Abstract
In this study, the presence of antibiotics (ANB) residues was evaluated in poultry meat purchased from German and Lithuanian markets. In addition, the antimicrobial activity of 13 lactic acid bacteria (LAB) strains, 2 essential oils (EO) (Thymus vulgaris and Origanum vulgare L.), and their compositions were tested for the purpose of inhibiting antibiotic-resistant Salmonella spp. ANB residues were found in 3 out of the 20 analyzed poultry meat samples: sample no. 8 contained enrofloxacin (0.46 μg/kg), sample no. 14 contained both enrofloxacin and doxycycline (0.05 and 16.8 μg/kg, respectively), and sample no. 18 contained enrofloxacin (2.06 μg/kg). The maximum residue limits (MRLs) for the sum of enrofloxacin and ciprofloxacin and for doxycycline in the poultry muscle are 100 μg/kg. Finally, none of the tested poultry meat samples exceeded the suggested MRLs; however, the issue of ANB residues still requires monitoring of the poultry industry in Germany, Poland, and Lithuania, despite the currently established low ANB concentrations. These findings can be explained by the increased use of alternatives to ANB in the poultry industry. Our results showed that an effective alternative to ANB, which can help to reduce the occurrence of antibiotic-resistant salmonella, is a composition containing 1.0% of thyme EO and the following LAB strains: Lactobacillus plantrum LUHS122, Enteroccocus pseudoavium LUHS242, Lactobacillus casei LUHS210, Lactobacillus paracasei LUHS244, Lactobacillus plantarum LUHS135, Lactobacillus coryniformins LUHS71, and Lactobacillus uvarum LUHS245, which can be recommended for poultry industry as components of feed or for the treatment of surfaces, to control the contamination with Salmonella strains. However, it should be mentioned that most of the tested LAB strains were inhibited by thyme EO at the concentrations of 0.5 and 1.0%, except for LUHS122, LUHS210, and LUHS245. Finally, it can be noted that the agents responsible for the inhibitory effect on Salmonella are not the viable LAB strains but rather their metabolites, and further studies are needed to identify which metabolites are the most important.
Key words: poultry, meat, antibiotic residues, antimicrobial activity, Salmonella
Introduction
The European Union (EU) imposed a complete ban of all antibiotics (ANB) as growth promoters (GP) in animal feed since January 2006, and according to the regulations by Food and Drug Administration (FDA), ANB cannot be used for growth-promoting purposes across the United States of America (USA) from 2017. The restriction of ANB use in animal feed is a controversial global issue because the presence of ANB in feed formulations is known to promote the growth of broilers (Gadde et al., 2018, Wealleans et al., 2018) which is explained with the timely control of infections in poultry farms (Singer and Hofacre, 2006). However, the exposure to ANB can lead to the spread of drug-resistant infections in humans and animals, which are projected to cause 10 million human deaths the loss of 100 trillion USD by 2050 if the current trends in ANB consumption will continue (O’Neill, 2014, Mellor et al., 2019). The widespread clinical and agricultural use of antimicrobials has facilitated the emergence of antimicrobial resistance in bacteria (Laxminarayan and Heymann, 2012). Some opportunistic and pathogenic bacteria are more virulent than others. Thus, over 100,000 cases of enterocolitis in the EU, causing annual losses of €3 billion, are attributed to non-typhoidal Salmonella infections, of which Salmonella enterica subsp. enterica serovar Typhimurium is the second most common serovar (EFSA, 2017). It has been reported that poultry and its products are a potential source of resistant Salmonella strains (De Oliveira et al., 2005, Singh et al., 2010, Velasquez et al., 2018). The control of Salmonella in poultry production is very complicated, because birds can be exposed to Salmonella not only from wild birds but also from flies (Wales et al., 2010, Andrés et al., 2013). Also, it should be mentioned that the presence of pathogenic bacteria in the microbiota of broilers is an important biosafety factor in the poultry industry (Clavijo et al., 2019).
Salmonella is a common pathogen that can survive and pass through the technological steps of poultry production (Vinueza-Burgos et al., 2019). Human gastrointestinal infections caused by Salmonella usually are associated with the consumption of poultry products; therefore, the control of this type of pathogens is of great importance (Wegener et al., 2003). Three possible routes of Salmonella contamination in chicken meat have been identified, including initial presence, cross-contamination from broilers carrying Salmonella that have been slaughtered on the same day, and contamination from resident flora in the slaughterhouse, with the last route being the most common (Shang et al., 2019).
However, the treatment of poultry with ANB is not an acceptable solution, as the use of ANB promotes the resistance of pathogenic strains, as well as ANB residues can directly affect the human immune system, growth, and metabolism processes (Muhammad et al., 2019). To reduce the health risks due to ANB use, a search for alternatives continues. It has been suggested that xylanase and amylase produced by Aspergillus niger during solid state fermentation of apple pomace can be used as alternatives to ANB GP in poultry feed (Suresh et al., 2019). Also, the use of probiotics (PRO) has been suggested to reduce the presence of ANB in poultry farming (Patterson and Burkholder, 2003, Gaggia et al., 2010). Most PRO are bacteria that already exist in the digestive tract of animals and have the properties of bacterial community stabilizers or antimicrobials against undesirable bacterial species (de Vrese and Schrezenmeir, 2008, Kabir, 2009). Our previous studies have shown that lactic acid bacteria (LAB) can inhibit methicillin-resistant Staphylococcus aureus (Bartkiene et al., 2019). In addition, LAB has various properties, which are desirable in poultry farms. For example, phosphatase excreted by LAB can lead to improvement of phosphate digestion (Neveling et al., 2020). The LAB, possessing PRO properties, showed ability to attach to intestinal epithelial cells and to reduce pathogens colonization, as well as to increase growth performance and improve the immune system of the poultry (Soomro et al., 2019, Mohammadreza et al., 2020, Salehizadeh et al., 2020). In addition to aforementioned probiotic properties, LAB can reduce mycotoxins in feed (Haquea et al., 2020).
Also, our previous studies showed strong antimicrobial properties of some essential oils (EO), which do not inhibit LAB, while inhibiting pathogenic bacteria (Bartkiene et al., 2018a, Bartkiene et al., 2019). Essential oils typically contain a combination of volatiles that produce cumulative antimicrobial effects. Essential oils have a great potential as alternatives to ANB in poultry industry and are generally favoured as natural antimicrobials that are less toxic and free from residues (Zhai et al., 2018).
Finally, although LAB and EO are well known for their antimicrobial properties in the poultry industry, studies regarding the antimicrobial activity of these very different agents are scarce. For this reason, we set out to test our hypothesis that these antimicrobials with different mechanisms of action can produce a synergic antimicrobial effect. In this study, the presence of ANB residues was evaluated in poultry meat purchased from the German and Lithuanian markets. In addition, the antimicrobial activity of 13 different LAB strains, 2 Eos, and their compositions against ANB-resistant Salmonella spp. was tested.
Materials and methods
Poultry Meat Samples, Salmonella and Lactic Acid Bacteria Strains, Essential Oils
A total of 20 poultry meat samples were purchased from different hypermarkets and central markets in Germany and Lithuania (Table 1). The obtained meat samples originated from different countries: Germany (purchased in Germany), Lithuania, Latvia, Poland, and France (purchased in Lithuania).
Table 1.
Poultry meat samples.
| No. | Type of poultry | Country of origin | The country of retail purchase |
|---|---|---|---|
| 1 | |||
| 2 | |||
| 3 | |||
| 4 | |||
| 5 | |||
| 6 | Germany | ||
| 7 | Germany | ||
| 8 | |||
| 9 | |||
| 10 | Chicken | ||
| 11 | Latvia | ||
| 12 | Lithuania | ||
| 13 | Poland | ||
| 14 | Poland | ||
| 15 | Lithuania | Lithuania | |
| 16 | Lithuania | ||
| 17 | Lithuania | ||
| 18 | Lithuania | ||
| 19 | Lithuania | ||
| 20 | France |
The Salmonella strains were isolated from raw poultry products (chicken) in the Northern region of Kazakhstan in years 2018-2019 (the project was supported by the Ministry of Education and Science of the Republic of Kazakhstan, Project number AP05131447). All isolates belonged to the Enteritidis serotype of Salmonella enterica. Susceptibility testing was performed using disk-diffusion method at the Kostanay State University (Kazakhstan) according to clinical breakpoints set by EUCAST (whenever possible) and the applicable national standard. The Salmonella resistance profiles are given in Table 2.
Table 2.
The antibiotic–resistant profile of Salmonella.
| Salmonella strains | Antibiotics1 |
|---|---|
| Salmonela K2 | AMP, KAN, NEO, TET, DOXY, CIP |
| Salmonela K5 | AMP, KAN, NEO, GEN, DOXY |
| Salmonela K43 | AMP, DOXY, CIP, SXT, FUR |
| Salmonela K72 | FUR |
| Salmonela K76 | DOXY, FUR |
AMP = ampicillin; KAN = kanamycin; NEO = neomycin; GEN = gentamicin; DOXY = doxycycline; CIP = ciprofloxacin; SXT = sulfamethoxazole/trimethoprim; FUR = nitrofurantoin.
The LAB strains (Leuconostoc mesenteroides LUHS225, Lactobacillus plantarum LUHS122, Enteroccocus pseudoavium LUHS242, Lactobacillus casei LUHS210, Lactobacillus curvatus LUHS51, Lactobacillus farraginis LUHS206, Pediococcus pentosaceus LUHS183, Pediococcus acidilactici LUHS29, Lactobacillus paracasei LUHS244, L. plantarum LUHS135, Lactobacillus coryniformis LUHS71, Lactobacillus brevis LUHS173, and Lactobacillus uvarum LUHS245) were acquired from the Lithuanian University of Health Sciences collection (Kaunas, Lithuania). The LAB strains were selected according to their inhibiting properties against pathogenic and opportunistic bacterial strains (Bartkiene et al., 2018b, Bartkiene et al., 2019, Lele et al., 2018). The tested LAB strains were grown in the MRS medium (Biolife, Italy) at 30°C. Two percent of the MRS solution (v/v) in which the strains were multiplied were inoculated into fresh medium and propagated for 18 h. The multiplied LAB samples were used for the determination of their antimicrobial activities against the aforementioned Salmonella strains.
The EO of thyme (Thymus vulgaris) and oregano (Origanum vulgare L.) were purchased from Sigma-Aldrich (Saint-Louis, MO, USA).
Evaluation of Antibiotic Residues in Poultry Meat Samples by UHPLC-MS/MS Method
The following antibiotics were analyzed in this study: cephalosporins (cefacetrile, cefalexin, cefoperazone, cefalonium, cefaprim, cefazolin, cefquinome, ceftiofur), penicillins (amoxicillin, ampicillin, benzylpenicillin, cloxacillin, dicloxacillin, nafcillin, oxacillin, phenoxymethylpenicillin, penicillin V), quinolones (ciprofloxacin, danofloxacin, difloxacin, enrofloxacin, flumequine, marbofloxacin, nalidixic acid, norfloxacin, orbifloxacin, oxolinic acid, sarafloxacin), sulfonamides (sulfachloropyridazine, sulfadimethoxine, sulfadimidine, sulfadoxine, sulfamerazine, sulfamethizole, sulfathiazole, sulfamonomethoxine, sulfanilamide), tetracyclines (chlortetracycline, doxycycline, oxytetracycline, tetracycline), macrolides and lincosamides (erythromycin A, josamycin, kitasamycin, lincomycin, neospiramycin, pirlimycin, spiramycin, tildipirosin, tilmicosin, tylosin A, tulathromycin A), and other antibiotics (thiamphenicol, bacitracin, novobiocin, rifaxamin, tiamulin, tylvalosin, valnemulin, and trimethoprim).
The analyses were performed according to a previously published method by Reinholds et al., (2016). According to this method, a 2 g sample was weighed into a 15 mL centrifuge tube. Quality control samples were fortified with the appropriate volume of standard solution to obtain levels corresponding to 10% of EU MRLs for muscles. Then 3 mL of acetonitrile was added to each sample. The samples were vigorously shaken for 20 min and centrifuged for 15 min at 4,500 rpm. The supernatant was collected and loaded onto a Phree phospholipid removal tube (1 mL) that was preconditioned with 0.5 mL of acetonitrile. The obtained extracts (2 mL) were collected into clean sample tubes, while the Phree tubes were washed with additional 0.3 mL of acetonitrile. The combined acetonitrile extracts were evaporated to dryness under nitrogen stream at 55°C. The residues were dissolved in 1 mL of 0.1% formic acid solution in water/methanol (90:10, v/v). The samples were then filtered through 0.22 μm centrifuge filters at 3,000 rpm and transferred to autosampler vials for further analysis. A 10 μL aliquot of each sample was injected into the UHPLC-MS/MS system.
The obtained low-level concentrations of enrofloxacin and ciprofloxacin were confirmed using the method described by Pugajeva et al., 2018. According to that method, a sample of muscle tissue (10 g) was spiked with 50 μL of 0.01 μg L−1 internal standard solution (concentration in samples was 0.05 μg/kg). The analytes were extracted by adding 20 mL of acetonitrile, then shaken for 20 min, and sonicated for 10 min in ultrasonic bath. After centrifugation at 4,000 rpm for 10 min, 15 mL of the supernatant was transferred into another centrifuge tube and evaporated under nitrogen stream at 50°C. The sample was reconstituted in 5 mL of water and centrifuged for 10 min at 4,000 rpm at 4°C. The supernatant was loaded into a Strata X cartridge (500 mg/6 mL) previously conditioned with methanol (5 mL) and deionised water (5 mL). The column was washed with aqueous 50% methanol solution. The elution of analytes was achieved with 5 mL of 1% ammonia solution in methanol. The eluate was evaporated to dryness under nitrogen stream at 50°C. The residue was dissolved in aqueous 50% methanol solution (200 μL), then transferred into a vial for UHPLC-MS/MS analysis.
Chromatographic separation of target compounds was achieved using an UltiMate 3,000 UHPLC system (Thermo Scientific, Waltham, MA, USA). The separation was performed on a 100 mm × 2.1 mm i.d., 1.9 μm Hypersil Gold analytical column (Thermo Scientific). The mobile phase component A was water and the component B was methanol, both containing 0.1% of formic acid. The flow rate was 300 μL min−1. The effective gradient began at the initial mobile phase composition of 90% A and 10% B. The percentage of mobile phase component B was linearly raised from 10 to 30% until 4.0 min, then maintained for 1.0 min. From 5.0 min to 10 min, the percentage of component B was linearly raised up to 95% and was held constant until 10.5 min. Then the percentage of component B was sharply decreased to 10% over 0.5 min and was kept at this level until 15 min. The column and sample temperatures were 30°C and 10°C, respectively.
The UHPLC system was coupled to a Thermo Scientific TSQ Quantiva mass spectrometer equipped with a heated electrospray ionization probe used in the positive ionization mode. Sample analysis was performed in the selected reaction monitoring (SRM) mode, by selecting one precursor and 2 product ions for each compound with a dwell time of 100 ms per channel, using resolution of 0.7 FWHM for Q1 and Q3 and setting the collision gas (argon) pressure at 1.5 mTorr. The following general ionization source parameters were applied: spray voltage 4.0 kV, vapouriser temperature 320°C, ion transfer tube temperature 280°C, sheath gas (N2) 40 arbitrary units (arb), auxiliary gas (N2) 15 (arb), and sweep gas (N2) 5 (arb). The data processing was carried out with TraceFinderEFS software (Thermo Fisher Scientific).
Evaluation of Lactic Acid Bacteria and Essential Oils Antimicrobial Properties Against Salmonella Strains
An agar well diffusion assay was used for testing the antimicrobial activity of LAB. For this purpose, 0.5 McFarland turbidity suspension of each Salmonella strain was inoculated onto the surface of cooled Mueller Hinton Agar (Oxoid, UK) using sterile cotton swabs. Wells with 6 mm diameter were punched in the agar and filled with 50 μL of the tested LAB suspension. The antimicrobial activity against the tested bacteria was determined by measuring the DIZ (mm). The experiments were repeated 3 times and the average value of DIZ was calculated.
In addition, the minimal inhibitory concentrations (MIC) of the LAB and EO against the aforementioned Salmonella strains were determined according to the Clinical and Laboratory Standards Institute (CLSI) microdilution method (CLSI, 2015). Minimal inhibitory concentration was defined as the concentration of LAB or EO that inhibited visible microbial growth. Two concentrations of LAB and 4 concentrations of EO were tested against the Salmonella strains (suspension of 0.5 McFarland turbidity): (i) 0.5 mL LAB + 0.1 mL of Salmonella suspension, (ii) 0.5 mL LAB + 0.01 mL of Salmonella suspension, (iii) 0.01 mL EO + 0.01 mL of Salmonella suspension, (iv) 0.02 mL EO + 0.1 mL of Salmonella suspension, (v) 0.05 mL EO + 0.01 mL of Salmonella suspension, (vi) 0.1 mL EO + 0.1 mL of Salmonella suspension. The experiments were performed in triplicate.
Evaluation of Essential Oil Antimicrobial Properties Against Lactic Acid Bacteria
The LAB strains selected for the highest antimicrobial activity were multiplied in MRS broth (Biolife, Italy) at 30°C. Then, 500 μL of the selected LAB strains in 10 mL of physiological solution were added. The LAB strains diluted with physiological solution were tested as (I) control; (II) with 50 μL of T. vulgaris EO; (III) with 100 μL of T. vulgaris EO. Count of LAB was determined after 0 and 24 hr of cultivation at 30°C. The LAB counts were determined on MRS agar (Liofilchem, Roseto degli Abruzzi, Teramo, Italy) using standard plate count techniques (ISO 15214:1998). The plates were incubated at 30°C for 72 h under anaerobic conditions (using an AnaeroGen atmosphere generation system, Oxoid).
Results and discussion
Antibiotic Residues in Poultry Meat Samples
Antibiotic residues detected in poultry meat samples are shown in Table 3. Among the different classes of antimicrobials, some of them are used for broad applications. For instance, fluoroquinolones and sulphonamides are used as GP as well as drugs against a broad spectrum of both gram-positive and gram-negative microorganisms (Jiang et al., 2013). In this study, antibiotic residues were found in 3 of the 20 poultry meat samples analyzed: enrofloxacin (0.46 μg/kg) was found in the sample no. 8, enrofloxacin and doxycycline (0.05 and 16.8 μg/kg, respectively) were found in the sample no. 14, and enrofloxacin (2.06 μg/kg) was found in the sample no. 18. Our previous studies showed that 37 of 40 samples contained residues of enrofloxacin in the concentration range of 3.3–1,126 ng/kg (Pugajeva et al., 2018). Because finding that ANB can promote the growth of animals, various ANBs have been added to animal feed at subtherapeutic doses. Although this practice has been beneficial for animal productivity, there is a concern about long-term effects or the environment and the public health. The frequent use of ANB in animal feed has led to the dissemination of ANB-resistant strains of poultry pathogens, such as Salmonella, Campylobacter, and Escherichia coli (Suresh et al., 2018). Also, the use of ANB as a GP in animal feed, which leads to their residues in meat, can cause allergic reactions, as well as technological problems during fermentation of certain meat products (Pavlov et al., 2005). The European Centre for Disease Prevention and Control (ECDC) states that ANB resistance continues to be a serious public health threat worldwide, and the European Commission (EC) decided in 2006 to ban all commonly used ANB-GP in animal feed due to concerns about the potential for ANB-resistant strains of bacteria and ANB residues in meat products. For this reason, there has been considerable interest in alternatives to ANB. To reduce the risk of anti-bacterial resistance, the European Union (EU) applied a “precautionary principle” model by banning certain antimicrobial GP (Kriebel et al., 2001). For those ANB that are not banned, maximum residue limits (MRL) of ANB have been set by EU countries and the USA to ensure the safety of consumers. According to the definition by EU authorities, the MRL is the maximal legally acceptable amount of pharmacologically active substances and their metabolites in foodstuffs originating from animals. The MRLs are calculated with reference to the acceptable daily intake (ADI), which includes a large safety margin in the calculation, and the ADI for meat is about 500 g per person (Mungroo and Neethirajan, 2014). The requirements of those regulations can be met by relying on a withdrawal period, which is the time period between the last doses of any pharmacologically active substance administered to the animal and the time at which the residue level in tissues or products must not exceed the MRL. Withdrawal periods promote consumer safety by ensuring that the MRL is not exceeded (European Commission, 2001, National Office of Animal Health Ltd. (NOAH). 2016). Although efforts have been made to harmonize MRLs worldwide under the aegis of World Trade Organization (WTO) and the Codex Alimentarius, MRLs still vary from one geographical location to another. In fact, MRLs in a particular animal product may differ from one country to another depending on the local food safety regulatory agencies and drug usage patterns (APVMA, 2014). Acceptable daily intake is also a key requirement that is established on the basis of the no observable effect level, as identified from toxicological studies, divided by a safety factor (often 100) (European Commission, 2001). The MRLs for the sum of enrofloxacin and ciprofloxacin and for doxycycline in the poultry muscle are 100 μg/kg. According to the results of this study, the problem with ANB residues is still relevant in the poultry industry of Germany, Poland, and Lithuania. However, in comparison with our previous results, ANB residues were found at lower amounts. These findings can be explained by improved control of food quality and the increased use of alternatives to ANB in the poultry industry.
Table 3.
Antibiotic residues in poultry meat samples.
| No. | Type of poultry | Country of origin | The country of retail purchase | Enrofloxacin |
Doxycycline |
|---|---|---|---|---|---|
| μg/kg | |||||
| 8 | Chicken | Germany | Germany | 0.46 ± 0.03 | nd |
| 14 | Poland | Lithuania | 0.05 ± 0.01 | 16.80 ± 0.13 | |
| 18 | Lithuania | 2.06 ± 0.05 | nd | ||
Values are mean ± SD of 3 replicate analyses (n = 3).
Abbreviation: nd, not detected.
Lactic Acid Bacteria, Essential Oils, and Their Composition of Antimicrobial Properties Against Salmonella Strains
The inhibition zones (IZ) caused by LAB against the tested Salmonella strains, as well as the MIC of the tested LAB strains and Eos, and the IZ of their combinations are shown in Table 4, Table 5, Table 6, respectively.
Table 4.
The inhibition zones (mm) caused by lactic acid bacteria (LAB) against the tested Salmonella strains.
| Salmo-nella strains | Diameter of inhibition zone, mm |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LAB strains1 | |||||||||||||
| 225 | 122 | 242 | 210 | 51 | 206 | 183 | 29 | 244 | 135 | 71 | 173 | 245 | |
| K2 | nd | 14.3 ±1.2b | 12.3 ±0.3a | 10.3 ±0.5a | nd | 10.2 ±0.6a | 11.0 ±0.9a | 12.1 ±0.6a | 11.3 ±0.3a | 14.2 ±0.2b,c | 11.0 ±0.5a | nd | 14.3 ±0.5b |
| K5 | nd | 12.1 ±0.9a | 12.0 ±0.3a | 12.0 ±1.0a | nd | 14.3 ±0.7c | 11.0 ±0.4a | 12.0 ±0.3a | 12.1 ±0.5a | 13.3 ±0.3b | 11.0 ±0.3a | nd | 14.0 ±0.3b |
| K43 | nd | 13.2 ±0.4a | 13.3 ±0.2b | 11.2 ±0.9a | nd | nd | 11.0 ±0.6a | 12.3 ±0.5a | 13.2 ±0.3b | 12.4 ±0.5a | 12.3 ±0.2b | nd | 14.0 ±0.5b |
| K72 | nd | 13.3 ±0.5a | 11.3 ±0.9a | 10.0 ±0.7a | nd | 12.3 ±1.0b | 12.3 ±0.9a | 12.0 ±0.3a | 13.3 ±0.3b | 14.0 ±0.6b | 11.5 ±0.3a | nd | 12.3 ±0.6a |
| K76 | nd | 12.1 ±1.1a | 11.0 ±0.7a | 11.3 ±1.2a | nd | 12.0 ±0.7b | nd | nd | 11.0 ±0.3a | 13.1 ±0.3b | 12.3 ±0.3b | nd | 14.0 ±0.4b |
a–cMean values with different letters are significantly different (P ≤ 0.05).
Values are mean ± SD of 3 replicate analyses (n = 3).
Abbreviation: nd, not detected.
225 = Leuconostoc mesenteroides LUHS225; 122 = Lactobacillus plantrum LUHS122; 242 = Enteroccocus pseudoavium LUHS242; 210 = Lactobacillus casei LUHS210; 51 = Lactobacillus curvatus LUHS51; 206 = Lactobacillus farraginis LUHS206; 183 = Pediococcus pentosaceus LUHS183; 29 = Pediococcus acidilactici LUHS29; 244 = Lactobacillus paracasei LUHS244; 135 = Lactobacillus plantarum LUHS135; 71 = Lactobacillus coryniformins LUHS71; 173 = Lactobacillus brevis LUHS173; 245 = Lactobacillus uvarum LUHS245.
Table 5.
The minimal inhibitory concentrations (MIC) of the lactic acid bacteria (LAB) strains and essential oils (EO) against the tested Salmonella strains.
| Salmonella strains | MIC |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LAB strains1 | |||||||||||||
| 0.5 mL LAB +0.01 mL pathogen | |||||||||||||
| 225 | 122 | 242 | 210 | 51 | 206 | 183 | 29 | 244 | 135 | 71 | 173 | 245 | |
| K2 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| K5 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| K43 | - | - | - | - | - | - | - | + | - | - | - | - | - |
| K72 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| K76 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 0.5 mL LAB +0.1 mL pathogen | |||||||||||||
| K2 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| K5 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| K43 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| K72 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| K76 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| EOs1 |
||||||||
|---|---|---|---|---|---|---|---|---|
| 0.1% Eos +0.01 mL pathogen |
0.2% Eos +0.01 mL pathogen |
0.5% Eos +0.01 mL pathogen |
1% Eos +0.01 mL pathogen |
|||||
| Thy | Ore | Thy | Ore | Thy | Ore | Thy | Ore | |
| K2 | + | + | + | + | + | + | - | + |
| K5 | + | + | - | + | - | + | - | + |
| K43 | + | + | - | + | - | + | - | + |
| K72 | + | + | + | + | + | + | - | + |
| K76 | + | + | - | + | + | + | - | + |
Values are mean ± SD of 3 replicate analyses (n = 3).
(-) ‒ the pathogens did not grow, (+) – the pathogens grow.
225 = Leuconostoc mesenteroides LUHS225; 122 = Lactobacillus plantrum LUHS122; 242 = Enteroccocus pseudoavium LUHS242; 210 = Lactobacillus casei LUHS210; 51 = Lactobacillus curvatus LUHS51; 206 = Lactobacillus farraginis LUHS206; 183 = Pediococcus pentosaceus LUHS183; 29 = Pediococcus acidilactici LUHS29; 244 = Lactobacillus paracasei LUHS244; 135 = Lactobacillus plantarum LUHS135; 71 = Lactobacillus coryniformins LUHS71; 173 = Lactobacillus brevis LUHS173; 245 = Lactobacillus uvarum LUHS245; Thy = Thymus vulgaris; Ore = Origanum vulgare L.
Table 6.
The inhibition zones (mm) of the lactic acid bacteria (LAB) strains and thyme (Thy) essential oil (EO) compositions against the tested Salmonella strains.
| Salmonella strains | Inhibition zone, mm |
||||
|---|---|---|---|---|---|
| LAB strains composition | LAB strains and Thy EO composition (0.1% EO) | LAB strains and Thy EO composition (0.2% EO) | LAB strains and Thy EO composition (0.5% EO) | LAB strains and Thy EO composition (1% EO) | |
| K2 | nd | nd | nd | 13.0 ± 0.2 | 14.2 ± 0.3 |
| K5 | 10.0 ± 0.3 | nd | nd | 12.5 ± 0.3 | 15.0 ± 0.2 |
| K43 | 11.0 ± 0.1 | nd | nd | 11.2 ± 0.1 | 15.4 ± 0.5 |
| K72 | 10.5 ± 0.4 | nd | nd | 12.0 ± 0.3 | 14.1 ± 0.3 |
| K76 | 10.0 ± 0.2 | 10.0 ± 0.1 | 10.0 ± 0.3 | 13.5 ± 0.2 | 14.0 ± 0.4 |
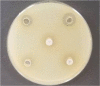
| Images | ||
 |
 |
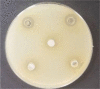 |
| 1—LAB strains and Thy EO composition (0.5% EO); 2—LAB strains and Thy EO composition (1.0% EO); 3—LAB strains and Thy EO composition (0.2% EO); 4—LAB strains and Thy EO composition (0.1% EO); 5—LAB strains composition | Salmonella K2 | Salmonella K5 |
 |
 |
 |
| Salmonella K43 | Salmonella K72 | Salmonella K76 |
Lactic acid bacteria composition consists of LUHS122, LUHS242, LUHS210, LUHS244, LUHS135, LUHS71, LUHS245 strains (122 = Lactobacillus plantarum LUHS122; 242 = Enteroccocus pseudoavium LUHS242; 210 = Lactobacillus casei LUHS210; 244 = Lactobacillus paracasei LUHS244; 135 = Lactobacillus plantarum LUHS135; 71 = Lactobacillus coryniformins LUHS71; 245 = Lactobacillus uvarum LUHS245).
Values are mean ± SD of 3 replicate analyses (n = 3).
Abbreviation: nd, not detected.
When comparing the IZ caused by LAB against Salmonella, the LAB strains L. mesenteroides LUHS225, L. curvatus LUHS51, and L. brevis LUHS173 did not inhibit the tested Salmonella strains. Furthermore, L. farraginis LUHS206 did not exhibit antimicrobial activity against Salmonella K43, while P. pentosaceus LUHS183 and P. acidilactici LUHS29 did not exhibit antimicrobial activity against the Salmonella strain K76 (Table 4). However, the other tested LAB strains inhibited all of the tested Salmonella strains and the highest IZ was caused by the LAB strains LUHS122, LUHS135, and LUHS245 against the Salmonella strain K2 (the average IZ diameter was 14.3 mm), LAB strains LUHS206 and LUHS245 against the Salmonella strain K5 (the average IZ diameter was 14.2 mm), LAB strain LUHS245 against the Salmonella strain K43 (the average IZ diameter was 14.0 mm), LAB strain LUHS135 against the Salmonella strain K72 (the average IZ diameter was 14.0 mm), and LAB strain LUHS245 against the Salmonella strain K76 (the average IZ diameter was 14.0 mm).
When comparing the MIC of the LAB strains and EO against the tested Salmonella strains, it was found that all of the tested LAB strains at both test concentrations inhibited Salmonella, except for 0.5 mL of LUHS29 + 0.01 mL of Salmonella strain K43 suspension (Table 5). Comparing the MICs of the tested EO, the oregano EO did not inhibit Salmonella strains at any of the tested concentrations, while the thyme EO at 0.2% concentration inhibited the Salmonella strains K2 and K72, at 0.5% concentration inhibited the Salmonella strains K2, K72, and K76, and at 1.0% inhibited all of the tested Salmonella strains.
Further experiments were performed with the LAB strains LUHS122, LUHS242, LUHS210, LUHS244, LUHS135, LUHS71, and LUHS245 in combination with different concentrations of thyme EO, which had previous shown the highest antimicrobial activity against Salmonella (Table 6). It should be mentioned that it is very important to reduce the necessary concentration of EO, because EO possess very intense flavors that may not be palatable for animals and thus negatively affect the feed consumption. When comparing the antimicrobial properties of LAB and EO combination with the effects of LAB alone, the addition of EO at the concentrations of 0.1 and 0.2% reduced the antimicrobial properties of the mixture (the strains K5, K43, and K76 were not inhibited, while the inhibition of strain K76 remained similar in comparison with pure LAB). However, the addition of EO at the concentrations of 0.5 and 1.0% enhanced the antimicrobial properties of the LAB mixture, compared to LAB strains alone, and the antimicrobial activity was further improved by increasing the concentration of EO (the IZ diameters resulting from 0.5 and 1.0% of EO in combination with LAB were on average 12.4 and 14.5 mm, respectively). It should be mentioned that the Salmonella strain K2 was not inhibited by LAB strains alone or in mixtures with EO at the concentrations of 0.1 and 0.2%; however, increasing the concentration of EO to 0.5 and 1.0% suppressed this strain (the IZ diameters were 13.0 and 14.2 mm for LAB in combination with 0.5 and 1.0% of EO, respectively).
At the last stage of this experiment, the antimicrobial properties of thyme EO at the selected concentrations were tested against LAB strains (Table 7). It was established that most of the LAB strains were inhibited by thyme EO at 0.5 and 1.0% concentrations, except for LUHS122, LUHS210, and LUHS245. By using 0.5% of thyme EO, the counts of LAB strains LUHS122, LUHS210, and LUHS245 were reduced by 26.5, 16.7, and 27.8%, respectively. When using 1.0% of thyme EO, the counts of LAB strains LUHS122, LUHS210, and LUHS245 were reduced by 29.2, 44.7, and 43.2%, respectively. Finally, it could be assumed Salmonella inhibition was not caused directly by the the viable cells of LAB strains, but rather their metabolites and further studies will be needed to identify which metabolites are the most important.
Table 7.
The effect of Thymus vulgaris (Thy) essential oil (EO) influence on lactic acid bacteria (LAB) inhibition.
| LAB strains1 |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LUHS 122 | LUHS 244 | LUHS 210 | LUHS 242 | LUHS 245 | LUHS 135 | LUHS 71 | LUHS 183 | LUHS 51 | LUHS 29 | LUHS 225 | LUHS 206 | LUHS 173 | |
| log10 cfu mL−1 | |||||||||||||
| 0.5 mL LAB | 8.26 ± 0.03 | 8.32 ± 0.04 | 7.47 ± 0.02 | 7.99 ± 0.07 | 7.30 ± 0.06 | 7.09 ± 0.05 | 7.35 ± 0.04 | 7.59 ± 0.01 | 7.62 ± 0.06 | 7.50 ± 0.02 | 7.61 ± 0.03 | 6.22 ± 0.02 | 7.93 ± 0.04 |
| 0.5 mL LAB + Thy EO composition (0.5% EO) | 6.07 ± 0.6 | nd | 6.22 ± 0.06 | nd | 5.27 ± 0.01 | nd | nd | nd | nd | nd | nd | nd | nd |
| 0.5 mL LAB + Thy EO composition (1.0% EO) | 5.85 ± 0.06 | nd | 4.13 ± 0.04 | nd | 4.15 ± 0.03 | nd | nd | nd | nd | nd | nd | nd | nd |
Abbreviation: nd, not detected.
LUHS122 = Lactobacillus plantrum; LUHS244 = Lactobacillus paracasei; LUHS210 = Lactobacillus casei; LUHS242 = Enteroccocus pseudoavium; LUHS245 = Lactobacillus uvarum; LUHS135 = Lactobacillus plantarum; LUHS71 = Lactobacillus coryniformins; LUHS206 = Lactobacillus farraginis; LUHS29 = Pediococcus acidilactici; LUHS183 = Pediococcus pentosaceus; LUHS225 = Leuconostoc mesenteroides; LUHS173 = Lactobacillus brevis; LUHS51 = Lactobacillus curvatus.
The desirable properties of probiotics (PRO) in poultry have been recognized since the study by Rantala and Nurmi (1973), who observed that the bacteria from the gut of mature birds can be used for the protection of young chicks from infection. Baba et al. (1991) published their findings that the composition of several PRO strains is more effective at reducing Salmonella colonization in chicks than any individual PRO strain. Later, it was published that PRO comprising 29 bacterial strains also reduced the amount of recoverable Salmonella from chicks (Corrier et al., 1990). Furthermore, anaerobic PRO extracted from ceca suppressed Salmonella (Impey et al., 1984) or Salmonella and Campylobacter (Blankenship et al., 1993, Stern et al., 2001, Higgins et al., 2007).
Thomas et al. (2019) published that culture supernatants from Lactobacillus ingluviei strain UMNPBX19 and Lactobacillus salivarius strain UMNPBX2 exhibited antimicrobial activity against Salmonella. A study by Adetoye et al. (2018) demonstrated in vitro suppression of Salmonella by intestinal LAB from cattle (Lactobacillus amylovorus C94 and L. salivarius C86). The data published by Burkholder et al. (2019) suggested a protective effect of L. acidophilus, L. rhamnosus, and L. casei against Salmonella enterica Javiana. Ahmed et al. (2019) concluded that Lactobacillus species with PRO properties can be used in poultry feed formulation for their health benefits to combat gastrointestinal infections. In their study, 6 of 21 Lactobacillus strains showed good antimicrobial activities against S. aureus, Salmonella typhimurium, and E. coli. Our results are in agreement with the aforementioned studies that demonstrated the ability of some LAB strains to suppress Salmonella. However, the antimicrobial activity mechanisms of LAB can be explained in different ways. The data published by Zhu et al. (2019) indicate that the main mechanism of LAB activity against Salmonella infection is mediated by short-chain fatty acids excreted by the Lactobacillus johnsonii L531 strain used. Other authors have described how the surface proteins of Lactobacillus kefiri strains 8,321 and 83,113 and L. plantarum strain 83,114 can be used as alternative means for the control of Salmonella biofilm formation in the poultry industry (Merino et al., 2019). Also, LAB can produce various inhibitory compounds such as bacteriocins, organic acids, hydrogen peroxide, diacetyl, and carbon dioxide that are known to inhibit pathogenic microorganisms (Vieco-Saiz et al., 2019). Enzymes excreted by LAB improve the rates of nutrient absorption, as well as stimulate the immune system of animals. It was demonstrated that nisin and beta-lactams excreted by LAB can inhibit the Salmonella enterica serovar Typhimurium (Rishi et al., 2014, Singh et al., 2014). It should be mentioned that the heterofermentative LAB can produce other metabolites: organic acids, ethanol, diacetyl, hydrogen peroxide (H2O2), and so on (Schnürer and Magnusson, 2005, Elshaghabee et al., 2016). Results of this study showed that not the viable LAB strains but their metabolites were the most important in Salmonella inhibition, and further studies are needed to identify which metabolites are the most important.
Organic acids excreted by LAB reduce pH, creating unfavorable local microenvironment for pathogens, resulting in their inhibition and death (Surendran et al., 2017, Zhitnitsky et al., 2017, Dittoe et al., 2018). As demonstrated by Wang et al. (2015), lactic acid concentrations of 0.5% (v/v) could completely inhibit the growth of Salmonella spp. However, these acids do not affect animal epithelial cells (Allen and Flemström, 2005). The presence of ethanol excreted from LAB was shown to result in bacterial cell death due to plasma membrane leakage (Ingram, 1989). It was described that Lb. plantarum, Lb. helveticus, Lb. bulgaricus, Ent. faecalis, and mainly Leuc. mesenteroides and Lc. lactis biovar diacetylactis are the most common LAB species producing diacetyl (García-Quintáns et al., 2008, Singh, 2018), which interferes with arginine utilization by reacting with the arginine-binding protein of gram-negative bacteria (Lindgren and Dobrogosz, 1990). Also, LAB can create anaerobic environment by excreting CO2, and aerobic bacteria cannot propagate in such environment (Singh, 2018). Some strains of LAB are able to produce hydrogen peroxide (H2O2), which can inhibit pathogens devoid of catalase at low quantities via superoxide anion chain reaction enhancing toxic oxidation (Mitchell et al., 2015). However, the antibacterial activity of H2O2 depends on its concentration, pH, temperature, and other factors (Surendran et al., 2017).
According to Sadia Ashraf et al. (2018), phytochemicals also can provide alternative options for the treatment of antibiotic-resistant Salmonella, and it was concluded that Nigella sativa has the necessary in vitro activity against S. enetrica and thus can be used as a therapeutic agent. In a study with extracts of natural compounds, it was shown that some phenolic type natural products possessed evident antibacterial ability against pathogenic bacteria, but not against LAB. The most common phenolic compounds (carvacrol, trans-cinnamaldehyde, p-coumaric acid, eugenol, gallic acid, and rosmarinic acid) exhibit strong antibacterial effects against pathogenic bacteria that are mainly responsible for the antibacterial activity of EO (Chak-LunChan et al., 2018). It was reported that a combination of EO obtained from Syzygium aromaticum and Cinnamomum zeylanicum inhibited both S. Enteritidis and S. Typhimurium isolates. Such antimicrobial activity has been attributed to the main EO compounds: cinnamaldehyde and eugenol (Ismail et al., 2017). Cinnamaldehyde and eugenol are able to inhibit the production of essential bacterial enzymes due to the presence of a carbonyl group that binds and inactivates them and/or causes damage to the bacterial cell wall (Di Pasqua et al., 2007). The presence of cinnamaldehyde and eugenol may enhance the antibacterial effect, as suggested by Burt (2004). Essential oils from A. triphylla, Cinnamomum citratus, L. cubeba, and M. piperita showed no relevant activity against Salmonella; however, other authors have described in vitro antibacterial activity of EO from S. aromaticum and C. zeylanicum against paratyphoid Salmonella strains (Simitzis et al., 2014, Thanissery et al., 2014, Abbes et al., 2018). It has been reported that the EO of cinnamon (C. zeylanicum) and thyme (T. vulgaris) produced the highest activity, with 22.5–38.5 mm inhibition zones against 5 Salmonella serotypes (Olaimat et al., 2019). In a different application, the EO of thyme in combination with cold plasma treatment led to a higher antibacterial activity of plasma-treated nanofibers (Lin et al., 2019). Essential oils could be applied for the purposes of facility disinfection, as well as added to chicken feed to prevent intestinal colonization with pathogens (Ebani et al., 2019). The antimicrobial activity data for EO showed that thymol, eugenol, and carvacrol exhibit strong antimicrobial activity against both E. coli and S. typhimurium (Franz and Baser, 2010, Hippenstiel et al., 2011, Bassole and Juliani, 2012). Thymol, eugenol, and carvacrol have similar chemical structures and exert synergic antimicrobial effects (Bassole and Juliani, 2012), but it is necessary to optimize their formulation (Zhai et al., 2018). In conclusion, it must be pointed out that although there are several viable approaches for pathogen control on meat and eggs in the conventional poultry industry, the selection of acceptable antibacterials is much more limited for organic poultry producers (Arsi et al., 2019). The findings of this study provide useful data regarding effective strategies for pathogen control at organic farms.
Conclusions
The problem with ANB residues is still highly relevant in the poultry industries of Germany, Poland, and Lithuania, despite the fact that only low ANB concentrations were established (0.46 μg/kg of enrofloxacin in sample no. 8, 0.05 and 16.8 μg/kg of enrofloxacin and doxycycline, respectively, in sample no.14, and 2.06 μg/kg of enrofloxacin in sample no.18). For this reason, there is an ongoing search for new alternatives to ANB in the poultry industry. The most effective composition for the control of Salmonella tested in this study consists of thyme EO (1.0%) with the following LAB strains: LUHS122, LUHS242, LUHS210, LUHS244, LUHS135, LUHS71, and LUHS245. However, it should be mentioned that most of the tested LAB strains were inhibited by thyme EO at the concentrations of 0.5 and 1.0%, except for LUHS122, LUHS210, and LUHS245. Finally, it can be noted that further studies are needed to identify the particular metabolites of LAB that are the most effective agents for the control of Salmonella spp.
Acknowledgments
Part of this research is supported by the Baltic-German University Liaison Office by the German Academic Exchange Service (DAAD) with funds from the Foreign Office of the Federal Republic Germany.
Conflict of Interest Statement: The authors declare that they have no conflicts of interest.
Footnotes
Supplementary data associated with this article can be found in the online version at http://doi.org/10.1016/j.psj.2020.05.002.
Supplementary data
References
- Abbes C., Mansouri A., Landoulsi A. Synergistic effect of the Lactoperoxidase system and cinnamon essential oil on total flora and Salmonella growth inhibition in raw milk. J. Food Qual. 2018;3:1–6. [Google Scholar]
- Adetoye A., Pinloche E., Bolanle A.A., Ayeni F.A. Characterization and anti-salmonella activities of lactic acid bacteria isolated from cattle faeces. BMC Microbiol. 2018;18:96. doi: 10.1186/s12866-018-1248-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed Z., Vohra M.S., Khan M.N., Ahmed A., Khan T.A. Antimicrobial role of Lactobacillus species as potential probiotics against enteropathogenic bacteria in chickens. J. Infect Dev. Ctries. 2019;13:130–136. doi: 10.3855/jidc.10542. [DOI] [PubMed] [Google Scholar]
- Allen A., Flemström G. Gastroduodenal mucus bicarbonate barrier: protection against acid and pepsin. Am. J. Physiol. Cell Physiol. 2005;288:1–19. doi: 10.1152/ajpcell.00102.2004. [DOI] [PubMed] [Google Scholar]
- Andrés S., Vico J.P., Garrido V., Grilló M.J. Epidemiology of subclinical salmonellosis in wild birds from an area of high prevalence of pig salmonellosis: phenotypic and genetic profiles of Salmonella isolates. Zoonoses Public Health. 2013;60:355–365. doi: 10.1111/j.1863-2378.2012.01542.x. [DOI] [PubMed] [Google Scholar]
- APVMA Veterinary Drug Residues in Food Commodities and Overseas Trade. Accessed Oct. 2020. 2014. https://apvma.gov.au/node/669
- Arsi K., Donoghue D.J., Venkitanarayanan K., Donoghue A.M. Food Safety in Poultry Meat Production. Springer; Cham: 2019. Reducing Foodborne pathogens in organic poultry: Challenges and Opportunities; pp. 25–46. [Google Scholar]
- Ashraf S., Anjum A.A., Ahmad A., Firyal S., Sana S., Latif A.A. In vitro activity of Nigella sativa against antibiotic resistant Salmonella enterica. Environ. Toxicol. Pharmacol. 2018;58:54–58. doi: 10.1016/j.etap.2017.12.017. [DOI] [PubMed] [Google Scholar]
- Baba E., Nagaishi S., Fukata T., Arakawa A. The role of the intestinal microflora on the prevention of Salmonella colonisation in gnotobiotic chickens. Poult. Sci. 1991;70:1902–1907. doi: 10.3382/ps.0701902. [DOI] [PubMed] [Google Scholar]
- Bartkiene E., Ruzauskas M., Lele V., Zavistanaviciute P., Bernatoniene J., Jakstas V., Ivanauskas L., Zadeike D., Klupsaite D., Viskelis P., Bendoraitiene J. Development of antimicrobial gummy candies with addition of bovine colostrum, essential oils and probiotics. Int. J. Food Sci. Tech. 2018;53:1227–1235. [Google Scholar]
- Bartkiene E., Zavistanaviciute P., Lele V., Ruzauskas M., Bartkevics V., Bernatoniene J., Gallo P., Tenore G.C., Santini A. Lactobacillus plantarum LUHS135 and paracasei LUHS244 as functional starter cultures for the food fermentation industry: Characterisation, mycotoxin-reducing properties, optimisation of biomass growth and sustainable encapsulation by using dairy by-products. LWT. 2018;93:649–658. [Google Scholar]
- Bartkiene E., Lele V., Sakiene V., Zavistanaviciute P., Ruzauskas M., Bernatoniene J., Jakstas V., Viskelis P., Zadeike D., Juodeikiene G. Improvement of the antimicrobial activity of lactic acid bacteria in combination with berries/fruits and dairy industry by-products. J. Sci. Food Agric. 2019;99:3992–4002. doi: 10.1002/jsfa.9625. [DOI] [PubMed] [Google Scholar]
- Bassole I.H.N., Juliani H.R. Essential oils in combination and their antimicrobial properties. Molecules. 2012;17:3989–4006. doi: 10.3390/molecules17043989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship L.C., Bailey J.S., Cox N.A., Stern N.J., Brewer R., Williams O. Two-step mucosal competitive exclusion flora treatment to diminish Salmonellae in commercial broiler chickens. Poult. Sci. 1993;72:1667–1672. doi: 10.3382/ps.0721667. [DOI] [PubMed] [Google Scholar]
- Burkholder K.M., Fletcher D.H., Gileau L., Kandolo A. Lactic acid bacteria decrease Salmonella enterica Javiana virulence and modulate host inflammation during infection of an intestinal epithelial cell line. Pathog. Dis. 2019;77:3. doi: 10.1093/femspd/ftz025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt S. Essential oils: their antimicrobial properties and potential applications in foods—a review. Int. J. Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Chan C.L., Gan R.Y., Shah N.P., Corke H. Polyphenols from selected dietary spices and medicinal herbs differentially affect common food-borne pathogenic bacteria and lactic acid bacteria. Food Contr. 2018;92:437–443. [Google Scholar]
- Clavijo V., Flórez M.J.V. The gastrointestinal microbiome and its association with the control of pathogens in broiler chicken production: a review. Poult. Sci. 2018;97:1006–1021. doi: 10.3382/ps/pex359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLSI (Clinical and Laboratory Standards Institute) 10th ed. Vol. 32. CLSI; Wayne, PA: 2015. Approved Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; p. 2. (CLSI Document M07-A10). [Google Scholar]
- Corrier D.E., Hinton A., Jr., Ziprin R.L., Beier R.C., DeLoach J.R. Effect of dietary lactose on cecal pH, bacteriostatic volatile fatty acids, and Salmonella typhimurium colonization of broiler chicks. Avian Dis. 1990;34:617–625. [PubMed] [Google Scholar]
- De Oliveira S.D., Flores F.S., Dos Santos L.R., Brandelli A. Antimicrobial resistance in Salmonella enteritidis strains isolated from broiler carcasses, food, human and poultry-related samples. Int. J. Food Microbiol. 2005;97:297–305. doi: 10.1016/j.ijfoodmicro.2004.04.022. [DOI] [PubMed] [Google Scholar]
- de Vrese M., Schrezenmeir J. Probiotics, prebiotics, and synbiotics. In: Stahl U., Donalies U.E., Nevoigt E., editors. Food Biotechnology. Advances in Biochemical Engineering/Biotechnology. Vol. 111. Springer; Berlin, Heidelberg: 2008. [Google Scholar]
- Di Pasqua R., Betts G., Hoskins N., Edwards M., Ercolini D., Mauriello G. Membrane toxicity of antimicrobial compounds from essential oils. J Agric Food Chem. 2007;55:4863–4870. doi: 10.1021/jf0636465. [DOI] [PubMed] [Google Scholar]
- Dittoe D.K., Ricke S.C., Kiess A.S. Organic acids and potential for modifying the avian gastrointestinal tract and reducing pathogens and disease. Front. Vet. Sci. 2018;5:216. doi: 10.3389/fvets.2018.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebani V.V., Nardoni S., Bertelloni F., Tosi G., Massi P., Pistelli L., Mancianti F. In vitro antimicrobial activity of essential oils against Salmonella enterica serotypes enteritidis and typhimurium strains isolated from poultry. Molecules. 2019;24:900. doi: 10.3390/molecules24050900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshaghabee F.M.F., Bockelmann W., Meske D., Vrese M.D., Walte H.G., Schrezenmeir J., Heller K.J. Ethanol production by selected intestinal microorganisms and lactic acid bacteria growing under different nutritional conditions. Front. Microbiol. 2016;7:47. doi: 10.3389/fmicb.2016.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission Establishment of Maximum Residue Limits (MRLs) for Residues of Veterinary Medicinal Products in Foodstuffs of Animal Origin. 2001. https://ec.europa.eu/health/veterinary-use/maximum-residue-limits/developments_en Accessed Oct. 2020.
- European Food Safety Authority and European Centre for Disease Prevention and Control The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2016. EFSA J. 2017;15:5077. doi: 10.2903/j.efsa.2017.5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz C., Baser K. Windisch. Essential oils and aromatic plants in animal feeding-a European perspective. A review. Flavour Fragr. J. 2010;25:327–340. [Google Scholar]
- Gadde U.D., Oh S., Lillehoj H.S., Lillehoj E.P. Antibiotic growth promoter’s virginiamycin and bacitracin methylene disalicylate alter the chicken intestinal metabolome. Sci. Rep. 2018;8:3592. doi: 10.1038/s41598-018-22004-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gaggia F., Mattarelli P., Biavati B. Probiotics and prebiotics in animal feeding for safe food production. Int. J. Food Microbiol. 2010;141:S15–S28. doi: 10.1016/j.ijfoodmicro.2010.02.031. [DOI] [PubMed] [Google Scholar]
- García-Quintáns N., Repizo G., Martín M., Magni C., López P. Activation of the diacetyl/acetoin pathway in Lactococcus lactis subsp. lactis bv. diacetylactis CRL264 by acidic growth. Appl. Environ. Microbiol. 2008;74:1988–1996. doi: 10.1128/AEM.01851-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haquea M.A., Wanga Y., Shenc Z., Lia X., Saleemid M.K., Hea C. Mycotoxin contamination and control strategy in human, domestic animal and poultry: a review. Microb. Pathogenesis. 2020;142:104095. doi: 10.1016/j.micpath.2020.104095. [DOI] [PubMed] [Google Scholar]
- Higgins J.P., Higgins S.E., Vicente J.L., Wolfenden A.D., Tellez G., Hargis B.M. Temporal effects of lactic acid bacteria probiotic culture on Salmonella in Neonatal broilers. Poult. Sci. 2007;86:1662–1666. doi: 10.1093/ps/86.8.1662. [DOI] [PubMed] [Google Scholar]
- Hippenstiel F., Abdel-Wareth A., Kehraus S., Südekum K. Effects of selected herbs and essential oils, and their active components on feed intake and performance of broilers-a review. Arch. Geflügelk. 2011;75:226–234. [Google Scholar]
- Impey C.S., Mead G.C., George S.M. Evaluation of treatment with defined and undefined mixtures of gut microorganisms for preventing Salmonella colonization in chicks and Turkey poults. Food Microbiol. 1984;1:143–147. [Google Scholar]
- Ingram L.O. Ethanol tolerance in bacteria. Crit. Rev. Biotechnol. 1989;9:305–319. doi: 10.3109/07388558909036741. [DOI] [PubMed] [Google Scholar]
- Ismail M., Kemegne G.A., Njayou F.N., Penlap V., Mbacham W.F., Kamdem S.L.S. Chemical composition, antibiotic promotion and in vivo toxicity of Piper nigrum and Syzygium aromaticum essential oil. Afr. J. Biochem. Res. 2017;11:58–71. [Google Scholar]
- ISO . International Organization for Standardization; Geneva, Switzerland: 1998. Microbiology of Food and Animal Feeding Stuffs - Horizontal Method for the Enumeration of Mesophilic Lactic Acid Bacteria - Colony-count Technique at 30 Degrees C; p. 15214. [Google Scholar]
- Jiang W., Wang Z., Beier R.C., Jiang H., Wu Y., Shen J. Simultaneous determination of 13 fluoroquinolone and 22 sulfonamide residues in milk by a dual-colorimetric enzyme-linked immunosorbent assay. Anal. Chem. 2013;85:1995–1999. doi: 10.1021/ac303606h. [DOI] [PubMed] [Google Scholar]
- Kabir S.M.L. The role of probiotics in the poultry industry. Int. J. Mol. Sci. 2009;10:3531–3546. doi: 10.3390/ijms10083531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriebel D., Tickner J., Epstein P., Lemons J., Levins R., Loechler E.L., Quinn M., Rudel R., Schettler T., Stoto M. The precautionary principle in environmental science. Environ. Health Perspect. 2001;109:871–876. doi: 10.1289/ehp.01109871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laxminarayan R., Heymann D.L. Challenges of drug resistance in the developing world. BMJ. 2012;344:e1567. doi: 10.1136/bmj.e1567. [DOI] [PubMed] [Google Scholar]
- Lele V., Ruzauskas M., Zavistanaviciute P., Laurusiene R., Rimene G., Kiudulaite D., Tomkeviciute J., Nemeikstyte J., Stankevicius R., Bartkiene E. Development and characterization of the gummy–supplements, enriched with probiotics and prebiotics. Cyta-J Food. 2018;16:580–587. [Google Scholar]
- Lin L., Xue L., Haiying C. Cold plasma treated thyme essential oil/silk fibroin nanofibers against Salmonella Typhimurium in poultry meat. Food Packaging Shelf. 2019;21:2214–2894. [Google Scholar]
- Lindgren S.E., Dobrogosz W.J. Antagonistic activities of lactic acid bacteria in food and feed fermentations. FEMS Microbiol. 1990;87:149–164. doi: 10.1111/j.1574-6968.1990.tb04885.x. [DOI] [PubMed] [Google Scholar]
- Mellor K.C., Petrovska L., Thomson N.R., Harris K., Reid S.W.J., Mather A.E. Antimicrobial resistance Diversity suggestive of Distinct Salmonella typhimurium sources or selective pressures in food-production animals. Front. Microbiol. 2019;10:708. doi: 10.3389/fmicb.2019.00708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino L., Trejo F.M., De Antoni G., Golowczyc M.A. Lactobacillus strains inhibit biofilm formation of Salmonella sp. isolates from poultry. Food Res. Int. 2019;123:258–265. doi: 10.1016/j.foodres.2019.04.067. [DOI] [PubMed] [Google Scholar]
- Mitchell C., Fredricks D., Agnew K., Hitti J. Hydrogen peroxideproducing lactobacilli are associated with lower levels of vaginal interleukin-1b, independent of bacterial vaginosis. Sex. Transm. Dis. 2015;42:358–363. doi: 10.1097/OLQ.0000000000000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadreza K., Seyyed-Hamed H., Faramin J., Mehran N., Alireza S., Isam T.K., Vito L., Vincenzo T. Effects of dietary chicory (chicorium intybus L.) and probiotic Blend as natural feed Additives on performance traits, Blood Biochemistry, and gut microbiota of broiler chickens. Antibiotics. 2020;9:5. doi: 10.3390/antibiotics9010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad J., Khan S., Su J.Q., Hesham A.E.L., Ditta A., Nawab J., Ali A. Antibiotics in poultry manure and their associated health issues: a systematic review. J. Soils Sediments. 2020;20:486–497. [Google Scholar]
- Mungroo N.A., Neethirajan S. Biosensors for the detection of antibiotics in poultry industry - a review. Biosensors. 2014;4:472–493. doi: 10.3390/bios4040472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Office of Animal Health Ltd. (NOAH). Maximum Residue Limits (MRLs) and the Safety of Food from Animals. 2016. http://www.noah.co.uk/issues/briefingdoc/09-mrls.htm Accessed Sep. 2020.
- Neveling D.P., Ahire J.J., Laubscher W., Rautenbach M., Dicks L.M.T. Genetic and phenotypic characteristics of a multi-strain probiotic for broilers. Curr. Microbiol. 2020;77:369–387. doi: 10.1007/s00284-019-01797-3. [DOI] [PubMed] [Google Scholar]
- O’Neill J. Tackling a Crisis for the Health and Wealth of Nations; 2014. Antimicrobial Resistance. [Google Scholar]
- Olaimat A.N., Al-Holy M.A., Abu Ghoush M.H., Al-Nabulsi A.A., Osaili T.M., Holley R.A. Inhibitory effects of cinnamon and thyme essential oils against Salmonella spp. in hummus (chickpea dip) J. Food Process. Pres. 2019;43:13925. [Google Scholar]
- Patterson J.A., Burkholder K.M. Application of prebiotics and probiotics in poultry production. Poult. Sci. 2003;82:627–631. doi: 10.1093/ps/82.4.627. [DOI] [PubMed] [Google Scholar]
- Pavlov A., Lashev L., Rusev V. Studies on the residue levels of tobramycin in stored poultry products. Trakia J. Sci. 2005;3:20–22. [Google Scholar]
- Pugajeva I., Avsejenko J., Judjallo E., Bērziņš A., Bartkiene E., Bartkevics V. High occurrence rates of enrofloxacin and ciprofloxacin residues in retail poultry meat revealed by an ultra-sensitive mass-spectrometric method, and antimicrobial resistance to fluoroquinolones in Campylobacter spp. Food Addit Contam A. 2018;35:1107–1115. doi: 10.1080/19440049.2018.1432900. [DOI] [PubMed] [Google Scholar]
- Rantala M., Nurmi E. Prevention of the growth of Salmonella infantis in chicks by the flora of the alimentary tract of chickens. Br. Poult. Sci. 1973;14:627–630. doi: 10.1080/00071667308416073. [DOI] [PubMed] [Google Scholar]
- Reinholds I., Pugajeva I., Perkons I., Bartkevics V. The application of phospholipid removal columns and ultra-high performance liquid chromatography—tandem quadrupole mass spectrometry for quantification of multi-class antibiotics in aquaculture samples. J. Pharm. Biomed. Anal. 2016;128:126–131. doi: 10.1016/j.jpba.2016.05.002. [DOI] [PubMed] [Google Scholar]
- Rishi P., Preet Singh A., Garg N., Rishi M. Evaluation of nisin–b-lactam antibiotics against clinical strains of Salmonella enterica serovar. Typhi. J. Antibiot. 2014;67:807–811. doi: 10.1038/ja.2014.75. [DOI] [PubMed] [Google Scholar]
- Salehizadeh M., Modarressi M.H., Mousavi S.N., Ebrahim M.T. Evaluation of lactic acid bacteria isolated from poultry feces as potential probiotic and its in vitro competitive activity against Salmonella typhimurium. Vet. Res. Forum. 2020;11:67–75. doi: 10.30466/vrf.2018.84395.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnürer J., Magnusson J. Antifungal lactic acid bacteria as biopreservatives. Trends Food Sci. Technol. 2005;16:70–78. [Google Scholar]
- Shang K., Wei B., Jang H.K., Kang M. Phenotypic characteristics and genotypic correlation of antimicrobial resistant (AMR) Salmonella isolates from a poultry slaughterhouse and its downstream retail markets. Food Control. 2019;100:35–45. [Google Scholar]
- Simitzis P.E., Bronis M., Charismiadou M.A., Mountzouris K.C., Deligeorgis S.G. Effect of cinnamon (Cinnamomum zeylanicum) essential oil supplementation on lamb growth performance and meat quality characteristics. Animal. 2014;8:1554–1560. doi: 10.1017/S1751731114001335. [DOI] [PubMed] [Google Scholar]
- Singer R.S., Hofacre C.L. Potential impacts of antibiotic use in poultry production. Avian Dis. 2006;50:161–172. doi: 10.1637/7569-033106R.1. [DOI] [PubMed] [Google Scholar]
- Singh A.P., Preet S., Rishi P. Nisin/beta-lactam adjunct therapy against Salmonella enterica serovar Typhimurium: a mechanistic approach. J. Antimicrob. Chemother. 2014;69:1877–1887. doi: 10.1093/jac/dku049. [DOI] [PubMed] [Google Scholar]
- Singh S., Yadav A.S., Singh A.,S.M., Bharti P. Prevalence of Salmonella in chicken eggs collected from poultry farms and marketing channels and their antimicrobial resistance. Food Res. Int. 2010;43:2027–2030. [Google Scholar]
- Singh V.P. Recent approaches in food bio-preservation-a review. Open Vet. J. 2018;8:104–111. doi: 10.4314/ovj.v8i1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soomro R.N., Abd El-Hack M.E., Shah S.S., Taha A.E., Alagawany M., Swelum A.A., Hussein E.O.S., Ba-Aawdh H.A., Saadeldin I., El-Edel M.A. Impact of restricting feed and probiotic supplementation on growth performance, mortality and carcass traits of meat-type quails. Anim. Sci. J. 2019;90:1388–1395. doi: 10.1111/asj.13290. [DOI] [PubMed] [Google Scholar]
- Stern N.J., Cox N.A., Bailey J.S., Berrang M.E., Musgrove M.T. Comparison of mucosal competitive exclusion and competitive exclusion treatment to reduce Salmonella and Campylobacter spp. colonization in broiler chickens. Poult. Sci. 2001;80:156–160. doi: 10.1093/ps/80.2.156. [DOI] [PubMed] [Google Scholar]
- Surendran N.M., Amalaradjou M.A., Venkitanarayanan K. Antivirulence properties of probiotics in combating microbial Pathogenesis. Adv. Appl. Microbiol. 2017;98:1–29. doi: 10.1016/bs.aambs.2016.12.001. [DOI] [PubMed] [Google Scholar]
- Suresh G., Das R.K., Kaur-Brar S., Rouissi T., Avalos Ramirez A., Chorfi Y., Godbout S. Alternatives to antibiotics in poultry feed: molecular perspectives. Crit. Rev. Microbiol. 2018;44:318–335. doi: 10.1080/1040841X.2017.1373062. [DOI] [PubMed] [Google Scholar]
- Suresh G., Santos D.U., Rouissi T., Satinder K.B., Mehdi Y., Godbout S., Chorfi Y., A Ramirez A. Production and in-vitro evaluation of an enzyme formulation as a potential alternative to feed antibiotics in poultry. Process. Biochem. 2019;80:9–16. [Google Scholar]
- Thanissery R., Kathariou S., Smith D.P. Rosemary oil, clove oil, and a mix of thyme-orange essential oils inhibit Salmonella and Campylobacter in vitro. J. Appl. Poult. Res. 2014;23:23–221. [Google Scholar]
- Thomas J.V., Nair D.V.T., Noll S., Johnson T.J., Cardona C., Johny A.K. Effect of Turkey-Derived beneficial bacteria Lactobacillus salivarius and Lactobacillus ingluviei on a Multidrug-resistant Salmonella Heidelberg strain in Turkey poults. J. Food Prot. 2019;82:435–440. doi: 10.4315/0362-028X.JFP-18-286. [DOI] [PubMed] [Google Scholar]
- Velasquez C.G., Macklin K.S., Kumar S., Bailey M., Ebner P., Oliver H.F. Prevalence and antimicrobial resistance patterns of Salmonella isolated from poultry farms in southeastern United States. Poult. Sci. 2018;97:2144–2152. doi: 10.3382/ps/pex449. [DOI] [PubMed] [Google Scholar]
- Vieco-Saiz N., Belguesmia Y., Raspoet R., Auclair E., Gancel F., Kempf I., Drider D. Benefits and Inputs from lactic acid bacteria and their bacteriocins as alternatives to antibiotic growth promoters during food-animal production. Front. Microbiol. 2019;10:57. doi: 10.3389/fmicb.2019.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinueza-Burgos C., Baquero M., Medina J., De Zutter L. Occurrence, genotypes and antimicrobial susceptibility of Salmonella collected from the broiler production chain within an integrated poultry company. Int. J. Food Microbiol. 2019;299:1–7. doi: 10.1016/j.ijfoodmicro.2019.03.014. [DOI] [PubMed] [Google Scholar]
- Wales A.D., Carrique-Mas J.J., Rankin M., Bell B. Review of the carriage of zoonotic bacteria by arthropods, with special reference to Salmonella in mites, flies and litter beetles. Zoonoses Public Health. 2010;57:299–314. doi: 10.1111/j.1863-2378.2008.01222.x. [DOI] [PubMed] [Google Scholar]
- Wang L., Liu C., Chen M., Ya T., Huang W., Gao P., Zhang H. A novel Lactobacillus plantarum strain P-8 activates beneficial immune response of broiler chickens. Int. Immunopharmacol. 2015;29:901–907. doi: 10.1016/j.intimp.2015.07.024. [DOI] [PubMed] [Google Scholar]
- Wealleans A.L., Li W., Romero L.F., Mathis G., Lumpkins B. Performance and cost-benefit improvements following supplementation with a combination of direct-fed microbials and enzymes to broiler chickens raised with or without ionophores. J. Appl. Poult. 2018;27:23–32. [Google Scholar]
- Wegener H.C., Hald T., Wong D.L.F., Madsen M., Korsgaard H., Bager F., Gerner-Smidt P., Mølbak K. Salmonella control programs in Denmark. Emerg. Infect. Dis. 2003;9:774–780. doi: 10.3201/eid0907.030024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai H., Liu H., Wang S., Wu J., Kluenter A.M. Potential of essential oils for poultry and pigs. Anim. Nutr. 2018;4:179–186. doi: 10.1016/j.aninu.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhitnitsky D., Rose J., Lewinson O. The highly synergistic, broad spectrum, antibacterial activity of organic acids and transition metals. Sci. Rep. 2017;7:445454. doi: 10.1038/srep44554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu A., Zhi W., Qiu Y., Wei L., Tian J., Pan Z., Kang X., Gu W., Duan L. Surveillance study of the prevalence and antimicrobial resistance of Salmonella in pork from open markets in Xuzhou, China. Food Cont. 2019;98:474–480. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


