(1,35) = 6.097, P = 0.019). (F) The precision of the activity onset (cycle-to-cycle variation) was reduced by the treatment (F(1, 35) = 41.644, P < 0.001) and genotype (F(1,35) = 29.686, P < 0.001). Overall, DLaN negatively impacted most measures of activity rhythms and the mutant mice exhibited more activity in the day and increased variability of activity onset which resulted in an overall reduction in the strength of the daily rhythm.
Table 1.
Rhythms in locomotor activity and sleep behavior in Cntnap2 KO mice were disrupted by dim light at night (DLaN).
| Cntnap2 KO | Cntnap2 KO + DLaN | WT | WT + DLaN | Genotype | Treatment | |
|---|---|---|---|---|---|---|
| Locomotor Activity rhythms | ||||||
| Power (% variance) | 29.5 ± 2.1 | 21.6 ± 1.8* | 39.2 ± 2.1 | 26.6 ± 1.9* | F = 13.789; P < 0.001 | F = 26.780; P < 0.001 |
| Cage Activity (a.u./hr) | 139.2 ± 11.6 | 84.1 ± 9.9* | 172.6 ± 11.6 | 93.1 ± 10.9* | F = 3.716; P = 0.063 | F = 37.428; P < 0.001 |
| Activity in day (%) | 20.8 ± 1.9 | 26.1 ± 1.8* | 15.9 ± 1.0 | 23.1 ± 1.1* | F = 6.097; P = 0.019 | F = 15.346; P < 0.001 |
| Onset Variability (min) | 25.7 ± 2.1 | 48.8 ± 6.2* | 7.1 ± 2.1 | 29.4 ± 6.5* | F = 29.686; P < 0.001 | F = 41.644; P < 0.001 |
| Fragmentation | 9.8 ± 0.9 | 11.6 ± 0.9 | 9.0 ± 0.9 | 11.3 ± 0.9 | F = 1.173; P = 0.287 | F = 2.888; P = 0.089 |
| Sleep Behavior rhythms | ||||||
| Daily sleep (min) | 908.6 ± 43.8 | 1042.6 ± 49.4* | 943.5 ± 52.8 | 1018.0 ± 54.7* | F = 0.011; P = 0.914 | F = 4.762; P = 0.036 |
| Sleep in day (min) | 590.9 ± 17.6 | 564.6 ± 33.1 | 622.0 ± 27.8 | 569.4 ± 50.3 | F = 0.274; P = 0.604 | F = 1.318; P = 0.258 |
| Sleep in night (min) | 317.7 ± 28.2 | 478.0 ± 49.8* | 321.5 ± 32.3 | 448.6 ± 53.2* | F = 0.091; P = 0.764 | F = 11.533; P = 0.002 |
| Bouts in day (#) | 3.7 ± 0.5 | 3.9 ± 0.2 | 4.3 ± 0.2 | 3.3 ± 0.5 | F = 1.118; P = 0.299 | F = 14.492; P < 0.001 |
| Bouts in night (#) | 6.3 ± 0.4 | 5.7 ± 0.4 | 5.8 ± 0.6 | 6.1 ± 0.5 | F = 0.067; P = 0.797 | F = 1.966; P = 0.172 |
| Bout duration day | 177.4 ± 26.7 | 207.5 ± 33.0 | 229.0 ± 54.7 | 161.2 ± 27.1 | F = 1.581; P = 0.217 | F = 1.329; P = 0.257 |
| Bout duration night | 47.7 ± 4.8 | 89.8 ± 15.8* | 54.4 ± 3.9 | 82.7 ± 9.2* | F = 0.007; P = 0.979 | F = 14.676; P < 0.001 |
| Sleep onset (ZT) | 21.2 ± 2.4 | 22.2 ± 0.6* | 24.4 ± 0.2 | 23.2 ± 0.2* | F = 5.639; P = 0.023 | F = 20.617; P < 0.001 |
Comparisons of age-matched WT and Cntnap2 KO mice under LD or DLaN regimen (n = 8/group). Values are shown as averages ± SEM. Data were analyzed with a two-way ANOVA using genotype and treatment as factors. The Holm-Sidak test for multiple comparisons was used when appropriate.
indicates significant differences between LD controls and those exposed to DLaN.
P values < 0.05 were considered significant and are shown in bold.
3.2. Nighttime light exposure alters the temporal pattern of sleep in Cntnap2 KO mice
We sought to determine whether loss of Cntnap2 had an impact on the amount or temporal pattern of sleep behavior measured with video recording in combination with an automated mouse tracking analysis software system (Wang et al., 2017, 2018). Both Cntnap2 KO (n = 10) and WT controls (n = 10) exhibited clear rhythms in sleep under both lighting conditions (Fig. 2A, B; Table 1). Further analysis showed significant effects of time and treatment in the mutants while, in the WT mice, there were significant effects of time but not treatment. Post-hoc analysis by Multiple Comparison Procedures (Holm-Sidak method) revealed significant changes in sleep caused by DLaN exposure at a number of phases during the night in both genotypes (Fig. 2A, B). Quantitative analysis of all the sleep rhythms (2-way ANOVA; Table 1) found that the total amount of sleep (per 24 h-day; Fig. 2C) was not affected by genotype but did increase with treatment. Analysis of the same animals before and after the exposure confirmed that DLaN significantly increased the total amount of sleep in the mutant and mice. Significant day/night differences in the amount of sleep behavior (Fig. 2D) were seen in Cntnap2 KO and WT with higher amounts of sleep during the day. In contrast, the treated groups lost the day/night difference. Sleep fragmentation as measured by the total number of sleep bouts (Fig. 2E; Table 1) was not affect by genotype or treatment. However, the maximum sleep bout length (Fig. 2F) was increased by treatment but not genotype. Post-hoc analysis indicated that DLaN increased the duration of sleep in individual bouts in the Cntnap2 KO and WT mice. Finally, sleep onset (Fig. 2G, H) was altered by both genotype and treatment (Table 1). Thus, the Cntnap2 KO mice exhibited a disruption in sleep onset, but other parameters were no different from WT mice. Both genotypes exhibited disrupted temporal patterns in sleep behavior driven by the DLaN treatment.
Fig. 2.
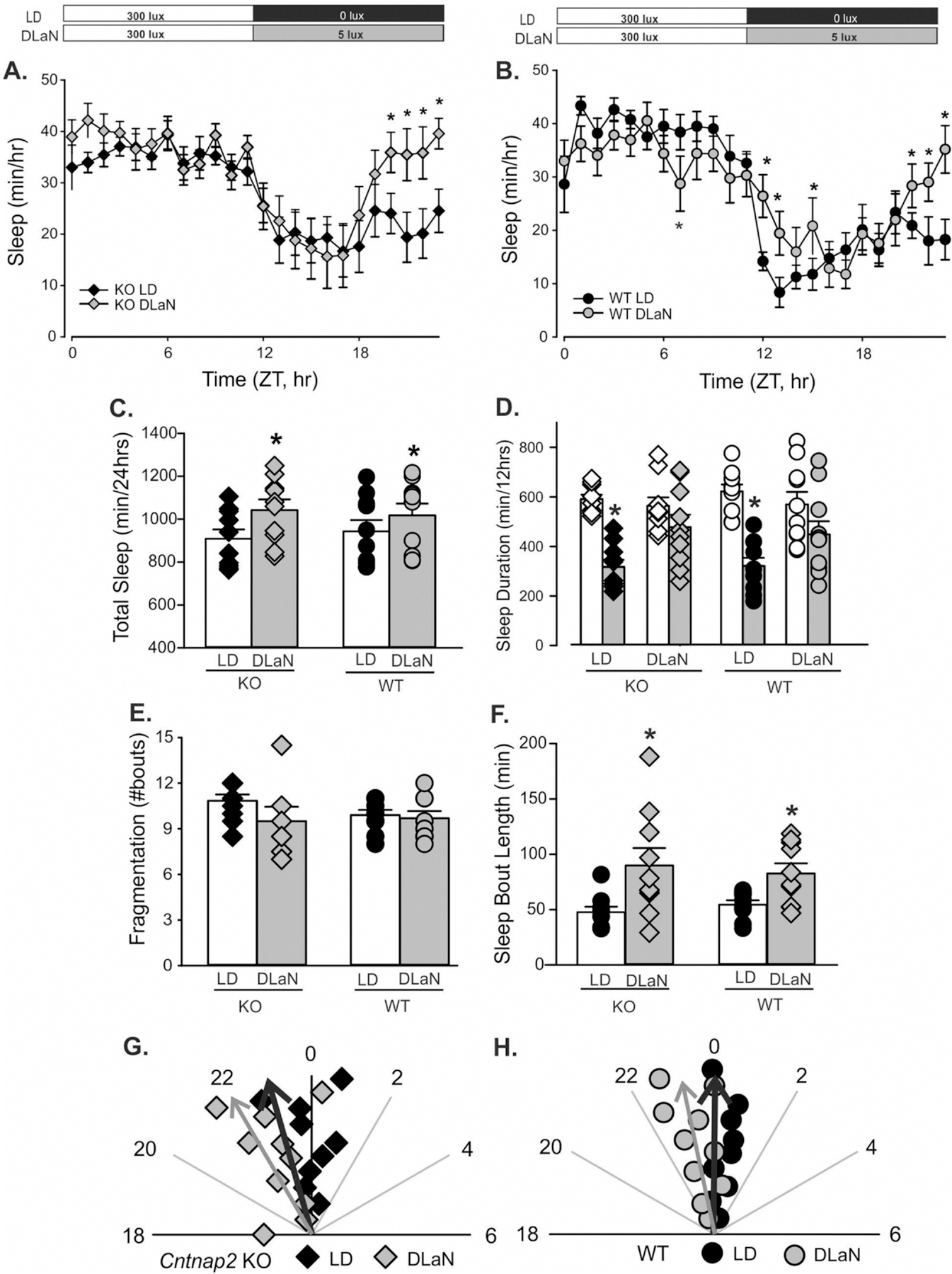
Rhythms in sleep behavior are altered by DLaN. Waveforms of daily rhythms in sleep behavior under LD and DLaN in both Cntnap2 KO (A) and WT (B) mice. The sleep waveform (1 h bins) of each genotype using a 2-way ANOVA for repeated measures with genotype and time as factors. All Cntnap2 KO (n = 10) and WT controls (n = 10) exhibited clear rhythms in sleep under both lighting conditions. The Cntnap2 KO exhibited significant effects of time (F(23, 479) = 8.426, P < 0.001) and treatment (F(1, 479) = 7.279, P = 0.024). The WT mice exhibited significant effects of both time (F(23, 432) = 16.133, P < 0.001) but not treatment (F(1, 432) = 0.838, P = 0.360). There were significant interactions between treatment and time (F(23, 479) = 2.812, P < 0.001). Post-hoc analysis by Multiple Comparison Procedures (Holm-Sidak method) indicated significant reductions in activity caused by DLaN exposure at a number of phases during the night in both genotypes. Significant (P < 0.05) differences between the genotypes are indicated with an asterisk over the treated values. Several aspects of sleep behavior were measured from each genotype (Cntnap2 KO, WT) under each lighting condition (LD, DLaN) and the resulting data analyzed with 2-way ANOVA. Histograms show means ± SEM with the values from individual animals overlaid. Significant (P < 0.05) differences because of treatment are indicated with an asterisk (*) over the treated values while the number sign (#) indicates a genotypic difference. (C) Total sleep was increased by treatment (F(1, 39) = 4.762, P = 0.036) but there were no effects of genotype (F(1,39) = 0.011, P = 0.914). (D) Day/night differences in the amount of sleep were disrupted by treatment. Both WT (t(9) = 8.960, P < 0.001) and Cntnap2 KO (Z(9) = 2.805, P = 0.002) exhibited increased sleep in the day than in the night. These differences were lost in both WT (t(9) = 1.347, P = 0.211) and KO (t(9) = 1.228, P = 0.251) mice under DLaN. (E) The number of sleep bouts was unaltered by treatment (F(1, 39) = 1.880, P = 0.179) or genotype (F(1,39) = 0.440, P = 0.511). (F) The sleep bout length was increased by treatment (F(1, 39) = 14.676, P < 0.001) but not genotype (F(1,39) = 0.001, P = 0.979). (G, H) Polar plots show the time of sleep onset for the mice measured under each condition. The numbers show the phases (ZT) with ZT 0 showing the time of light-onset. The onset of sleep was altered by both treatment (F(1, 39) = 20.617, P < 0.001) and genotype (F(1,39) = 5.6, P = 0.023). The vector indicates the peak phase and the amplitude relative to WT controls. Overall, DLaN increased sleep behavior by shifting the onset of sleep to an earlier phase. This change had the effect of eliminating the normal day/night difference in sleep duration.
3.3. Daytime neural activity in the central clock is compromised in the Cntnap2 KO mice
A characteristic property of SCN neurons is that these cells generate a circadian rhythm of neural activity with higher spontaneous activity during the day and low activity during the night. This neural activity is critical for the synchrony of cells within the SCN circuit as well as the ability of this nucleus to drive outputs throughout the body. Thus, we measured the SFR of the dorsal SCN neurons in Cntnap2 KO and WT. Each of these cells was determined to be within the dorsal region of the SCN by directly visualizing the location of the cell with infrared DIC video microscopy. First, we found that the Cntnap2 mutants exhibited a reduced daytime firing rate compared to WT controls (Fig. 3A) which was accompanied by a shift in the distribution of SFR with the mutants exhibiting more cells firing below 2 Hz (Fig. 3B). In a second set of experiments, we measured the SFR from Cntnap2 KO and WT mice held under a normal LD or DLaN for 2 weeks prior to the preparation of the brain slice. With this data set, we observed that the Cntnap2 KO mice exhibited a reduced SFR and the DLaN treatment did not have any obvious impact of the daytime or nighttime firing rate (Fig. 3C). In WT mice, the normal day/night rhythm as lost when the mice were held under DLaN (Fig. 3C). The SFR is a direct measure of the output of the SCN and our data indicate that the Cntnap2 KO mice exhibit a modestly reduced spontaneous neural activity.
Fig. 3.
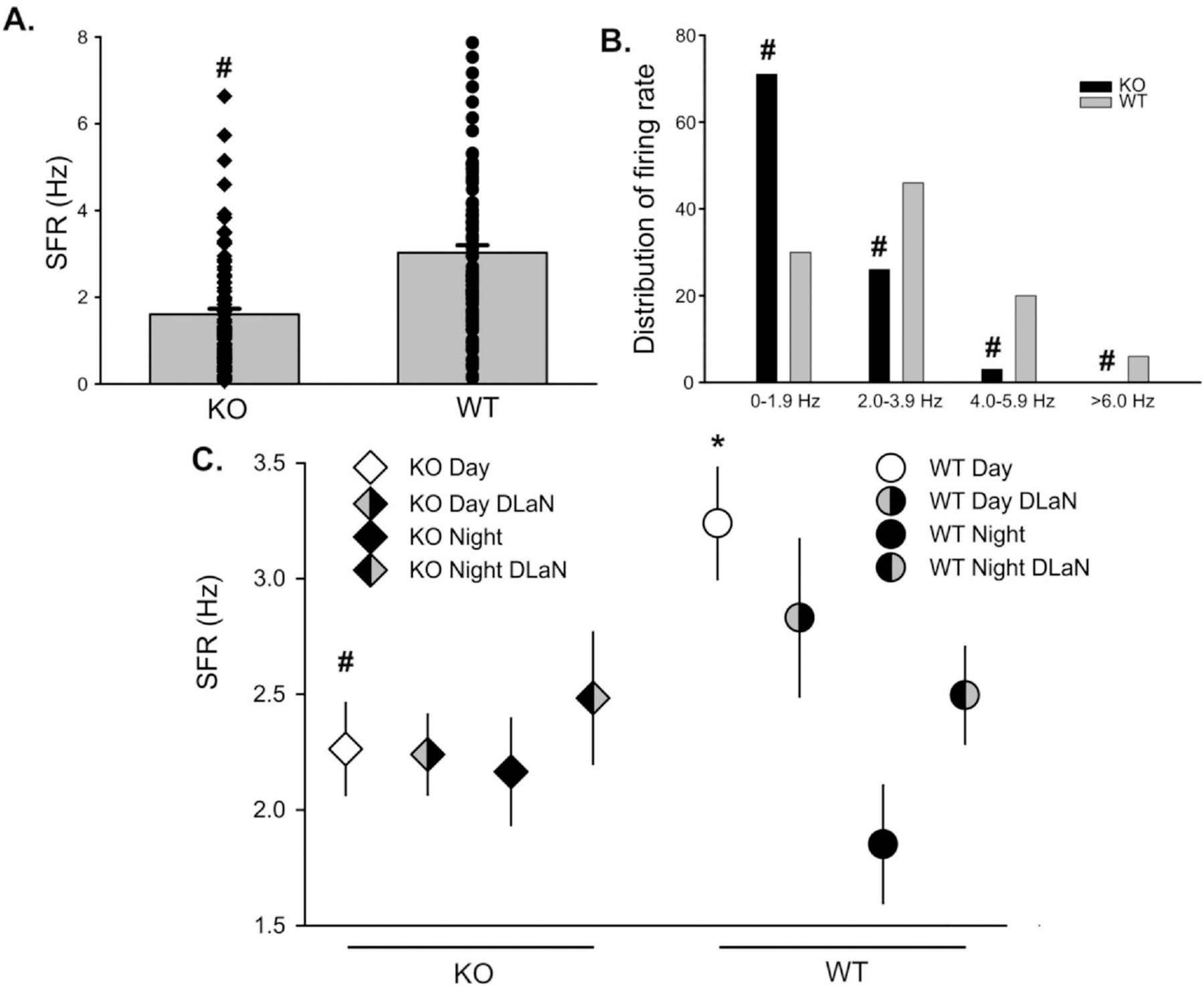
DLaN did not alter the daytime spontaneous frequency rate (SFR) of SCN neurons measured in a brain slice preparation. (A) Extracellular recording techniques indicated that SCN neurons from the Cntnap2 KO (n = 101) exhibited a modestly reduced daytime (ZT 5–7) firing rate compared to WT (n = 102) controls (Mann-Whitney Rank Sum test with U = 2499, P < 0.001). (B) The distribution of SFR was shifted lower in the mutants with highly significant changes in the number of neurons firing at the 0–2 Hz rate (Z score = −6.050, P = 0.001). In a separate experiment (C), cell-attached recording techniques were used to measure the SFR of Cntnap2 KO and WT mice in brain slices prepared from mice held in normal LD and under DLaN. In WT mice (one-way ANOVA), there were significant differences (H = 19.744, P < 0.001) driven by a day/night difference in firing rate (Q = 4.418; P < 0.001). In Cntnap2 KO, the day/night difference was lost (H = 0.338; P < 0.953). If we consider only day-time firing rate, the WT exhibits significantly higher SFR (H = 11.722; P = 0.029) than the KO. There was no significant day/night difference in SFR under DLaN. In short, DLaN did not produce changes in SCN firing rate that we could measure in the slice preparation, but the Cntnap2 KO did show a reduced SCN output.
3.4. DLaN shifted the phase and amplitude of the PER2:LUC rhythms in the Cntnap2 KO mice
Next, we used in vitro imaging of tissue from Cntnap2 KO and WT PER2:LUC mice to examine the impact of DLaN on bioluminescence rhythms. For both genotypes, we observed robust daily rhythms from the SCN, the hippocampus and liver under control conditions (Figs. 4, 5; Table 2; Fig. S5). The SCN was clearly impacted by the DLaN treatment (Fig. 4). In the central clock, the amplitude was reduced by treatment, but there were no differences between the genotypes. The peak phase varied with both treatment and genotype (Table 2). The Cntnap2 KO exhibited a significant phase advance, while the phase of the WT SCN was unaltered (Fig. 4E, F). In the hippocampus (Fig. 5), the KO mice exhibited a strong reduction in amplitude that was not seen in the WT (Fig. 5c, Table 2). The peak phase varied with treatment but not genotype (Table 2). The phase advances (4–6 h) were of larger magnitude than what we observed in the SCN. In the liver (Table 2, Fig. S5), the amplitude was not altered by treatment or genotype. The DLaN treatment also advanced the phase of the liver rhythms. There was no significant effect of genotype or treatment on the endogenous tau (free-running period) measured from any of the three tissues (Table 2). Overall, our data indicated that the Cntnap2 KO still presents robust PER2:LUC rhythms, but DLaN altered the amplitude and phase of these rhythms in a tissue-specific manner. The KO exhibited more sensitivity to the impact of treatment on phase in the SCN.
Fig. 4.
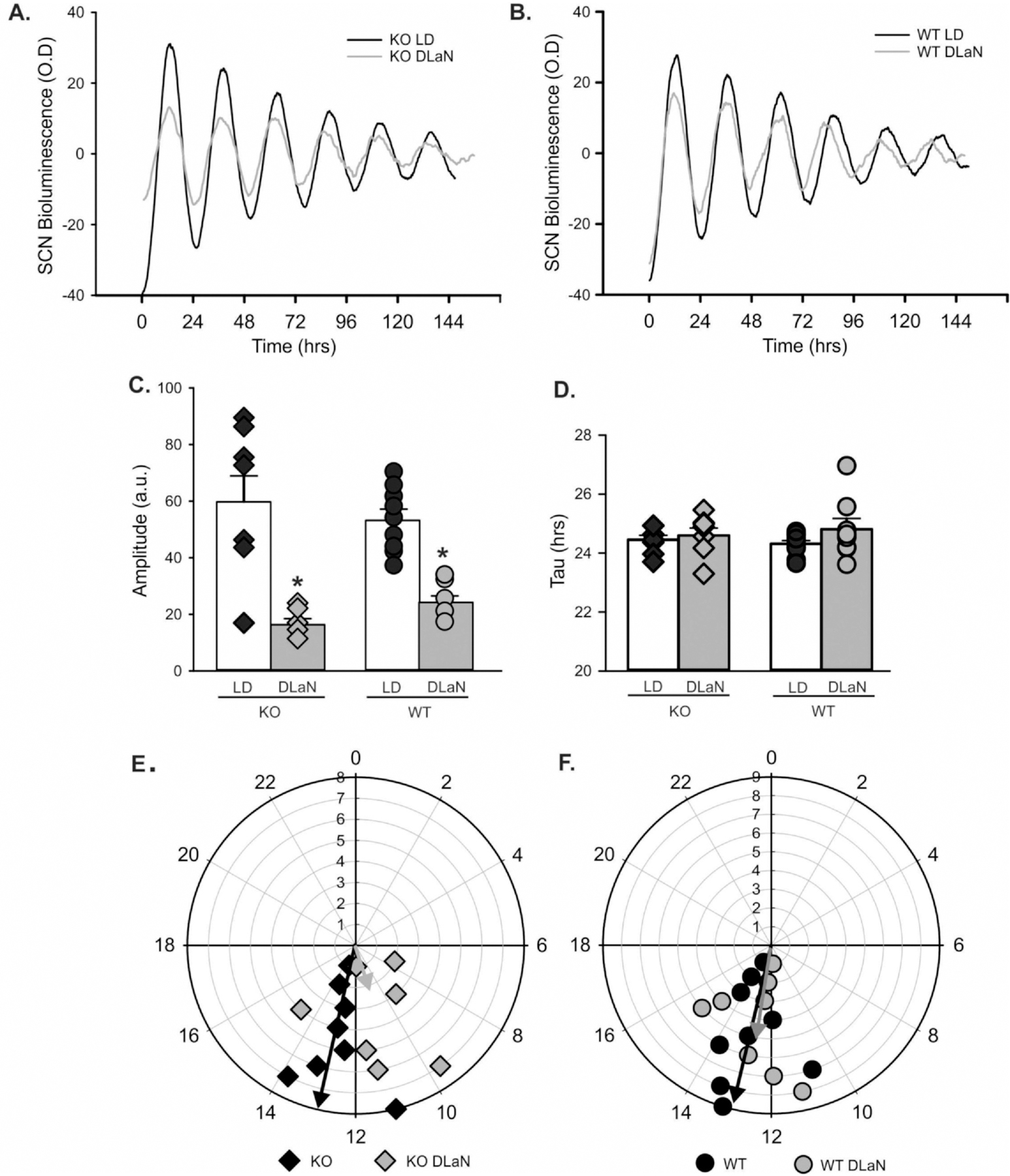
DLaN alters the PER2:LUC bioluminescence rhythms measured in the SCN. Representative examples of bioluminescence rhythms measured from the Cntnap2 KO (A) and WT (B) SCN under LD and DLaN conditions. Several parameters were measured from the bioluminescence rhythms and analyzed with 2-way ANOVA. Histograms show means ± SEM with the values from individual animals overlaid. Significant (P < 0.05) differences because of treatment are indicated with an asterisk (*) over the treated values while the number sign (#) indicates a genotypic difference. (C) The amplitude was reduced by treatment (F(1,31) = 47.458, P < 0.001) but there was no differences between the genotypes (F(1,31) = 0.014, P = 0.907). (D) There was no difference in the tau (free-running period) measured by treatment (F(1,31) = 1.838, P = 0.186) or genotype (F(1,31) = 0.024, P = 0.879). (E, F) Polar plots show the peak phase of the rhythms measured under each condition. The numbers show the phases (ZT) with ZT 0 showing the time of light-onset. The peak phase varied with both treatment (F(1,31) = 4.726, P = 0.038) and genotype (F(1,31) = 4.424, P = 0.045). The vector indicates the peak phase and the amplitude relative to WT controls. Overall, DLaN decreased the amplitude of rhythms in both genotypes and caused a phase advance in the mutants only.
Fig. 5.
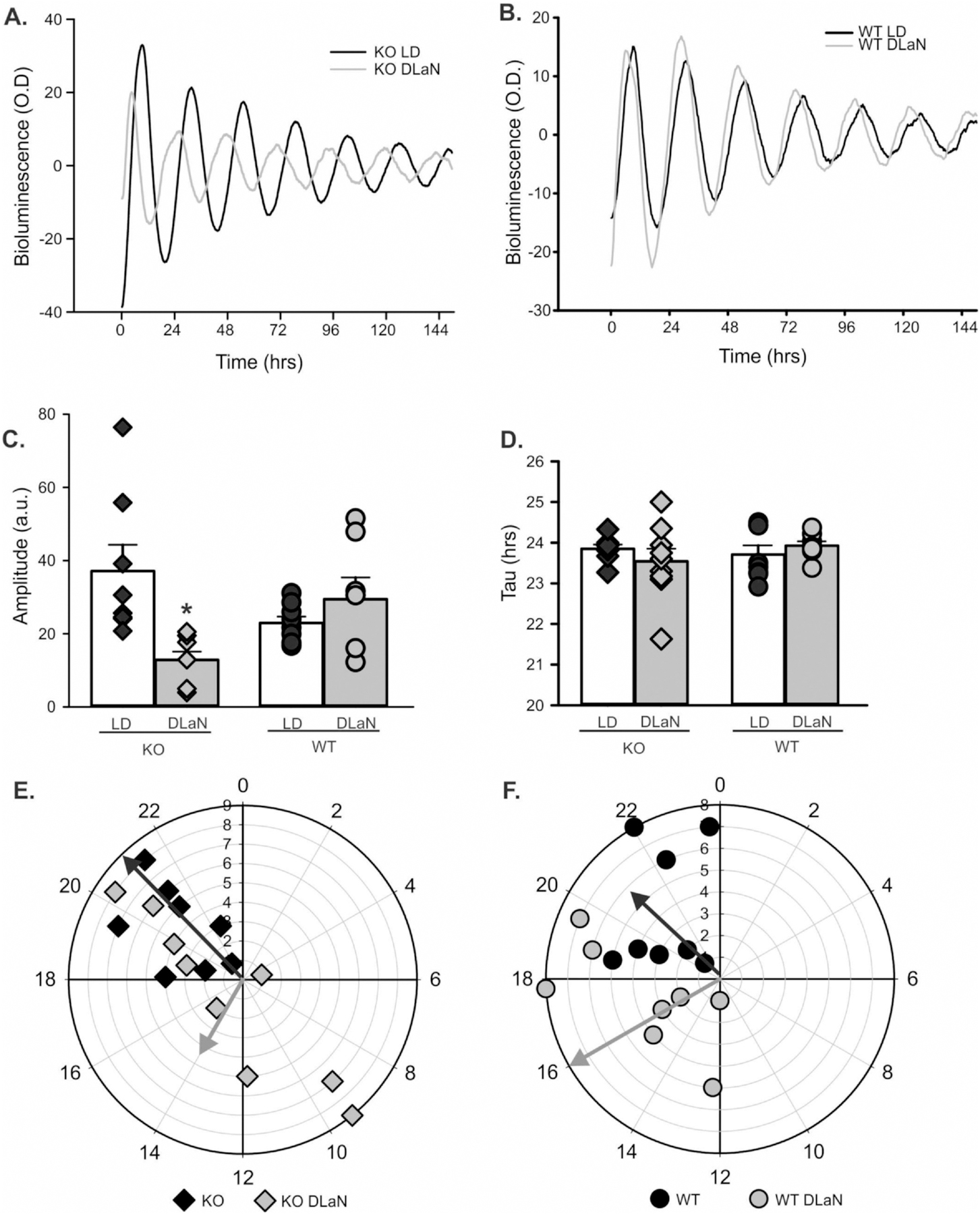
DLaN alters the PER2:LUC bioluminescence rhythms measured in the hippocampus. Representative examples of bioluminescence rhythms measured from the Cntnap2 KO (A) and WT (B) hippocampus under LD and DLaN conditions. Several parameters were measured from the bioluminescence rhythms and analyzed with 2-way ANOVA. Histograms show means ± SEM with the values from individual animals overlaid. Significant (P < 0.05) differences because of treatment are indicated with an asterisk (*) over the treated values while the number sign (#) indicates a genotypic difference. (C) Overall, the amplitude of the rhythms was not altered by treatment (F(1,32) = 3.566, P = 0.069) or genotype (F(1,32) = 0.065, P = 0.800). However, the treatment did dramatically reduce the amplitude of the KO rhythms, an effect that was significant in post-hoc tests. (D) There was no difference in the tau (free-running period) measured by treatment (F(1,32) = 0.205, P = 0.654) or genotype (F(1,32) = 0.629, P = 0.450). (E, F) Polar plots show the peak phase of the rhythms measured under each condition. The numbers show the phases (ZT) with ZT 0 showing the time of light-onset. The peak phase varied with treatment (F(1,32) = 20.108, P < 0.001) but not genotype (F(1,32) = 0.602, P = 0.444). The vector indicates the peak phase and the amplitude relative to WT controls. Overall, DLaN caused a phase advance in both genotypes and decreased the amplitude in the mutants only.
Table 2.
DLaN alters the peak phase and amplitude of the PER2:LUC rhythms measured in SCN, hippocampus, and liver.
| Cntnap2 KO | Cntnap2 KO + DLaN | WT | WT + DLaN | Genotype | Treatment | |
|---|---|---|---|---|---|---|
| SCN | ||||||
| Tau | 24.4 ± 0.2 | 24.6 ± 0.2 | 24.3 ± 0.2 | 24.8 ± 0.2 | F = 0.024; P = 0.879 | F = 1.838; P = 0.186 |
| Amplitude | 59.8 ± 5.2 | 16.5 ± 5.6* | 53.3 ± 4.9 | 24.3 ± 5.2* | F = 0.014; P = 0.907 | F = 47.458; P < 0.001 |
| Phase | 12.8 ± 0.5 | 10.9 ± 0.6* | 13.1 ± 0.5 | 12.8 ± 0.5 | F = 4.424; P = 0.045 | F = 4.726; P = 0.038 |
| Damping rate | −0.33 ± 0.03 | −0.29 ± 0.04 | −0.30 ± 0.02 | −0.36 ± 0.04 | F = 0.540;P = 0.469 | F = 0.211; P = 0.105 |
| Hippocampus | ||||||
| Tau | 23.8 ± 0.2 | 23.5 ± 0.2 | 23.8 ± 0.2 | 23.9 ± 0.2 | F = 0.629; P = 0.450 | F = 0.205 P = 0.654 |
| Amplitude | 37.2 ± 4.7 | 12.9 ± 4.4* | 22.9 ± 4.7 | 29.5 ± 5.0 | F = 0.065; P = 0.800 | F = 3.566; P = 0.069 |
| Phase | 20.6 ± 1.2 | 14.4 ± 1.2* | 20.9 ± 1.2 | 16.0 ± 1.2* | F = 0.602; P = 0.444 | F = 20.108; P < 0.001 |
| Damping rate | −0.30 ± 0.02 | −0.27 ± 0.06 | −0.35 ± 0.04 | −0.323 ± 0.06 | F = 1.355; P = 0.254 | F = 0.344; P = 0.562 |
| Liver | ||||||
| Tau | 23.0 ± 0.2 | 22.9 ± 0.2 | 23.0 ± 0.2 | 23.4 ± 0.2 | F = 1.502; P = 0.752 | F = 0.444; P = 0.510 |
| Amplitude | 276.2 ± 50.6 | 243.7 ± 47.7 | 248.3 ± 45.2 | 232.9 ± 50.6 | F = 0.158; P = 0.694 | F = 0.242; P < 0.626 |
| Phase | 16.6 ± 0.7 | 15.0 ± 0.7 | 17.8 ± 0.6 | 15.72 ± 0.7* | F = 2.002; P = 0.167 | F = 7.903; P = 0.008 |
| Damping rate | −0.22 ± 0.07 | −0.12 ± 0.05 | −0.14 ± 0.04 | −0.17 ± 0.05 | F = 0.116; P = 0.736 | F = 0.518; P = 0.477 |
Comparisons of age-matched WT and Cntnap2 KO mice under standard LD or DLaN regimen (n = 7–9/group). Data were analyzed with a two-way ANOVA using genotype and treatment as factors. The Holm-Sidak test for multiple comparisons was used when appropriate.
indicates significant differences between LD controls and those exposed to DLaN.
P values < 0.05 were considered significant and are shown in bold.
3.5. Nighttime light exposure alters social behavior in Cntnap2 KO mice
A key symptom in ASD is difficulty with social interactions, which has been demonstrated in both juvenile (Peñagarikano et al., 2011) and adult (Thomas et al., 2017) Cntnap2 KO mice. When the three-chamber social approach test was administered to adult mice during the night (ZT 14–16), Cntnap2 KO mice under LD conditions did not exhibit a preference for the stranger chamber, whilst under DLaN, they actually preferred the object (Fig. 6A). WT typically spent more time in the chamber with a stranger mouse than in the one containing the object (Fig. 6B, Table 3). This social preference was eliminated by DLaN (Fig. 6B). The chamber dwell times were converted to a social preference index by normalizing the chamber preference to the total time spent in the two chambers of interest (Fig. 6C). The two-way ANOVA indicated that there were significant effects of both genotype and treatment. Post hoc analysis indicated that DLaN decreased preference for the stranger mouse in both WT and Cntnap2 KO, but the negative social effects of the exposure were stronger in the Cntnap2 KO mice. There were no genotypic or treatment differences in the time spent in the center chamber (Table 3). These findings indicate that exposure to DLaN disrupted social behavior in both genotypes, with the mutants being more susceptible.
Fig. 6.
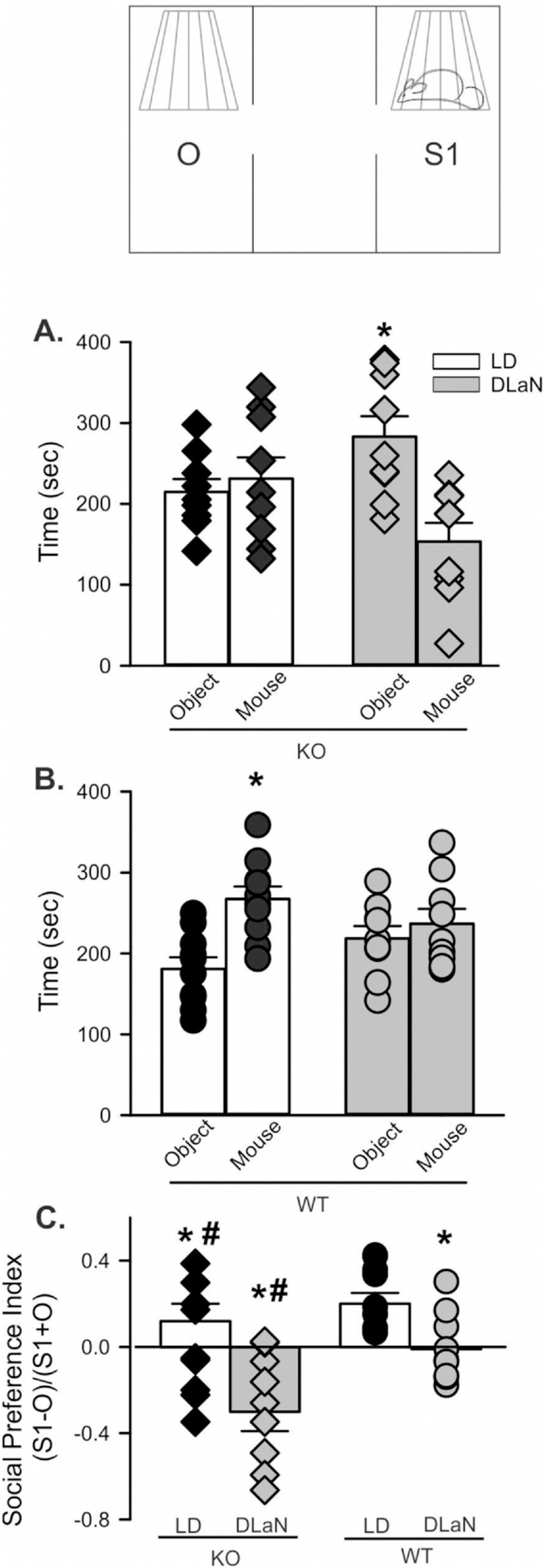
Social preference is reduced by DLaN. Performance on the 3-chamber social test was assessed in mice of each genotype (Cntnap2 KO, WT) under each lighting condition (LD, DLaN) and the resulting data analyzed with 2-way ANOVA. During the social preference stage of the three chamber test, mice freely explored the arena with a novel object (O) in one chamber, and an age- and sex-matched WT stranger mouse (S1) in the other chamber while dwell time is measured. (A) Cntnap2 KO mice do not show a preference for the mouse over an object (t(8) = 0.533, P = 0.601). Under DLaN, the mutants actually prefer the object and avoid the mouse (t(8) = 3.791, P = 0.002). (B) WT mice prefer to spend time with the stranger mouse over the object (t(9) = 4.391, P < 0.001). This preference is lost under DLaN conditions (t(8) = 0.751, P = 0.464). (C) Normalizing the difference between chamber dwell times by time spent in both chambers produced the social preference index. This index shows significant effects of genotype (F(1,36) = 11.757, P = 0.002) and treatment (F(1, 36) = 13.892, P < 0.001). Significant (P < 0.05) differences because of treatment are indicated with an asterisk (*) over the treated values while the number sign (#) indicates a genotypic difference. DLaN reduced social performance in both genotypes with the mutant mice appearing to avoid the test mouse.
Table 3.
DLaN impacted social and grooming behavior.
| Cntnap2 KO | Cntnap2 KO + DLaN | WT | WT + DLaN | Genotype | Treatment | |
|---|---|---|---|---|---|---|
| Three chambered social test | ||||||
| Center chamber (sec) | 153.9 ± 19.1 | 163.6 ± 19.7 | 154.5 ± 15.4 | 144.8 ± 13.3 | F = 0.265; P = 0.610 | F = 0.001; P = 0.999 |
| Social chamber (sec) | 231.2 ± 26.3.6 | 164.4 ± 15.8* | 271.3 ± 15.1 | 236.6 ± 16.8 | F = 8.066 P = 0.008 | F = 6.669; P = 0.014 |
| Object chamber (sec | 218.6 ± 13.8 | 283.0 ± 22.9* | 166.8 ± 11.7 | 218.6 ± 13.8 | F = 8.111; P = 0.008 | F = 9.326; P = 0.004 |
| Social Index | 0.02 ± 0.09 | −0.26 ± 0.07* | 0.24 ± 0.5 | 0.04 ± 0.06* | F = 11.757; P = 0.002 | F = 13.892; P < 0.001 |
| Grooming | ||||||
| Grooming (sec) | 26.7 ± 2.0 | 66.0 ± 14.2* | 8.6 ± 1.7 | 13.7 ± 1.8 | F = 21.986; P < 0.001 | F = 8.231; P = 0.007 |
| Distance (m) | 47.1 ± 3.1 | 38.1 ± 2.0 | 39.4 ± 2.3 | 34.6 ± 1.8 | F = 3.603; P = 0.065 | F = 5.846; P = 0.020 |
Two-way ANOVA followed by Holm-Sidak’s multiple comparisons test was used to evaluate the effects of genotype and time, and their interaction. Degrees of freedom are reported in parentheses. Data are reported as the mean ± SEM. P values < 0.05 were considered significant and are shown in bold.
3.6. Nighttime light exposure aggravates repetitive behavior in Cntnap2 KO mice
A hallmark of ASD in humans is repetitive behavior, which is recapitulated in the Cntnap2 mutants in the form of increased grooming behavior during the day (Peñagarikano et al., 2011; Thomas et al., 2017). We examined grooming behavior in the night (ZT 14–16) for each of the four groups and observed that the amount of grooming was significantly impacted by both genotype and treatment (Fig. 7A, Table 3). Post hoc analysis indicated that the mutants exhibited more grooming behavior than WT mice and that this aberrant grooming behavior was exacerbated by DLaN treatment. WT mice housed under DLaN did not display increased grooming compared to WT mice in LD housing. The DLaN exposure decreased exploratory behavior as measured by distance traveled during the test (Fig. 7B, Table 3). There were no genotypic or treatment differences in the time spent in the center chamber (Fig. 7C). In the absence of DLaN, the Cntnap2 KO exhibited high levels of spontaneous grooming during the day but not in the night (Fig. S6). Therefore, the DLaN exposure drove a large increase in repetitive behavior in the Cntnap2 KO but not in WT mice.
Fig. 7.
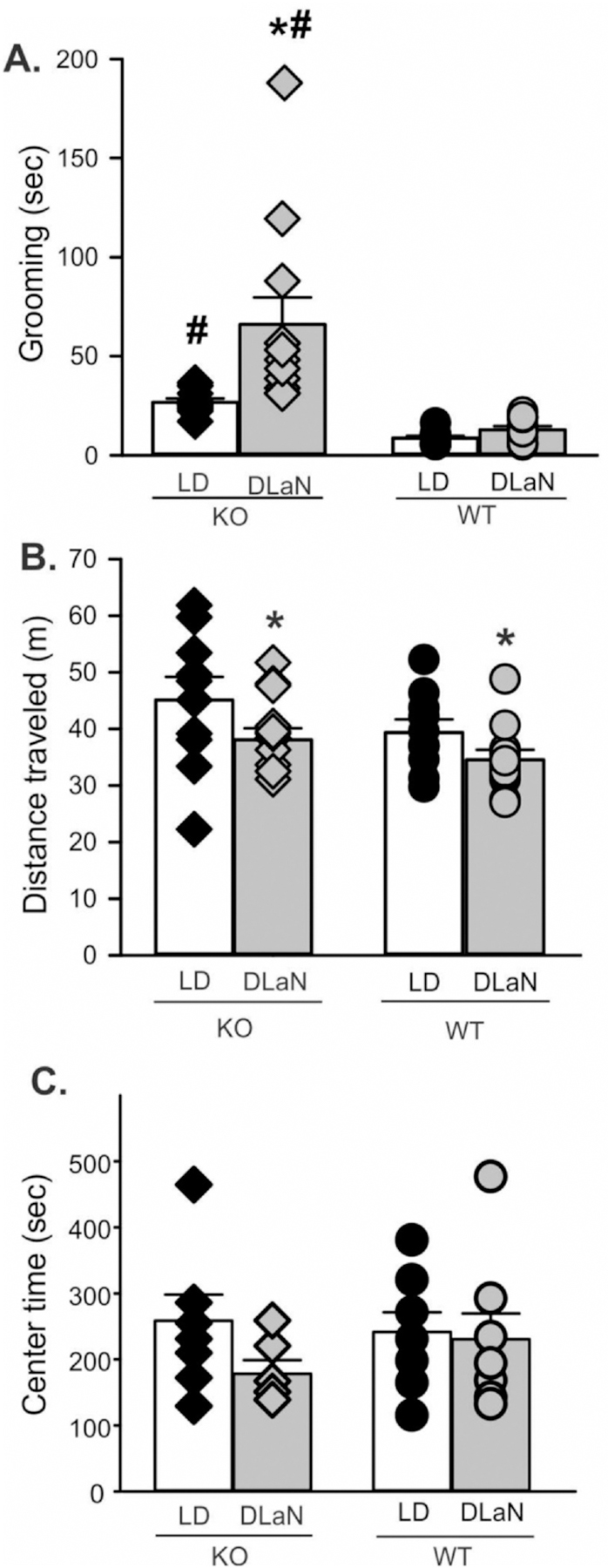
Repetitive and exploratory behavior are altered by DLaN. Grooming and movement was assessed in a novel arena from mice of each genotype (Cntnap2 KO, WT) under each lighting condition (LD, DLaN) and the resulting data analyzed with 2-way ANOVA. Histograms show means ± SEM with the values from individual animals overlaid. Significant (P < 0.05) differences because of treatment are indicated with an asterisk (*) over the treated values while the number sign (#) indicates a genotypic difference. (A) Grooming behavior was significantly increased by genotype (F(1,43) = 21.986, P < 0.001) and treatment (F(1, 43) = 8.231, P = 0.007). There was a significant interaction between the two factors (F(1, 43) = 5.296, P = 0.027). (B) Novel arena exploration was decreased by the treatment (F(1, 43) = 5.846, P = 0.020) but not genotype (F(1,43) = 3.603, P = 0.065). (C) The time spent in the center of the open field did not vary with genotype (F(1, 29) = 0.254, P = 0.619 or treatment (F(1,29) = 1.688, P = 0.205). There was no significant interaction between the two factors (F(1, 29) = 0.981, P = 0.331).
3.7. Treatment with melatonin ameliorates the effects of nighttime light exposure on activity rhythms and repetitive behavior
Melatonin has been proposed as a safe and effective treatment to help with the sleep/wake disruptions common in neurodevelopmental disabilities (Goldman et al., 2014; Kawabe et al., 2014; Schwichtenberg and Malow, 2015). Levels of melatonin are normally high during the night and low during the day. Therefore, we sought to determine whether nightly treatment with melatonin (3 mg/kg) would improve the excessive grooming behavior seen in the Cntnap2 mutant exposed to DLaN. WT and KO mice housed under DLaN conditions were treated daily (ZT 11.5) with melatonin or vehicle for 2 weeks (Fig. 8A, Table 4). Significant effects were seen for both genotype and treatment as well as their interaction. Post hoc analysis revealed that melatonin treatment in the night significantly reduced grooming behavior in the mutant mice. As a control for the time of administration, another group of KO mice was treated with melatonin in the day (ZT 23.5). No amelioration in excessive grooming behavior was observed (Fig. 8A).
Fig. 8.
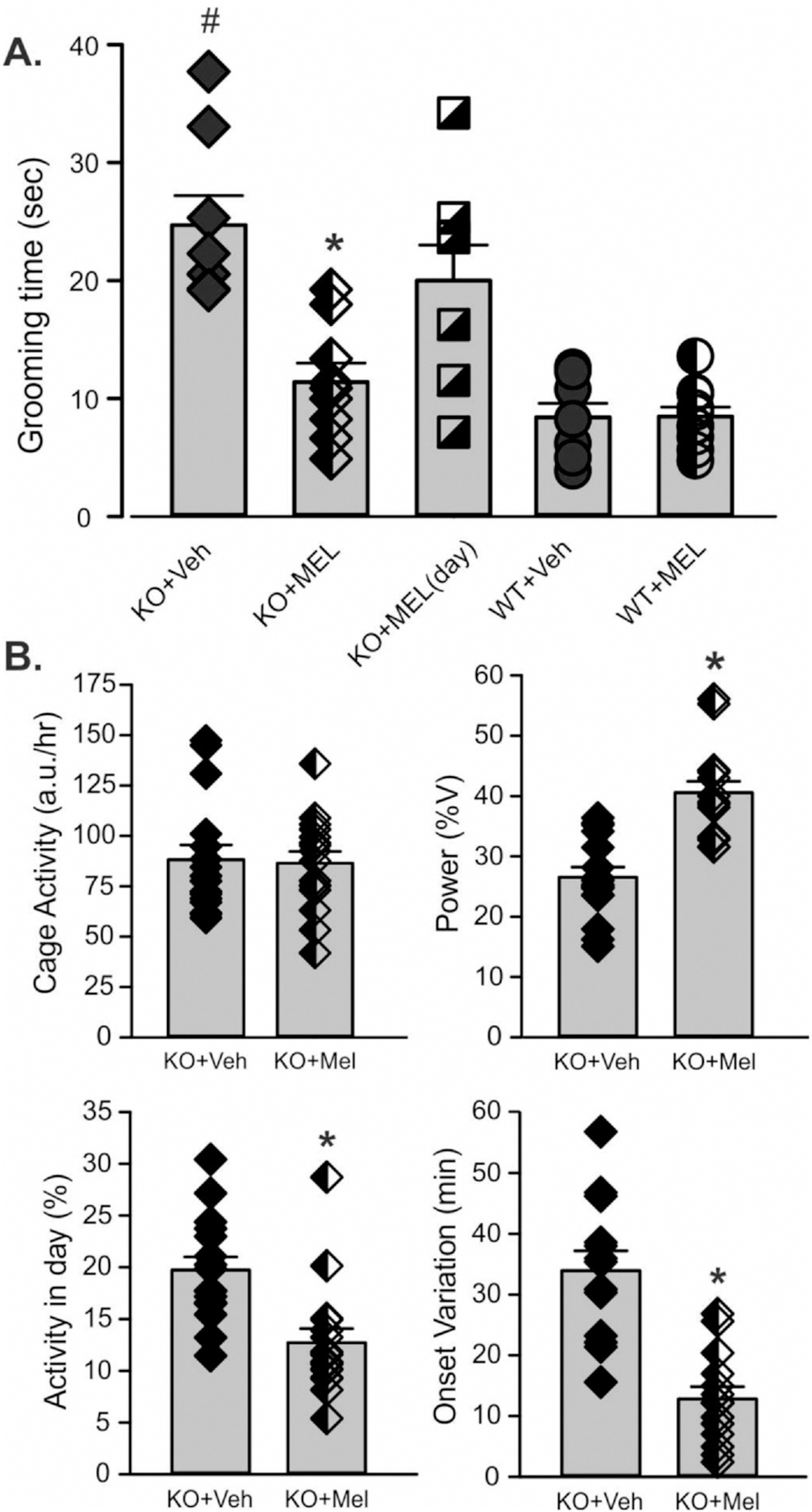
Treating mice with melatonin counteracts the effects of DLaN on repetitive behavior. Both WT and Cntnap2 KO mice were housed under DLaN for 2 weeks. During the same 2 weeks, mice were treated with either vehicle or a melatonin (3.0 mg/kg), given daily at ZT 11.5 (30 min before the night phase). (A) Grooming was assessed in a novel arena and the resulting data analyzed with 2-way ANOVA. Histograms show means ± SEM with the values from individual animals overlaid. A separate group of Cntnap2 KO mice treated with melatonin at ZT 23.5 are shown as squares. Half-tone symbols indicate mice that were treated with melatonin. Significant (P < 0.05) differences because of treatment are indicated with an asterisk (*) over the treated values while the number sign (#) indicates a genotypic difference. Grooming behavior was significantly increased by genotype (F(1,34) = 25.338, P < 0.001) and reduced by the melatonin treatment (F(1, 34) = 7.074, P = 0.012). There was a significant interaction between the two factors (F(1, 34) = 11.615, P = 0.002). The mutant mice treated with melatonin at dawn were not different from vehicle controls (t(13) = 1.070, P = 0.304). (B) The impact of melatonin treatment on cage activity of a separate group of Cntnap2 KO mice under DLaN was assessed. Compared to vehicle controls, melatonin did not alter activity levels (t(30) = 0.181, P = 0.857). Melatonin treatment did improve the power (t(30) = 3.605, P = 0.001) and precision (U(30) = 25, P = 0.001) of the activity rhythm. Treatment with melatonin effectively reduced the repetitive behavior and improved the activity rhythms of the Cntnap2 KO mice under DLaN.
Table 4.
Melatonin effective counter-measure for DLaN-evoked grooming behavior.
| Grooming | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Cntnap2 KO DLaN vehicle | Cntnap2 KO DLaN melatonin | WT DLaN vehicle | WT DLaN melatonin | Genotype | Treatment | |
| Grooming (sec) | 27.7 ± 2.5 | 11.4 ± 1.6 | 8.4 ± 1.1 | 8.5 ± 1.1 | F = 25.338; P < 0.001 | F = 7.074; P = 0.012 |
| Activity rhythms | ||||||
|
| ||||||
| Cntnap2 KO LD vehicle | Cntnap2 KO DLaN melatonin | WT LD vehicle | WT DLaN melatonin | Genotype | Treatment | |
|
| ||||||
| Power (% variance) | 29.6 ± 2.3 | 40.6 ± 1.9 | 26.2 ± 2.1 | 33.7 ± 1.6 | F = 3.816; P < 0.058 | F = 12.350; P = 0.001 |
| Activity (a.u./24 h) | 2116.1 ± 173.6 | 2075.8 ± 139.4 | 2864.4 ± 249.7 | 4200.4 ± 484.8 | F = 37.432; P < 0.001 | F = 7.614; P = 0.009 |
| Daytime activity (%) | 18.5 ± 1.3 | 12.7 ± 1.4 | 16.2 ± 1.6 | 11.7 ± 0.8 | F = 0.939; P = 0.338 | F = 9.649; P = 0.003 |
| Onset Variability (min) | 30.1 ± 3.9 | 12.8 ± 2.0 | 43.3 ± 1.8 | 36.7 ± 3.8 | F = 15.366; P < 0.001 | F = 9.099; P = 0.005 |
| Fragmentation | 11.8 ± 0.4 | 10.7 ± 0.5 | 8.6 ± 0.5 | 9.4 ± 0.6 | F = 14.213; P < 0.001 | F = 0.089; P = 0.763 |
Two-way ANOVA followed by Holm-Sidak’s multiple comparisons test was used to evaluate the effects of genotype and treatment. Data are reported as the mean ± SEM. P values < 0.05 were considered significant and are shown in bold.
Finally, we determined the effects of the same nightly melatonin treatment on activity rhythms in the Cntnap2 KO exposed to DLaN. Cohorts of mutant mice were held under DLAN and treated with either melatonin (3 mg/kg) or vehicle (Fig. 8B, Table 4). Melatonin significantly improved the power of the daily rhythms by decreasing inappropriate activity during the day as well as reducing the cycle-to-cycle variation in activity onset. Therefore, melatonin successfully rescued the excessive grooming and disrupted activity rhythms seen in the Cntnap2 KO under DLaN.
4. Discussion
Cntnap2 homozygous null (Cntnap2 KO) mice were chosen because of their strong construct validity and the human genetic evidence that implicate this gene in autism. Recessive loss-of-function mutations in Cntnap2 cause Pitt-Hopkins-like syndrome (Peippo and Ignatius, 2012; Zweier et al., 2009), symptoms of which include severe intellectual disability, lack of speech, and seizures. In addition, a homozygous single base pair deletion, resulting in a truncated protein, causes Recessive Symptomatic Focal Epilepsy, which presents with autistic symptoms as well as cortical dysplasia and epilepsy (Strauss et al., 2006). The Cntnap2 KO mice have the first exon of Cntnap2 gene replaced by a neo gene that results in the absence of the Cntnap2 protein in the brains in homozygous mice (Poliak et al., 1999).
One of the key assessments in this study was the evaluation of the mutant mice compared to WT controls. We found that Cntnap2 KO mice have weaker daily cage activity rhythms, are more day-active and have less precision in activity onset than WT mice (Fig. 1; Table 1). Previous work with this model reported acute hyperactivity when the mice were placed in a testing field in the light phase of the LD cycle (Peñagarikano et al., 2011; Brunner et al., 2015; Thomas et al., 2017). We also observed some evidence for acute hyperactivity in the mutants when the mice were placed in an open arena (Fig. 7) as has also been seen with home cage activity (Angelakos et al., 2019). However, when the activity rhythms are measured over a 24-h cycle, the Cntnap2 KO mice show reduced locomotor activity, especially during the night. (Fig. 1; Thomas et al., 2017; Angelakos et al., 2019). We found that sleep behavior was relatively unaffected in the Cntnap2 KO (Fig. 2; Table 1) with overall sleep duration or fragmentation unaltered. Previous analysis on electroencephalogram (EEG)-defined sleep in the Cntnap2 KO mice enabled the authors to parse vigilance states into wake, rapid eye movement (REM) and non-REM sleep. They were able to demonstrate blunted rhythms in each of the states along with fragmented bouts of wakefulness (Thomas et al., 2017). The authors found a reduced spectral power of the EEG in the alpha (9–12 Hz) range (Thomas et al., 2017), suggesting a disruption in the coherence in the electrical activity of thalamic pacemaker cells that are thought to be responsible for the alpha waves.
The alterations in the amplitude of the diurnal rhythms in activity, sleep (NREM, REM) and core body temperature could be most simply explained if the Cntnap2 mutation caused disruption in the circadian timing system. The Cntnap2 transcript is highly expressed in the SCN as well as the paraventricular nucleus in the adult mouse (Lein et al., 2007). However, behaviorally, there does not appear to be an alteration in free-running circadian period (Tau) when activity is measured in constant darkness (Angelakos et al., 2019). Similarly, we did not see a difference in Tau between the WT (23.67 ± 0.07, n = 14) and mutant (23.53 ± 0.17, n = 8) mice when housed in constant darkness (DD). The free-running period, as measured by locomotor behavior in DD, is an extremely sensitive marker of output of the core molecular clock that drives circadian oscillations (Takahashi et al., 2008). Thus, the behavioral data do not suggest a disruption in the core clock in the Cntnap2 KO mice. In addition, we did not observe anomalies in the morphology of the SCN or in the number of cells expressing the peptides VIP and AVP (Fig. S7). We went on to measure PER2-driven bioluminescence from the SCN. Using this direct read out of the molecular clock (Yoo et al., 2004), we found that the key circadian parameters of amplitude, phase and free-running period did not vary between the genotypes (Figs. 4, 5; Table 3). We did see a reduction in daytime SFR measured from the mutant SCN (Fig. 3) so there may be some circadian disruption that could explain the deficits in the diurnal rhythms. Collectively, this work indicates that the Cntnap2 KO mice exhibit disruptions in the temporal patterning of activity and sleep which fits with a general pattern of deficits seen in mouse models of monogenic neurological disorders (Angelakos et al., 2019; Brown et al., 2019; Shi and Johnson, 2019; Takumi et al., 2019).
Social deficits were observed in the Cntnap2 KO as measured by the 3-chamber test (Fig. 6; Table 3), where they had decreased preference for spending time in close vicinity to a stranger mouse compared to WT. However, the time that the mutant mice spent investigating the stranger mouse was not different from the controls. Prior work with this model found robust social deficits in juvenile Cntnap2 mutants (Peñagarikano et al., 2011) that responded positively to treatment with oxytocin (Peñagarikano et al., 2015). Working with slightly older mice, another group observed social deficits in one test (urine-open field) but not in the 3-chamber or reciprocal social interaction test (Brunner et al., 2015). Another study using adult mutants found reduced social interactions when the KO mice were allowed to interact in a cage (Thomas et al., 2017). The reasons for these differences among the different laboratories’ measurements of social behavior are not clear. The strongest phenotype that we observed with the Cntnap2 KO model was increased repetitive behavior. The mutant mice spent more time grooming (Fig. 7; Table 3) than WT controls. This repetitive behavior has been shown in prior work in the Cntnap2 model (Peñagarikano et al., 2011; Thomas et al., 2017) and can be normalized by treatment with Risperidone (Peñagarikano et al., 2011), demonstrating the predictive validity of the model.
Genetic studies have revealed heterogeneity in ASD, predicting hundreds of rare genetic associations (Iossifov et al., 2012; O’Roak et al., 2012; Sanders et al., 2012). Despite evidence of genetic risk factors, environmental risk factors are very likely to be playing an important role in the symptoms and serving as a “second hit”. Nightly light exposure is a common environmental perturbation and has been shown to cause a number of ill effects (Bedrosian et al., 2016; Bedrosian et al., 2011; Borniger et al., 2014; Fonken et al., 2013; Fonken et al., 2012; Fonken and Nelson, 2011; Lucassen et al., 2016). Therefore, we investigated the impact of dim light at night on locomotor activity, sleep, PER2:LUC bioluminescence rhythms, social and grooming behavior in both WT and mutant mice. The DLaN treatment had a dramatic effect on measures of locomotor activity rhythms (Fig. 1; Fig. S2) and the temporal pattern of sleep (Fig. 2). For example, the normal day/night rhythms in the amount of sleep is lost under DLaN treatment, and the amount of time sleeping in the day and night were not significantly different in either genotype. The DLaN treatment did not altered SFR (Fig. 3C) although the day/night rhythm in SFR was lost under DLaN exposure. DLaN also dramatically reduced the amplitude of the PER2:LUC rhythm measured at the level of the SCN (Fig. 4). Since we do not have single cell resolution for the bioluminescence, we cannot resolve whether the reduction in amplitude is driven by a loss of synchrony in the cell population or damping of the single cell oscillation. Based on prior work examining the impact of constant light on SCN neurons (Ohta et al., 2005), we would expect that DLaN desynchronizes clock neurons but does not compromise their ability to generate circadian rhythms. Finally, DLaN reduced social behavior in both genotypes (Fig. 6) and increased grooming in the mutants (Fig. 7).
We expected that the mutants may be more sensitive to the effects of circadian disruption and found some evidence for selective vulnerability in the Cntnap2 model i.e. DLaN produced significantly worse effects in the KO compared to WT controls. This selective vulnerability was seen with changes in the amplitude and phase of the PER2 rhythms measured in the hippocampus, reduced social index, and increase time spent grooming. In our hands, the impact on activity onset and grooming were particularly robust. In the present study, using a shorter circadian disruption of 2 weeks, the negative impact of the light at night proved to be reversible when conditions of true darkness were restored (Fig. S4). It remains to be determined if longer durations under DLaN lead to permanent deficits. Circadian disruptions have been observed in a number of neurodevelopmental disorders (Angelakos et al., 2019; Brown et al., 2019; Shi and Johnson, 2019; Takumi et al., 2019), and it will be important to explore whether there is a selectively vulnerable developmental period for long-term effects of DLaN on behavior (e.g. Borniger et al., 2014; Logan and McClung, 2019).
The mechanisms underlying the negative impact of DLaN are not known and could be mediated by multiple factors. Based on recent work, our assumption is that the effects of DLaN are mediated by intrinsically photosensitive retinal ganglion cells (ipRGCs) (Lazzerini Ospri et al., 2017). For example, work from the Hattar laboratory indicates that ipRGCs that project to the SCN mediate the effects of light on learning (Fernandez et al., 2018). Mood regulation by light, on the other hand, requires an SCN-independent pathway linking ipRGCs to the perihabenular nucleus (PHb). The PHb is anatomically integrated in a distinct circuit with the limbic system and appears to be both necessary and sufficient for driving the effects of light on affective behavior. An important area for future work will be to determine which of these ipRGC pathways underlie the impact of DLaN observed in the present study. Notably, recent work on the mouse forebrain has found evidence that a variety of the genes coding for transcripts linked to synaptic transmission are regulated by the circadian timing system, while the proteome is more highly influenced by the sleep/wake cycle (Noya et al., 2019). Many of these same synaptic transcripts have been shown to be down-regulated in cortical regions of patients with neurodevelopmental disorders (Gandal et al., 2018). Therefore, by altering both the circadian clock and sleep behavior, DLaN would be expected to have a major impact on synaptic transmission throughout the forebrain.
In this study, we show that treatment with melatonin improves some of the symptoms seen in the Cntnap2 model, with striking improvements in repetitive behaviors i.e. excessive grooming (Fig. 8A; Table 4). In addition, nightly melatonin improved the power and precision of cage activity rhythms (Fig. 8B). Thus, two key parameters in which the Cntnap2 mutants show selective vulnerability to DLaN are both improved and, in the case of grooming behavior, returned to WT levels. Exogenous melatonin treatment has also been proposed as a treatment for circadian rhythm disorders (e.g. Burke et al., 2013; Riemersma-van der Lek et al., 2008; Schroeder and Colwell, 2013) and is particularly appealing for use in young and adolescent patients (Eckerberg et al., 2012), as most indications are that it is well-tolerated. Perhaps for this reason, melatonin is one of the most commonly used medications for sleep disturbances in children and adolescents with developmental disabilities (Rossignol and Frye, 2014; Schwichtenberg and Malow, 2015). For example, a survey of ASD patients found that medications for sleep were prescribed in 46% of 4–10-year-olds given a sleep diagnosis, with melatonin being the most commonly used medication (Malow et al., 2016). Mostly relying on self-reports by patients and family members, the studies to date have found consistent evidence that sleep latency is improved in many of the patients taking melatonin (Andersen et al., 2008; Garstang and Wallis, 2006; Giannotti et al., 2006; Malow et al., 2012; Wirojanan et al., 2009; Wright et al., 2011). There has been at least one study which found that treatment with the melatonin receptor agonist Ramelteon improved insomnia and behavioral symptoms in a small number of ASD patients (Kawabe et al., 2014). Recent work examined the association between repetitive behaviors and sleep problems in children with ASD. Patients with repetitive behaviors, but not restrictive behaviors like the insistence on routine, experienced significant reported sleep problems even after controlling for anxiety (Hundley et al., 2016). This suggests that melatonin may be better suited as an intervention for the subset of ASD patients with repetitive behaviors and sleep problems (Schroder et al., 2019). Understandably, the work in patients is focused on treating the symptoms rather than understanding the underlying biology. With animal models, it is possible to explore the underlying mechanisms and develop improved therapeutics with caveats. For example, in the context of the present work, it is worth noting that many commonly used mouse strains like the C57 line do not produce melatonin (Ebihara et al., 1986).
The Cntnap2 model has strong construct validity, as mutations in this gene are found in humans with autism. Nevertheless, not all mouse models can recapitulate all of the autism domain deficits due to the broad spectrum of causes and symptoms in ASD. In our hands, the locomotor and repetitive behavior phenotypes were most robust in the adult mouse and were the focus of our study. Dim light at night is a mild environmental disruptor of circadian rhythms and one that may be commonly experienced by patients with ASD. At least two studies have shown that these young patients are more exposed to light at night via electronic screens than age-matched controls (Engelhardt et al., 2013; Mazurek et al., 2016). This light exposure by itself has been shown to delay sleep in healthy young people (Chang et al., 2015; Gronli et al., 2016; Wood et al., 2013). Our work raises the possibility that sleep and circadian disruption may be an environmental factor that makes at least some of the core symptoms of developmental disabilities worse. While this remains to be rigorously tested, the data suggests that patients and caregivers may benefit from living in a temporally structured environment with particular attention paid to lighting conditions. While melatonin levels would be suppressed by light exposure during the night, melatonin supplements or melatonin receptor agonists may prove to be an appropriate countermeasure.
Supplementary Material
Acknowledgments
we would like to acknowledge the intellectual support and encouragement of Dr. D.H. Geschwind. Mr. Shuichi Kojima helped with the morphological analysis and Ms. Sarah Brown helped with the behavioral experiments. Dr. Kathy Tamai provided editorial assistance. Financial support came from the UCLA Center for Autism Research and Treatment (CART) and UCLA Clinical and Translational Science Institute (NIH UL1TR001881, PI Dubinett) for a pilot award titled “Circadian Disruption in the CNTNAP2 mouse model of autism spectrum disorder.” Core equipment used in this study was supported by the National Institute of Child Health Development under award number: 5U54HD087101.
Abbreviations:
- ANOVA
analysis of variance
- AVP
arginine vasopressin
- ASD
autism spectrum disorders
- DD
constant darkness
- Cntnap2 KO
contactin associated protein-like 2 knock out
- DLaN
dim light at night
- EEG
electroencephalogram
- ipRGCs
intrinsically photosensitive retinal ganglion cells
- LD
light:dark
- PER2
PERIOD2
- REM
rapid eye movement
- SFR
spontaneous firing rate
- SEM
standard error of the mean
- SCN
suprachiasmatic nucleus
- VIP
vasoactive intestinal peptide
- (WT)
wild-type
- ZT
Zeitgeber Time
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nbd.2020.105064.
Declaration of competing interest
Dr. Colwell received research funding from Takeda Pharmaceuticals from 2015 to 2017. The authors of this work have no financial interests related to this manuscript.
References
- Andersen IM, Kaczmarska J, McGrew SG, Malow BA, 2008. Melatonin for insomnia in children with autism spectrum disorders. J. Child Neurol 23 (5), 482–485. 10.1177/0883073807309783. [DOI] [PubMed] [Google Scholar]
- Angelakos CC, Tudor JC, Ferri SL, Jongens TA, Abel T, 2019. Home-cage hypoactivity in mouse genetic models of autism spectrum disorder. Neurobiol. Learn. Mem 165, 107000. 10.1016/j.nlm.2019.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arking DE, Cutler DJ, Brune CW, Teslovich TM, West K, Ikeda M, Rea A, Guy M, Lin S, Cook EH, Chakravarti A, 2008. A common genetic variant in the neurexin superfamily member CNTNAP2 increases familial risk of autism. Am. J. Hum. Genet 82 (1), 160–164. 10.1016/j.ajhg.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakkaloglu B, O’Roak BJ, Louvi A, Gupta AR, Abelson JF, Morgan TM, Chawarska K, Klin A, Ercan-Sencicek AG, Stillman AA, Tanriover G, Abrahams BS, Duvall JA, Robbins EM, Geschwind DH, Biederer T, Gunel M, Lifton RP, State MW, 2008. Molecular cytogenetic analysis and resequencing of contactin associated protein-like 2 in autism spectrum disorders. Am. J. Hum. Genet 82 (1), 165–173. 10.1016/j.ajhg.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedrosian TA, Fonken LK, Walton JC, Haim A, Nelson RJ, 2011. Dim light at night provokes depression-like behaviors and reduces CA1 dendritic spine density in female hamsters. Psychoneuroendocrinology 36 (7), 1062–1069. 10.1016/j.psyneuen.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Bedrosian TA, Fonken LK, Nelson RJ, 2016. Endocrine effects of circadian disruption. Annu. Rev. Physiol 78, 109–131. 10.1146/annurev-physiol-021115-105102. [DOI] [PubMed] [Google Scholar]
- Borniger JC, McHenry ZD, Abi Salloum BA, Nelson RJ, 2014. Exposure to dim light at night during early development increases adult anxiety-like responses. Physiol. Behav 133, 99–106. 10.1016/j.physbeh.2014.05.012. [DOI] [PubMed] [Google Scholar]
- Brown LA, Fisk AS, Pothecary CA, Peirson SN, 2019. Telling the time with a broken clock: quantifying circadian disruption in animal models. Biology (Basel) 8 (1), 18. 10.3390/biology8010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner D, Kabitzke P, He D, Cox K, Thiede L, Hanania T, Sabath E, Alexandrov V, Saxe M, Peles E, Mills A, Spooren W, Ghosh A, Feliciano P, Benedetti M, Luo Clayton A, Biemans B, 2015. Comprehensive analysis of the 16p11.2 deletion and null Cntnap2 mouse models of autism spectrum disorder. PLoS One 10 (8). 10.1371/journal.pone.0134572. e0134572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke TM, Markwald RR, Chinoy ED, Snider JA, Bessman SC, Jung CM, Wright KP Jr., 2013. Combination of light and melatonin time cues for phase advancing the human circadian clock. Sleep 36 (11), 1617–1624. 10.5665/sleep.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang AM, Aeschbach D, Duffy JF, Czeisler CA, 2015. Evening use of light-emitting eReaders negatively affects sleep, circadian timing, and next-morning alertness. Proc. Natl. Acad. Sci. U. S. A 112 (4), 1232–1237. 10.1073/pnas.1418490112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeisler CA, 2013. Perspective: casting light on sleep deficiency. Nature 497 (7450), S13. 10.1038/497S13a. [DOI] [PubMed] [Google Scholar]
- Devnani PA, Hegde AU, 2015. Autism and sleep disorders. J. Pediatr. Neurosci 10 (4), 304–307. 10.4103/1817-1745.174438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominoni D, Quetting M, Partecke J, 2013. Artificial light at night advances avian reproductive physiology. Proc. Biol. Sci 280 (1756). 10.1098/rspb.2012.3017.20123017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebihara S, Marks T, Hudson DJ, Menaker M, 1986. Genetic control of melatonin synthesis in the pineal gland of the mouse. Science 231 (4737), 491–493. 10.1126/science.3941912. [DOI] [PubMed] [Google Scholar]
- Eckerberg B, Lowden A, Nagai R, Akerstedt T, 2012. Melatonin treatment effects on adolescent students’ sleep timing and sleepiness in a placebo-controlled crossover study. Chronobiol. Int 29 (9), 1239–1248. 10.3109/07420528.2012.719962. [DOI] [PubMed] [Google Scholar]
- Elrod MG, Hood BS, 2015. Sleep differences among children with autism spectrum disorders and typically developing peers: a meta-analysis. J. Dev. Behav. Pediatr 36 (3), 166–177. 10.1097/DBP.0000000000000140. [DOI] [PubMed] [Google Scholar]
- Engelhardt CR, Mazurek MO, Sohl K, 2013. Media use and sleep among boys with autism spectrum disorder, ADHD, or typical development. Pediatrics 132 (6), 1081–1089. 10.1542/peds.2013-2066. [DOI] [PubMed] [Google Scholar]
- Falchi F, Cinzano P, Duriscoe D, Kyba CCM, Elvidge CD, Baugh K, Portnov BA, Rybnikova NA, Furgoni R, 2016. The new world atlas of artificial night sky brightness. Sci. Adv 2 (6), e1600377. 10.1126/sciadv.1600377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez DC, Fogerson PM, Lazzerini Ospri L, Thomsen MB, Layne RM, Severin D, Zhan J, Singer JH, Kirkwood A, Zhao H, Berson DM, Hattar S, 2018. Light affects mood and learning through distinct retina-brain pathways. Cell 175 (1). 10.1016/j.cell.2018.08.004. 71–84.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher SP, Schwartz MD, Wurts-Black S, Thomas AM, Chen TM, Miller MA, Palmerston JB, Kilduff TS, Morairty SR, 2016. Quantitative electroencephalographic analysis provides an early-stage Indicator of disease onset and progression in the zQ175 knock-in mouse model of Huntington’s disease. Sleep 39 (2), 379–391. 10.5665/sleep.5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK, Nelson RJ, 2011. Illuminating the deleterious effects of light at night. F1000 Med. Rep 3 (18). 10.3410/M3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK, Kitsmiller E, Smale L, Nelson RJ, 2012. Dim nighttime light impairs cognition and provokes depressive-like responses in a diurnal rodent. J. Biol. Rhythm 27 (4), 319–327. 10.1177/0748730412448324. [DOI] [PubMed] [Google Scholar]
- Fonken LK, Aubrecht TG, Melendez-Fernandez OH, Weil ZM, Nelson RJ, 2013. Dim light at night disrupts molecular circadian rhythms and increases body weight. J. Biol. Rhythm 28 (4), 262–271. 10.1177/0748730413493862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandal MJ, Zhang P, Hadjimichael E, Walker RL, Chen C, Liu S, Won H, van Bakel H, Varghese M, Wang Y, Shieh AW, Haney J, Parhami S, Belmont J, Kim M, Moran Losada P, Khan Z, Mleczko J, Xia Y, Dai R, Wang D, Yang YT, Xu M, Fish K, Hof PR, Warrell J, Fitzgerald D, White K, Jaffe AE, PsychENCODE Consortium, Peters MA, Gerstein M, Liu C, Iakoucheva LM, Pinto D, Geschwind DH, 2018. Transcriptome-wide isoform-level dysregulation in ASD, schizophrenia, and bipolar disorder. Science 362 (6420). 10.1126/science.aat8127. pii, eaat8127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garstang J, Wallis M, 2006. Randomized controlled trial of melatonin for children with autistic spectrum disorders and sleep problems. Child Care Health Dev 32 (5), 585–589. 10.1111/j.1365-2214.2006.00616.x. [DOI] [PubMed] [Google Scholar]
- Giannotti F, Cortesi F, Cerquiglini A, Bernabei P, 2006. An open-label study of controlled-release melatonin in treatment of sleep disorders in children with autism. J. Autism Dev. Disord 36 (6), 741–752. 10.1007/s10803-006-0116-z. [DOI] [PubMed] [Google Scholar]
- Goldman SE, Adkins KW, Calcutt MW, Carter MD, Goodpaster RL, Wang L, Shi Y, Burgess HJ, Hachey DL, Malow BA, 2014. Melatonin in children with autism spectrum disorders: endogenous and pharmacokinetic profiles in relation to sleep. J. Autism Dev. Disord 44 (10), 2525–2535. 10.1007/s10803-014-2123-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronli J, Byrkjedal IK, Bjorvatn B, Nodtvedt O, Hamre B, Pallesen S, 2016. Reading from an iPad or from a book in bed: the impact on human sleep. A randomized controlled crossover trial. Sleep Med 21, 86–92. 10.1016/j.sleep.2016.02.006. [DOI] [PubMed] [Google Scholar]
- Hundley RJ, Shui A, Malow BA, 2016. Relationship between subtypes of restricted and repetitive behaviors and sleep disturbance in autism spectrum disorder. J. Autism Dev. Disord 46 (11), 3448–3457. 10.1007/s10803-016-2884-4. [DOI] [PubMed] [Google Scholar]
- Iossifov I, Ronemus M, Levy D, Wang Z, Hakker I, Rosenbaum J, Yamrom B, Lee YH, Narzisi G, Leotta A, Kendall J, Grabowska E, Ma B, Marks S, Rodgers L, Stepansky A, Troge J, Andrews P, Bekritsky M, Pradhan K, Ghiban E, Kramer M, Parla J, Demeter R, Fulton LL, Fulton RS, Magrini VJ, Ye K, Darnell JC, Darnell RB, Mardis ER, Wilson RK, Schatz MC, McCombie WR, Wigler M, 2012. De novo gene disruptions in children on the autistic spectrum. Neuron 74 (2), 285–299. 10.1016/j.neuron.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabe K, Horiuchi F, Oka Y, Ueno S, 2014. The melatonin receptor agonist ramelteon effectively treats insomnia and behavioral symptoms in autistic disorder. Case Rep. Psychiatry 2014, 561071. 10.1155/2014/561071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuljis DA, Loh DH, Truong D, Vosko AM, Ong ML, McClusky R, Arnold AP, Colwell CS, 2013. Gonadal- and sex-chromosome-dependent sex differences in the circadian system. Endocrinology 154 (4), 1501–1512. 10.1210/en.2012-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzerini Ospri L, Prusky G, Hattar S, 2017. Mood, the circadian system, and melanopsin retinal ganglion cells. Annu. Rev. Neurosci 40, 539–556. 10.1146/annurev-neuro-072116-031324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee FY, Wang HB, Hitchcock ON, Loh DH, Whittaker DS, Kim YS, Aiken A, Kokikian C, Dell’Angelica EC, Colwell CS, Ghiani CA, 2018. Sleep/wake disruption in a mouse model of BLOC-1 deficiency. Front. Neurosci 12, 759. 10.3389/fnins.2018.00759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein E, Hawrylycz M, Ao N, et al. , 2007. Genome-wide atlas of gene expression in the adult mouse brain. Nature 445 (7124), 168–176. 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- Logan RW, McClung CA, 2019. Rhythms of life: circadian disruption and brain disorders across the lifespan. Nat. Rev. Neurosci 20 (1), 49–65. 10.1038/s41583-018-0088-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh DH, Kudo T, Truong D, Wu Y, Colwell CS, 2013. The Q175 mouse model of Huntington’s disease shows gene dosage- and age-related decline in circadian rhythms of activity and sleep. PLoS One 8 (7), e69993. 10.1371/journal.pone.0069993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucassen EA, Coomans CP, van Putten M, de Kreij SR, van Genugten JH, Sutorius RP, de Rooij KE, van der Velde M, Verhoeve SL, Smit JW, Lowik CW, Smits HH, Guigas B, Aartsma-Rus AM, Meijer JH, 2016. Environmental 24-hr cycles are essential for health. Curr. Biol 26 (14), 1843–1853. 10.1016/j.cub.2016.05.038. [DOI] [PubMed] [Google Scholar]
- Malow B, Adkins KW, McGrew SG, Wang L, Goldman SE, Fawkes D, Burnette C, 2012. Melatonin for sleep in children with autism: a controlled trial examining dose, tolerability, and outcomes. J. Autism Dev. Disord 42 (8), 1729–1737. author reply 1738. 10.1007/s10803-011-1418-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malow BA, Katz T, Reynolds AM, Shui A, Carno M, Connolly HV, Coury D, Bennett AE, 2016. Sleep difficulties and medications in children with autism spectrum disorders: a registry study. Pediatrics 137 (Suppl. 2), S98–S104. 10.1542/peds.2015-2851H. [DOI] [PubMed] [Google Scholar]
- Mazurek MO, Sohl K, 2016. Sleep and behavioral problems in children with autism spectrum disorder. J. Autism Dev. Disord 46 (6), 1906–1915. 10.1007/s10803-016-2723-7. [DOI] [PubMed] [Google Scholar]
- Mazurek MO, Engelhardt CR, Hilgard J, Sohl K, 2016. Bedtime electronic media use and sleep in children with autism spectrum disorder. J. Dev. Behav. Pediatr 37 (7), 525–531. 10.1097/DBP.0000000000000314. [DOI] [PubMed] [Google Scholar]
- Nord AS, Roeb W, Dickel DE, Walsh T, Kusenda M, O’Connor KL, Malhotra D, McCarthy SE, Stray SM, Taylor SM, Sebat J, Network SP, King B, King MC, McClellan JM, 2011. Reduced transcript expression of genes affected by inherited and de novo CNVs in autism. Eur. J. Hum. Genet 19 (6), 727–731. 10.1038/ejhg.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noya SB, Colameo D, Brüning F, Spinnler A, Mircsof D, Opitz L, Mann M, Tyagarajan SK, Robles MS, Brown SA, 2019. The forebrain synaptic transcriptome is organized by clocks but its proteome is driven by sleep. Science 366 (6462). 10.1126/science.aav2642. pii, eaav2642. [DOI] [PubMed] [Google Scholar]
- Ohta H, Yamazaki S, McMahon DG, 2005. Constant light desynchronizes mammalian clock neurons. Nat. Neurosci 8 (3), 267–269. 10.1038/nn1395. [DOI] [PubMed] [Google Scholar]
- O’Roak BJ, Deriziotis P, Lee C, Vives L, Schwartz JJ, Girirajan S, Karakoc E, Mackenzie AP, Ng SB, Baker C, Rieder MJ, Nickerson DA, Bernier R, Fisher SE, Shendure J, Eichler EE, 2011. Exome sequencing in sporadic autism spectrum disorders identifies severe de novo mutations. Nat. Genet 43 (6), 585–589. 10.1038/ng.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Roak BJ, Vives L, Girirajan S, Karakoc E, Krumm N, Coe BP, Levy R, Ko A, Lee C, Smith JD, Turner EH, Stanaway IB, Vernot B, Malig M, Baker C, Reilly B, Akey JM, Borenstein E, Rieder MJ, Nickerson DA, Bernier R, Shendure J, Eichler EE, 2012. Sporadic autism exomes reveal a highly inter-connected protein network of de novo mutations. Nature 485 (7397), 246–250. 10.1038/nature10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peippo M, Ignatius J, 2012. Pitt-Hopkins syndrome. Mol. Syndromol 2 (3–5), 171–180. 10.1159/000335287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peñagarikano O, Abrahams BS, Herman EI, Winden KD, Gdalyahu A, Dong H, Sonnenblick LI, Gruver R, Almajano J, Bragin A, Golshani P, Trachtenberg JT, Peles E, Geschwind DH, 2011. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell 147 (1), 235–246. 10.1016/j.cell.2011.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peñagarikano O, Lázaro MT, Lu XH, Gordon A, Dong H, Lam HA, Peles E, Maidment NT, Murphy NP, Yang XW, Golshani P, Geschwind DH, 2015. Exogenous and evoked oxytocin restores social behavior in the Cntnap2 mouse model of autism. Sci. Transl. Med 7 (271). 10.1126/scitranslmed.3010257. 271ra8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliak S, Gollan L, Martinez R, Custer A, Einheber S, Salzer JL, Trimmer JS, Shrager P, Peles E, 1999. Caspr2, a new member of the neurexin superfamily, is localized at the juxtaparanodes of myelinated axons and associates with K+ channels. Neuron 24 (4), 1037–1047. 10.1016/s0896-6273(00)81049-1. [DOI] [PubMed] [Google Scholar]
- Raap T, Pinxten R, Eens M, 2015. Light pollution disrupts sleep in free-living animals. Sci. Rep 5, 13557. 10.1038/srep13557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemersma-van der Lek RF, Swaab DF, Twisk J, Hol EM, Hoogendijk WJ, Van Someren EJ, 2008. Effect of bright light and melatonin on cognitive and non-cognitive function in elderly residents of group care facilities: a randomized controlled trial. JAMA 299 (22), 2642–2655. 10.1001/jama.299.22.2642. [DOI] [PubMed] [Google Scholar]
- Robinson-Shelton A, Malow BA, 2016. Sleep disturbances in neurodevelopmental disorders. Curr. Psychiatry Rep 18 (1), 6. 10.1007/s11920-015-0638-1. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Merrow M, 2016. The circadian clock and human health. Curr. Biol 26 (10), R432–R443. 10.1016/j.cub.2016.04.011. [DOI] [PubMed] [Google Scholar]
- Rossignol DA, Frye RE, 2014. Melatonin in autism spectrum disorders. Curr. Clin. Pharmacol 9 (4), 326–334. 10.2174/15748847113086660072. [DOI] [PubMed] [Google Scholar]
- Rybnikova NA, Haim A, Portnov BA, 2016. Does artificial light-at-night exposure contribute to the worldwide obesity pandemic? Int. J. Obes 40 (5), 815–823. 10.1038/ijo.2015.255. [DOI] [PubMed] [Google Scholar]
- Sanders SJ, Murtha MT, Gupta AR, Murdoch JD, Raubeson MJ, Willsey AJ, Ercan-Sencicek AG, DiLullo NM, Parikshak NN, Stein JL, Walker MF, Ober GT, Teran NA, Song Y, El-Fishawy P, Murtha RC, Choi M, Overton JD, Bjornson RD, Carriero NJ, Meyer KA, Bilguvar K, Mane SM, Sestan N, Lifton RP, Gunel M, Roeder K, Geschwind DH, Devlin B, State MW, 2012. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature 485 (7397), 237–241. 10.1038/nature10945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder CM, Malow BA, Maras A, Melmed RD, Findling RL, Breddy J, Nir T, Shahmoon S, Zisapel N, Gringras P, 2019. Pediatric prolonged-release melatonin for sleep in children with autism spectrum disorder: impact on child behavior and Caregiver’s quality of life. J. Autism Dev. Disord 49 (8), 3218–3230. 10.1007/s10803-019-04046-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder AM, Colwell CS, 2013. How to fix a broken clock. Trends Pharmacol. Sci. 34 (11), 605–619. 10.1016/j.tips.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwichtenberg AJ, Malow BA, 2015. Melatonin treatment in children with developmental disabilities. Sleep Med. Clin 10 (2), 181–187. 10.1016/j.jsmc.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi SQ, Johnson CH, 2019. Circadian biology and sleep in monogenic neurological disorders and its potential application in drug discovery. Curr. Opin. Behav. Sci 25, 23–30. 10.1016/j.cobeha.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson TJ, Visser ME, Arnold W, Barrett P, Biello S, Dawson A, Denlinger DL, Dominoni D, Ebling FJ, Elton S, Evans N, Ferguson HM, Foster RG, Hau M, Haydon DT, Hazlerigg DG, Heideman P, Hopcraft JG, Jonsson NN, Kronfeld-Schor N, Kumar V, Lincoln GA, MacLeod R, Martin SA, Martinez-Bakker M, Nelson RJ, Reed T, Robinson JE, Rock D, Schwartz WJ, Steffan-Dewenter I, Tauber E, Thackeray SJ, Umstatter C, Yoshimura T, Helm B, 2015. Disrupted seasonal biology impacts health, food security and ecosystems. Proc. Biol. Sci 282 (1817), 20151453. 10.1098/rspb.2015.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss KA, Puffenberger EG, Huentelman MJ, Gottlieb S, Dobrin SE, Parod JM, et al. , 2006. Recessive symptomatic focal epilepsy and mutant contactin-associated protein-like 2. N. Engl. J. Med 354 (13), 1370–1377. 10.1056/NEJMoa052773. [DOI] [PubMed] [Google Scholar]
- Takahashi JS, Shimomura K, Kumar V, 2008. Searching for genes underlying behavior: lessons from circadian rhythms. Science 322 (5903), 909–912. 10.1126/science.1158822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takumi T, Tamada K, Hatanaka F, Nakai N, Bolton PF, 2019. Behavioral neuroscience of autism. Neurosci. Biobehav. Rev (18), 30372–30375. 10.1016/j.neubiorev.2019.04.012. pii S0149–7634. [DOI] [PubMed] [Google Scholar]
- Thomas AM, Schwartz MD, Saxe MD, Kilduff TS, 2017. Cntnap2 knockout rats and mice exhibit epileptiform activity and abnormal sleep-wake physiology. Sleep 40 (1). 10.1093/sleep/zsw026. [DOI] [PubMed] [Google Scholar]
- Veatch OJ, Maxwell-Horn AC, Malow BA, 2015. Sleep in autism Spectrum disorders. Curr. Sleep Med. Rep 1 (2), 131–140. 10.1007/s40675-015-0012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker WH 2nd, Borniger JC, Gaudier-Diaz MM, Hecmarie Meléndez-Fernández O, Pascoe JL, Courtney DeVries A, Nelson RJ, 2019. Acute exposure to low-level light at night is sufficient to induce neurological changes and depressive-like behavior. Mol. Psychiatry 25 (5), 1080–1093. 10.1038/s41380-019-0430-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H-B, Whittaker DS, Truong D, Mulji AK, Ghiani CA, Loh DH, Colwell CS, 2017. Blue light therapy improves circadian dysfunction as well as motor symptoms in two mouse models of Huntington’s disease. Neurobiol. Sleep Circadian Rhythms 2, 39–52. 10.1016/j.nbscr.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HB, Loh DH, Whittaker DS, Cutler T, Howland D, Colwell CS, 2018. Time-restricted feeding improves circadian dysfunction as well as motor symptoms in the Q175 mouse model of Huntington’s disease. eNeuro 5 (1). 10.1523/ENEURO.0431-17.2017. pii, ENEURO.0431–17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker DS, Loh DH, Wang HB, Tahara Y, Kuljis D, Cutler T, Ghiani CA, Shibata S, Block GD, Colwell CS, 2018. Circadian-based treatment strategy effective in the BACHD mouse model of Huntington’s disease. J. Biol. Rhythm 33 (5), 535–554. 10.1177/0748730418790401. [DOI] [PubMed] [Google Scholar]
- Wirojanan J, Jacquemont S, Diaz R, Bacalman S, Anders TF, Hagerman RJ, et al. , 2009. The efficacy of melatonin for sleep problems in children with autism, fragile X syndrome, or autism and fragile X syndrome. J. Clin. Sleep Med 5 (2), 145–150. [PMC free article] [PubMed] [Google Scholar]
- Wood B, Rea MS, Plitnick B, Figueiro MG, 2013. Light level and duration of exposure determine the impact of self-luminous tablets on melatonin suppression. Appl. Ergon 44 (2), 237–240. 10.1016/j.apergo.2012.07.008. [DOI] [PubMed] [Google Scholar]
- Wright B, Sims D, Smart S, Alwazeer A, Alderson-Day B, Allgar V, et al. , 2011. Melatonin versus placebo in children with autism spectrum conditions and severe sleep problems not amenable to behaviour management strategies: a randomised controlled crossover trial. J. Autism Dev. Disord 41 (2), 175–184. 10.1007/s10803-010-1036-5. [DOI] [PubMed] [Google Scholar]
- Yang M, Silverman JL, Crawley JN, 2011. Automated three-chambered social approach task for mice. Curr. Protoc. Neurosci 10.1002/0471142301.ns0826s56. Chapter 8, Unit-8.26. [DOI] [PMC free article] [PubMed]
- Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS, 2004. PERIOD2:LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc. Natl. Acad. Sci. U. S. A 101 (15), 5339–5346. 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweier C, de Jong EK, Zweier M, Orrico A, Ousager LB, Collins AL, et al. , 2009. CNTNAP2 and NRXN1 are mutated in autosomal-recessive Pitt-Hopkins-like mental retardation and determine the level of a common synaptic protein in Drosophila. Am. J. Hum. Genet 85 (5), 655–666. 10.1016/j.ajhg.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


