Abstract
Suspected and confirmed cases should be treated in a designated hospital with effective isolation and protective conditions. The isolation condition of suspected cases should be the highest, and treatment should be carried out in a single room instead of mixed accommodation. Only confirmed cases should be admitted to the same ward, and critically ill patients should be admitted to ICU as soon as possible. At this stage, asymptomatic infected persons should also be isolated for observation. If a severe epidemic occurs in the area and medical resources are limited, mild cases and asymptomatic infected persons can be treated and observed at home, but registration and management should be carried out by the local disease prevention and control institutions and community health service centers, so as to guide, observe, and treat the quarantine at home. Moreover, the referral and transfer of severe patients should be safe, evaluated well, and no problems should be caused on the way.
Keywords: ICU, Lopinavir, Ritonavir, HFNC, Respiratory therapists, Isolation
1. Site of care
Suspected and confirmed cases should be treated in a designated hospital with effective isolation and protective conditions. The isolation condition of suspected cases should be the highest, and treatment should be carried out in a single room instead of mixed accommodation. Only confirmed cases should be admitted to the same ward, and critically ill patients should be admitted to ICU as soon as possible. At this stage, asymptomatic infected persons should also be isolated for observation. If a severe epidemic occurs in the area and medical resources are limited, mild cases and asymptomatic infected persons can be treated and observed at home, but registration and management should be carried out by the local disease prevention and control institutions and community health service centers, so as to guide, observe, and treat the quarantine at home. Moreover, the referral and transfer of severe patients should be safe, evaluated well, and no problems should be caused on the way.
2. General care
Rest in bed, strengthen supportive treatment, adequate caloric intake, pay attention to water and electrolyte balance, and maintain internal environment stability. At the same time, close follow-up should be conducted to observe patients' respiration, monitor blood oxygen saturation, and observe changes in body temperature. Attention should be paid to those patients who have changed from mild to severe or critical, especially the elderly, obese, patients with diabetes, hypertension, coronary heart disease, COPD, etc. According to the conditions, blood routine, urine routine, c-reactive protein, biochemical indicators (liver enzyme, cardiac enzyme, renal function, etc.), and coagulation function should be monitored when necessary, as well as arterial blood gas analysis and chest imaging being performed if required.
3. Antivirals
What is fundamental to an infectious disease? The bottom line is to treat the cause of disease. For COVID-19, antiviral therapy is a top priority, in both mild and severe cases. Without treatment and intervention for its cause, other treatments are very passive, so everyone is trying their best to suppress the virus at work. In fact, what we have observed is that the existence and detoxification times of coronavirus in the body are very long, and a number of body autopsies show a large number of virus particles and virus inclusion bodies in the alveolar cavity and pulmonary septum of the patient. Can other treatments work without clearing away the virus in severe patients? In addition, if the inflammation is due to excessive inflammation caused by the virus, if you do not get rid of the virus, can only immunosuppressive agents be effective? Respiratory support is an excellent tool to provide patients with valuable recovery time. But not every patient is so lucky. Lung damage in some patients is so severe that even ECMO cannot reverse it. This should remind us that etiology is the most important part of the treatment.
At present, there is no effective antiviral drug. α-interferon inhalation can be tried (adults 5 million IU each time, adding 2 mL of sterilized water for injection, twice a day) as a treatment course of at least 5 days, but no immediate effect of α-interferon atomization has been observed so far. Notably, the interferon atomization must be performed with an air compression pump, because it can make the particles smaller—such as PM2.5 or PM5 levels—so that they can reach the alveoli. Lopinavir (200 mg) and ritonavir (50 mg) were originally used for the combination treatment of HIV, being HIV protease inhibitors and active peptide inhibitors, respectively, and the latter can increase the drug concentration of the former. Because lopinavir/ritonavir is a protease inhibitor with the ability to inhibit the formation of viral nucleic acids and has been used to treat HIV, it is also considered as a candidate drug for treating COVID-19, and has been published. This study is the first clinical trial for the treatment of COVID-19 published in the world's top medical journal since the start of the outbreak. It is also one of the few clinical trials of a drug published during the outbreak of a new infectious disease, including SARS, in the last 20 years. The accompanying NEJM editorial hailed Chinese researchers as heroes for conducting rigorous clinical trials in the midst of such a difficult outbreak. After a rigorous and scientific RCT study, it has been proved that this drug has a certain effect, but its effect is not significant. In terms of safety evaluation, the lopinavir/ritonavir group had a higher incidence of gastrointestinal adverse events. According to the results of this study, more than 40% of patients who received lopinavir/ritonavir did not have their viral nucleic acid turn negative after the 2 weeks of observation, suggesting that the antiviral effect of lopinavir/ritonavir may be limited or the course of the treatment is insufficient. On the other hand, the virus may be infectious for a long time. Some clinical observations have shown that the average time of virus turning negative is 20 days—not as short as we expected. Moreover, we initially observed that the nucleic acid turns negative after more than 30 days. In the future, detailed observations should be made, including multisite sampling, to see if viral nucleic acids persist for a longer time. This is the disease pattern that we need to understand further. Does the course of lopinavir/ritonavir treatment need to be extended? An extension may be necessary until viral nucleic acids become negative. According to data from the clinical study, lopinavir/ritonavir is effective, has some side effects, but can be tolerated; in addition, the course of the treatment may need to be longer.
Avoiding adverse reactions is quite important for the use of lopinavir/ritonavir; these include liver function damage, diarrhea, and nausea in patients with obvious gastrointestinal symptoms. It is also necessary to avoid interactions with other clinical drugs, especially to avoid combined use of simvastatin and erythromycin, which may be used by some elderly patients complicated with chronic obstructive pulmonary disease, hypertension, hyperlipidemia, and other diseases. It is necessary to pay attention to the interaction between drugs so as not to aggravate liver function damage as combined utilities. When lopinavir/ritonavir is used in combination with erythromycin, it may cause some symptoms of arrhythmias with prolonged QT intervals.
Until the results of a rigidly designed, randomized, double-blind trial are known we cannot predict how effective or ineffective remdesivir will be, or how much it can suppress the COVID-19 virus. Whether remdesivir is ultimately shown to be effective or not will have direct implications for clinical treatment.
4. Glucocorticoids
At present, there is great controversy about the application of hormones: some think that hormones are effective, while others think that hormones do more harm than good. Although there is controversy, it is undeniable that hormones are commonly used in the treatment of viral pneumonia. Evidence-based medicine for the treatment of viral pneumonia is controversial. Most of these studies are retrospective studies with poor comparability and insufficient evidence levels. As we all know, the level of evidence in retrospective studies is inadequate and its comparability is poor. A good study requires large-scale, multicenter, randomized, double-blind, controlled studies, but it is too difficult to do according to this design. Hormone dosage, timing, usage, and duration vary. For example, patients have increased mortality after using hormones, mainly because many patients are already very sick when using hormones, while mildly ill patients do not need to use hormones. This leads to an unscientific conclusion that the mortality of patients who use hormones is high. The high mortality rate is not caused by hormone treatment, but it may be caused by severe illness. At the present stage in the fever clinic and ward, because some patients cannot be admitted to the hospital in time, or they come to see the doctor when their condition is very serious, these patients may not be able to get timely treatment, and may die within 2 or 3 days. At this time, although doctors use hormones, the effect has not been revealed. If we do research at this time, we will find that the mortality rate of these patients increased after the use of hormones. So, at this time, our conclusion may not be scientific. In addition, if the usage and dosage of hormones are not standardized, the conclusions of the study may also be biased. For patients with novel coronavirus pneumonia, how should hormones be used? From our experience, hormones should not be used in patients with early, mild symptoms, such as within 7 days of infection, or between 7 and 10 days, as this will accelerate the replication of the virus. When the patients are at the peak of the disease—that is, if the patients have sustained high fever, obvious dyspnea, hypoxemia, and obvious progression of chest imaging lesions after 7–10 days—it may be the best time to use steroids. In some patients with COVID-19, the imaging manifestations are of organizing pneumonia, which is sensitive to hormones, and this is a very good time to use steroids. As described below, there are two cases of hormone treatment to help patients survive from the period of hypoxemia.
Case 1: 77-year-old, male, admitted to hospital on January 10, 2020. He became ill in January. He was admitted to hospital after 1 week of fatigue and 4 days of fever. Past history: diabetes for 22 years, treated by long-term oral metformin; hypertension history for 20 years. Fig. 5.1 shows his chest image on January 9, 2020, with multiple ground-glass shadows in both lungs.
Fig. 5.1.
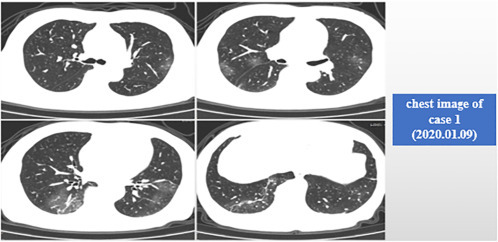
Chest image of case 1 (January 9, 2020).
By January 14, 2020, the ninth day of disease onset, the patient's chest CT showed a significant progression in ground-glass shadows (Fig. 5.2 ). The study found that many cases tend to progress during 7–10 days from onset of the disease, or even after 10 days, but the degree of progression varies. Some patients presented with increased dyspnea and respiratory failure, while others presented with increased imaging findings, but no obvious respiratory failure. The patient's chest CT scan on January 9 and January 14 showed significant exacerbation within 5 days, clinically manifested with fever, dyspnea, and hypoxemia. So for this patient, we applied the ventilator in time.
Fig. 5.2.
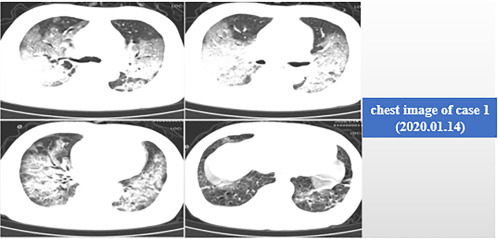
Chest image of case 1 (January 14, 2020).
As shown in Fig. 5.3 , from January 10, 2020, we applied methylprednisolone with an initial dose of 60 mg per day, but the condition did not improve. So we increased the hormone level to 80 mg per day (40 mg per time, twice a day), but the patient's condition continued to deteriorate. On January 14, the patient developed respiratory failure. We applied methylprednisolone to 160 mg per day (80 mg per time, twice a day), and the patient's condition gradually controlled and remained stable. Then we gradually reduced the dose of hormone.
Fig. 5.3.
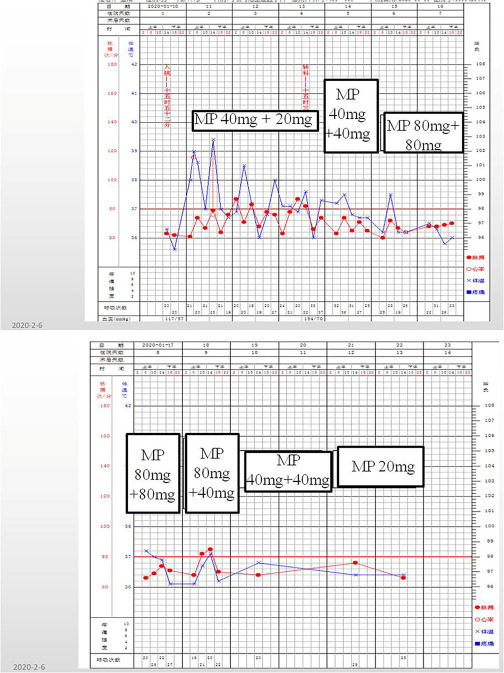
Hormone utilization and changes of vital signs in case 1 (January 10, 2020). MP: methylprednisolone.
Fig. 5.4 is the chest CT on January 18, 2020. After 4 days of treatment, including increased hormone doses, use of a noninvasive ventilator, and other antiinfective and antiviral treatments, the inflammatory exudation began to decrease. After overcoming this difficult period, the patient recovered and was discharged from hospital on January 29.
Fig. 5.4.
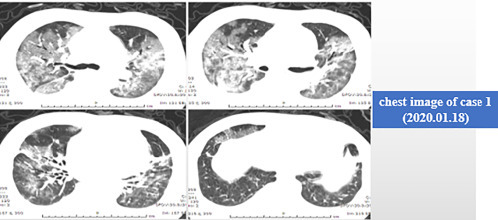
Chest CT image of case 1 (January 18, 2020).
Case 2: 77-year-old, male, with fever for 10 days, and admitted to hospital on January 7, 2020. The oxygen pressure on admission was 65 mmHg and the patient was immediately transferred to ICU and treated with noninvasive mechanical ventilation. Fig. 5.5 is the chest CT image on January 7, 2020, with ground-glass shadows in both lungs, characterized as organizing pneumonia. These patients responded well to the hormone. The patient was given methylprednisolone 80 mg per day for 7 days, and the dyspnea was quickly relieved. The patient's body temperature dropped and the condition was stabilized. A chest CT scan was performed on January 19, showing obvious absorption of the lesion (Fig. 5.6 ).
Fig. 5.5.

Chest CT image of case 2 (January 7,2020).
Fig. 5.6.
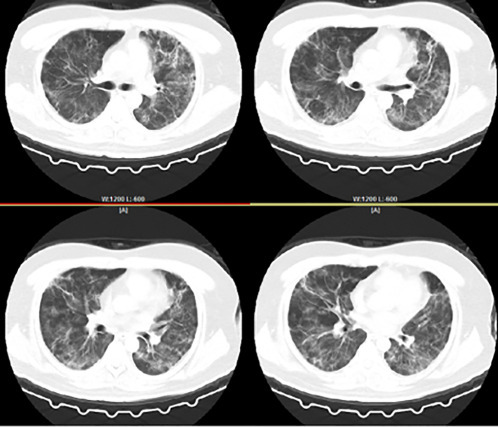
Chest CT image of case 2 (January 19, 2020).
Hormone use, if appropriate, can be beneficial for patients. Hormones will inhibit the immune function. Therefore, in the early stages of the disease, immunosuppressive agents should not be used. After 7–10 days, hormones can be added in cases with persistent fever, obvious dyspnea, hypoxemia, progressive imaging progression, or SOPs-like changes. Before using hormones, doctors must evaluate the cellular and humoral immune function of patients, and must evaluate the viral load, the patient's lymphocyte, T cell subsets, and NK cells. After comprehensive assessment, we can use hormones. If the patient's cellular immune system is compromised and the virus is still replicating, glucocorticoids should be used with care. The changes of lymphocyte, c-reactive protein, oxygenation index, and imaging manifestations should be closely observed after glucocorticoid administration. Generally, the period of glucocorticoids treatment should be 3–5 days, then the dosage should be reduced slowly after the improvement of illness, and the administration can be stopped. Large doses and prolonged time of usage should be avoided.
5. Respiratory support
5.1. General principles
According to the change of arterial oxygen saturation or peripheral capillary oxygen saturation, effective oxygen therapy should be given in time, including oxygen delivery by nasal catheter or face mask, and high-flow nasal cannula (HFNC) therapy, with noninvasive or invasive mechanical ventilation if necessary.
The respiratory support methods for severe COVID-19 patients are basically high-flow nasal cannula (HFNC) oxygen therapy, noninvasive mechanical ventilation, and invasive mechanical ventilation at the early stage, and extracorporeal membrane oxygenation (ECMO) at the advanced stage.
5.2. HFNC
High-flow nasal cannula is an effective way to correct patient's hypoxia. Patients with mild ARDS (oxygenation index 200–300 mmHg) or mild COVID-19 are recommended for use of HFNC, but its efficiency in improving hypoxia is inferior to that of a bilevel positive airway pressure (BiPAP) ventilator. Making a choice between HFNC and noninvasive ventilation depends mainly on patients’ tolerance and response to oxygen therapy: HFNC should be considered when patients are intolerant of a BiPAP ventilator or show rapid improvement in hypoxia once receiving HFNC therapy. Noninvasive ventilation is preferred when applicable and available.
Initial setting for HFNC: FiO2 is set to 100%, with a slow increase in flow rate from 30 L/min to 50 L/min. Consider using the ROX index [SpO2/(FiO2*RR)] to predict the success of HFNC.
-
(1)
Evaluate once every 2 h. An ROX index higher than 3.85 predicts a low risk of endotracheal intubation and high probability of success of HFNC, and therefore HFNC treatment is recommended to be continued.
-
(2)
If the ROX index at 2 h is less than 2.85, immediate endotracheal intubation should be considered.
-
(3)
If the ROX index at 2 h ranges between 2.85 and 3.85, continue HFNC treatment and reevaluate the ROX index at 6 h: continue HFNC treatment if the index at 6 h > 3.85, and perform immediate endotracheal intubation if < 3.85. Evaluate the Rox index again at 12 h: an index of > 4.88 indicates great probability of success of HFNC, and an index of < 4.88 suggests immediate endotracheal intubation.
The ROX index is used to determine optimal timing of the switch from HFNC to endotracheal intubation. It is safer to observe for the first 2 h, because longer observation time indicates possible worse effect of HFNC, which may lead to a delayed intubation. Additionally, it is necessary for the medical staff to observe at the bedside for a period of time to calculate the ROX index. Whether the endotracheal intubation timing of critical COVID-19 patients can be determined based on the ROX index only needs further observation and verification. The best threshold still needs to be explored. In general, the efficacy of HFNC on severe ARDS is extremely limited. The timing of endotracheal intubation should not be delayed due to a transient improvement in oxygenation index resulted from HFNC treatment.
5.3. Noninvasive mechanical ventilation
Attention should be paid to the use of noninvasive ventilators. Some hold that if a 2-h noninvasive ventilation does not yield an improvement in the oxygenation index, one should immediately change to invasive mechanical ventilation. Others insist that we should only consider invasive ventilation when noninvasive ventilation has proved to have no effect. Actually, appropriate use and careful adjustment could ensure effective noninvasive ventilation and save patients’ lives.
Respiratory failure in COVID-19 patients has resulted from ARDS or interstitial pneumonia. Invasive mechanical ventilation may attenuate ventilation/perfusion ratio imbalance, but could not improve diffusing capacity due to interstitial involvement. Worse, oxygenation was often observed when switching noninvasive ventilation to invasive ventilation. Inability to extubate within a short period may lead to prolonged invasive mechanical ventilation, and therefore increase the risk of ventilation-associated pneumonia, which contributes to high mortality. Some studies found that the mortality rate of COVID-19 patients using invasive mechanical ventilation is more than 90%; however, the use of a noninvasive ventilator requires medical staff to stay bedside for repeated adjustment, including checking the appropriateness of parameters and absence of gas leakage. Most noninvasive ventilation failure is associated with inadequacy of adjustment and premature discontinuation after a first failed attempt.
Here is a representative case. A 70-year-old female patient with COVID-19 was admitted for hospitalization. Her blood oxygen saturation dropped below 80% and stayed around 80% for more than 2 days after receiving noninvasive ventilation. Rather than performing intubation on this patient, according to the previous point of view, our nurse stayed at the bedside to adjust carefully the parameters of the noninvasive ventilator. With the help of antiviral drugs and steroids, blood oxygen saturation improved to 90% on the fourth day postnoninvasive ventilation, and even reached 97%–98% on the seventh day. If the patient had been intubated, it's very likely that she could not have been extubated within such a short period and a series of complications might have appeared. Therefore, time and effort should be put into ensuring successful application of noninvasive ventilation.
It is worth noting that patients with shortness of breath may not be able to cooperate with the noninvasive ventilator, so it is necessary to evaluate the patient in advance. If the patient is at risk of the disease progressing, early application of noninvasive ventilation should be considered. In some cases, patients' blood oxygen saturation on nasal catheter may reach 94%–95%, which seems to be a good condition. However, if a noninvasive ventilator is applied when the patient's respiratory rate is not that fast, it's easier to achieve good tolerance and patient-ventilator synchrony, so that when the patient's condition deteriorates, the ventilator will take effect. We need to raise awareness of the importance of introducing a noninvasive ventilator early.
Regarding initial settings of noninvasive ventilation, a patient with strong spontaneous respiratory drive has high minute ventilation, and therefore a PEEP of 5–8 cmH2O is sufficient, and it's not recommended to have the patient on a high level of PEEP at the early stage, such as 10 cmH2O, 12 cmH2O, or 14 cmH2O. An air leak that occurs around the mask will aggravate the patient's condition due to insufficient oxygen supply, and should be attached with utmost care. Another critical parameter is oxygen concentration. The recommended initial FiO2 for noninvasive ventilation, if applicable, is 1.0, with a gradual decrease, rather than an increase starting from 0.5 or 0.6, because patients’ early demand for oxygen supply is great.
There are many predictors for noninvasive ventilation failure, and special attention should be paid to changes in states of consciousness and hemodynamic abnormalities: loss of consciousness or occurrence of irritability after noninvasive ventilation indicates the urgent need for endotracheal intubation; a significant drop in blood pressure or hemodynamic instability when receiving noninvasive ventilation also requires timely intubation. After receiving noninvasive ventilation, some patients still maintain consciousness, and present attenuated dyspnea and increased oxygenation index, with normal hemodynamic parameters. However, severe respiratory muscle fatigue develops in those patients, and weaning from noninvasive ventilation for a short period of time to eat and drink may induce a rapid decrease in oxygen saturation, which is slow to recover even when noninvasive ventilation is continued. Such patients should be actively assessed for the necessity of invasive mechanical ventilation.
A series of studies on ARDS have shown that high tidal volume under noninvasive ventilation is an independent risk factor for predicting noninvasive ventilation failure, though no consensus has been reached on the threshold value, which ranges from 8 mL/kg, through 9 mL/kg, to 9.5 mL/kg of ideal body weight. Minute ventilation and tidal volume are two important factors for prediction. Minute ventilation is calculated as tidal volume (12 mL/kg of ideal body weight) times of measured respiratory rate. If the minute ventilation is maintained around 12 L, the tidal volume ranges between 8 and 10 mL/kg of ideal body weight, the respiratory rate is below 25 breathing per minute, and the oxygenation index is stable, noninvasive ventilation should continue to be used; otherwise, endotracheal intubation should be considered.
5.4. Invasive mechanical ventilation
5.4.1. The lung protective ventilation strategy should be strictly enforced
The initial setting of tidal volume is 6 mL/kg of ideal body weight, regardless of the ventilator mode, pressure-controlled or volume-controlled ventilation. We are inclined to choose pressure-controlled ventilation for COVID-19 patients, because 70%–80% of patients present excessive purulent and bloody airway secretions after endotracheal intubation. If it is not possible to clean the airway rapidly after endotracheal intubation, increased airway resistance will render it difficult to achieve an inhaling peak pressure of < 42 cmH2O or plateau pressure of < 30 cmH2O under volume-controlled ventilation.
Plateau pressure should be limited to < 30 cmH2O. If the plateau pressure exceeds 30 cmH2O, gradually reduce the tidal volume at a speed of 1 mL/kg of body weight, until the plateau pressure is < 30 cmH2O or the minimum of tidal volume is reduced to 4 mL/kg of body weight. In the meantime, ensure adequate alveolar ventilation and increase the respiratory rate to improve clearance of carbon dioxide (usually 5 breaths/min per change). In addition, pay attention to the ventilator waveform. Ideally, the patient's end expiratory flow will return to zero, which indicates that there is no hyperventilation or excessive gas trapping. The presence of end expiratory flow reflects incomplete expiration, which requires a reduction in respiratory rate or an adjustment of the inspiration expiration ratio to extend expiration time.
5.4.2. FiO2 is initially set at 100%
Initial PEEP settings are suggested in the ARDSnet FiO2-PEEP table. PEEP corresponding to a FiO2 of 6 L/min or 0.7 L/min is chosen as the initial setting, which should be 20 × FiO2 ± 2. In some cases, especially those having delayed intubation, lung injury will aggravate if the initial PEEP setting is too high, and therefore it is generally at a level of 10–12 cmH2O. It is not necessary to achieve a blood oxygen saturation (SpO2) at 100%, instead, SpO2 maintained at 88%–95% with early partial pressure of oxygen no less than 60 mmHg is sufficient for patients’ needs. Previously, we observed that the partial pressure of oxygen in a patient who underwent emergency intubation as a rescue measure with a FiO2 of 100% increased from 20 mmHg to 50 mmHg at the early stage, and gradually increased to 70–80 mmHg after 2 or 3 h. Patience is required for management of this kind of case.
Early use of prone ventilation does work. Many patients who had an oxygenation index of 50 mmHg showed significant improvement within 12 h and even reached an oxygenation index of 250 mmHg after endotracheal intubation with early prone ventilation. Almost all patients who were given early prone ventilation after intubation presented significantly improved in terms of their oxygenation index, but the efficacy of prone ventilation gradually declines after 96 h. In our department, prone ventilation is required to be initiated 3 h postendotracheal intubation when oxygen supply is stable, and should last at least 18 h a day.
After endotracheal intubation for invasive ventilation, pay attention to conservative fluid management and initiate prone ventilation when required.
Ensure adequate sedation and analgesia at an early stage. Considering the insufficient oxygen supply for ARDS lungs, achieving a deep sedation state helps to reduce the patient's whole body oxygen consumption. Additionally, early adequate sedation and analgesia can reduce the inflammatory storm brought on by excessive spontaneous breathing and avoid ventilator-induced lung injury.
Other aspects mainly include blood glucose management, blood pressure management, nutrition support, and so on.
5.5. ECMO
The principle of initiating ECMO is that the initiating timing should be based on static compliance (Cstat) and dynamic compliance (Cdyn). If possible, measure esophageal pressure and transpulmonary pressure.
The timing of ECMO initiation currently set by us is not necessarily accurate and is only for reference. For patients undergoing prone ventilation for 24 h, if the oxygenation index has no improvement and stays below 100 mmHg, and CO2 retention occurs with a PaCO2 of > 50 mmHg, initiating ECMO will be considered. In addition, if the patient develops an early and persistent intractable metabolic acidosis with pH < 7.2, ECMO initiation is also suggested. A large body of literature suggests that for patients receiving mechanical ventilation for more than 7 days with high levels of oxygen delivery, ECMO is of little significance and therefore is not recommended.
Once connected to the ECMO, the ventilator settings must be adjusted: respiratory rate is suggested to be at 8–10 breaths/min and no more than 20 breaths/min; adjust PEEP based on patient-specific changes in condition; if possible, reduce FiO2 to < 0.4 to avoid oxygen toxicity. Pay attention to management of all kinds of catheters to avoid bloodstream infection, especially for immunosuppressed patients.
5.6. Respiratory support and respiratory therapists in the treatment of severely and critically ill patients
The focus of respiratory support includes ordinary oxygen therapy (administration of oxygen via nasal cannula or face mask), delivering high flow oxygen through a nasal cannula, noninvasive ventilation, invasive ventilation, and ECMO. Airway management should also be given significant importance, including sputum suction and management of artificial airways. Currently, respiratory therapists (RTs) are urgently needed in critical care areas to help doctors and nurses to carry out more specialized respiratory support.
In the treatment of novel coronavirus pneumonia, RTs should play an important role in providing respiratory treatment, and pay special attention to ensure each treatment is standardized. Currently, there is still much room for improvement in oxygen supplement methods, including usage of nasal catheter, HFNC, and especially noninvasive ventilation. The efficacy of noninvasive ventilation depends largely on the users’ depth of knowledge and skillfulness in device settings. Clinical judgment should be adapted to the ability and level of your team in providing respiratory support, and you should identify the optimal timing for treatment.
In terms of airway management, COVID-19 patients are very likely to present airway mucus obstruction, with pathological changes of peripheral small-airway sputum bolt. Wetting the airway with water, which helps to remove phlegm, is of critical importance. In terms of application of apophlegmatic drugs, combining intravenous and oral medications is suggested, such as N-acecysteine and anningpai (eucalyptol, limonene, pinene) enteric soft capsules for oral route, and ambroxol for intravenous route.
6. Convalescent plasma therapy
In addition to drugs, another potential antiviral treatment is convalescent plasma therapy, a relatively old therapy. Convalescent plasma is an important therapeutic option for humans to deal with emerging infectious diseases, especially when there is limited research on novel drug development. Convalescent plasma should theoretically be effective, as plasma derived from recently recovered patients contains certain neutralizing antibodies, which may be able to fight, neutralize, and eliminate the virus. The application of plasma therapy in emerging infectious diseases, which currently include SARS, avian influenza, MERS, and others, has a history of more than 100 years. Some clinical trials have suggested that convalescent plasma therapy is effective, while no obvious effect was observed in studies on Ebola virus disease. No specific treatment for COVID-19 is currently available, and therefore convalescent plasma treatment could be an important option for rescuing severe patients.
Compared with the convalescent plasma therapy of the 19th century, that of the 21st century has contemporary methods of observation and treatment that have earned it more attention at the level of plasma antibodies, such as receptor binding domain antibodies and neutralizing antibodies. Convalescent plasma preparation requires strict procedures to ensure the safety of plasma and adequate titer of plasma neutralizing antibodies. Some biopharmaceutical enterprises that are qualified to prepare plasma have actively participated in developing convalescent plasma for clinical treatment. Ruijin Hospital assisted Wuhan frontline hospitals to carry out a plasma treatment study in 10 severe COVID-19 cases. After convalescent plasma transfusion, all patients’ clinical symptoms improved within 24–48 h, and significant improvements in inflammatory indicators and pulmonary radiological images were observed, especially the obvious absorption of ground-glass opacity.
However, this study is exploratory and moderate patients as well as severe patients getting better do not necessarily need plasma therapy. Plasma therapy is recommended to be used in severe patients with progression, especially those without ARDS. The occurrence of ARDS indicates the patient has been severely ill and transfusion of convalescent plasma with high titer of neutralizing antibodies may still not work. The suggested optimal timing for convalescent plasma transfusion is early after the patient progresses to the severe or critical stage. Transfusion before the occurrence of ARDS helps to prevent progression to critical illness. Meanwhile, strictly prepared plasma is required by Chinese laws and regulations on blood transfusion. Relevant biopharmaceutical enterprises must comply fully with China's plasma preparation technical standards to ensure the quality of plasma and patients' safety. Another controlled study of convalescent plasma treatment is currently recruiting patients in Wuhan. It is hoped that 50 patients will be enrolled for convalescent plasma therapy and 50 patients for SARS-CoV-2 nonimmune plasma therapy. This study aims to obtain further medical evidence of plasma therapy, and provide an objective and comprehensive evaluation on its efficacy among COVID-19 patients, especially those in early, severe, or critical conditions.
Recently, the National Health Commission published the "Guidelines on Convalescent Plasma Therapy in COVID-19 Patients (Trial First Edition)," providing eligibility requirements and criteria for both recipients and donors, with the aim of ensuring the appropriate and standard application of plasma therapy.
7. Cytokine-targeted therapy
Considering the cytokine storm in COVID-19 patients, a clinical study on talizumab—a monoclonal antibody that specifically binds to the interleukin-6 receptor—was conducted with the joint efforts of the Chinese University of Science and Technology, School of Life Sciences and Medicine and its affiliated hospitals. In the published data of the current phase 1 clinical trial, the body temperature of 11/14 COVID-19 patients returned to normal within 24 ho with improvement in the oxygenation index; four patients presented absorption of lesions in chest CT images, and one critical case has been successfully weaned off from endotracheal intubation. The follow-up data of 14 patients will be updated in the future.
8. Venous thromboembolism prophylaxis and treatment
Hypercoagulation and hyperfibrinolysis are common in COVID-19 patients, as evidenced by clinical presentations and pathological findings of thrombosis. No contraindications means a certain degree of indication of anticoagulant therapy. Treatment regimens could be made based on a patient's disease severity. A patient with a confirmed diagnosis of VTE could be given a treatment dosage, and those without VTE could be given a prophylactic dosage, and adjustments made according to age and other hemorrhagic risks. It is necessary to monitor the risk of heparin-induced thrombocytopenia (HIT) when administering heparin.
9. Antibacterial treatment
Principally, patients with mild COVID-19 do not need antibacterial therapy, or any special treatment. They are mostly self-limiting within 2 weeks after symptomatic treatment. It is estimated that more than 90% of patients with mild forms of the disease will improve or become self-healing, and therefore they do not require antibacterial drugs.
The progression of COVID-19 is relatively slow, unlike influenza, which may progress to severe pneumonia requiring hospitalization 3–4 days after onset of illness. The average intubation time of severe COVID-19 cases is 11 days. Secondary bacterial infection is rare among patients receiving invasive ventilation for 2 or 3 days, but it might occur in patients under ventilation for more than 3 days, such as carbapenem-resistant Enterobacteriaceae (CRE, such as Klebsiella pneumoniae), Acinetobacter baumannii, and Staphylococcus aureus. In general, patients admitted for a short period of time are not likely to have bacterial infection. It is recommended to avoid unnecessary or inappropriate use of antimicrobial drugs at the early stage of hospitalization, especially combination use of broad-spectrum antibiotics.
Novel coronavirus pneumonia is also a community-acquired pneumonia, and the recommendations on antimicrobial treatment in "Guidelines on Diagnosis and Treatment of Community-Acquired Pneumonia in Adults," issued in 2016, should be followed. For patients with confirmed diagnosis of novel coronavirus pneumonia and suspected infections of other pathogens, it is not inappropriate to empirically use antimicrobial drugs. Therefore, from a practical point of view, try not to use antimicrobial drugs for patients with mild cases without obvious evidence of bacterial infection. When bacterial infection cannot be excluded, it is recommended to decide empiric treatment according to local epidemiological resistance profiles and avoid using broad-spectrum antimicrobial drugs. For the treatment of patients with severe forms of the disease, empirically use antibiotics to ensure broad-spectrum of coverage after rapid collection of diverse specimens, followed by discontinuing or deescalating antibiotics based on microbiological culture results and switching to narrower-spectrum antibiotics to target the identified pathogens.
Secondary bacterial infection tends to occur after viral infection, so bacterial monitoring should be strengthened during COVID-19 treatment. If there is any evidence of secondary bacterial infection, such as expectoration of purulent or yellow sputum, or elevated procalcitonin, prescribe antimicrobial drugs and carry out personalized treatment according to the type of infection, comorbidities, and liver and kidney function; try not to use broad-spectrum antimicrobial drugs. Inappropriate and overextended use of broad-spectrum antibiotics might lead to secondary fungal infection. When suspecting secondary fungal infection, pathogen monitoring including G test and GM test should be considered. For patients with lymphopenia, such as those with lymphocyte count below 300–400/uL, it should be considered whether to take measures for prevention of Pneumocystis Carinii pneumonia. When dealing with infections, remember to take all important issues into account, including etiological aspects.
10. Standards for discontinuation of isolation and hospital discharge
The following four criteria need to be met for a patient to be eligible for hospital discharge or discontinuation of isolation:
① Body temperature returns to normal for more than 3 days.
② An obvious improvement in respiratory symptoms is observed.
③ Chest CT imaging shows a marked improvement in acute exudative lesions.
④ Two consecutive negative results of nucleic acid test are obtained in respiratory specimens (the sampling interval is greater than 1 day ).
Patients are allowed to go home and return to work or school according to the fifth version of the “Guidelines on Diagnosis and Treatment of Covid-19,” which was issued by the National Health Commission. However, according to the sixth version, patients who have met the standards of discharge may still not be able to go home as required. For example, patients who have been discharged from the hospital and returned to their community or place of residence must be further quarantined for another 2 weeks. This has two main purposes: first, to restore their own resistance, and second, to avoid transmitting the virus to others in cases where patients are still contagious within this 2 weeks.


