Abstract
The objective of this study was to investigate the effects of dietary iron (Fe) on reproductive performance of Chinese Yellow broiler breeder hens during the egg-laying period. A total of 480, 55-wk-old hens were balanced for laying rate and then randomly allotted into 5 groups, each with 6 replicates (8 cages for each replicate with 2 birds per cage). The trial was for 10 wk. Birds were fed diet with 44, 58, 72, 86, or 100 mg/kg Fe contained feed. Laying performance, biochemical indices and reproductive hormones in plasma, egg quality, ovarian and oviductal variables, tibial breaking strength, and hatching performance were determined. The key performance variables hematocrit, hatchability of live embryos, and tibial breaking strength were selected for analysis by quadratic polynomial (QP) and broken-line (BL) regressions to better determine optimal dietary Fe level. Qualified egg (excluding those with double-yolk, soft-shell, cracked, very small malformed, etc.) rate tended to decrease with the lowest and highest dietary Fe levels. Hematocrit was affected (P = 0.003) by dietary Fe, along with linear (P = 0.017) and quadratic (P = 0.002) effect. There was a significant effect (P = 0.034) of dietary Fe level on tibial breaking strength of breeder hens with a quadratic (P = 0.044) effect. Breeder hens fed inadequate (44 mg/kg diet) or excess (100 mg/kg) Fe both had lower (P < 0.05) tibial breaking strength compared to that of hens fed 86 mg/kg Fe. Hatchability of live embryos was affected (P = 0.004) by diet; with both linear (P = 0.014) and quadratic (P = 0.001) effects. Maximal hatching of live embryos occurred with diets of breeder hens containing 72 mg/kg Fe. From the QP and BL models fitted to hematocrit, tibial breaking strength, and hatchability variables, the optimal dietary Fe level for Chinese Yellow broiler breeder hens in the laying period was 70–90 mg/kg. The daily Fe fed (allowance) was about 8–11 mg.
Key words: iron, breeder hen, reproductive performance, laying, hatchability
INTRODUCTION
Iron (Fe) is an essential trace mineral for all living organisms, and it plays an important role in oxygen and electron transport as well as in DNA synthesis (Bothwell et al., 1979). This element is widely spread on earth and is present in almost all feed ingredients used in commercial poultry diets (NRC, 1994).
Limited research data focus on Fe nutrition of poultry, particularly for broiler breeders (Bess et al., 2012; Taschetto et al., 2017). Available recommendations of dietary Fe of broiler breeder vary widely, from 20 to 140 mg/kg (Leeson and Summers, 2009; Cobb-vantress, 2013; Aviagen, 2016; Taschetto et al., 2017). Recommended amount of Fe currently available in the literature are based on statistical analyses and from deriving estimates utilizing diverse models (Taschetto et al., 2017). It is noteworthy that current references for Fe use are mainly targeted on fast-growing broiler breeder lines such as Arbor Acres, Ross, and Cobb. Research on proper supplementation of Fe in diet of slower-growing Chinese Yellow broiler breeder hens is lacking, though China is the third largest producer of chickens, with approximately 4 billion Chinese Yellow broilers produced every year.
Pollack et al. (2011) indicated that Fe levels are associated with hormone levels in the body. Iron is essential for oxygen transport, DNA synthesis, and energy production (Mackenzie et al., 2008). Hormones and other physiological variables including energy metabolism strictly control egg production in chickens (Onagbesan et al., 2006). The coordinated activity of the hypothalamus–pituitary–gonadal axis maintains the production of reproductive endocrine hormones including luteinizing hormone (LH) and follicle-stimulating hormone (FSH) to initiate and maintain ovarian follicle growth and ovulation for egg production (Furr et al., 1973; Etches et al., 1984). Lewis et al. (2005) suggested that plasma LH and FSH concentrations during the rearing period might be useful predictors of egg production rate in chickens. On the other hand, Fe deficiency may potentiate the absorption and accumulation of Mn. Manganese acts centrally to stimulate LH secretion (Pine et al., 2005); thus, Fe may affect reproductive hormones. To the authors' knowledge, effects of dietary Fe on reproductive hormones of hens have not been reported.
Hens maintained in cages had a lower bone breaking strength and a lower tibial ash than those kept in floor pens (Rowland et al., 1968). Several factors that may lead to osteoporosis or bone fragility include a deficiency of sex hormones, calcium deficiency, and malnutrition (Rowland and Harms, 1970), but a potential effect of Fe deficiency on tibial breaking strength is unknown.
Laying performance, hematocrit and hemoglobin of hens, and egg quality and hatchability of eggs are influenced by dietary Fe content in highly selected Western breeds (Morck and Austic, 1981; Taschetto et al., 2017). It was hypothesized here that laying performance, biochemical indices, and reproductive hormones in plasma, egg quality, ovarian and oviductal variables, tibial breaking strength, and hatching performance could be affected by dietary Fe levels in Chinese Yellow breeder hens. To test this hypothesis, these variables were measured in birds fed 5 levels of dietary Fe and regressions were fitted for key performance variables. The optimal dietary Fe level of the breeder hens in the laying period was determined using quadratic polynomial (QP) and broken line (BL, 2-slope BL or BL with plateau) models.
MATERIALS AND METHODS
Chickens and Husbandry
A total of 480, 55-wk-old Chinese Yellow broiler breeder hens (Lingnan, an improved local breed, heavy body weight line) were obtained from Guangdong Wiz Agricultural Science & Technology Co., Ltd. (Guangzhou, China). Birds were balanced for laying rate and then randomly allotted into 5 groups, each with 6 replicates (8 cages for each replicate with 2 birds per cage). All experimental methods conformed to guidelines established by the Guangdong Academy of Agricultural Sciences Institutional Animal Care and Use Committee. The breeder hens were under study for 10 wk until the trial was finished at 64 wk of age. All birds received 16 h of daily lighting from 06:00 AM to 10:00 PM. Room temperature and humidity were recorded daily.
Diets
The basal diet was formulated to meet or exceed the nutritional requirements of breeder hens (Ministry of Agriculture, China, 2004) with no addition of Fe (calculated 45.4 mg/kg, analyzed 44.0 mg/kg). Calcium and non-phytate phosphorus contents in the basal diet were 3.0 and 0.41%, respectively. The composition of the basal diet is shown in Table 1. Additional Fe (0, 14, 28, 42, and 56 mg/kg Fe of diet) was added as FeSO4 • H2O (Guangdong Newland Feed Science & Technology Co., Ltd, Guangzhou, China) and final contents were measured by atomic absorption spectrophotometry. All breeder hens received about 120 g diet (as needed, consistently increased or decreased by a few grams based on egg production) every day and had ad libitum access to fresh water. The water was filtered to eliminate possible source of Fe before drinking by breeder hens. The apparatus for removing Fe from water was bought from Guangzhou Chenxing Environmental Protection Technology Co., Ltd. (Guangzhou, China). Iron in water was absorbed by silica sand and further eliminated by ion exchange. All birds were fed the basal diet for 2 wk for partial depletion of iron stored in the body. After this 2-wk adaption, feeding with the experimental diets started and continued for 8 wk.
Table 1.
Composition of the basal diet.
| Ingredient, % | Value | Nutritional level2 | Value |
|---|---|---|---|
| Corn | 65.0 | ME, Kcal/kg | 2795 |
| DDGS | 10.0 | CP, % | 16.0 |
| Soybean protein concentrate | 13.6 | Lysine, % | 0.80 |
| Soybean oil | 0.91 | Methionine, % | 0.40 |
| L-Lysine HCl | 0.01 | Calcium, % | 3.00 |
| DL-Methionine | 0.26 | Non-phytate phosphorus, % | 0.41 |
| Tryptophan | 0.01 | Fe, mg/kg3 | 45.4 |
| Calcium carbonate (analytically pure) | 6.27 | Fe, mg/kg4 | 44.0 |
| Dicalcium phosphate (food-grade) | 1.75 | ||
| Salt (NaCl) | 0.25 | ||
| Maize cob meal | 0.94 | ||
| Premix1 | 1.00 |
Provided per kg of diet: vitamin A, 15,000 IU (retinyl acetate, Guangdong Newland Feed Science & Technology Co., Ltd., Guangzhou, China); vitamin D3, 3,600 IU (cholecalciferol); vitamin E, 47 IU (dl-α-tocopheryl acetate, Guangdong Newland Feed Science & Technology Co., Ltd., Guangzhou, China); vitamin K, 6 mg; thiamine, 3 mg; riboflavin, 9 mg; niacin, 60 mg; pantothenic acid, 16 mg; vitamin B6, 6mg; folic acid, 1.5 mg; cobalamin, 0.03 mg; biotin, 0.06 mg; 50% choline chloride, 900 mg; CuSO4•5H2O, 28 mg; ZnSO4•H2O, 210 mg; MnSO4•H2O, 280 mg; NaSeO3, 0.60 mg; Ca(IO3)2•H2O, 1.46 mg; ethoxyquin, 150 mg; calcium propanoate, 1.00 g; maize cob meal (carrier), 4.61 g.
Values were calculated based on the data provided by Feeding Standard of Chicken (Ministry of Agriculture, China, 2004).
Calculated Fe content based on Fe analyses in Corn, DDGS, soybean protein concentrate, calcium carbonate, dicalcium phosphate and corn gluten meal.
Iron was analyzed by atomic absorption spectrophotometry.
Measurements
Laying Performance.
Mortality was checked daily and dead birds were recorded to adjust estimates of egg laying rate and egg mass as appropriate. Number of total laid eggs, defective eggs (including those with double-yolk, soft-shell, cracked, very small malformed, etc.) and total egg weight were recorded daily. At 64 wk age (end of trial), egg laying rate, average egg weight, egg mass (egg weight of each breeder laid per day), and qualified egg rate (1-total defective eggs/total eggs laid) were calculated. Qualified eggs met the criteria described by Xu et al. (2010).
Sampling.
Two birds, representative of average egg production in each replicate, and having laid eggs the previous day (to standardize status in the laying cycle), were individually weighed and 7 mL blood was sampled from the brachial vein into evacuated tubes containing EDTA-K2 (1 mg/mL blood). Two milliliters of non-clotted blood were held to measure hematocrit and hemoglobin. The remainder (5 mL) was held on ice for <1 h and then centrifuged at 860 × g for 15 min at 4°C and plasma aliquots were kept at −80°C until analysis. The birds were electrically stunned and exsanguinated to obtain tissues. Ovarian and oviductal weights were weighed and recorded. Ovarian index (%) = 100 × ovarian weight/live weight, and oviductal index (%) = 100 × oviductal weight/live weight. Oviductal length was measured with a ruler. The number of dominant follicles with diameter greater than 8 mm was recorded. The tibia was dissected from the right leg and its breaking strength was determined in an Instron Universal Testing Machine with 50-kg-load cell at 50-kg-load range with a crosshead speed of 50 mm/min (Park et al., 2003).
Biochemical Indices and Reproductive Hormones in Plasma.
Concentration of Fe, malondialdehyde (MDA) in plasma was measured using kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Hematocrit was measured with a precision ESR (erythrocyte sedimentation rate) tube (inside diameter, 5 mm; length, 112 mm).
Concentrations in plasma of the reproductive hormones, LH, FSH, progesterone, and estradiol were determined with kits (Guangzhou KingMed Center for Clinical Laboratory Co., Ltd., Guangzhou, China).
Egg Quality.
At completion of the feeding (64 wk of age) of the breeder hens, 2 eggs per replicate, representative of the mean egg weight, were used to measure related indices of egg quality. Egg shape index, the ratio of vertical length to diameter at mid-length, was calculated from measurements made with a Vernier caliper. Shell strength was determined with an Egg Force Reader (EFR-01, Orka, Ramat HaSharon, Israel). Eggshell was separated from albumen and yolk, washed to remove residual albumen and dried at 65°C for 4 h, and then weighed. Eggshell proportion (%) = 100 × eggshell weight/egg weight. Shell thickness, yolk color, and Haugh unit were measured using an egg multi tester EMT-5200 (Robotmation Co., Ltd., Tokyo, Japan). Shell thickness was calculated as the average thickness at the blunt end, sharp end, and middle of eggs. The yolk color was determined according to the La Roche scale (scores 1–15) (Zita et al., 2013).
Hatching.
During the last 2 wk, all breeder hens were artificially inseminated every 3 d with 30 μL pooled semen. Fifty qualified eggs (55 to 70 g) from each replicate were collected in the last week, weighed individually, and incubated under standard conditions for hatching. On the 5th day after the start of incubation, unfertilized eggs were recorded and eliminated. On the 18th day, eggs with dead embryos also were recorded and eliminated. The number of hatched chicks on day 21 and 22 was recorded and hatchling weight per replicate was recorded.
Statistical Analysis
The effects of dietary Fe treatment were assessed by one-way GLM ANOVA procedures of SAS (version 8.0) with replicates as the experimental unit for each variable. When needed for normality and homogeneity of variance, data were transformed. When the main effect was significant (P < 0.05), linear and quadratic effects of Fe content were determined. For key performance variables, optimal dietary Fe level of the breeder hens in the laying period was determined using QP (Pesti et al., 2009) and broken-line (BL, 2-slope BL or BL with plateau) (Kapš and Lamberson, 2004) regression models. The QP model (Y = α + β × Fe + γ × (Fe)2) had Y as the dependent variable; α was the intercept; β was the linear coefficient; and γ was the quadratic coefficient. The maximum response for Fe was defined as Fe = – β/(2 × γ). The 2-slope BL model (Y = α + β × Fe, Fe ≤ γ; Y = δ + ɛ × Fe, Fe > γ) had Y as the dependent variable; α and δ were both intercepts; and β and ɛ were slopes of the 2 lines. The Fe level at the break point (γ) was considered as providing maximum responses. The BL with plateau model (Y = α + β × Fe, Fe ≤ γ; Y = α + β × γ, Fe > γ) had Y as the dependent variable; α was the intercept; β was the slope of line; and the value (α + β × γ) was the plateau. The Fe level at the break point (γ) was considered to be that providing maximum responses.
RESULTS
Laying Performance of Chinese Broiler Breeder Hens
Egg laying rate, egg weight, and egg mass of the breeder hens were not influenced (P > 0.10) by the different levels of Fe fed (Table 2). Qualified egg rate tended to decrease (P = 0.085) with the lowest and highest dietary Fe levels.
Table 2.
Effects of different dietary iron on laying performance of Chinese Yellow broiler breeders.
| Dietary Fe content, mg/kg |
P-value | ||||||
|---|---|---|---|---|---|---|---|
| Variable | 44 | 58 | 72 | 86 | 100 | SEM1 | Fe level |
| Egg laying rate, % | 45.8 | 48.8 | 47.0 | 48.0 | 45.8 | 1.22 | 0.366 |
| Egg weight, g | 64.8 | 64.8 | 65.3 | 64.4 | 64.6 | 0.454 | 0.725 |
| Egg mass, g/d | 29.7 | 31.6 | 30.6 | 30.9 | 29.6 | 0.801 | 0.374 |
| Qualified egg rate, % | 94.9 | 96.6 | 96.6 | 95.4 | 95.2 | 0.513 | 0.085 |
Standard error of the mean from ANOVA (n = 6).
Biochemical Variables
Hematocrit was affected (P = 0.003) by dietary Fe, along with linear (P = 0.017) and quadratic (P = 0.002) effect. Hematocrit was the greatest with 86 mg/kg Fe (Table 3). The same dietary level of Fe resulted in highest blood hemoglobin content and differences (P = 0.055) among the diets were observed. Plasma Fe concentration were highest in breeder hens fed 100 mg/kg Fe, but no significant (P = 0.866) effects were demonstrated. The same dietary level of Fe resulted in highest plasma MDA content and differences (P = 0.094) among the diets were demonstrated.
Table 3.
Biochemical indices in plasma of Chinese Yellow broiler breeders fed diets with different iron contents.
| Dietary Fe content, mg/kg |
P-value1 |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | 44 | 58 | 72 | 86 | 100 | SEM1 | Fe level | Linear | Quadratic |
| Iron, mg/L | 5.22 | 5.37 | 5.27 | 5.22 | 5.71 | 0.365 | 0.866 | ||
| Hematocrit2, % | 27.3 | 30.1 | 30.4 | 33.1 | 29.9 | 0.899 | 0.003 | 0.017 | 0.002 |
| Hemoglobin2, g/L | 106 | 103 | 110 | 115 | 100 | 3.51 | 0.055 | ||
| MDA3, nmol/mL | 2.49 | 3.12 | 3.31 | 3.66 | 4.06 | 0.395 | 0.094 | ||
Standard error of the mean from ANOVA (n = 6), linear and quadratic contrasts examined only when Fe level was significant.
Measured in whole blood.
MDA: Malondialdehyde.
Reproductive Hormone
Of the reproductive hormones measured (Table 4), only concentrations of LH were affected (P = 0.041) by different levels of dietary Fe; highest values occurred with 100 mg/kg Fe with a quadratic (P = 0.007) effect.
Table 4.
Reproductive hormones in plasma of Chinese Yellow broiler breeders fed diets with different iron contents.
| Dietary Fe content, mg/kg |
P-value1 |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Hormone2 | 44 | 58 | 72 | 86 | 100 | SEM1 | Fe level | Linear | Quadratic |
| LH, mIU/mL | 4.82 | 4.53 | 4.63 | 4.71 | 5.23 | 0.148 | 0.041 | 0.104 | 0.007 |
| FSH, mIU/mL | 2.02 | 1.98 | 1.98 | 2.25 | 1.65 | 0.179 | 0.241 | ||
| Progesterone, ng/mL | 0.140 | 0.224 | 0.167 | 0.145 | 0.148 | 0.025 | 0.605 | ||
| Estradiol, pg/mL | 626 | 799 | 899 | 882 | 722 | 102 | 0.328 | ||
Standard error of the mean from ANOVA (n = 6), linear and quadratic contrasts examined only when Fe level was significant.
LH: luteinizing hormone; FSH: follicle-stimulating hormone.
Egg Quality
Relevant indices of egg quality, viz. egg shape index, shell strength, shell thickness, eggshell proportion, yolk color score, and Haugh unit are presented in Table 5. There was a significant (P = 0.037) effect of dietary Fe on egg shape index with a quadratic (P = 0.027) effect. Shell strength was affected (P = 0.037) by dietary Fe with both linear (P = 0.004) and quadratic (P = 0.013) effects. The highest value of egg shape index occurred with 86 mg/kg Fe. Egg yolk color was affected (P = 0.028) by dietary Fe.
Table 5.
Egg quality of Chinese Yellow broiler breeders fed diets with different iron contents.
| Dietary Fe content, mg/kg |
P-value1 |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | 44 | 58 | 72 | 86 | 100 | SEM1 | Fe level | Linear | Quadratic |
| Egg shape index | 1.31 | 1.33 | 1.34 | 1.35 | 1.30 | 0.010 | 0.037 | 0.873 | 0.027 |
| Shell strength, kgf | 3.45 | 3.53 | 3.10 | 3.11 | 2.78 | 0.174 | 0.037 | 0.004 | 0.013 |
| Shell thickness, mm | 0.322 | 0.311 | 0.306 | 0.308 | 0.302 | 0.006 | 0.154 | ||
| Eggshell proportion, % | 8.65 | 8.37 | 8.23 | 8.42 | 8.37 | 0.143 | 0.409 | ||
| Yolk color score | 8.58 | 9.17 | 8.70 | 8.83 | 8.80 | 0.120 | 0.028 | 0.805 | 0.410 |
| Haugh unit | 68.3 | 72.1 | 69.9 | 69.7 | 67.6 | 1.79 | 0.464 | ||
Standard error of the mean (n = 6), linear and quadratic contrasts examined only when Fe level was significant.
Ovarian and Oviductal Variables
The relevant variables, ovarian weight, ovarian index, oviductal weight, oviductal index, oviductal length, and number of dominant follicles are presented in Table 6; none was affected (P > 0.10) by dietary Fe content.
Table 6.
Ovarian variables of Chinese Yellow broiler breeders fed diets with different iron content.
| Dietary Fe content, mg/kg |
P-value |
||||||
|---|---|---|---|---|---|---|---|
| Variable | 44 | 58 | 72 | 86 | 100 | SEM1 | Fe level |
| Ovarian weight, g | 48.3 | 48.3 | 48.4 | 50.0 | 43.7 | 2.39 | 0.426 |
| Ovarian index, % | 1.49 | 1.49 | 1.61 | 1.60 | 1.48 | 0.089 | 0.738 |
| Oviductal weight, g | 56.8 | 56.3 | 60.3 | 58.0 | 54.1 | 2.38 | 0.509 |
| Oviductal index, % | 1.94 | 1.78 | 1.79 | 1.78 | 1.73 | 0.095 | 0.502 |
| Oviductal length, cm | 65.0 | 63.6 | 66.3 | 63.4 | 66.8 | 1.43 | 0.300 |
| Number of dominant follicles | 4.75 | 4.58 | 4.70 | 4.92 | 4.42 | 0.231 | 0.339 |
Standard error of the mean (n = 6).
Tibial Breaking Strength
There was a significant effect (P = 0.034) of dietary Fe level on tibial breaking strength of breeder hens with a quadratic (P = 0.044) effect (Figure 1). Breeder hens fed inadequate (44 mg/kg diet) or excess (100 mg/kg) Fe both had lower (P < 0.05) tibial breaking strength compared to that of hens fed 86 mg/kg Fe.
Figure 1.
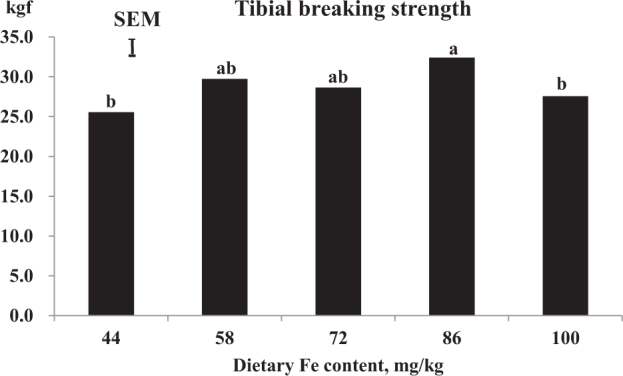
Effect of dietary iron on tibial breaking strength. Means not sharing the same letter differ (P < 0.05); the vertical bar shown is the SEM from the error mean square of the ANOVA (n = 6). Tibial breaking strength was affected (P = 0.034) by dietary Fe with a quadratic (P = 0.044) effect.
Hatching Performance
Some relevant data on hatching performance, fertility rate, hatchability, and birth weight of chicks are presented in Table 7. Of the variables examined, only the proportion of live embryos that hatched was affected (P = 0.004) by dietary Fe; with both linear (P = 0.014) and quadratic (P = 0.001) effects. Maximal hatching percentage, based on numbers of fertilized eggs or live embryos, occurred with diets of breeder hens containing 72 mg/kg Fe.
Table 7.
Hatching performance of Chinese Yellow broiler breeders fed diets with different iron content.
| Dietary Fe content, mg/kg |
P-value1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | 44 | 58 | 72 | 86 | 100 | SEM1 | Fe level | Linear | Quadratic |
| Hatching egg weight, g | 62.5 | 62.4 | 62.3 | 62.2 | 61.5 | 0.459 | 0.571 | ||
| Fertility rate, % | 94.0 | 97.2 | 96.0 | 94.7 | 92.7 | 1.10 | 0.095 | ||
| Proportion of live embryos | 95.7 | 96.2 | 97.4 | 95.8 | 94.1 | 1.48 | 0.635 | ||
| Hatchability of fertilized eggs, % | 82.4 | 84.0 | 89.4 | 87.6 | 83.5 | 2.06 | 0.090 | ||
| Hatchability of live embryos, % | 83.8 | 87.7 | 93.2 | 91.5 | 90.0 | 1.49 | 0.004 | 0.014 | 0.001 |
| Hatching weight of chicks, g | 42.1 | 41.9 | 41.4 | 41.7 | 40.5 | 0.461 | 0.140 | ||
Standard error of the mean (n = 6), linear and quadratic contrasts examined only when Fe level was significant.
Regression Analyses
The data of hematocrit, hatchability of live embryos, and tibial breaking strength were selected for further analysis by QP and BL regressions related to the dietary Fe level (Figure 2, Figure 3, Figure 4 and Table 8). According to the maximum dietary Fe response from regression models in Table 8 and the average daily feed allowance of 119 g, the daily Fe fed allowance of Chinese Yellow broiler breeder hens in laying period were calculated (Table 9). Hematocrit indicated Fe deficiency when hens were fed the basal diet without additional Fe supplementation. The optimal Fe concentration estimated for hematocrit was 80.4 mg/kg (9.57 mg/d) based on QP regression or 86.4 mg/kg (10.28 mg/d) based on the 2-slope BL model. The optimal Fe levels that maximized tibial breaking strength of hens using QP and 2-slope BL regression models were 77.4 mg/kg (9.21 mg/d) and 89.0 mg/kg (10.59 mg/d), respectively. A maximum response for hatchability of live embryos was observed at 90.5 mg/kg (10.77 mg/d) and 72.0 mg/kg (8.57 mg/d) for QP and BL with plateau models, respectively.
Figure 2.
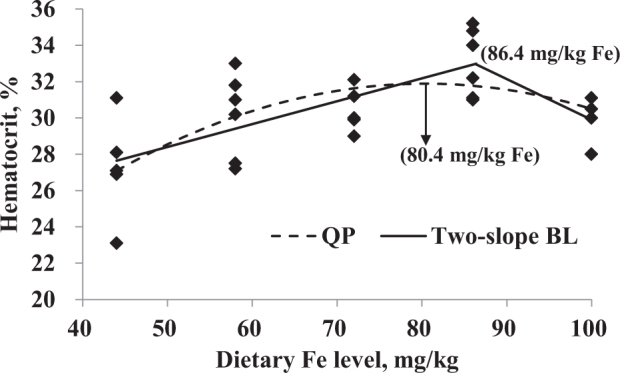
Hematocrit (%) in blood of broiler breeder hens fed diets supplemented with Fe. Regression equations obtained using the increasing dietary Fe in the current study (44, 58, 72, 86, and 100 mg/kg). Dashed line is the quadratic polynomial (QP) regression (Y = 8.46 + 0.583 × X − 0.003627 × X2; the maximum response arrow pointing at 80.4 mg/kg Fe, R2 = 0.407, P = 0.002); solid line is the 2-slope broken-line estimation [Y = 22.1 + 0.126 × X (X ≤ 86.4) and Y = 52.3 − 0.224 × X (X > 86.4), the maximum response at 86.4 mg/kg Fe, R2 = 0.475, P < 0.001].
Figure 3.
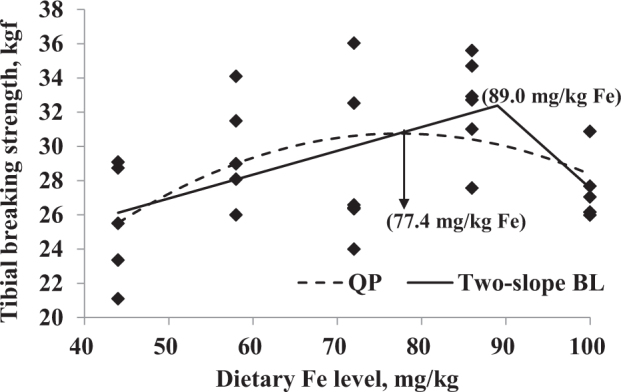
Tibial breaking strength (kgf) of broiler breeder hens fed diets supplemented with Fe. Regression equations obtained using the increasing dietary Fe in the current study (44, 58, 72, 86, and 100 mg/kg). Dashed line is the quadratic polynomial (QP) regression (Y = 2.68 + 0.725 × X − 0.00469 × X2; the maximum response arrow pointing at 77.4 mg/kg Fe, R2 = 0.229, P = 0.002); solid line is the 2-slope broken-line estimation [Y = 20.0 + 0.139 × X (X ≤ 89.0) and Y = 71.5 − 0.439 × X (X > 89.0), the maximum response at 89.0 mg/kg Fe, R2 = 0.294, P < 0.001].
Figure 4.
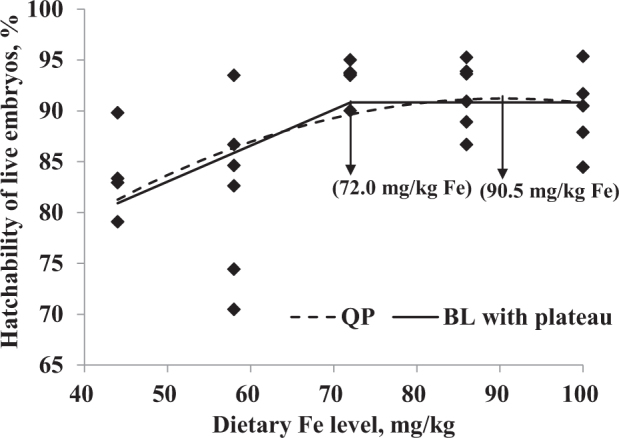
Hatchability of live embryos (%) of broiler breeder hens fed diets supplemented with Fe. Regression equations obtained using the increasing dietary Fe in the current study (44, 58, 72, 86, and 100 mg/kg). Dashed line is the quadratic polynomial (QP) regression (Y = 53.5 + 0.834 × X − 0.00461 × X2; the maximum response arrow pointing at 90.5 mg/kg Fe, R2 = 0.291, P = 0.019); solid line is the broken-line with plateau estimation [Y = 65.3 + 0.354 × X (X ≤ 72.0) and Y = 90.8 (X > 72.0), the maximum response arrow pointing at 72.0 mg/kg Fe, R2 = 0.337, P = 0.009].
Table 8.
Dose response regressions for Chinese Yellow broiler breeder hens fed diets with different iron content.
| Variable | Model | Regression equation1 | Maximum dietary Fe response, mg/kg | P-value | R2 |
|---|---|---|---|---|---|
| Hematocrit, % | QP2 | Y = 8.46 + 0.583 × X − 0.003627 × X2 | 80.4 | 0.002 | 0.407 |
| 2-slope BL3 | Y = 22.1 + 0.126 × X (X ≤ 86.4) | 86.4 | <0.001 | 0.475 | |
| Y = 52.3 − 0.224 × X (X > 86.4) | |||||
| Tibial breaking strength, kgf | QP | Y = 2.68 + 0.725 × X − 0.00469 × X2 | 77.4 | 0.044 | 0.229 |
| 2-slope BL | Y = 20.0 + 0.139 × X (X ≤ 89.0) | 89.0 | 0.015 | 0.294 | |
| Y = 71.5 − 0.439 × X (X > 89.0) | |||||
| Hatchability of live embryos, % | QP | Y = 53.5 + 0.834 × X − 0.00461 × X2 | 90.5 | 0.019 | 0.291 |
| BL with plateau4 | Y = 65.3 + 0.354 × X (X ≤ 72.0) | 72.0 | 0.009 | 0.337 | |
| Y = 90.8 (X > 72.0) |
Regression equations obtained using the analyzed Fe in the trial diets (44, 58, 72, 86, and 100 mg/kg).
QP: Quadratic polynomial; QP model: Y = α + β × X + γ × X2, where Y is the response variable, X is the dietary Fe, α is the intercept; β and γ are the linear and quadratic coefficients respectively. The maximal response was obtained by – β / (2 × γ).
BL: Broken line; 2-slope BL model: Y = α + β × Fe, Fe ≤ γ; Y = δ + ϵ × Fe, Fe > γ, where Y is the response variable, X is the dietary Fe, both α and δ are intercepts, and both β and ϵ are slopes of lines. The Fe level at the break point (γ) was considered as the one providing the maximal response.
BL with plateau model: Y = α + β × Fe, Fe ≤ γ; Y = α + β × γ, Fe > γ, where Y is the response variable, X is the dietary Fe, α is the intercept, β is the slope of line, the value (α + β × γ) is the plateau. The Fe level at the break point (γ) was considered as the one providing the maximal response.
Table 9.
Daily iron fed allowance of Chinese Yellow broiler breeder hens.
| Variable | Model | Optimal dietary iron level, mg/kg | Daily iron fed allowance, mg |
|---|---|---|---|
| Hematocrit, % | QP1 | 80.4 | 9.57 |
| 2-slope BL2 | 86.4 | 10.28 | |
| Tibial breaking strength, N | QP | 77.4 | 9.21 |
| 2-slope BL | 89.0 | 10.59 | |
| Hatchability of live embryos, % | QP | 90.5 | 10.77 |
| BL with plateau3 | 72.0 | 8.57 |
QP: Quadratic polynomial; QP model: Y = α + β × X + γ × X2, where Y is the response variable, X is the dietary Fe, α is the intercept; β and γ are the linear and quadratic coefficients respectively. The maximal response was obtained by − β / (2 × γ).
BL: Broken line; 2-slope BL model: Y = α + β × Fe, Fe ≤ γ; Y = δ + ϵ × Fe, Fe > γ where Y is the response variable, X is the dietary Fe, both α and δ are intercepts, both β and ϵ are slopes of lines. The Fe level at the break point (γ) was considered as the one providing the maximal response.
BL with plateau model: Y = α + β × Fe, Fe ≤ γ; Y = α + β × γ, Fe > γ where Y is the response variable, X is the dietary Fe, α is the intercept, β is the slope of line, the value (α + β × γ) is the plateau. The Fe level at the break point (γ) was considered as the one providing the maximal response.
DISCUSSION
Laying performance of broiler breeder hens is a commercially important aspect of reproductive performance. Supplementary Fe did not improve egg laying rate, egg weight, and egg mass in the current study. These results with Chinese Yellow breeders are consistent with the lack of significant effect of dietary Fe on egg production and egg mass of Cobb 500 broiler breeder hens (Abbasi et al., 2015). In contrast, Taschetto et al. (2017) investigated Fe requirements of Cobb 500 slow-feathering broiler breeder hens during the egg-laying period and found that egg production responded with a significant increase as Fe was added. Depletion of Fe (only 24.6 mg/kg Fe in the basal diet) was of longer duration (11 wk) may lead to an extreme lack of the element in breeder hens (Taschetto et al., 2017). In laying hens, a significant effect of dietary Fe on egg production was also observed (Morck and Austic, 1981).
The minimum hematocrit in hens fed 44 mg/kg Fe here are indicative of Fe deficiency. Hematocrit and hemoglobin are often considered to be key indices reflecting presence or absence of Fe deficiency (Morck and Austic, 1981; Bess et al., 2012; Taschetto et al., 2017). Effects of Fe supplementation for breeder hens were studied by Taschetto et al. (2017), also using QP and BL models. These authors showed that hematocrit in blood increased with graded dietary levels from 24.6 to 99.6 mg/kg of Fe. They showed maximal responses of hematocrit occurring between 130 and 135 mg/kg dietary Fe, which exceeded those (70 to 90 mg/kg dietary Fe) found in the present study. Breeder hens fed 100 mg/kg dietary Fe in this study may ingest excess Fe because this level caused the decreased hematocrit, highest blood MDA, a lipid peroxidation product, and poor egg quality (lowest egg shape index and shell strength). Excess ingested Fe may induce oxidative damage and produce highly reactive hydroxyl radicals (Gutteridge, 1994; Troost et al., 2003; Troost et al., 2006) and MDA (Rimbach et al., 1997; Carrier et al., 2001; Lund et al., 2001). In the previous study with Chinese Yellow broilers, high doses of dietary Fe increased production of MDA and oxidative stress occurred (Gou et al., 2018).
Differences in egg laying performance have long been considered to relate to differences in plasma levels of reproductive hormones such as LH, FSH, and progesterone (Wilson, 1978; Yu et al., 1992; Bruggeman et al., 1999; Lovell et al., 2001). In the current study, breeder hens consuming the highest quantities of Fe had highest plasma concentrations of LH. The LH levels were not related to egg laying rate here, agreeing with Onagbesan et al. (2006), but not with earlier reports from egg-type hens (Tanabe et al., 1981; Wang and Johnson, 1993). Hassan et al. (2016) demonstrated that enhanced egg production may be due to increased serum concentrations of FSH and estradiol, along with increased ovarian follicle number, but that was not observed in the current study. Iron deffiency or Fe excess in the current study could not result in atrophy or hypertrophy of the functionally mature ovary and oviduct of the hens. Therefore, ovarian and oviductal variables were not affected by dietary Fe content.
Another indicator reflecting dietary Fe deficiency or excess in the present study was tibial breaking strength. Rats fed an iron-deficient diet demonstrated anemia as reflected by significantly lower hematocrit readings and developed decreased bone mass and increased fragility (Medeiros et al., 2002). Analogously, decreased hematocrit level was observed in excess aluminum (Al) intoxicated rats, which induced a negative effect on bone tissue, affecting collagen synthesis and matrix mineralization (Martínez et al., 2011). Minerals such as calcium (Ca) and phosphorus are usually considered to have the major impact on tibial quality (Bishop et al., 2000; Onyango et al., 2003; Han et al., 2013), while zinc, manganese, and copper have recently received more attention (Aksu et al., 2011; El-Hussein et al., 2012; Favero et al., 2013). Chen et al. (2015) demonstrated that tibial breaking strength of laying ducks was decreased with decreasing dietary calcium, but tibial weight and length were not affected. Here, with breeder hens, tibial weight, length, and even tibial perimeter were not affected by dietary Fe (data not shown), but tibial breaking strength was reduced when hens were fed insufficient or excess dietary Fe. Maximal tibial breaking strength here required dietary Fe 77.4 (QP) and 89.0 mg/kg (2-slope BL), so in the range of 70 to 90 mg/kg.
Morck and Austic (1981) demonstrated that hatchability of fertile eggs laid by White Leghorn hens was significantly affected by dietary Fe. Likewise, in the current study with Chinese Yellow broiler breeder hens, the significant effect of dietary Fe on hatchability of live embryos was observed. A deficient Fe status may lead to embryonic malformations and delayed development even causing post-hatch death (Abbasi et al., 2015). Iron is essential for oxygen transport and energy production (Mackenzie et al., 2008). Lower percentage of haematocrit in hens along with lower hatchability were also observed (Ebrahem et al., 2014). In the current study, among hens fed the basal diet with inadequate dietary Fe, many embryos that were alive on the 18th day of incubation died between the 19th to 22th day, during the hatching period; some of the chicks may have had delayed development and lacked sufficient energy to be able to pip. The QP and BL estimates, with plateau adjustments, derived here occurred with maximum hatchability at 90.5 and 72.0 mg/kg dietary Fe, also within or close to the 70 to 90 mg/kg range. These values of dietary Fe requirement exceeded that of 55 mg/kg required for maximal hatchability of fertile eggs laid by White Leghorns, obtained by Morck and Austic (1981).
These data from Chinese Yellow broiler breeder hens in the laying period indicate that a dietary deficiency of the trace element Fe is reflected by lowest blood hematocrit, in blood, a negative impact upon egg shape index and yolk color score, and notably led to the lowest tibial breaking strength and hatchability of live embryos. Similarly, excess dietary Fe, was reflected in highest plasma content of the lipid peroxidative product MDA and led to poor hematocrit, impaired egg shape index, and shell strength, with even lower hatchability of live embryos. Therefore, from the QP and BL models of the key performance variables hematocrit, tibial breaking strength and hatchability, the optimal dietary Fe levels were 70 to 90 mg/kg, and daily Fe fed allowance was 8 to 11 mg.
ACKNOWLEDGMENTS
This research was supported by the National Key Research & Development Program (2018YFD0500600), the earmarked fund for Modern Agro-industry Technology Research System (CARS-41) from Ministry of Agriculture, Natural Science Foundation from Guangdong province (2017A030310096), the Scientific and Technological Project of Guangdong Province (2017B020202003), the Scientific and Technological Project of Guangzhou city (201804020091), the “Twelve-Five’ National Science and Technology Support Program (2014BAD13B02) and the Presidential Foundation of the Guangdong Academy of Agricultural Sciences (201807B, 201620, 201805, 201809B, 201908). The authors thank W. Bruce Currie, Cornell University, Ithaca, NY, for suggestions on the manuscript.
REFERENCES
- Abbasi M., Zaghari M., Ganjkhanlo M., Khalaji S. Is dietary iron requirement of broiler breeder hens at the late stage of production cycle influenced by phytase supplementation? J. Appl. Anim. Res. 2015;43:166–176. [Google Scholar]
- Aksu T., Özsoy B., Aksu D.S., Yoruk M.A., Gul M. The effects of lower levels of organically complexed zinc, copper and manganese in broiler diets on performance, mineral concentration of tibia and mineral excretion. Kafkas. Univ. Vet. Fak. Derg. 2011;17:141–146. [Google Scholar]
- Aviagen . Aviagen Group; Huntsville, AL, USA: 2016. ROSS 308 Parent Stock: Nutrition Specifications. [Google Scholar]
- Bess F., Vieira S.L., Favero A., Cruz R.A., Nascimento P.C. Dietary iron effects on broiler breeder performance and egg iron contents. Anim. Feed Sci. Technol. 2012;178:67–73. [Google Scholar]
- Bishop S.C., Fleming R.H., McCormack H.A., Flock D.K., Whitehead C.C. Inheritance of bone characteristics affecting osteoporosis in laying hens. Br. Poult. Sci. 2000;41:33–40. doi: 10.1080/00071660086376. [DOI] [PubMed] [Google Scholar]
- Bothwell T.H., Charlton R.W., Cook J.D., Finch C.A. Blackwell Scientific; Oxford: 1979. Iron Metabolism in Man; pp. 7–81. [Google Scholar]
- Bruggeman V., Onagbesan O., D'Hondt E., Buys N., Safi M., Vanmontfort D., Berghman L., Vandesande F., Decuypere E. Effects of timing and duration of feed restriction during rearing on reproductive characteristics in broiler breeder females. Poult. Sci. 1999;78:1424–1434. doi: 10.1093/ps/78.10.1424. [DOI] [PubMed] [Google Scholar]
- Carrier J., Aghdassi E., Platt I., Cullen J., Allard J.P. Effect of oral iron supplementation on oxidative stress and colonic inflammation in rats with induced colitis. Aliment. Pharmacol. Ther. 2001;15:1989–1999. doi: 10.1046/j.1365-2036.2001.01113.x. [DOI] [PubMed] [Google Scholar]
- Chen W., Zhao F., Tian Z.M., Zhang H.X., Ruan D., Li Y., Wang S., Zheng C.T., Lin Y.C. Dietary calcium deficiency in laying ducks impairs eggshell quality by suppressing shell biomineralization. J. Exp. Biol. 2015;218:3336–3343. doi: 10.1242/jeb.124347. [DOI] [PubMed] [Google Scholar]
- Cobb-vantress . Cobb Vantress Inc.; Siloam Springs, AR: 2013. Breeder Management Supplement. [Google Scholar]
- Ebrahem M., Kersten S., Valenta H., Breves G., Beineke A., Hermeyer K., Dänicke S. Effects of feeding deoxynivalenol (DON)-contaminated wheat to laying hens and roosters of different genetic background on the reproductive performance and health of the newly hatched chicks. Mycotoxin Res. 2014;30:131–140. doi: 10.1007/s12550-014-0197-z. [DOI] [PubMed] [Google Scholar]
- El-Hussein O.M., Hashish S.M., Ali R.A., Arafa S.A., El- Samee L.D.A., Olemy A.A. Effects of feeding organic zinc, manganese and copper on broiler growth, carcass characteristics, bone quality and mineral content in bone, liver and excreta. Int. J. Poult. Sci. 2012;11:368–377. [Google Scholar]
- Etches R.J., Petitte J.N., Anderson-Langmuir C.E. Interrelationships between the hypothalamus, pituitary gland, ovary, adrenal gland, and the open period for LH release in the hen (Gallus domesticus) J. Exp. Zool. 1984;232:501–511. doi: 10.1002/jez.1402320317. [DOI] [PubMed] [Google Scholar]
- Favero A., Vieira S., Angel C., Bos-Mikich A., Lothhammer N., Taschetto D., Cruz R., Ward T. Development of bone in chick embryos from Cobb 500 breeder hens fed diets supplemented with zinc, manganese, and copper from inorganic and amino acid-complexed sources. Poult. Sci. 2013;92:402–411. doi: 10.3382/ps.2012-02670. [DOI] [PubMed] [Google Scholar]
- Furr B.J., Bonney R.C., England R.J., Cunningham F.J. Luteinizing hormone and progesterone in peripheral blood during the ovulatory cycle of the hen Gallus domesticus. J. Endocrinol. 1973;57:159–169. doi: 10.1677/joe.0.0570159. [DOI] [PubMed] [Google Scholar]
- Gou Z.Y., Li L., Fan Q.L., Lin X.J., Jiang Z.Y., Zheng C.T., Ding F.Y., Jiang S.Q. Effects of oxidative stress induced by high dosage of dietary iron ingested on intestinal damage and caecal microbiota in Chinese Yellow broilers. J. Anim. Physiol. Anim. Nutr. 2018;102:924–932. doi: 10.1111/jpn.12885. [DOI] [PubMed] [Google Scholar]
- Gutteridge J.M. Hydroxyl radicals, iron, oxidative stress, and neurodegeneration. Ann. N. Y. Acad. Sci. 1994;738:201–213. doi: 10.1111/j.1749-6632.1994.tb21805.x. [DOI] [PubMed] [Google Scholar]
- Han J.C., Qu H.X., Wang J.Q., Yao J.H., Zhang C.M., Yang G.L., Cheng Y.H., Dong X.S. The effects of dietary cholecalciferol and 1α-hydroxycholecalciferol levels in a calcium- and phosphorus-deficient diet on growth performance and tibia quality of growing broilers. J. Anim. Feed. Sci. 2013;22:158–164. [Google Scholar]
- Hassan M.R., Sultana S., Choe H.S., Ryu K.S. Effect of monochromatic and combined light colour on performance, blood parameters, ovarian morphology and reproductive hormones in laying hens. Ital. J. Anim. Sci. 2016;12:359–364. [Google Scholar]
- Kapš M., Lamberson W. 1st ed. Cromwell Press; Trowbridge: 2004. Biostatistics for Animal Science. [Google Scholar]
- Leeson S., Summers J.D. 3rd ed. Nottingham University Press; Guelph: 2009. Commercial Poultry Nutrition. [Google Scholar]
- Lewis P.D., Ciacciariello M., Ciccone N.A., Sharp P.J., Gous R.M. Lighting regimens and plasma LH and FSH in broiler breeders. Br. Poult. Sci. 2005;46:349–353. doi: 10.1080/00071660500098509. [DOI] [PubMed] [Google Scholar]
- Lovell T.M., Knight P.G., Groome N.P., Gladwell R.T. Changes in plasma inhibin A levels during sexual maturation in the female chicken and the effects of active immunization against inhibin alpha-subunit on reproductive hormone profiles and ovarian function. Biol. Reprod. 2001;64:188–196. doi: 10.1095/biolreprod64.1.188. [DOI] [PubMed] [Google Scholar]
- Lund E.K., Fairweather-Tait S.J., Wharf S.G., Johnson I.T. Chronic exposure to high levels of dietary iron fortification increases lipid peroxidation in the mucosa of the rat large intestine. J. Nutr. 2001;131:2928–2931. doi: 10.1093/jn/131.11.2928. [DOI] [PubMed] [Google Scholar]
- Mackenzie E.L., Iwasaki K., Tsuji Y. Intracellular iron transport and storage: from molecular mechanisms to health implications. Antioxid. Redox. Signal. 2008;10:997–1030. doi: 10.1089/ars.2007.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez M.D.P., Olivera M.I., Dmytrenko G., Conti M.I. Aluminum bone toxicity in immature rats exposed to simulated high altitude. J. Bone Miner. Metab. 2011;29:526–534. doi: 10.1007/s00774-010-0254-4. [DOI] [PubMed] [Google Scholar]
- Medeiros D.M., Plattner A., Jennings D., Stoecker B. Bone morphology, strength and density are compromised in iron-deficient rats and exacerbated by calcium restriction. J. Nutr. 2002;132:3135–3141. doi: 10.1093/jn/131.10.3135. [DOI] [PubMed] [Google Scholar]
- Ministry of Agriculture, China . Standards Press of China; Beijing: 2004. Feeding Standard of Chicken. [Google Scholar]
- Morck T.A., Austic R.E. Iron requirements of White Leghorn hens. Poult. Sci. 1981;60:1497–1503. doi: 10.3382/ps.0601497. [DOI] [PubMed] [Google Scholar]
- NRC . National Academy Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Onagbesan O.M., Metayer S., Tona K., Williams J., Decuypere E., Bruggeman V. Effects of genotype and feed allowance on plasma luteinizing hormones, follicle-stimulating hormones, progesterone, estradiol levels, follicle differentiation, and egg production rates of broiler breeder hens. Poult. Sci. 2006;85:1245–1258. doi: 10.1093/ps/85.7.1245. [DOI] [PubMed] [Google Scholar]
- Onyango E., Hester P., Stroshine R., Adeola O. Bone densitometry as an indicator of percentage tibia ash in broiler chicks fed varying dietary calcium and phosphorus levels. Poult. Sci. 2003;82:1787–1791. doi: 10.1093/ps/82.11.1787. [DOI] [PubMed] [Google Scholar]
- Park S.Y., Birkhold S.G., Kubena L.F., Nisbet D.J., Ricke S.C. Effect of storage condition on bone breaking strength and bone ash in laying hens at different stages in production cycles. Poult. Sci. 2003;82:1688–1691. doi: 10.1093/ps/82.11.1688. [DOI] [PubMed] [Google Scholar]
- Pesti G., Vedenov D., Cason J., Billard L. A comparison of methods to estimate nutritional requirements from experimental data. Br. Poult. Sci. 2009;50:16–32. doi: 10.1080/00071660802530639. [DOI] [PubMed] [Google Scholar]
- Pine M., Lee B., Dearth R., Hiney J.K., Dees W.L. Manganese acts centrally to stimulate luteinizing hormone secretion: a potential influence on female pubertal development. Toxicol. Sci. 2005;85:880–885. doi: 10.1093/toxsci/kfi134. [DOI] [PubMed] [Google Scholar]
- Pollack A.Z., Schisterman E.F., Goldman L.R., Mumford S.L., Albert P.S., Jones R.L., Wactawski-Wende J. Cadmium, lead, and mercury in relation to reproductive hormones and anovulation in premenopausal women. Environ. Health Perspect. 2011;119:1156–1161. doi: 10.1289/ehp.1003284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimbach G., Markant A., Most E., Pallauf J. Liver and colon oxidant status in growing rats fed increasing levels of dietary iron. J. Trace Elem. Med. Biol. 1997;11:99–104. doi: 10.1016/S0946-672X(97)80033-8. [DOI] [PubMed] [Google Scholar]
- Rowland L.O., Harms R.H., Wilson H.R., Ahmed E.M., Waldroup P.W., Fry J.L. Influence of various dietary factors on bone fragility of caged layers. Poult. Sci. 1968;47:507–511. [Google Scholar]
- Rowland L., Harms R. The effect of wire pens, floor pens and cages on bone characteristics of laying hens. Poult. Sci. 1970;49:1223–1225. [Google Scholar]
- Tanabe Y., Nakamura T., Tanase H., Doi O. Comparisons of plasma LH, progesterone, testosterone and estradiol concentrations in male and female chickens (Gallus domesticus) from 28 to 1141 days of age. Endocrinol. Jpn. 1981;28:605–613. doi: 10.1507/endocrj1954.28.605. [DOI] [PubMed] [Google Scholar]
- Taschetto D., Vieira S.L., Angel C.R., Stefanello C., Kindlein L., Ebbing M.A., Simões C.T. Iron requirements of broiler breeder hens. Poult. Sci. 2017;96:3920–3927. doi: 10.3382/ps/pex208. [DOI] [PubMed] [Google Scholar]
- Troost F.J., Brummer R.J., Haenen G.R., Bast A., van Haaften R.I., Evelo C.T., Saris W.H. Gene expression in human small intestinal mucosa in vivo is mediated by iron-induced oxidative stress. Physiol. Genomics. 2006;25:242–249. doi: 10.1152/physiolgenomics.00114.2005. [DOI] [PubMed] [Google Scholar]
- Troost F.J., Saris W.H., Haenen G.R., Bast A., Brummer R.J. New method to study oxidative damage and antioxidants in the human small bowel: effects of iron application. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;285:G354–G359. doi: 10.1152/ajpgi.00422.2002. [DOI] [PubMed] [Google Scholar]
- Wang S.Y., Johnson P.A. Increase in ovarian alpha-inhibin gene expression and plasma immunoreactive inhibin level is correlated with a decrease in ovulation rate in the domestic hen. Gen. Comp. Endocrinol. 1993;91:52–58. doi: 10.1006/gcen.1993.1103. [DOI] [PubMed] [Google Scholar]
- Wilson S.C. Relationship between plasma concentration of luteinising hormone and intensity of lay in the domestic hen. Br. Poult. Sci. 1978;19:643–650. doi: 10.1080/00071667808416524. [DOI] [PubMed] [Google Scholar]
- Xu H., Shen X., Zhou M., Fang M., Zeng H., Nie Q., Zhang X. Effector prediction in host-pathogen interaction based on a Markov model of a ubiquitous EPIYA motif. BMC Genomics. 2010;11:1–17. doi: 10.1186/1471-2164-11-S3-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M.W., Robinson F.E., Etches R.J. Effect of feed allowance during rearing and breeding on female broiler breeders: 3. Ovarian steroidogenesis. Poult. Sci. 1992;71:1762–1767. doi: 10.3382/ps.0711762. [DOI] [PubMed] [Google Scholar]
- Zita L., Ledvinka Z., Klesalová L. The effect of the age of Japanese quails on certain egg quality traits and their relationships. Vet. Arhiv. 2013;83:223–232. [Google Scholar]


