Abstract
Effects of the in ovo injection of vitamin D3 (D3) and 25-hydroxycholecalciferol (25OHD3) on broiler embryo serum 25OHD3 concentrations, hatchability, and hatchling somatic characteristics were determined. Eggs from a 35-wk-old commercial Ross 708 broiler breeder flock were set in a single-stage incubator with 11 treatments represented on each of 8 incubator tray levels (blocks). Each treatment group within a flat on each tray level contained 30 eggs. Control treatments were noninjected and diluent injected. Vitamin treatments were commercial diluent containing 0.6 μg D3, 0.6 μg 25OHD3, 0.6 μg D3 + 0.6 μg 25OHD3, 1.2 μg D3, 1.2 μg 25OHD3, 1.2 μg D3 + 1.2 μg 25OHD3, 2.4 μg D3, 2.4 μg 25OHD3, or 2.4 μg D3 + 2.4 μg 25OHD3. At 432 h of incubation (hoi), 50-μL solution volumes were injected. Blood samples were collected at 462 hoi for serum 25OHD3 analysis, and hatchability of injected live embryonated eggs (HI) was determined at 492 and 516 hoi. At 516 hoi, hatchling yolk-free BW and weights of the liver and yolk sac were determined. Percentage of yolk moisture and dry mater was calculated. At 492 and 516 hoi, HI did not differ between treatments. Embryos that received 1.2 μg or more of either vitamin D3 source alone or in combination had higher serum 25OHD3 concentrations than those that were injected with diluent alone or diluent containing 0.6 μg of D3. Hatchlings that received 1.2 or 2.4 μg of 25OHD3 had higher percentage of yolk dry matter or lower percentage of yolk moisture levels than noninjected controls and those that received D3 alone at any level. These results indicate that the in ovo injection of either vitamin D3 source at levels equal to or higher than 1.2 μg resulted in serum 25OHD3 concentrations that were higher than that of noninjected controls. In addition, the in ovo injection of 1.2 μg or higher of either vitamin D3 source did not negatively affect broiler HI or chick quality.
Key Words: broiler, in ovo injection, percentage yolk dry matter, serum 25OHD3, vitamin D3 source
Introduction
In ovo vaccination is used commercially to deliver a particular vaccine between 17.5 and 19.25 D of incubation (doi) into the amniotic sac surrounding the broiler embryo (Williams, 2011). In ovo injection is widely used in the US commercial broiler industry and has allowed for the direct administration of particular nutrients or vaccines to embryos. It is less labor intensive and is relatively less stressful for the embryo in comparison with the vaccination of hatchlings (Williams, 2007). It also uniformly delivers vaccines with limited contamination for the initiation of an early immune response in broilers (Williams, 2007, Salmanzadeh, 2012). The poultry industry commercially uses in ovo injection against Marek's disease. In addition, several laboratories have conducted research to determine effects of the in ovo injection of various nutrients including glucose (Salmanzadeh, 2012, Salmanzadeh, 2012) and L-ascorbic acid (Zhang et al., 2018), to increase the hatchability and BW and reduce the feed conversion ratio of broilers.
Vitamin D3 (D3) and 25-hydroxycholecalciferol (25OHD3) are both involved in calcium and phosphorous absorption and bone mineralization and have regulatory functions for the immune system and in the development of muscle in broiler chickens (Rama-Rao et al., 2006, Morris et al., 2014, Vignale et al., 2015). Dietary D3 is absorbed in the upper portion of the small intestine and is then hydroxylated to 25OHD3 by 25 hydroxylase in the liver before being converted to the biologically active form of D3 (1, 25-dihydroxycholecalciferol [1,25(OH)2D3]) by 1 α-hydroxylase in the kidney (Henry, 1980). An increase in broiler hatchability was observed when serum 25OHD3 levels increased in response to in ovo injection of 25OHD3 (Bello et al., 2013). In comparison with D3, the inclusion of 25OHD3 has been shown to elevate serum 25OHD3 levels in broilers (Yarger et al., 1995). In addition, the inclusion of 25OHD3 in drinking water of broiler breeders has been shown to decrease early embryo mortality and elevate 4-day-old broiler serum 25OHD3 levels and to subsequently decrease feed conversation ratio from 15 to 27 D after hatch in comparison with D3 at the same level of inclusion (Saunders-Blades and Korver, 2014). The breast meat yield and performance of broilers has also been reported to increase when their serum 25OHD3 levels were increased in response to dietary supplementation with 25OHD3. This response to 25OHD3 has been attributed to its longer half-life (Smith and Goodman, 1971, Hollis and Wagner, 2013) and its higher rate of absorption in the intestine (Bar et al., 1980) in comparison with D3.
The in ovo injection of 0.60 μg of 25OHD3 has likewise been shown to influence the hatchability and yolk characteristics of broilers (Bello et al., 2013, Bello et al., 2015). Embryos that received an in ovo injection of 0.60 μg of 25OHD3 exhibited higher hatchability and serum 25OHD3 levels than embryos from a diluent-injected control group (Bello et al., 2013). The in ovo injection of 20 ng of vitamin D3 at 12 doi increased the blood calcium levels of chicken embryos (Mansour et al., 2017). However, the effects of D3 and 25OHD3 alone or in combination on broiler hatchability and chick quality have not been well delineated in previous research. Therefore, the objective of this study was to investigate the effects of the in ovo injection of vitamin D3 and 25OHD3 across a broad dosage range, alone or in combination, on broiler hatchability and chick quality.
Material and methods
General
Both preliminary and main experiment protocols of this study were approved by the Institutional Animal Care and Use Committee of Mississippi State University. Eggs were collected from 35-wk-old commercial Ross 708 broiler breeder hens and stored under commercial conditions (12.8°C and 10.4°C dry- and wet-bulb temperatures, respectively) for 24 h (Zhang et al., 2018). The eggs were gradually warmed at room temperature (23.9°C dry bulb) for 4 h before being set. In both trials, prespecified concentrations of D3 (ROVIMIX D3 500; DSM Nutritional Products Inc., Parsippany, NJ) or 25OHD3 (ROVIMIX Hy-D 1.25%; DSM Nutritional Products Inc., Parsippany, NJ) were dissolved in distilled sterile water. Commercial MD vaccine diluent (Merial Co., Athens, GA) in each injector infusion bag (400 mL total volume) was removed and replaced with 15.3 mL of D3 or 3.8 mL of 25OHD3 in distilled sterile water.
At 18 doi, 50-μL volumes of the in ovo injection treatments were applied to those eggs that were preassigned to a specific treatment. At 462 h of incubation (hoi), in the preliminary and main studies, blood samples were collected from the chorioallantoic vasculature. Blood was collected from 4 eggs in each of the 3 treatment groups on each of the 3 incubator tray levels (total eggs 36) in the preliminary study and from 4 eggs in each of the 11 treatment groups on each of the 8 incubator tray levels (total eggs 352) in the main study. At 462 hoi, blood samples from live embryonated eggs were collected from the chorioallantoic vasculature, and serum was extracted as specified by Peebles et al. (1996). Serum samples within each replicate group in the preliminary and main studies were randomly selected and pooled, and the 25OHD3 concentrations of 3 replicate serum samples were analyzed by RIA (DSM Nutritional Products; Parsippany, NJ) as per the protocol described by Hollis et al. (1993).
Preliminary Experiment Design, Sampling, and Data Collection
A total of 270 Ross × Ross 708 broiler hatching eggs from 35-wk-old broiler breeder hens were randomly set in a single-stage NMC2000 incubator (NatureForm Incubator Co., Jacksonville, FL). The eggs were incubated at 37.5°C (dry-bulb temperature) and 28.9°C (wet-bulb temperature). Thirty eggs were assigned to each of 3 treatment groups that were randomly represented on each of 3 incubator tray levels. Each tray level served as a replicate unit (block) for each treatment. All eggs were candled at 288 and 430 hoi to remove infertile eggs and early-dead embryos. The in ovo injection treatments were applied by hand injection at 432 hoi following the procedures described by Embrex Inc. (2002). The applied treatments included a commercial diluent-injected control group and 2 vitamin treatment groups in which commercial diluent contained either 1.2 μg of D3 or 1.2 μg of 25OHD3. At hatch (502 hoi), hatchling BW and hatchability of injected live embryonated eggs (HI) were also determined.
Main Experiment Design, Sampling, and Data Collection
A total of 2,640 Ross × Ross 708 broiler hatchling eggs from 35-wk-old broiler breeder hens were randomly set in a single-stage incubator (Chick Master Incubator Company, Medina, Ohio). The eggs were incubated at temperatures of 37.2°C (dry bulb) and 28.8°C (wet bulb) that followed a multistage profile recommended by the company. Thirty eggs were assigned to each of 11 treatment groups that were randomly assigned to each of 8 incubator tray levels. Each tray level served as a replicate unit (block) for each treatment. Incubator air temperature and relative humidity were recorded every 15 min using HOBO ZW Series wireless data loggers (Onset Computer Corporation, Bourne, MA) during the 21 doi period. Eggs were candled at 288 and 430 hoi to remove eggs that were infertile or that contained dead embryos. Control treatments were noninjected and diluent-injected. Vitamin treatments in diluent were 0.6 μg D3, 0.6 μg 25OHD3, 0.6 μg D3 + 0.6 μg 25OHD3, 1.2 μg D3, 1.2 μg 25OHD3, 1.2 μg D3 + 1.2 μg 25OHD3, 2.4 μg D3, 2.4 μg 25OHD3, and 2.4 μg D3 + 2.4 μg 25OHD3. At 432 hoi, the prespecified in ovo-injected treatments were applied using an Inovoject M (Zoetis, Parsippany, NJ) multiegg injection machine. At the same time, 1 egg from each of the 11 treatment groups on each of the 8 incubator tray levels (total eggs 88) were injected with colloidal coomassie brilliant blue G-250 dye (Genlantis, San Diego, CA) and immediately euthanized for embryo staging analysis. The embryo staging analysis was performed to determine the location of the dye and the developmental stage of the embryo as per the procedure described by Avakian (2006).
At 492 and 516 hoi, HI was determined, and at 516 hoi, hatch residue analysis was conducted as described by Ernst et al. (2004), for determination of postinjection embryonic mortality. Hatchling BW, yolk-free BW, relative liver weight (RLW), and relative yolk sac weight were determined for 1 chick from each of the 11 treatment groups on each of the 8 incubator tray levels (88 total chicks) at 516 hoi. Yolk sac samples were collected and stored at −20°C in sealed containers for subsequent yolk moisture analysis. Percentages of yolk moisture (PYM) and dry matter (PYDM) were determined by drying yolk samples at 37.7°C for 4 D. The samples were cooled for 2 h at room temperature before being weighed.
Statistical Analysis
Randomized complete block experimental designs were used in both the preliminary and main studies, with incubator tray level serving as the blocking factor and with all treatments randomly represented on each of 8 tray levels. A one-way ANOVA using the MIXED procedure of SAS 9.4, version 9.4 (SAS Institute Inc., Cary, NC). was used to analyze all variables within each individual time period separately. Means separations were performed by Fisher's protected least significant difference (Steel and Torrie, 1980). Pairwise differences between means were considered significant at P ≤ 0.05. The following model was used for analysis of the data:
where μ was the population mean; Bi was incubator tray level (i = 1 to 8); Tj was treatment (j = 1 to 11); and Eij was the residual error.
In the main study, hatch and sample data were further tested by contrast analysis using the MIXED procedure of SAS 9.4 (SAS Institute Inc., Cary, NC). The effects of injection dosage across vitamin D3 source (0.6, 1.2, 2.4, and 4.8 μg) and vitamin D3 source (D3, 25OHD3, and D3 + 25OHD3) across dosage of injection were tested (Table 1). Pairwise differences between means for contrast analysis were significant considered at P ≤ 0.1, P ≤ 0.05, and P ≤ 0.001.
Table 1.
Descriptions of contrast types and the corresponding treatment means compared in the main study. Eggs were injected at 18 D of incubation with vitamin D3 (D3) or 25-hydroxycholecalciferol (25OHD3) alone at 0.6, 1.2, and 2.4 μg dosages or in combination at 1.2, 2.4, and 4.8 μg dosages.
| Contrast type | Treatment means compared |
|---|---|
| Vitamin D3 types across dosage | |
| D3 alone compared with 25OHD3 alone | D3 vs. 25OHD3 |
| D3 alone compared with the combination of D3 and 25OHD3 | D3 vs. D3 + 25OHD3 |
| 25OHD3 alone compared with the combination of D3 and 25OHD3 | 25OHD3 vs. D3 + 25OHD3 |
| Dosage across vitamin D3 types | |
| 0.6 μg compared with 1.2 μg | 0.6 vs. 1.2 |
| 0.6 μg compared with 2.4 μg | 0.6 vs. 2.4 |
| 0.6 μg compared with 4.8 μg | 0.6 vs. 4.8 |
| 1.2 μg compared with 2.4 μg | 1.2 vs. 2.4 |
| 1.2 μg compared with 4.8 μg | 1.2 vs. 4.8 |
| 2.4 μg compared with 4.8 μg | 2.4 vs. 4.8 |
Results and disscussion
Preliminary Experiment
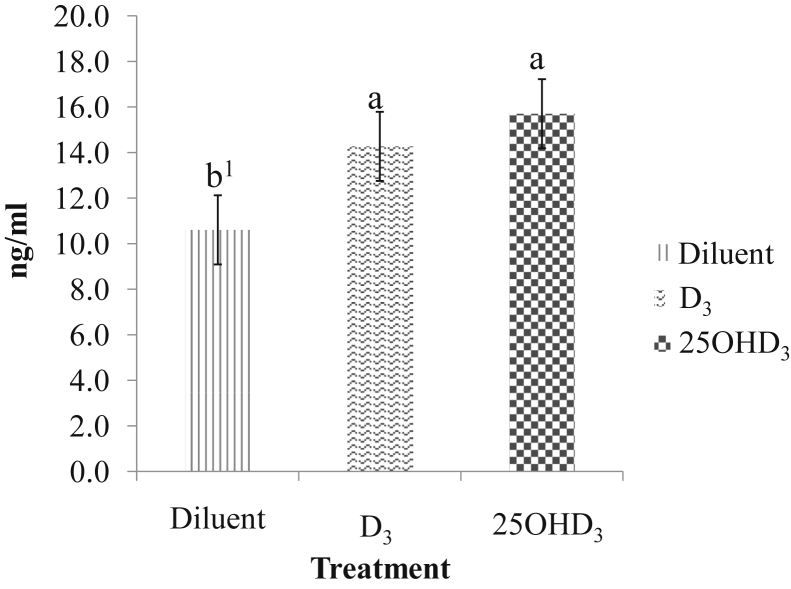
In the preliminary trial, it was observed that the injection of 1.2 μg of either D3 or 25OHD3 significantly increased serum levels of 25OHD3 in the embryos at 19.25 doi (Figure 1). These results indicate that the in ovo injection of 1.2 μg of either D3 or 25OHD3 is capable of increasing the circulating levels of 25OHD3 in broiler embryos. No significant differences were observed between treatments for HI (90.6% ± 2.68, diluent; 83.3% ± 10.67, D3; and 90.3% ± 4.76, 25OHD3) and hatchling BW (40.2 g ± 1.47, diluent; 42.3 g ± 1.17, D3; and 42.0 g ± 0.49, 25OHD3). The low HI values in the preliminary trial were likely a result of the small number of units of treatment replication, and the low HI in 1 replicate unit of the D3-injected treatment skewed the mean of that treatment. Consequently, with the large amount of variation among the units of replication, nonsignificant differences in HI and their corresponding SEM were noted between treatments. A larger scale study with greater numbers of replicate units per treatment may be required to reveal possible treatment differences for HI. Therefore, this issue was addressed in the main study.
Figure 1.
Serum 25-hydroxylvitamin D3 (25OHD3) concentrations at 19.25 D of incubation in embryos that received 50 μl of in ovo-injected diluent, 50 μl of diluent containing 1.2 μg of vitamin D3 (D3) or 1.2 μg of 25OHD3. a,bMeans with no common superscript differ significantly (P ≤ 0.05). 1SD bar.
Main Experiment
The sites of injections in the main study were confirmed to be 2.27, 93.18, and 4.55% in the air cell, amnion, and embryo, respectively. Embryonic serum 25OHD3 concentrations were lower in embryos in the 0.6 μg of D3 treatment and in the diluent-injected and noninjected control groups in comparison with all other treatments (Table 2). Across injection dosage, serum 25OHD3 concentrations were greater in embryos that received 25OHD3 alone or in combination with D3 than those that received D3 alone (Table 3). In addition, across source of vitamin D3, serum 25OHD3 concentrations were greater in embryos that were injected at dosages equal to or greater than 1.2 μg (Table 3). There were no significant treatment effects on BW, yolk-free BW, RLW, or relative yolk sac weight at 516 hoi or on HI at 492 and 516 hoi (Table 4).
Table 2.
Serum 25-hydroxycholecalciferol (25OHD3) concentrations of embryos at 462 h of incubation (hoi) after in ovo injection of a 50 μl solution volume at 432 hoi.
| Treatment | Serum 25OHD3 concentration (ng/mL) |
|---|---|
| Noninjected | 8.61b |
| Diluent1 | 9.74b |
| Vitamin D32 | |
| 0.6 | 8.83b |
| 1.2 | 11.24a |
| 2.4 | 11.60a |
| 25OHD33 | |
| 0.6 | 10.95a |
| 1.2 | 12.10a |
| 2.4 | 12.29a |
| Vitamin D3 + 25OHD34 | |
| 1.2 | 11.41a |
| 2.4 | 10.96a |
| 4.8 | 12.20a |
| Source of variation | |
| Pooled SEM | 0.301 |
| P-value | 0.001 |
a,bMeans within control, vitamin D3, 25OHD3, and vitamin D3 + 25OHD3 categories with no common superscript differ significantly (P < 0.05).
Eggs injected with 50 μl commercial MD diluent at 432 hoi.
Eggs injected with 50 μl commercial MD diluent containing vitamin D3 at 0.6, 1.2, and 2.4 μg at 432 hoi.
Eggs injected with 50 μl commercial MD diluent containing 25OHD3 at 0.6, 1.2, and 2.4 μg at 432 hoi.
Eggs injected with 50 μl commercial MD diluent containing a combination of D3 and 25OHD3 at 1.2, 2.4, and 4.8 μg at 432 hoi.
Table 3.
Contrast analyses for serum 25-hydroxycholecalciferol (25OHD3) concentrations at 462 h of incubation (hoi), postinjection hatchability of live embryonated eggs at 492 and 516 hoi, and hatchling somatic variables. Contrasts included vitamin D3 (D3), 25OHD3, and their combination across injection and dosages (0.6, 1.2, 2.4, and 4.8 μg across vitamin D3 types).
| Serum 25OHD31 | H-4922 | H-5163 | BW4 | YFBW5 | RLW6 | RYW7 | PYM8 | PYDM9 | |
|---|---|---|---|---|---|---|---|---|---|
| Contrasts of vitamin D3 types across dosage | |||||||||
| D3 vs. 25OHD3 | ∗∗∗ | ns | ns | ns | ns | ns | ns | ∗∗ | ∗∗ |
| D3 vs. D3 + 25OHD3 | ∗∗∗ | † | ns | ns | ns | ns | ns | ns | ns |
| 25OHD3 vs. D3 + 25OHD3 | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| Contrasts of dosages across vitamin D3 types | |||||||||
| 0.6 vs. 1.2 | ∗∗∗ | ns | ns | ns | ns | ns | ns | ns | ns |
| 0.6 vs. 2.4 | ∗∗∗ | ∗ | ns | ns | ns | ns | ns | ns | ns |
| 0.6 vs. 4.8 | ∗∗∗ | ∗ | ns | ns | ns | ns | ns | † | † |
| 1.2 vs. 2.4 | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| 1.2 vs. 4.8 | ns | ns | ns | ns | ns | † | ns | ∗ | ∗ |
| 2.4 vs. 4.8 | ns | ns | ns | ns | ns | ∗∗ | ns | ns | ns |
†Treatment means for the same variable with no common superscript differ significantly (P ≤ 0.1).
∗Treatment means for the same variable with no common superscript differ significantly (P ≤ 0.05).
∗∗Treatment means for the same variable with no common superscript differ significantly (P ≤ 0.01).
∗∗∗Treatment means for the same variable with no common superscript differ significantly (P ≤ 0.005).
Abbreviation: ns = not significant.
Serum concentration of 25OHD3 was determined at 462 hoi.
Hatchability of live embryonated eggs at 492 hoi.
Hatchability of live embryonated eggs at 516 hoi.
Hatching BW at 516 hoi.
Yolk-free BW at 516 hoi.
Relative liver weight at 516 hoi.
Relative yolk sac weight at 516 hoi.
Percentage of yolk moisture at 516 hoi.
Percentage of yolk dry matter at 516 hoi.
Table 4.
Hatchability of live embryonated eggs at 492 (H-492) and 516 (H-516) h of incubation (hoi) and hatchling BW, yolk-free BW (YFBW), relative yolk sac weight (RLW), percentage of yolk moisture (RYM), and percentage of yolk dry matter (PYDM) in noninjected and diluent-injected (50 μL) control groups and eggs injected with diluent containing 0.6, 1.2, 2.4, or 4.8 μg of vitamin D3 (D3) or 25-hydroxycholecalciferol (25OHD3) alone or in combination in the main study.
| H-492 | H-516 | BW | YFBW | RLW | RYW | PYM | PYDM | |
|---|---|---|---|---|---|---|---|---|
| -----%------- | ------G------ | -------------%------------- | ||||||
| Treatment | ||||||||
| Noninjected | 90.35 | 97.55 | 41.74 | 40.73 | 2.490 | 11.34 | 46.60a | 53.40c |
| Diluent1 | 83.35 | 94.19 | 42.75 | 41.93 | 2.191 | 11.84 | 41.58a,b | 58.42a,b |
| D32 | ||||||||
| 0.6 | 75.96 | 96.76 | 42.46 | 41.61 | 2.28 | 12.49 | 43.55a | 56.46b |
| 1.2 | 84.45 | 94.81 | 41.18 | 40.45 | 2.351 | 12.75 | 43.35a | 56.65b |
| 2.4 | 90.01 | 95.29 | 44.65 | 42.28 | 2.471 | 12.45 | 42.69a | 57.31b |
| 25OHD33 | ||||||||
| 0.6 | 83.5 | 94.71 | 42.18 | 40.80 | 2.212 | 10.18 | 31.93a,b | 68.07a,b |
| 1.2 | 86.82 | 96.8 | 42.50 | 41.73 | 2.142 | 12.20 | 31.26c | 68.74a |
| 2.4 | 92.20 | 94.81 | 41.23 | 41.21 | 2.431 | 12.56 | 37.34a,b | 62.66a,b |
| D3 + 25OHD34 | ||||||||
| 1.2 | 89.5 | 97.51 | 42.15 | 41.21 | 2.251 | 11.56 | 41.55a,b | 58.46a,b |
| 2.4 | 87.8 | 94.11 | 41.58 | 39.75 | 2.361 | 12.28 | 33.00b | 66.00a |
| 4.8 | 93.94 | 95.7 | 43.21 | 41.44 | 1.970 | 12.50 | 40.04a,b | 59.95a,b |
| Source of variation | ||||||||
| Pooled SEM | 0.391 | 0.495 | 1.039 | 1.127 | 0.144 | 1.19 | 3.669 | 3.669 |
| P-value | 5.236 | 1.321 | 0.499 | 0.938 | 0.320 | 0.679 | 0.030 | 0.030 |
a,bTreatment means for the same variable with no common superscript differ significantly (P < 0.05).
Eggs injected with 50 μl commercial diluent at 432 hoi.
Eggs injected with 50 μl commercial diluent containing vitamin D3 at 0.6, 1.2, and 2.4 μg/egg at 432 hoi.
Eggs injected with 50 μl commercial diluent containing 25OHD3 at 0.6, 1.2, and 2.4 μg/egg at 432 hoi.
Eggs injected with 50 μl commercial diluent containing a combination of D3 and 25OHD3 at 1.2, 2.4, and 4.8 μg/egg at 432 hoi.
Across vitamin D3 source, HI at 492 hoi was greater for chicks that received 2.4 or 4.8 μg dosages than those received the 0.6 μg dosage (Table 3). In addition, the 25OHD3 and D3 treatment combination resulted in a higher HI at 492 hoi than the injection of D3 alone across dosage level. Across vitamin D3 source, birds that received the 4.8 μg dosage had a significantly greater RLW than those that received the 2.4 μg dosage, and birds that received the 4.8 μg dosage tended (P = 0.064) to have a greater RLW than those that received the 1.2 μg dosage (Table 3). Across injection dose, the in ovo injection of 25OHD3 alone resulted in a higher PYDM and a lower PYM of the broiler chicks at hatch in comparison with those that received D3 alone (Table 3). Across vitamin D3 source, the 4.8 μg dose resulted in a lower PYDM and a higher PYM in comparison with the 2.4 μg dose (Table 3). Chicks in the noninjected control group had a lower PYDM and higher PYM as compared with those in any of the other treatment groups. In addition, those that received 1.2 or 2.4 μg of D3 alone had a significantly lower PYDM and higher PYM relative to those that received 1.2 μg of 25OHD3 or the combination of D3 and 25OHD3 at 2.4 μg (Table 4). There were no significant treatment effects for any of the hatch residue variables, but embryonic mortalities in eggs that were administered a 2.4 μg dosage of either D3 or 25OHD3 alone tended (P = 0.099) to have fewer late dead mortalities than those that received 1.2 μg of either D3 or 25OHD3 alone (Table 5).
Table 5.
Effects of in ovo injection treatment (noninjected, diluent injected, and injected with diluent containing vitamin D3 [D3] or 25-hydroxycholecalciferol [25OHD3] or the combination of D3 + 25OHD3 [50 μL]) and their dosages, on hatch residue analysis variables (late dead embryo, pipped dead and live embryo, and dead chick at 516 h of incubation (hoi).
| Late dead1 | Pipped dead2 | Pipped live3 | Dead chick4 | |
|---|---|---|---|---|
| -------------------- (%) ----------------------------- | ||||
| Treatment | ||||
| Noninjected | 3.44 | 0.34 | 0 | 0 |
| Diluent5 | 3.83 | 1.35 | 0 | 1.02 |
| D36 | ||||
| 0.6 | 3.90 | 0.35 | 0.36 | 0.37 |
| 1.2 | 5.40 | 0.34 | 0 | 0.38 |
| 2.4 | 2.16 | 1.80 | 1.03 | 0.34 |
| 25OHD37 | ||||
| 0.6 | 4.17 | 0.74 | 0.66 | 1.03 |
| 1.2 | 5.48 | 0.35 | 0.36 | 0.36 |
| 2.4 | 1.71 | 1.03 | 0.68 | 1.03 |
| D3 + 25OHD38 | ||||
| 1.2 | 4.48 | 0 | 0 | 0 |
| 2.4 | 6.48 | 0.69 | 0 | 0.35 |
| 4.8 | 3.17 | 1.06 | 0 | 1.39 |
| Source of variation | ||||
| Pooled SEM | 0.099 | 0.529 | 0.149 | 0.424 |
| P-value | 1.114 | 0.404 | 0.322 | 0.473 |
Dead embryos that had not externally pipped at 516 hoi.
External pipped dead (chick pipped shell and was dead) at 516 hoi.
External pipped alive (chick pipped shell and was alive) at 516 hoi.
Dead chicks that were found at 516 hoi.
Eggs injected with 50 μl commercial diluent at 432 hoi.
Eggs injected with 50 μl commercial diluent containing vitamin D3 at 0.6, 1.2, and 2.4 μg/egg at 432 hoi.
Eggs injected with 50 μl commercial diluent containing 25OHD3 at 0.6, 1.2, and 2.4 μg/egg at 432 hoi.
Eggs injected with 50 μl commercial diluent containing a combination of D3 and 25OHD3 at 1.2, 2.4, and 4.8 μg/egg at 432 hoi.
The objective of the main study was to investigate the effects of the in ovo injection of various levels of 2 vitamin D3 sources on broiler hatchability and hatchling characteristics. The importance and requirement of vitamin D3 for chicken embryonic development is well known, and the presence of vitamin D3 in eggs is very important in the support of embryo calcium metabolism during incubation (Narbaitz, 1987). A deficiency in vitamin D3 has further been shown to reduce hatchability and increase late embryo mortality (Stevens et al., 1984). During the last stage of embryonic growth, calcium is mainly absorbed from the yolk, as only small amounts of calcium are absorbed directly from the eggshell (Noy and Sklan, 2001). Vitamin D increases yolk calcium mobilization by increasing the level of vitamin D–dependent calcium-binding protein and calbindin-D28K in the yolk sac (Tuan and Suyama, 1996). The maximum activity of 1-α hydroxylase during embryogenesis is observed at 17 doi and dramatically decreases between 19 doi and hatch (Turner et al., 1987). In addition, it has been suggested that the activity of 25-hydroxylase is low during the first 10 D of posthatch life owing to a lack in the conversation D3 to 25OHD3 during that period (Saunders-Blades and Korver, 2014). Consequently, altered serum 25OHD3 levels in broilers have not been observed to occur in response to dietary D3 supplementation at 2,500 IU/kg of feed. Effects on early posthatch broiler performance have subsequently not been observed (Saunders-Blades and Korver, 2014). In comparison with D3, 25OHD3 has a longer half-life, which is approximately 2–3 wk in duration (Smith and Goodman, 1971). Conversely, the half-life of D3 is only approximately 12–24 h (Smith and Goodman, 1971, Haddad et al., 1993). In addition, as compared with dietary D3 at the same level of inclusion, 25OHD3 is mainly stored in the liver, as well as in white and red muscles in pigs (Burild et al., 2016). These results indicate that 25OHD3 persists for a much longer period of time in the blood, which provides adequate time for it to subsequently be converted to the active form vitamin D3 or for it to be stored for later usage.
Effects of the in ovo injection of 25OHD3 alone at various dosage levels on the hatchability, hatching chick quality, and the posthatch production and performance of broilers have been investigated (Gonzales et al., 2013, Bello et al., 2013, Bello et al., 2014a, Bello et al., 2014b, Bello et al., 2014c, Bello et al., 2015, Mansour et al., 2017). However, those effects on the broiler embryo have not been investigated when D3 is administrated at 18 doi by amniotic in ovo injection alone or in combination with 25OHD3. It is well documented that the dietary or in ovo use of 25OHD3 increases serum 25OHD3 concentrations in broiler embryos and hatchlings (Bello et al., 2013, Saunders-Blades and Korver, 2014). Similar to that of these previous studies, various levels of injected 25OHD3 alone or in combination with D3 increased serum 25OHD3 concentrations in the broiler embryos of the present study. However, a 1.2 μg level or higher of D3 was required to cause an increase in serum 25OHD3 concentrations in comparison with that of noninjected or diluent-injected controls.
In agreement with the current results, Gonzales et al. (2013) similarly reported that the in ovo injection of 25OHD3 did not affect overall hatchability. Conversely, Bello et al. (2013) observed that the in ovo injection of 25OHD3 increased HI at 20 and 21 doi as compared with diluent-injected controls. The difference in 25OHD3 sources and volumes of injection that were used in the present study and in the study by Bello et al. (2013) may be the basis for the inconsistencies in their results. An in ovo injection of a 100 μl volume of solution in which the crystalline form of 25OHD3 was suspended was used in the study performed by Bello et al. (2013). However, 50 μl volumes of solutions containing water-soluble forms of both vitamin D3 sources were in ovo injected in the present study. Another reason for the discrepancy in the results of the studies may be related to their very different HI percentages that were observed in the diluent-injected control groups. The diluent-injected treatment group in this study resulted in an 83% HI in comparison with a 90% HI in the 25OHD3-injected treatment groups in the study conducted by Bello et al. (2013). Furthermore, HI was 94% for the diluent-injected control groups in the present study, which was in the same range as that for the groups that received in ovo injections of the 2 vitamin D3 sources (Table 4).
The practice of in ovo injection of 25OHD3 or D3 alone or in combination has not been examined in the past. However, the dietary combination of D3 with 25OHD3 increased BW, bone Ca, and P contents (Papešová et al., 2008) and increased bone mineralization (Fritts and Waldroup, 2003), protein synthesis, and satellite cell activity and size in broiler chickens (Hutton et al., 2014). The novel observation in this study was that across level of injection, a combination of D3 and 25OHD3 increased HI at 492 hoi in comparison with D3 alone. In ovo injection of the D3 and 25OHD3 combination could, therefore, be more effective in increasing HI in comparison with D3 alone. The reason for this incremental increase in HI at 492 hoi in response to the in ovo injection of the combination of D3 and 25OHD3 in comparison with D3-injected embryos is not clear. Further study is required to determine the possible synergic effects between these in ovo-injected vitamin D3 sources on the neonatal performance of broilers.
Broilers are resistant to high levels of dietary D3 (50,000 IU per kg of feed) and suffer no apparent negative effects on their growth and the mineralization of their bones (Baker et al., 1998). However, hypervitaminosis of vitamin D3 was reported when broilers were fed D3 at 2.5 mg/kg of BW, which is equivalent to 100,000 IU (Morrissey et al., 1977). Vitamin D3 toxicity in chickens leads to the deposition of calcium in the soft tissues, resulting in renal tubular calcification, reduced performance, and reduced egg production (NRC, 1987; Terry et al., 1999). It has been established that elevated levels of 1,25(OH)2D3 can retard mineral deposition and can reduce cell survival and liver function (Pande et al., 2015). In an in vitro study, 2.4 and 24 mmol dosages of 1,25(OH)2D3 resulted in decreased cell proliferation and mineral deposition in chicken bone marrow–derived mesenchymal stem cells (Pande et al., 2015). However, in this study, the in ovo injection of a combination of D3 and 25OHD3 at a more moderate but relatively high dosage (4.8 μg) resulted in no negative effects on HI and chick quality as compared with diluent-injected and noninjected control groups. However, effects of the in ovo injection of vitamin D3 sources at levels higher than 4.8 μg have not been tested to determine their possible effects on posthatch performance. Bello et al. (2013) reported that broiler hatching eggs that received in ovo injections of 5.4 μg of 25OHD3 resulted in broilers having higher PYM and lower PYDM values in comparison with those that received treatment levels that ranged between 0.3 and 1.2 μg. In addition, in ovo injection of 0.6 μg of 25OHD3 has been shown to increase PYM (Bello et al., 2013) and bone quality (Bello et al., 2014b) in broilers in comparison with those injected with diluent alone. Similarly, the 2 vitamin D3 sources used together at higher doses of injection in the present study resulted in a higher PYM and a lower PYDM in broilers in comparison with those in response to 0.60 and 1.20 μg levels. A higher PYM may indicate that the in ovo injection of D3 or 25OHD3 alone or in combination at 4.8 μg or higher may negatively affect chick quality at hatch and their posthatch performance.
In conclusion, these findings showed that when in ovo injected, D3 and 25OHD3 at dosages between 0.60 to 4.8 μg alone or in combination increased the serum 25OHD3 concentrations of broiler embryos in comparison with those belonging to noninjected and diluent-injected control groups. Nevertheless, these vitamin D3 sources at those dosages did not affect the HI and hatchling BW of the broilers. The subsequent increased circulating levels of 25OHD3 may have the potential to improve broiler performance. However, the combination of the D3 and 25OHD3 at the 4.8 μg level resulted in an increased RLW and PYM and reduced PYDM, which may lead to a subsequent reduction in posthatch chick performance. Further research is needed to determine the effects of the various vitamin D3 sources alone or in combination on posthatch broiler performance and meat yield.
Acknowledgments
This publication is a contribution of the Mississippi Agriculture and Forestry Experiment Station. This material is based upon work that is supported by the National Institute of Food and Agriculture, United States Department of Agriculture, Hatch project under accession number 329260. Use of trade names in this publication does not imply endorsement by Mississippi Agricultural and Forestry Experiment Station of these products, nor similar ones not mentioned.
The authors express their appreciation for the financial support of the United States Department of Agriculture (USDA grant no. 58-6406-4-016), DSM Nutritional Products Inc., Zoetis Animal Health Co., Merial Select Inc., and for the assistance of the graduate and undergraduate students of the Mississippi State University Poultry Science Department. Special also thanks to Dr. Bradley Turner, Dr. April Waguespack Levy, and Dr. David Smith for their invaluable assistance.
Conflict of Interest Statement: The authors did not provide a conflict of interest statement.
References
- Avakian A.P. Understanding in ovo vaccination. Int. Hatchery Pract. 2006;20:15–17. [Google Scholar]
- Baker D.H., Biehl R.R., Emmert J.L. Vitamin D3 requirement of young chicks receiving diets varying in calcium and available phosphorus. Br. Poult. Sci. 1998;39:413–417. doi: 10.1080/00071669888980. [DOI] [PubMed] [Google Scholar]
- Bar A., Sharvit M., Noff D., Edelstein S., Hurwitz S. Absorption and excretion of cholecalciferol and of 25-hydroxycholecalciferol and metabolites in birds. J. Nutr. 1980;110:1930–1934. doi: 10.1093/jn/110.10.1930. [DOI] [PubMed] [Google Scholar]
- Bello A., Zhai W., Gerard P.D., Peebles E.D. Effects of the commercial in ovo injection of 25-hydroxycholecalciferol on the hatchability and hatching chick quality of broilers. Poult. Sci. 2013;92:2551–2559. doi: 10.3382/ps.2013-03086. [DOI] [PubMed] [Google Scholar]
- Bello A., Bricka R.M., Gerard P.D., Peebles E.D. Effects of commercial in ovo injection of 25-hydroxycholecalciferol on broiler bone development and mineralization on days 0 and 21 posthatch. Poult. Sci. 2014;93:1053–1058. doi: 10.3382/ps.2013-03608. [DOI] [PubMed] [Google Scholar]
- Bello A., Hester P.Y., Gerard P.D., Zhai W., Peebles E.D. Effects of commercial in ovo injection of 25-hydroxycholecalciferol on bone development and mineralization in male and female broilers12. Poult. Sci. 2014;93:2734–2739. doi: 10.3382/ps.2014-03981. [DOI] [PubMed] [Google Scholar]
- Bello A., Zhai W., Gerard P.D., Peebles E.D. Effects of the commercial in ovo injection of 25-hydroxycholecalciferol on broiler posthatch performance and carcass characteristics. Poult. Sci. 2014;93:155–162. doi: 10.3382/ps.2013-03389. [DOI] [PubMed] [Google Scholar]
- Bello A., Nascimento M., Pelici N., Womack S.K., Zhai W., Gerard P.D., Peebles E.D. Effects of the in ovo injection of 25-hydroxycholecalciferol on the yolk and serum characteristics of male and female broiler embryos. Poult. Sci. 2015;94:734–739. doi: 10.3382/ps/pev017. [DOI] [PubMed] [Google Scholar]
- Burild A., Lauridsen C., Faqir N., Sommer H.M., Jakobsen J. Vitamin D3 and 25-hydroxyvitamin D3 in pork and their relationship to vitamin D status in pigs. J. Nutr. Sci. 2016;5:e3–e9. doi: 10.1017/jns.2015.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embrex, Inc. Embrex, Inc.; Research Triangle Park, NC: 2002. Manual in Ovo Vaccination Guide. IOM-001/2.1. [Google Scholar]
- Ernst R.A., Bradley F.A., Abbott U.K., Craig R.M. University of California, Division of Agriculture and Natural Resources; 2004. Egg Candling and Breakout Analysis for Hatchery Quality Assurance and Analysis of Poor Hatches; pp. 1–9. pub. 8134. [Google Scholar]
- Fritts C.A., Waldroup P.W. Effect of source and level of vitamin D on live performance and bone development in growing broilers. J. Appl. Poult. Res. 2003;12:45–52. [Google Scholar]
- Gonzales E., Cruz C.P., Leandro N.S.M., Stringhini J.H., Brito A.B. In ovo supplementation of 25(OH)D3 to broiler embryos. Rev. Bras. Cienc. Avic. 2013;15:199–202. [Google Scholar]
- Haddad J.G., Matsuoka L.Y., Hollis B.W., Hu Y.Z., Wortsman J. Human plasma transport of vitamin D after its endogenous synthesis. J. Clin. Invest. 1993;91:2552–2555. doi: 10.1172/JCI116492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry H.L. Measurement of the chicken kidney 25-hydroxyvitamin D3 1-hydroxylase and 25-hydroxyvitamin D3 24-hydroxylase. Methods Enzymol. 1980;67:445–449. doi: 10.1016/s0076-6879(80)67054-2. [DOI] [PubMed] [Google Scholar]
- Hollis B.W., Kamerud J.O., Selvaag S.R., Lorenz J.D., Napoli J.L. Determination of vitamin D status by radioimmunoassay with a 1251-labeled tracer. Clin. Chem. 1993;39:529–533. [PubMed] [Google Scholar]
- Hollis B.W., Wagner C.L. The role of the parent compound vitamin D with respect to metabolism and function: why clinical dose intervals can affect clinical outcomes. J. Clin. Endocrinol. Metab. 2013;98:4619–4628. doi: 10.1210/jc.2013-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton K.C., Vaughn M.A., Litta G., Turner B.J., Starkey J.D. Effect of vitamin D status improvement with 25-hydroxycholecalciferol on skeletal muscle growth characteristics and satellite cell activity in broiler chickens. J. Anim. Sci. 2014;92:3291–3299. doi: 10.2527/jas.2013-7193. [DOI] [PubMed] [Google Scholar]
- Mansour D.S., El-Senosi Y.A., Mohamed M.I., Amer M.M., Elaroussi M.A. Effects of injecting vitamin D3 or an active metabolite in ovo on chick embryonic development and calcium homeostasis. W. J. Pharm. Pharm. Sci. 2017;6:1454–1467. [Google Scholar]
- Morris A., Shanmugasundaram R., Lilburn M.S., Selvaraj R.K. 25-hydroxycholecalciferol supplementation improves growth performance and decreases inflammation during an experimental lipopolysaccharide injection. Poult. Sci. 2014;93:1951–1956. doi: 10.3382/ps.2014-03939. [DOI] [PubMed] [Google Scholar]
- Morrissey R.L., Cohn R.M., Empson R.N., Jr., Greene H.L., Taunton O.D., Ziporin Z.Z. Relative toxicity and metabolic effects of cholecalciferol and 25-hydroxycholecalciferol in chicks. J. Nutr. 1977;107:1027–1034. doi: 10.1093/jn/107.6.1027. [DOI] [PubMed] [Google Scholar]
- Narbaitz R., Tsang C.P., Grunder A.A. Effects of vitamin D deficiency in the chicken embryo. Calcif. Tissue Int. 1987;40:109–113. doi: 10.1007/BF02555714. [DOI] [PubMed] [Google Scholar]
- Noy Y., Sklan D. Yolk and exogenous feed utilization in the post-hatch chicks. Poult. Sci. 2001;80:1490–1495. doi: 10.1093/ps/80.10.1490. [DOI] [PubMed] [Google Scholar]
- NRC. Natl. Acad. Press; Washington, DC: 1987. Vitamin Tolerance of Animals. [Google Scholar]
- Pande V.V., Chousalkar K.C., Bhanugopan M.S., Quinn J.C. Super pharmacological levels of calcitriol (1,25-(OH)2D3) inhibits mineral deposition and decreases cell proliferation in a strain dependent manner in chicken mesenchymal stem cells undergoing osteogenic differentiation in vitro. Poult. Sci. 2015;94:2784–2796. doi: 10.3382/ps/pev284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papešová L., Fučíková A., Pípalová M., Tupý P. The synergic effect of vitamin D3 and 25-hydroxycholecalciferol/calcidiol in broiler diet. Sci. Acta Boch. 2008;3:273–277. [Google Scholar]
- Peebles E.D., Cheaney J.D., Vaughn K., Latour M.A., Smith T.W., Haynes R.L., Boyle C.R. Changes in gonadal weights, serum lipids and glucose during maturation in the juvenile Northern Bobwhite quail (Colinus virginianus) Poult. Sci. 1996;75:1411–1416. doi: 10.3382/ps.0751411. [DOI] [PubMed] [Google Scholar]
- Rama-Rao S.V., Raju M.V.L.N., Panda A.K., Shyam Sunder G., Sharma R.P. Effect of high concentrations of cholecalciferol on growth, bone mineralization, and mineral retention in broiler chicks fed suboptimal concentrations of calcium and nonphytate phosphorus. J. Appl. Poult. Res. 2006;15:493–501. [Google Scholar]
- Salmanzadeh M. The effects of in-ovo injection of glucose on hatchability, hatching weight and subsequent performance of newly-hatched chicks. Rev. Bras. Cienc. Avic. 2012;41:71–158. [Google Scholar]
- Saunders-Blades J., Korver D.R. The effect of maternal vitamin D source on broiler hatching egg quality, hatchability, and progeny bone mineral density and performance. Poult. Sci. 2014;23:773–783. doi: 10.3382/ps/pev002. [DOI] [PubMed] [Google Scholar]
- Smith J.E., Goodman D.S. The turnover and transport of vitamin D and of a polar metabolite with the properties of 25-hydroxycholecalciferol in human plasma. J. Clin. Invest. 1971;50:2159–2167. doi: 10.1172/JCI106710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel R.G.D., Torrie J.H. 2nd ed. McGraw-Hill; New York, NY: 1980. Principles and Procedures of Statistics. A Biometrical Approach. [Google Scholar]
- Stevens V.I., Blair R., Salmon R.E., Stevens J.P. Effect of varying levels of dietary vitamin D3 on Turkey hen egg production, fertility and hatchability, embryo mortality and incidence of embryo malformations. Poult. Sci. 1984;63:760–764. doi: 10.3382/ps.0630760. [DOI] [PubMed] [Google Scholar]
- Terry M., Lanenga M., McNaughton J.L., Stark L.E. Safety of 25-hydroxyvitamin D3 as a source of vitamin D3 in layer poultry feed. Vet. Hum. Toxicol. 1999;41:312–316. [PubMed] [Google Scholar]
- Tuan R.S., Suyama E. Developmental expression and vitamin D regulation of calbindin-D28K in chick embryonic yolk sac endoderm. J. Nutr. Apr. 1996;126:1308S–1316S. [PubMed] [Google Scholar]
- Turner R.T., Graves J.S., Bell N.H. Regulation of 25-hydroxyvitamin D3 metabolism in chick embryo. Am. J. Physiol. 1987;252:E38–E43. doi: 10.1152/ajpendo.1987.252.1.E38. [DOI] [PubMed] [Google Scholar]
- Vignale K., Greene E.S., Caldas J.V., England J., Boonsinchai N., Sodsee P., Pollock E.D., Dridi S., Coon C.N. 25-Hydroxycholecalciferol enhances male broiler breast meat yield through the mTOR pathway. J. Nutr. 2015;145:855–863. doi: 10.3945/jn.114.207936. [DOI] [PubMed] [Google Scholar]
- Williams C.J. In ovo vaccination for disease prevention. Int. Poult. Prod. 2007;15:7–9. [Google Scholar]
- Williams C.J. In ovo vaccination and chick quality. Int. Hatch. Prac. 2011;19:7–13. [Google Scholar]
- Yarger J.G., Sunders C.A., McNaughton J.L., Quarles C.L., Hollis B.W., Gray R.W. Comparison of dietary 25-hydroxycholecalciferol and cholecalciferol in broiler chickens. Poult. Sci. 1995;74:1159–1167. doi: 10.3382/ps.0741159. [DOI] [PubMed] [Google Scholar]
- Zhang H., Elliott K.E.C., Durojaye O.A., Fatemi S.A., Peebles E.D. Effects of in ovo administration of L-ascorbic acid on broiler hatchability and its influence on the effects of pre-placement holding time on broiler quality characteristics. Poult. Sci. 2018;97:1941–1947. doi: 10.3382/ps/pey040. [DOI] [PubMed] [Google Scholar]